Abstract
The autonomic nervous system (ANS) is profoundly affected by high intensity exercise. However, evidence is less clear on ANS recovery and function following prolonged bouts of high intensity exercise, especially in non-endurance athletes. Therefore, this study aimed to investigate the relationships between duration and intensity of acute exercise training sessions and ANS recovery and function in Division I football athletes. Fifty, male football athletes were included in this study. Subjects participated in 135 days of exercise training sessions throughout the 25-week season and wore armband monitors (Warfighter Monitor, Tiger Tech Solutions) equipped with electrocardiography capabilities. Intensity was measured via heart rate (HR) during an ‘active state’, defined as HR ≥ 85 bpm. Further, data-driven intensity thresholds were used and included HR < 140 bpm, HR < 150 bpm, HR < 160 bpm, HR ≥ 140 bpm, HR ≥ 150 bpm and HR ≥ 160 bpm. Baseline HR and HR recovery were measured and represented ANS recovery and function 24h post-exercise. Linear regression models assessed the relationships between time spent at the identified intensity thresholds and ANS recovery and function 24h post-exercise. Statistical significance set at α < 0.05. Athletes participated in 128 training sessions, totaling 2735 data points analyzed. Subjects were predominantly non-Hispanic black (66.0%), aged 21.2 (±1.5) years and average body mass index of 29.2 (4.7) kg⋅(m2)−1. For baseline HR, statistically significant associations between duration and next-day ANS recovery were observed at HR < 140 bpm (β = −0.08 ± 0.02, R2 = 0.31, p < 0.001), HR above 150 and 160 bpm intensity thresholds (β = 0.25 ± 0.02, R2 = 0.69, p < 0.0000 and β = 0.59 ± 0.06, R2 = 0.71, p < 0.0000). Similar associations were observed for HR recovery: HR < 140 bpm (β = 0.15 ± 0.03, R2 = 0.43, p < 0.0000) and HR above 150 and 160 bpm (β = −0.33 ± 0.03, R2 = 0.73, p < 0.0000 and β = −0.80 ± 0.06, R2 = 0.71, p < 0.0000). The strengths of these associations increased with increasing intensity, HR ≥ 150 and 160 bpm (baseline HR: β range = 0.25 vs 0.59, R2: 0.69 vs 0.71 and HR recovery: β range = −0.33 vs −0.80, R2 = 0.73 vs 0.77). Time spent in lower intensity thresholds, elicited weaker associations with ANS recovery and function 24h post-exercise, with statistical significance observed only at HR < 140 bpm (β = −0.08 ± 0.02, R2 = 0.31, p < 0.001). The findings of this study showed that ANS recovery and function following prolonged high intensity exercise remains impaired for more than 24h. Strength and conditioning coaches should consider shorter bouts of strenuous exercise and extending recovery periods within and between exercise training sessions.
Keywords: Exercise training, Overtraining, Sports, Strength, Conditioning, Collegiate football
Key Points.
-
•
The autonomic nervous system may require more than 24h of recovery following prolonged bouts of high-intensity exercise in collegiate football athletes.
-
•
The “apparent restoration” of the autonomic nervous system 24h following, prolonged less strenuous exercise may be indicative of full recovery or parasympathetic hyperactivity.
-
•
Strength and conditioning coaches must strategically design exercise training programs to allow for sufficient recovery of the autonomic nervous system, especially following prolonged bouts of high-intensity exercise.
1. Introduction
Performing high intensity exercise requires optimal function of the autonomic nervous system (ANS) [[1], [2], [3]]. This type of training is ubiquitously integrated into the exercise training programs of elite, collegiate-level athletes given the effectiveness it has on enhancing sports performance [4]. Importantly, the ANS is profoundly affected by acute bouts of high intensity exercise, requiring periods of rest to restore optimal function [[5], [6], [7]]. Sport performance professional organizations, such as the National Strength and Conditioning Association, recommend that coaches prescribe, at minimum, 24h in between high intensity exercise sessions [8,9]. Significant challenges exist however, in implementing this recommendation in sport exercise training programs as athletes often train at high frequencies (5 to 6 days/week) and long durations (>120 min/session). Unfortunately, athletes are not always given the suggested 24h of rest between intense exercise sessions. Therefore, understanding the influence of the intensity and duration of collegiate level exercise training sessions and its impact on the ANS is important to understand, especially, for sports requiring fast, powerful, and change-in-direction movements like football [10].
Well-established evidence shows an inverse relationship between intensity and duration with experts recommending shorter durations for higher intensity exercise and vice versa [[11], [12], [13]]. The suggested duration at which an individual athlete performs exercise at any given level of intensity is governed by which metabolic pathways are activated during the exercise [14], availability of energy substrates [15,16] and current fitness level [17]. At the collegiate level, exercise training programs challenge this physiological relationship with athletes performing exercise at high levels of intensity for prolonged durations, likely imposing supraphysiological disturbances [18,19]. Available studies, albeit mostly conducted among endurance-type athletes (e.g., runners, cyclists), found significant deficits in ANS function in the immediate post-exercise period (0 to 12h) following prolonged, high intensity exercise [20]. Specifically, endurance athletes experienced delayed parasympathetic reactivation [21], depressed heart rate variability [22] and prolonged central and peripheral fatigue [23]. The few studies examining ANS recovery ≥24h after prolonged bouts of high intensity exercise found most athletes were fully recovered within 72h [24]. A significant limitation of the current literature is that these studies have only been conducted on endurance athletes [25]. Thus, a void exists in the literature regarding non-endurance type sports. Endurance athletes typically sustain continuous, workloads with intermittent bouts of high intensity, utilize different skeletal muscle fiber types, rely mostly on mitochondrial respiration for energy production [8] etc. Conversely, for sports like collegiate football, athletes often perform bouts of high intensity exercise at higher frequency and execute more forceful and powerful movements within training sessions. These athletes exhibit greater skeletal muscle damage and faster substrate depletion during training sessions that typically last longer than 2h and are implemented multiple times each week [26]. As such, the impact on ANS recovery for athletes participating in more strength- and power-focused sports is unclear.
