Several critical care syndromes, such as sepsis, are characterized by hyperinflammation, which is instigated not only by any underlying infectious agent but also by the release of danger-associated molecular patterns (DAMPs) from injured tissue and dying cells (1). In addition to proinflammatory activity, DAMPS can be prothrombotic and directly cytotoxic. Indeed, circulating levels of many DAMPs are associated with severity and progression of critical illness, including sepsis and trauma-induced organ injury in patients (2). The ability of some DAMPs to generate sepsis-like organ injury when injected in vivo suggests a central role in the disease process (3).
Histones are basic proteins that form the building blocks of the nucleosome complex in which negatively charged DNA is spooled around two copies of the four histones: H2A, H2B, H3, and H4. As such, they play crucial roles in the packaging and arranging of DNA into functional units (4, 5). Through modification of histone–DNA physical interactions, they play indispensable roles within the nucleus in regulating DNA transcription and replication (6). However, histones are readily released from the nucleus of activated or injured cells into the extracellular environment, where they can have significant roles in host protection through activation of innate immune cells and direct cytotoxicity toward pathogens (7).
Extracellular histones are derived via two main processes: inducible programmed cell death triggered by microbial or endogenous mediators (e.g., cytokine, death factors) and more abrupt necrotic cell death through physical trauma and ischemia-associated loss of cell integrity (8). Several distinct forms of programmed necrosis have been associated with infection and injury, contributing to and amplifying the inflammatory response (9). A specific type of programmed necrosis in neutrophils, termed NETosis, generates a citrullinated isoform histone structure that enables their release embedded within web-like stands of DNA that constitute neutrophil extracellular traps (NETs). Thus, histones released via multiple processes may be a central component of so-called necroinflammation, a major driver of exaggerated inflammatory and coagulation cascades associated with end-organ dysfunction (10). Despite their potentially harmful effects, relatively little is known about the molecular and physical determinants of extracellular histone activity or their potential regulation by endogenous homoeostatic pathways.
In this issue of the Journal, Augusto and colleagues (pp. 176–187) highlight the functional and translational role for CLU (clusterin), an extracellular chaperone protein and scavenger of extracellular histones (11). First, they show CLU has privileged binding to histones and, importantly, that it can neutralize the in vitro proinflammatory, cytotoxic, and platelet-aggregating effects of histones. Moreover, the histone-neutralizing activity of normal human plasma was reduced markedly by predepletion of CLU. Crucially, CLU was active against various histone compositions, including citrullinated histones (CitHis) released through neutrophil NETosis. This is a key aspect of the study, as the concept of enhancing histone clearance being beneficial irrespective of origin (from cell death mediated by tissue injury or immune cell activation) enables future translational application into all sterile and nonsterile causes of acute tissue injury and inflammation.
Levels of circulating nucleosomes and CLU–histone complexes increased in mice injected with endotoxin. Furthermore, mice showed reduced free CLU levels, and CLU supplementation led to lower inflammation and increased survival. Murine in vivo studies using CLU−/− whole-body knockouts showed higher IL-6 and tumor necrosis factor-α release, but not IL1-β, with histone injection, suggesting a specific role for CLU in modulating histone-mediated inflammation. Indeed, CLU supplementation within this histone-induced inflammation model improved survival. Finally, investigations into more clinically translational in vivo mouse model of cecal ligation and puncture showed that CLU deficiency has a significant mortality impact, whereas CLU supplementation improves survival.
A major strength of this study is its extensive evaluation of CLU activity and dynamics in patients with sepsis. Nucleosomes and CLU–histone complexes are significantly increased in patients with sepsis, together with citrullinated H3. The authors demonstrate that nucleosomes are lower in sepsis survivors over the first week of sepsis. Indeed, consistent with in vivo mouse data, circulating levels of CLU are reduced in patients with sepsis, with nonsurvivors having far lower levels than survivors. Moreover, there is a greater increase in CLU over time in survivors compared with nonsurvivors of sepsis. Interestingly, monocytes from patients with sepsis have increased expression of CLU mRNA, suggesting that reduced levels of circulating CLU are a function of increased consumption rather than reduced production. Furthermore, CLU supplementation of patient plasma neutralized its cytotoxic and inflammatory effects on monocytes.
