Abstract
Background
To evaluate whether contrast sensitivity is associated with lower extremity physical function in cognitively intact older adults.
Methods
Cross-sectional analysis of the relationship of binocular and worse eye log contrast sensitivity (LCS) to expanded Short Physical Performance Battery (eSPPB) and its components (gait speed, narrow walking speed, chair stand pace, and balance) in 192 cognitively healthy older adults. The association of LCS with postural sway and gait was also tested with tasks that further challenged functional reserve.
Results
Mean age was 76.4 years with 56% identifying as female and over 98.5% having good corrected visual acuity. Lower LCS was significantly associated with worse performance on the eSPPB, 4-M gait speed, narrow walking speed, and balance time in unadjusted and adjusted models. The relationship between worse eye LCS and larger postural sway was 3 times greater on a foam surface (beta 1.07, 95% CI [0.35, 1.80]) than a firm surface (beta 0.35, 95% CI [0.05, 0.65]), and both were robust to adjustment for confounders; similar findings were observed with binocular LCS. Lower binocular LCS had a greater decremental effect on gait velocity during the fast pace (beta −0.58, 95% CI [−0.90, −0.27]) than the usual pace (Beta −0.39 [−0.63, −0.15]) gait task.
Conclusions
These findings suggest that cognitively unimpaired older adults without significant visual acuity impairment can have subtle preclinical deficits in contrast sensitivity and physical function that could place them at risk of mobility and balance issues. Future studies should determine whether this subset of older adults may benefit from targeted intervention to prevent disability.
Keywords: Balance, Cognitive aging, Contrast sensitivity, Gait, Physical function
Walking and the maintenance of postural stability are complex tasks that rely on the coordination of multiple visual, vestibular, and somatosensory inputs (1). It is thought that vision plays an increasingly central role in mobility as patients age (2). Thus, age-related and pathologic declines in visual function over time can place older adults at a particularly increased risk of falls (3,4). Visual impairment (VI) has also been associated with slower gait speed (5,6), and both impairments in vision (7–10) and gait (11–15) have been shown to predict cognitive impairment. Patients with cognitive dysfunction are also at increased fall risk (16), but the exact relationship between visual, cognitive, and physical function is not well understood (17,18).
A majority of the literature examining the association between vision and physical function does not consider that cognitive function may be an important confounder (5,6,19–21) or a mediator of this association (22). In addition, most of the populations studied had at least moderate impairment in central visual acuity, which is most likely explained by more advanced concomitant pathologic eye disease (5,6,19–21). However, according to the 2019 Global Burden of Disease Study, nearly half of the cases of VI worldwide are related to milder decrements in visual acuity (23). Moreover, there is increasing literature documenting age-related declines in other visual measures such as contrast sensitivity (CS) (5,6,20,24). Although CS is not routinely collected in clinical practice, poor CS has been associated with slower gait speed, mobility impairment, and falls in several population-based studies (3,6,25,26), but in these studies, patients also had a high co-prevalence of poor visual acuity.
However, in the Health, Aging and Body Composition study, we found that lower performance on the Short Physical Performance Battery (SPPB) was significantly associated with impaired CS, but no other measures of visual function in a multivariable model that included visual acuity, stereoacuity, and self-reported visual function while controlling for other sociodemographic and clinical covariates (26). CS is the ability to perceive differences in shades of light and dark and is central to pattern recognition and depth perception, which may explain the observed relationship with mobility and balance. Some studies suggest impaired CS may be more widespread than impaired central visual acuity (20,25), and that deficits in CS may also be upstream of acuity loss or visual field damage, making it a potential marker of very early disease or even age-related changes (24). Since CS deficits may precede VA deficits and cognitive deficits, we hypothesized that CS might be a potential earlier biomarker for physical dysfunction, which could suggest possible targets for upstream intervention before the onset of disability. To test this hypothesis, we sought to examine whether CS dysfunction was associated with mild decrements in physical function in a cohort of cognitively healthy older adults with good visual acuity. Such mild deficits may be subtle, so it is possible that a predisposition to dysfunction may not be evident unless the system is placed under duress. Thus, we subjected participants to a challenge task for both gait and balance to determine whether such a challenge elicited a stronger association between physical performance and CS that could suggest preclinical dysfunction.
Method
We conducted a cross-sectional analysis of tests of visual and physical function that were measured during the baseline visit of the Brain Networks and Mobility (B-NET) study (NCT03430427). B-NET is an ongoing prospective cohort study of community-dwelling older adults ≥ 70 years old recruited from Forsyth County, North Carolina, and surrounding regions. All study participants signed a written informed consent and were able to communicate with study personnel. Exclusion criteria for participation in B-NET were the following: major uncorrected hearing or vision problems, being a single or double amputee, having musculoskeletal implants severe enough to impede functional testing (eg, joint replacements), dependency on a walker or another person to ambulate, recent surgery or hospitalization within the prior 6 months, serious or uncontrolled chronic disease (eg, Stage 3 or 4 cancer, Stage 3 or 4 heart failure, uncontrolled angina, liver failure or cirrhosis of the liver, respiratory disease requiring the use of oxygen, renal failure requiring dialysis, diagnosis of schizophrenia, bipolar, or other psychotic disorders), alcoholism (>21 drink per week), clinical manifestation of a neurologic disease affecting mobility, prior traumatic brain injury with residual deficits, history of brain tumors, seizures within the last year, being unwilling or unable to have an MRI brain scan, plans to relocate within the next 2 years, participation in a behavioral intervention trial, or evidence of cognitive impairment. Cognitive function was assessed with a battery of tests including the Montreal Cognitive Assessment (MoCA), Trail-Making Test A and B, Auditory Verbal Learning Test, Digit Symbol Coding, Craft Story Immediate and Delayed Recall, Word Fluency by Letter (F and L), and Category (animals, vegetables). Participants with MoCA scores of <21 were excluded, and scores >26 were deemed eligible. For participants with MoCA scores between 21 and 26, the study neuropsychologist (L.D.B.) reviewed all available cognitive test data to exclude those with suspected mild cognitive impairment (MCI). The study was conducted in accordance with the Declaration of Helsinki with study approval by the Institutional Review Board of the Wake Forest School of Medicine.
