Abstract
Background
At present, it is not known whether targeting plus immunotherapy combined with transarterial chemoembolization (TACE) can improve the efficacy of hepatocellular carcinoma (HCC). The aim of this retrospective experiment was to explore the difference in clinical efficacy between antiangiogenic drugs plus PD-1 inhibitors combined with and without TACE.
Methods
Clinical data of 145 patients with HCC who received anti-angiogenesis therapy plus PD-1 inhibitor combined with TACE (TACE-P-T) (n = 62) or anti-angiogenesis therapy combined with PD-1 inhibitor (P-T) (n = 83) in China from October 2018 to December 2022 were collected and reviewed. We used propensity matching (PSM) to create two groups with comparable baseline scores, compared their median survival time (mOS) and median progression-free survival time (mPFS), and performed subgroup analysis.
Results
Before PSM, the mOS and mPFS of patients were 20.3 and 5.0 months in the triple therapy group and 13.6 and 7.4 months in the control group, respectively. After PSM, the mOS and mPFS of patients were 19.7 and 6.6 months in the triple treatment group and 10.5 and 3.7 months in the control group, respectively. Therefore, the TACE-P-T group showed better survival outcomes than P-T. In the subgroup analysis, compared with the control group, the mOS was 10.7 vs 20.3 months in the alpha fetoprotein (AFP) (≥ 400ng/mL/<400ng/mL) group, 29.3 vs 7.4 months in the alkaline phosphatase (ALP) (≥ 125u/L/< 125u/L) group and 10.5 vs 20.0 months in the Portal vein invasion (PVTT) group.
Conclusion
Antiangiogenic therapy combined with PD-1 inhibitors combined with TACE has significant survival benefits for HCC patients.
Keywords: HCC, PD-1 inhibitors, antiangiogenic therapy, TACE
Introduction
The incidence of liver cancer has been increasing in many countries in recent decades. As the most common histological type of liver cancer, hepatocellular carcinoma (HCC) accounts for most liver cancer diagnoses and deaths.1 Chronic viral hepatitis and progressive liver cirrhosis are the main causes of HCC.2 Based on Barcelona staging (BCLC), the most suitable candidate for TACE is with intermediate-stage HCC (BCLC-B). Patients with stage BCLC-C have found survival benefits in a large number of studies, including a large longitudinal cohort study of 18,031 patients from 14 countries and regions, documenting the experience of patients with HCC in the real world and finding that TACE is the first-line treatment for patients with HCC near stage BCLC-C.3 So far, the only trial that has reached the main end point for the treatment of advanced HCC between 2003 and 2020 is the TACTICS trial,4 which compared the efficacy of TACE plus sorafenib with TACE alone. The median mPFS of TACE plus sorafenib was 25.2 months and TACE alone was 13.5 months (HR0.59).
At the same time, targeted drugs, mainly including sorafenib and lenvatinib, have been approved as first-line therapies for HCC. In 2020, the combination of atirizumab and bevacizumab showed a better survival rate than sorafenib, the median OS (MOS) is prolonged to 19.2 months, and the objective response rate (ORR) is prolonged to 27.3%. Becoming the new FDA-approved first-line standard regimen for unresectable or metastatic HCC.5,6
The recently developed combination strategy of TACE and PD-1/PD-L1 plus tyrosine kinase inhibitor (TKI) shows promising survival benefits for HCC patients, especially those with high risk of tumor recurrence, TACE failure or refractory disease after treatment.7 As a local inducer of immunogenic cell death in HCC, TACE has a positive effect on immunotherapy.8 Combining the latest treatments for advanced disease and systemic treatments with TACE is a promising approach to the treatment of intermediate diseases. Immune-based therapy in combination with TACE may be beneficial because TACE increases antigen release and immune recognition.9 Therefore, it is necessary to explore the efficacy of TACE combined with PD-1 inhibitor and tyrosinase inhibitor in the treatment of HCC.
