Abstract
Background
There is a lack of consensus among studies on the association between proton pump inhibitor (PPI) use and cognitive impairment. This association is not well studied among minority populations, including among Puerto Ricans. Therefore, we sought to examine this association among Boston-area Puerto Ricans.
Methods
The Boston Puerto Rican Health Study is an ongoing longitudinal cohort that enrolled 1499 Boston-area Puerto Rican adults, aged 45–75 years at baseline. Complete outcome and exposure data was available for 1290 baseline participants. Covariate-adjusted linear regression and linear mixed effects models were used to examine the association between PPI use, and global cognition, executive function, and memory cross-sectionally and longitudinally over ~12.7 years of follow-up. Furthermore, we examined the cross-sectional association between long-term PPI use (continuous use of ~6.2 years) and global cognition, executive function, and memory.
Results
Among 1 290 participants at baseline, 313 (24.3%) self-reported PPI use. Baseline PPI use was not associated with baseline global cognition, executive function, or memory. Baseline PPI use also did not alter the trajectory of global cognition, executive function, or memory over ~12.7 years of follow-up. Long-term PPI use was not associated with global cognition, executive function, or memory over ~12.7 years of follow-up.
Conclusion
In this study of Boston-area Puerto Ricans, we did not observe an association between PPI use and global cognition, executive function, or memory either cross-sectionally or over 12.7 years of follow-up.
Keywords: Boston-area Puerto Ricans, Cognitive function, Proton pump inhibitors
It is estimated that 6.5 million adults aged 65 years and older live with Alzheimer’s dementia in the United States (1). The etiopathology of cognitive impairment is attributed to a complex combination of genetic and environmental risk factors, including inappropriate medication use (2).
Proton pump inhibitors (PPIs) are used to treat acid-related gastrointestinal (GI) disorders, such as gastro-esophageal reflux disease, Helicobacter pylori infection, peptic ulcers, dyspepsia, Zollinger–Ellison syndrome, and GI symptoms associated with corticosteroid use, antiplatelet therapy, and chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs) (3,4). PPIs are among the most highly prescribed medications worldwide, and there is increasing long-term and off-label utilization, with evidence suggesting 25%–70% of PPI users do so without appropriate indication (4,5). Long-term PPI use may be associated with side effects, including vitamin B12 deficiency, fractures, Clostridium difficile infection, and small intestinal bacterial overgrowth (2–4,6,7).
Several studies have reported an association between PPI use and cognitive impairment (3,6,8–24). One of the driving hypothesis used to explain this potential association is related to the malabsorption of vitamin B12 due to suppression of gastric acid by PPI use (8). Gastric acid is essential for the absorption of vitamin B12 and PPIs suppress gastric secretion by forming irreversible disulfide bonds with cysteine residues on the proton pumps of gastric parietal cells, inhibiting ATPase-mediated gastric acid pumps (25). Vitamin B12 and folate are coenzymes in the synthesis of neurotransmitters such as serotonin and catecholamine, and vitamin B12 deficiency may contribute to the demyelination of neurons and cognitive impairment (26).
PPIs are reported to cross the blood–brain barrier (BBB) based on their pharmacokinetic properties (27). It is hypothesized that PPI use may contribute to cognitive impairment due to the homology between gastric and central nervous system (CNS) proton pumps (9,14). Vacuolar-type ATPase (V-ATPase) proton pumps in the CNS are involved in acid–base and potassium homeostasis and maintain the proton gradient required for neurotransmitter packaging in synaptic vesicles. Studies utilizing animal models report that PPIs penetrate the CNS (9,27,28) and prevent degradation of fibrillary amyloid-β (Aβ) by affecting proton pumps of microglial lysosomal vacuoles. An acidic lysosomal environment is required to degrade Aβ plaques, and it is reported that PPIs decrease lysosomal pH by blocking V-ATPase pumps, thus influencing the degradation of fibrillary Aβ. Therefore, it is hypothesized that long-term PPI use is associated with neurodegeneration and cognitive impairment (9,28–30). It is also hypothesized that PPIs inhibit acetylcholine biosynthesizing enzyme, which may increase the risk of dementia (15).
Although studies have examined the association between PPI use and cognitive impairment (3,6,8–24), there is a lack of consensus. While some report no association between PPI use and cognitive function (10,22), risk of dementia (16–18), or conversion from mild cognitive impairment (MCI) to dementia due to Alzheimer’s disease (AD) (13), others report an association between PPI use increased risk of dementia (11,12,19), particularly with long-term PPI use (20), and increased odds of AD and non-AD dementia among PPI users (21). This lack of consensus on the association between PPI use and cognitive impairment or dementia motivated a recent study utilizing the Food and Drug Administration Adverse Event Reporting System database (FAERS/AERS), which reported increased odds of memory impairment with PPI use (23). In contrast, however, another recent analysis utilizing the FAERS/AERS reported no association between short- and long-term PPI use and dementia (24). Additionally, some studies report on PPI use and impairment in specific cognitive domains assessed by tests for spatial orientation, immediate and short-term recall, visual memory, attention, or executive function (10,20,31). A recent review observed that the current evidence on the association between PPI use and risk of dementia is incomplete, highlighting the need to further test this association (32)
To the best of our knowledge, there are no studies reporting on the association between PPI use and cognitive function in Puerto Ricans. Hispanics and Latinos, including Puerto Ricans, are an under-studied population, and are at an increased risk of cognitive impairment and dementia due to distinct lifestyle characteristics and risk factors such as the high prevalence of diabetes and cardiovascular diseases (33). Recent estimates suggest that the largest increase in Alzheimer’s disease and related dementias (ADRD), by 2060, will be among Hispanics and Latinos (34). Therefore, we sought to examine the association between PPI use and cognitive function, including global cognition, and the domains of executive function and memory both cross-sectionally and longitudinally, over ~12.7 years of follow-up among Boston-area Puerto Ricans (35).
Method
Data Collection
This analysis was conducted within the Boston Puerto Rican Health Study (BPRHS), an ongoing longitudinal cohort (35) that enrolled 1 499 self-identified Puerto Rican adults residing in the Boston, Massachusetts, metropolitan area aged 45–75 years at baseline. Of these, 1 250 participants completed wave-2 (mean 2.2 years from baseline ± 0.61 year), 980 participants completed wave-3 (mean 6.2 years from baseline, standard deviation [SD] ± 0.98 year), and 638 participants completed wave-4 (mean 12.7 years from baseline ±1.18 years). Participants were recruited through door-to-door enumeration and community approaches. One participant per household was randomly recruited for the study. Participants with serious health conditions or Mini-Mental State Examination (MMSE) scores ≤10 were excluded (35). Study protocols were approved by the Institutional Review Boards at Tufts Medical Center and the University of Massachusetts Lowell.
