Abstract
Adeno-associated virus type 5 (AAV5) is distinct from other dependovirus serotypes based on DNA hybridization and serological data. To better understand the biology of AAV5, we have cloned and sequenced its genome and generated recombinant AAV5 particles. The single-stranded DNA genome is similar in length and genetic organization to that of AAV2. The rep gene of AAV5 is 67% homologous to AAV2, with the majority of the changes occurring in the carboxyl and amino termini. This homology is much less than that observed with other reported AAV serotypes. The inverted terminal repeats (ITRs) are also unique compared to those of the other AAV serotypes. While the characteristic AAV hairpin structure and the Rep DNA binding site are retained, the consensus terminal resolution site is absent. These differences in the Rep proteins and the ITRs result in a lack of cross-complementation between AAV2 and AAV5 as measured by the production of recombinant AAV particles. Alignment of the cap open reading frame with that of the other AAV serotypes identifies both conserved and variable regions which could affect tissue tropism and particle stability. Comparison of transduction efficiencies in a variety of cells lines and a lack of inhibition by soluble heparin indicate that AAV5 may utilize a distinct mechanism of uptake compared to AAV2.
The adeno-associated viruses (AAV) are members of the parvovirus family and are helper virus dependent for replication and gene expression. Of the six serotypes of AAV which have been identified in primates, only three (AAV type 2 [AAV2], AAV3, and AAV5) have been isolated from humans. While AAV4 can infect human and rat cells in culture, serological data suggest that its natural host is the African green monkey (2). Several of these serotypes have been recently cloned, expanding our knowledge of basic AAV biology.
The most extensively studied of these serotypes is AAV type 2 (AAV2). The genome of AAV2 consists of a single strand of DNA 4,680 nucleotides in length (29, 44). The terminal sequence is organized as interrupted palindromes that can form a characteristic hairpin structure which is energetically stable. Two large open reading frames (ORFs) have been identified within the genome. The left ORF encodes the nonstructural replication initiator polypeptides (Rep). The Rep proteins regulate AAV DNA replication, transcription, and the accumulation of single-stranded progeny genomes (4, 5, 8, 20, 32, 39, 46, 47). The importance of the Rep proteins is emphasized by their high degree of conservation. The Rep ORFs of AAV2, AAV3, AAV4, and AAV6 are approximately 90% identical, with most of the changes being conserved amino acid substitutions. The Rep ORF can encode four different polypeptides from two promoters, with a splice variant in each.
The p5-promoted Rep proteins (Rep78 and Rep68) can bind to DNA in a sequence-specific manner and nick duplex inverted terminal repeats (ITRs) in a site- and strand-specific manner. This cleavage reaction occurs at the terminal resolution site (trs) within the ITR and permits the replication of the ends of the linear genome. The DNA-nicking activity requires the binding of the Rep proteins to tandem repeats of a GAGY motif present in the ITRs, structures at the ends of the viral genome (6, 8, 34, 36, 42). DNA binding and terminal resolution are central to the preferential integration of the AAV genome into the AAVS1 locus on the q arm of human chromosome 19 (26, 38, 48, 50).
The ITR serves as the origin of viral DNA replication. Within the ITR, two elements are described which are central for this function: a GAGC repeat motif and the trs. While a number of sequence differences within the ITR have been reported in the different serotypes, the ability to fold into a hairpin conformation and the existence of a Rep binding site and trs are conserved.
Two additional Rep proteins are produced from an internal promoter in the Rep ORF at map unit 19 and are referred to by their apparent molecular masses: Rep52 and Rep40. While these two proteins lack the DNA binding ability of Rep68 and Rep78 they have recently been shown to possess ATP-dependent DNA helicase activity (40). All four of the Rep proteins possess nucleoside triphosphate (NTP) binding activities, DNA and RNA helicase activities (22, 23, 25, 51), and nuclear localization signals (25, 54).
In addition to the role of Rep proteins in the life cycle of AAV, Rep expression results in a characteristic phenotype. Overexpression of Rep68 and Rep78 can either negatively or positively affect transcription of heterologous promoters, inhibit cellular transformation by papillomavirus or by adenovirus E1a plus an activated ras oncogene (17–19, 24, 27, 28, 40), and inhibit progression through the cell cycle (16, 53). While some of these activities may be mediated by the direct interaction of Rep with DNA, other effects may result from interactions with cellular regulatory proteins. Rep52 and Rep78 have recently been shown to bind to the cyclic AMP-dependent protein kinase (PKA) and to a novel PKA-like kinase (PrKX). This interaction results in an inhibition of the protein kinase activity and an alteration in gene expression in vivo (11).
The AAV2 virion is a nonenveloped, icosahedral particle approximately 25 nm in diameter. It consists of three related proteins referred to as VP1, VP2, and VP3. These proteins are found in a ratio of approximately 1:1:10, respectively (3), and are each derived from the right-hand ORF. These proteins differ from each other as a result of alternative splicing and from the use of an atypical start codon. The cap ORF is less conserved among serotypes than the rep ORF. Protein sequence alignment of the different serotypes indicates blocks of divergent and conserved sequences. Comparison with the X-ray crystallographic structure determined for canine parvovirus indicates that some of these sequences may exist on the exterior of the virion, thus accounting for the altered tissue tropism among the different serotypes (10, 33, 35) and the hemagglutination activity of AAV4. Recent work has demonstrated that heparan sulfate proteoglycans (HSP) can play a role in virus binding and uptake, presumably through an interaction with the capsid proteins (45).
AAV has a number of characteristics which make it an attractive vector for gene therapy (21). Transduction with AAV vectors results in stable, long-term transgene expression in vivo in skeletal muscle, photoreceptors, liver, and central nervous system neuronal cells (14, 31, 43, 52). The ITRs have been shown to be the only cis element required for rescue, replication, packaging, and integration (37). Since no viral genes are included in the recombinant particle, a host immune response directed against viral genes expressed in vivo is prevented. In contrast to other viral vectors, AAV is naturally replication defective and is classified as nonpathogenic.