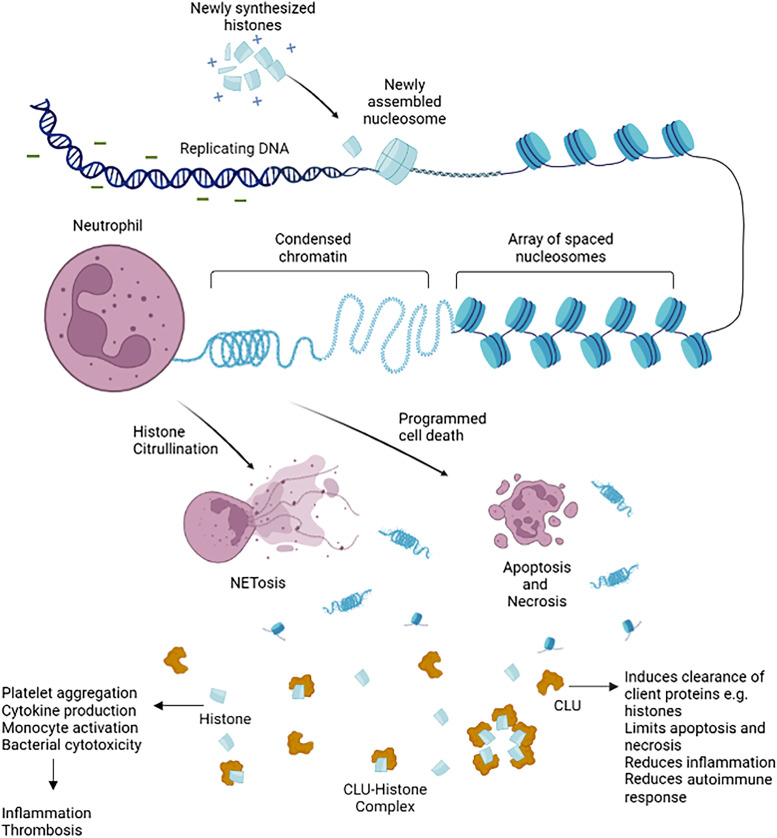
The authors nicely highlight the functional and translational relevance of CLU not only as a histone scavenger promoting systemic clearance but also as a direct regulator of histone activity (Figure 1). CitHis, released during NETosis, have emerged as major DAMPs in sepsis (12). Elevated concentrations of circulating CitHis have been reported in critically ill patients and patients with sepsis and are associated with the severity of the sepsis (13). Alternatively, histones released by accidental necrosis have been implicated in the development of acute respiratory distress syndrome associated with extrapulmonary traumatic injury (14). The potential for the neutralization of neutrophil-dependent injury processes or, more specifically, whether CLU affects the activity of NETs with histone–DNA complexes, requires further investigation.
Figure 1.
Histones are basic proteins whose positive charges associate with DNA, enabling the negatively charged DNA to spool around them. They form memberships of chromatin beads called nucleosomes, with each nucleosome bead consisting of a histone octamer composed of two copies of each histone protein: H2A, H2B, H3, and H4. They are coiled within the cell, forming condensed chromatin material. Extracellular histones are released by cell death in response to infection and inflammation as chromatin material, nucleosome, or individual histones. CLU (Clusterin), an extracellular chaperone protein, forms bonds to misfolded or excess proteins, including histones, forming complexes. CLU–histone complexes neutralize histone activity. In addition, these complexes aid the clearance of extracellular histones by binding to cell surface receptor(s); they are internalized by receptor-mediated endocytosis and trafficked to autophagosomes for degradation. NET = neutrophil extracellular trap.
