Abstract
In this study, we have developed a new ultrasonic synthesis method of dibenzoepines using olanzapine and quetiapine, which are well-known drugs for the treatment of schizophrenia and bipolar disorder. The method is based on the N-alkylation reaction of the piperazine fragment in tricyclic compounds with methyl iodide or 2-(2-chloroethoxy)ethanol as the alkylating agent, respectively. The synthesis reactions were carried out in an ultrasonic bath with solvents such as acetonitrile or dimethylformamide in the presence of potassium or sodium carbonate or sodium hydroxide and metal-free, ecological phase transfer catalyst at a temperature of 40–50 °C. This allowed us to obtain olanzapine in 1 h (Y = 67%), and quetiapine in 3 h (Y = 72%). An ultrasonic reactor (Qsonica Q700) was used in the synthesis of olanzapine and made it possible to shorten the reaction time to 10 min and obtain 90% yield with very high purity. The developed method allows obtaining compounds in mild conditions and in a short time, thanks to which the process is more ecological than others described in the literature.
Keywords: Ultrasound-assisted synthesis, Dibenzoazepine, Phase transfer catalysis, N-alkylation, Olanzapine, Quetiapine
1. Introduction
In the 1960s, the search for new effective neuroleptic drugs in the group of tricyclic systems resulted in obtaining substances with new, unique, "atypical", and previously unknown properties. One of them was clozapine (1), the first such drug on the market, which was introduced in 1971. It was characterized by a very pronounced antipsychotic activity, but at the same time, it did not induce the catalepsy symptoms characteristic of tricyclic neuroleptics. As a result, a hypothesis was formulated that this compound did not cause extrapyramidal symptoms. Unfortunately, a few years later, it was observed that clozapine (1) showed side effects in the hematopoietic system – agranulocytosis. This caused the drug to be withdrawn from therapy. However, the search for a drug that would exhibit at least similar antipsychotic effects bore no results and therefore clozapine (1) was reintroduced on the market in 1989 [[1], [2], [3], [4]].
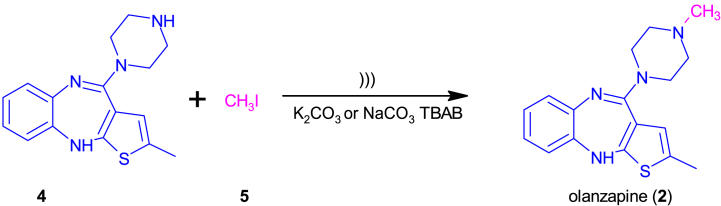
Clozapine (1) gave rise to new SDA drugs – serotonin/dopamine antagonists, which, in addition to blocking dopamine receptors, are characterized by a strong blockade of the 5-HT2A receptor. In the following years, it was possible to develop other drugs from the dibenzoepine derivatives group, such as olanzapine (2) and quetiapine (3), which are currently very important drugs available on the market (Fig. 1) [ [5,6]]. The dibenzoepine derivatives include compounds from the group of tricyclic neuroleptics, whose basic element is a seven-membered heterocyclic system (epin), fused with two aromatic rings. The key element in binding to receptors is the piperazine moiety adjacent to the tricyclic system.
Fig. 1.
Structures of benzodiazepines: clozapine (1), olanzapine (2), and quetiapine (3).
Olanzapine (2-methyl-4-(4-methylpiperazin-1-yl)-10H-benzo[b]thieno [2,3-e][1,4]diazepine) (2) has antipsychotic, anti-manic, and mood-stabilizing effects [ [[7], [8], [9], [10]]]. It is widely used in psychiatry to treat schizophrenia, severe manic episodes in the course of bipolar disorder [11,12]. The mechanism of action of olanzapine (2) is a consequence of blocking the D2 receptor and even stronger 5-HT2A blocking. In addition, olanzapine strongly blocks histamine (H1) and muscarinic receptors [ [[13], [14], [15], [16]]]. It is worth noting that the application of olanzapine (2) does not cause the side effect of clozapine (1), i.e. agranulocytosis. This is probably because olanzapine (2) is more active, so a lower therapeutic dose can be used. In addition, it may also be important that olanzapine (2) is less lipophilic, which contributes to limited metabolism and thus, only small amounts of the metabolite responsible for this side effect are formed [17].
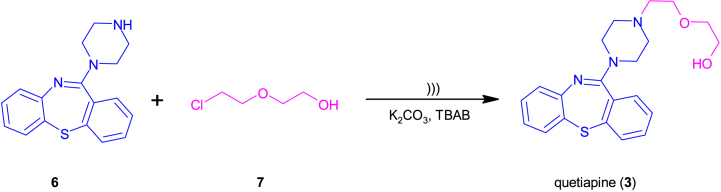
Quetiapine (2-[2-(4-dibenzo[b,f] [1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol) (3) is used in the treatment of schizophrenia and relapse prevention and the treatment and prevention of the recurrence of bipolar disorder [ [[18], [19], [20], [21], [22], [23]]]. Its antipsychotic effect is mainly due to the blockade of dopaminergic and serotonergic 5-HT2A receptors. It also blocks the H1 receptor and has a low affinity for muscarinic receptors. Interestingly, the activity of quetiapine (3) is due to its active metabolite, i.e. norquetiapine (NKWE) [ [[24], [25], [26], [27], [28], [29]]].