A comparison of human AAV2, AAV3, and AAV5 by DNA Southern blot hybridization indicates a high degree of homology between AAV2 and AAV3 compared to that between both types 2 and 3 and AAV5 (1). In addition, serological data have shown that seroconversion occurs in early childhood with AAV2 and AAV3 but not until 15 to 20 years of age with AAV5 (15), further suggesting that AAV5 is a more distantly related member of the AAV family. While the seroconversion pattern for AAV2 and AAV3 closely follows that of adenovirus, the later seroconversion for AAV5 temporally occurs closer to and follows that for herpes simplex virus (HSV). This suggests that HSV, instead of adenovirus, may serve as the natural helper virus for AAV5. While adenovirus type 12 has been shown to function as a helper virus, the circumstantial association of AAV5 and HSV suggests an altered tissue tropism for AAV5 compared to that of types 2 and 3. To better understand the nature of AAV5 and to determine its usefulness as a vector for gene transfer, the viral genome was cloned and sequenced and recombinant virus was produced using the AAV5 rep and cap genes.
MATERIALS AND METHODS
Cell culture and virus propagation.
cos and HeLa cells were maintained as monolayer cultures in D10 medium (Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum, 100 μg of penicillin/ml, 100 U of streptomycin/ml, and 1× Fungizone) as recommended by the manufacturer (GIBCO, Gaithersburg, Md.). All other cell types were grown under standard conditions as previously reported. AAV5 stocks were a kind gift from Ursula Bantel-Schaal. Recombinant virus was produced, and titers were determined as previously described (9).
DNA cloning and sequencing.
Viral DNA was isolated by digestion of concentrated virus with proteinase K at 50°C for 1 h, followed by phenol-chloroform extraction and ethanol precipitation. The DNA was annealed by heating to 95°C for 5 min, followed by slow cooling to 65°C for 18 h.
The full-length genome of AAV5 was constructed by reassembling overlapping clones using unique restriction sites. The right ITR was cloned by creating a blunt end of the viral DNA with T4 DNA polymerase and then digesting it with XhoI. A 700-bp fragment was isolated and cloned into the SalI-SmaI sites of plasmid NEB193 (NEB). The DNA sequence was determined by using an ABI 373A automated sequencer and FS dye-terminator chemistry (ABI). Both strands of the plasmid were sequenced and confirmed by sequencing of a second clone. Because of the high degree of secondary structure within the ITR this region was sequenced using radiolabeled dye-terminator chemistry (Amersham). The packaging plasmids were assembled by subcloning a large fragment of the AAV5 genome (260 to 4448 nucleotides) into the XbaI site of pUC/SV40ori (9). Two different helper plasmids were produced by this cloning strategy. 5RepCapA has the simian virus 40 origin (SV40ori) fragment adjacent to the p5 promoter at the 5′ end of the AAV5 RepCap fragment, and 5RepCapB has the SV40ori adjacent to the polyadenylation signal at the 3′ end. The helper plasmid SV40ori(−) contains the AAV5 RepCap fragment but lacks the SV40ori.
The AAV5 vector plasmid which contains the AAV5 ITRs and the nucleus-localized beta-galactosidase gene with a Rous sarcoma virus (RSV) promoter was constructed by the seamless cloning system (Stratagene). The plasmid was assembled by ligating the following fragments in the indicated order: pAV2 (29) backbone sequence 4934 to 7412; AAV5 ITR sequence 1 to 188; beta-galactosidase expression cassette from pAAVRnLacZ (9), nucleotides 300 to 4340; and AAV5 ITR sequence 188 to 1. The final plasmid is referred to as pAAV5LacZ and was confirmed by sequencing.
Sequence analysis.
The presence of potential transcription factor binding sites was analyzed with the TESS computer program (University of Pennsylvania). DNA sequence alignments were performed with the Align program (Scientific and Educational Software Inc.). Protein alignments were done using the Palign program (PcGene), and the phyolgenetic trees were assembled with the CLUSTAL program (PcGene).
Transduction of cells.
Exponentially growing cells (2 × 104) were plated in each 2-cm well of a 12-well plate, transduced with serial dilutions of virus in 800 μl of medium, and incubated for 48 to 60 h at 37°C. After this period, the cells were fixed and stained for beta-galactosidase activity overnight at 37°C with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (ICN Biomedicals). No endogenous beta-galactosidase activity was visible after 24 h of incubation in X-Gal solution. Infectious titers were determined by counting the number of positive cells in the different dilutions, using a calibrated microscope (ocular diameter, 3.1 mm2) and then multiplying by the area of the well and the dilution of the virus. Titers were determined by the average number of cells in a minimum of 10 fields per well.
Heparin competition.
Serial dilutions of dialyzed virus were incubated in 400 μl of medium supplemented with 20 μg of heparin/ml (Sigma H-3149) for 1 h at room temperature and then added to 2 × 104 cos cells in a 12-well plate. The cells were incubated with the virus for 1 h, after which the virus was removed and the cells were rinsed three times with medium. One milliliter of medium was added to the cells, and the cells were incubated for 48 to 60 h, followed by fixing and staining as described above.
Nucleotide sequence accession number.
The sequence of AAVs is available through GenBank under accession no. AF085716.
