Abstract
Seizure is associated with pathological changes of hippocampus, but the mechanism by which hippocampal neuronal apoptosis promotes epilepsy is unclear. Our previous study showed that the expression of NHE-1 was increased in epileptic model rats. Therefore, this study further explores the effect of NHE-1 on hippocampal cells apoptosis and seizure in lithium chloride-pilocarpine epileptic model rats. First, we established a lithium chloride-pilocarpine induced epileptic rat model and detected the expression of NHE-1, calpain1 and apoptosis in the hippocampus. Then, we further down-regulated NHE-1 to observe the expression of calpain1 and apoptosis in the hippocampus, as well as its effect on seizures in rats. We found that the expression of NHE-1 and calpain1 and apoptosis in the hippocampus was significant increased in the model group. After down-regulating NHE-1, the expression of calpain1 was decreased, and hippocampal cell apoptosis was alleviated. In addition, down-regulation of NHE-1 reduced the frequency and duration of seizures in epileptic rats. Therefore, hippocampal NHE-1 overexpression is closely related to the development of neuronal apoptosis in a rat model of epilepsy, and downregulating NHE-1 expression can reduce cell apoptosis. Moreover, the NHE-1/calpain1 signaling pathway may be an important mechanism leading to hippocampal cell apoptosis.
Keywords: Epilepsy, Lithium chloride-pilocarpine model, Sodium-hydrogen exchanger-1, Calpain1, Hippocampal, Apoptosis
1. Introduction
Epilepsy is a common chronic central nervous system disease caused by abnormal discharge of brain neurons. According to epidemiological data, there are about 70 million epileptic patients worldwide [1]. Currently, treatment of patients with epilepsy is mainly based on anti-seizure medications. However, approximately one-third of patients are considered to have drug-resistant epilepsy (DRE) [2,3]. Several studies have reported that postoperative specimens from patients with DRE have characteristic pathological features, such as neuronal loss, hippocampal sclerosis, glial cell proliferation, and synaptic remodeling. Among these features, hippocampal sclerosis is one of the most common pathological changes of DRE [[4], [5], [6], [7]]. Neuronal loss and hippocampal sclerosis are closely related to neuronal apoptosis and necrosis. It has been reported that recurrent seizures led to neuronal loss through apoptosis and necrosis, resulting in hippocampal sclerosis, which in turn promotes the progression of epilepsy [8,9]. Therefore, we believe that reducing apoptosis can also complement anti-epileptic drug therapy.
Sodium hydrogen exchanger-1 (NHE-1), is widely distributed in the nervous system and is a cell membrane protein [10,11]. The main function of NHE-1 is to prevent excessive intracellular acidification, regulate intracellular pH, and maintain the intracellular acid-base balance by exchanging intra- and extracellular levels of H+ and Na+ [12]. It is activated by many components, such as growth factors, hormones, mechanical stress, intracellular acidification, and hypoxia [13]. Under acidosis and hypoxic conditions, as NHE-1 becomes activated, H+ is eliminated, Na+ is internalized into the cell, and Na+ overload stimulates the Na+/Ca2+exchanger (NCX) to increase intracellular Ca2+ concentration, which triggers a series of downstream molecular changes in calcium signaling [14,15]. Previous research has reported that NHE-1 was increased in the hippocampal CA1 region after ischemia-reperfusion injury in a gerbil model, and that ischemic injury to neurons in the hippocampal CA1 region was reduced after the use of NHE-1 inhibitors [16]. NHE-1 inhibitors have also been reported to reduce neuronal death after cerebral ischemia, and to have a neuroprotective effect on ischemic-hypoxic brain injury [17].
Calpain has been found to be overexpressed in postoperative specimens from patients with DRE [18]. Calpain constitutes a group of calcium-activated protein hydrolases that are involved in apoptosis of epileptic neurons [19]. These studies suggest that NHE-1 and calpain may participate in the development of epilepsy. However, the mechanism of NHE-1 in the development of epilepsy models is unclear. Combined with previous studies, we hypothesized that seizures may activate NHE-1, which leads to neuronal apoptosis by activation of calpain. Based on this possibility, in the present study, we determined the effect of modulating NHE-1 on apoptosis of hippocampal neurons.
2. Experimental design
2.1. Expression characteristics of NHE-1, calpain1, and apoptosis in model groups
Thirty healthy adult male Sprague Dawley (SD) rats were selected and randomly divided into a control group (n = 15) and a lithium chloride-pilocarpine epileptic model group (n = 15), three rats died during the model construction, finally rats of the model group were 12. Epileptic seizures in rats were recorded using 24h continuous video monitoring. Immunohistochemistry, immunofluorescence, RT-qPCR, and western blotting were used to detect the expressions of NHE-1 and calpain1 in the hippocampus of rats, and cell apoptosis was detected by hematoxylin & eosin (HE) staining, TUNEL staining, and electron microscopy.
2.2. Transfection of lentivirus in SD rats
Fifteen healthy adult male SD rats were randomly divided into five groups: a control group, LV-empty group, LV-NHE-1 7 d, LV-NHE-1 14 d, LV-NHE-1 21 d, each group consisted of three rats. Control group was injected with phosphate-buffered saline (PBS). LV-empty group was shRNA injected with lentivirus empty vector (GENE, Shanghai, China) into the hippocampus. LV-NHE-1 7 d, LV-NHE-1 14 d and LV-NHE-1 21 d were bilaterally stereotaxically injected with NHE-1-silencing shRNA (GENE, Shanghai, China) into the hippocampus.
2.3. Effects of NHE-1 downregulation on calpain1 and apoptosis
Sixty adult healthy male SD rats were randomly assigned to the following four groups by a random number table: LV-NHE-1 with epilepsy group (LV-NHE-1+EP, n = 15), LV-empty with epilepsy group (LV-empty + EP, n = 15), the epilepsy group (EP, n = 15), and the control group (n = 15). Rats in the LV-NHE-1+EP and LV-empty + EP group rats were bilaterally stereotaxically injected with NHE-1-silencing shRNA lentivirus (GENE, Shanghai, China) and shRNA lentivirus empty vector (GENE, Shanghai, China)) into the hippocampus. The EP group and control group were injected with the same dose of PBS. According to the lentivirus transduction efficiency, epilepsy models were established by lithium chloride-pilocarpine after 2 weeks of lentiviral transduction. In establishing an epilepsy model, three rats died in the LV-NHE-1+EP group, two rats died in the LV-empty + EP group, three rats died in the EP group. Epileptic seizures in rats were recorded using 24-h continuous video monitoring. The kindling rate and spontaneous recurrent seizures for 2 weeks were recorded in rats after successful models were obtained. The hippocampal tissues were obtained after 2 weeks and used for immunoblotting, RT-qPCR analysis, immunohistochemistry, and TUNEL staining.