Sociodemographic and Clinical Data
Sociodemographic and clinical data were acquired at the baseline visit including age, sex, race, education, and smoking status. Body mass index (BMI) was calculated using the participant’s measured height and body mass (kg/m2). Cerebrovascular disease was defined as a history of stroke or brain hemorrhage, and if participants were hospitalized for the event they were excluded from the study. Diabetes mellitus was defined as fasting blood glucose ≥ 126 mg/dL, self-reported diabetes mellitus or high blood sugar, or use of diabetic medications; participants with fasting glucose >250 mg/dL were excluded. Cardiovascular disease was defined by a history of a heart attack, coronary, or myocardial infarction in the preceding 30 days requiring hospitalization overnight, or a history of heart failure. Hypertension was indicated by self-report of hypertension, use of anti-hypertensive medications, or a systolic blood pressure >130 mmHg at the baseline visit. Depressive symptoms were assessed with the 20-item Center for Epidemiologic Study-Depression Scale (CES-D) (27).
Visual Function Testing
Participants were asked to report if they had cataracts, glaucoma, age-related macular degeneration, or problems with their retina, retinopathy, or other retinal disease or changes. They were also asked to rate their eyesight: “At the present time, would you say your eyesight (with glasses or contact lenses, if you wear them) is excellent, good, fair, poor, very poor, completely blind, or do not know.” For the purpose of analysis, this question was converted into a binary variable of fair/poor versus good/excellent eyesight.
Participants were instructed to stand at 4-M and read the ETDRS eye chart while wearing corrective lenses (if applicable) with both eyes open. Both logarithm of the minimum angle of resolution (logMAR) visual acuity and Snellen visual acuity were recorded, and a binary variable of worse than 20/40 versus 20/40 or better was created for analysis purposes.
CS testing was completed using a Pelli-Robson eye chart which was placed at a distance of 5 feet and participants were asked to wear their corrective lenses if they had them. The total number of letters read correctly was recorded for both monocular and binocular conditions and converted to log contrast sensitivity (LCS) (28). LCS was further categorized as moderate impairment (<1.55 log units) versus not impaired (≥1.55 log units). This cutoff of <1.55 log units for moderate impairment was based a priori on the threshold used in prior longitudinal cohort studies for adults older than 60 years of age and represents the ability to read fewer than 34 letters on the Pelli-Robson chart (9,25,26,29). Variables were created for binocular testing conditions and for the worse eye.
Mobility Function Testing
eSPPB
In order to address ceiling effects that could limit the value of the traditional SPPB in a well-functioning cohort such as B-NET, an expanded Short Physical Performance Battery (eSPPB) was adapted from the original test described by Guralnik et al. (30). The eSPPB increased the challenge to participants’ physical function assessments for balance and added a narrow gait assessment. For standing balance time, participants were asked to hold a side-by-side posture for 10 seconds, and then the semi-tandem, tandem, and 1-leg positions for 30 seconds each. If participants were unable to hold the semi-tandem stand for 30 seconds, then they were asked to hold a short tandem stand for 10 seconds instead of 30 seconds. After the measurement of the usual 4-M gait speed (m/s), participants were asked to keep their steps in between 2 parallel lines marked 20 cm apart for measurement of a 4-m narrow walking speed. Chair stand pace was also captured as the number of times a participant could stand up from a seated position during a 5-second period. For each subcomponent, continuous scores were calculated based on the proportion of the best possible score, which distinguishes it from the SPPB which reports ranges of performance as a categorical measure. The resulting overall eSPPB score ranges from 0 to 4, rather than the traditional 12-point right-skewed categorical score distribution of the SPPB. The higher scores reflect better performance.
Postural sway
An Advanced Mechanical Technology Incorporated AccuSway biomechanics force platform was used to measure postural sway using center-of-pressure (COP) trajectory data collected at 100 Hz. Postural sway was measured under 2 conditions: while standing on the firm force plate surface and while standing on an Airex foam pad placed atop the force plate. Participants were asked to stand comfortably on either the firm or foam surface for a series of five 30-second trials while standing barefoot in an upright closed stance position with feet abducted 10°. For both conditions, postural sway performance was assessed by the area (inch2) of the 95% confidence ellipse encompassing the path of the COP, with higher values representing worse performance.
Gait speed
Gait speed was assessed while making 4 passes at usual pace and 4 passes at fast pace over a 4-m instrumented mat (GAITRite System, Sparta, NJ), which provides data on gait velocity in cm/s. The velocity was converted to m/s for ease of interpretation.
Statistical Methods
Descriptive analyses were performed and chi-square test, Fisher’s exact test, t test, or Wilcoxon Rank Sum test was used as applicable to evaluate the association of each of the clinical and sociodemographic variables with worse eye LCS < 1.55 versus LCS ≥ 1.55. Next, separate bivariate linear regression analyses were constructed with each of the vision variables (ie, binocular continuous LCS, worse eye continuous LCS continuous, binocular LCS < 1.55, worse eye LCS < 1.55) as potential predictors of interest and expanded SPPB as the primary outcome for mobility function. The association of each vision predictor with the individual tests that contribute to the eSPPB (ie, gait speed, narrow walking speed, chair stand pace, and balance time) was also evaluated. In addition, the aforementioned clinical and sociodemographic variables were evaluated against eSPPB and its components to determine potential covariates to include in multivariable models; those with a p < .05 were adjusted for in subsequent multivariable regression analysis.
Next, to further examine the relationship of LCS to mobility function under usual and more challenging conditions, gait and balance were each assessed using data from the GAITRite mat and AccuSway force platform, respectively. Separate bivariate and multivariable linear regression models were constructed with each of the LCS variables and gait speed under usual pace and fast pace conditions. Similar analyses were run with LCS and postural sway using the area (inch2) of the 95% confidence ellipse encompassing the path of the COP on either the firm or the foam surface as the outcome.
The same covariates were used in all of the multivariable analyses. Model 1 presents an unadjusted bivariate analysis with LCS as the main predictor variable. Model 2 adjusted for covariates that had a p value of <0.05 in bivariate analyses with eSPPB—namely, BMI, hypertension, and diabetes mellitus; in addition, sex was included due to its known association with strength and borderline association with LCS. Since age is collinear with most age-related covariates, the decision was made to add age only to the final model to determine whether the association between vision and physical functions was the same for 2 people of the same age or if the effect was related to aging.
Results
Only 3 participants in the study had a visual acuity worse than 20/40, and fewer than 10% (n = 19) of participants had LCS < 1.55 with binocular testing. Baseline clinical and sociodemographic variables were thus stratified by worse eye LCS < 1.55 (N = 86), which was significantly associated with age and diabetes mellitus (p < .05) but only borderline associated with sex (p < .10; Table 1). There was no significant association of self-reported quality of eyesight with LCS, cataract, or glaucoma (p > .05), but there was a significant association with self-reported retinopathy and macular degeneration. There was no significant association of MoCA scores with LCS (p = .3689).