Considering the wide and universal use of olanzapine (2) and quetiapine (3) and the constant growth of interest in these compounds, the current task is the search for the synthesis methods that will allow obtaining these substances in an ecological way, e.g. under mild reaction conditions, with short reaction times, and also with high yield and purity.
As described in the current literature, the methods of olanzapine synthesis by the N-alkylation reaction of demethylolanzapine involve the synthesis with the use of dimethyl sulphate in the presence of sodium hydroxide in methanol or a mixture of methanol and dichloromethane [30]. Moreover, this synthesis can be carried out in the presence of potassium carbonate in methanol (Y = 60%) or acetone (Y = 46%) [31]. There are other N-alkylation reactions of demethylolanzapine described in the patent literature, for example, with methyl iodide in tetrahydrofuran as the solvent [31]. The reaction is favored by the alkaline environment; therefore, the process is carried out in the presence of such agents as triethylamine, t-BuONa, NaOH, NaH. The highest reaction efficiency (99%) and the shortest process time (2 h) are obtained with t-BuOK and by conducting the process at a temperature of 0 °C [31]. Another known method of obtaining olanzapine involves the N-methylation reaction of demethylolanzapine under solvent-free conditions in the presence of microwave irradiation [32].
As for quetiapine, its synthesis most often requires the reaction of norquetiapine hydrochloride and 2-(2-chloroethoxy)ethanol. It is carried out in the presence of sodium carbonate, sodium iodide, tetrabutylammonium bromide (TBAB) in toluene at the temperature of 115–120 °C for 17 h. The expected product is obtained with a yield of 98.2% [33]. There are also known quetiapine synthesis reactions in solvents such as butan-1-ol or dimethylformamide (DMF) or reactions without TBAB [33]. The synthesis can also be carried out in water [34] and in 1-methyl-pyrrolidin-2-one and toluene [35].
Literature data from recent years shows that the use of ultrasound in the synthesis of heterocyclic compounds increases the yield and reaction rate, makes reactions more energy-efficient, and also improves the efficiency of phase transfer catalysts [[36], [37], [38], [39], [40], [41], [42], [43]]].
With these advantages in mind, it was decided to test the possibility of using ultrasound to support the synthesis of olanzapine and quetiapine. A method based on the N-alkylation of piperazine was chosen, because it is a very common element in bioactive compounds. This suggests that this method will be universal also in the synthesis of other compounds. In the syntheses, tetrabutylammonium bromide (TBAB) was used as the phase transfer catalyst because it is environmentally friendly, very stable, non-volatile, non-flammable, and non-corrosive as well as inexpensive and easily commercially available [ [44,45]]. Literature data on the use of TBAB confirms that it has very beneficial effects on alkylation reactions [ [[46], [47], [48], [49], [50]]]. Moreover, numerous experiments in N-alkylation reactions of imides and substituted piperazines have proved that it is often the best option among various PTC catalysts [ [[51], [52], [53], [54]]].
2. Materials and methods
The progress of all reactions was monitored by TLC (thin layer chromatography) using chloroform-methanol (80:20) as eluent for olanzapine (2) or methanol-toluen (80:20) for quetiapine (3). TLC plates on aluminum silica gel 60 F254 (Merck) plates were used. TLC spots were detected by absorbing them in ultraviolet (UV) light (λ = 254 nm) and in an iodine-saturated chamber. Moreover, the progress of the reaction was also assessed by HPLC (high-performance liquid chromatography) (Knauer), XTerraRP C18 4.6 × 150 5 μm column, mobile phase CH3CN/H2O/triethylamine 40/40/0.1; flow 1 ml/min) to compare the retention times with the standard. UPLC-MS (ultra-performance liquid chromatography-tandem mass spectrometry) analyses were also performed (Waters Acquity UPLC coupled with a Waters TQD mass spectrometer, ESI-tandem quadrupole electrospray ionization mode), PDA detector, Acquity UPLC BEH C18 column, 1.7, 2.1 × 100 mm (Waters Corporation, Milford, MA, USA), mobile phase: methanol:water + formic acid (4:6 + 0.1%, v/v).
Olanzapine (2) and quetiapine (3) standards, demethylolanzapine (4), norquetiapine (5) as well as other reagents and solvents were purchased from Sigma-Aldrich and TCI (used without purification).
2.1. General procedure for the synthesis of olanzapine (2) – in an ultrasonic bath
0.98 g (3.3 mmol) of demethylolanzapine (4) 0.31 cm3 (5 mmol) of methyl iodide (5), 0.097 g (0.3 mol) of TBAB, 1.38 g (10 mmol) of K2CO3 or Na2CO3 and 50 cm3 DMF* or CH3CN. The reaction was carried out in the presence of ultrasound in an ultrasonic bath (operating conditions: temperature 50 °C, power 80 W, continuous mode, F = 40 kHz). The progress of the reaction was monitored by TLC and HPLC. After 1 h, the product was isolated by adding water, cooling it to 10 °C, and filtering (with DMF) or extracting (with CH3CN). Additionally, the residue obtained after the extraction was purified by maceration in methanol.
* In the case of the reaction with a reduced amount of solvent (Table 1), 10 cm3 was used.
Table 1.