Moreover, evidence is further limited on the ANS response to subsequent exercise performance 24h post prolonged, high intensity exercise. Knowing how the ANS recovers and functions following exercise training sessions is critical to strength and conditioning coaches for designing and implementing effective training programs aimed at enhancing sports performance. The dearth of evidence on these relationships predisposes non-endurance athletes to unknown levels of ANS deterioration, functional overreaching, and overtraining [27]. Therefore, this study aimed to investigate the relationships between time spent at varying levels of intensity during acute bouts of exercise training and the influence on ANS recovery and function in Division-I football athletes. Our first and second hypotheses were that exercise performed at longer durations with higher intensities would lead to athletes eliciting higher baseline heart rates (HR) and lower HR recoveries due to reductions in ANS recovery and function. These outcomes would reflect inadequate ANS recovery and function 24h following prolonged bouts of higher intensity exercise.
2. Methods
2.1. Study design
This prospective study tracked ANS recovery and function in a sample of male, Division-I collegiate football athletes over a 25-week period. The ANS outcomes were assessed using baseline HR and HR recovery measured 24h following training. These cardiac-based metrics represented ANS recovery and function, respectively.
2.2. Subjects
Subjects were recruited from a Division I collegiate football team located in the southeastern region of the State of Florida. The athletes were participating in a 25-week, aerobic, speed, strength, agility, and power-focused training program during preseason through regulation play. The prospective participants were recruited from a pre-selected group of athletes the coaches identified as “starters”, which were athletes that competed in nearly every regulation game and for most of its duration. Fifty healthy, male football athletes participated in this study. On average the athletes were 21.2 (±1.5) years old, weighed 103.0 (±20.2) kg and were 187.5 (±6.6) cm tall. Additionally, the average body mass index was 29.2 (4.7) kg⋅(m2)−1 and ranged between 23.7 kg (m2)−1 to 44.9 kg (m2)−1. The racial/ethnic composition of the sample was 66.0% non-Hispanic black, 18.0% Caucasian, and 12.0% Hispanic. Prior to any measurements, the athletes were informed of the benefits and risks of the study and voluntarily consented to the study. All study protocols followed the ethical principles defined in the declaration of Helsinki and were approved by the university's Institutional Review Board (IRB #20191223).
2.3. Procedures
2.3.1. Exercise training sessions
Fifty football athletes completed pre- and in-season training from May through December of 2022. The off-, pre- and in-seasons lasted 27 weeks, of which, 25 weeks were broken into four separate training blocks. During the first 10 weeks (i.e., off-season), athletes participated in 2, 4-week training programs (Training Camp I and II) separated by one week of rest following each program. Next, the athletes participated in their 4-week pre-season training program (Pre-Season Camp) followed by their in-season training for the remaining 13 weeks, see Fig. 1. The duration of the exercise training sessions averaged 161.1 (±40.6) minutes and ranged from 90.1 to 339.6 min. The intensity of the exercise training sessions varied daily and between each athlete. All athletes were exposed to the same training sessions which occurred during football practices and included strength- & power-focused resistance exercises, short-distance sprint intervals, aerobic training, and agility training.
Fig. 1.
The 25-week football training and measurement schematic.
2.3.2. Intensity of acute exercise training sessions
In this study, the intensity at which the athletes performed during acute exercise training sessions was represented by the athletes' HR in an ‘active state’. An ‘active state’ was defined as HR values measuring above 85 beats per minute (bpm). Periods where HR values ≤ 85 bpm, were defined as “non-active”. The times spent in “non-active” states were excluded from the analyses. Importantly, this study did not impose any categorical expressions of exercise intensity previously established in the scientific literature (e.g., % HR reserve, %HRmax, %VO2reserve, %VO2max). These intensity thresholds rely on many assumptions likely not applicable to this sample of collegiate athletes [[28], [29], [30]]. The continuous expression of intensity used in this study allowed for increased flexibility and factoring in of inter-individual variability [31]. Importantly, the exercise intensity metric factored in all activities performed during training sessions (e.g., sprints, resistance training).
2.3.2.1. HR measurement
Participants were fitted with armband monitors equipped with temperature, electrocardiography (ECG), photoplethysmography (PPG), and inertial measurement unit (IMU) capabilities (Warfighter Monitor (WFM), Tiger Tech Solutions Inc, Miami FL). The WFM armbands were previously validated in several diverse subpopulations [32]. Monitors were placed on the posterior aspect of the left upper arm, secured with an elastic band, and worn for the duration of each acute exercise training session. Although the WFM device collected several biometric parameters, only HR and duration were analyzed.
2.3.3. ANS recovery to acute exercise training sessions
2.3.3.1. Next-day baseline HR
Next-day baseline HR represented ANS recovery. Baseline HR was measured in the early morning, following at least 4 min of inactivity, per established protocols [33]. Specifically, baseline HR was measured prior to the start (0600–0700) of the following day's exercise training session. Each athlete was required to remain nearly motionless in a seated position for a period of 5 min to collect a “resting” baseline HR.