RESULTS
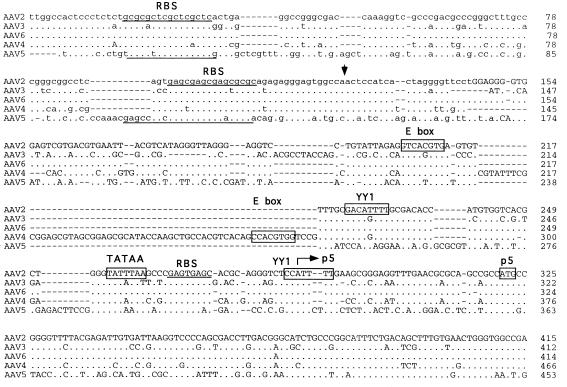
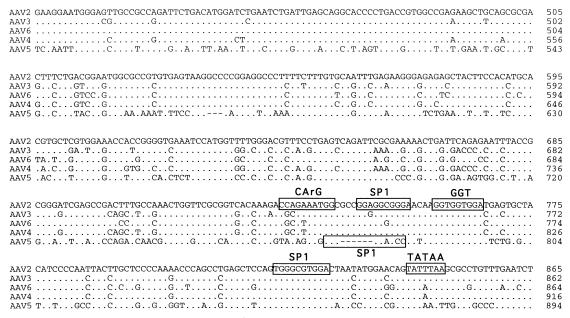
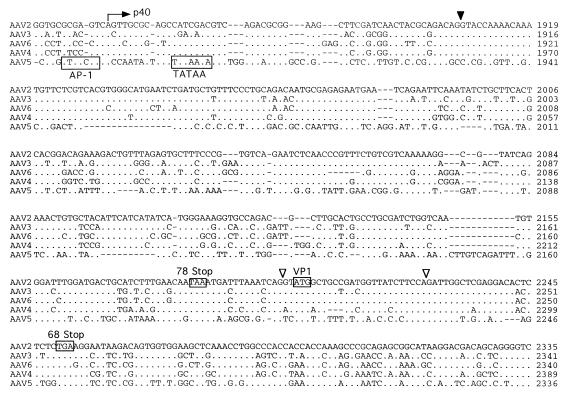
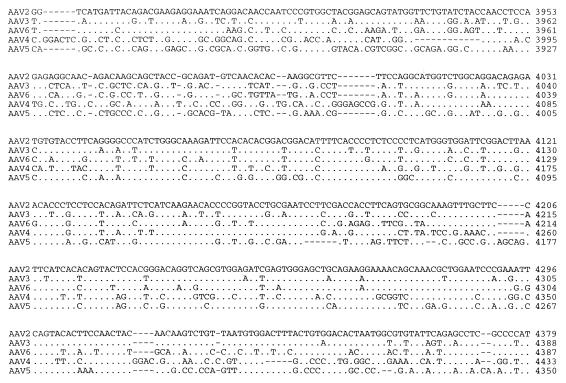
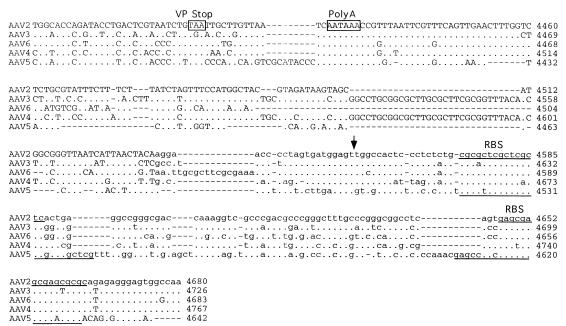
A seed stock of AAV5 produced by coinfection of adherent cos cells was expanded by passage through HeLa cells in suspension cultures. The viral particles were isolated by CsCl isopycnic gradient centrifugation. DNA dot blots of the gradient fractions indicated that the highest concentration of viral genomes was contained in fractions with a buoyant density of 1.385 to 1.420 g/cm3, which is similar to that of AAV2. Analysis of annealed virion DNA obtained from these fractions indicated a major species of 4.6 kb in length, which upon restriction analysis with BamHI or EcoRI gave bands similar in size to those previously reported (1). Additional restriction endonuclease mapping indicated a unique BssHII site at one end of the viral genome. This site was used in cloning the major fragment of the viral genome. Additional overlapping clones were isolated, the sequence of the entire viral genome was determined, and two distinct ORFs were identified (Fig. 1).
FIG. 1.
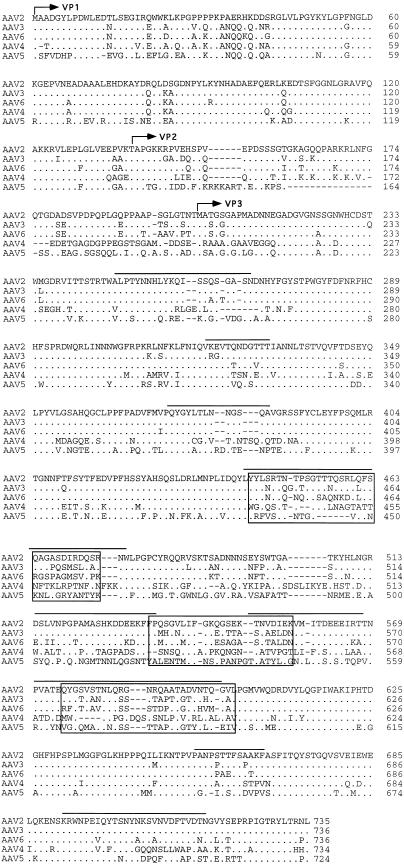
Sequence of the AAV5 genome. The genomes of AAV2, AAV3, AAV4, AAV5, and AAV6 were aligned by using the Align program (Scientific and Educational Software). The sequence of the ITR region is presented in lowercase type, and the rest of the genome is shown in capitals. trs are indicated by vertical arrows. Proposed transcription factor binding sites are boxed, with the name of the site above, as is the single polyadenylation site. Identified promoters are indicated by horizontal arrows, with their corresponding AAV2 map position indicated. Initiation (ATG) and termination (STOP) codons are marked. Splice donor and acceptor sites are indicated by solid and open triangles, respectively.
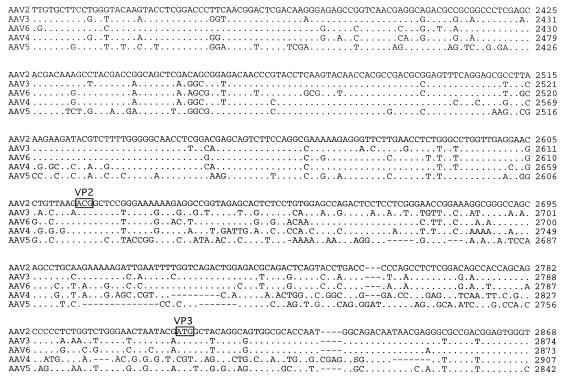
Computer analysis indicated that the left-hand ORF was approximately 67% homologous to that of the AAV2 Rep ORF (Fig. 2). In contrast, the rep gene sequences of AAV2, AAV3, AAV4, and AAV6 were greater than 90% similar. The central portion of the AAV5 Rep ORF (amino acids [aa] 322 to 470), which is present in all four Rep proteins, has the greatest homology (90%) with the Rep ORFs of the other cloned AAV serotypes. Although some sequence differences were found in this region, data from functional studies of AAV2 Rep predict that these changes will not affect the biochemical activities of AAV5. The amino terminus (aa 1 to 322) of the p5-promoted Rep proteins (Rep68 and Rep78) was 62% similar between AAV2 and AAV5. The carboxyl-terminal portion (aa 470 to 610) of the unspliced Rep polypeptides (Rep78 and Rep52) was 26% similar. The amino terminus of Rep is necessary for DNA binding and for trs endonuclease activities. Although the specific amino acids involved in DNA binding have not been identified, AAV2 tyrosine 152 is conserved and is important for trs endonuclease activity (49). An amphipathic helix region important for Rep multimerization (aa 159 to 179) (41) was also conserved in AAV5. The carboxyl termini of Rep78 and Rep52 encode a Zn finger motif which is conserved among the AAV serotypes. This region is required for interaction with the cellular kinases PrKX and PKA (11), causing the inhibition of kinase activity.