Assessment of Cognitive Function
A comprehensive battery of culturally-appropriate cognitive tests were utilized, based on participant choice, in either Spanish (98%) or English, at baseline, wave-2, and wave-4, by research assistants trained and supervised by a clinical psychologist, as described previously (35,36). General cognitive function was assessed with the MMSE, with scores ranging from 0 to 30 and higher scores indicating better cognitive function (37). Verbal memory was evaluated using a 16-word list learning test (38). Digit span forward and backward tests were administered to assess attention and working memory (38). Executive function was evaluated by the Stroop test involving the naming of colors (38), and verbal fluency test involved naming as many words as possible, starting with a given letter (38). Clock drawing (39) and figure copying tests assessed visuo-spatial function and organization (40). A composite global cognitive score (GCS) was computed as the mean of z-scores for each of the following individual tests: MMSE, word list learning, recognition, percentage retention, Stroop, letter fluency, digit span forward and backward, clock drawing, and weighted figure copying, as described previously (36,41). Additionally, principal components analysis with varimax rotation was used to derive factors covering 2 composite variables for the cognitive domains of memory and executive function, as described previously (36,42).
Assessment of Proton Pump Inhibitor Use
Medication data from baseline, wave-2, and wave-3 were utilized for analyses. Medication data were self-reported by 1 494 participants at baseline, 1 252 participants at wave-2, and 939 participants at wave-3. At the time of writing, PPI use and other medication information at wave-4 were not available. Prescription and over-the-counter medication use were ascertained by careful assessment of medication use via examination of medicine containers. Having reported medication use at baseline, participants were asked to self-report on subsequent medication use, and ascertainment was repeated by examination of medicine containers at waves 2 and 3 (35). PPIs reported in the BPRHS were omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole. Our primary exposure measure was any PPI use, that is, participants self-reporting utilization of any PPIs listed earlier were classified as users, and those reporting none were classified as nonusers.
Covariates
Detailed methodology for data collection and covariate assessment at baseline through subsequent waves have been described previously (35). Covariate data at baseline and wave-3 were used in our analyses. Participants provided information on age, education level, family history, and income level. Questionnaires were designed based on questions from the National Health and Nutrition Examination Survey (NHANES) III (43), the Hispanic Health (44,45) and Nutrition Examination Survey, and the National Health Interview Survey Supplement on Aging (46). Dietary intake was assessed with a semiquantitative food-frequency questionnaire (FFQ) specifically designed for this population (35). Mediterranean diet was assessed from FFQ and categorized (range of 0–9) based on intake of 9 dietary components: grains, vegetables, fruits, legumes/pulses/nuts, fish, olive oil, meat/poultry, dairy, and alcohol (35,41). Medications such as platelet aggregation inhibitor use, aspirin, NSAID, and multivitamin supplement use were ascertained by careful assessment via examination of containers (35) and categorized as binary variables. At each time point, participants self-reported health conditions, information on health-insurance, and self-rated health status (35). Smoking and alcohol use frequency, history, and type were assessed. Alcohol use was categorized as nondrinker (none within the past year), moderate drinker, or heavy drinker, based on the average amount of alcohol consumed. Smoking status was categorized as nonsmoker (lifetime use of <100 cigarettes), past smoker, or current smoker. Physical activity score was based on a modified questionnaire, as described previously (35), and computed as the sum of hours spent on activities over 24-hours, and multiplied by weights for a rate of oxygen consumption associated with each activity. Body mass index (BMI) was computed using weight (kg) divided by height (m) squared (35). BMI of 25–29.9 was classified as overweight, 30–39.9 as obese classes I and II, and ≥40 as extremely obese. Cholesterol was analyzed from ethylenediaminetetraacetic acid plasma with an enzymatic endpoint reaction (35). Diabetes was defined as fasting plasma glucose ≥126 mg/dL or use of diabetes medication and categorized as a binary variable. Hypertension was defined as blood pressure ≥140/90 mmHg or use of hypertension medication and categorized as a binary variable. Anemia, categorized as a binary variable, was defined by both hemoglobin and hematocrit concentration based on recommendations by the World Health Organization (hemoglobin concentration male <13g/dL and female <12g/dL defined as anemic) and the Center for Disease Control (hematocrit concentration male <39.9% and female <35.7% defined as anemic) (35). Depression was evaluated by the Center for Epidemiological Studies Depression (CES-D) score, and participants with scores ≥16 were classified as likely depressed (35,47). C-reactive protein (CRP) was measured in serum using the Immulite 1000 High Sensitive CRP kit (Seimens Medical Solutions Diagnostics, Los Angeles, CA) (35). For assessment of apolipoprotein E ε4 status, genomic DNA was isolated from blood using the QIAamp DNA Blood mini kit (Qiagen, Hilden, Germany), and genotyping was performed to identify genetic polymorphisms (Applied Biosystems TaqMan SNP genotyping system, Foster City, USA) (35).
Statistical Analysis
Analyses were performed using R, version 4.1.0 (R Foundation for Statistical Computing). Descriptive statistics and baseline univariate differences in demographics among PPI users versus PPI nonusers were evaluated by chi-square, Mann–Whitney–Wilcoxon, Fisher, or T-tests, as appropriate.
Associations between PPI exposure and cognitive outcomes were analyzed cross-sectionally at baseline, adjusting for relevant covariates. Covariates included factors known to affect both the exposure and outcome, as well as those reported in previous studies. Multivariable linear regression (MLR) models were adjusted for age, sex/estrogen status, smoking status, education, apolipoprotein E ε4 status, alcohol frequency, BMI (model 2), and additionally for depression score (CES-D), diabetes, hypertension, and stroke (model 3). The final model was additionally adjusted for anemia, histamine type-2 receptor antagonist (H2RA), platelet aggregation inhibitor, aspirin, NSAID, multivitamin use, and Mediterranean diet score (model 4). Previous studies, including in the BPRHS, have reported that the Mediterranean diet is associated with cognitive function (41). H2RA use is reported to be associated with gastric acid reduction and cognitive function (10,17). H2RAs reported in the BPRHS include cimetidine, famotidine, nizatidine, and ranitidine. H2RA, platelet aggregation inhibitor, aspirin, NSAID, and multivitamin use were self-reported and categorized as binary variables. We did not adjust for cholinergic medication use, as there were only 6 baseline users.
Next, we defined long-term PPI use as self-reported PPI use at all 3 time-points, from baseline through wave-3 (mean 6.2 years ± 0.98 year) and examined cross-sectional associations between long-term PPI use (continuous utilization of ~6.2 years) and cognitive function scores at wave-4 (mean 12.7 years from baseline), adjusting for covariates at wave-3 as described earlier. Cognitive function at wave-3 was not available in the BPHRS.