2.3.4. ANS function 24H post-acute exercise training
2.3.4.1. Next-day HR recovery
HR recovery was measured during the next-day's exercise training session to track ANS function following acute bouts of exercise. HR recovery was defined as the reduction in HR during 30-s rest intervals representing localized parasympathetic activation. HR recovery was measured within the first 30-s of rest as during this period HR exhibits the greatest rate of change [34]. HR recovery was quantified for all rest intervals occurring throughout the training session, and then averaged.
Importantly, baseline HR and HR recovery were measured 24h following a training session. As such, baseline HR and HR recovery were not measured following one or more rest days. Including rest days would likely dilute the association and not accurately represent the acute influence of exercise training intensity on short term ANS recovery and function, see Fig. 1.
2.4. Statistical analyses
This study investigated the relationships between the time spent at varying levels of intensity during acute bouts of exercise training and the influence on ANS recovery and function within 24h post-exercise. The duration (in minutes) of time spent in an ‘active state’ and intensity (HR, bpm) served as the primary independent variables. Given the established inverse relationship between intensity and duration, the analyses were stratified by intensity thresholds including time spent in HR < 140 bpm, HR < 150 bpm, HR < 160 bpm and HR > 140 bpm, HR > 150 bpm, and HR > 160 bpm. Next-day baseline HR and HR recovery served as the primary outcome variables and represented ANS recovery and function within 24h post-exercise training, respectively. The conditional distributions for each association were tested for normality using the Kolmogrov-Smirnov test and all p-values fell below 0.05. All associations were quantified using linear regression models and were performed separately for each intensity threshold and outcome variable. For all models, β coefficients and standard errors were estimated, and the a priori threshold for statistical significance was set at α = 0.05. Statistical analyses was performed in MATLAB, version 2021b (MathWorks, Natick, MA).
3. Results
Table 1 displays the information on the duration and time spent above and below different HR values during exercise training sessions. Further, it shows the athletes’ ANS recovery and function 24h post-exercise with baseline HR and HR recovery, respectively. A total of 128 exercise training sessions lasting, on average, 161.1 (40.6) minutes were assessed. The average time spent in each intensity threshold decreased with increasing HR ranges from 112.3 (36.0) minutes at HRs between 85 and 139 bpm to 5.1 (10.0) minutes at HRs ≥160 bpm. It should be noted that the range for time spent in specific intensities was considerably wide. For ANS recovery 24h post exercise, the athletes, on average, elicited a baseline HR of 61.4 (±8.6) bpm, ranging between 44.8 and 118.2 bpm. In response to acute exercise the following day, the athletes, on average, exhibited HR recovery values of 30.6 (6.0) beats, ranging between 11.2 and 49.6 beats during 30-s rest intervals.
Table 1.
Duration of acute exercise training sessions, time spent in various intensity thresholds, and next-day ANS recovery and function.
| Mean (SD) | Median (min, max) | |
|---|---|---|
| No. of Training Sessions | 128 | |
| Duration of Sessions (min) | 161.1 (40.6) | 157.1 (90.1, 339.6) |
| Time Spent in Intensity Thresholds | ||
| HR 85–139 bpm | 112.3 (36.0) | 109.4 (17.1, 273.2) |
| HR 140–149 bpm | 26.3 (15.6) | 24.3 (0.0, 105.4) |
| HR 150–159 bpm | 17.5 (10.4) | 16.2 (0.0, 70.2) |
| HR ≥ 160 bpm | 5.1 (10.0) | 1.0 (0.0, 98.7) |
| ANS Recovery | ||
| Baseline HR (bpm) | 61.4 (8.6) | 60.1 (44.8, 118.2) |
| ANS Function | ||
| HR Recovery (bpm) | 30.6 (6.0) | 31.0 (11.2, 49.6) |
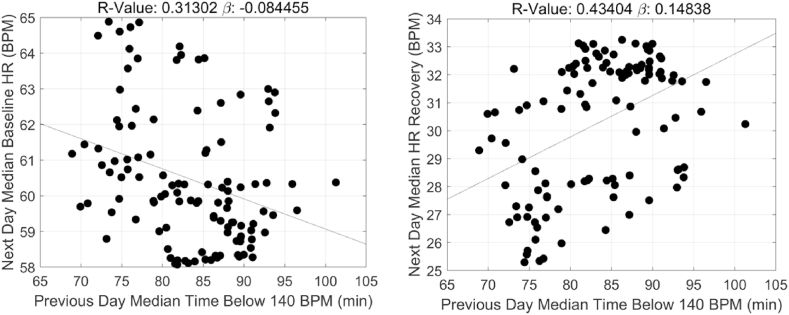
Adjusted linear regression and correlation coefficients representing the relationships between the time spent at varying intensity threshold during acute bouts of exercise training and the influence on ANS recovery and function 24h post-exercise is presented in Table 2. For baseline HR, statistically significant associations with next-day recovery were observed for the 3 of the 4 intensity thresholds including HR < 140 bpm (β = −0.08 ± 0.02, R2 = 0.31, p < 0.001), HR above 150 and 160 bpm (β = 0.25 ± 0.02, R2 = 0.69, p < 0.0000 and β = 0.59 ± 0.06, R2 = 0.71, p < 0.0000). Similar associations were HR recovery including HR < 140 bpm (β = 0.15 ± 0.03, R2 = 0.43, p < 0.0000), HR above 150 and 160 bpm (β = −0.33 ± 0.03, R2 = 0.73, p < 0.0000 and β = −0.80 ± 0.06, R2 = 0.71, p < 0.0000). At lower intensity thresholds, specifically HRs <140 bpm, duration at this intensity negatively and positively associated with both baseline HR and HR recovery 24h post exercise training, respectively. Conversely, higher intensity thresholds, specifically HRs ≥150 bpm and HRs ≥160 bpm were positively and negatively associated with baseline HR and HR recovery 24h post exercise training, respectively. Moreover, the strengths of the associations between duration and ANS recovery and function increased with increasing intensity, HR ≥ 150 and 160 bpm (baseline HR: β range = 0.25 vs 0.59, R2: 0.69 vs 0.71 and HR recovery: β range = −0.33 vs −0.80, R2 = 0.73 vs 0.77). Graphical representations of these relationships appear in Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7.