Table 1.
Baseline Sociodemographic and Clinical Variables Stratified by Log Contrast Sensitivity in the Worse Eye
| Total (N = 192) | Log Contrast Sensitivity ≥ 1.55 in Worse Eye (N = 106) | Log Contrast Sensitivity <1.55 in Worse Eye (N = 86) | p Value† | |
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age, mean ± SD (range) | 76.4 ± 4.7 (70.3, −90.7) | 75.5 ± 4.2 (70.3, 89.2) | 77.6 ± 5.1 (70.5, 90.7) | .0013* |
| Sex | .0998 | |||
| Male | 84 (43.75) | 52 (49.1) | 32 (37.2) | |
| Female | 108 (56.25) | 54 (50.9) | 54 (62.8) | |
| Race | .9752 | |||
| Black | 18 (9.38) | 10 (9.4) | 8 (9.3) | |
| Non-Black | 174 (90.63) | 96 (90.6) | 78 (90.7) | |
| Education | .2193 | |||
| Postgraduate | 49 (25.52) | 30 (28.3) | 19 (22.1) | |
| College | 116 (60.42) | 65 (61.3) | 51 (59.3) | |
| High school | 27 (14.06) | 11 (10.4) | 16 (18.6) | |
| Clinical variables | ||||
| Hypertension | .2712 | |||
| Yes | 154 (80.21) | 82 (77.4) | 72 (83.7) | |
| No | 38 (19.79) | 24 (22.6) | 14 (16.3) | |
| Diabetes mellitus | .0069* | |||
| Yes | 41 (21.35) | 15 (14.2) | 26 (30.2) | |
| No | 151 (78.65) | 91 (85.8) | 60 (69.8) | |
| Cardiovascular disease | .5879 | |||
| Yes | 3 (1.56) | 1 (0.9) | 2 (2.3) | |
| No | 189 (98.44) | 105 (99.1) | 84 (97.7) | |
| Cerebrovascular disease | .2459 | |||
| Yes | 7 (3.65) | 2 (1.9) | 5 (5.8) | |
| No | 185 (96.35) | 104 (98.1) | 81 (94.2) | |
| Body mass index (kg/m2), mean ± SD (range) | 28.4 ± 5.6 (15.7, 59.8) | 28.1 ± 5.3 (17.8, 57.5) | 28.8 ± 6.0 (15.7, 59.8) | .4251 |
| Center for Epidemiologic Study Depression Scale, mean ± SD (range) | 4.3 ± 5.3 (0, 34) | 3.9 ± 5.2 (0, 30) | 4.9 ± 5.3 (0, 34) | .1919 |
| Smoking status | .7359 | |||
| Current smoker | 13 (6.81) | 6 (5.7) | 7 (8.1) | |
| Former smoker | 91 (47.64) | 52 (49.5) | 39 (45.3) | |
| Nonsmoker | 87 (45.55) | 47 (44.8) | 40 (46.5) | |
| Montreal Cognitive Assessment, mean ± SD (range) | 25.6 ± 2.2 (21, 30) | 25.8 ± 2.30 (21, 30) | 25.5 ± 2.07 (21, 29) | .3689 |
| Vision variables | ||||
| Visual acuity | .053 | |||
| Worse than 20/40 | 3 | 0 | 3 | |
| Better than or equal to 20/40 | 189 | 106 | 83 | |
| Log contrast sensitivity, continuous, binocular, mean ± SD (range) | 1.7 ± 0.1 (1.35, 2.05) | 1.8 ± 0.1 (1.50, 2.05) | 1.6 ± 0.1 (1.35, 1.95) | <0.0001* |
| Log contrast sensitivity, continuous, worse eye, mean ± SD (range) | 1.5 ± 0.2 (0.95, 1.9) | 1.6 ± 0.1 (1.55, 1.9) | 1.4 ± 0.1 (0.95, 1.5) | <.0001* |
| Self-rated eyesight‡ | .0899 | |||
| Fair/poor | 19 (9.9) | 7 (6.6) | 12 (14.0) | |
| Excellent/good | 173 (90.1) | 99 (93.4) | 74 (86.0) | |
| Self-reported cataract | .1330 | |||
| Yes | 142 (74.74) | 74 (70.5) | 68 (80.0) | |
| No | 48 (25.26) | 31 (29.5) | 17 (20.0) | |
| Self-reported glaucoma | .1330 | |||
| Yes | 16 (8.42) | 7 (6.7) | 9 (10.6) | |
| No | 174 (91.58) | 98 (93.3) | 76 (89.4) | |
| Self-reported retinopathy or retinal disease | .0010* | |||
| Yes | 29 (15.59) | 8 (7.8) | 21 (25.3) | |
| No | 157 (84.41) | 95 (92.2) | 62 (74.7) | |
| Self-reported age-related macular degeneration | .0250* | |||
| Yes | 26 (14.13) | 9 (8.9) | 17 (20.5) | |
| No | 158 (85.87) | 92 (91.1) | 66 (79.5) |
Notes: n(%) for categorical variable, mean (SD) for continuous variable, median (Q1–Q3) for skewed continuous variable. SD = standard deviation.
† p Value based on chi2 or Fisher’s exact test for categorical variable, t test for continuous variable.
‡Self-reported “Don’t know” and treated as missing for glaucoma (N = 1), retinopathy (N = 5), age-related macular degeneration (N = 7).
*p < .05.
Supplementary Table 1 presents bivariate analyses of potential sociodemographic and clinical covariates with eSPPB and its components of gait speed, narrow walking speed, chair stand pace, and standing balance time. In terms of potential covariates, only age, hypertension, diabetes mellitus, and BMI were significantly associated across outcomes (p < .05), and so were included in subsequent multivariable models. Since MoCA scores were not significantly associated with study outcomes or LCS, and exploratory adjustment did not impact study findings, it was not retained in final models.