3. Materials and methods
3.1. Animals and ethics
A total of 105 male SD rats, 6–8 weeks (200−240 g), were provided by the Laboratory Animal Center of Guizhou Medical University (Guizhou, China). The animals were housed in a room with a temperature of 22 ± 2 °C, humidity of 50–70%, and a 12-h light/dark cycle. Rats were raised in single cages and provided with standard chow diet and water ad libitum. All animals care and experiments were conducted in accordance with the Guidelines for Animal Experiments of Guizhou Medical University.
3.2. Modeling of lithium chloride-pilocarpine
According to the lithium chloride-pilocarpine model [20], rats were first intraperitoneally (i.p.) injected with lithium chloride (127 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) and pilocarpine (35 mg/kg, Sigma-Aldrich) 16–18 h later. Before 30 min pilocarpine injection, rats were given atropine (1 mg/kg, Aladdin-Holdings Group, Beijing, China) to block the peripheral effects of pilocarpine [21]. Rat convulsions were graded according to the Racine scale [22]. When grade IV or V appeared, it was considered as a generalized seizure, and regarded as status epilepticus (SE). When the duration of onset was 60 min or more, the kindling was deemed successful and stimulation was immediately stopped. Seizures were terminated using diazepam (7.5 mg/kg, i. p.) when rats experienced class IV seizures for 60 min. The control group was treated (i.p.) with the same dosage of saline.
3.3. Stereotactic intrahippocampal injection
The rats were anesthetized using diazepam using peritoneal injections, and fixed on the brain stereo-positioning instrument. According to the standard rat stereotaxic atlas [23], we set the anterior fontanel as the origin, 3.0 mm backward of the anterior fontanelle, 2.2 mm aside the sagittal slit, and 3.5 mm under the dura mater. A microsampler with a capacity of 5 μL was fixed to the microinjector pump of the stereotyper, and then injected slowly at a rate of 0.3 μL/min, with a total of 3 μL injected at each point. After completion, the scalp was carefully sutured and disinfected, and the rats were placed on a bench with a constant temperature of 37 °C until they awakened. All rats were given penicillin (0.3 mL/100 g) i. p. For the first 3 days after lentivirus injection to prevent infection.
3.4. HE staining
After the rats were anesthetized, they were intracardially perfused with fixative (4% paraformaldehyde). Then, the brain tissue was collected and soaked in fixative for 12 h. Specimens were dehydrated and immersed in wax and were then sliced to a thickness of 5 μm for subsequent experiments. Then, paraffin sections were dewaxed with xylene and dehydrated with gradient alcohol. The sections were then washed with flowing water. The rat sections were stained with hematoxylin (Sigma-Aldrich) for 5 min, and washed with running water, differentiated with 1% hydrochloric acid ethanol for 3 s, and washed again in water. Slices were then stained with eosin for 2 min and then washed with running water for 30 s, followed by washing with distilled water for 3 s. Sections were then dehydrated with alcohol gradient, and were mounted with neutral gum and observed using a light microscope.
3.5. Transmission electron microscopy
Fresh hippocampal tissues were fixed with 2.5% glutaraldehyde for 24 h. Samples were then rinsed three times with 0.1 M phosphate buffer for 15 min each, and fixed with 1% phosphoric acid buffer at room temperature for 2 h. The samples were then washed with running 0.1 M phosphate buffer. Samples were then dehydrated through a series of graded alcohols for 15 min each. The tissues were treated with acetone:812 embedding agent (1:1), and with 812 embedding agent, both for overnight, and then baked at 60 °C for 4 h for polymerization of the embedding resin. Subsequently, ultrathin sections of 60–80 nm were cut. The slices were stained using 2% uranium acetate saturated alcohol solution and lead citrate for 15 min, respectively, and then dried overnight at room temperature. Slices were observed and captured as images using transmission electron microscopy.
3.6. Immunocytochemistry
Preparation of tissue sections was the same as for the previously described for HE staining. EDTA repair solution was used for antigen retrieval of tissue sections. Then, the sections were washed with PBS (pH 8.0). The sections were then incubated with 3% hydrogen peroxide to block endogenous peroxidase. Subsequently, the slides were incubated with normal goat serum (Jinqiao Bio-Technology, Beijing, China) to block nonspecific antigens for 30 min at 37 °C, then the slides were incubated with rabbit anti-rat NHE-1 (1:100; Abcam, Cambridge, MA, USA) and rabbit anti-rat calpain1 (1:100; Abcam) overnight at 4 °C. After washing three times with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody for 2 h at room temperature. Staining was performed using a chromogenic substrate, 3,3′-diaminobenzidine (DAPI) (Sigma-Aldrich), followed by counterstaining with Harris hematoxylin (Sigma-Aldrich), dehydration, and mounting. Images were captured using a fluorescence microscope and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
3.7. Immunofluorescence
Preparation of tissue sections was the same as the previously described HE staining. Paraffin sections were dewaxed, rinsed, boiled, and then treated with normal goat serum (Boster Biological Engineering, Wuhan, China) for 30 min at 37 °C, then mixed primary antibodies were added dropwise and incubated overnight at 4 °C. The mixed primary antibodies were rabbit anti-rat NHE-1 (1:100; Abcam), rabbit anti-rat calpain1 (1:100; Abcam), mixed with mouse anti-rat Neun antibody (1:100; MilliporeSigma, Darmstadt, Germany). The next day, specimens were incubated with mixed antibodies of FITC-labeled goat anti-rabbit secondary antibody (1:100; Boster Biological Engineering) and TRITC-labeled goat anti-mouse secondary antibody (1:100; Boster Biological Engineering) mixed antibodies for 1 h at 37 °C in the dark. After rinsing with PBS, for nuclear staining, the slides were incubated with 4ʹ,6-diamidino-2-phenylindole (Shanghai Biyuntian Biological Co.,Ltd, Shanghai, China) in the dark, then mounted with 50% glycerol. Finally, the images were captured using a fluorescence microscope and analyzed using ImageJ software.
3.8. Western blotting
Total protein from hippocampal tissues was extracted and protein concentration was determined using a BCA kit (Shanghai Biyuntian Biological Co.,Ltd, Shanghai, China). Specifically, 40 μg of total protein was resolved by SDS-PAGE (8%), and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Burlington, MA, USA). The PVDF membranes were blocked by incubating with 5% bovine serum albumin at room temperature for 1.5 h, then the PVDF membrane was incubated with the corresponding primary antibodies overnight at 4 °C, including rabbit polyclonal anti-NHE-1 (1:1000; Abcam), rabbit monoclonal anti-calpain1 (1:1000; Abcam), and rabbit polyclonal anti-GAPDH (1:1000; Hangzhou Goodhere Biotechnology, Hangzhou, China). The next day, the primary antibodies were washed, and the NHE-1, calpain1, and GAPDH PVDF membranes were incubated in HRP-linked goat anti-rabbit secondary antibody (1:50,000; Boster Biological Engineering, Wuhan, China) for 2 h at room temperature. Finally, the protein bands were developed with ECL reagent (Pulilai Gene Technology, Beijing, China). Film grayscale values were analyzed with BandScan, and GAPDH was used as an internal reference.