Table 2.
Adjusted linear regression coefficients for the relationships between time spent in different intensity thresholds and next-day ANS recovery and function.
| Slope (β) | SE | Adjusted R2 | 95% CI | p-value | |
|---|---|---|---|---|---|
| Baseline HR (bpm) | |||||
| Time Spent in Intensity Thresholds | |||||
| HR Below 140 bpm | −0.08 | 0.02 | 0.31 | −0.13, −0.04 | 0.001 |
| HR Below 150 bpm | −0.003 | 0.02 | 0.01 | −0.05, 0.04 | 0.89 |
| HR Above 150 bpm | 0.25 | 0.02 | 0.69 | 0.20, 0.29 | <0.0000 |
| HR Above 160 bpm | 0.59 | 0.06 | 0.71 | 0.48, 0.70 | <0.0000 |
| HR Recovery (bpm) | |||||
| Time Spent in Intensity Thresholds | |||||
| HR Below 140 bpm | 0.15 | 0.03 | 0.43 | 0.09, 0.21 | <0.0000 |
| HR Below 150 bpm | 0.04 | 0.03 | 0.14 | −0.02, 0.10 | 0.15 |
| HR Above 150 bpm | −0.33 | 0.03 | 0.73 | −0.39, −0.27 | <0.0000 |
| HR Above 160 bpm | −0.80 | 0.06 | 0.77 | −0.92, −0.66 | <0.0000 |
*SE = standard error, CI = confidence interval, HR = heart rate, bpm = beats per minute, “time spent” represents the number of minutes spent in each intensity threshold.
Fig. 2.
Adjusted linear regression correlations between time spent in (a) HRs below 140 bpm and (b) baseline HR and HR recovery 24h post-exercise, in a sample of Division I male, football athletes.
Fig. 3.
Adjusted linear regression correlations between time spent in (a) HRs below 150 bpm and (b) baseline HR and HR recovery 24h post-exercise, in a sample of Division I male, football athletes.
Fig. 4.
Adjusted linear regression correlations between time spent in (a) HRs below 160 bpm and (b) baseline HR and HR recovery 24h post-exercise, in a sample of Division I male, football athletes.
Fig. 5.
Adjusted linear regression correlations between time spent in (a) HRs above 140 bpm and (b) baseline HR and HR recovery 24h post-exercise, in a sample of Division I male, football athletes.
Fig. 6.
Adjusted linear regression correlations between time spent in (a) HRs above 150 bpm and (b) baseline HR and HR recovery 24h post-exercise, in a sample of Division I male, football athletes.
Fig. 7.
Adjusted linear regression correlations between time spent in (a) HRs above 160 bpm and (b) baseline HR and HR recovery 24h post-exercise, in a sample of Division I male, football athletes.
4. Discussion
This study aimed to investigate the relationships between duration and intensity of exercise training on next-day ANS recovery and function in a sample of Division-I collegiate football athletes. The major finding of this study was that at certain intensity thresholds, duration significantly and negatively impacted next-day ANS recovery. Specifically, prolonged exposure to higher intensities elicited higher baseline HRs 24h post-exercise. Similarly, prolonged exposure to higher intensities also impaired ANS recovery during 30-s rest intervals, 24h later. Conversely, sustained exercise at lower intensity thresholds (HRs <140 bpm) did not perturb next-day ANS recovery, rather positive associations were observed.
The most novel aspect of this study was observing specific intensity thresholds at which exercise training duration impaired next-day ANS recovery. The authors found sustaining exercise (≥2 min) at HRs above 150 bpm negatively influenced ANS recovery, demonstrated by elevated baseline HRs 24h post-exercise. Moreover, slower cardiac deceleration was also observed during 30-s rest intervals, indicating reduced HR recovery. These negative associations strengthened for exercise sustained at HRs above 160 bpm (Baseline HR: R2 = 0.69 vs 0.71; β = 0.24 vs 0.59 & HR recovery: R2 = 0.73 vs 0.77; β = −0.33 vs −0.80). In support, other studies previously demonstrated impaired ANS function following higher intensity exercise including deficits in parasympathetic reactivation [35], proprioception [36], motor skills and learning [37]. Uniquely however, this study focused on ANS recovery 24h post exercise, where former studies measured its recovery in the immediate and short-term post-exercise period [20]. In doing so, the current study showed the ANS requires more than 24h to fully recover from exercise sustained at higher intensities. This observation raises significant concern for collegiate level athletes training for sports like football that require fast, powerful, dynamic movements. Often, for these sports, many sessions of strenuous exercise are performed each week. As such, it is imperative for strength and conditioning professionals to factor in recovery time when programming workouts to avoid deficits in ANS recovery and optimize physical performance.
