Abstract
Background
We examined the cross-sectional and longitudinal relationships of motor functions with depression in older adults with type 2 diabetes (T2D).
Methods
Participants (n = 984) were from the longitudinal Israel Diabetes and Cognitive Decline (IDCD) study. They were initially cognitively normal and underwent evaluations of motor functions (grip strength and gait speed) and of depression (using the 15-item version of the Geriatric Depression Scale [GDS]) approximately every 18 months. We applied Hierarchical Linear Mixed Models (HLMM) to investigate the associations between motor functions and depression adjusting for sociodemographic, cardiovascular factors, overall cognitive score, and subjective report of exhaustion.
Results
Participants’ baseline characteristics were 72 (±5) years of age (59.6% males), 13 (±4) years of education, Mini-Mental Status Exam (MMSE) score of 28.01 (±1.78), and a GDS score of (2 ± 2.00), consistent with normal cognitive status and lack of major affective symptomatology. Slower gait speed at baseline was associated with higher GDS scores (p = .001) and with their increase over time (p = .049). A decrease in walking speed from baseline was associated with an increase in GDS scores (p = .015). Lower grip strength at baseline was associated with higher GDS scores (p = .002), but not with trajectories in GDS scores over time. A faster decrease in grip strength from baseline was associated with a faster increase in GDS scores (p = .022).
Conclusions
Both gait speed and grip strength are cross-sectionally associated with depression. However, only gait speed and its decrease over time can potentially be used to predict incident depression symptoms, thus facilitating the introduction of depression prevention strategies.
Keywords: Depression, Gait speed, Geriatric depression scale, Grip strength, Motor function, Type 2 diabetes
Symptoms of depression, especially at sub-syndromal levels, are highly prevalent in old age, reaching prevalence rates of 10.4% for minor depression (1) and up to 27% for depression symptoms (2). In this age group, even at sub-syndromal levels, symptoms of depression are associated with higher morbidity, mortality, and poor cognitive and functional outcomes. Response rates to antidepressant medications are lower in older adults compared to younger age groups (3), potentially reflecting the different pathophysiological mechanisms underlying depression in this age group (4). The substantial burden of depression in old age, together with the limited availability of qualified professionals and the partial efficacy of currently available interventions, led to an increasing awareness of the need for preventive interventions in this age group (5,6). Depression prevention strategies in old age have been shown to be effective in improving functional status, disease outcomes, and quality of life (7).
The role of old-age depression in health-related outcomes is further accentuated in the presence of chronic diseases and specifically in type 2 diabetes (T2D). The prevalence of T2D increases with age, reaching rate of 21.4% in individuals aged ≥65 years (8). Older adults with T2D constitute ~50% of the adult population with diabetes mellitus (9), and are at increased risk for depression (10), which in turn is associated with worse diabetes, health, and cognitive-related outcomes (11). Depression in T2D is prone to chronicity (12) and recurrence (13). Moreover, some of the antidepressant medications may exacerbate glucose dysregulation (12), stressing the importance of depression prevention treatments in older adults with T2D. However, there is a shortage in objective tools for the prediction of depression in old age and specifically in T2D (14). Motor functions, commonly measured via walking speed and handgrip strength, are important markers of biological aging (15) and potent predictors of brain-related outcomes, such as cognitive impairment (16), and depression (17–19). Low gait speed, and low grip strength (17–19) are associated with higher levels of depression (20), lower response to antidepressant medications (21), and depression chronicity (22). However, studies on the association of motor function with subtle forms of depression, which are the most prevalent forms in old age, are still relatively scarce, and specifically so in distinct clinical populations who are prone for depression such as T2D.
Given the high risk of older adults with T2D to both impaired motor function (23) and to depression, we examined the longitudinal relationship of gait speed and grip strength and the changes in their trajectories, with trajectories of depression symptoms in n = 984 older adults with T2D, participants of the Israel Diabetes and Cognitive Decline (IDCD) study.
Method
Participants are patients with T2D aged ≥65, engaged in the IDCD study, a collaboration of the Icahn School of Medicine at Mount Sinai, NY, the Sheba Medical Center, Israel, and the Maccabi Healthcare Services (MHS), Israel. The study was approved by all 3 IRB committees and all participants signed informed consent.
The IDCD is a longitudinal study, investigating the relationship between long-term T2D characteristics and cognitive decline. Its methods have been described in depth elsewhere (24). Briefly, IDCD participants were randomly selected from the ~11,000 older adults with T2D listed in the diabetes registry of MHS, the second-largest HMO in Israel. The diabetes registry was established in 1998 to facilitate T2D management and improve its treatment. Entry criteria to the registry are any of the following: (i) HbA1c > 55.7s mmol/mol (7.25%), (ii) glucose >200 mg/dl on 2 exams more than 3 months apart, (iii) purchase of anti-diabetic medication twice within 3 months supported by an HbA1c > 47.4 mmol/mol (6.5%) or glucose > 125 mg/dl within half a year, (iv) diagnosis of T2D (ICD9 code) by a general practitioner, internist, endocrinologist, ophthalmologist, or diabetes advisor, supported by an HbA1c > 47.4 mmol/mol (6.5%), or glucose > 125 mg/dl within half a year. The MHS diabetes registry collects detailed information on laboratory, medication, and medical diagnoses of its subjects, including diagnosis of depression and purchase of antidepressant medications prior to recruitment to the IDCD study.
Eligibility criteria to the IDCD were the following: being listed in the MHS diabetes registry, T2D diagnosis, living in the central area of Israel, age ≥65 years, being identified as cognitively normal at baseline (based on a multidisciplinary weekly consensus conference), absence of major medical, psychiatric, or neurological diagnoses that may significantly affect cognitive performance, having ≥3 HbA1c measurements in the diabetes registry, fluency in Hebrew, and availability of an informant.