To examine the association between baseline PPI use and trajectory of cognitive function over ~12.7 years of follow-up (wave-4), we used linear mixed effects models (LMM; nlme package in R). For cognitive function, level-1 data (observations) included 3 measures of cognitive function collected at baseline, wave-2, and wave-4 follow-up. We used continuous autoregressive 1 correlation structure to fit random effects due to unevenly spaced time-points, assuming a higher correlation between adjacent times and a lower correlation between time points spaced further apart, and assuming more variability in scores with progressing time. Random effects were fitted with random slopes and intercepts. Baseline PPI use was the main effect. Time was a continuous variable for years from baseline through wave-4 follow-up. Baseline PPI use and all baseline covariates were treated as fixed effects. Random effects were modeled for participant study ID (intercept) and time (slope). To examine whether global cognitive score trajectory, executive function, or memory scores varied by baseline PPI use, we added an interaction term between PPI use and time. The β-coefficient of these interaction terms describes the association of baseline PPI use on changes in global cognitive score, memory, and executive function over time. LMM was performed adjusting for baseline covariates included in models 1–4, as described earlier for MLR.
We examined whether age at baseline modified the association between PPI use and cognitive function/cognitive trajectory by adding a multiplicative term for age×PPI use to our fully adjusted multivariate regression models. We assessed effect modification by age in all 3 analyses conducted in this study: cross-sectional analyses at baseline, long-term PPI use in relation to wave-4 cognition, and cognitive trend over 4 waves of follow-up.
We examined whether PPI use predicted loss to follow-up (possibility of attrition bias) in our study using logistic regression, estimating, among all participants at baseline, the odds of loss to follow-up at wave-4 and according to a category of PPI use.
Results
Among the total 1 499 baseline participants, we observed an increasing utilization of PPIs from baseline (25.2%) to wave-3 (35.5%). We also observed an increasing utilization of non-aspirin NSAIDs from baseline (32.9%) to wave-3 (38.3%), aspirin/acetyl salicylic acid use from baseline (28.3%) to wave-3 (45.0%), and multivitamin use from baseline (19.7%) to wave-3 (27.2%). There was a slight decrease in the utilization of H2RAs from baseline (7.81%) to wave-3 (6.14%).
Complete cognitive function data for GCS, executive, and memory function were available for 1 294 participants at baseline, 1 060 at wave-2, and 470 at wave-4. After excluding participants with missing cognitive function scores and PPI use exposure data (Figure 1), our analysis was based on 1 290 participants at baseline, 1 057 at wave-2 (mean 2.2 years from baseline ± 0.61 year), and 404 at wave-4 (mean 12.7 years from baseline ±1.18 years).
Figure 1.
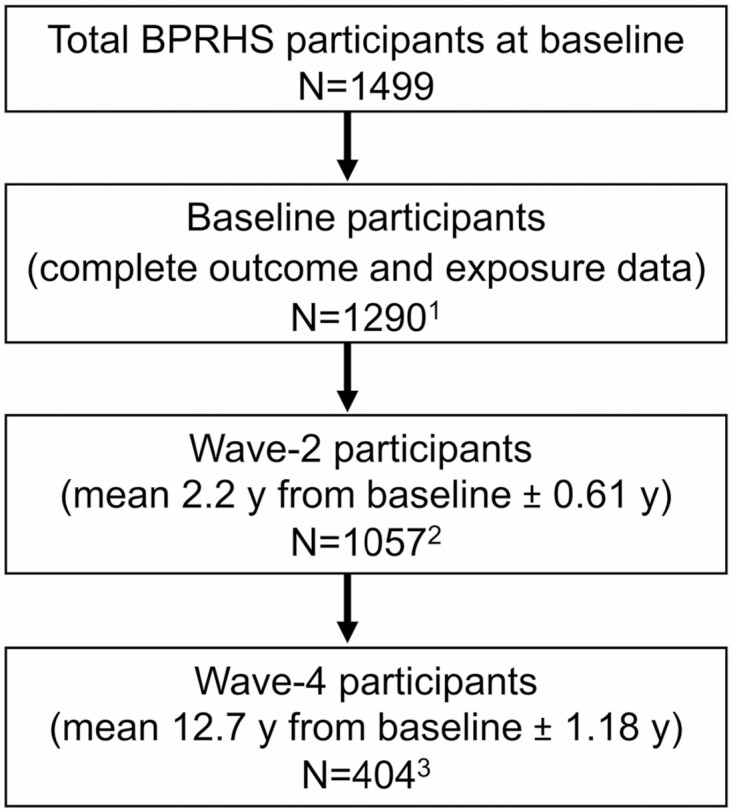
Boston Puerto Rican Health Study (BPRHS) participant flow chart. 1Baseline participants with complete data on baseline global cognition, executive function, and memory scores and baseline PPI use. 2Wave-2 participants with complete data on wave-2 global cognition, executive function, and memory scores and wave-2 PPI use. 3Wave-4 participants with complete data on wave-4 global cognition, executive function, and memory scores and wave-3 PPI use. PPI = proton pump inhibitor.
Among 1 290 baseline participants, 313 (24.3%) self-reported PPI use, 18 (1.4%) were concomitant H2RA users, 118 (37.7%) were concomitant non-aspirin NSAID users, 110 (35.1%) were concomitant acetyl salicylic acid (aspirin) users, and 113 (36.1%) were concomitant multivitamin users. At baseline, compared to nonusers, PPI users were older (median 58 vs 55 years, p < .01), more likely to be postmenopausal female without estrogen use (63.6% vs 54.0%, p < .01), and to have less education years (47.3% vs 39.9%, p = .025), less alcohol use (64.9% vs 51.1%, p < .01), low physical activity (p < .01), overweight BMI (63.3% vs 53.3%, p < .01), increased prevalence of gastrointestinal disorders (p < .01), hypertension (p = .005), stroke (p = .02), arthritis (p < .01), anemia (p = .01), depressive symptomatology (p < .01), increased intake of acetyl salicylic acid (aspirin; 35.1% vs 24.9%, p < .01), non-aspirin NSAIDs (37.7% vs 30.9%, p = .03), and multivitamins (36.1% vs 28.5%, p = .01), and higher serum C-reactive protein concentration (3.95 mg/L vs 3.50 mg/L, p = .04) (Table 1). Among 404 participants who completed all 3 waves of the study and had complete outcome and exposure data, 106 reported PPI use from baseline through wave-3 (mean PPI use 6.2 years ± 0.98 year) and were defined as long-term PPI users. Among the 106 long-term PPI users, 3 (2.8%), 47 (49.8%), 50 (47.2%), and 68 (64.2%) reported concomitant H2RA, non-aspirin NSAID, acetyl salicylic acid (aspirin) or multivitamin use with PPI use, respectively.
Table 1.