The observed insufficient recovery of the ANS following the acute exposure to longer periods of high intensity exercise is supported by physiological evidence. Following the performance of sustained, high intensity exercise, athletes exhibit significant skeletal muscle damage [38], depleted glycogen stores [39], high concentrations of lactic acid [40] and at durations longer than 2 h, elevated levels of ammonia consequent to increased reliance on proteolysis for energy supply [19]. While many of these physiological changes revert to baseline within 60 min post-exercise, the full repletion of glycogen stores and repair of damaged skeletal muscle requires between 24 and 48h [41]. Depriving athletes of an adequate recovery period following acute, bouts of strenuous exercise may negatively affect their exercise performance during subsequent training sessions [42]. With insufficient recovery time, athletes likely remain in the post-exercise catabolic state characterized by proteolysis [43], pro-inflammatory phase, incomplete repair of skeletal muscle [41] and partially repleted energy stores [44]. Consequently, athletes may generate less force and power, reach central and peripheral fatigue more quickly [45,46], thus, reducing the effectiveness of training sessions. Since the ANS regulates many of these physiological processes [47], the sustained deterioration of the ANS observed in this study 24h post, high strenuous exercise, is intuitive.
Interestingly, the current study also observed that acute bouts of exercise sustained at lower intensities influenced ANS recovery. Specifically, prolonged exercise (65 to 105 min) at HRs ≤140 bpm resulted in lower baseline HRs 24h post-exercise potentially suggesting sufficient recovery of the ANS. Additionally, faster cardiac deceleration was observed during 30-s rest intervals, suggesting adequate HR recovery post 24h. Consistent physiological evidence also supports our observation that acute bouts of sustained exercise at lower intensities associates with next-day ANS recovery and function. Less strenuous workloads impose a lower physiological demand compared to high strenuous workloads with studies showing greater reliance on oxidative metabolic [16], increased free fatty acid utilization [48], slower rate of glycogen depletion [49], reduced skeletal muscle damage [17], etc. As such, a 24-h post-exercise period may be sufficient for full recovery of the ANS following prolonged, less strenuous exercise. However, at durations exceeding 2h, metabolic pathways shift as skeletal muscles augment their reliance on blood glucose for energy [50,51]. This shift increases the competition between the skeletal muscles and brain for sufficient energy, potentially facilitating the onset of central and peripheral fatigue. Moreover, proteolysis within the skeletal muscle begins supplementing blood glucose levels via gluconeogenesis, possibly resulting in a sustained catabolic state post-exercise (24–48h) [52].
Our observation that prolonged bouts of less strenuous exercise (HR ≤ 140 bpm) and ANS recovery highlights two exercise programming related concepts. First, although the ANS recovery window is shorter for acute bouts of sustained, less strenuous exercise, strength and conditioning coaches must still use caution by strategically placing these types of training sessions. This is especially true for weekly regimens, including multiple sessions of high strenuous exercise. For example, scheduling this type of session 24h post-strenuous exercise may be inappropriate as the ANS is not yet likely fully recovered. As shown in this study, athletes exhibited reduced ability to adequately recover from acute exercise bouts, at any level, on the following day. Thus, implementing sustained, less strenuous bouts of exercise may prolong the catabolic state, further delaying the full recovery of the ANS and the effectiveness of subsequent training sessions. In support, Sherman et al., 1984 examined “active recovery” in runners following the completion of a marathon and found that runners engaging in an “active recovery” consisting of 20–45 min of running at 50–60% of VO2max, were not fully recovered 7 days post-race [53]. Conversely, runners not engaging in an “active recovery” were fully recovered 3 days post-race. These findings strongly emphasize the prioritizing of including sufficient recovery periods between training sessions, especially following strenuous training sessions.
Second, while 24h appears to be a sufficient recovery period for the ANS previously exposed to an acute, sustained bout of less strenuous exercise, this does not suggest that athletes should train at this level for longer periods of time nor at high frequency throughout each training week. It is possible that deterioration of the ANS manifests cumulatively over several consecutive training sessions as indicated in the study conducted Sherman et al., 1984. In support, one study found that over a 5-week training period at moderate intensity (1 week), overload (3 weeks) and tapering (1 week) resulted in functional overreaching among triathletes and was attributed to parasympathetic hyperactivity. Importantly, parasympathetic hyperactivity was most pronounced when analyzed across several weeks as opposed to 1, 7-day period [54]. Thus, it is conceivable that the “apparent restoration” of the ANS 24h post-exercise at lower intensities could reflect parasympathetic hyperactivity, further strengthening the emphasis on coaches strategically and appropriately sequencing of exercise training sessions [55].
The current study has several strengths. First, the study employed a prospective cohort study design in a “real-world” sport setting. As such, the findings authentically reflect the training regimens implemented in collegiate football players rather than those performed in laboratory-based settings. Second, the “physiological workload” more commonly known as “intensity” in the sports science field was quantified using HRs elicited during training and analyzed on a continuum rather than categorically using pre-defined intensity classifications. This allowed for increased flexibility in examining the associations between exercise duration, intensity and ANS recovery and function, likely yielding findings that more accurately represent the inter-individual variability of the study sample. Third, this study utilized a large amount of data. The data were from 50 athletes that performed 128 training sessions throughout a standard collegiate football season totaling 2735 individual sets, thus, providing a comprehensive insight to the physiological loads imposed on the ANS. This study also possesses weaknesses warranting attention. First, because the athletes immediately participated in subsequent training sessions, we were only able to determine if 24h, for each athlete, was a sufficient recovery period for the ANS. Thus, for athletes requiring longer than 24h, we were unable to identify a more precise recovery window. Second, the study included only males playing one collegiate sport which reduces the generalizability of our findings to other sports implementing similar training programs and female athletes. Lastly, other factors potentially influencing the ANS recovery and function were not controlled for including nutrition patterns, hydration status, quality/quantity of sleep and post-training recovery methods such as cold-water immersion, massage, etc.