In recent years, with the advances in the evaluation of the efficacy of PD-1 inhibitors alone or PD-1 inhibitors combined with targeted therapy in HCC, the combination of atrizumab and bevacizumab has been shown to improve overall survival relative to sorafenib, leading to its approval by the FDA as treatment regimen for HCC. Recently, duvalizumab plus trimeriumab and atrizumab plus capotinib showed better OS and PFS compared with sorafenib, respectively. Therefore, the combination of TACE and PD-1 inhibitors represent a promising treatment option for HCC. Marinelli studies have proved that TACE can be safely combined with programmed cell death 1 blockade and may lead to significant delays in tumor progression and disease staging in specific patients.10 Meanwhile, for the inevitable angiogenesis after TACE, targeted antivascular therapy seems to be the optimal choice.11 According to the study by Chen, targeted drugs can reduce tumor PD-L1 level and regulatory T cell (Treg) differentiation, and improve the efficacy of anti-PD-1 by blocking FGF receptor 4 (FGFR4).12 Therefore, we have reason to infer that TACE combined with PD-1 inhibitors and targeted inhibitors can provide better survival benefits than targeted immunotherapy, which was the focus of this study.
Materials and Methods
Patients
This study was approved by the institutional review committee of our university. Clinical data of 145 patients with HCC who received TACE-P-T or P-T in 4 hospitals in China from October 2018 to January 2023 were collected and reviewed, including 62 cases and 83 cases that received triple therapy and targeted combined immunotherapy, respectively. The inclusion criteria were as follows: a) pathological diagnosis of HCC; b) ECOGPS score of 0–2; c) tumor recurrence after radical resection or ablation; d) at least one measurable lesion, according to the revised solid tumor response assessment criteria (MRECIST); and e) at least one cycle of PD-1 inhibitors plus targeted therapy with or without TACE. The exclusion criteria were as follows: a) missing or incomplete follow-up information; b) number of tumors > 5 or diffuse HCC; c) other malignant tumors; d) severe ascites or hepatic encephalopathy; and e) severe heart, lung, kidney or blood coagulation dysfunction.
This study was approved by the Ethics Committee of the affiliated Hospital of Southwest Medical University (Grant No.: KY2020254). Since this is a retrospective study, no signed individual informed consent was obtained.
TACE Procedure
The TACE method has also been reported in our previous studies.13 Briefly, TACE was performed by experienced interventional departments or hepatobiliary surgeons by delivering lipiodol emulsions (10–20 mL) and one or more chemotherapeutic drugs such as cisplatin, cisplatin and mitomycin C, or fluorouracil. After inserting the microcatheter into a selected tumor blood supply vessel that circulates to the farthest end of the hepatic artery, it was embolized with gelatin sponge particles or other embolic material if necessary.
Use of PD-1 Inhibitors and Targeted Drugs
The appropriate dose of PD-1 inhibitors and targeted drugs was determined based on the patient’s height and weight. All patients were injected with PD-1 inhibitors every three weeks and took targeted drugs daily until an intolerable toxic reaction or progressive disease occurred. Drug use was discontinued if severe treatment-related adverse events occurred.
Treatment Protocol
TACE is recommended for patients with HCC with PVTT without refractory ascites or hepatic encephalopathy with an ECOG score of 0.2. TACE is not recommended for patients with hepatocellular carcinoma whose liver function grade is 3–4, liver function insufficiency and liver function grade 3 or 4. Before patients receive TACE, doctors recommend target immunotherapy. If the patient agrees to the recommendation, PD-1 combined with targeted therapy will be given after the first TACE. Patients who refuse to receive PD-1 combined with targeted therapy only receive TACE.
Follow Up
All patients underwent a physical examination every 3–6 weeks after the first treatment, including physical examination, routine blood and biochemical examination, and chest and abdomen enhanced CT or MRI enhanced scan. The last follow-up was conducted on January 17, 2023. The interval from the beginning of treatment to the progression of the disease was PFS. The interval from the beginning of treatment to death or the last follow-up was OS.
Statistical Analysis
Categorical data were expressed as a percentage (proportion of patients). All categorical variables were analyzed using C2 test and McNemar analysis. PSM was used to identify two groups with similar baseline confounders and match variables, including age, sex, tumor size, tumor number, platelet level, alkaline phosphatase (ALP), Child-Pugh score, alpha-fetoprotein (AFP) level, white blood cell level, BCLC stage, portal vein invasion, hepatitis B virus infection, extrahepatic metastasis and lymph node metastasis. PFS and OS were estimated using Kaplan-Meier method and logarithmic test. Cox analysis was used to identify the prognostic factors affecting OS and PFS. In this study, SPSS for Windows 26.0 was used for statistical analysis. Double-tail p < 0.05 was considered to be significant.