FIG. 2.
(Top) Rep functional domains. Within the rep gene, two promoters, located at map units 5 and 19, direct the expression of four proteins, Rep78 and Rep68 and Rep52 and Rep40, respectively. Two proteins are produced from each promoter, one spliced, Rep68 and Rep40, and one unspliced, Rep78 and Rep52. Within the rep ORF, several functional domains have been identified and are indicated at the top. These include DNA binding (DB), trs (Y), NTP binding pocket (NTP), nuclear localization domain (NLS), zinc finger (Zn), and splice sites, with their relative amino acid positions indicated below. (Bottom) Comparison of rep ORF. The rep ORFs of AAV2, AAV3, AAV4, AAV6, and AAV5 were compared by using the Palign program (Pcgene). Identical amino acids are indicated by a dot. Dashes indicate gaps in the sequence added by the alignment program. The initiator codon of the p5 Rep proteins Rep78 and Rep68 and the p19 Rep proteins Rep52 and Rep40 are indicated by a horizontal arrow. Tyrosine 152 which has been shown to be important in trs activity is indicated by an asterisk. The NTP binding site is overlined, and lysine 340 is in bold. Regions necessary for multimerization are indicated above the sequence. Basic amino acids of the nuclear localization signal (NLS) are underlined and indicated above. A vertical arrow indicates the point of divergence for the Rep78 and Rep52 proteins from Rep68 and Rep40 as a result of the splicing event. The coordinating cystine and histidine amino acids which form the zinc finger structure in the carboxy terminus are indicated by dots.
Similarities between the AAV2 and AAV5 p5 promoters were also found. Core transcriptional elements such as the TATAA box and Rep and USF binding sites were conserved (Fig. 1). However, the YY1 sites at positions +1 and −60 relative to the transcription start site were not found, suggesting that AAV5 gene expression is regulated differently from that of AAV2. The organization and sequence of the p19 promoters were conserved. The presence and relative position of transcription factor binding sites within the p40 promoter of AAV5 were retained but were shifted in their alignment compared to those of the other serotypes (Fig. 1).
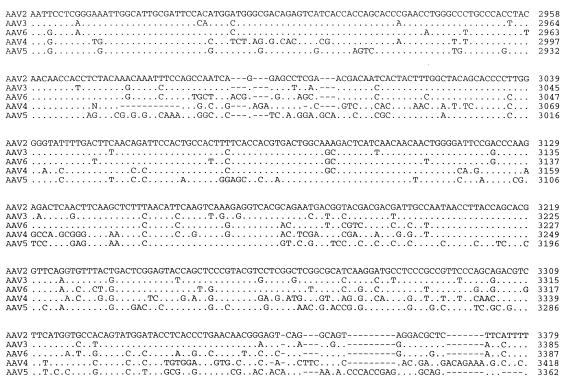
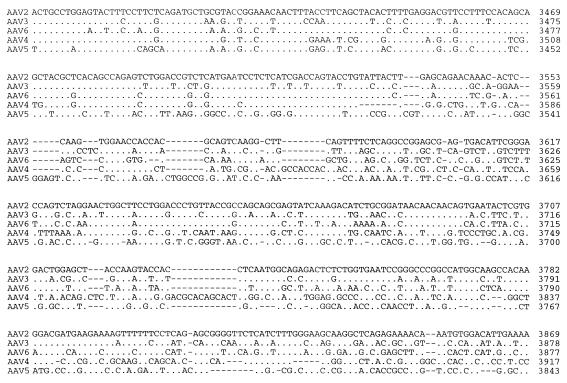
The right ORF of AAV5, which encodes the three viral capsid proteins, was found to be approximately 56% identical to the capsid ORF of AAV2 (Fig. 3). As with the sequences of the other reported AAV serotypes, the related structural elements can be organized into blocks of similar and divergent sequence. By using the published three-dimensional structure of the canine parvovirus and computer-aided sequence comparisons, a number of these divergent regions have been shown to be on the exterior of the virus, thus suggesting an altered tissue tropism (Fig. 3).
FIG. 3.
Comparison of cap ORF. The capsid ORFs of AAV2, AAV3, AAV4, AAV5, and AAV6 were compared, using the Palign program (Pcgene). Identical amino acids are indicated by a single dot. Dashes indicate gaps in the sequence added by the alignment program. The theoretical initiator codons of VP2 and VP3 are indicated by horizontal arrows and identified. Regions which have been proposed to be on the surface of AAV are overlined (3a). Divergent regions are boxed.
The VP1 ORF of AAV5 has approximately 60% homology with those of AAV2, AAV3, and AAV6 and the goose dependovirus (59%). A phylogenetic analysis (CLUSTAL) of the VP1 ORF indicated that AAV5 is equally divergent from AAV2, AAV3, and AAV6 and goose dependovirus (data not shown). Furthermore, the rep ORF of AAV5 has a slightly greater homology with the other AAV serotypes (66%) than with the goose dependovirus (57%) (data not shown).
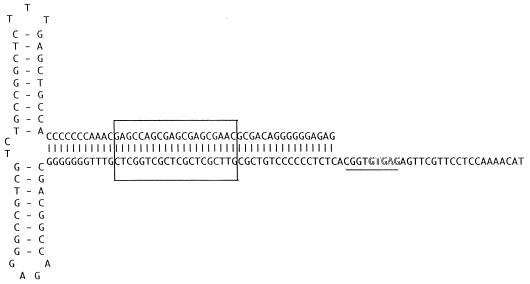
The ITR of AAV5 was cloned as a fragment from the right end of the genome. The AAV5 ITR is only 58% homologous with the AAV2 ITR but still retained the ability to fold into a hairpin structure (Fig. 4). As with the rep ORF, this percent homology was much lower than that observed with the other cloned AAV serotypes (2, 3, 4, and 6). Two sequence elements within the stem region of the hairpin have been found to be critical for the function of the ITR as an origin of viral replication. A repeat motif of GAGC/T serves as the recognition site of Rep78 and Rep68 and is conserved between AAV2 and AAV5. The AAV2 trs recognition motif (GGTTGA) located downstream of the Rep binding site is conserved among AAV serotypes 3, 4, and 6 but has only weak homology with that of AAV5 serotypes. This suggests that the ITRs and Rep proteins of AAV5 will not complement those of the other known AAV serotypes.