5. Conclusions
In conclusion, the findings of this study emphasize concepts critical in designing and implementing effective exercise training programs in collegiate football athletes. First, our study showed that performing bouts of exercise at HRs ≥150 bpm for longer than 2 min negatively impacts the ANS. Specifically, ANS recovery and function remains impaired for more than 24h and is present in rested and exercise states. Second, the slower cardiac deceleration observed during next-day rest intervals following acute bouts of high intensity exercise, raises significant concerns for collegiate sport exercise training programs that require 5 to 6 daily exercise sessions per week. The high frequency of training, regardless of workload, presents challenges in providing athletes adequate recovery periods between sessions. Therefore, strength and conditioning coaches should consider design strategies that reduce prolonged bouts of strenuous exercise and lengthen rest and recovery periods within [56] and between exercise training sessions. We recommend that future studies focus on more precisely defining the time to full recovery of the ANS and investigate the potential cumulative effects of performing several consecutive training sessions at varying intensity on ANS recovery and function.
Statements and declarations
Funding
The authors have no funding to disclose.
Availability of data and material
HR, HR recovery and duration data are available upon request.
Ethics approval
University's Institutional Review Board (IRB #20191223).
Consent to participate
Prior to any measurements, the athletes were informed of the benefits and risks of the study and voluntarily consented to the study.
Consent for publication
Not applicable.
Code availability
Limited code is available upon request.
Author contributions
The authors each contributed to the development of this manuscript in the following ways, conceived and designed the experiments: SHW, ER, HLW, MJW, SL, KB, JG and LAF; performed the experiments: SC, EDW, SH, KB, JG and DH; analyzed and interpreted the data: MJW, HLW, and SMM; contributed reagents, materials, analysis tools or data: HLW, MJW, and SHW; wrote the paper: SMM, HLW, and MJW.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Samantha M McDonald reports financial support was provided by TigerTech Solutions, Inc. S. Howard Wittels reports financial support was provided by TigerTech Solutions, Inc. Eva D Wittels reports financial support was provided by TigerTech Solutions. Stephanie Hendricks reports financial support was provided by TigerTech Solutions, Inc. Michael Joseph Wishon reports financial support was provided by TigerTech Solutions, Inc. Dustin Hecocks reports financial support was provided by TigerTech Solutions, Inc. Stephanie Chong reports financial support was provided by TigerTech Solutions, Inc. Harrison L Wittels reports financial support was provided by TigerTech Solutions, Inc. Harrison L. Wittels, the founder of TigerTech Solutions, possesses trade secret protection for the Warfighter Monitor used to collect the data analyzed and presented in the current manuscript.
Acknowledgements
The authors would like to sincerely thank all the athletes, coaches, and university staff for their participation in this study and the scientific contributions to enhancing sports performance.
References
- 1.Jensen J.L., Marstrand P.C.D., Nielsen J.B. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J. Appl. Phys. 2005;99:1558–1568. doi: 10.1152/japplphysiol.01408.2004. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell J.H., Kaufman M.P., Iwamoto G.A. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu. Rev. Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 3.Dela F., Mohr T., Jensen C.M.R., Haahr H.L., Secher N.H., Biering-Sørensen F., et al. Cardiovascular control during exercise. Circulation. 2003;107:2127–2133. doi: 10.1161/01.CIR.0000065225.18093.E4. [DOI] [PubMed] [Google Scholar]
- 4.Laursen P.B., Jenkins D.G. The scientific basis for high-intensity interval training: optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002;32:53–73. doi: 10.2165/00007256-200232010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kamijo K., Nishihira Y., Hatta A., Kaneda T., Wasaka T., Kida T., et al. Differential influences of exercise intensity on information processing in the central nervous system. Eur. J. Appl. Physiol. 2004;92:305–311. doi: 10.1007/s00421-004-1097-2. [DOI] [PubMed] [Google Scholar]
- 6.Smith M., Tallis J., Miller A., Clarke N.D., Guimarães-Ferreira L., Duncan M.J. The effect of exercise intensity on cognitive performance during short duration treadmill running. J. Hum. Kinet. 2016;51:27–35. doi: 10.1515/hukin-2015-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart G.M., Yamada A., Haseler L.J., Kavanagh J.J., Chan J., Koerbin G., et al. Influence of exercise intensity and duration on functional and biochemical perturbations in the human heart. J. Phys. 2016;594:3031–3044. doi: 10.1113/JP271889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strength National. fourth ed. Human Kinetics; Champaign, IL: 2016. Conditioning Association. NSCA's Essentials of Strength and Conditioning. [Google Scholar]
- 9.Kellmann M., Bertollo M., Bosquet L., Brink M., Coutts A.J., Duffield R., et al. Recovery and performance in sport: consensus statement. Int. J. Sports Physiol. Perform. 2018;13:240–245. doi: 10.1123/ijspp.2017-0759. [DOI] [PubMed] [Google Scholar]