IDCD participants’ recruitment procedure has been described in detail previously (24). In short, the MHS diabetes registry is thoroughly screened for detection of potential participants, excluding anyone with an ICD code for dementia, treatment with cholinesterase inhibitors, or with a major psychiatric or neurological condition (eg, schizophrenia or Parkinson’s disease) that might affect cognitive performance. Depression is not an exclusion criterion. The MHS team contacts potential participants and asks for their participation after determining Hebrew fluency and the availability of an informant. Consenting individuals undergo a comprehensive cognitive assessment (described below). All participants’ data, including the results of the cognitive battery administered, the score of the Clinical Dementia Rating (CDR) scale (described below), and the results of the affective assessments, are discussed by a multidisciplinary consensus conference team in order to define cognitive status (ie, cognitively normal, MCI, or dementia and their subtypes). If the participant is cognitively normal at baseline, follow-up interviews, identical on procedures, and content to the baseline assessment, are performed at 18 months intervals. Participants diagnosed as MCI or dementia at baseline, are not included in the study, however, those who convert from a status of normal cognition to MCI during follow-up, continue their participation in the study until conversion to dementia.
Assessment of Depression Symptoms
The presence of depression symptoms is evaluated using the 15-item version of the Geriatric Depression Scale (GDS) (25), a self-reported, easily administered scale. This questionnaire is suitable for depression screening in large-scale studies of aging populations and is advantageous in the context of older adults with T2D since it has little focus on somatic symptoms which could be confounded by diabetes symptoms such as neuropathy (25). GDS data are collected at every IDCD visit.
Assessment of Motor Function
Motor function was assessed using 2 performance-based measures of motor ability, as follows: (i) Gait speed: Two lines were marked on the floor to define 3 m. Participants were instructed to stand up and walk 3 m at their usual pace after the instruction “Go.” The time (in seconds) required to walk 3 m was recorded. (ii) Grip strength: A hydraulic hand dynamometer (Jamar; Lafayette Instrument Co, Lafayette, Indiana), which displays isometric grip force ranging from 0 to 90 kg, was used to measure grip strength bilaterally. Three trials of grip strength from each hand were averaged for a composite grip strength score (26).
Assessment of Subjective Exhaustion
During every IDCD visit, participants were asked to rate whether the following two statements regarding exhaustion were relevant for them: “during the last week, I felt that everything that I did was an effort for me” and “I could not bring myself to action.” For each question, participants had to choose one of the following answers: seldom or never (less than 1 day), not frequently (1–2 days), often or quite often (3–4 days), most of the time (5–7 days).
Cognitive Assessment
A broad neuropsychological battery is administered to IDCD participants (detailed in ref. (24)), and is used to calculate scores in the cognitive domains of episodic memory, attention/ working memory, executive functions, semantic categorization, and overall cognition.
Clinical Dementia Rating Scale
A numeric scale derived from clinician rating of cognition and daily function in the domains of memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care, with composite scores ranging from 0 to 3 (0 = cognitively normal; 0.5 = questionable dementia, ≥1 = increasing degrees of severity of dementia) (27). IDCD participants with a CDR≥ 0.5 at baseline were not included in this study.
Brain MRI Procedures
A random sub-sample of IDCD participants had a brain magnetic resonance imaging (MRI) scan. Scans were performed using a 3 Tesla scanner (GE, Signa HDxt, v16VO2). High-resolution (1 mm3) images were acquired using a 3D inversion recovery prepared spoiled gradient-echo (FSPGR) T1-weighted sequence (TR/TE = 7.3/2.7s, 20o flip angle, TI 450 ms). The T1 weighted anatomical images for each subject were processed using the Voxel Based Morphometry [VBM (15)] toolbox, developed by Gaser (http://www.fil.ion.ucl.ac.uk/spm/ext/#VBMtools) and implemented in Statistical Parametric Mapping (SPM8) software. This procedure included automated iterative skull stripping, segmentation of the images into gray matter (GM), white matter (WM), and cerebrospinal fluid probability images, and spatial normalization of the GM images to a customized GM template in standard Montreal Neurological Institute space. In order to optimize signal to noise, the GM maps were smoothed using an 8 mm Gaussian kernel. GM probability maps were thresholded at 0.1 to minimize the inclusion of incorrect tissue types. Total intracranial volume (TICV) was calculated using the segmented and thresholded images (TICV = GM+WM+CSF).
Statistical Analysis
We used Pearson correlations for continuous variables and t-test for dichotomous variables in order to explore the relationship between the motor function variables (gait speed and grip strength) and possible confounders which may affect the association between motor function and depression. We applied Hierarchical Linear Mixed Models (HLMM) to investigate the main and longitudinal effects of the association between baseline motor function and GDS scores (see below) and the main and longitudinal effects of the association between the change in motor function and the change in GDS scores between measurements (see below).
The use of HLMM allowed us to include a random intercept, assuming each subject had a different baseline GDS, and a random slope for time, assuming that the change in GDS scores over time can vary between different subjects.
The main effects represent the cross-sectional associations of motor functions with GDS scores. The longitudinal effects of the models examined the associations of motor functions or of change in motor functions with GDS scores or changes in GDS scores over time. For example, if a decrease in motor functions (ie, weaker hand grip) between successive measurements was associated with an increase in GDS scores (ie, more depressive symptoms), then a significant negative interaction with time would suggest that this effect increased over time. Similarly, if an increase in change in motor function (ie, greater decline in gait speed between successive measurements) was associated with an increase in GDS scores change (ie, a greater increase in depressive symptoms between successive measurements), then a significant negative interaction with time would suggest that this effect increased over time. All models were adjusted for factors that have previously been reported to affect depression or motor function, that is, sociodemographic characteristics (age, sex, and education) (28,29) measured at the IDCD baseline, cardiovascular risk factors measured by Maccabi (diabetes duration, and the mean of all measures for each participant for total cholesterol, creatinine, HbA1c, triglycerides, systolic and diastolic blood pressure, and BMI) (30) exhaustion and global cognition (31).
To examine the potential effect of brain pathology (brain atrophy and white matter hyperintensities [WMH]) on the relationships between motor functions and depression, we conducted an exploratory analysis additionally adjusting to total GM volume, total intracranial volume, and WMH’s volume.
We applied a sensitivity analysis to reduce the potential contribution of pre-existing major affective disorder to the results, excluding participants with a diagnosis of depression or those purchasing antidepressant medications based on MHS medical records.