3.9. RT-qPCR
Total RNA was extracted from rat hippocampal tissue using TRIzol reagent (Ambion, Austin, TX, USA). The extraction procedure was performed according to the manufacturer's instructions. Complementary DNA synthesis was conducted using a reverse transcription reagent kit (Ambion, Austin, TX, USA) according to the manufacturer's instructions. The amplification reaction was performed in a CFX96 Real-Time PCR assay system (Bio-Rad, Hercules, CA, USA). The reaction conditions were: 1) 95 °C, 30 s; 2) 95 °C, 5 s; 3) 60 °C, 30 s; 4) 39 cycles between steps 2−3, and 5) a final melting curve at 65 °C–95 °C (increment: 0.5 °C/5 s). Primers used in this study were the following: rat NHE-1, forward: 5′-GTAACCTGCAGAAAACCCGG-3′, reverse: 5′-TCGATGGTGATGACAGGGAG-3'; rat calpain1: forward: 5 ′-TGAGGGTCAAGATGGAGGAC-3′, reverse: 5′-AGGTGCCCTCGTAAAATGTG-3'; rat GAPDH, forward: 5′-ACAGCAACAGGGTGGTGGAC-3′, reverse: 5′- TTTGAGGGTGCAGCGAACTT-3'. Prism software (GraphPad, San Diego, CA, USA) was used for assessing relative RNA levels.
3.10. TUNEL staining
Preparation of tissue sections was the same as the previously described HE staining. Paraffin sections were dewaxed in xylene and alcohol. Then, 100 μL of Proteinase K solution at a concentration of 20 μg/mL was added dropwise to each sample and incubated for 20 min at room temperature. Then, 100 μL of 1 × Equilibration Buffer was added dropwise to each sample and incubated for 15 min at room temperature. The TdT reaction buffer (ddH2O, 5 × Equilibration Buffer, Alexa Fluor 647-12-dUTP Labeling Mix/488 Labeling Mix) (Shanghai Yisheng Bio-Technology/VazymeBiotech, Shanghai, China), and recombinant TdT Enzyme were added to the tissue and washed with PBS. The samples were then incubated with DAPI (Biyuntian Culture Communication, Biyuntian, China) for 10 min and washed three times for 5 min with PBST. An anti-fluorescence quencher was used to seal the sections. Finally, the images were observed and captured using a fluorescence microscopy.
3.11. Statistical analysis
Prism 8.0 software (GraphPad) was used for data analysis, and the Kolmogorov–Smirnov test and chi-square test (Levene's test) were used to test the normality of the metrological data. The metrological data conforming to the normal distribution and homogeneity of variance were expressed as the mean ± standard deviation, and one-way analysis of variance, followed by the least significant difference test for comparisons among groups. For two groups of data, an independent samples t-test method was used. Differences less than 0.05 (P < 0.05) were considered statistically significant.
4. Results
4.1. Apoptosis in the hippocampus of the model group
To characterize neuronal changes in the hippocampal CA1 region of epileptic rats, we determined the morphology of hippocampal neurons in the control and model groups using HE staining, and found that neurons in the hippocampal CA1 region of model rats were structurally disordered, with sparse cell arrangements, large numbers of neuronal losses and degenerations were visible, and glial cell proliferation was observed (Fig. 1A). In the control group, the hippocampal neurons were abundant, with normal structures and ordered cell arrangements (Fig. 1A).
Fig. 1.
Morphological changes of hippocampus in epileptic rats. (A) Hematoxylin and eosin staining, compared with the control group. The model showed structural disorder of neurons, with white arrows denoting neuronal degeneration and losses, and black arrows denoting glial hyperplasia. (B) Ultramicroscopic pathological features, the hippocampal area of the model group showed wrinkled nuclei, deepened chromatin, and integrated blocks at the edges, significantly reduced organelles, and degenerated and wrinkled mitochondria. (C) TUNEL staining, immunofluorescence of hippocampal cells by TUNEL (red). Nuclei were labeled with 3,3′-diaminobenzidine (blue), and pink denotes apoptosis-positive cells. (D) Percentage of TUNEL-positive apoptotic cells in each group. Data are expressed as the mean ± SD,**P < 0.01 compared with the control group.
The microscopic pathological changes of apoptotic cells in brain tissues of the rat hippocampal region were examined using electron microscopy. The results showed that nuclei of the control group had regular morphology, uniform distribution of chromatin, and abundant organelles. The distribution of the rough endoplasmic reticulum was regular, and the lumen of the reticulum was uniform. The mitochondria were well-developed and densely arranged (Fig. 1B). In the model group, the nucleus showed fixation changes, the nuclear membrane was wrinkled, the chromatin was clumped and border set, irregular chromatin rings were formed in the nucleus, the organelles were reduced, the mitochondria were degenerated and wrinkled, and the rough endoplasmic reticulum was swollen (Fig. 1B).
TUNEL staining was performed to detect in situ apoptosis of cells in the hippocampus of rats in both groups. The results showed that scattered apoptosis-positive cells were seen in the control group, while a large number of apoptosis-positive cells were seen in the hippocampus of model rats. After calculating the percentages of apoptotic cells in both groups, apoptosis positive cells in the hippocampus of rats in the model group were significantly increased, when compared with the control group (Fig. 1C–D, P < 0.01).
4.2. Localization of NHE-1 and calpain1 in hippocampal tissues of epileptic rats
Immunofluorescence was used to detect the NHE-1 and calpain1 expression and distribution in rats. Double-labeled immunofluorescence revealed that NHE-1 and calpain1 were expressed in neuronal cell membranes and the cell plasma, respectively, and co-localized with Neun, a marker of mature neurons in brain tissues of epilepsy models (Fig. 2). The optical density in the images was analyzed using ImageJ. The results showed NHE-1 fluorescence intensity was significantly higher in the model group than that of the control group (Fig. 2A–C, P < 0.01), but its Neun fluorescence intensity was decreased, when compared with the control group (Fig. 2A–D, P < 0.01). Similarly, calpain1 fluorescence was significantly enhanced in the model group than the control group (Fig. 2B–E, P < 0.01), but its Neun fluorescence intensity was also decreased (Fig. 2B–F, P < 0.01).
Fig. 2.
Expressions of NHE-1 and calpain1 in the hippocampus of epileptic rats (immunofluorescence). (A) Expression of NHE-1 (green) in the hippocampal region of the model group and neurons (red) were co-expressed. (B) Expression of calpain1 (green) in the hippocampus of the model group and neurons (red) were co-expressed. (C–D) The mean optical density expression of NHE-1 in the hippocampal CA1 region of the model group was higher than that of the control (P < 0.01); the Neun-positive cells in the model group were significantly lower than that of the control (P < 0.01). (E–F) The mean optical density of calpain1 in the hippocampal CA1 region was significantly increased in the model group, when compared with the control (P < 0.01), and Neun positivity was significantly decreased in the model compared with the control group (P < 0.01). Data are expressed as the mean ± SD,**P < 0.01 compared with the control group.