Result
Study Population
In this study, a total of 145 patients who met the inclusion and exclusion criteria received PD-1 inhibitors combined with antiangiogenic therapy with or without TACE. This study was approved by the Ethics Committee of the affiliated Hospital of Southwest Medical University (Grant No.: KY2020254).
Before PSM, the triple therapy group differed from the control group only in extrahepatic metastasis. Finally, a total of 92 patients were enrolled in the matched cohort with no difference in baseline variables between the two groups (Table 1).
Table 1.
Baseline Characteristics of the Patients Before and After PSM
| Variable | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Triple Therapy Group | Control Group | P | Triple Therapy Group | Control Group | P | |
| Patients | 62 | 83 | 46 | 46 | ||
| Male sex | 55(0.89) | 71(0.86) | 0.576 | 41(0.89) | 42(0.91) | 0.726 |
| Age ≥ 65 years | 12(0.19) | 37(0.45) | 0.141 | 9(0.20) | 11(0.24) | 0.613 |
| Child–Pugh | 0.975 | 0.769 | ||||
| A | 48(0.77) | 65(0.78) | 35(0.76) | 32(0.70) | ||
| B | 13(0.21) | 17(0.20) | 10(0.22) | 13(0.28) | ||
| C | 1(0.02) | 1(0.01) | 1(0.02) | 1(0.02) | ||
| Number of tumors ≥ 2 | 46(0.74) | 60(0.72) | 0.798 | 36(0.78) | 34(0.74) | 0.625 |
| Tumor diameter> 5cm | 48(0.77) | 59(0.71) | 0.580 | 34(0.74) | 33(0.72) | 0.815 |
| AFP | 38(0.61) | 40(0.48) | 0.118 | 27(0.59) | 27(0.59) | 1 |
| ALP levels ≥ 125 U/L | 32(0.52) | 51(0.61) | 0.236 | 27(0.59) | 25(0.54) | 0.674 |
| Platelet count ≥ 100 × 109/L | 42(0.68) | 63(0.76) | 0.277 | 32(0.70) | 30(0.65) | 0.656 |
| ALT levels ≥ 40 U/L | 29(0.47) | 45(0.54) | 0.375 | 24(0.52) | 22(0.48) | 0.677 |
| Leukocyte ≥ 4 × 109/L | 52(0.84) | 81(0.98) | 0.781 | 39(0.85) | 40(0.87) | 0.765 |
| BCLC stage | 0.731 | 0.926 | ||||
| A | 6(0.10) | 6(0.07) | 3(0.07) | 4(0.09) | ||
| B | 9(0.15) | 8(0.10) | 5(0.11) | 6(0.13) | ||
| C | 46(0.74) | 68(0.82) | 37(0.80) | 35(0.76) | ||
| D | 1(0.02) | 1(0.01) | 1(0.02) | 1(0.02) | ||
| Portal vein invasion | 28(0.45) | 43(0.52) | 0.428 | 25(0.54) | 24(0.52) | 0.834 |
| HBV | 46(0.74) | 58(0.70) | 0.568 | 34(0.74) | 35(0.76) | 0.810 |
| Lymph node metastasis | 26(0.42) | 41(0.49) | 0.373 | 21(0.46) | 22(0.48) | 0.834 |
| Extrahepatic metastases | 14(0.23) | 32(0.39) | 0.041 | 13(0.28) | 15(0.33) | 0.650 |
Abbreviations: PSM, propensity score matching; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus.
Survival
Before PSM, 24 cases and 45 patients in the triple therapy group and target immunity group died before the follow-up deadline, respectively. The median follow-up time was 18.4 months. In terms of survival benefits, the mOS and mPFS of patients were 20.3 (95% CI 17.1-NA) and 7.4 (95% CI5.3–13.9) months in the triple therapy group and 13.6 (95% CI 10.4–21.6) months and 5.0 (95% CI, 3.33–9.33) months in the control group (p < 0.05; p = 0.28), respectively (Figure 1a and b).