All the statistical analyses were performed using the computed environment R version 3.5.2. We used “nlme” and “effects” packages for the HLMM. We defined a significance level of p = .05 in all statistical tests.
Results
Sample Characteristics and Associations of Demographic and Clinical Variables With Motor Function at Baseline
Table 1 shows demographic, health, and brain-related characteristics of the total sample (n = 984). Participants’ baseline characteristics were 72 (±5) years of age (59.6% males), 13 (±4) years of education, Mini-Mental State Examination (MMSE) score of 28.01 (±1.78), consistent with normal cognitive status, and a GDS score of (2 ± 2.00), consistent with lack of major affective symptomatology. Table 2 shows the associations of demographic and clinical variables with motor variables at baseline. Slower gait speed (longer time to walk 3 m) was associated with male gender (p < .001), fewer years of education (p < .001), worse cognitive functioning (p < .001), higher BMI (p < .001), lower creatinine values (p = .032), higher exhaustion (p < .001), lower GM (p < .001), intracranial (ICV) (p < .001) volumes and with a higher load of WMH (p < .001). Lower grip strength at baseline was associated with female gender (p < .001), older age (p < .001), worse cognitive functioning (p < .001), lower diastolic blood pressure (p < .001), higher triglyceride (p < .001), lower creatinine (p < .001), higher Hba1c (p = .015), higher exhaustion (p < .001), lower GM (p < .001), and lower ICV (p < .001).
Table 1.
Sample Demographic and Cardiovascular Characteristics (n = 984)
| Min | Max | Mean/n (n%) | SD | ||
|---|---|---|---|---|---|
| Sex | Male | 588 (59.6) | |||
| Female | 396 (40.4) | ||||
| Age at baseline | 62 | 85 | 72 | 5 | |
| Years of education | 0 | 26 | 13 | 4 | |
| Number of assessments | 1 | 6 | 2.52 | 0.87 | |
| MMSE at baseline | 13 | 30 | 28.01 | 1.78 | |
| GDS score at baseline | 0 | 14 | 2 | 2 | |
| Overall cognition baseline | −25.2 | 18 | 0.76 | 7.22 | |
| Diabetes duration | 0.33 | 19.90 | 9.79 | 4.39 | |
| BMI at baseline | 18 | 50 | 28.87 | 4.28 | |
| Systolic BP | 99.93 | 171.4 | 134.3 | 9.65 | |
| Diastolic BP | 58.69 | 95.68 | 76.89 | 4.86 | |
| Cholesterol | 93.50 | 267.1 | 177.8 | 25.08 | |
| Triglyceride | 41.44 | 707.4 | 155.8 | 60.77 | |
| Creatinine | 0.50 | 3.2 | 0.99 | 0.24 | |
| Hba1c | 3.93 | 10.03 | 6.77 | 0.75 | |
| Frailty effort | 0 | 3 | 0.31 | 0.75 | |
| Frailty activate self | 0 | 3 | 0.14 | 0.55 | |
| Gray matter | 376 | 655 | 514 | 49 | |
| Intracranial volume | 1022 | 1749 | 1331 | 135 | |
| White matter hyperintensities | 0 | 76 | 13.1 | 14.2 | |
| Gait speed at baseline | 1.22 | 18.22 | 4.32 | 2.35 | |
| Grip strength at baseline | 6 | 83 | 29 | 10 |
Note: BMI = body mass index; BP = blood pressure; GDS = Geriatric Depression Scale; MMSE = Mini-Mental State Examination.
Table 2.
Associations Between Demographic and Clinical Variables to Motor Function at Baseline
| Sex | Age at baseline | Years of education | Overall cognition score at baseline | Diabetes duration | BMI At baseline |
SBP | DBP | Cholesterol | Triglyceride | creatinine | Hba1c | frailty effort | frailty-activate self | GM | ICV | LPM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gait speed at baseline | .184*** | .097** | −.155*** | −.148*** | −.044 | 0.167*** | .050 | −.058 | .044 | .027 | −.069* | −.013 | 0.137*** | 0.172*** | −0.139*** | −0.167*** | 0.156*** |
| Grip strength at baseline | −.675*** | −.148*** | .120*** | .184*** | −.011 | −0.40 | −.020 | .154*** | −.229*** | −.116*** | .391*** | −.081* | −0.177*** | −0.70*** | 0.329*** | 0.460*** | −0.27 |
Notes: DBP = diastolic blood pressure; GM = gray matter; ICV = intracranial volume; LPM = lesion probability map; SBP = systolic blood pressure.
*p < .05, **p < .01, ***p < .001.
Cross-sectional and Longitudinal Associations of Motor Function With Depression Symptoms
Gait speed
Slower gait speed at baseline was associated with more depression symptoms (β = 0.140, p < .001, Table 3, Figure 1A) and with a greater increase in depression symptoms over time (β = 0.001 for the interaction of gait speed with time, p = .046). (Table 3, Figure 1B). Adjusting for sociodemographic and cardiovascular variables as well as for global cognition and exhaustion did not alter the results (p = .001 and p = .049, respectively; Table 3).
Table 3.