FIG. 4.
AAV5 ITR. The sequence of the ITR is shown in the hairpin conformation. The putative Rep binding site is boxed, and the region homologous to the AAV2 trs is underlined, with the sequence differences in the trs outlined.
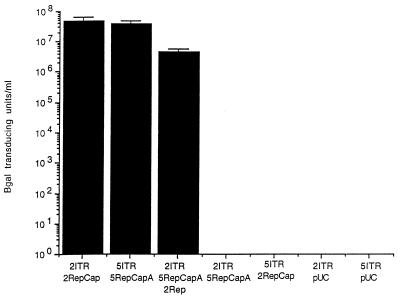
We tested the ability of AAV rep and cap gene products to replicate and package a type 5 ITR genome containing a beta-galactosidase reporter gene and conversely, the ability of AAV5 Rep and Cap to replicate and package an AA2 ITR beta-galactosidase construct. Recombinant particles were packaged by either using type 2 Rep and Cap (2RepCap) or type 5 Rep and Cap (5RepCapA) expression plasmids as previously described (9). As shown in Fig. 5, a combination of the 2ITR and 5ITR with their respective AAV2 and AAV5 expression plasmids produced high titers of virus, but a combination of the 2ITR with the 5RepCapA expression plasmid or the 5ITR with the 2RepCap expression plasmid produced no detectable transduction events. Likewise, no transducing units were detected with a combination of 2ITR or 5ITR with pUC. However, cotransfection of the 2ITR vector with the 5RepCapA expression plasmid and a plasmid expressing the Rep78 protein of AAV2 (2Rep) produced some transducing particles (Fig. 5). This result suggests that the AAV2 DNA could be packaged into an AAV5 capsid. The lack of cross-complementation between AAV2 and AAV5 is a distinct result from that of other serotypes of AAV which have shown cross-complementation of the Rep and ITRs (10).
FIG. 5.
Complementation assay. Recombinant AAV particles were produced in cos cells by using plasmids which contain the rep and cap genes of AAV2 (2RepCap), AAV5 (5RepCapA), or AAV2 Rep alone (2Rep) and a nucleus-localized beta-galactosidase gene with an RSV promoter and either AAV2 ITRs (2ITR) or AAV5 ITRs (5ITR). After electroporation, the cos cells were infected with adenovirus and cleared viral lysate was prepared and used to transduce cos cells.
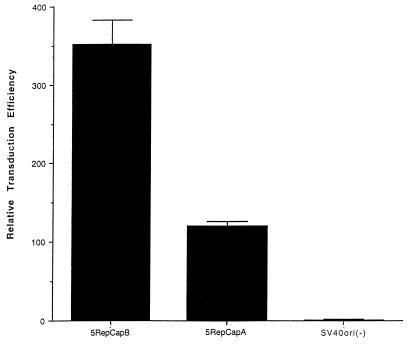
The inclusion of SV40ori in the AAV2 RepCap packaging (expression) plasmid has been shown to improve the yield of recombinant particles 50- to 100-fold (9). The inclusion of this element in the AAV5 packaging plasmid also resulted in an increase in the number of particles generated (Fig. 6). Comparing relative transduction efficiencies of particles generated using a packaging plasmid lacking the SV40ori [SVori(−)] versus one that includes it (5RepCapA) resulted in greater than a 100-fold increase in the relative number of transducing particles produced (Fig. 6). The orientation of SV40ori relative to the RepCap fragment also had an effect on the yield of transducing particles. Positioning the SV40ori next to the poly(A) signal (5RepCapB) resulted in a 2.9-fold increase in transducing particles compared to that produced by placement of the SVori adjacent to the p5 promoter (5RepCapA) (Fig. 6). It has not been determined whether the difference in particle yields results from replication of the helper plasmid, i.e., increased copy number of the plasmid or enhanced expression.
FIG. 6.
Effect of SV40ori on particle production. cos cells were electroporated with a vector plasmid containing AAV5 ITRs and a nucleus-localized beta-galactosidase gene with an RSV promoter (AAV5RnlacZ) and one of three helper plasmids: AAV5RepCapA, AAV5RepCapB, and SV40ori(−). Cells were infected with adenovirus, and at 48 h postinfection, clear viral lysate was prepared and serial dilutions of the virus were used to transduce cos cells. The relative transduction efficiencies are reported compared to the transduction efficiency of the SV40ori(−) helper plasmid.
After purification of recombinant AAV5 and AAV2 particles (rAAV5 and rAAV2) by CsCl isopycnic density gradient centrifugation, quantitation by DNA dot blot hybridization indicated that a greater number of AAV5 particles were generated per producer cell than AAV2 particles. In a comparative study using packaging plasmids with the SV40ori in the same orientation relative to the RepCap fragment, 1.2 × 1012 rAAV5 particles were produced from 8 × 107 cos cells compared to 1 × 1011 rAAV2 particles. These results were similar to those observed with AAV4, in which the yield of recombinant AAV4 particles was approximately 10-fold greater than that of AAV2 (10).