Figure 1.
Kaplan-Meier plots: The triple therapy group exhibited longer mOS and mPFS than that of the control group before (a and b) and after (c and d) PSM.
After PSM, the mOS and mPFS were 19.7 (95% CI, 13.9-NA) and 6.6 (95% CI, 5.33–15.8) months in the triple treatment group and 10.5 (95% CI, 5.0–16.6) and 3.7 (95% CI, 2.7–6.4) months in the control group was (p < 0.05; p = 0.065) (Figure 1c and d).
Prognostic Factors Analysis
To further determine the independent risk factors affecting OS and PFS, we carried out univariate and multivariate cox analysis after PSM. The results showed that AFP ≥ 400 ng/mL, ALP ≥ 125u/L and triple therapy were independent risk factors for mOS after PSM (p < 0.05) (Table 2). For mPFS after PSM, there are no variables as independent risk factors (Table 3).
Table 2.
Analyses of Prognostic Factors for Survival After PSM
| Factor | Overall Survival | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex (male/female) | 0.496(0.193–0.273) | 0.145 | ||
| Age (≥65/<65 years) | 1.337(0.72–2.484) | 0.357 | ||
| Child-Pugh class (A/B/C) | 0.481 | |||
| A | 1.000 | |||
| B | 1.281(0.694–2.365) | 0.429 | ||
| C | 2.088(0.498–8.756) | 0.314 | ||
| Number of tumors (≥2/<2) | 1.608(0.848–3.049) | 0.146 | ||
| Tumor diameter (≥5/<5 cm) | 1.466(0.775–2.774) | 0.239 | ||
| AFP (≥400/<400 ng/mL) | 2.435(1.307–4.537) | 0.005 | 2.046(1.068–3.918) | 0.031 |
| ALP (≥125/<125 U/L) | 3.248(1.755–6.014) | <0.001 | 2.579(1.341–4.96) | 0.005 |
| Platelet (<100,000/≥100,000/mL) | 1.546(0.829–2.883) | 0.171 | ||
| ALT (≥40/<40U/L) | 1.112(0.639–1.935) | 0.708 | ||
| Leukocyte (<4000/≥4000/mL) | 1.758(0.697–4.432) | 0.232 | ||
| HBV (positive/negative) | 1.913(0.974–3.758) | 0.06 | ||
| Portal vein invasion (yes/no) | 2.08(1.147–3.77) | 0.016 | 1.5(0.797–2.822) | 0.208 |
| BCLC | 0.065 | |||
| A | 1.000 | |||
| B | 1.245(0.175–8.883) | 0.827 | ||
| C | 4.298(1.025–18.014) | 0.046 | ||
| D | 6.979(0.959–50.804) | 0.055 | ||
| Lymph node metastasis (yes/no) | 1.335(0.765–2.329) | 0.31 | ||
| Extrahepatic metastases (yes/no) | 1.135(0.636–2.025) | 0.668 | ||
| Triple therapy (Yes/No) | 0.477(0.271–0.842) | 0.011 | 0.385(0.215–0.689) | 0.001 |
Abbreviations: PSM, propensity score matching; HR, hazard ratio; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine transaminase; HBV, hepatitis B virus; BCLC, Barcelona ClinicLiver Cancer.
Table 3.