Association of Contrast Sensitivity With Expanded SPPB
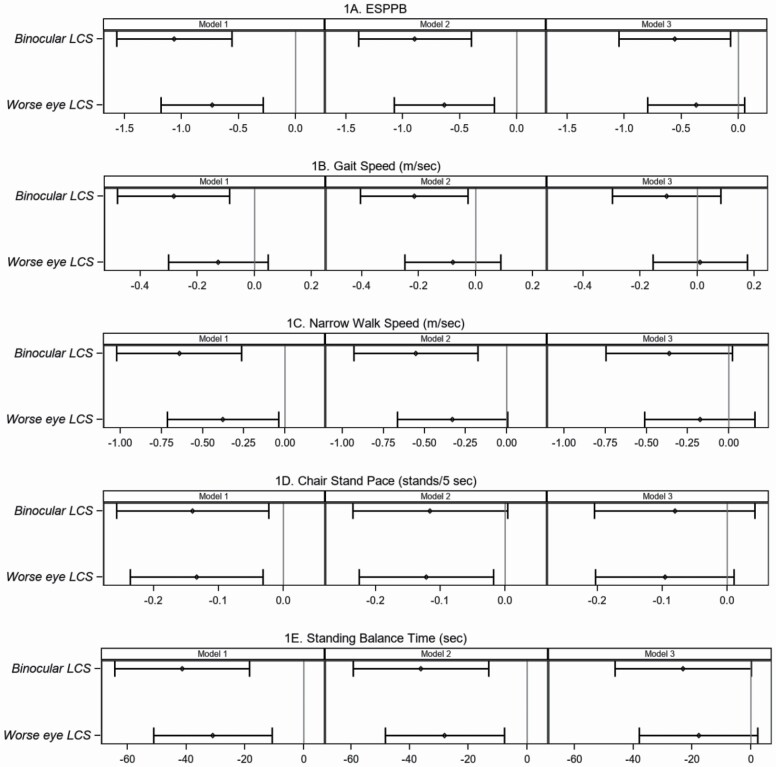
In Table 2, bivariate and multivariable models are presented for the association between LCS and expanded SPPB and its components. Binocular LCS was significantly associated with eSPPB and all components. For example, a loss of 1 unit of LCS (~3 1/3 lines) was associated with an eSPPB that was −1.06 units lower, a −0.28 m/s slower gait speed, −0.64 m/s slower narrow walk speed, −0.14 fewer chair stands per seconds, and −41.41-second shorter balance time. Notably, the decremental relationship between lower LCS and gait speed was twice as great for the narrow walk speed test as compared to the standard 4-M gait speed test. Binocular LCS remained significantly associated with eSPPB after adjusting for BMI, diabetes, hypertension, sex, and age (Model 3); it also remained associated with gait speed, narrow walk speed, and standing balance time adjusting for all covariates except age (Model 2). Similarly, LCS in the worse eye was associated with eSPPB, narrow walk speed, chair stand pace, and balance time, and remained significantly associated after adjusting for all covariates, except age (Figure 1). Similar trends were observed when eSPPB was examined against moderately impaired CS (LCS < 1.55; Supplementary Table2; Supplementary Figure 1).
Table 2.
Multivariable Analyses for the Relationship Between (A) Log Contrast Sensitivity and Expanded SPPB and Its Components—Gait Speed, Narrow Walk, Chair Stand Pace, and Standing Balance; (B) Log Contrast Sensitivity and Postural Sway and Gait Velocity Under Usual and Challenge Conditions
| (A) Relationship Between Log Contrast Sensitivity and Expanded SPPB and Its Components—Gait Speed, Narrow Walk, Chair Stand Pace, and Standing Balance | |||||
|---|---|---|---|---|---|
| Predictor | |||||
| Log Contrast Sensitivity, Worse Eye | Log Contrast Sensitivity, Binocular | ||||
| Outcome | Model† | Raw Beta‡ (95% CI) | p Value | Raw Beta‡ (95% CI) | p Value |
| Expanded SPPB | 1 | −0.73 (−1.18, −0.28) | .0016* | −1.06 (−1.57, −0.56) | <.0001* |
| 2 | −0.63 (−1.07, −0.20) | .0048* | −0.89 (−1.39, −0.40) | .0005* | |
| 3 | −0.37 (−0.80, 0.06) | .0885 | −0.56 (−1.05, −0.07) | .0256* | |
| Gait speed (m/s) | 1 | −0.13 (−0.30, 0.05) | .1537 | −0.28 (−0.48, −0.09) | .0051* |
| 2 | −0.08 (−0.25, 0.09) | .3526 | −0.22 (−0.41, −0.03) | .0261* | |
| 3 | 0.01 (−0.15, 0.18) | .8992 | −0.11 (−0.30, 0.08) | .2700 | |
| Narrow walk speed (m/s) | 1 | −0.38 (−0.71, −0.04) | .0286* | −0.64 (−1.02, −0.26) | .0010* |
| 2 | −0.33 (−0.66, 0.01) | .0535 | −0.55 (−0.93, −0.17) | .0044* | |
| 3 | −0.18 (−0.51, 0.16) | .3007 | −0.36 (−0.75, 0.02) | .0633 | |
| Chair stand pace (stands/s) | 1 | −0.13 (−0.24, −0.03) | .0107* | −0.14 (−0.26, −0.02) | .0197* |
| 2 | −0.12 (−0.23, −0.02) | .0223* | −0.12 (−0.24, 0.00) | .0579 | |
| 3 | −0.10 (−0.20, 0.01) | .0771 | −0.08 (−0.20, 0.04) | .1988 | |
| Standing balance time (s) | 1 | −30.92 (−51.12, −10.71) | .0029* | −41.41 (−64.31, −18.50) | .0005* |
| 2 | −28.07 (−48.39, −7.75) | .0071* | −36.13 (−59.22, −13.04) | .0023* | |
| 3 | −17.83 (−37.97, 2.30) | .0823 | −23.11 (−46.30, 0.09) | .0509 | |
| (B) Relationship Between Log Contrast Sensitivity and Postural Sway and Gait Velocity Under Usual and Challenge Conditions | |||||
| Force plate postural sway 95% ellipse area (inch2)—firm, N = 188 | 1 | 0.35 (0.05, 0.65) | .0229* | 0.31 (−0.03, 0.66) | .0724 |
| 2 | 0.38 (0.08, 0.68) | .0134* | 0.29 (−0.05, 0.63) | .0966 | |
| 3 | 0.35 (0.04, 0.66) | .0267* | 0.24 (−0.11, 0.60) | .1800 | |
| Force plate postural sway 95% ellipse area (inch2)—foam, N = 187 | 1 | 1.07 (0.35, 1.80) | .0039* | 1.05 (0.22, 1.87) | .0130* |
| 2 | 1.08 (0.39, 1.78) | .0025* | 0.86 (0.07, 1.66) | .0335* | |
| 3 | 0.91 (0.19, 1.63) | .0130* | 0.62 (−0.20, 1.44) | .1389 | |
| GAITRite mat, gait velocity, usual pace (m/s), N = 155 | 1 | −0.24 (−0.44, −0.03) | .0227* | −0.39 (−0.63, −0.15) | .0015* |
| 2 | −0.21 (−0.41, −0.01) | .0359* | −0.34 (−0.57, −0.11) | .0042* | |
| 3 | −0.15 (−0.34, 0.05) | .1483 | −0.25 (−0.49, −0.81) | .0429* | |
| GAITRite mat, gait velocity, fast pace (m/s), N=154 | 1 | −0.33 (−0.60, −0.06) | .0178* | −0.58 (−0.90, −0.27) | .0004* |
| 2 | −0.26 (−0.52, 0.00) | .0533 | −0.48 (−0.78, −0.18) | .0021* | |
| 3 | −0.12 (−0.38, 0.13) | .3490 | −0.29 (−0.59, 0.02) | .0624 |
Notes: CI = Confidence interval; SPPB = Short Physical Performance Battery.