Final Hierarchical Linear Models Predicting GDS Depression Scores by Motor Function
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | β Coefficient | SE | p Value | β Coefficient | SE | p Value | β Coefficient | SE | p Value |
| Gait speed* | 0.140 | 0.030 | <.001 | 0.080 | 0.031 | .001 | 0.042 | 0.033 | .197 |
| Gait speed time† | 0.001 | 0.001 | .046 | 0.001 | 0.001 | .049 | 0.001 | 0.0006 | .026 |
| Gait speed change | 0.050 | 0.019 | .007 | 0.037 | 0.015 | .015 | 0.045 | 0.018 | .015 |
| Gait speed change time | 0.003 | 0.002 | .049 | 0.002 | 0.001 | .221 | 0.001 | 0.002 | .534 |
| Grip strength | −0.043 | 0.007 | <.001 | −0.030 | 0.010 | .002 | −0.057 | 0.009 | .053 |
| Grip strength time | −0.0001 | 0.0001 | .491 | −0.0002 | 0.0001 | .076 | −0.0001 | 0.0001 | .689 |
| Grip strength change | −0.001 | 0.007 | .831 | 0.007 | 0.005 | .222 | −0.001 | 0.007 | .822 |
| Grip strength change time | −0.0008 | 0.0005 | .105 | −0.0009 | 0.0004 | .022 | −0.0007 | 0.0004 | .111 |
Notes: Model 1 includes only main and longitudinal effects of the motor variables. Model 2 is adjusted to age, sex, education, diabetes duration, BMI at baseline, systolic and diastolic blood pressure, total cholesterol, triglycerides, creatinine, HbA1c, total cognitive score, and frailty. Model 3 is a sensitivity analysis examining the main and longitudinal effects of motor variables on depression symptoms while adjusting for age, sex, education, diabetes duration, BMI at baseline, systolic and diastolic blood pressure, total cholesterol, triglycerides, creatinine, HbA1c, total cognitive score, and frailty.
*Main effect
†Longitudinal effect
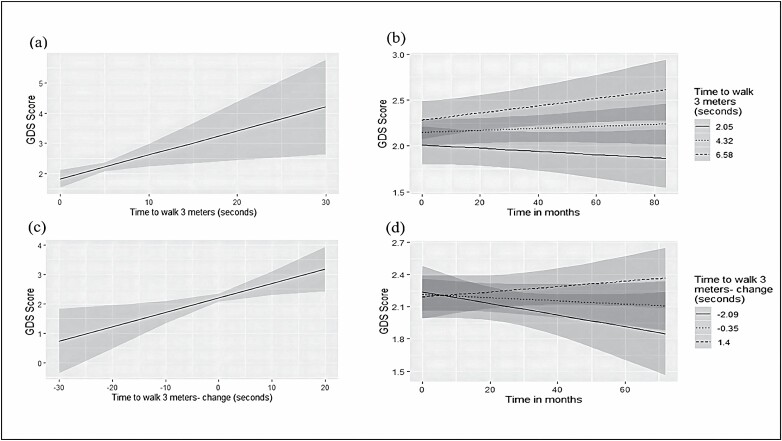
Figure 1.
Plots for the associations between gait speed (time in seconds to walk 3 m, higher scores representing lower gait speed) with Geriatric Depression Scale (GDS) score: (A) association between baseline gait speed and baseline GDS scores; (B) association between baseline gait speed and change in GDS score over time; (C) association between change in gait speed over time with change in GDS score over time; (D) association between extent of change in gait speed over time with extent of change in GDS score over time.
The main effect of change in gait speed on GDS scores was strongly significant such that an increase in walking time (ie, decrease in walking speed) from baseline was associated with an increase in GDS scores (β = 0.050, p = .007; Table 3; Figure 1C). This effect remained significant after adjusting for sociodemographic, and cardiovascular variables as well as global cognition and exhaustion (p = .015). Adding the time term into the model, also resulted in a significant effect over time (β = 0.003, p = .049), such that a greater increase over time in walking time was associated with an accelerated increase in GDS scores over time. However, this association did not withstand adjustment for the covariates (p = .221; Figure 1D).
We also examined whether depressive symptoms at baseline predict motor decline: higher GDS scores at baseline were not associated with the trajectory of gait speed over time (β = −0.003, p = .726). Adjusting for sociodemographic and cardiovascular variables as well as for global cognition and exhaustion did not alter the results (p = .789).
To reduce the potential contribution of pre-existing major affective disorder to the results, the analysis was repeated for n = 690 participants after excluding those with a diagnosis of depression or those purchasing antidepressant medications based on MHS medical records. In the fully adjusted models (controlling for sociodemographic, and cardiovascular variables as well as global cognition and exhaustion), apart from gait speed at baseline, which was no longer associated with depression symptoms (β = 0.042; p = .197), all the remaining findings regarding the relationship of gait speed with depression, were essentially unaltered; lower gait speed at baseline was associated with increase in the number of depression symptoms over time (β = 0.001, p = .026). Change in gait speed from baseline was associated with an increase in depression symptoms over time (β = 0.045, p = .015). The degree of decrease over time in gait speed was not associated with an accelerated increase in GDS scores over time (β = 0.001, p = .534; Table 3, Model 3). Additionally, the number of depression symptoms at baseline did not predict a change in gate speed (β = 0.001, p = .747).
Lastly, we repeated the analysis in n = 213 participants for whom brain MRI data were available, additionally adjusting the models for GM and WMHs’ volume. Lower gait speed at baseline was associated with more depressive symptoms (β = 0.144, p = .025), and with greater increase in depressive symptoms over time (β = 0.004, p < .001). The main effect for change in gait speed turned nonsignificant after adjusting for the MRI components (β = 0.009, p = .795). Adding the time term into the model did not alter the results (β = −0.0006, p = .804; Supplementary Table 1).
Grip strength
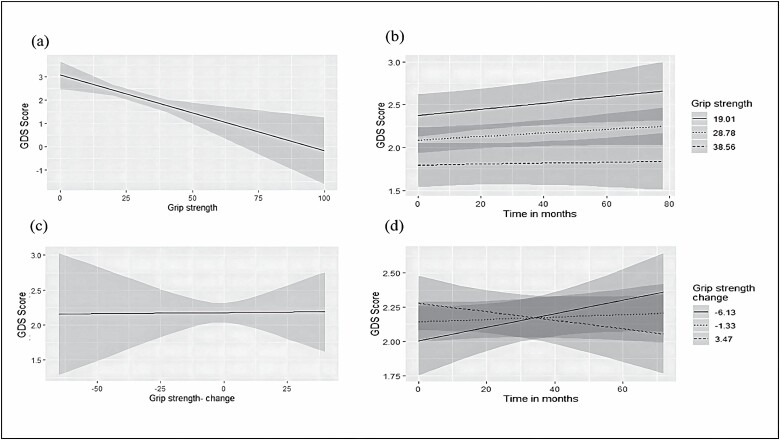
Lower grip strength at baseline was associated with more depression symptoms (β = −0.043, p < .001). This association remained significant after adjustment for sociodemographic and cardiovascular variables as well as for overall cognitive function and exhaustion (p = .002; Table 3; Figure 2A). However, the association of grip strength at baseline with the slope of GDS score over time was nonsignificant (β = −0.0001, p = .491; Table 3; Figure 2B) with no change after adjustment for the above-mentioned covariates (p = .076).