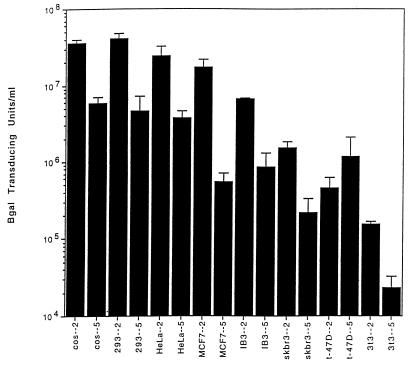
The sequence divergence in the capsid protein ORF implies that the tissue tropisms of AAV5 and AAV2 may differ. Tissue tropism was assessed in part by transducing a variety of cell lines with serially diluted, purified rAAV5 or rAAV2 containing the gene for nucleus-localized beta-galactosidase. As shown in Fig. 7, cos cells transduced with an equivalent number of rAAV2 or rAAV5 particles resulted in a sixfold higher transducing titer of rAAV2 than rAAV5. Similarly, 293, HeLa, and IB3 cells were transduced more efficiently with rAAV2 than with rAAV5 (nine-, seven-, and eightfold, respectively). However, transduction of MCF7 cell lines with rAAV2 was approximately 32 times higher than that obtained with rAAV5. NIH 3T3, skbr3, and t-47D cell lines exhibited poor transduction efficiencies for both vectors.
FIG. 7.
Titers for rAAV2LacZ and rAAV5LacZ. Different cell lines were transduced with an equal number of particles in serial dilutions of either rAAV2 or rAAV5 expressing LacZ (see Materials and Methods) and are expressed as transducing units per milliliter.
Previous work has shown that adenovirus can increase the transduction efficiency of AAV2 (12, 13). As a control for adenovirus effects on transduction levels, cos, HeLa, 293, MCF7, NIH 3T3, and IB3 cells were coinfected with rAAV and adenovirus and stained for beta-galactosidase activity after 24 h. The transduction efficiency of rAAV increased in the presence of adenovirus; however, there was no increase in the relative transduction efficiencies between rAAV2 and rAAV5 in any of the cell lines (data not shown). This suggests that the difference in transduction efficiencies was not the result of adenovirus contamination.
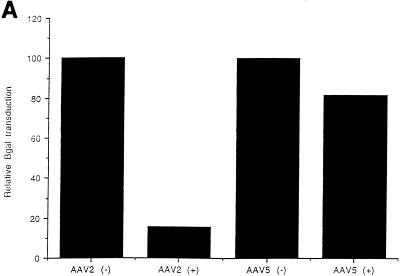
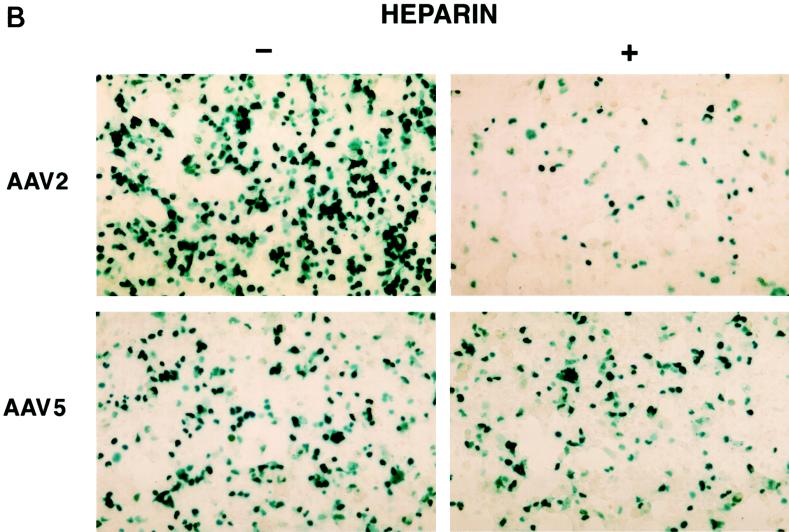
The large difference in transduction efficiencies for AAV2 and AAV5 in MCF7 cells compared to cos, HeLa, and 293 cells suggests that distinct virus uptake mechanisms may exist for AAV2 and AAV5. A recent publication demonstrated that HSP on the surfaces of cells are involved in AAV2 transduction (45). Incubation of soluble heparin with rAAV2 was shown to inhibit transduction by blocking viral binding. Since the transduction data suggested a difference in tissue tropism for AAV5 and AAV2, the sensitivity of AAV5 transduction to heparin was determined. At a multiplicity of infection (MOI) of 100, a final concentration of 20 μg of heparin/ml had a minimal effect on AAV5 transduction as shown in Fig. 8A. In contrast, this amount of heparin inhibited 90% of the AAV2 transduction. Even at an MOI of 1,000, a strong inhibition of AAV2 transduction was observed, with a minimal effect on AAV5 (Fig. 8B). These data support the hypothesis developed from the tissue tropism study that AAV2 and AAV5 may utilize distinct cell surface molecules for binding and uptake.
FIG. 8.
Heparin competition. (A) Effect of soluble heparin sulfate on AAV5 and AAV2 transduction as determined by preincubation of rAAV5 virus (MOI, 100) containing the beta-galactosidase gene with 20 μg of heparin sulfate/ml prior to transduction of cos cells. Free virus was washed from the cells after a 1-h incubation and stained for beta-galactosidase activity. Transduction efficiency was then normalized to the heparin-minus condition. (B) Effect of heparin sulfate on AAV5 and AAV2 transduction at an MOI of 1,000.
DISCUSSION
With the cloning and sequencing of AAV3, AAV4, AAV5, and AAV6, our understanding of the AAV family has increased significantly. The high degree of conservation of the rep ORF between AAV2, AAV3, AAV4, and AAV6 emphasizes the importance of this gene to the biology of the virus. While the rep ORF of AAV5 is significantly more divergent than in other serotypes, the core region (aa 322 to 470) which is common to all four Rep proteins is highly conserved among the serotypes (90% similar). Furthermore, the ITRs of each serotype are similar in length and symmetry, retaining the ability to form a characteristic hairpin structure. Mutational analysis has demonstrated that the ability to form the terminal hairpin structure is vital to AAV replication (30); conservation of this motif among the five AAV serotypes supports these findings.
The divergent sequences among the serotypes have also been informative. Comparison of the capsid ORFs of the different serotypes has identified a number of divergent sequence blocks within a framework of conserved regions. Some of these divergent sequences may lie on the exterior of the viral particle, thus accounting for the altered tissue tropism, antibody response, and hemagglutination activity that has been reported in the different serotypes. Furthermore, the divergence in the AAV5 Rep and ITR may be useful in defining the DNA binding domain, as well as amino acids that are involved in trs activity.