†Beta is for decrease in log contrast sensitivity.
‡Model 1: unadjusted bivariate analysis; Model 2: Model 1 + multivariable adjustment for body mass index, sex, hypertension, diabetes mellitus; Model 3: Model 2 + multivariable adjustment for age (10 y).
*p < .05.
Figure 1.
Association of moderately impaired log contrast sensitivity with the expanded short physical performance battery and its components. Association between binocular or worse eye log contrast sensitivity and (A) expanded Short Physical Performance Battery (eSPPB), (B) gait speed (m/s), (C) narrow walk speed (m/s), (D) chair stand pace (chair stands per 5 seconds), (E) Standing balance time (seconds). Left Panel—Model 1 is unadjusted bivariate analysis between binocular or worse eye log contrast sensitivity and each measure of physical function. Middle Panel—Model 2 is multivariable analysis adjusted for sex, body mass index, diabetes mellitus, and hypertension. Right Panel—Model 3 is multivariable analysis adjusted for age (10 years), sex, body mass index, diabetes mellitus, and hypertension. Plots show differences in the beta coefficient (point estimate with 95% confidence intervals [horizontal lines]) in physical function outcomes compared for a 1-unit decrease in log contrast sensitivity.
Association of Contrast Sensitivity With Postural Sway and Gait Velocity Under Simple and Complex Conditions
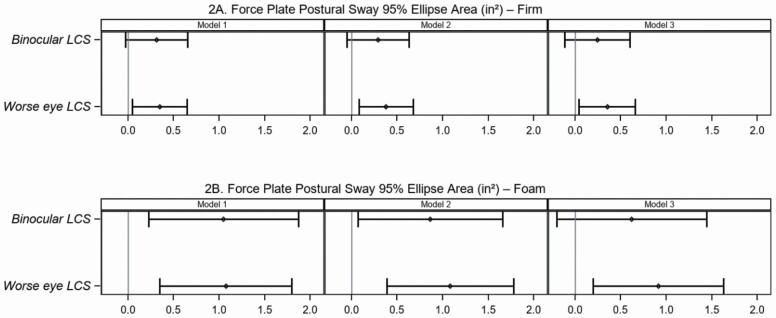
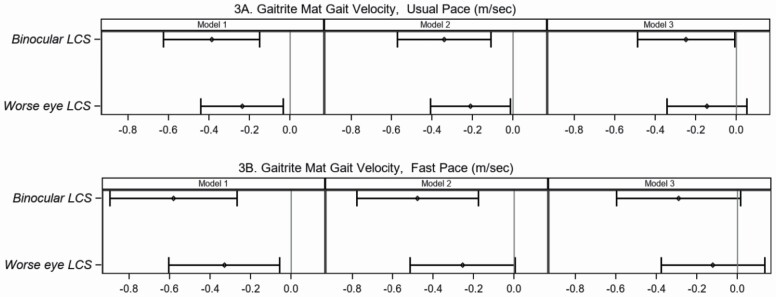
In Table 2, multivariable models are presented for the association of LCS with postural sway, and GAITRite mat measures acquired under simple and more complex conditions. In general, the strength of the association of LCS with postural sway was more pronounced when standing on the foam surface relative to the firm surface (Figure 2; Supplementary Figure 2; Supplementary Table 3). Participants with a 1-unit decline in binocular LCS demonstrated a larger 95% ellipse area, and the strength of this relationship was 3 times greater when standing on the foam (beta 1.05, 95% CI [0.22, 1.87]) versus firm surface (beta 0.31, 95% CI [0.03, 0.66]). Similarly, the association of LCS in the worse eye with postural sway was 3 times greater when standing on the foam surface (beta 1.07, 95% CI [0.35, 1.80]) compared to the firm surface (beta 0.35, 95% CI [0.05, 0.65]), and this association was robust to adjustment for confounders including age. For a 1-unit decline in binocular LCS, there was a greater decremental effect on fast pace (beta −0.58 m/s, 95% CI [−0.90, −0.27]) than usual pace (beta −0.39 m/s, 95% CI [−0.63, −0.15]), which was attenuated after adjusting for all confounders including age (Figure 3). This relationship was less pronounced when using the worse eye LCS variable, or the dichotomous variables (Supplementary Table 3; Supplementary Figure 3).
Figure 2.
Association between binocular or worse eye log contrast sensitivity and postural sway on a firm and foam surface. Association between binocular or worse eye log contrast sensitivity and force plate postural sway 95% ellipse area (inch2) on (A) firm surface or (B) foam surface. Left Panel—Model 1 is unadjusted bivariate analysis between binocular or worse eye log contrast sensitivity and each measure of postural sway. Middle Panel—Model 2 is multivariable analysis adjusted for sex, body mass index, diabetes mellitus, and hypertension. Right Panel—Model 3 is multivariable analysis adjusted for age (10 years), sex, body mass index, diabetes mellitus, and hypertension. Plots show differences in the Beta coefficient (point estimate with 95% confidence intervals [horizontal lines]) in postural sway compared for a 1-unit decrease in log contrast sensitivity.
Figure 3.
Association between binocular or worse eye log contrast sensitivity and gait velocity under usual pace or fast pace conditions. Association between binocular or worse eye log contrast sensitivity and GAITRite mat gait velocity (m/s) at (A) usual pace or (B) fast pace. Left Panel—Model 1 is unadjusted bivariate analysis between binocular or worse eye log contrast sensitivity and each measure of gait velocity. Middle Panel—Model 2 is multivariable analysis adjusted for sex, body mass index, diabetes mellitus, and hypertension. Right Panel—Model 3 is multivariable analysis adjusted for age (10 years), sex, body mass index, diabetes mellitus, and hypertension. Plots show differences in the Beta coefficient (point estimate with 95% confidence intervals [horizontal lines]) in gait velocity compared for a 1-unit decrease in log contrast sensitivity.