Synthesis of olanzapine (2) and quetiapine (3).
| No. | Substrates | Molar ratio | Process conditions | Power [W] | Reaction conditions | Time | Yield % | |
|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 5 | 1 (4): 2.4 (5) | conventional, - 10 °C | – | THF, Et3N | 4 h | 82 (Mesar et al., 2008) |
| 2 | 4 | 5 | 1 (4): 2.4 (5) | conventional, - 15 °C | – | THF, NaOH | 4 h | 98 (Mesar et al., 2008) |
| 3 | 4 | 5 | 1 (4): 2.4 (5) | conventional, - 10 °C | – | THF, NaH | 3 h | 98 (Mesar et al., 2008) |
| 4 | 4 | 5 | 1 (4): 2.4 (5) | conventional, - 10 °C | – | THF, t-BuOK | 2 h | 98 (Mesar et al., 2008) |
| 5 | 6 | 7 | 1 (4): 1.3 (5) | conventional, 115–120 °C | – | n-butanol, Na2CO3, NaI, TBAB | 24 h | 60 (Diller et al., 2004) |
| 6 | 6 | 7 | 1 (4): 1.3 (5) | conventional, 115–120 °C | – | toluene, Na2CO3, NaI, TBAB | 24 h | 72 (Diller et al., 2004) |
| 7 | 6 | 7 | 1 (4): 1.3 (5) | conventional, 100 °C | – | water, Na2CO3, NaI | 9 h | 80 (Ashok et al., 2006) |
| 8 | 4 | 5 | 1 (4): 1.5 (5) | Ultrasound Bath, Frequency 40 kHz, continuous | 80 | DMFa, K2CO3, TBAB | 60 | 40 |
| 9 | 4 | 5 | 1 (4): 1.5 (5) | Ultrasound Bath, Frequency 40 kHz, continuous | 80 | DMF, K2CO3, TBAB | 1 h | 44 |
| 10 | 4 | 5 | 1 (4): 1.5 (5) | Ultrasound Bath, Frequency 40 kHz, continuous | 80 | DMF, K2CO3 | 2 h | 15 |
| 11 | 4 | 5 | 1 (4): 1.5 (5) | Ultrasound Bath, Frequency 40 kHz, continuous | 80 | CH3CNa, K2CO3,TBAB | 1 h | 67 |
| 12 | 4 | 5 | 1 (4): 1.5 (5) | Ultrasound Bath, Frequency 40 kHz, continuous | 80 | CH3CN, K2CO3, TBAB | 1 h | 49 |
| 13 | 4 | 5 | 1 (4): 1.5 (5) | Ultrasound Bath, Frequency 40 kHz, continuous | 80 | DMFa, Na2CO3, TBAB | 1 h | 50 |
| 14 | 6 | 7 | 1 (6): 1.5 (7) | Ultrasound Bath, Frequency 40 kHz, continuous | 80 | DMF, K2CO3, TBAB | 2 h | 72 |
| 15 | 6 | 7 | 1 (6): 1.5 (7) | Ultrasound Bath, Frequency 40 kHz, continuous | 80 | CH3CN, K2CO3, TBAB | 2 h | 50 |
| 16 | 4 | 5 | 1 (4): 1.5 (5) | QSonica Q700, Amplitude 60%, pulse mode 5:1 | 50–60 | DMF, K2CO3, TBAB | 10 min | 70 (93% purity) |
| 17 | 4 | 5 | 1 (4): 1.5 (5) | QSonica Q700, Amplitude 60%, pulse mode 5:1 | 35–40 | CH3CN, K2CO3, TBAB | 10 min | 90 (100% purity) |
| 18 | 4 | 5 | 1 (4): 1.5 (5) | QSonica Q700, Amplitude 60%, pulse mode 5:1 | 45–50 | H2O, K2CO3, TBAB | 10 min | 12 (91% purity) |
| 19 | 4 | 5 | 1 (4): 3 (5) | QSonica Q700, Amplitude 60%, pulse mode 5:1 | 50–60 | CH3CN, K2CO3, TBAB | 10 min | 25 (91% purity) |
| 20 | 4 | 5 | 1 (4): 3 (5) | QSonica Q700, Amplitude 60%, pulse mode 5:1 | 50–60 | CH3CN, NaOH, TBAB | 10 min | 25 (91% purity) |
Reactions with reduced amount of solvent.
Rf (TLC) 0.62 (chloroform-methanol 80:20), tM (UPLC) = 2.76 min, MS = 312, IR 3237, 3178, 3100, 3050, 2942, 2922, 2839, 2804, 1587, 1558, 1454, 1411, 1360, 1281, 1266, 1220, 1146, 1029, 1003, 970, 780, 760 cm-1, 1H NMR (400 MHz, DMSO) δ 7.61 (s, 1H), 6.81 (s, 3H), 6.71 (s, 1H), 6.34 (s, 1H), 3.35 (d, J = 10.9 Hz, 4H), 2.37 (s, 4H), 2.27 (s, 3H), 2.21 (s, 3H).
2.2. General procedure for the synthesis of quetiapine (3) – in an ultrasonic bath
0.25 g (0.84 mmol) of norquetiapine (4), 0.27 cm3 (1.27 mmol) of 2-(2-chloroethoxy) ethanol, 0.027 g (0.084 mmol) of TBAB, 0.348 g (2.52 mmol) of K2CO3 and 2 cm3 of DMF or CH3CN were placed in a flask. The reaction was carried out in the presence of ultrasound in an ultrasonic bath (operating conditions: temperature 50 °C, power 80 W, continuous mode, F = 40 kHz). The progress of the reaction was monitored by TLC and HPLC. After 3 h from the start of the reaction, water was added to the reaction mixture and then the mixture was extracted with two portions of methylene chloride and concentrated to give a solid, which was then dried.