Demographic Characteristics at Baseline (N = 1 290) in the Boston Puerto Rican Health Study
| PPI User (N = 313) |
PPI Nonuser (N = 977) |
p | |
|---|---|---|---|
| Age (years), median (range) | 58 (45–74) | 55 (45–75) | <.01* |
| Male, n (%) | 67 (21.4) | 306 (31.3) | |
| Female premenopausal or postmenopausal with estrogen use, n (%) | 47 (15.0) | 143 (14.6) | |
| Female postmenopausal with no estrogen use, n (%) | 199 (63.6) | 528(54.0) | <.01† |
| Education below eighth grade, n (%) | 148 (47.3) | 389 (39.9) | |
| Education eighth grade and above, n (%) | 165 (52.7) | 585 (60.1) | .03† |
| Nonsmoker, n (%) | 142 (45.4) | 433 (44.3) | |
| Past smoker, n (%) | 109 (34.8) | 291 (29.8) | |
| Current smoker, n (%) | 61 (19.5) | 250 (25.6) | .10† |
| Alcohol non-drinker, n (%) | 203 (64.9) | 499 (51.1) | |
| Alcohol moderate drinker, n (%) | 90 (28.8) | 380 (38.9) | |
| Alcohol heavy drinker, n (%) | 16 (5.1) | 87 (8.9) | <.01† |
| Physical activity score, median (min, max) | 29.3 (24.3, 53.0) | 30.8 (24.8, 62.6) | <.01* |
| BMI < 25, n (%) | 34 (10.9) | 133 (13.7) | |
| BMI ≥ 25 and <30, n (%) | 80 (25.7) | 319 (33.0) | |
| BMI ≥ 30, n (%) | 197 (63.3) | 516 (53.3) | <.01† |
| Cholesterol (mg/dL), median (min, max) | 186 (89, 375) | 182 (87, 355) | .64* |
| Gastrointestinal disorder, n (%) | 203 (65.1) | 202 (20.8) | <.01‡ |
| Hypertension, n (%) | 231 (73.8) | 628 (64.3) | .005† |
| Stroke, n (%) | 19 (6.1) | 29 (3.0) | .02‡ |
| Type 2 diabetes, n (%) | 131 (41.9) | 346 (35.4) | .11† |
| Arthritis, n (%) | 197 (63.1) | 442 (45.4) | <.01‡ |
| Anemia, n (%) | 61 (19.9) | 130 (13.7) | .01‡ |
| Medication, supplement, and diet | |||
| Acetyl salicylic acid (aspirin), n (%) | 110 (35.1) | 243 (24.9) | <.01‡ |
| Nonsteroidal anti-inflammatory drugs (NSAIDs),§n (%) | 118 (37.7) | 302 (30.9) | .03‡ |
| Platelet aggregation inhibitors, n (%) | 15 (4.8) | 28 (2.9) | .14‡ |
| H2 receptor antagonists, n (%) | 18 (5.8) | 76 (7.8) | .28‡ |
| Multivitamin supplement, n (%) | 113 (36.1) | 278 (28.5) | .01‡ |
| Mediterranean diet score, mean (±SD) | 4.82 (± 1.5) | 4.99 (± 1.4) | .96‖ |
| Neuropsychiatric scores | |||
| Depression scores (CES-D), mean (±SD)¶ | 22.9(± 13.3) | 19.2 (± 13.1) | <.01‖ |
| Biomarkers | |||
| C-reactive protein (mg/L), median (min, max) | 3.95 (0.1, 56.6) | 3.50 (0, 127) | .04* |
| Apolipoprotein E Ɛ4 (at least 1 copy), n (%) | 53 (16.9) | 187 (19.1) | .63† |
Notes: PPI = proton pump inhibitor; BMI = body mass index; SD = standard deviation.
*Mann–Whitney–Wilcoxon test.
†Fisher test.
‡χ² test.
§Non-aspirin NSAIDs.
‖ T-test.
¶Center for Epidemiological Studies-Depression (CES-D).
Cross-Sectional Association Between Baseline PPI Use and Baseline Cognitive Function
At baseline, in multivariable analyses adjusted for relevant covariates, PPI use was not associated with global cognitive function (fully adjusted model 4, β = −0.006, p = .84), executive function (model 4, β = −0.037, p = .53), or memory (model 4, β = 0.021, p = .75; Table 2).
Table 2.
Association Between Baseline PPI Use and Baseline Cognitive Function Among 1290 Participants in the Boston Puerto Rican Health Study
| N | β baseline PPI use (95% CI) | p baseline PPI use | p baseline PPI use×age * | |
|---|---|---|---|---|
| Global cognitive score | ||||
| Model 1† | 1 290 | −0.057 (−0.119, 0.006) | .075 | |
| Model 2‡ | 1 278 | −0.009 (−0.066, 0.049) | .77 | |
| Model 3§ | 1 275 | 0.007 (−0.050, 0.064) | .81 | |
| Model 4‖ | 1 234 | −0.006 (−0.064, 0.052) | .84 | .08 |
| Executive function | ||||
| Model 1† | 1 290 | −0.191 (−0.318, −0.063) | .0035 | |
| Model 2‡ | 1 278 | −0.065 (−0.179, 0.049) | .26 | |
| Model 3§ | 1 275 | −0.026 (−0.140, 0.088) | .65 | |
| Model 4‖ | 1 234 | −0.037 (−0.153, 0.079) | .53 | .07 |
| Memory score | ||||
| Model 1† | 1 290 | 0.001 (−0.129, 0.131) | .99 | |
| Model 2‡ | 1 278 | 0.017 (−0.109, 0.144) | .79 | |
| Model 3§ | 1 275 | 0.049 (−0.077, 0.176) | .45 | |
| Model 4‖ | 1 234 | 0.021 (−0.108, 0.1503) | .75 | .71 |
Notes: PPI = proton pump inhibitor; CI = confidence interval; BMI = body mass index; CES-D = Center for Epidemiological Studies Depression; H2RA = histamine type-2 receptor antagonist; NSAID = nonsteroidal anti-inflammatory drug.
*Interaction: baseline PPI use and baseline age.
†Model 1: Univariate (n = 1 290).
‡Model 2: Adjusted for age (continuous), sex/estrogen status (categorical: male; female premenopausal or postmenopausal with estrogen use and female postmenopausal with no estrogen use), smoking (categorical: nonsmoker/lifetime use of <100 cigarettes, past smoker, and current smoker), education (categorical: education <eighth grade and eighth grade and above), Apolipoprotein E Ɛ4 (categorical: no copies, at least 1 copy, and missing), alcohol frequency (categorical: nondrinker/none within past year, moderate drinker, or heavy drinker), and BMI (categorical: overweight, obese, and extremely obese).
§Model 3: Adjusted for model 2+ depression score (CES-D; continuous), diabetes (categorical: yes/no), hypertension (yes/no), and stroke (yes/no).
‖Model 4: Adjusted for model 3+ anemia (yes/no), H2RA (yes/no), platelet aggregation inhibitors (yes/no), aspirin (yes/no), NSAID (yes/no), multivitamin (yes/no), and Mediterranean diet score (range 0–9).