Discussion
Understanding risk factors that are present in the very early stages of age-related functional decline and that may also contribute to the progression to disability is a critically important public health goal in our aging population. Older adults who are cognitively intact are an important cohort to consider in this respect when evaluating the relationship between visual and mobility dysfunction. Cognitive reserve may protect against mobility dysfunction (31), and conversely, clinically significant cognitive impairment may be downstream of both visual (7–10) and physical dysfunction (11–14). Moreover, there is evidence to suggest that with aging, the importance of visual perception increases as a compensation for the down weighting of vestibular information (2). In this study, all included participants were cognitively intact at baseline and underwent visual testing. Therefore, the B-NET study provides an ideal cohort to begin to explore whether potentially early biomarkers of visual dysfunction, like CS, are related to mild decrements in physical function independent of cognitive performance. In addition, this analysis is unique in its examination of the relationship of not just binocular, but also worse eye LCS, on balance and gait.
Postural stability has been shown to decline with increasing age (32,33), which may place one at an increased risk of falls (25). Maintaining good postural control is a complex process that incorporates visual, vestibular, and somatosensory information; deficits in any one of these inputs can precipitate postural instability. However, in older adults without overt dysfunction, subtle deficits in balance might only be unmasked by challenges to the system’s functional reserve. One such challenge could be subtle visual dysfunction due to impaired CS in the setting of good visual acuity. In our study, we found that worse LCS was significantly associated with lower extremity physical dysfunction on the eSPPB. Further exploration of the individual tests that contribute to eSPPB demonstrated that lower LCS was associated with significantly shorter standing balance times. The balance test in the expanded SPPB presents increasingly difficult tasks for the participant to complete and is more challenging than the traditional balance test for SPPB. Moreover, the stronger relationship of LCS with gait speed on the narrow walking test compared to the standard 4-M gait test may also be explained by the task’s challenge to balance. Further trials such as standing on a compliant or foam surface can also destabilize postural control (32). To that end, we found that the strength of the decremental association between lower LCS and larger postural sway area increased over threefold when standing on the foam surface compared to the firm surface. Lord and colleagues previously reported an association between postural sway and multiple visual measures including binocular CS, high and low contrast visual acuity, depth perception, and stereopsis, when standing with eyes open on a compliant foam surface, but failed to find any association on a firm surface (34). Similarly, another study demonstrated that older adults exhibited greater postural sway on a foam but not firm surface when exposed to low light levels (35), but did not find a significant association with binocular CS. The fact that we examined the additional challenge of impaired CS in the worse eye CS, which was more prevalent than binocular impairment, may explain why we also detected an association with balance on the firm surface in those with asymmetric visual dysfunction. Such asymmetric visual input may be important to consider when assessing factors that impact balance and that could place older adults at increased fall risk.
Participants with a lower LCS also demonstrated a slower gait speed, with both the narrow walk and the GAITRite mat assessments being more sensitive to dysfunction than the standard gait speed from eSPPB. Gait becomes an increasingly complex cognitive task as one ages with greater reliance on higher order executive function (1). In the Ginkgo Evaluation of Memory Study, deficits in gait speed were shown to predict early cognitive decline, with certain tasks like fast-paced walking being more sensitive to this relationship (11). Fast pace is of particular interest because it challenges an individual’s functional reserve, and may better approximate real-life walking in public. Other groups have also shown that decremental performance in fast walking predicts future cognitive performance (15) and dementia (11). Similarly, moderately impaired binocular CS (LCS < 1.55) has also been shown to predict both future walking limitation (25) and decline in cognitive performance on the Modified Mini-Mental State Examination (3MS) (9). The fact that visual function has been associated with both future cognitive and physical dysfunction, whereas gait dysfunction has been shown to predict cognitive decline, raises the question as to whether such mild impairments in visual function may be an upstream early risk factor for subsequent physical and cognitive decline. In the cognitively intact B-NET population, participants with worse LCS had slower gait speed, and this relationship was stronger when the functional reserve was challenged by requiring a fast pace or narrow gait. Since the B-NET cohort was cognitively healthy at baseline, future investigation should consider whether impaired LCS predicts longitudinal change in gait and cognitive performance and their relationship over time.
A strength of this study is the fact that these associations of CS with mobility and balance were detected in a cohort with good central acuity without substantial self-reported visual dysfunction. Since participants may not have been cognizant of experiencing any visual issues, this suggests that the observed visual decrements were essentially preclinical. Previous studies have demonstrated associations between more advanced VI and either cognition (7–10) or physical performance (5,6,19–21), but in these studies, participants also had poor visual acuity and frequently self-reported experiencing poor visual function. However, CS is associated with more domains of cognitive impairment than visual acuity or stereoacuity (36). In patients with low vision, CS has also been shown to be more important to orientation mobility than visual acuity (37). More recently, we have shown that CS contributes to balance and gait independent of visual acuity, stereoacuity, and self-reported vision in the Health, Aging and Body Composition study, suggesting it is more important to physical function than many other aspects of vision (26). These findings are of particular importance because CS is not currently assessed in clinical practice, yet can be easily and quickly evaluated using a simple and inexpensive eye chart that has been widely validated in multiple cohort studies (9,38,39). In recent years, new computerized assessments of CS are also being developed for iPads, computers, and virtual reality platforms, though these are more expensive and have not been as widely tested for reliability and validity as the Pelli-Robson chart (40). Moreover, in B-NET, we have demonstrated that differences in LCS may be a marker of subtle balance and gait dysfunction in those without cognitive impairment or visual complaints. Such findings may be upstream of more permanent mobility and cognitive dysfunction. Whether LCS can be used to identify cognitively intact older adults at risk of future cognitive or mobility disability shall be considered in future longitudinal follow-ups.
Limitations
A limitation of the study is the small sample size which limited the exploration of different thresholds for LCS impairment. However, moderate impairment (LCS < 1.55) was explored and the general relationships with physical function were similar. The cross-sectional nature of the baseline data set also precludes the assessment of risk. Because the study was not originally designed to investigate the role of vision, best-corrected visual acuity and ophthalmic examination data were not collected. Those with self-report of very severe visual dysfunction were likewise excluded from participation because of key visual tasks central to the main study hypothesis that required good visual acuity to participate. Thus, the prevalence of visual dysfunction and more advanced eye pathology is likely lower than in the general population. Although it is known that age-related eye disease can be responsible for CS impairment, there was a higher prevalence of self-reported cataract than impairment in LCS in this cohort, and the prevalence of other age-related eye diseases was substantially lower than the prevalence of moderate impairment in LCS. Nevertheless, there was an association between self-reported retinal disease and LCS, which may explain some of the etiology of visual dysfunction. The incorporation of diagnostic testing and ophthalmic examination to distinguish underlying pathology versus age-related dysfunction will be important in future work.