Rf (TLC) 0.75 (metanol:toluen 80:20), tM (UPLC) = 4.76 min, MS = 383, IR: 3372, 2959, 2872, 1614, 1573, 1562, 1472, 1447, 1429, 1301, 1278, 1119, 1040, 1019, 870, 769, 748 cm-1
1H NMR (400 MHz, CDCl3) δ 7.53 (dd, J = 7.3, 3.9 Hz, 1H), 7.43–7.30 (m, 4H), 7.20 (ddd, J = 15.2, 7.4, 1.5 Hz, 1H), 7.08 (dd, J = 8.0, 1.2 Hz, 1H), 6.93 (dtd, J = 16.5, 7.6, 1.4 Hz, 1H), 4.30 (s, 1H), 3.99 (s, 1H), 3.80–3.75 (m, 2H), 3.75–3.70 (m, 3H), 3.68–3.61 (m, 4H), 3.38–3.14 (m, 2H), 2.81–2.62 (m, 4H).
2.3. General procedure for the synthesis of olanzapine (2) – in QSonica Q700 reactor
0.98 g (3.3 mmol) of demethylolanzapine (4) 0.31–0.62 cm3 (5–10 mmol) of methyl iodide (5), 0.097 g (0.3 mol) of TBAB, 1.38 g (10 mmol) of K2CO3 or NaOH and 50 cm3 DMF, CH3CN or H2O. The reaction was carried out in the presence of ultrasound in the QSonica Q700 sonicator reactor (amplitude 60%, power 40–60 W in a pulse mode with a pulse duration of 60 s and a break of 12 s, 40–50 °C). The progress of the reaction was monitored by TLC and HPLC. After 10 min from the start of the reaction, water was added to the reaction mixture and the product was filtered off. The crude product was triturated in methanol to yield olanzapine. The methanol obtained after maceration was then concentrated to crystallize olanzapine, yielding an additional second portion of the product.
3. Results
Our preliminary research, which aimed to show the possibilities of using ultrasound in the synthesis of olanzapine (2) or quetiapine (3), was conducted in an ultrasonic bath (80 W, 40 KHz, 50 °C) by the most commonly described method of synthesis, i.e. the N-alkylation reaction (Scheme 1, Scheme 2).
Scheme 1.
The synthesis of olanzapine (2) in the presence of ultrasound.
Scheme 2.
The synthesis of quetiapine (3) in the presence of ultrasound.
In the case of the synthesis of olanzapine, the reactions were carried out with demethylolanzapine (2-methyl-4-(1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine) (4) and methyl iodide (5) in the presence of a tetrabutylammonium bromide (TBAB) as a phase transfer catalyst and potassium or sodium carbonate in a solvent such as dimethylformamide or acetonitrile.
The experiments showed that olanzapine (2) could be obtained in an ultrasonic bath from demethylolanzapine (4) within an hour by N-alkylation with methyl iodide (5) in a solvent such as dimethylformamide. The yield was in the range of 40–44% using potassium carbonate and 50% using sodium carbonate. When the synthesis was carried out in acetonitrile, the yield was in the range of 49–67% (Table 1, no. 8–13). During the first study, we checked the effect of TBAB on the efficiency and speed of the process in the reaction using DMF and potassium carbonate. In the results, it can be observed that the reaction occurs three times less efficiently without a catalyst despite longer time (Table 1, no. 10). The reactions were carried out without heating and spontaneous heating of the ultrasonic bath water to 50 °C was observed after time. In one of the first tests, the effect of raising the temperature to 80 °C was evaluated, but neither higher efficiency nor shorter reaction time was observed. This was also accompanied by a problem with evaporation of water from the ultrasonic bath, so this option was discarded.
In the next stage of the study, the possibility of synthesizing quetiapine (3) under similar conditions (Table 1, no. 9–10) was assessed. In this case, the reaction was slower and had to be carried out for 3 h. After the first hour, only a small amount of product was observed in the reaction mixture and it was necessary to run it for 3 h to obtain a yield of 72% in dimethylformamide and 50% yield in acetonitrile.
The obtained results encouraged conducting a further study to continue the synthesis of the olanzapine (2) with the Qsonica Q700 sonicator reactor (Church Hill Rd, Newtown, CT, USA) (amplitude 60%, power 40–60 W in pulse mode with pulse duration of 60 s and a break of 12 s, 40–50 °C) (Table 1, no. reaction 16–20). The first reactions were carried out in the same conditions as in the case of an ultrasonic bath, i.e. with acetonitrile and dimethylformamide as solvents, and in the presence of potassium carbonate and TBAB with the same molar ratio of reagents. It turned out that when the Qsonica reactor was used, the expected product was obtained after 10 min, i.e. six times faster and with much higher efficiency (Table 1, no. 16–17). The effect of replacing the solvent with water was checked, but this resulted in a drastic reduction in efficiency to 25% (Table 1, no. 18).