Long-Term PPI Use and Wave-4 Cognitive Function
There was no association between long-term PPI use (continuous utilization from baseline to ~6.2 years) and wave-4 (~12.7 years) global cognitive score in MLR models, after adjusting for relevant covariates (fully adjusted model 4, β = 0.021, p = .75; Table 3). There were also no associations between long-term PPI use and executive function (model 4, β = 0.054, p = .72), and memory (model 4, β = 0.098, p = .53; Table 3).
Table 3.
Association Between Long-Term PPI Use (Mean 6.2 Years Use From Baseline) and Cognitive Function at Wave-4 (Mean 12.7 years) Follow-up Among 404 Participants in the Boston Puerto Rican Health Study
| N | β long-term PPI use (95% CI) | p long-term PPI use | p long-term PPI use×age * | |
|---|---|---|---|---|
| Global cognitive score | ||||
| Model 1† | 404 | −0.045 (−0.173, 0.084) | .5 | |
| Model 2‡ | 379 | 0.037 (−0.084, 0.158) | .55 | |
| Model 3§ | 364 | 0.054 (−0.072, 0.180) | .4 | |
| Model 4‖ | 311 | 0.021 (−0.112, 0.154) | .75 | .36 |
| Executive function | ||||
| Model 1† | 404 | 0.091(−0.388, 0.196) | .52 | |
| Model 2‡ | 379 | 0.101 (−0.164, 0.366) | .45 | |
| Model 3§ | 364 | 0.12 (−0.159, 0.391) | .41 | |
| Model 4‖ | 311 | 0.054 (−0.238, 0.345) | .72 | .24 |
| Memory score | ||||
| Model 1† | 404 | 0.062 (−0.209, 0.332) | .66 | |
| Model 2‡ | 379 | 0.077 (−0.198, 0.352) | .58 | |
| Model 3§ | 364 | 0.11 (−0.176, 0.399) | .45 | |
| Model 4‖ | 311 | 0.098 (−0.211, 0.406) | .53 | .402 |
Notes: Age and all covariates at wave-3. PPI = proton pump inhibitor; CI = confidence interval; BMI = body mass index; CES-D = Center for Epidemiological Studies Depression; H2RA = histamine type-2 receptor antagonist; NSAID = nonsteroidal anti-inflammatory drug.
*Interaction: baseline PPI use and baseline age.
†Model 1: Univariate (n = 404).
‡Model 2: Adjusted for age (continuous), sex/estrogen status (categorical: male; female premenopausal or postmenopausal with estrogen use and female postmenopausal with no estrogen use), smoking (categorical: nonsmoker/lifetime use of <100 cigarettes, past smoker, and current smoker), education (categorical: education <eighth grade and eighth grade and above), Apolipoprotein E Ɛ4 (categorical: no copies, at least 1 copy, and missing), alcohol frequency(categorical: nondrinker/none within past year, moderate drinker, or heavy drinker), and BMI (categorical: overweight, obese, and extremely obese).
§Model 3: Adjusted for model 2+ depression score (CES-D; continuous), diabetes (categorical: yes/no), hypertension (yes/no), and stroke (yes/no).
‖Model 4: Adjusted for model 3+ anemia (yes/no), H2RA (yes/no), platelet aggregation inhibitors (yes/no), aspirin (yes/no), NSAID (yes/no), multivitamin (yes/no), and Mediterranean diet score (range 0–9).
Longitudinal Trajectory Analyses
Among 1 290 baseline participants with the complete outcome and exposure data, 480 participants (37.2%) were lost to follow-up at wave-4 (~12.7 years from baseline). Baseline PPI use did not predict odds of loss to follow-up by wave-4 (odds ratio [OR] = 0.83, p = .16), leading us to conclude that attrition bias was not a major factor in these analyses.
In longitudinal linear mixed effects models adjusted for age, sex/estrogen status, smoking status, education, apolipoprotein E ε4 status, alcohol use, BMI, depression score, diabetes, hypertension, stroke, anemia, H2RA, platelet aggregation inhibitor, aspirin, NSAID, multivitamin use, and Mediterranean diet score, baseline PPI use was not associated with the trajectory of the global cognitive score (fully adjusted model 4, β = 0.001, p = .74), executive function (model 4, β = 0.0004, p = .94) or memory (model 4, β = 0.011, p = .17) over ~12.7 years of follow-up (Table 4). The ~12 years trend in GCS according to PPI exposure is summarized in Supplementary Figure 1.
Table 4.
Longitudinal Association Baseline PPI Use and Cognitive Function Over Wave-4 (Mean 12.7 Years) Follow-up in the Boston Puerto Rican Health Study
| Model 1* | Model 2† | Model 3‡ | Model 4§ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |
| Global cognitive score | ||||||||||||
| PPI | −0.052 | −0.110, 0.007 | .084 | −0.005 | −0.059, 0.049 | .85 | 0.009 | −0.044, 0.064 | .72 | −0.001 | −0.056, 0.053 | .96 |
| Time | −0.002 | −0.005, 0.002 | .32 | −0.002 | −0.004, 0.001 | .29 | −0.002 | −0.005, 0.001 | .22 | −0.003 | −0.006, −0.0004 | .09 |
| PPI: time | −0.0006 | −0.007, 0.006 | .85 | −0.0007 | −0.006, 0.005 | .8 | 0.0002 | −0.006, 0.007 | .95 | 0.001 | −0.005, 0.008 | .74 |
| N observations | 2 685 | 2 660 | 2 655 | 2 586 | ||||||||
| N groups | 1 290 | 1 278 | 1 275 | 1 234 | ||||||||
| AIC‖ | 2 584 | 2 341 | 2 343 | 2 283 | ||||||||
| BIC¶ | 2 637 | 2 465 | 2 501 | 2 481 | ||||||||
| LL# | −1 283 | −1 149 | −1 144 | −1 107 | ||||||||
| Executive function | ||||||||||||
| PPI | −0.19 | −0.31, −0.066 | .003 | −0.059 | −0.17, 0.0503 | .29 | −0.017 | −0.13, 0.092 | .76 | −0.027 | −0.138, 0.084 | .63 |
| Time | −0.028 | −0.034, −0.022 | .00 | −0.027 | −0.033, −0.021 | .00 | −0.027 | −0.033, −0.021 | .00 | −0.028 | −0.034, −0.022 | .00 |
| PPI: time | 0.0003 | −0.012, 0.013 | .96 | −0.0005 | −0.013, 0.012 | .94 | −0.0004 | −0.013, 0.012 | .94 | 0.0004 | −0.012, 0.013 | .94 |
| N observations | 2 685 | 2 660 | 2 655 | 2 586 | ||||||||
| N groups | 1 290 | 1 278 | 1 275 | 1 234 | ||||||||
| AIC‖ | 6 321 | 5 918 | 5 890 | 5 745 | ||||||||
| BIC¶ | 6 374 | 6 042 | 6 049 | 5 943 | ||||||||
| LL# | −3 151 | −2 938 | −2 918 | −2 838 | ||||||||
| Memory score | ||||||||||||
| PPI | −0.017 | −0.13, 0.10 | .77 | −0.002 | −0.12, 0.11 | .97 | 0.024 | −0.088, 0.14 | .68 | −0.0004 | −0.114, 0.113 | .99 |
| Time | 0.008 | 0.0002, 0.016 | .044 | 0.006 | −0.002, 0.014 | .14 | 0.006 | −0.003, 0.014 | .17 | 0.004 | −0.004, 0.012 | .32 |
| PPI: time | 0.007 | −0.009, 0.023 | .39 | 0.008 | −0.008, 0.024 | .32 | 0.008 | −0.008, 0.024 | .31 | 0.011 | −0.005, 0.026 | .17 |
| N observations | 2 685 | 2 660 | 2 655 | 2 586 | ||||||||
| N groups | 1 290 | 1 278 | 1 275 | 2 586 | ||||||||
| AIC‖ | 7 219 | 7 052 | 7 037 | 1 234 | ||||||||
| BIC¶ | 7 272 | 7 175 | 7 196 | 7 073 | ||||||||
| LL# | −3 600 | −3 505 | −3 492 | −3 403 |
Notes: Linear mixed effects models to examine longitudinal association baseline PPI use and cognitive function over wave-4 follow-up (mean 12.7 years from baseline). Cognitive function scores (baseline to mean 12.7 years from baseline); PPI use and all covariates at baseline. Estimates for models with and without interaction are from the same model. PPI = proton pump inhibitor; CI = confidence interval; CES-D = Center for Epidemiological Studies Depression; H2RA = histamine type-2 receptor antagonist; NSAID = nonsteroidal anti-inflammatory drug.