4.3. NHE-1 and calpain1 were over expressed in hippocampal tissues of epileptic rats
Immunohistochemical results showed that cells containing brownish-yellow granules in hippocampal neurons were NHE-1 and calpain1 staining positive cells. Stronger fluorescence intensity of NHE-1 and calpain1 were detected in the hippocampal region of rats of the model group. NHE-1 mainly distributed in neuronal cell membranes and calpain1 distributed in neuronal cell plasma (Fig. 3A and B). Statistical analysis showed that the mean optical density values of NHE-1 and calpain1 in the model group rats were significantly higher than those in the control groups (Fig. 3C–D, P < 0.05, P < 0.01). The mRNA levels of NHE-1 and calpain1 in hippocampal tissues of control and model groups were detected by RT-qPCR. The results showed that the expressions of NHE-1 and calpain1 mRNA in hippocampal tissues of rats in the model group were significantly higher than that in the control group (Fig. 3E–F, P < 0.01). The protein results of NHE-1 and calpain1 were also similar to those of mRNA, which showed that the expression of NHE-1 and calpain1 proteins was significantly higher in the hippocampal tissue of rats in the model group than in the control group (Fig. 3G-H-I, P < 0.01).
Fig. 3.
Expression of NHE-1 and calpain1 in epileptic rats. (A–B) NHE-1 and calpain1expression were measured by immunohistochemistry; the model group had stronger positive staining for NHE-1 and calpain1 in the CA1 region of the hippocampus. Black arrows denote NHE-1 and calpain1positive cells. (C–D) Statistical plots of NHE-1 and calpain1 expressions in the hippocampal CA1 region. (E–F) NHE-1 and calpain1 mRNA levels were detected by RT-qPCR; the mRNA expression of both NHE-1 and calpain1 was increased in the control group, when compared with the model. (G) NHE-1 and calpain1 protein expressions in the hippocampus of the control and model rats. (H–I) The respective bar graph shows that the NHE-1 and calpain1 protein levels were upregulated in the model rats, when compared with the controls. Data are expressed as the mean ± SD,**P < 0.01 compared with the control group.
4.4. Lentiviral transfection efficiency
The efficiency of lentiviral transfection interfering with the NHE-1 gene was tested to determine the effective time of lentiviral downregulation of NHE-1, and this time was used to further develop a lithium chloride-pilocarpine epilepsy model. The expression of NHE-1 in the hippocampal region of rats in each of the above groups was detected by immunoblotting, and the results showed that in the LV-NHE-1 group, there was no significant difference in hippocampal NHE-1 expression in the 7-d subgroup compared with the control group (Fig. 4A and B, P > 0.05), and hippocampal NHE-1 expressions in both the 14-d and 21-d subgroups were downregulated, when compared with the control group (Fig. 4A–B, P < 0.01), while the differences in NHE-1 expressions between the 14-d and 21-d groups were not statistically significant (Fig. 4A–B, P > 0.05). Therefore, the lithium chloride-pilocarpine epilepsy model was established after 2 weeks of lentiviral transfection.
Fig. 4.
Lentiviral transfection and NHE-1 expression inhibition efficiency. (A) Western blot bands; (B) semi-quantitative analysis of bands. Data are expressed as the mean ± SD. NHE-1 expressions in three subgroups of LV-NHE-1 compared to the controls,**P < 0.01.
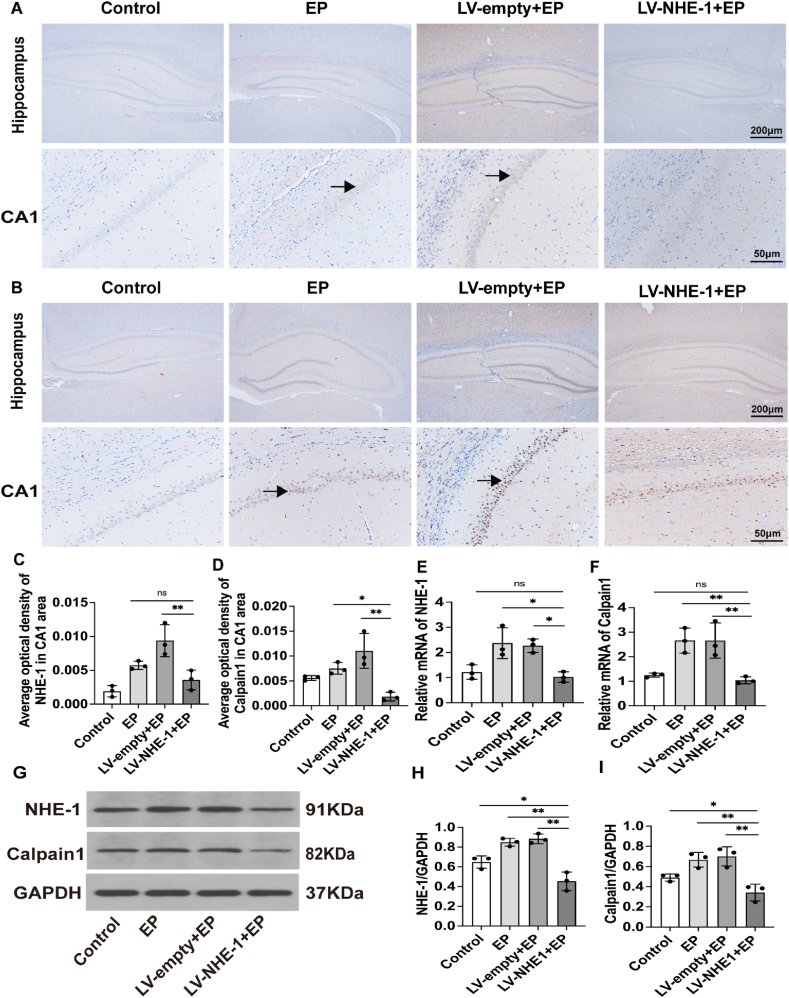
4.5. Calpain1 expression was decreased by lentiviral interference with NHE-1
To evaluate the expressions of NHE-1 and calpain1 in the hippocampal region of the epileptic rat model after shRNA interference of NHE-1, immunohistochemistry was used to detect the localizations and expressions of NHE-1 and calpain1 in the hippocampal region of rats in each group. Weaker NHE-1 and calpain1 immunoreactivities were detected in the hippocampus of rats in the LV-NHE-1+EP group, while stronger NHE-1 and calpain1 immunoreactivities were detected in the hippocampus of rats in the LV-empty + EP and EP groups (Fig. 5A-B-C-D, P < 0.01). mRNA levels in hippocampal tissues of rats in each group were detected by RT-qPCR and protein levels in hippocampal tissues were detected by Western blotting. The RT-qPCR results showed (Fig. 5E and F) that the expressions of NHE-1 and calpain1 mRNA levels in hippocampal tissues of rats in the LV-NHE-1+EP group was significantly lower than that in the LV-empty + EP group (Fig. 5E–F, P < 0.05, P < 0.01). Furthermore, LV-NHE-1 and calpain1 mRNA expressions in hippocampal tissues of rats in the NHE-1+EP group was also lower than that in the EP group (Fig. 5E–F, P < 0.05, P < 0.01).