In the course of the experiments, it was noticed that it was very important to monitor the progress of the reaction and finish it in a timely manner. When the reaction was carried out for too long or when a large excess of methyl iodide (5) was used (Table 1, no. 19–20), a by-product was formed in the reaction mixture. The by-product was a methyl derivative of olanzapine 1,1-dimethyl-4-(2-methyl-10H-thieno[2,3-b][1,5]benzodiazepin-4-yl)piperazin-1-ium (8) (Fig. 2), which is known in the literature [42].
Fig. 2.
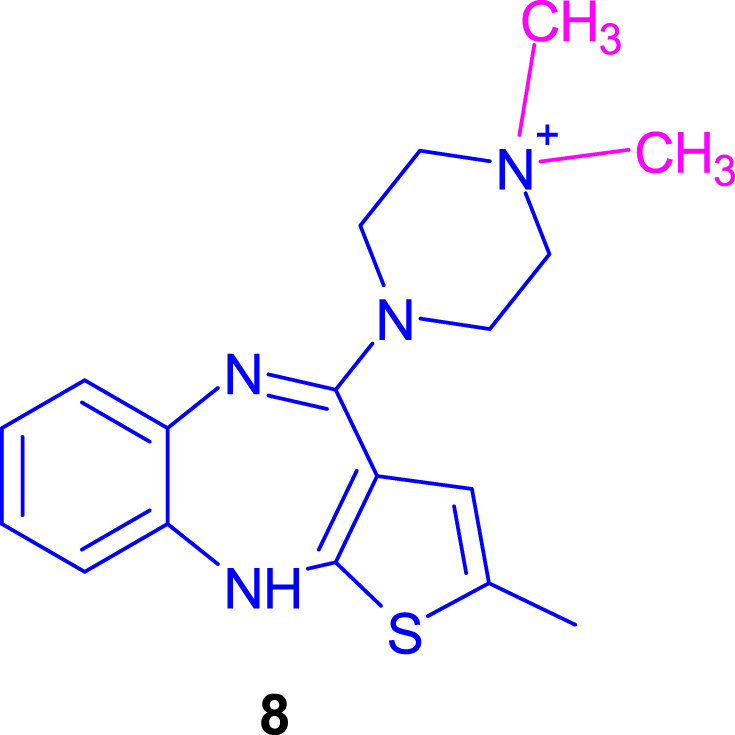
By-product 1,1-dimethyl-4-(2-methyl-10H-thieno[2,3-b][1,5]benzodiazepin-4-yl)piperazin-1-ium (8).
A by-product resulting from N-methylation at position 10 in demethylolanzapine (4) was not observed, which is consistent with the literature data.
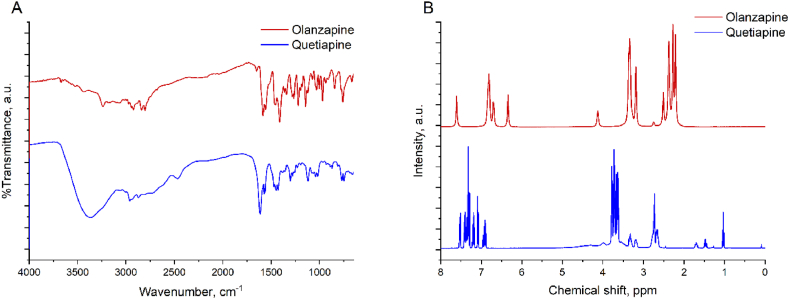
The structures of the compounds obtained were confirmed using FT-IR (Fig. 3. A), 1H NMR (Fig. 3. B) and MS.
Fig. 3.
Spectra FT-IR (A), 1H NMR (B). Olanzapine (2) is marked with a red line and quetiapine (3) with a blue line.
Interestingly, the method developed in the N-alkylation of piperazine, present in the structure of olanzapine or quetiapine, can be successfully adopted in an analogous reaction in the synthesis of other APIs (Active Pharmaceutical Ingredients) from the family of long-chain arylpiperazines such as trazodone, aripiprazole, flibanserin with the yield of 71–85% [54].
4. Discussion
The results show that in the case of using the QSonica Q700 reactor, it is possible to obtain olanzapine (2) in the presence of potassium carbonate and in acetonitrile as a solvent after 10 min with a 90% yield of very high purity (100%) (Table 1, no. 9). High yield and purity can be also achieved with dimethylformamide (Table 1, no. 8). When water is used as a solvent, in this case, olanzapine (2) is obtained with only 25% yield (Table 1, no. 10). The use of a threefold excess of the alkylating agent 5 results in the formation of a by-product 8 and, as mentioned earlier, in this case, it is only possible to obtain olanzapine at 25% yield (Table 1, no. 11). Then, it was checked if using a stronger base, i.e. sodium hydroxide instead of potassium carbonate would make it possible to obtain a higher yield of olanzapine. However, the result was exactly the same (Y = 25%) (Table 1, no.12). Our research has also proved that the method can be also used in the N-alkylation of piperazine in long-chain arylpiperazine derivatives, which are a very important group of bioactive compounds.
5. Conclusion
The conducted experiments have proved that the use of ultrasound in the synthesis of heterocyclic compounds such as the benzodiazepines, i.e. olanzapine or quetiapine, is a very effective synthesis option. The use of an ultrasonic bath (80 W, 40 kHz, 50 °C) makes it possible to obtain olanzapine within 1 h with a yield of up to 67% and quetiapine within 3 h with a yield of 72%. When an ultrasonic reactor is used (amplitude 60%, power 40–60 W in a pulse mode with pulse duration of 60 s and a break of 12 s, 40–50 °C), it is possible to shorten the synthesis time to 10 min and obtain olanzapine with a high yield of 90% and very high purity. If we take into consideration that many of the syntheses of olanzapine and quetiapine described in the literature concern reactions that last many hours and often require extreme conditions, we believe that the developed synthesis method is a very advantageous green alternative, e.g. due to lower energy consumption in the process.