*Model 1: Univariate.
†Model 2: Adjusted for age (continuous), sex/estrogen status (categorical: male; female premenopausal or postmenopausal with estrogen use and female postmenopausal with no estrogen use), smoking (categorical: nonsmoker/lifetime use of <100 cigarettes, past smoker, and current smoker), education (categorical: education <eighth grade and eighth grade and above), Apolipoprotein E Ɛ4 (categorical: no copies, at least 1 copy, and missing), alcohol frequency (categorical: nondrinker/none within past year, moderate drinker, or heavy drinker), and BMI (categorical: overweight, obese, and extremely obese).
‡Model 3: Adjusted for model 2+ depression score (CES-D; continuous), diabetes (categorical: yes/no), hypertension (yes/no), and stroke (yes/no).
§Model 4: Adjusted for model 3+ anemia (yes/no), H2RA (yes/no), platelet aggregation inhibitors (yes/no), aspirin (yes/no), NSAID (yes/no), multivitamin (yes/no), and Mediterranean diet score (range 0–9).
‖AIC = Akaike Information Criterion.
¶BIC = Bayesian Information Criterion.
#LL = log-likelihood.
We did not observe the interaction between age and PPI in cross-sectional (Tables 2 and 3) and longitudinal analyses (fully adjusted GCS β = 0.001, p = .24; executive function β = −0.0003, p = .77; and memory β = −0.0005, p = .67). In cross-sectional analyses stratified on age, we did not observe differing affects of PPI on cognitive function at baseline: (GCS β = 0.03; p = .46 and β = −0.05; p = .28 for participants <60 years vs 60 years or older at baseline, respectively). Results for executive function and memory were similar to GCS (not presented).
Discussion
We examined the association between PPI use and cognitive function in a population of older Puerto Rican adults living in the mainland United States, both cross-sectionally and longitudinally. Some studies have reported an increased risk of cognitive impairment or dementia with PPI use (11,12,19–21,24), while others report no association between PPI use and dementia risk (16) or, conversely, less cognitive impairment and dementia risk with PPI use (13,17,18). The differences in results may be due to variations in study design, age of the study cohort, neuroprotective factors, confounding variables, cognitive panel and methods of measuring cognition, assessment of PPI use, and utilization of specific types of PPIs. Most studies that reported a positive association between PPI use and cognition or dementia were based on claims (12,19) or other types of public health care systems data (21). Claims-based retrospective cohort data are generally not collected for the specific purpose of epidemiological research, they may lack information on key participant characteristics, such as education level (12). In contrast, studies that report no association between PPI and cognition all adjusted for education levels (10,13,16,22) were prospective cohort (10,16) or clinical registry-based studies (22). On the other hand, in the prospective studies that report no association between PPI use and cognition, PPI use was assessed based on self-reports (10,13), which may introduce bias.
In our baseline cohort, compared to PPI nonusers, PPI users were likely to be older, postmenopausal female participants with less education, and poorer health status, as indicated by high comorbidity, high serum CRP concentration, and polypharmacy. Compared to PPI nonusers, PPI users also reported low alcohol intake, high multivitamin, acetyl salicylic acid (aspirin), and non-aspirin NSAID use. A previous study has reported reduced odds of cognitive impairment among older women utilizing NSAIDs (48).
Among healthy individuals, negative associations between PPI use and visual memory, executive, working, and planning function scores are reported (31), and because several studies have observed an association between PPI use and specific cognitive domains (10,20,31), we examined the association between PPI use with executive function and memory, in addition to global cognitive function. Accumulation of Aβ in the parietal cortex is associated with impairment in executive and attention domains, and accumulation of Aβ in the medial temporal lobe is reported to be associated with memory impairment (31). Although we initially observed a negative baseline cross-sectional association between PPI use and executive function, and a trend toward a negative association between PPI use and global cognitive score in univariate analysis, these were attenuated after adjusting for relevant covariates. Our results are in agreement with a study based on the Danish National Patient Register, which also reported no association between daily PPI use (based on dose from the prescription registry) and cognitive function among middle-aged and older individuals (22). Another study, with 13 864 female participants, also reported no association between PPI use (categorized by duration, from 1 to 14 years) and composite global cognitive or memory scores (10). That study, however, reported a detrimental association between PPI use and psychomotor speed and attention (10).
Strengths and Limitations
Strengths of our study include a focus on an under-studied minority population, detailed data on exposure, outcome, and key covariates for ~12.7 years of follow-up, and careful assessment of medication use via examination of medicine containers. According to recent data (49), Puerto Ricans are the second-largest Hispanic group in the United States, and our study is relevant to the health of this growing population.
Our study also has several limitations. These include loss to follow-up, which could introduce bias into our analyses; however, PPI use did not predict a loss to follow-up (attrition bias), in our study. The relatively young age of our cohort (mean 56.1 years) may also potentially explain the lack of association. However, our participants suffer from overlapping risk factors for cognitive decline and AD/ADRD, including high BMI (55.3% ≥30), low education (42.6% < eighth grade), prevalent diabetes (40%), and hypertension (66.6%), putting our study participants at substantial risk of cognitive decline and AD/ADRD. We also examined the effect, and did not observe, effect modification by age in our analyses.