Fig. 5.
Expressions of NHE-1 and calpain1 in epileptic rats after lentiviral transfection. (A–B) The LV-NHE-1+EP group had lighter positive staining for NHE-1 in the CA1 region of the hippocampus; black arrows denote NHE-1 positive cells. Calpain1 in the LV-NHE-1+EP group stained less positively in the CA1 region of the hippocampus; black arrows denote calpain1 positive cells. (C–D) Statistical plots of NHE-1 and calpain1 expressions in the hippocampal CA1 region. (E–F) NHE-1 and calpain1 mRNA levels were detected by RT-qPCR, which revealed that the mRNA expressions of both NHE-1 and calpain1 were decreased in the LV-NHE-1+EP group, when compared with LV-empty + EP and EP group. (G) NHE-1 and calpain1 protein expressions in the hippocampus after lentiviral transfection. (H–I) The intensities of NHE-1 and calpain1 bands were decreased in the LV-NHE-1+EP group, when compared with the LV-empty + EP and EP groups. Data are expressed as the mean ± SD,**P < 0.01 compared with the controls.
NHE-1 and calpain1 protein expression levels were similar to mRNA expression levels, and the Western blot results showed (Fig. 5G-H-I) that NHE-1 and calpain1 protein expressions in hippocampal tissues of rats in the LV-NHE-1+EP group were significantly decreased, when compared with the LV-empty + EP group (Fig. 5G–I, P < 0.01), and LV-NHE-1+EP group NHE-1 and calpain1 protein expressions in rat hippocampal tissues were lower than that in the EP group (Fig. 5G–I, P < 0.01).
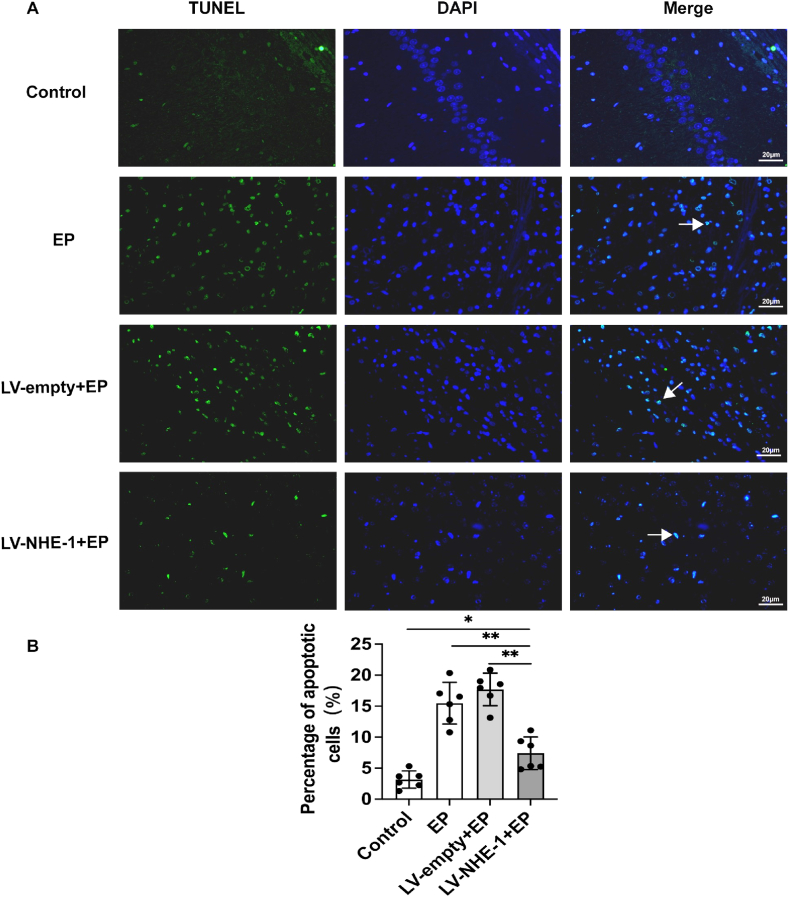
4.6. Alleviation of apoptotic response in epileptic rats after lentiviral transfection
Two weeks before establishment of the lithium chloride-pilocarpine epilepsy model, rats in the LV-NHE-1+EP group were stereotaxically injected with a lentivirus that interfered with the NHE-1 in the hippocampus, while the LV-empty + EP group was injected with an empty vector that did not interfere with the NHE-1. The rats were then euthanized two weeks after behaviors recording. Histological evaluation of apoptosis in the hippocampus showed a significant decrease in apoptosis-positive cells in the LV-NHE-1+EP group, when compared with the LV-empty + EP and EP groups (Fig. 6A and B, P < 0.01). Apoptotic cells of neurons were increased in the LV-empty + EP and EP group rats, when compared with the control group (Fig. 6A–B, P < 0.05). These results showed that downregulation of NHE-1 in the hippocampal region decreased calpain1 expression, while hippocampal apoptosis was significantly attenuated in epileptic rats.
Fig. 6.
Changes in apoptosis in each group of epileptic rats after lentiviral downregulation of NHE-1. (A) Immunofluorescence was measured using TUNEL (green), nuclei were labeled with 3,3′-diaminobenzidine (blue), and white arrows denote apoptosis-positive cells. (B) Percentage of TUNEL-positive apoptotic cells in each group; apoptotic cells were significantly reduced in the LV-NHE-1+EP group, when compared with the EP and LV-empty + EP groups. Data are expressed as the mean ± SD, *P < 0.05, **P < 0.01 compared with the LV-NHE-1+EP group.
4.7. Decreased seizure frequency before and after lentiviral downregulation of NHE-1
A total of 30 SD rats were used in the first study, with 15 of which were used for lithium chloride-pilocarpine modeling. Twelve rats were successfully modeled, and three rats died of modeling failure. A total of 27 rats survived. Most of the surviving model rats had grades 2−3 seizures lasting several minutes during the first 3 days, which lasted for several minutes. Thereafter, the rats experienced a latency period of 2–3 weeks with few epileptic seizures. After the latency period, the rats entered a chronic spontaneous phase.