The great advantage of the developed method is the short time and mild reaction conditions. The reactions are carried out at room temperature and the maximum temperature observed during the synthesis is 50 °C. An additional benefit is the fact that the obtained compounds are characterized by a very high purity. This allows eliminating costly purification procedures, which often require toxic solvents.
Both olanzapine and quetiapine are sold all over the world and this demands their production in very large quantities. The presented modifications in the syntheses, if introduced in the industry, could significantly affect the ecology of the production of these drugs. An important advantage of the developed method is the fact that it is universal and can be also successfully used in the synthesis of other APIs.
The developed method, which was tested on the example of the synthesis of olanzapine and quetiapine, can be also used in the synthesis of other compounds with a piperazine system. Our experiments prove that with appropriate adaptation, i.e. appropriate selection of a PTC or solvent, this method can be also successfully applied in other piperazine N-alkylation reactions.
Author contribution statement
Jolanta Jaskowska: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Anna Karolina Drabczyk; Damian Kułaga; Przemysław Zaręba; Zbigniew Majka; Przemysław Jodłowski: Performed the experiments.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Jolanta Jaskowska reports financial support was provided by Cracow University of Technology. Jolanta Jaskowska has patent #PL239444 (B1) issued to Assignee.
References
- 1.Crilly J. The history of clozapine and its emergence in the US market: a review and analysis. Hist. Psychiatr. 2007;18:39–60. doi: 10.1177/0957154X07070335. [DOI] [PubMed] [Google Scholar]
- 2.Solanki R.K., Singh P., Swami M., Mukesh K. Clozapine: current perspective. Indian J. Soc. Psychiatry. 2007;49:271–276. doi: 10.4103/0019-5545.37668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meltzer H.Y. Treatment-resistant schizophrenia--the role of clozapine. Curr. Med. Res. Opin. 1997;14:1–20. doi: 10.1185/03007999709113338. [DOI] [PubMed] [Google Scholar]
- 4.Latif Z., Jabbar F., Kelly B. Clozapine and blood dyscrasia - a review. Psychiatrist. 2011;35:27–29. doi: 10.1192/pb.bp.109.026054. [DOI] [Google Scholar]
- 5.Leucht S., Cipriani A., Spineli L., Mavridis D., Orey D., Richter F., Samara M., Barbui C., Engel R.R., Geddes J.R., Kissling W., Stapf M.P., Lässig B., Salanti G., Davis J.M. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Revuelta M., Pelayo-Terán J.M., Juncal-Ruiz M., Vázquez-Bourgon J., Suárez-Pinilla P., Romero-Jiménez R., Setién Suero E., Ayesa-Arriola R., Crespo-Facorro B. Antipsychotic treatment effectiveness in first episode of psychosis: PAFIP 3-year follow-up randomized clinical trials comparing haloperidol, olanzapine, risperidone, aripiprazole, quetiapine, and ziprasidone. Int. J. Neuropsychopharmacol. 2020;23:217–229. doi: 10.1093/ijnp/pyaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bánki C.M. Olanzapine: a second generation antipsychotic drug and an "atypical" mood stabilizer? Psychiatr. Hung. 2007;22:311–320. [PubMed] [Google Scholar]
- 8.McCormack P.L., Wiseman L.R. Spotlight on olanzapine in bipolar I disorder. CNS Drugs. 2005;19:553–555. doi: 10.2165/00023210-200519060-00006. [DOI] [PubMed] [Google Scholar]
- 9.Tohen M., Wang W.V., Leboyer M., Jen K.Y. Variables as mediators or moderators in predicting relapse to any type of mood episode in a bipolar maintenance study. J. Clin. Psychiatry. 2012;73:e913–e917. doi: 10.4088/JCP.10m06737. [DOI] [PubMed] [Google Scholar]
- 10.Geoffroy P.A., Bellivier F., Henry C. Treatment of manic phases of bipolar disorder: critical synthesis of international guidelines. Encephale. 2014;40:330–337. doi: 10.1016/j.encep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Cipriani A., Rendell J., Geddes J.R. Olanzapine in long-term treatment for bipolar disorder. Cochrane Database Syst. Rev. 2009;21:CD004367. doi: 10.1002/14651858.CD004367.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Cipriani A., Rendell J., Geddes J.R. Olanzapine in the long-term treatment of bipolar disorder: a systematic review and meta-analysis. J. Psychopharmacol. 2010;24:1729–1738. doi: 10.1177/0269881109106900. [DOI] [PubMed] [Google Scholar]
- 13.Harvey R.C., James A.C., Shields G.E. A systematic review and network meta-analysis to assess the relative efficacy of antipsychotics for the treatment of positive and negative symptoms in early-onset schizophrenia. CNS Drugs. 2016;30:27–39. doi: 10.1007/s40263-015-0308-1. [DOI] [PubMed] [Google Scholar]