Also, the study was restricted to cognitively unimpaired individuals, so it may not reflect performance in adults with dementia or MCI. Although there were some participants with intermediate MoCA scores, they were only included in the study if they did not have MCI or dementia based on a large suite of cognitive tests evaluated by the study neuropsychologist. The primary reason for selecting a lower MoCA cutoff was due to recent literature about MoCA cutoffs in diverse samples. In particular, Sachs et al. showed that nearly 60% of participants in SPRINT who were adjudicated as cognitively normal during 4 years of follow-up had MoCA scores below the traditional 26 cutoff and 29% scored below 23 (41). Moreover, a disproportionate number of participants falling below the 26 cutoff were from communities underrepresented in research, such as Blacks, Hispanics, and those with less than a college degree. For this reason, we based the exclusion of MCI participants on the larger battery of cognitive tests. We also did not adjust for MoCA in the final models as there was no significant association of MoCA with LCS or eSPPB, and exploratory adjustment for MoCA did not impact study findings of the association between LCS and physical performance. The B-NET cohort is also not representative of the population in general and may not be broadly generalizable given the number of inclusion and exclusion criteria applied to these community volunteers. However, this uniquely healthy cohort of older adults enabled a focused examination of the role of CS on very mild gait and balance dysfunction independent of cognitive impairment, which prior studies on visual and physical function have not considered. Exploration of the relationship of CS to longitudinal declines in cognitive and physical function over time will be considered in future investigations.
Conclusion
In conclusion, we have demonstrated that impairment in CS is associated with poor balance and mild gait dysfunction in a cohort of cognitively intact older adults. The association of LCS with balance and gait was more pronounced when participants were required to perform tasks that challenged their functional reserve, either by standing on a foam as opposed to a firm surface, or when walking in a narrow gait or at a fast pace. Impaired CS may be an early marker of impending physical or cognitive dysfunction, and the complex relationship among these 3 conditions should be considered in future longitudinal studies. These results also identify a potential subset of higher functioning older adults for whom specific prevention interventions may be developed to reduce the risk of future disability.
Supplementary Material
Acknowledgments
The authors of this manuscript receive support from the Claude Pepper Older Americans Independence Center. All authors contributed to the design, analysis, or analytic review, and writing or editing of this manuscript.
Contributor Information
Atalie C Thompson, Wake Forest Claude D. Pepper Center, Winston-Salem, North Carolina, USA; Department of Surgical Ophthalmology, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA; Department of Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Haiying Chen, Wake Forest Claude D. Pepper Center, Winston-Salem, North Carolina, USA; Department of Biostatistics, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Michael E Miller, Wake Forest Claude D. Pepper Center, Winston-Salem, North Carolina, USA; Public Health Sciences, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Christopher C Webb, Wake Forest Claude D. Pepper Center, Winston-Salem, North Carolina, USA; Department of Biostatistics, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Jeff D Williamson, Wake Forest Claude D. Pepper Center, Winston-Salem, North Carolina, USA; Department of Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Anthony P Marsh, Wake Forest Claude D. Pepper Center, Winston-Salem, North Carolina, USA; Department of Health and Exercise Science, Wake Forest University, Winston-Salem, North Carolina, USA.
Christina E Hugenschmidt, Wake Forest Claude D. Pepper Center, Winston-Salem, North Carolina, USA; Department of Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Laura D Baker, Department of Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA; Public Health Sciences, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Paul J Laurienti, Wake Forest Claude D. Pepper Center, Winston-Salem, North Carolina, USA; Department of Radiology, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Stephen B Kritchevsky, Wake Forest Claude D. Pepper Center, Winston-Salem, North Carolina, USA; Department of Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Funding
This research was supported by the National Institutes of Health (R01AG052419, 3R01AG052419-02S1), Wake Forest Claude D Pepper Center (P30AG021332), and the Wake Forest Clinical and Translational Science Awards (UL1TR001420). A.C.T. is also supported by the NEI K23EY030897. The sponsor had no role in the design or execution of this study.
Conflict of Interest
A.C.T. is a consultant for Topcon Medical Inc. which had no relationship to this study. The other authors declare no conflict of interest.
References
- 1. Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164(4):541–548. doi: 10.1007/s00221-005-2280-3 [DOI] [PubMed] [Google Scholar]
- 2. Alberts B, Selen LPJ, Medendorp WP. Age-related reweighting of visual and vestibular cues for vertical perception. J Neurophysiol. 2019;121(4):1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lord SR. Visual risk factors for falls in older people. Age Ageing. 2006;35(Suppl 2):ii42–ii45. doi: 10.1093/ageing/afl085 [DOI] [PubMed] [Google Scholar]
- 4. Knudtson MD, Klein BE, Klein R. Biomarkers of aging and falling: the Beaver Dam Eye Study. Arch Gerontol Geriatr. 2009;49(1):22–26. doi: 10.1016/j.archger.2008.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. West SK, Rubin GS, Broman AT, Muñoz B, Bandeen-Roche K, Turano K. How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophthalmol. 2002;120(6):774–780. [DOI] [PubMed] [Google Scholar]
- 6. Klein BE, Klein R, Lee KE, Cruickshanks KJ. Performance-based and self-assessed measures of visual function as related to history of falls, hip fractures, and measured gait time: the Beaver Dam Eye Study. Ophthalmology. 1998;105(1):160–164. doi: 10.1016/s0161-6420(98)91911-x [DOI] [PubMed] [Google Scholar]