After downregulation of NHE-1 expression of seizures in epileptic rats, kindling time was recorded in the establishment of a pilocarpine rat model and 2 weeks of epileptic seizures after the onset of successful chronic spontaneous kindling. We found that the time to successful kindling was prolonged in the LV-NHE-1+EP group, when compared with the LV-empty + EP and EP groups during the establishment of the pilocarpine rat model (Fig. 7B, P < 0.01). The seizure grades in rats were recorded every 10 min during the establishment of the pilocarpine model, according to the Racine scale, and the results showed that the LV-NHE-1+EP group had lower seizure levels than the LV-empty + EP group at all time points within 1 h of establishing the epilepsy model (Fig. 7A). After the successful seizure model in epileptic rats was accomplished, the seizure process was recorded. The LV-NHE-1+EP group had fewer seizures during the chronic spontaneous phase than the LV-empty + EP and EP groups, and their seizure duration was shorter than that of the LV-empty + EP and EP groups, with all differences being statistically significant (Fig. 7C–D, P < 0.05).
Fig. 7.
Behavioral changes in rats after downregulation of NHE-1. (A) During the establishment of the model in rats, the seizure grades at all time points within 1 h were lower in the LV-NHE-1+EP group, when compared with the LV-empty + EP group. (B) Differences in the latency of successful ignition during epilepsy modeling in each group of rats; the ignition latency in the LV-NHE-1+EP group was prolonged compared with the LV-empty + EP and EP groups. (C–D) During the chronic spontaneous period, the frequency and duration of seizures were reduced in the LV-NHE-1+EP group, when compared with the LV-empty + EP and the EP groups. Data are expressed as the mean ± SD, **P < 0.01; *P < 0.05 compared with the LV-NHE-1+EP group.
5. Discussion
Recurrent seizures can cause neuron injury. Neuronal apoptosis and necrosis are important pathological features of neuronal damage after epilepsy [8]. Study results reporting neuronal death after status epilepticus (SE) with typical apoptotic features have been confirmed by morphological or biochemical methods in human patients with temporal lobe epilepsies, as well as in various models of SE such as electrical or chemical ignition, or local or systemic administration of epileptogenic agents [[24], [25], [26]]. In the present study, we observed upregulation of NHE-1 and calpain1 expressions in the hippocampal region, and significant neuronal apoptosis in a pilocarpine-induced epilepsy rat model. We also observed that inhibition of NHE-1 expression by lentivirus downregulated calpain1 and alleviated the hippocampal apoptotic reaction. In addition, downregulation of NHE-1 followed by prolonged pilocarpine exposure in rats resulted in some alleviation of seizure frequency and seizure grade in the chronic spontaneous phase.
Calpain is a calcium-dependent protein hydrolase that is hyperactivated by increased intracellular calcium levels under pathological conditions, and substrates of cellular regulatory necrosis, apoptosis, and autophagy pathways are affected by calpain [27,28]. In our study, calpain1 expression increased in the hippocampal region of model rats during the chronic spontaneous phase, and TUNEL staining showed significant apoptosis in the hippocampal region of model rats, suggesting that apoptosis in the hippocampal region was correlated with increased calpain1 expression. This is consistent with the findings of Gao and Geng [29], which showed that calpain1 played an important role in neuronal injury during SE. However, the upstream regulators of calpain1 leading to neuronal apoptosis are unknown.
NHE-1 is involved in the neuronal apoptotic response in ischemic brain diseases and neurodegenerative diseases, and inhibition of NHE-1 can prevent neuronal cell apoptosis and exert neuroprotective effects [30]. Our previous study found that NHE-1 and apoptosis-related protein Bax expressions were increased in postoperative brain tissue specimens from patients with temporal lobe epilepsy, and neuronal loss and necrosis were significant [31]. However, the mechanism of NHE-1 involved in neuronal apoptosis in epilepsy has not been reported. In the present study, we confirmed that NHE-1 played an important role in the apoptosis of epileptic neurons. We used different methods, involving histology and molecular biology, to confirm that the expressions of NHE-1 and calpain1 were significantly increased in the hippocampal region of the epileptic rat model. TUNEL staining in this study showed that the apoptosis of hippocampal cells in the model group was clear, the apoptosis of hippocampal cells and elevated expressions of NHE-1 and calpain1 in hippocampal neurons were consistent. Previous studies showed that calpain1 promotes neuronal apoptosis in epileptic rats. In addition, NHE-1 mainly distributed in neuronal cell membranes and calpain1 distributed in neuronal cell plasma. Hence, the upregulation of NHE-1 and calpain1 expressions may be related to hippocampal neuronal apoptosis. This may reveal the involvement of NHE-1 and calpain1 in the development of hippocampal neuron apoptosis in epileptic rats.
Hence, we then further determined the effect of NHE-1 alteration on calpain1 and apoptosis, and used the lentiviral local interference protein expression technique to decrease NHE-1 protein expression in the hippocampal region. RT-qPCR and Western blot results showed that the expression of calpain1 also decreased after lentiviral intervention in hippocampal NHE-1. TUNEL staining showed that apoptosis-positive cells in the hippocampal region were significantly increased in the LV-empty + EP and EP groups, indicating that hippocampal cell apoptosis was increased during the chronic spontaneous phase after SE, while apoptosis-positive cells in the hippocampal region of the LV-NHE-1+EP group were significantly less than those in the LV-empty + EP and EP groups, suggesting that after lentiviral interference of NHE-1, apoptotic cells in the hippocampus of rats were significantly reduced. This result is consistent with the downregulation of NHE-1 and calpain1 expression as shown by RT-qPCR, Western blot, and immunohistochemistry. Therefore, it can be hypothesized that the expressions of NHE-1 and calpain1 and hippocampal cells apoptosis are positively correlated in the epileptic rat model, and NHE-1 may affect calpain1 expression, while calpain1 participates in the occurrence of apoptosis by affecting downstream pathways such as those of caspase and the bcl2 family.
Dong et al. [32] reported that NHE-1 activation increased intracellular Na+ concentration, which in turn stimulated NCX, leading to an increase in intracellular Ca2+ concentration and promoting mitochondrial damage and cell death. Cengiz [17] used a NHE-1 inhibitor to treat neuronal degeneration and apoptosis in hippocampal tissue of ischemic-hypoxic brain injury, and found that it played a neuronal protective role by inhibiting NHE-1, NCX, and calcium overload. It has been observed that during lipopolysaccharide-induced apoptosis in vascular endothelial cells, with increased Ca2+ activity, NHE-1 expression increased and calpain1 expression was also increased [33]. Previous studies have reported that ischemia induced acidosis, causing excessive activation of NHE-1, leads to intracellular Na+ overload, followed by activation of NCX, resulting in excessive Ca2+ intracellular entry [34,35], with the excess Ca2+ eventually leading to cell death [36]. Combined with previous studies, the results showed that calpain1 promotes apoptosis by activating caspase and the bcl2 family, while the activation of calpain1 requires the involvement of intracellular Ca2+. Depending on the primary function of NHE-1, its activation may lead to abnormal intracellular calcium aggregation. We observed that after repeated seizures, both NHE-1 and calpain1 expressions were upregulated in the hippocampal region of rats, and apoptosis was significant. It was therefore suggested that NHE-1 and calpain1 were closely related to the occurrence of apoptosis. The possible reasons were the following: repeated seizures could cause neurons to be in a state of ischemia and hypoxia, resulting in a decrease in intra-neuronal pH and acidosis, followed by activation of NHE-1, leading to intracellular Na+ overload, and excessive Na+ stimulated NCX activity, which increases intracellular Ca2+ concentrations that activate calpain1, eventually leading to apoptosis. We therefore hypothesize that NHE-1 may take part in apoptosis by regulating the expression of calpain1.