- 14.Pagsberg A.K., Tarp S., Glintborg D., Stenstrøm A.D., Fink-Jensen A., Correll C.U., Christensen R. Acute antipsychotic treatment of children and adolescents with schizophrenia-spectrum disorders: a systematic review and network meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:191–202. doi: 10.1016/j.jaac.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Osser D.N., Roudsari M.J., Manschreck T. The psychopharmacology algorithm project at the Harvard South Shore Program: an update on schizophrenia. Harv. Rev. Psychiatr. 2013;21:18–40. doi: 10.1097/HRP.0b013e31827fd915. [DOI] [PubMed] [Google Scholar]
- 16.Duggan L., Fenton M., Rathbone J., Dardennes R., El‐Dosoky A., Indran S. Olanzapine for schizophrenia. Cochrane Database Syst. Rev. 2005;2:CD001359. doi: 10.1002/14651858.CD001359.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng W., Kennar R., Uetrecht J. Effect of clozapine and olanzapine on neutrophil kinetics: implications for drug-induced agranulocytosis. Chem. Res. Toxicol. 2014;27:1104–1108. doi: 10.1021/tx500183x. [DOI] [PubMed] [Google Scholar]
- 18.Maan J.S., Ershadi M., Khan I. StatPearls Publishing; Treasure Island (FL): 2022. Saadabadi A. Quetiapine. [PubMed] [Google Scholar]
- 19.El-Khalili N. Update on extended release quetiapine fumarate in schizophrenia and bipolar disorders. Neuropsychiatric Dis. Treat. 2012;8:523–536. doi: 10.2147/NDT.S14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Jurdi R.K., Dixit L.A., Sajatovic M. Role of extended release quetiapine in the management of bipolar disorders. Neuropsychiatric Dis. Treat. 2010;24:29–35. doi: 10.2147/NDT.S4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabe M., Dixit L.A., Sajatovic M. NPJ Schizophr. 2021;7:1–11. doi: 10.2147/ndt.s4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishi T., Ikuta T., Sakuma K., Okuya M., Iwata N. Efficacy and safety of antipsychotic treatments for schizophrenia: a systematic review and network meta-analysis of randomized trials in Japan. J. Psychiatr. Res. 2021;138:444–452. doi: 10.1016/j.jpsychires.2021.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y., Wen S.W., Li M., Sun Z., Yuan X., Retnakaran R., Zhang R., Zhai D. Dose-response association of acute-phase quetiapine treatment with risk of new-onset hypothyroidism in schizophrenia patients. Br. J. Clin. Pharmacol. 2021;87:4823–4830. doi: 10.1111/bcp.14928. [DOI] [PubMed] [Google Scholar]
- 24.Maneeton N., Maneeton B., Woottiluk P., Likhitsathian S., Suttajit S., Boonyanaruthee V., Srisurapanont M. Quetiapine monotherapy in acute treatment of generalized anxiety disorder: a systematic review and meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2016;12:259–276. doi: 10.2147/DDDT.S89485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suttajit S., Srisurapanont M., Maneeton N., Maneeton B. Quetiapine for acute bipolar depression: a systematic review and meta-analysis. Drug Des. Deve.l Ther. 2014;25:827–838. doi: 10.2147/DDDT.S63779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutton P., Taylor P., Mulligan L., Tully S., Moncrieff J. Quetiapine immediate release v. placebo for schizophrenia: systematic review, meta-analysis and reappraisal. Br. J. Psychiatry. 2015;206:360–370. doi: 10.1192/bjp.bp.114.154377. [DOI] [PubMed] [Google Scholar]
- 27.Sparshatt A., Jones S., Taylor D. Quetiapine: dose-response relationship in schizophrenia. CNS Drugs. 2008;22:69–72. doi: 10.2165/00023210-200822010-0004. [DOI] [PubMed] [Google Scholar]
- 28.Miodownik C., Lerner V. Quetiapine: efficacy, tolerability and safety in schizophrenia. Expert Rev. Neurother. 2006;6:983–992. doi: 10.1586/14737175.6.7.983. [DOI] [PubMed] [Google Scholar]
- 29.Ågren R. Worldwide antipsychotic drug search intensities: pharmacoepidemological estimations based on Google Trends data. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-92204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel H.V., Ray A.K., Patel P.B., Patel M.R. 2004. Process of Preparation of Olanzapine Form I. Patent USA US20040048854 A1. [Google Scholar]
- 31.Mesar T., Copar A., Sturm H., Ludescher J. 2008. Synthesis of 2-Methyl-4-(4-Methyl-1-Piperazinly)-10h-Thieno(2,3-B) (1,5) Benzodiazepine and Salts Thereof. Patent USA US20080161557A1. [Google Scholar]
- 32.Jaśkowska J., Kułaga D., Majka Z. A new solvent-free method for the synthesis of olanzapine and its derivatives. Przemys. Chem. 2016;95:1918–1920. doi: 10.15199/62.2016.10.12. [DOI] [Google Scholar]
- 33.Diller D., Dolitzky B.Z. 2004. Synthesis of Quetiapine and Pharmaceutically Acceptable Salts Thereof. Patent WO2004076431A1. [Google Scholar]