Analyses of Prognostic Factors for Progression-Free Survival After PSM
| Factor | Progression-Free Survival | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex (male/female) | 1.058(0.456–2.455) | 0.896 | ||
| Age (≥65/<65 years) | 0.927(0.53–1.621) | 0.791 | ||
| Child-Pugh class (A/B/C) | 0.753 | |||
| A | 1 | |||
| B | 1.056(0.256–4.358) | 0.94 | ||
| C | 1.286(0.299–5.527) | 0.736 | ||
| Number of tumors (≥2/<2) | 1.597(0.918–2.781) | 0.098 | ||
| Tumor diameter (≥5/<5 cm) | 1.784(1.027–3.097) | 0.04 | 1.062(0.55–2.051) | 0.858 |
| AFP (≥400/<400 ng/mL) | 0.818(0.51–1.31) | 0.403 | ||
| ALP (≥125/<125 U/L) | 1.739(1.073–2.819) | 0.025 | 1.202(0.705–2.049) | 0.499 |
| Platelet (<100,000/≥100,000/mL) | 1.658(0.982–2.799) | 0.059 | ||
| ALT (≥40/<40U/L) | 1.119(0.701–1.787) | 0.637 | ||
| Leukocyte (<4000/≥4000/mL) | 1.604(0.767–3.355) | 0.209 | ||
| HBV (positive/negative) | 1.264(0.738–2.165) | 0.394 | ||
| Portal vein invasion (yes/no) | 1.758(1.086–2.845) | 0.022 | 1.149(0.673–1.962) | 0.611 |
| BCLC | 0.016 | 0.204 | ||
| A | 1 | 1 | ||
| B | 0.457(0.083–2.525) | 0.369 | 0.627(0.099–3.984) | 0.621 |
| C | 0.378(0.069–2.079) | 0.264 | 0.495(0.079–3.095) | 0.452 |
| D | 1.404(0.342–5.766) | 0.638 | 1.504(0.322–7.019) | 0.604 |
| Lymph node metastasis (yes/no) | 1.302(0.816–2.077) | 0.268 | ||
| Extrahepatic metastases (yes/no) | 0.88(0.528–1.466) | 0.624 | ||
| Triple therapy (Yes/No) | 0.644(0.401–1.033) | 0.068 | ||
Abbreviations: PSM, propensity score matching; HR, hazard ratio; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine transaminase; HBV, hepatitis B virus; BCLC, Barcelona Clinic Liver Cancer.
Subgroup Analysis
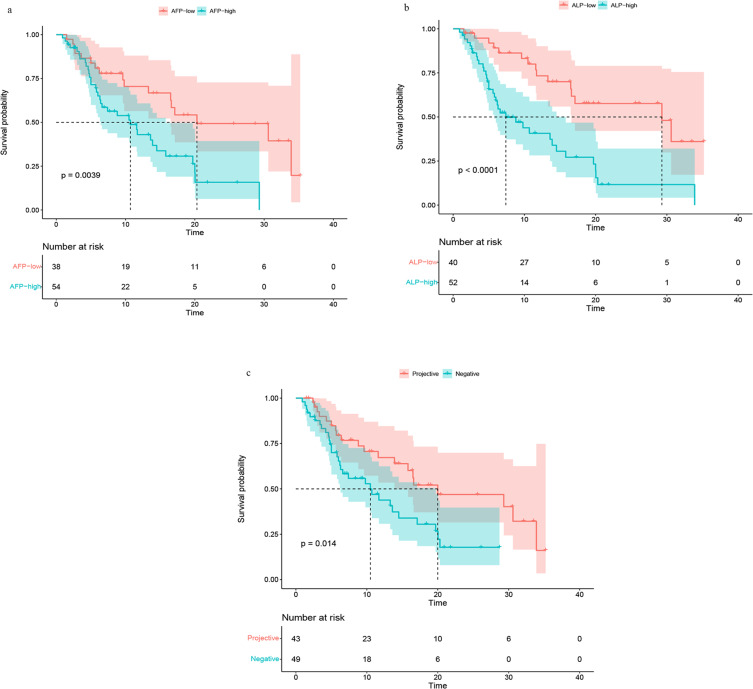
Subgroup analysis showed that compared with the control group, the mOS was lower in the AFP (≥ 400ng/mL/<400ng/mL) group (10.7 (95% CI, 6.4–15.8) vs 20.3 (95% CI, 16.5-NA) months) (Figure 2a), but higher and the mOS was in the ALP (≥ 125u/L/< 125u/L) group (29.3 (95% CI, 16.6-NA) vs.7.4 (95% CI, 5.8–14.5) months) (Figure 2b). In PVTT group, mOS was 10.5 (95% CI, 6.3–19.7) vs 20.0 (95% CI, 13.9-NA) (Figure 2c).
Figure 2.
Survival curve of AFP (≥ 400ng/mL/<400ng/mL) group (a), ALP (≥ 125u/L/< 125u/L) group (b), PVTT group (c).