- 10.Pincivero D.M., Bompa T.O. A physiological review of American football. Sports Med. 1997;23:247–260. doi: 10.2165/00007256-199723040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Zuniga J.M., Berg K., Noble J., Harder J., Chaffin M.E., Hanumanthu V.S. Physiological responses during interval training with different intensities and duration of exercise. J. Strength Condit Res. 2011;25(5):1279–1284. doi: 10.1519/JSC.0b013e3181d681b6. [DOI] [PubMed] [Google Scholar]
- 12.Tschakert G., Hofmann P. High-intensity intermittent exercise: methodological and physiological aspects. Int. J. Sports Physiol. Perform. 2013;8:600–610. doi: 10.1123/ijspp.8.6.600. [DOI] [PubMed] [Google Scholar]
- 13.Tschakert G., Handl T., Weiner L., Birnbaumer P., Mueller A., Groeschl W., et al. Exercise duration: independent effects on acute physiologic responses and the need for an individualized prescription. Phys. Rep. 2022;10 doi: 10.14814/phy2.15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calbet J.A.L., Martín-Rodríguez S., Martin-Rincon M., Morales-Alamo D. An integrative approach to the regulation of mitochondrial respiration during exercise: focus on high-intensity exercise. Redox Biol. 2020;35 doi: 10.1016/j.redox.2020.101478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essén B. Intramuscular substrate utilization during prolonged exercise. Ann. N. Y. Acad. Sci. 1977;301:30–44. doi: 10.1111/j.1749-6632.1977.tb38183.x. [DOI] [PubMed] [Google Scholar]
- 16.Hargreaves M., Spriet L.L. Skeletal muscle energy metabolism during exercise. Nat Metab. 2020;2:817–828. doi: 10.1038/s42255-020-0251-4. [DOI] [PubMed] [Google Scholar]
- 17.Ertel K.A., Hallam J.E., Hillman A.R. The effects of training status and exercise intensity on exercise-induced muscle damage. J. Sports Med. Phys. Fit. 2020;60:449–455. doi: 10.23736/S0022-4707.19.10151-X. [DOI] [PubMed] [Google Scholar]
- 18.Gottschall J.S., Davis J.J., Hastings B., Porter H.J. Exercise time and intensity: how much is too much? Int. J. Sports Physiol. Perform. 2020;15:808–815. doi: 10.1123/ijspp.2019-0208. [DOI] [PubMed] [Google Scholar]
- 19.Hansen M., Trappe T., Crameri R.M., Qvortrup K., Kjaer M., Langberg H. Myofibrillar proteolysis in response to voluntary or electrically stimulated muscle contractions in humans. Scand. J. Med. Sci. Sports. 2009;19:75–82. doi: 10.1111/j.1600-0838.2007.00766.x. [DOI] [PubMed] [Google Scholar]
- 20.Coates A.M., Hammond S., Burr J.F. Investigating the use of pre-training measures of autonomic regulation for assessing functional overreaching in endurance athletes. Eur. J. Sport Sci. 2018;18:965–974. doi: 10.1080/17461391.2018.1458907. [DOI] [PubMed] [Google Scholar]
- 21.Holt AC, Plews DJ, Oberlin-Brown KT, Merien F, Kilding AE. Cardiac Parasympathetic and anaerobic performance recovery after high-intensity exercise in rowers. Int. J. Sports Physiol. Perform.. 14:331–338. [DOI] [PubMed]
- 22.Burma J.S., Copeland P.V., Macaulay A., Khatra O., Smirl J.D. Effects of high-intensity intervals and moderate-intensity exercise on baroreceptor sensitivity and heart rate variability during recovery. Appl. Physiol. Nutr. Metabol. 2020;45:1156–1164. doi: 10.1139/apnm-2019-0810. [DOI] [PubMed] [Google Scholar]
- 23.Coote J.H. Recovery of heart rate following intense dynamic exercise. Exp. Physiol. 2010;95:431–440. doi: 10.1113/expphysiol.2009.047548. [DOI] [PubMed] [Google Scholar]
- 24.Stanley J., Peake J.M., Buchheit M. Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Med. 2013;43:1259–1277. doi: 10.1007/s40279-013-0083-4. [DOI] [PubMed] [Google Scholar]
- 25.Seiler S., Jøranson K., Olesen B.V., Hetlelid K.J. Adaptations to aerobic interval training: interactive effects of exercise intensity and total work duration. Scand. J. Med. Sci. Sports. 2013;23:74–83. doi: 10.1111/j.1600-0838.2011.01351.x. [DOI] [PubMed] [Google Scholar]
- 26.Jalilvand F., Chapman D., Lockie R. Strength and conditioning considerations for collegiate american football. J. Aust. Streng. Cond. 2019;27(2):72–85. [Google Scholar]
- 27.Kreher J.B., Schwartz J.B. Overtraining syndrome: a practical guide. Sport Health. 2012;4:128–138. doi: 10.1177/1941738111434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robergs R., Landwehr R. The surprising history of the “HRmax=220-age” equation. J. Exer. Phys. Online. 2002;5(2):1–11. [Google Scholar]
- 29.Esco M.R., Chamberlain N., Flatt A.A., Snarr R.L., Bishop P.A., Williford H.N. Cross-validation of age-predicted maximal heart rate equations among female collegiate athletes. J. Strength Condit Res. 2015;29(11):3053–3059. doi: 10.1519/JSC.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 30.Cunha FA da, Farinatti P. de TV., Midgley A.W. Methodological and practical application issues in exercise prescription using the heart rate reserve and oxygen uptake reserve methods. J. Sci. Med. Sport. 2011;14:46–57. doi: 10.1016/j.jsams.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Hadzi-Pavlovic D. Correlations II: categorizing continuous data. Acta Neuropsychiatr. 2007;19:129–130. [Google Scholar]