- 34.Ashok K., Dharmendra S., Swapnali H.P., Ganesh D.M., Uttamrao A.S., Balasaheb G.J., Ragneshkumar R. 2006. Industrial Preparation of 11-[4-{2-(2-hydroxyethoxy Ethyl}-1-Piperazinyl] Dibenzo [b,f]-1[1, 4] Thiazepine. Patent WO2006077602A1. [Google Scholar]
- 35.Holkar A.G., Pise A.C. 2007. Processes for the Preparation of Thiazepines. Patent WO2007020011. [Google Scholar]
- 36.Majhi S. Applications of ultrasound in total synthesis of bioactive natural products: a promising green tool. Ultrason. Sonochem. 2021;77 doi: 10.1016/j.ultsonch.2021.105665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee B. Recent developments on ultrasound assisted catalyst-free organic synthesis. Ultrason. Sonochem. 2017;35:1–14. doi: 10.1016/j.ultsonch.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 38.Shen M., Zhou J., Elhadidy M., Xianyu Y., Feng J., Liu D., Ding T. Cyclodextrin metal–organic framework by ultrasound-assisted rapid synthesis for caffeic acid loading and antibacterial application. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma A., Priya A., Kaur M., Singh A., Kaur G., Banerjee B. Ultrasound-assisted synthesis of bioactive S-heterocycles. Synth. Commun. 2021;51:3209–3236. doi: 10.1080/00397911.2021.1970775. [DOI] [Google Scholar]
- 40.Pagadala R., Kasi V., Shabalala N.G., Jonnalagadda S.B. Ultrasound-assisted multicomponent synthesis of heterocycles in water – a review. Arab. J. Chem. 2022;15 doi: 10.1016/j.arabjc.2021.103544. [DOI] [Google Scholar]
- 41.Sudha S., Pasha M.A. Catalyst-free ultrasound assisted novel one pot pseudo five component synthesis of aryl-bis-[1H-pyrazol-5-ol-4-yl]methanes, het(aryl)-bis-[1H-pyrazol-5-ol-4-yl]methanes and their 1-phenyl derivatives in aqueous medium. Green Synth. Catal. 2022;3:190–193. doi: 10.1016/j.gresc.2022.03.011. [DOI] [Google Scholar]
- 42.Mohammadi A., Amini M., Hamedani M.P., Torkabadi H.H., Walker R.B. Study of the formation of artifacts following dichloromethane reaction with some nitrogenous drugs. Asian J. Chem. 2008;20:5573–5580. [Google Scholar]
- 43.Charette A.B., Chinchilla R., Nájera C. Encyclopedia of Reagents for Organic Synthesis. JohnWiley & Sons, Ltd.; Chichester, West Sussex, UK: 2007. Tetrabutylammonium bromide. [Google Scholar]
- 44.Banik B.K., Banerjee B., Kaur G., Saroch S., Kumar R. Tetrabutylammonium bromide (TBAB) catalyzed synthesis of bioactive heterocycles. Molecules. 2020;14:1–24. doi: 10.3390/molecules25245918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C., Hang T., Zhang H. Microwave-promoted N-alkylation of acridones without solvent. Synth. Commun. 2003;33:451–456. doi: 10.1081/SCC-120015776. [DOI] [Google Scholar]
- 46.Keenan T., Jean A., Arseniyadis S. Phase-transfer-catalyzed alkylation of hydantoins. ACS Org. Inorg. Au. 2022;2:312–317. doi: 10.1021/acsorginorgau.1c00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torosyan G.H. The selective N-alkylation of monoethanolamine in PTC condition. MOJ Biorg. Inorg. Chem. 2018;2:19–21. doi: 10.15406/mojboc.2018.02.00049. [DOI] [Google Scholar]
- 48.Ren B., Yana N., Gana L. Regioselective alkylation of carbohydrates and diols: a cheaper iron catalyst, new applications and mechanism. RSC Adv. 2017;7 doi: 10.1039/c7ra10220h. [DOI] [Google Scholar]
- 49.Bogdał D., Pielichowski J., Boroń A. Remarkable fast microwave-assisted N-alkylation of phthalimide in dry media. Synlett. 1996;9:873–874. doi: 10.1055/s-1996-5587. [DOI] [Google Scholar]
- 50.Jaśkowska J., Kowalski P. N-Alkylation of imides using phase transfer catalysts under solvent-free conditions. J. Het. Chem. 2008;45:1371–1375. doi: 10.1002/jhet.5570450519. [DOI] [Google Scholar]
- 51.Jaśkowska J., Drabczyk A., Kułaga D., Zaręba P., Majka Z. Solvent-free microwave-assisted synthesis of aripiprazole. Curr. Chem. Lett. 2018;7:81–86. doi: 10.5267/j.ccl.2018.08.002. [DOI] [Google Scholar]
- 52.Jaśkowska J., Zaręba P., Śliwa P., Pindelska E., Satała G., Majka Z. Microwave-Assisted synthesis of trazodone and its derivatives as new 5-HT1A ligands: binding and docking studies. Molecules. 2019;24:1–19. doi: 10.3390/molecules24081609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaśkowska J., Drabczyk A., Michorczyk P., Kułaga D., Zaręba P., Jodłowski P., Majka Z., Jakubski J., Pindelska E. Mechanochemical synthesis method for drugs used in the treatment of CNS diseases under PTC conditions. Catalysts. 2022;12:1–15. doi: 10.3390/catal12050464. [DOI] [Google Scholar]
- 54.Jaśkowska J., Drabczyk A., Kułaga D., Zaręba P., Jodłowski P. 2021. Method of Producing Long-Chain Arylpiperazines. Patent PL239445. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.