Figure 2.
Plots for the associations between grip strength (in kilograms, higher scores representing higher grip strength) with Geriatric Depression Scale (GDS) score: (A) association between baseline grip strength and baseline GDS scores; (B) association between baseline gait speed and change in GDS score over time; (C) association between change in gait speed over time with change in GDS score over time; (D) association between extent of change in gait speed over time with extent of change in GDS score over time.
The main effect of change in grip strength on change in GDS score from baseline was not significant (β = −0.001, p = .831; Table 3; Figure 2C), that is, a decrease in hand grip strength was not associated with increase in depression over time. Adding the time term into the model, also resulted in a nonsignificant effect (β = −0.001, p = .105; Table 3; Figure 2D), that is, the degree of change in grip strength was not associated with the degree of change in depression. Adjustment for sociodemographic and cardiovascular variables as well as for overall cognitive performance and exhaustion, did not alter the results for the main effect of change in grip strength (p = .963) but the time term turned significant (p = .022), suggesting that a greater decrease over time in grip strength is associated with an accelerated increase in GDS scores over time.
GDS scores at baseline were not associated with trajectories of grip strength over time (β = −0.001, p = .989). Adjusting for sociodemographic and cardiovascular variables as well as for overall cognitive performance and exhaustion did not alter the results (p = .783).
After excluding patients with a diagnosis of depression or those purchasing antidepressant medications, grip strength was not associated with the number of depression symptoms in any of the models.
In an exploratory analysis, additional adjustment for GM and WMHs’ turned the association between grip strength at baseline and depression symptoms to nonsignificant (β = −0.022, p = .213). The association between grip strength at baseline and the change over time in depression symptoms remained nonsignificant (β = −0.0003, p = .152) as well as the association between the change in grip strength and depression symptoms (β = 0.011, p = .314). The association between the change in grip strength and the degree of change in depression symptoms remained significant (β = −0.002, p = .020; Supplementary Table 1).
Discussion
This study extends the current knowledge on the associations of motor function and depression by examining these relationships specifically in older adults with T2D, who are at high risk of depression and compromised motor function (32,33). The study additionally innovates by examining the associations of concomitant changes in motor function with trajectories of depression symptoms over time. We show that in older adults with T2D who are initially cognitively normal, both slower gait speed and weaker grip strength were associated with a higher number of depression symptoms at baseline. However, gait speed and grip strength differ in their longitudinal relationships with the number of depression symptoms. Specifically, slower gait speed at baseline and its decrease during follow-up, were associated with an increase in the number of depression symptoms over time. These effects withstood adjustment for sociodemographic, cardiovascular, cognitive function, and self-rated exhaustion variables. In contrast, grip strength at baseline or decrease in grip strength over time was not associated with longitudinal trends in depression. Only the rate of change in grip strength was associated with an accelerated increase in the number of depression symptoms. The results were minimally affected in a sensitivity analysis excluding IDCD participants with a clinical diagnosis of depression or those receiving anti-depression medications based on MHS medical records, such that the baseline relationship of gait speed with baseline depression was no longer significant. The longitudinal relationships between gait speed and depression were not affected, suggesting that the contribution of a small segment of the IDCD cohort with preexisting depression, to the associations found was minimal. The present results support the potential of grip strength and gait speed measurement as objective markers of coexistent symptoms of depression. Gait speed, is also a predictor of an increase in the number of incident depression symptoms and thus may be developed as a marker for incipient depression, which can enable the introduction of depression-prevention interventions. Such a tool is especially important in the context of subtle symptoms of depression, which are the most prevalent in old age (1,2), but commonly underdiagnosed (34).
Previous studies examining the relationships of motor functions with depression, generally demonstrate an association of worse motor functions with higher depression scores, but findings vary by the population studied, the type of motor function examined, the type and severity of depression-related outcome, and the study design (cross-sectional or longitudinal). In the majority of these studies, only one of the motor functions was measured, limiting the ability to compare their strength as predictors of depression-related outcomes. Slower gait speed was cross-sectionally associated with elevated symptoms of depression (20) and with increased risk for incident depression (14) in older adults. Similarly, in middle-aged (mean age 41 years (35)) and “young old” populations (mean = 66 years (18)), lower handgrip strength was associated with higher cotemporary depression scores (18,35), and with increased incidence of depression at follow-up (18). Both slow gait speed and weak grip strength were highly comorbid with major depression or with a persistent depressive disorder in nondemented older adults (36). Moreover, in these patients, worse motor function was associated with higher severity of depression (36). Despite these apparent consistencies, it is important to take into consideration that the role of motor functions in depression may differ be the overall clinical context, as demonstrated by the association of handgrip strength with depression in individuals with metabolic disease (eg, diabetes) but not in those with arthritis (37). Thus, motor functions are potential simple and objective markers for depression symptoms and for their trajectory over time, however, their clinical implementation should be differentially adapted to different co-morbidities.
There are several possible explanations for our findings. Cerebrovascular pathology is associated with slower gait speed (38), weaker grip strength (35), and with old-age depression. The risk for incident stroke increases with decreasing gait speed (39), potentially pointing to this motor function as a marker of subclinical cerebrovascular pathology. Cerebrovascular pathology, more prominent in the context of diabetes, largely affects frontal-subcortical circuits, which are crucial for emotional regulation and motor functionality in late life (14). Grip strength has also been associated with hippocampal volume (35), which in turn, plays a role in the onset and course of depression (40). Thus, motor functions may reflect disrupted integrity of brain areas responsible for coordination, balance, strength as well as for higher mental function (15) and affect, and for the interconnections between them (15). We examined the potential contribution of GM and WMH’s volume in a small sample of IDCD participants for whom brain MRI was available. When these variables were added to the statistical model, the associations between changes in gait speed and changes in depression over time were no longer significant, however, the relationship of baseline gait speed with cross-sectional and longitudinal depression scores were unaltered. Similarly, the association of grip strength at baseline with co-temporary depression scored were no longer significant, but faster decrease in grip strength was still associated with accelerated worsening in depression symptoms. GM and WMHs may therefore underlie the relationships found, but only to a certain extent. The small number of IDCD participants for who brain MRI was available prevents a deeper exploration of this hypothesis.