In addition, we found that during the establishment of the lithium chloride-pilocarpine epilepsy model, the LV-NHE-1+EP group exhibited prolonged latency of SE and reduced seizure levels, and during chronic spontaneous periods, the number and duration of epileptic seizures were decreased in the LV-NHE-1+EP group, when compared with the LV-empty + EP and EP groups, indicating that the severity of seizures could be reduced after lentiviral intervention with NHE-1. This is consistent with previous studies. Ou-Yang et al. [37] reported that seizures and severities were significantly alleviated and cognition was improved in epileptic rats after treatment with the NHE-1 inhibitor, amiloride, with a possible mechanism being a reduction in glutamate release and NMDA receptor activation. By knocking-down NHE-1 expression in excitatory glutamatergic neurons and inhibitory GABAergic neurons in mice, it was reported that NHE-1 mice with knockdown of inhibitory GABAergic neurons exhibited seizures and ataxia, while NHE-1 knockdown of excitatory glutamatergic neurons had no seizures. The possible mechanism was impairment of synaptic GABA vesicle release leading to seizures. This study suggested that NHE-1 was involved in seizures by modulating inhibition of interneurons to affect network excitability [38]. Inhibition of NHE-1 lead to intracellular acidification, which in turn attenuates NMDA receptor activation and glutamatergic excitatory transmission [39]. Thus, NHE1 may affect seizures by regulating synaptic membrane receptors and neurotransmitters.
However, in the involvement of NHE-1 in apoptosis-related pathways, we only determined the expression characteristics of NHE-1 and calpain1, and did not further examine the expression characteristics of NCX, Na+, and Ca2+ concentrations. We will explore further interaction of NHE-1 and NCX, and expressions of Na+ and Ca2+ in future experiments.
In summary, we suggest that NHE-1, which is upregulated in expression in epileptic model rats, may be involved in the onset and development of epilepsy by exacerbating neuronal apoptosis, and the NHE-1/calpain1 signaling pathway may be an important mechanism leading to apoptosis.
Ethics statement
The studies were reviewed and approved by the Ethics Committee of Guizhou Medical University (No.1800140).
Author contributions
Shuang Peng, Xuling Wu, Qian Zheng: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Lan Ye, Xiangang Mo, Zhanhui Feng: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jianwei Xu, Dongjun Xie, Mengyun Zhou, Mingwei Wang, Yongran Cheng: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data, Wrote the paper.
FUNDING
This work was supported by the National Natural Science Foundation of China (No. 81860248, No. 81960224), the Science Foundation of China Association Against Epilepsy (No. 2018016), the Science and Technology Fund of Guizhou Health Commission (No. gzwjkj2020-2-005, No. gzwkj2021-024), the Cultivation Project of the National Natural Science Foundation of China (No. 19NSP051), and the Basic Research Program of Guizhou Province (Qiankehe basic-ZK[2023]395 and 324).
Data availability statement
All data can be obtained by contacting the corresponding authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Contributor Information
Lan Ye, Email: frogyl266@163.com.
Xiangang Mo, Email: moxiangang123@126.com.
Zhanhui Feng, Email: h9450203@126.com.
References
- 1.Tatum W.O.t. Epilepsy & behavior reports. Epilepsy Behav Rep. 2019;12 doi: 10.1016/j.ebr.2019.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher R.S., Acevedo C., Arzimanoglou A., et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 3.Singh A., Trevick S. The epidemiology of global epilepsy. Neurol. Clin. 2016;34:837–847. doi: 10.1016/j.ncl.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Caciagli L., Bernasconi A., Wiebe S., et al. A meta-analysis on progressive atrophy in intractable temporal lobe epilepsy: time is brain? Neurology. 2017;89:506–516. doi: 10.1212/WNL.0000000000004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Z.H., Hao J., Ye L., et al. Overexpression of mu-calpain in the anterior temporal neocortex of patients with intractable epilepsy correlates with clinicopathological characteristics. Seizure. 2011;20:395–401. doi: 10.1016/j.seizure.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henshall D.C., Murphy B.M. Modulators of neuronal cell death in epilepsy. Curr. Opin. Pharmacol. 2008;8:75–81. doi: 10.1016/j.coph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Meraz M.L., Niquet J., Wasterlain C.G. Distinct caspase pathways mediate necrosis and apoptosis in subpopulations of hippocampal neurons after status epilepticus. Epilepsia. 2010;51(3):56–60. doi: 10.1111/j.1528-1167.2010.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Han Y., Du J., et al. Alterations of apoptosis and autophagy in developing brain of rats with epilepsy: changes in LC3, P62, Beclin-1 and Bcl-2 levels. Neurosci. Res. 2018;130:47–55. doi: 10.1016/j.neures.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Lu J.Q., Steve T.A., Wheatley M., et al. Immune cell infiltrates in hippocampal sclerosis: correlation with neuronal loss. J. Neuropathol. Exp. Neurol. 2017;76:206–215. doi: 10.1093/jnen/nlx001. [DOI] [PubMed] [Google Scholar]
- 10.Guan X., Luo L., Begum G., et al. Elevated Na/H exchanger 1 (SLC9A1) emerges as a marker for tumorigenesis and prognosis in gliomas. J. Exp. Clin. Cancer Res. 2018;37:255. doi: 10.1186/s13046-018-0923-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng T., Shi Y., Xiong Z.G., et al. Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke? Prog. Neurobiol. 2014;115:189–209. doi: 10.1016/j.pneurobio.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H., Carney K.E., Falgoust L., et al. Emerging roles of Na(+)/H(+) exchangers in epilepsy and developmental brain disorders. Prog. Neurobiol. 2016;138–140:19–35. doi: 10.1016/j.pneurobio.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang H.B., Gao X., Nepomuceno R., et al. Na(+)/H(+) exchanger in the regulation of platelet activation and paradoxical effects of cariporide. Exp. Neurol. 2015;272:11–16. doi: 10.1016/j.expneurol.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araujo I.M., Carreira B.P., Pereira T., et al. Changes in calcium dynamics following the reversal of the sodium-calcium exchanger have a key role in AMPA receptor-mediated neurodegeneration via calpain activation in hippocampal neurons. Cell Death Differ. 2007;14:1635–1646. doi: 10.1038/sj.cdd.4402171. [DOI] [PubMed] [Google Scholar]