Sequence analysis, tissue tropism, and the heparin sensitivity studies identify AAV5 as a distinct virus among the characterized dependoviruses. Although some of the p5 promoter elements are retained in AAV serotypes 2 through 6, other conserved elements are absent in AAV5, suggesting alternative mechanisms of transcriptional regulation. Conversely, the conservation of some transcriptional elements within the p40 promoter, despite the divergence in their sequences and relative positions, indicates the importance of these sites in the regulation of expression.
The rep ORF of AAV5 is distinct from that of AAV serotypes 2, 3, 4, and 6, and no AAV2 ITR-containing genomes can be packaged into type 5 capsids. However, transducing particles are produced if AAV2 rep ORF is added, suggesting that the lack of complementation is not at the level of viral assembly, e.g., viral DNA packaging, but at the level of viral DNA replication. The absence of an AAV2 trs consensus motif supports this idea and has been confirmed by in vitro replication data (7). Unexpectedly, production of rAAV5 particles using standard packaging systems is approximately 10- to 50-fold more efficient than that for rAAV2, which may facilitate its use as a vector for gene therapy.
AAV2 transduction is inefficient in the absence of HSP and is inhibited by soluble heparin (45). Although the role of heparan sulfate binding in AAV2 transduction is not understood, it may be that a second interaction is responsible for AAV2 uptake, with HSP acting to concentrate the virus on the cell surface through an electrostatic interaction. If an interaction between the virion and a less abundant but higher affinity receptor(s) is necessary, the weaker interaction with HSP would promote entry. Though the nature of this additional interaction is speculative, the fact that AAV5 transduction is not neutralized by soluble heparin is evidence that AAV5 may utilize a distinct pathway for uptake.
AAV5 is distinct among the dependent parvoviruses in that it was originally isolated from a patient sample instead of from laboratory stocks of adenovirus. Sequence analysis and biochemical results define AAV5 as unique among the dependoviruses. The AAV5 ITR and Rep protein are unique and do not complement those of AAV2. Moreover, AAV5 transduction is not sensitive to heparin, which inhibits AAV2. These distinct activities of AAV5 and their linkage to the viral sequence will be of importance with regard to the analysis of the viral protein structure and function and the development of chimeric vectors.
ACKNOWLEDGMENT
We thank Ursula Bantel-Schaal for her kind gift of AAV5 stocks.
REFERENCES
- 1.Bantel-Schall U, zur Hausen H. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology. 1984;134:52–63. doi: 10.1016/0042-6822(84)90271-x. [DOI] [PubMed] [Google Scholar]
- 2.Blacklow N R, Hoggan M D, Rowe W P. Serologic evidence for human infection with adeno-associated viruses. J Natl Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- 3.Buller R M L, Rose J A. Characterization of adenovirus-associated virus-induced polypeptides in KB cells. J Virol. 1978;25:3331–3338. doi: 10.1128/jvi.25.1.331-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Chapman M S, Rossmann M G. Structure, sequence, and function correlations among parvoviruses. Virology. 1993;194:491–508. doi: 10.1006/viro.1993.1288. [DOI] [PubMed] [Google Scholar]
- 4.Chejanovsky N, Carter B J. Mutagenesis of an AUG codon in the adeno-associated virus rep gene: effects on viral DNA replication. Virology. 1989;173:120–128. doi: 10.1016/0042-6822(89)90227-4. [DOI] [PubMed] [Google Scholar]
- 5.Chejanovsky N, Carter B J. Replication of a human parvovirus nonsense mutant in mammalian cells containing an inducible amber suppressor. Virology. 1989;171:239–247. doi: 10.1016/0042-6822(89)90531-x. [DOI] [PubMed] [Google Scholar]
- 6.Chiorini J A, Wiener S M, Kotin R M, Owens R A, Kyöstiö S R M, Safer B. Sequence requirements for stable binding and function of adeno-associated virus type 2 Rep68 protein on the ITR. J Virol. 1994;68:7448–7457. doi: 10.1128/jvi.68.11.7448-7457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiorini, J. A., S. A. Afione, and R. M. Kotin. 1999. Submitted for publication.
- 8.Chiorini J A, Weitzman M D, Owens R A, Urcelay E, Safer B, Kotin R M. Biologically active Rep proteins of adeno-associated virus type 2 produced as fusion proteins in Escherichia coli. J Virol. 1994;68:797–804. doi: 10.1128/jvi.68.2.797-804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiorini J A, Wendtner C M, Urcelay E, Safer B, Hallek M, Kotin R M. High-efficiency transfer of the T cell co-stimulatory molecule B7-2 to lymphoid cells using high-titer recombinant adeno-associated virus vectors. Hum Gene Ther. 1995;6:1531–1541. doi: 10.1089/hum.1995.6.12-1531. [DOI] [PubMed] [Google Scholar]
- 10.Chiorini J A, Yang L, Lui Y, Safer B, Kotin R M. Cloning and characterization of AAV type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiorini J A, Zimmermann B, Yang L, Smith R H, Ahearn A, Herberg F, Kotin R M. Inhibition of PrKX, a novel protein kinase, and the cAMP-dependent protein kinase, PKA, by the regulatory proteins of adeno-associated virus type 2. Mol Cell Biol. 1998;18:5921–5929. doi: 10.1128/mcb.18.10.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flannery J G, Zolotukhin S, Vaquero M I, LaVail M M, Muzyczka N, Hauswirth W W. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georg-Fries B, Biederlack S, Wolf J, zur Hausen H. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology. 1984;134:64–71. doi: 10.1016/0042-6822(84)90272-1. [DOI] [PubMed] [Google Scholar]
- 16.Hermanns J, Schulze A, Durr P, Kleinschmidt J A, Schmidt R, zur Hausen H. Infection of primary cells by adeno-associated virus type 2 results in a modulation of cell cycle-regulating proteins. J Virol. 1997;71:6020–6027. doi: 10.1128/jvi.71.8.6020-6027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermonat P L. The adeno-associated virus Rep78 gene inhibits cellular transformation induced by bovine papillomavirus. Virology. 1989;172:253–261. doi: 10.1016/0042-6822(89)90127-x. [DOI] [PubMed] [Google Scholar]
- 18.Hermonat P L. Inhibition of H-ras expression by the adeno-associated virus Rep78 transformation suppressor gene product. Cancer Res. 1991;51:3373–3377. [PubMed] [Google Scholar]
- 19.Hermonat P L. Inhibition of bovine papillomavirus plasmid DNA replication by adeno-associated virus. Virology. 1992;189:329–333. doi: 10.1016/0042-6822(92)90710-7. [DOI] [PubMed] [Google Scholar]