Depression and motor impairments may be both a cause and consequence of a physically inactive lifestyle (20). People who are more physically active, have better motor functions and are less prone for cerebrovascular pathology (35) and, therefore, depression. Alternatively, individuals with depression, are less likely to be engaged in physical activity and consequently, have lower muscle strength and slower and gait speed, and be more prone to cerebrovascular pathology.
Lastly, brain dopaminergic functionality, which is relevant for both motor functions and depression decreases with age and is disrupted in diabetes (41). Another potential mechanism of psychomotor slowing in the context of T2D, may involve the increased inflammatory process in this population (42), which has been demonstrated to modify the functionality of the basal ganglia and of the dopaminergic pathways, potentially presenting as anhedonia, fatigue, and psychomotor slowing (43).
The divergence, to some extent, in the relationships between walking speed and grip strength with longitudinal depression-related outcomes in the IDCD cohort may potentially suggest that the underlying mechanisms are not entirely overlapping, as reflected by the associations of walking speed, but not grip strength, with global WM fractional anisotropy in a cohort of 122 healthy older adults (44). The findings could also be explained by the different muscle groups and neurological systems supporting walking speed and hand grip strength (45). Others have demonstrated, in community-dwelling older adults, that both gait speed and grip strength at baseline are associated with cognitive decline over a 10-year follow-up. However, each of these motor functions predicted deterioration in a different cognitive test-gait speed predicted changes in the DSST while grip strength predicted changes in MMSE scores, suggesting that different motor functions affect different cognitive domains.
The longitudinal associations of depression with motor functions could theoretically be explained by the role of depression in the prediction of motor outcomes, however, in this study, depression scores at baseline were not associated with trajectories of motor function thereafter.
The strengths of the study include the large cohort of patients with a well-validated diagnosis of T2D and the availability of long-term information on numerous potential confounders. Moreover, diabetes per se and the confounders were directly measured rather than self-reported.
The main limitation of the study is the measurement of depression symptoms based on the GDS score rather than a clinical assessment. Nevertheless, this tool enables the measurement of subtle depression symptomatology, which is commonly missed in clinical assessment (usually directed toward detection of depression with clinical significance). Subtle depression symptoms comprise the most common form of depression in old age. The GDS has been widely used as a screening tool for depression (46), and in T2D, it has been associated with cognition (47), quality of life (48) and cerebral small vessel disease (49), indicating its relevance in predicting poor health outcomes. The results of the present study may not necessarily be generalizable to all patients with T2D, as IDCD participants are relatively well educated, cognitively normal, mostly without clinically significant affective symptoms, and with relatively well-controlled diabetes (mean HbA1c value of 6.8% [SD = 0.8%]). Also, the effect sizes detected are small. Nevertheless, they are consistent and endured adjustment for numerous factors. The findings could potentially be more pronounced in patients with more severe forms of depression-related outcomes. It is plausible that the present results are affected by factors not assessed in our study, such as life history of depression (age of onset, number, duration and severity of symptoms), comorbid anxiety, or other medical comorbidities. Nevertheless, the relationships remained significant after including a large number of demographic, T2D, and health-related factors in the statistical model. Data on clinical factors that may affect the relationship between motor functions and depression (eg, blood pressure, cholesterol, or kidney functions) were only available at baseline, limiting our ability to examine the effect of changes in these variables over time on the relationship of motor function and depression. Nevertheless, the inclusion of the baseline levels of these variables in the statistical models (Model 2, Table 3) did not affect the results. Self-rated quality of life, which may potentially be associated with motor function, depression and the inter-relationship between these factors, was not assessed in the IDCD.
The present findings innovate by demonstrating the relationship of two easily measurable and objective motor functions with subtle depression symptoms, specifically in a sample of T2D patients. These patients are prone to depression, which has a detrimental effect on disease outcomes, even in its mildest forms. The phenotypic expression (37) and mechanisms (50) underlying depression in old age, and specifically in T2D, may differ from the general population, and from other clinical settings (37) stressing the importance of examining this relationship specifically in older adults with T2D. In contrast to previous studies, which usually examined only one motor variable, in the IDCD, both motor functions-gait speed and grip strength, were measured longitudinally, revealing some divergence in their longitudinal relationships with depression symptom. The differences between distinct motor variables to serve as markers of incident depression symptoms, highlight the importance of further developing the optimal motor function measurement for the prediction of depression. The availability of such tools may facilitate the introduction of depression prevention strategies in an early window of time.
Supplementary Material
Contributor Information
Inbar Lavie, Sackler School of Medicine, Tel-Aviv University, Tel Aviv, Israel.
Michal Schnaider Beeri, The Joseph Sagol Neuroscience Center, Sheba Medical Center, Tel Hashomer, Ramat Gan, Israel; The Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York, USA; Baruch Ivcher School of Psychology, Interdisciplinary Center, Herzliya, Israel.
Yonathan Schwartz, The Joseph Sagol Neuroscience Center, Sheba Medical Center, Tel Hashomer, Ramat Gan, Israel.
Laili Soleimani, The Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Anthony Heymann, Department of Family Medicine, Tel Aviv University, Tel Aviv, Israel; Maccabi Healthcare Services, Israel.
Joseph Azuri, Department of Family Medicine, Tel Aviv University, Tel Aviv, Israel; Maccabi Healthcare Services, Israel.
Ramit Ravona-Springer, Sackler School of Medicine, Tel-Aviv University, Tel Aviv, Israel; The Joseph Sagol Neuroscience Center, Sheba Medical Center, Tel Hashomer, Ramat Gan, Israel; Psychiatric Division, Sheba Medical Center, Tel-Hashomer, Israel.