- 15.Ma J., Chen Z., Ma Y., et al. MicroRNA-19a attenuates hypoxia-induced cardiomyocyte apoptosis by downregulating NHE-1 expression and decreasing calcium overload. J. Cell. Biochem. 2020;121:1747–1758. doi: 10.1002/jcb.29411. [DOI] [PubMed] [Google Scholar]
- 16.Hwang I.K., Yoo K.Y., An S.J., et al. Late expression of Na+/H+ exchanger 1 (NHE1) and neuroprotective effects of NHE inhibitor in the gerbil hippocampal CA1 region induced by transient ischemia. Exp. Neurol. 2008;212:314–323. doi: 10.1016/j.expneurol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Cengiz P.K., Uluc K., Kendigelen P., Hagemann T., Akture E., Messing A., Ferrazzano P., Sun D. Inhibition of Na +/H + exchanger isoform 1 is neuroprotective in neonatal hypoxic ischemic brain injury. Antioxidants Redox Signal. 2011;14:1803–1813. doi: 10.1089/ars.2010.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng S.W., Yu Y.L., Dong L., Xu Z.C., Ye L., Feng Z.H. Expression of NHE1 in hippocampus of epileptic rat model kindled by lithium pilocarpine. J. Guangxi Med. Univ. 2021;46:16–21. [Google Scholar]
- 19.Lam P.M., Gonzalez M.I. Calpain activation and neuronal death during early epileptogenesis. Neurobiol. Dis. 2019;124:141–151. doi: 10.1016/j.nbd.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karyakin V.B., Vasil'ev D.S., Zhuravin I.A., et al. Early morphological and functional changes in the GABAergic system of hippocampus in the rat lithium-pilocarpine model of epilepsy. Dokl. Biol. Sci. 2017;472:4–7. doi: 10.1134/S0012496617010045. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed J., II, Che Has A.T. The evolution of the pilocarpine animal model of status epilepticus. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racine R.J., Gartner J.G., W M Burnham Epileptiform activity and neural plasticity in limbic structures. Brain Res. 1972;47:262–268. doi: 10.1016/0006-8993(72)90268-5. [DOI] [PubMed] [Google Scholar]
- 23.Yang P., Wang Z., Zhang Z., et al. The extended application of the Rat Brain in Stereotaxic Coordinates in rats of various body weight. J. Neurosci. Methods. 2018;307:60–69. doi: 10.1016/j.jneumeth.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Song Y Z.M., Cai F.C. Oxcarbazepine causes neurocyte apoptosis and developing brain damage by triggering Bax/Bcl-2 signaling pathway mediated caspase 3 activation in neonatal rats. Eur. Rev. Med. Pharmacol. Sci. 2018;221:250–261. doi: 10.26355/eurrev_201801_14126. [DOI] [PubMed] [Google Scholar]
- 25.Weng N., Sun J., Kuang S., et al. MicroRNA-451 aggravates kainic acid-induced seizure and neuronal apoptosis by targeting GDNF. Curr. Neurovascular Res. 2020;17:50–57. doi: 10.2174/1567202617666191223150510. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B., Zhang J.W., Wang W.P., et al. Effect of lamotrigine on epilepsy-induced cognitive impairment and hippocampal neuronal apoptosis in pentylenetetrazole-kindled animal model. Synapse. 2017;71 doi: 10.1002/syn.21945. [DOI] [PubMed] [Google Scholar]
- 27.Martensson L.B., Blom C.L., Dahlin L.B. Ca(2+) involvement in activation of extracellular-signal-regulated-kinase 1/2 and m-calpain after axotomy of the sciatic nerve. Neural Regen Res. 2017;12:623–628. doi: 10.4103/1673-5374.205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radak D K.N., Resanovic I., Jovanovic A., Sudar-Milovanovic E., Zafirovic S., Mousad S.A. Isenovic ER apoptosis and acute brain ischemia in ischemic stroke. Curr. Vasc. Pharmacol. 2017;15:115–122. doi: 10.2174/1570161115666161104095522. [DOI] [PubMed] [Google Scholar]
- 29.Gao H., Geng Z. Calpain I activity and its relationship with hippocampal neuronal death in pilocarpine-induced status epilepticus rat model. Cell Biochem. Biophys. 2013;66:371–377. doi: 10.1007/s12013-012-9476-5. [DOI] [PubMed] [Google Scholar]
- 30.Xing R., Liu X., Tian B., et al. Neuroprotective effect of Na(+)/H(+) exchangers isoform-1 inactivation against 6-hydroxydopamine-induced mitochondrial dysfunction and neuronal apoptosis in Parkinson's disease models. Drug Dev. Res. 2021;82:969–979. doi: 10.1002/ddr.21799. [DOI] [PubMed] [Google Scholar]
- 31.Wu X.L.D., Peng S., Ye L., Xu Z.C., Feng Z.H. The expression of NHE1 and apoptosis related protein in brain tissue of patients with lateral temporal lobe epilepsy. Chinese Journal of Clinical Anatomy. 2021;39:431–436. [Google Scholar]
- 32.Dong Z., Saikumar P., Weinberg J.M., et al. Calcium in cell injury and death. Annu. Rev. Pathol. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 33.Cui G.M., Zhao Y.X., Zhang N.N., et al. Amiloride attenuates lipopolysaccharide-accelerated atherosclerosis via inhibition of NHE1-dependent endothelial cell apoptosis. Acta Pharmacol. Sin. 2013;34:231–238. doi: 10.1038/aps.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cengiz P., Kintner D.B., Chanana V., et al. Sustained Na+/H+ exchanger activation promotes gliotransmitter release from reactive hippocampal astrocytes following oxygen-glucose deprivation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pignataro G., Sirabella R., Anzilotti S., et al. Does Na(+)/Ca(2)(+) exchanger, NCX, represent a new druggable target in stroke intervention? Transl Stroke Res. 2014;5:145–155. doi: 10.1007/s12975-013-0308-8. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q. TMBIM-mediated Ca(2+) homeostasis and cell death. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:850–857. doi: 10.1016/j.bbamcr.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ou-Yang T.P., Zhu G.M., Ding Y.X., et al. The effects of amiloride on seizure activity, cognitive deficits and seizure-induced neurogenesis in a novel rat model of febrile seizures. Neurochem. Res. 2016;41:933–942. doi: 10.1007/s11064-015-1777-9. [DOI] [PubMed] [Google Scholar]
- 38.Bocker H.T., Heinrich T., Liebmann L., et al. The Na+/H+ exchanger Nhe1 modulates network excitability via GABA release. Cerebr. Cortex. 2019;29:4263–4276. doi: 10.1093/cercor/bhy308. [DOI] [PubMed] [Google Scholar]
- 39.Ali A., Dua Y., Pillai K.K., Vohora D. Seizures and sodium hydrogen exchangers: potential of sodium hydrogen exchanger inhibitors as novel anticonvulsants. CNS Neurol. Disord.: Drug Targets. 2008;7:343–347. doi: 10.2174/187152708786441830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data can be obtained by contacting the corresponding authors.