Discussion
Based on results of the IMBrave1 trial, which showed that tirizumab combined with bevacizumab significantly improved survival rate compared with sorafenib, this combination therapy is presently used as the first choice for patients with Child-PughA liver function. Sorafenib and lenvatinib were listed as other recommended options for first-line systemic treatment. The combination of atirizumab and bevacizumab achieved a better response rate than sorafenib (27.3% vs 11.9%).14–16 This result suggests the need to explore combination therapies with more satisfactory clinical benefits. Therefore, based on limited clinical cases, we retrospectively compared the efficacy of TACE-P-T triple therapy and P-T combination therapy. The results showed that compared with P-T, TACE-P-T has significant survival benefits for patients with HCC.
In the present study, before PSM, compared with the control group, both the OS and PFS of triple therapy were higher (20.3 vs 13.6, p < 0.05; 7.4 vs 5.0, p = 0.28). Similarly, after PSM, the OS and PFS of triple therapy were higher (19.7 vs 10.5, p < 0.05; 6.6 vs 3.7, p = 0.065) compared with the control group. This suggests that TACE improves the efficacy of systemic therapies for HCC. This may be partly because TACE surgery creates a hypoxic environment, which can induce neovascularization by stimulating vascular endothelial growth factor (VEGF) and other angiogenic pathways and promote vascular remodeling and growth of residual survival tumors.17–19 TACE provides a potential pillar for effective systemic therapy to improve survival outcomes. TACE can upregulate tumor antigens and “danger” signals, which can make the tumor more responsive to immunotherapy.20,21
At present, the development of TACE has shown an increasing trend. Studies have shown that stable homogeneous iodide preparation technology (SHIFT) can fully disperse ICG (indocyanine green) into lipid iodine for transcatheter embolization combined with fluorescence guided resection.22 Carrier-free indocyanine green nanoparticles (nanoICG) were also developed and then dispersed into lipiodol using ultra-stable homogeneous lipid alcohol preparation technology (SHIFTnanoICG) for transcatheter arterial embolization combined with near-infrared fluorescence-guided precision hepatectomy.23 At the same time, targeted combined immunotherapy has attracted research attention as a potential advanced systemic therapy for HCC. According to the latest IMbrave150 data, the median OS of Atezolizumab plus bevacizumab was higher than that of the single targeted therapy group (19.2 months (95% CI 17.0–23.7) vs (13.4 months (95% CI 11.4–16.9).14–16 Therefore, for patients with HCC, a treatment strategy that combines TACE with targeted systemic therapy may further enhance the survival benefits of patients. Our results reveal the necessity of combining TACE with P-T to achieve synergistic effect.
Several studies have confirmed the efficacy and safety of the combination of PD-1 inhibitors and anti-angiogenesis therapy. Therefore, the combination of local therapy and target immunotherapy can enhance the efficacy of systemic treatments for tumors. Some studies have shown that the combination of TACE, external radiotherapy, internal radiotherapy and sorafenib or lenvatinib has an positive clinical effect.4,21,24–26 Su and others demonstrated that radiotherapy combined with PD-1 and targeted therapy has survival benefits,27 further proving the bright prospect of TACE combined with target immunotherapy.
Studies have shown that TACE combined with target immunotherapy can improve the patients’ OS and PFS to some extent, which may lead to tumor vascular normalization and enhance the efficacy of TACE by promoting the accumulation of lipiodol, anticancer agents and gelatin sponges. Target immunotherapy drugs may stabilize disease progression after each TACE treatment, resulting in prolonged PFS,4,10,28 which is consistent with our research.
Our research also has some limitations. First, although our study fully confirmed the significant clinical efficacy of anti-angiogenic therapy plus PD-1 inhibitors combined with TACE, the drugs used in the cases included were not completely uniform, which may lead to heterogeneity. Second, whereas PSM reduced confounding factors in this study, since this is a retrospective study, potential bias may still exist. Therefore, a well-designed prospective research is needed to further verify our conclusions.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Funding Statement
This work was supported by a grant from Sichuan Science and Technology Program Province (2022YFS0622).
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author (Lanpaoxiansheng @126.com).
Animal Research (Ethics)
This research did not involve animal experiments.