The identification of CLU as an endogenous regulator of histone DAMP activity provides yet another example of the host’s intrinsic ability to counter or quench ongoing proinflammatory and antimicrobial defense mechanisms to maintain homoeostatic control. Sepsis is a key example of a dysregulated response with a variety of immune defects, such as hyporesponsiveness and a profound loss of innate and adaptive immune cells (15). This study highlights a novel mechanism by which the body’s innate buffer system can become dysregulated, leading to uncontrolled inflammatory responses. Indeed, this paper is timely, given the increasingly available extracorporeal biosorption systems now available on the market with varying degrees of clinical efficacy. Indeed, therapies that augment naturally occurring processes may be implemented earlier and more safely to avoid the vicious cascade of necrolysis-induced inflammation that is associated with high morbidity and mortality.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202305-0935ED on June 13, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med . 2020;202:361–370. doi: 10.1164/rccm.201910-1911TR. [DOI] [PubMed] [Google Scholar]
- 2. Ma KC, Schenck EJ, Pabon MA, Choi AMK. The role of danger signals in the pathogenesis and perpetuation of critical illness. Am J Respir Crit Care Med . 2018;197:300–309. doi: 10.1164/rccm.201612-2460PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen B, Miller AL, Rebelatto M, Brewah Y, Rowe DC, Clarke L, et al. S100A9 induced inflammatory responses are mediated by distinct damage associated molecular patterns (DAMP) receptors in vitro and in vivo. PLoS One . 2015;10:e0115828. doi: 10.1371/journal.pone.0115828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venkatesh S, Workman JL. Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol . 2015;16:178–189. doi: 10.1038/nrm3941. [DOI] [PubMed] [Google Scholar]
- 5. Felsenfeld G, Groudine M. Controlling the double helix. Nature . 2003;421:448–453. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- 6. Wu D, Shi Y, Zhang H, Miao C. Epigenetic mechanisms of immune remodeling in sepsis: targeting histone modification. Cell Death Dis . 2023;14:112. doi: 10.1038/s41419-023-05656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li X, Ye Y, Peng K, Zeng Z, Chen L, Zeng Y. Histones: the critical players in innate immunity. Front Immunol . 2022;13:1030610. doi: 10.3389/fimmu.2022.1030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silk E, Zhao H, Weng H, Ma D. The role of extracellular histone in organ injury. Cell Death Dis . 2017;8:e2812. doi: 10.1038/cddis.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarhan M, Land WG, Tonnus W, Hugo CP, Linkermann A. Origin and consequences of necroinflammation. Physiol Rev . 2018;98:727–780. doi: 10.1152/physrev.00041.2016. [DOI] [PubMed] [Google Scholar]
- 10. Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med . 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Augusto JF, Beauvillain C, Poli C, Paolini L, Tournier I, Pignon P, et al. Clusterin neutralizes the inflammatory and cytotoxic properties of extracellular histones. Am J Respir Crit Care Med . 2023;208:176–187. doi: 10.1164/rccm.202207-1253OC. [DOI] [PubMed] [Google Scholar]
- 12. Tsourouktsoglou T-D, Warnatsch A, Ioannou M, Hoving D, Wang Q, Papayannopoulos V. Histones, DNA, and citrullination promote neutrophil extracellular trap inflammation by regulating the localization and activation of TLR4. Cell Rep . 2020;31:107602. doi: 10.1016/j.celrep.2020.107602. [DOI] [PubMed] [Google Scholar]
- 13. Tian Y, Li P, Wu Z, Deng Q, Pan B, Stringer KA, et al. Citrullinated histone H3 mediates sepsis-induced lung injury through activating caspase-1 dependent inflammasome pathway. Front Immunol . 2021;12:761345. doi: 10.3389/fimmu.2021.761345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med . 2013;187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Vught LA, Wiewel MA, Hoogendijk AJ, Frencken JF, Scicluna BP, Klein Klouwenberg PMC, et al. The host response in patients with sepsis developing intensive care unit-acquired secondary infections. Am J Respir Crit Care Med . 2017;196:458–470. doi: 10.1164/rccm.201606-1225OC. [DOI] [PubMed] [Google Scholar]