- 20.Hermonat P L, Labow M A, Wright R, Berns K I, Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984;51:329–333. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermonat P L, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im D-S, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61:447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- 23.Im D S, Muzyczka N. Partial purification of adeno-associated virus Rep78, Rep52, and Rep40 and their biochemical characterization. J Virol. 1992;66:1119–1128. doi: 10.1128/jvi.66.2.1119-1128.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khleif S N, Myers T, Carter B J, Trempe J P. Inhibition of cellular transformation by the adeno-associated virus rep gene. Virology. 1991;181:738–741. doi: 10.1016/0042-6822(91)90909-u. [DOI] [PubMed] [Google Scholar]
- 25.Kleinschmidt J A, Möhler M, Weindler F W, Heilbronn R. Sequence elements of the adeno-associated virus rep gene required for suppression of herpes-simplex-virus-induced DNA amplification. Virology. 1995;206:254–262. doi: 10.1016/s0042-6822(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 26.Kotin R M, Menninger J C, Ward D C, Berns K I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- 27.Labow M A, Berns K I. The adeno-associated virus rep gene inhibits replication of an adeno-associated virus/simian virus 40 hybrid genome in cos-7 cells. J Virol. 1988;62:1705–1712. doi: 10.1128/jvi.62.5.1705-1712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labow M A, Graf L H, Jr, Berns K I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987;7:1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laughlin C A, Tratschin J D, Coon H, Carter B J. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983;23:65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- 30.LeFebvre R B, Riva S, Berns K I. Conformation takes precedence over sequence in adeno-associated virus DNA replication. Mol Cell Biol. 1984;4:1416–1419. doi: 10.1128/mcb.4.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCown T J, Xiao X, Li J, Breese G R, Samulski R J. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 32.Mendelson E, Trempe J P, Carter B J. Identification of the trans-acting Rep proteins of adeno-associated virus by antibodies to a synthetic oligopeptide. J Virol. 1986;60:823–832. doi: 10.1128/jvi.60.3.823-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muramatsu S I, Mizukami H, Young N S, Brown K E. Nucleotide sequence and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221:208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- 34.Owens R A, Trempe J P, Chejanovsky N, Carter B J. Adeno-associated virus rep proteins produced in insect and mammalian expression systems: wild-type and dominant-negative mutant proteins bind to the viral replication origin. Virology. 1991;184:14–22. doi: 10.1016/0042-6822(91)90817-u. [DOI] [PubMed] [Google Scholar]
- 35.Rutledge E A, Halbert C L, Russell D W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan J H, Zolotukhin S, Muzyczka N. Sequence requirements for binding of Rep68 to the adeno-associated virus terminal repeats. J Virol. 1996;70:1542–1553. doi: 10.1128/jvi.70.3.1542-1553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samulski R J, Chang L S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samulski R J, Zhu X, Xiao X, Brook J D, Housman D E, Epstein N, Hunter L A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. . (Erratum, 11:1228, 1992.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senapathy P, Tratschin J D, Carter B J. Replication of adeno-associated virus DNA. Complementation of naturally occurring rep− mutants by a wild-type genome or an ori− mutant and correction of terminal palindrome deletions. J Mol Biol. 1984;179:1–20. doi: 10.1016/0022-2836(84)90303-6. [DOI] [PubMed] [Google Scholar]
- 40.Smith R H, Kotin R M. The Rep52 gene product of adeno-associated virus is a DNA helicase with 3′-to-5′ polarity. J Virol. 1998;72:4874–4881. doi: 10.1128/jvi.72.6.4874-4881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith R H, Spano A J, Kotin R M. The Rep78 gene product of adeno-associated virus (AAV) self-associates to form a hexameric complex in the presence of AAV ori sequences. J Virol. 1997;71:4461–4471. doi: 10.1128/jvi.71.6.4461-4471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder R O, Im D-S, Ni T, Xiao X, Samulski R J, Muzyczka N. Features of the adeno-associated virus origin involved in substrate recognition by the viral Rep protein. J Virol. 1993;67:6096–6104. doi: 10.1128/jvi.67.10.6096-6104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava A, Lusby E W, Berns K I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45:555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trempe J P, Carter B J. Alternate mRNA splicing is required for synthesis of adeno-associated virus VP1 capsid protein. J Virol. 1988;62:3356–3363. doi: 10.1128/jvi.62.9.3356-3363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trempe J P, Mendelson E, Carter B J. Characterization of adeno-associated virus rep proteins in human cells by antibodies raised against rep expressed in Escherichia coli. Virology. 1987;161:18–28. doi: 10.1016/0042-6822(87)90166-8. [DOI] [PubMed] [Google Scholar]
- 48.Urcelay E, Ward P, Wiener S M, Safer B, Kotin R M. Asymmetric replication in vitro from a human sequence element is dependent on adeno-associated virus Rep protein. J Virol. 1995;69:2038–2046. doi: 10.1128/jvi.69.4.2038-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker S L, Wonderling R S, Owens R A. Mutational analysis of the adeno-associated virus Rep68 protein: identification of critical residues necessary for site-specific endonuclease activity. J Virol. 1997;71:2722–2730. doi: 10.1128/jvi.71.4.2722-2730.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weitzman M D, Kyöstiö S R M, Kotin R M, Owens R A. Adeno-associated virus (AAV) Rep proteins mediate complex formation between AAV DNA and its integration site in human DNA. Proc Natl Acad Sci USA. 1994;91:5808–5812. doi: 10.1073/pnas.91.13.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wonderling R S, Kyöstiö S R M, Owens R A. A maltose-binding protein/adeno-associated virus Rep68 fusion protein has DNA-RNA helicase and ATPase activities. J Virol. 1995;69:3542–3548. doi: 10.1128/jvi.69.6.3542-3548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Q, Chen F, Trempe J P. Characterization of cell lines that inducibly express the adeno-associated virus Rep proteins. J Virol. 1994;68:4847–4856. doi: 10.1128/jvi.68.8.4847-4856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Q, Kadam A, Trempe J P. Mutational analysis of the adeno-associated virus rep gene. J Virol. 1992;66:6058–6069. doi: 10.1128/jvi.66.10.6058-6069.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]