Consent to Participate (Ethics)
This retrospective study was approved by the Ethics Committee of The Affiliated Hospital of Southwest Medical University (approval number KY2020254) and complied with the standards of the Declaration of Helsinki. Written informed consent was waived because of the retrospective study.
Data Confidentiality Statement
We promise that all patient data will be kept confidential.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest regarding the content of this paper.
References
- 1.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi: 10.1002/hep.31288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi: 10.1016/S0140-6736(22)01200-4 [DOI] [PubMed] [Google Scholar]
- 3.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi: 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi: 10.6004/jnccn.2021.0022 [DOI] [PubMed] [Google Scholar]
- 6.Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345. doi: 10.1200/JCO.20.02672 [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Zhao M, Arai Y, et al. Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of International Society of Multidisciplinary Interventional Oncology (ISMIO). Hepatobiliary Surg Nutr. 2021;10(5):661–671. doi: 10.21037/hbsn-21-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9(9):e003311. doi: 10.1136/jitc-2021-003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown ZJ, Hewitt DB, Pawlik TM. Combination therapies plus transarterial chemoembolization in hepatocellular carcinoma: a snapshot of clinical trial progress. Expert Opin Investig Drugs. 2022;31(4):379–391. doi: 10.1080/13543784.2022.2008355 [DOI] [PubMed] [Google Scholar]
- 10.Marinelli B, Kim E, D’Alessio A, et al. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study. J Immunother Cancer. 2022;10(6):e004205. doi: 10.1136/jitc-2021-004205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. 2020;21(21):8165. doi: 10.3390/ijms21218165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi C, Chen L, Lin Z, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;74(5):2544–2560. doi: 10.1002/hep.31921 [DOI] [PubMed] [Google Scholar]
- 13.Su K, Gu T, Xu K, et al. Gamma knife radiosurgery versus transcatheter arterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Hepatol Int. 2022;16(4):858–867. doi: 10.1007/s12072-022-10339-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi: 10.1016/j.jhep.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 16.Galle PR, Finn RS, Qin S, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, Phase 3 trial. Lancet Oncol. 2021;22(7):991–1001. doi: 10.1016/S1470-2045(21)00151-0 [DOI] [PubMed] [Google Scholar]
- 17.Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50(3):604–620. doi: 10.1016/j.jhep.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 19.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914–921. doi: 10.1111/j.1572-0241.2007.01712.x [DOI] [PubMed] [Google Scholar]
- 20.Palmer DH, Malagari K, Kulik LM. Role of locoregional therapies in the wake of systemic therapy. J Hepatol. 2020;72(2):277–287. doi: 10.1016/j.jhep.2019.09.023 [DOI] [PubMed] [Google Scholar]
- 21.Li H, Wu Z, Chen J, et al. External radiotherapy combined with sorafenib has better efficacy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Exp Med. 2022. doi: 10.1007/s10238-022-00972-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Cheng H, Dai Q, et al. A superstable homogeneous lipiodol-ICG formulation for locoregional hepatocellular carcinoma treatment. J Control Release. 2020;323:635–643. doi: 10.1016/j.jconrel.2020.04.021 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Cheng H, Chen H, et al. A pure nanoICG-based homogeneous lipiodol formulation: toward precise surgical navigation of primary liver cancer after long-term transcatheter arterial embolization. Eur J Nucl Med Mol Imaging. 2022;49(8):2605–2617. doi: 10.1007/s00259-021-05654-z [DOI] [PubMed] [Google Scholar]
- 24.Ricke J, Klümpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71(6):1164–1174. doi: 10.1016/j.jhep.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 25.Ding X, Sun W, Li W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127(20):3782–3793. doi: 10.1002/cncr.33677 [DOI] [PubMed] [Google Scholar]
- 26.Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4(5):661–669. doi: 10.1001/jamaoncol.2017.5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su K, Guo L, Ma W, et al. PD-1 inhibitors plus anti-angiogenic therapy with or without intensity-modulated radiotherapy for advanced hepatocellular carcinoma: a propensity score matching study. Front Immunol. 2022;13:972503. doi: 10.3389/fimmu.2022.972503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi: 10.1016/S2468-1253(17)30156-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author (Lanpaoxiansheng @126.com).