Some variables, including PPI use and health conditions, were based on self-report, potentially leading to misclassification. Information on a dose of PPI was not available, so we were unable to examine associations with dose. Duration PPI use prior to baseline was also not available in our study; therefore, we could only ascertain the duration of use during our study period. Furthermore, PPIs are extensively metabolized in the liver by the cytochrome P450 (CYP) enzymes, mainly CYP2C19 and CYP3A4. Omeprazole, lansoprazole, and pantoprazole are mainly metabolized by CYP2C19 (50), and based on CYP2C19 mutation status, slow metabolizers of PPIs are also at higher risk for vitamin B12 deficiency, which could potentially lead to cognitive impairment. We did not have information on CYP mutation status in the BPRHS. Our study focuses on a unique population of Puerto Ricans in the Boston metropolitan area, with unique medication and dietary patterns, including increasing utilization of acetyl salicylic acid (aspirin), non-aspirin NSAIDs, multivitamins, and PPIs over time. The increasing utilization of anti-inflammatory medications, including NSAIDs and use of multivitamins in this cohort may be neuroprotective and potentially explain the lack of association between PPI use and cognitive function. However, due to the focus on this unique cohort, generalizability of our findings to other populations may be limited.
In conclusion, in this study of Boston-area Puerto Ricans, we did not observe an association between PPI use and cognitive function, cross-sectionally or over time. Larger studies of longer duration, with better ascertainment of PPI dose and duration, are needed to further examine this potential association.
Supplementary Material
Acknowledgments
We thank Esther Jennings, Xiyuan Zhang, Liam Fouhy, and Fan Chen at the Center for Population Health, University of Massachusetts, Lowell.
Contributor Information
Deepika Dinesh, Center for Population Health, University of Massachusetts Lowell, Lowell, Massachusetts, USA; Department of Biomedical and Nutritional Sciences, University of Massachusetts Lowell, Lowell, Massachusetts, USA.
Jong Soo Lee, Center for Population Health, University of Massachusetts Lowell, Lowell, Massachusetts, USA; Department of Mathematical Sciences, University of Massachusetts Lowell, Lowell, Massachusetts, USA.
Tammy M Scott, Center for Population Health, University of Massachusetts Lowell, Lowell, Massachusetts, USA; Friedman School of Nutrition Science and Policy, Tufts University, Boston, Massachusetts, USA; Department of Psychiatry, School of Medicine, Tufts University, Boston, Massachusetts, USA.
Katherine L Tucker, Center for Population Health, University of Massachusetts Lowell, Lowell, Massachusetts, USA; Department of Biomedical and Nutritional Sciences, University of Massachusetts Lowell, Lowell, Massachusetts, USA.
Natalia Palacios, Center for Population Health, University of Massachusetts Lowell, Lowell, Massachusetts, USA; Department of Public Health, University of Massachusetts Lowell, Lowell, Massachusetts, USA; Department of Nutrition, Harvard University School of Public Health, Boston, Massachusetts, USA; Geriatric Research Education Clinical Center, Department of Veterans Affairs, ENRM VA Hospital, Bedford, Massachusetts, USA.
Funding
This work was supported by National Institutes of Health (NIH) grants P01 AG023394, P50 HL105185, and R01 AG055948 (to K.L.T.). N.P. receives funding from NIH grant R01 NS097723.
Conflict of Interest
None declared.
Author Contributions
The authors’ responsibilities were as follows—D.D., N.P., and K.L.T.: contributed to the design; D.D.: conducted the data analyses and drafted the manuscript; T.M.S.: contributed expertise regarding analyses of the cognitive function data; N.P., J.S.L., T.M.S., and K.L.T.: provided analytical feedback and revised the manuscript; K.L.T.: is the Principal Investigator of the Boston Puerto Rican Health Study and oversaw all data collection and documentation. All authors read, contributed to the writing of, and approved the final manuscript.
Sponsor’s Role
The sponsors of this research had no role in the design, methods, participant recruitment, data collections analysis, or preparation of article.
References
- 1. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700–789. doi: 10.1002/alz.12638 [DOI] [PubMed] [Google Scholar]
- 2. Panel AGSBCUE, Fick DM, Semla TP, et al. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 3. Batchelor R, Gilmartin JFM, Kemp W, Hopper I, Liew D. Dementia, cognitive impairment and proton pump inhibitor therapy: a systematic review. J Gastroenterol Hepatol. 2017;32(8):1426–1435. doi: 10.1111/jgh.13750 [DOI] [PubMed] [Google Scholar]
- 4. Scarpignato C, Gatta L, Zullo A, Blandizzi C. Effective and safe proton pump inhibitor therapy in acid-related diseases—a position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14(1):1–35. doi: 10.1186/s12916-016-0718-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf. 2019;10:2042098618809927. doi: 10.1177/2042098618809927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li M, Luo Z, Yu S, Tang Z. Proton pump inhibitor use and risk of dementia: systematic review and meta-analysis. Medicine. 2019;98(7):e14422. doi: 10.1097/md.0000000000014422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors—evidence and plausibility. Int J Mol Sci. 2019;20(20):52035203. doi: 10.3390/ijms20205203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther Adv Drug Saf. 2013;4(3):125–133. doi: 10.1177/2042098613482484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ortiz-Guerrero G, Amador-Muñoz D, Calderón-Ospina CA, López-Fuentes D, Nava Mesa MO. Proton pump inhibitors and dementia: physiopathological mechanisms and clinical consequences. Neural Plast. 2018;2018:1–9. doi: 10.1155/2018/5257285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lochhead P, Hagan K, Joshi AD, et al. Association between proton pump inhibitor use and cognitive function in women. Gastroenterology. 2017;153(4):971–979.e4. doi: 10.1053/j.gastro.2017.06.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419–428. doi: 10.1007/s00406-014-0554-0 [DOI] [PubMed] [Google Scholar]
- 12. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410–416. doi: 10.1001/jamaneurol.2015.4791 [DOI] [PubMed] [Google Scholar]
- 13. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969–1974. doi: 10.1111/jgs.14956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papazoglou A, Arshaad MI, Henseler C, et al. The Janus-like association between proton pump inhibitors and dementia. Curr Alzheimer Res. 2021;18(6):453–469. doi: 10.2174/1567205018666210929144740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar R, Kumar A, Nordberg A, Långström B, Darreh-Shori T. Proton pump inhibitors act with unprecedented potencies as inhibitors of the acetylcholine biosynthesizing enzyme—a plausible missing link for their association with incidence of dementia. Alzheimers Dement. 2020;16(7):1031–1042. doi: 10.1002/alz.12113 [DOI] [PubMed] [Google Scholar]