- 7. Ehrlich JR, Swenor BK, Zhou Y, Langa KM. The longitudinal association of vision impairment with transitions to cognitive impairment and dementia: findings from the aging, demographics and memory study. J Gerontol A Biol Sci Med Sci. 2021;76(12):2187–2193. doi: 10.1093/gerona/glab157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagarajan N, Assi L, Varadaraj V, et al. Vision impairment and cognitive decline among older adults: a systematic review. BMJ Open. 2022;12(1):e047929. doi: 10.1136/bmjopen-2020-047929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swenor BK, Wang J, Varadaraj V, et al. Vision impairment and cognitive outcomes in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2019;74(9):1454–1460. doi: 10.1093/gerona/gly244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng DD, Swenor BK, Christ SL, West SK, Lam BL, Lee DJ. Longitudinal associations between visual impairment and cognitive functioning: the Salisbury Eye Evaluation Study. JAMA Ophthalmol. 2018;136(9):989–995. doi: 10.1001/jamaophthalmol.2018.2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fitzpatrick AL, Buchanan CK, Nahin RL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62(11):1244–1251. doi: 10.1093/gerona/62.11.1244 [DOI] [PubMed] [Google Scholar]
- 12. Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the Health, Aging and Body Composition Study. Neuroepidemiology. 2007;29(3–4):156–162. doi: 10.1159/000111577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jayakody O, Breslin M, Srikanth VK, Callisaya ML. Gait characteristics and cognitive decline: a longitudinal population-based study. J Alzheimer’s Dis. 2019;71(s1):S5–S14. [DOI] [PubMed] [Google Scholar]
- 14. Chou MY, Nishita Y, Nakagawa T, et al. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019;19(1):186. doi: 10.1186/s12877-019-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deshpande N, Metter EJ, Bandinelli S, Guralnik J, Ferrucci L. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009;38(5):509–514. doi: 10.1093/ageing/afp093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaw FE. Falls in cognitive impairment and dementia. Clin Geriatr Med. 2002;18(2):159–173. doi: 10.1016/s0749-0690(02)00003-4 [DOI] [PubMed] [Google Scholar]
- 17. Allali G, Launay CP, Blumen HM, et al. Falls, cognitive impairment, and gait performance: results from the GOOD initiative. J Am Med Dir Assoc. 2017;18(4):335–340. doi: 10.1016/j.jamda.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel I, Turano KA, Broman AT, Bandeen-Roche K, Muñoz B, West SK. Measures of visual function and percentage of preferred walking speed in older adults: the Salisbury Eye Evaluation Project. Invest Ophthalmol Vis Sci. 2006;47(1):65–71. doi: 10.1167/iovs.05-0582 [DOI] [PubMed] [Google Scholar]
- 19. West SK, Munoz B, Rubin GS, et al. Function and visual impairment in a population-based study of older adults: the SEE Project Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1997;38(1):72–82. [PubMed] [Google Scholar]
- 20. Guo X, Arsiwala LT, Dong Y, et al. Visual function, physical function, and activities of daily living in two aging communities. Transl Vis Sci Technol. 2021;10(14):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swenor BK, Muñoz B, West SK. Does visual impairment affect mobility over time? The Salisbury Eye Evaluation Study. Invest Ophthalmol Vis Sci. 2013;54(12):7683–7690. doi: 10.1167/iovs.13-12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahoney JR, Verghese J. Does cognitive impairment influence visual-somatosensory integration and mobility in older adults? J Gerontol A Biol Sci Med Sci. 2020;75(3):581–588. doi: 10.1093/gerona/glz117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Global Health. 2021;9(2):e144–ee60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haegerstrom-Portnoy G. The Glenn A. Fry Award Lecture 2003: vision in elders—summary of findings of the SKI study. Optom Vis Sci. 2005;82(2):87–93. [DOI] [PubMed] [Google Scholar]
- 25. Swenor BK, Simonsick EM, Ferrucci L, Newman AB, Rubin S, Wilson V, Health, Aging and Body Composition Study. Visual impairment and incident mobility limitations: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2015;63(1):46–54. doi: 10.1111/jgs.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson AC, Miller ME, Webb C, Williamson JD, Kritchevsky SB. Relationship of self-reported and performance-based visual function with performance-based measures of physical function: the Health ABC study. J Gerontol A Biol Sci Med Sci. 2022; glac225. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 28. Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vision Sci. 1988;2:187–199. [Google Scholar]
- 29. Mäntyjärvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. J Cataract Refract Surg. 2001;27(2):261–266. doi: 10.1016/s0886-3350(00)00562-9 [DOI] [PubMed] [Google Scholar]
- 30. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 31. Holtzer R, Zhu X, Rosso AL, Rosano C. Cognitive reserve and risk of mobility impairment in older adults. J Am Geriatr Soc. 2022;70(11):3096–3104. doi: 10.1111/jgs.17979 [DOI] [PubMed] [Google Scholar]
- 32. Hsiao D, Belur P, Myers PS, Earhart GM, Rawson KS. The impact of age, surface characteristics, and dual-tasking on postural sway. Arch Gerontol Geriatr. 2020;87:103973. doi: 10.1016/j.archger.2019.103973 [DOI] [PubMed] [Google Scholar]
- 33. Sullivan EV, Rose J, Rohlfing T, Pfefferbaum A. Postural sway reduction in aging men and women: relation to brain structure, cognitive status, and stabilizing factors. Neurobiol Aging. 2009;30(5):793–807. doi: 10.1016/j.neurobiolaging.2007.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lord SR, Menz HB. Visual contributions to postural stability in older adults. Gerontology. 2000;46(6):306–310. doi: 10.1159/000022182 [DOI] [PubMed] [Google Scholar]
- 35. Dev MK, Wood JM, Black AA. The effect of low light levels on postural stability in older adults with age-related macular degeneration. Ophthalmic Physiol Opt. 2021;41(4):853–863. [DOI] [PubMed] [Google Scholar]
- 36. Varadaraj V, Munoz B, Deal JA, et al. Association of vision impairment with cognitive decline across multiple domains in older adults. JAMA Netw Open. 2021;4(7):e2117416. doi: 10.1001/jamanetworkopen.2021.17416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marron JA, Bailey IL. Visual factors and orientation-mobility performance. Am J Optom Physiol Opt. 1982;59(5):413–426. doi: 10.1097/00006324-198205000-00009 [DOI] [PubMed] [Google Scholar]
- 38. Rubin GS, Bandeen-Roche K, Huang GH, et al. The association of multiple visual impairments with self-reported visual disability: SEE project. Investig Ophthalmol Vis Sci. 2001;42(1):64–72. [PubMed] [Google Scholar]
- 39. Pedula KL, Coleman AL, Hillier TA, et al. Visual acuity, contrast sensitivity, and mortality in older women: study of osteoporotic fractures. J Am Geriatr Soc. 2006;54(12):1871–1877. doi: 10.1111/j.1532-5415.2006.00983.x [DOI] [PubMed] [Google Scholar]
- 40. Pelli DG, Bex P. Measuring contrast sensitivity. Vis Res. 2013;90:10–14. doi: 10.1016/j.visres.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sachs BC, Chelune GJ, Rapp SR, et al. Robust demographically-adjusted normative data for the Montreal Cognitive Assessment (MoCA): results from the systolic blood pressure intervention trial. Clin Neuropsychol. 2022;36(8):2237–2259. doi: 10.1080/13854046.2021.1967450 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.