- 32.Peck J., Wishon M.J., Wittels H., Lee S.J., Hendricks S., Davila H., et al. Single limb electrocardiogram using vector mapping: evaluation and validation of a novel medical device. J. Electrocardiol. 2021;67:136–141. doi: 10.1016/j.jelectrocard.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Speed C., Arneil T., Harle R., Wilson A., Karthikesalingam A., McConnell M., et al. Measure by measure: resting heart rate across the 24-hour cycle. PLOS Digit Health. 2023;2 doi: 10.1371/journal.pdig.0000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandi P.S., Spodick D.H. Recovery from exercise at varying workloads. Time course of responses of heart rate and systolic intervals. Br. Heart J. 1977;39:958–966. doi: 10.1136/hrt.39.9.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchheit M., Laursen P.B., Ahmaidi S. Parasympathetic reactivation after repeated sprint exercise. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H133–H141. doi: 10.1152/ajpheart.00062.2007. [DOI] [PubMed] [Google Scholar]
- 36.Saxton J.M., Clarkson P.M., James R., Miles M., Westerfer M., Clark S., et al. Neuromuscular dysfunction following eccentric exercise. Med. Sci. Sports Exerc. 1995;27:1185–1193. [PubMed] [Google Scholar]
- 37.Pearce A.J., Sacco P., Byrnes M.L., Thickbroom G.W., Mastaglia F.L. The effects of eccentric exercise on neuromuscular function of the biceps brachii. J. Sci. Med. Sport. 1998;1:236–244. doi: 10.1016/s1440-2440(09)60007-4. [DOI] [PubMed] [Google Scholar]
- 38.Howatson G., Milak A. Exercise-induced muscle damage following a bout of sport specific repeated sprints. J. Strength Condit Res. 2009;23(8):2419–2424. doi: 10.1519/JSC.0b013e3181bac52e. [DOI] [PubMed] [Google Scholar]
- 39.Vigh-Larsen J.F., Ørtenblad N., Spriet L.L., Overgaard K., Mohr M. Muscle Glycogen metabolism and high-intensity exercise performance: a narrative review. Sports Med. 2021;51:1855–1874. doi: 10.1007/s40279-021-01475-0. [DOI] [PubMed] [Google Scholar]
- 40.Baker J.S., Thomas N., Cooper S.M., Davies B., Robergs R.A. Exercise duration and blood lactate concentrations following high intensity cycle ergometry. Res. Sports Med. 2012;20:129–141. doi: 10.1080/15438627.2012.634723. [DOI] [PubMed] [Google Scholar]
- 41.Tiidus P.M. Human Kinetics; Champaign, IL: 2008. Skeletal Muscle Damage and Repair. [Google Scholar]
- 42.Byrne C., Twist C., Eston R. Neuromuscular function after exercise-induced muscle damage. Sports Med. 2004;34:49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- 43.Thompson D., Nicholas C., Williams C. Muscular soreness following prolonged intermittent high-intensity shuttle running. J. Sports Sci. 1999;17:387–395. doi: 10.1080/026404199365902. [DOI] [PubMed] [Google Scholar]
- 44.O'Reilly K.P., Warhol M.J., Fielding R.A., Frontera W.R., Meredith C.N., Evans W.J. Eccentric exercise-induced muscle damage impairs muscle glycogen repletion. J. Appl. Physiol. 1987;63:252–256. doi: 10.1152/jappl.1987.63.1.252. [DOI] [PubMed] [Google Scholar]
- 45.Byrne C., Eston R. Maximal-intensity isometric and dynamic exercise performance after eccentric muscle actions. J. Sports Sci. 2002;20:951–959. doi: 10.1080/026404102321011706. [DOI] [PubMed] [Google Scholar]
- 46.Gibala M.J., MacDougall J.D., Tarnopolsky M.A., Stauber W.T., Elorriaga A. Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J. Appl. Physiol. 1995;78:702–708. doi: 10.1152/jappl.1995.78.2.702. [DOI] [PubMed] [Google Scholar]
- 47.Fisher J.P., Young C.N., Fadel P.J. John Wiley & Sons, Ltd; 2015. Autonomic Adjustments to Exercise in Humans. Comprehensive Physiology [Internet] pp. 475–512. [DOI] [PubMed] [Google Scholar]
- 48.Knoepfli B., Riddell M.C., Ganzoni E., Burki A., Villiger B., von Duvillard S.P. Off seasonal and pre-seasonal assessment of circulating energy sources during prolonged running at the anaerobic threshold in competitive triathletes. Br. J. Sports Med. 2004;38:402–407. doi: 10.1136/bjsm.2002.003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vøllestad N.K., Blom P.C. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol. Scand. 1985;125:395–405. doi: 10.1111/j.1748-1716.1985.tb07735.x. [DOI] [PubMed] [Google Scholar]
- 50.Rapoport B.I. Metabolic factors limiting performance in marathon runners. PLoS Comput. Biol. 2010;6:1–13. doi: 10.1371/journal.pcbi.1000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahlborg G., Wahren J. Brain substrate utilization during prolonged exercise. Scand. J. Clin. Lab. Invest. 1972;29:397–402. doi: 10.3109/00365517209080256. [DOI] [PubMed] [Google Scholar]
- 52.Hawley J.A., Leckey J.J. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. 2015;45(1):S5–S12. doi: 10.1007/s40279-015-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherman W.M., Costill D.L., Fink W.J., Hagerman F.C., Armstrong L.E., Murray T.F. Effect of a 42.2-km footrace and subsequent rest or exercise on muscle glycogen and enzymes. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983;55:1219–1224. doi: 10.1152/jappl.1983.55.4.1219. [DOI] [PubMed] [Google Scholar]
- 54.Le Meur Y., Pichon A., Schaal K., Schmitt L., Louis J., Gueneron J., et al. Evidence of parasympathetic hyperactivity in functionally overreached athletes. Med. Sci. Sports Exerc. 2013;45:2061–2071. doi: 10.1249/MSS.0b013e3182980125. [DOI] [PubMed] [Google Scholar]
- 55.Laursen P.B. Training for intense exercise performance: high-intensity or high-volume training? Scand. J. Med. Sci. Sports. 2010;20(2):1–10. doi: 10.1111/j.1600-0838.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- 56.Pincivero D.M., Lephart S.M., Karunakara R.G. Effects of rest interval on isokinetic strength and functional performance after short-term high intensity training. Br. J. Sports Med. 1997;31:229. doi: 10.1136/bjsm.31.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
HR, HR recovery and duration data are available upon request.