- 16. Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc. 2018;66(2):247–253. doi: 10.1111/jgs.15073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooksey R, Kennedy J, Dennis MS, et al. Proton pump inhibitors and dementia risk: evidence from a cohort study using linked routinely collected national health data in Wales, UK. PLoS One. 2020;15(9):e0237676. doi: 10.1371/journal.pone.0237676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Booker A, Jacob LE, Rapp M, Bohlken J, Kostev K. Risk factors for dementia diagnosis in German primary care practices. Int Psychogeriatr. 2016;28(7):1059–1065. doi: 10.1017/S1041610215002082 [DOI] [PubMed] [Google Scholar]
- 19. Tai S-Y, Chien C-Y, Wu D-C, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006. doi: 10.1371/journal.pone.0171006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herghelegiu AM, Prada GI, Nacu R. Prolonged use of proton pomp inhibitors and cognitive function in older adults. Farmacia. 2016;64(2):262–267. [Google Scholar]
- 21. Torres-Bondia F, Dakterzada F, Galván L, et al. Proton pump inhibitors and the risk of Alzheimer’s disease and non-Alzheimer’s dementias. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-78199-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wod M, Hallas J, Andersen K, Rodríguez LAG, Christensen K, Gaist D. Lack of association between proton pump inhibitor use and cognitive decline. Clin Gastroenterol Hepatol. 2018;16(5):681–689. doi: 10.1016/j.cgh.2018.01.034 [DOI] [PubMed] [Google Scholar]
- 23. Makunts T, Alpatty S, Lee KC, Atayee RS, Abagyan R. Proton-pump inhibitor use is associated with a broad spectrum of neurological adverse events including impaired hearing, vision, and memory. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-53622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu B, Hu Q, Tian F, Wu F, Li Y, Xu T. A pharmacovigilance study of association between proton pump inhibitor and dementia event based on FDA adverse event reporting system data. Sci Rep. 2021;11(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(6):528–534. doi: 10.1007/s11894-008-0098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riggs KM, Spiro A 3rd, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996;63(3):306–314. doi: 10.1093/ajcn/63.3.306 [DOI] [PubMed] [Google Scholar]
- 27. Cheng F, Ho Y-F, Hung L, Chen C, Tsai T. Determination and pharmacokinetic profile of omeprazole in rat blood, brain and bile by microdialysis and high-performance liquid chromatography. J Chromatogr A. 2002;949(1–2):35–42. doi: 10.1016/s0021-9673(01)01225-0 [DOI] [PubMed] [Google Scholar]
- 28. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837. doi: 10.1371/journal.pone.0058837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fallahzadeh M, Borhani Haghighi A, Namazi M. Proton pump inhibitors: predisposers to Alzheimer disease? J Clin Pharm Ther. 2010;35(2):125–126. [DOI] [PubMed] [Google Scholar]
- 30. Majumdar A, Capetillo-Zarate E, Cruz D, Gouras GK, Maxfield FR. Degradation of Alzheimer’s amyloid fibrils by microglia requires delivery of ClC-7 to lysosomes. Mol Biol Cell. 2011;22(10):1664–1676. doi: 10.1091/mbc.E10-09-0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akter S, Hassan MR, Shahriar M, Akter N, Abbas MG, Bhuiyan MA. Cognitive impact after short-term exposure to different proton pump inhibitors: assessment using CANTAB software. Alzheimers Res Ther. 2015;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azhar M, Fiedler L, Espinosa PS, Hennekens CH. Proton pump inhibitors and risk of dementia: a hypothesis generated but not adequately tested. Am J Alzheimers Dis Other Demen. 2021;36:15333175211062413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. González HM, Tarraf W, Gouskova N, et al. Life’s simple 7’s cardiovascular health metrics are associated with Hispanic/Latino neurocognitive function: HCHS/SOL results. J Alzheimers Dis. 2016;53(3):955–965. doi: 10.3233/jad-151125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tucker KL, Mattei J, Noel SE, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao X, Scott T, Falcon LM, Wilde PE, Tucker KL. Food insecurity and cognitive function in Puerto Rican adults. Am J Clin Nutr. 2009;89(4):1197–1203. doi: 10.3945/ajcn.2008.26941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 38. Fortuny LAI, Romo DH, Heaton RK, Iii REP. Manual De Normas Y Procedimientos Para La Bateria Neuropsicologia. 1999.
- 39. Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS, Breuer J. Screening for Alzheimer’s disease by clock drawing. J Am Geriatr Soc. 1989;37(8):730–734. [DOI] [PubMed] [Google Scholar]
- 40. Beery K. The development test of visual-motor intergration manual. Modern Curriculum Press. 1989. [Google Scholar]
- 41. Palacios N, Lee JS, Scott T, et al. Circulating plasma metabolites and cognitive function in a Puerto Rican cohort. J Alzheimers Dis. 2020;76:1267–1280. doi: 10.3233/jad-200040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith CE, Tucker KL, Scott TM, et al. Apolipoprotein C3 polymorphisms, cognitive function and diabetes in Caribbean origin Hispanics. PLoS One. 2009;4(5):e5465. doi: 10.1371/journal.pone.0005465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McDowell M, Briefel R, Warren R, Buzzard I, Feskanich D, Gardner S.. The dietary data collection system. An automated interview and coding system for NHANES III. CBORD Group, Inc., Ithaca, New York; 1990:125–131. [Google Scholar]
- 44. Delgado JL, Johnson CL, Roy I, Trevino FM. Hispanic Health and Nutrition Examination Survey: methodological considerations. Am J Public Health. 1990;80(Suppl):6–10. doi: 10.2105/ajph.80.suppl.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McDowell M, Loria C. Cultural considerations in analyzing dietary data from the Hispanic Health and Nutrition Examination Survey. In National Nutrition Database Conference. 1989:43–46.
- 46. Block G, Subar A. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. 1992;92(8):969–977. [PubMed] [Google Scholar]
- 47. Sawyer Radloff L, Teri L. 6/Use of the center for epidemiological studies-depression scale with older adults. Clin Gerontol. 1986;5(1–2):119–136. doi: 10.1300/j018v05n01_06 [DOI] [Google Scholar]
- 48. Kang JH, Grodstein F. Regular use of nonsteroidal anti-inflammatory drugs and cognitive function in aging women. Neurology. 2003;60(10):1591–1597. doi: 10.1212/01.wnl.0000065980.33594.b7 [DOI] [PubMed] [Google Scholar]
- 49. Facts on Hispanics of Puerto Rican origin in the United States, 2017. Accessed December 01, 2021, https://www.pewresearch.org/hispanic/fact-sheet/u-s-hispanics-facts-on-puerto-rican-origin-latinos/
- 50. Mori H, Suzuki H. Role of acid suppression in acid-related diseases: proton pump inhibitor and potassium-competitive acid blocker. J Neurogastroenterol Motil. 2019;25(1):6–14. doi: 10.5056/jnm18139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