Funding
This work was supported by the National Institute of Aging (grants R01 AG053446 to M.S.B., P50 AG05138 to Mary Sano), and the Helen Bader Foundation and the Leroy Schecter Foundation Award (to M.S.B.).
Conflict of Interest
None declared.
Autor Contributions
I.L.: Formal analysis; M.S.B.: Lead supervision; Y.S.: Project administration; L.S.: Analysis supervision; A.H. Reviewer; J.A.: Reviewer; R.R.-S.: Lead conceptualization, lead writer, supervision.
References
- 1. Polyakova M, Sonnabend N, Sander C, et al. . Prevalence of minor depression in elderly persons with and without mild cognitive impairment: a systematic review. J Affect Disord. 2014;152(154):28–38. doi: 10.1016/j.jad.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 2. Blazer D, Hughes DC, George LK. The epidemiology of depression in an elderly community population. Gerontologist. 1987;27(3):281–287. doi: 10.1093/geront/27.3.281 [DOI] [PubMed] [Google Scholar]
- 3. Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP. Design makes a difference: a meta-analysis of antidepressant response rates in placebo-controlled versus comparator trials in late-life depression. Am J Geriatr Psychiatry. 2008;16(1):65–73. doi: 10.1097/jgp.0b013e3181256b1d [DOI] [PubMed] [Google Scholar]
- 4. Sheline YI, Pieper CF, Barch DM, et al. . Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stahl ST, Albert SM, Dew MA, Lockovich MH, ReynoldsCF, 3rd. Coaching in healthy dietary practices in at-risk older adults: a case of indicated depression prevention. Am J Psychiatry. 2014;171(5):499-505. doi: 10.1176/appi.ajp.2013.13101373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smits F, Smits N, Schoevers R, Deeg D, Beekman A, Cuijpers P. An epidemiological approach to depression prevention in old age. Am J Geriatr Psychiatry. 2008;16(6):444–453. doi: 10.1097/jgp.0b013e3181662ab6 [DOI] [PubMed] [Google Scholar]
- 7. Cuijpers P, Beekman AT, ReynoldsCF, 3rd. Preventing depression: a global priority. JAMA. 2012;307(10):1033–1034. doi: 10.1001/jama.2012.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Control. CfD. National Diabetes Statistics Report. US Department of Health and Human Services. 2020. [Google Scholar]
- 9.NHS Digital annual report and accounts 2017–18. National Diabetes Audit Report 1: Care Processes and Treatment Targets 2017–18. England. 2018. [Google Scholar]
- 10. Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 2015;3(6):461–471. doi: 10.1016/s2213-8587(15)00134-5 [DOI] [PubMed] [Google Scholar]
- 11. Petrak F, Herpertz S, Albus C, et al. . Cognitive behavioral therapy versus sertraline in patients with depression and poorly controlled diabetes: the diabetes and depression (DAD) study: a randomized controlled multicenter trial. Diabetes Care. 2015;38(5):767–775. doi: 10.2337/dc14-1599 [DOI] [PubMed] [Google Scholar]
- 12. Zanoveli JM, Morais H, Dias IC, Schreiber AK, Souza CP, Cunha JM. Depression associated with diabetes: from pathophysiology to treatment. Curr Diabetes Rev. 2016;12(3):165–178. doi: 10.2174/1573399811666150515125349 [DOI] [PubMed] [Google Scholar]
- 13. Nefs G, Pouwer F, Denollet J, Pop V. The course of depressive symptoms in primary care patients with type 2 diabetes: results from the Diabetes, Depression, Type D Personality Zuidoost-Brabant (DiaDDZoB) Study. Diabetologia. 2012;55(3):608–616. doi: 10.1007/s00125-011-2411-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stahl ST, Altmann HM, Dew MA, et al. . The effects of gait speed and psychomotor speed on risk for depression and anxiety in older adults with medical comorbidities. J Am Geriatr Soc. 2021;69(5):1265–1271. doi: 10.1111/jgs.17024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clouston SA, Brewster P, Kuh D, et al. . The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35:33–50. doi: 10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demnitz N, Esser P, Dawes H, et al. . A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164–174. doi: 10.1016/j.gaitpost.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith L, Firth J, Grabovac I, et al. . The association of grip strength with depressive symptoms and cortisol in hair: a cross-sectional study of older adults. Scand J Med Sci Sports. 2019;29(10):1604–1609. doi: 10.1111/sms.13497 [DOI] [PubMed] [Google Scholar]
- 18. Fukumori N, Yamamoto Y, Takegami M, et al. . Association between hand-grip strength and depressive symptoms: Locomotive Syndrome and Health Outcomes in Aizu Cohort Study (LOHAS). Age Ageing. 2015;44(4):592–598. doi: 10.1093/ageing/afv013 [DOI] [PubMed] [Google Scholar]
- 19. McDowell CP, Gordon BR, Herring MP. Sex-related differences in the association between grip strength and depression: Rrfrom the Irish Longitudinal Study on Ageing. Exp Gerontol. 2018;104:147–152. doi: 10.1016/j.exger.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 20. Marino FR, Lessard DM, Saczynski JS, et al. . Gait speed and mood, cognition, and quality of life in older adults with atrial fibrillation. J Am Heart Assoc. 19 2019;8(22):e013212. doi: 10.1161/JAHA.119.013212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belvederi Murri M, Triolo F, Coni A, et al. . Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687 [DOI] [PubMed] [Google Scholar]
- 22. Sanders JB, Bremmer MA, Comijs HC, van de Ven PM, Deeg DJH, Beekman ATF. Gait speed and processing speed as clinical markers for geriatric health outcomes. Am J Geriatr Psychiatry. 2017;25(4):374–385. doi: 10.1016/j.jagp.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 23. Ganmore I, Elkayam I, Ravona-Springer R, et al. . Deterioration in motor function over time in older adults with type 2 diabetes is associated with accelerated cognitive decline. Endocr Pract. 2020;26(10):1143–1152. doi: 10.4158/ep-2020-0289 [DOI] [PubMed] [Google Scholar]
- 24. Beeri MS, Ravona-Springer R, Moshier E, et al. . The Israel Diabetes and Cognitive Decline (IDCD) study: design and baseline characteristics. Alzheimers Dement. 2014;10(6):769–778. doi: 10.1016/j.jalz.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheikh JY, Yesavage JA. Geriatric Depression Scale (GDS): a recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 26. Fried LP, Tangen CM, Walston J, et al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 27. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412.22412–2412.22412. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 28. da Costa Dias FL, Teixeira AL, Guimaraes HC, et al. . The influence of age, sex and education on the phenomenology of depressive symptoms in a population-based sample aged 75+ years with major depression: the Pieta Study. Aging Ment Health. 2021;25(3):462–467. doi: 10.1080/13607863.2019.1698517 [DOI] [PubMed] [Google Scholar]
- 29. Ylli A, Miszkurka M, Phillips SP, Guralnik J, Deshpande N, Zunzunegui MV. Clinically relevant depression in old age: an international study with populations from Canada, Latin America and Eastern Europe. Psychiatry Res. 2016;241:236–241. doi: 10.1016/j.psychres.2016.04.096 [DOI] [PubMed] [Google Scholar]
- 30. de Toledo Ferraz Alves TC, Ferreira LK, Busatto GF. Vascular diseases and old age mental disorders: an update of neuroimaging findings. Curr Opin Psychiatry. 2010;23(6):491–497. doi: 10.1097/yco.0b013e32833e339c [DOI] [PubMed] [Google Scholar]
- 31. Ferri F, Deschenes SS, Power N, Schmitz N. Associations between cognitive function, metabolic factors and depression: a prospective study in Quebec, Canada. J Affect Disord. 2021;283:77–83. doi: 10.1016/j.jad.2021.01.039 [DOI] [PubMed] [Google Scholar]
- 32. Liang X, Jiang CQ, Zhang WS, et al. . Glycaemia and hand grip strength in aging people: Guangzhou Biobank Cohort Study. BMC Geriatr. 2020;20(1):399. doi: 10.1186/s12877-020-01808-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gorniak SL, Lu FY, Lee BC, Massman PJ, Wang J. Cognitive impairment and postural control deficit in adults with Type 2 diabetes. Diabetes Metab Res Rev. 2019;35(2):e3089. doi: 10.1002/dmrr.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morichi V, Dell’Aquila G, Trotta F, Belluigi A, Lattanzio F, Cherubini A. Diagnosing and treating depression in older and oldest old. Curr Pharm Des. 2015;21(13):1690–1698. doi: 10.2174/1381612821666150130124354 [DOI] [PubMed] [Google Scholar]
- 35. Gu Y, Li X, Zhang Q, et al. . Grip strength and depressive symptoms in a large-scale adult population: the TCLSIH cohort study. J Affect Disord. 2021;279:222–228. doi: 10.1016/j.jad.2020.08.023 [DOI] [PubMed] [Google Scholar]
- 36. Brown PJ, Roose SP, O’Boyle KR, et al. . Frailty and its correlates in adults with late life depression. Am J Geriatr Psychiatry. 2020;28(2):145–154. doi: 10.1016/j.jagp.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marconcin P, Peralta M, Ferrari G, et al. . The association of grip strength with depressive symptoms among middle-aged and older adults with different chronic diseases. Int J Environ Res Public Health. 2020;17(19):6942. doi: 10.3390/ijerph17196942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosano C, Kuller LH, Chung H, Arnold AM, LongstrethWT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53(4):649–654. doi: 10.1111/j.1532-5415.2005.53214.x [DOI] [PubMed] [Google Scholar]
- 39. McGinn AP, Kaplan RC, Verghese J, et al. . Walking speed and risk of incident ischemic stroke among postmenopausal women. Stroke. 2008;39(4):1233–1239. doi: 10.1161/strokeaha.107.500850 [DOI] [PubMed] [Google Scholar]
- 40. Firth JA, Smith L, Sarris J, et al. . Handgrip strength is associated with hippocampal volume and white matter hyperintensities in major depression and healthy controls: a UK Biobank Study. Psychosom Med. 2020;82(1):39–46. doi: 10.1097/PSY.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 41. Pignalosa FC, Desiderio A, Mirra P, et al. . Diabetes and cognitive impairment: a role for glucotoxicity and dopaminergic dysfunction. Int J Mol Sci. 2021;22(22). doi: 10.3390/ijms222212366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- 43. Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33(3):315–327. doi: 10.1016/j.yfrne.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanders AM, Richard G, Kolskar K, et al. . Linking objective measures of physical activity and capability with brain structure in healthy community dwelling older adults. Neuroimage Clin. 2021;31:102767. doi: 10.1016/j.nicl.2021.102767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee YH, Kim JS, Jung SW, et al. . Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol. 2020;21(1):166. doi: 10.1186/s12882-020-01831-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mackin RS, Insel P, Tosun D, et al. . The effect of subsyndromal symptoms of depression and white matter lesions on disability for individuals with mild cognitive impairment. Am J Geriatr Psychiatry. 2013;21(9):906–914. doi: 10.1016/j.jagp.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ravona-Springer R, Heymann A, Lin HM, et al. . Increase in number of depression symptoms over time is related to worse cognitive outcomes in older adults with type 2 diabetes. Am J Geriatr Psychiatry. 2021;29(1):1–11. doi: 10.1016/j.jagp.2020.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Argano C, Catalano N, Natoli G, Monaco ML, Corrao S. GDS score as screening tool to assess the risk of impact of chronic conditions and depression on quality of life in hospitalized elderly patients in internal medicine wards. Medicine (Baltim). 2021;100(26):e26346. doi: 10.1097/md.0000000000026346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rensma SP, van Sloten TT, Ding J, et al. . Type 2 diabetes, change in depressive symptoms over time, and cerebral small vessel disease: longitudinal data of the AGES-Reykjavik study. Diabetes Care. 2020;43(8):1781–1787. doi: 10.2337/dc19-2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(4):325–336. doi: 10.1016/s2213-8587(19)30405-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.