Abstract
Varicella-zoster virus (VZV) glycoprotein gI is a type 1 transmembrane glycoprotein which is one component of the heterodimeric gE:gI Fc receptor complex. Like VZV gE, VZV gI was phosphorylated in both VZV-infected cells and gI-transfected cells. Preliminary studies demonstrated that a serine 343-proline 344 sequence located within the gI cytoplasmic tail was the most likely phosphorylation site. To determine which protein kinase catalyzed the gI phosphorylation event, we constructed a fusion protein, consisting of glutathione-S-transferase (GST) and the gI cytoplasmic tail, called GST-gI-wt. When this fusion protein was used as a substrate for gI phosphorylation in vitro, the results demonstrated that GST-gI-wt fusion protein was phosphorylated by a representative cyclin-dependent kinase (CDK) called P-TEFb, a homologue of CDK1 (cdc2). When serine 343 within the serine-proline phosphorylation site was replaced with an alanine residue, the level of phosphorylation of the gI fusion protein was greatly reduced. Subsequent experiments with individually immunoprecipitated mammalian CDKs revealed that the VZV gI fusion protein was phosphorylated best by CDK1, to a lesser degree by CDK2, and not at all by CDK6. Transient-transfection assays carried out in the presence of the specific CDK inhibitor roscovitine strongly supported the prior results by demonstrating a marked decrease in gI phosphorylation while gI protein expression was unaffected. Finally, the possibility that VZV gI contained a CDK phosphorylation site in its endodomain was of further interest because its partner, gE, contains a casein kinase II phosphorylation site in its endodomain; prior studies have established that CDK1 can phosphorylate casein kinase II.
Varicella-zoster virus (VZV) is a member of the alphaherpesvirus subfamily, which is characterized by a relatively short replicative cycle as well as the capacity to establish latent infections in neuronal cells (2, 49). McGeoch and Cook have now divided the alphaherpesviruses into two genera: Simplexvirus and Varicellovirus (32). VZV is the prototype of the Varicellovirus genus. Two clinical syndromes have been etiologically related to human VZV infection. They include chicken pox in children following primary viral infection and herpes zoster in adults from reactivation of a latent viral infection in ganglia. In cell culture, VZV is notoriously cell associated; although titers of infectious virus are invariably low, VZV replicates best in cells which are themselves continuing to divide (16). To date, six VZV glycoproteins have been characterized (11, 15). They are gE, gB, gH, gI, gC, and gL, which are named after their herpes simplex virus (HSV) counterparts (7, 49). However, only five of them are membrane associated; VZV gL is a cytoplasmic glycoprotein (11).
VZV gE and gI were formerly called gpI and gpIV, respectively. Both of them are type 1 transmembrane glycoproteins. In virus-infected cells as well as in transiently transfected cells, gE and gI have been shown to associate with each other to form a heterodimer, and this complex behaves as an Fc receptor when it appears on the cell surface (28, 57). A recent study indicates that the amino-terminal end of the extracellular domain of gI is important for association with VZV gE (21). Like many other cell surface receptors, both gE and gI undergo endocytosis in a pattern mimicking the human transferrin receptor; in the presence of gI, the amount of internalized gE is greatly increased (1, 42, 43). Studies with mutant viruses indicate that VZV gE is essential for viral assembly in tissue culture while gI is dispensable. However, both gE and gI are required for the virus to spread cell to cell, and gI is important for the proper cytoplasmic distribution of gE (6, 29).
In addition, VZV gE and gI share another interesting feature of nonviral cell surface receptors: both are phosphorylated (15, 57). Monomeric high-mannose and mature forms of gE are phosphorylated on the endodomain by a serine protein kinase, while an underglycosylated dimeric gE complex is modified by a tyrosine protein kinase (15, 42, 58). A computer-assisted homology search followed by site-directed mutagenesis of the gE cytoplasmic tail defined a prototypic serine-threonine consensus sequence for casein kinase II (CKII) phosphorylation; the authenticity of this site was verified by blotting the attached kinase with CKII-specific antibody (41). In contrast to that of gE, phosphorylation of VZV gI is less well understood. Previous work carried out in our laboratory indicated that a tailless gI mutant was not phosphorylated; although the endodomain does not contain a CKII phosphorylation motif, a serine-proline sequence within the cytoplasmic tail was a potential phosphorylation site (56). Although the protein kinase phosphorylating gI remains to be determined, the fact that a serine-proline sequence is a cyclin-dependent kinase (CDK) consensus phosphorylation site is particularly interesting in light of the highly cell-associated nature of this reclusive herpesvirus.
MATERIALS AND METHODS
Viruses, cells, and antibodies.
The MeWo strain of human melanoma cells is a preferred cell substrate for propagation of VZV (16). HeLa cells were obtained from the American Type Culture Collection and grown in Eagle’s minimum essential medium; human embryonic kidney 293T cells were maintained in Dulbecco modified minimum essential medium (44). Human CEM and Jurkat lymphocyte cells were maintained as described previously (18, 37). The VZV-32 strain was isolated from a child with chicken pox (16). Characterization of the anti-gI monoclonal antibody (MAb) 6B5 has been described previously (28). Rabbit antiserum against VZV gI was prepared in our laboratory by immunizing a rabbit with gI expressed by recombinant vaccinia virus. Antibodies to CDK1, CDK2, and CDK6 were produced as described previously (18).
Labeling of VZV gI with [32P]orthophosphate.
MeWo cells were seeded into a 25-cm2 monolayer and grown to 75% confluence. The cells were inoculated with VZV-32 strain-infected cells at a ratio of 1 infected cell to 10 uninfected cells. When 50% of the cells displayed cytopathic effect, the medium was replaced with fresh medium containing 500 μCi of [32P]orthophosphate/ml (10 mCi/ml; Amersham Life Science). The radiolabeling was continued for an additional 2 days, after which the cells were harvested for preparation of cell lysates in radioimmunoprecipitation assay (RIPA) buffer. Immunoprecipitation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were carried out as described previously (56, 57).
Transfection of HeLa cells with vaccinia-pTM1 plasmids.
The pTM1 plasmid was received from B. Moss, National Institutes of Health, Bethesda, Md. (12, 36). We previously described plasmids pTM1-gI, pTM1-gI-S343A, pTM1-gI-P344A, and pTM1-gI-P345A, as well as the methodology for Lipofectin-mediated transfection of HeLa cells (56). Plasmids pTM1-gI-T322A and pTM1-gI-T322A/S343A were constructed by a recombination PCR method for site-directed mutagenesis as described previously (59). Plasmids pTM1-gI and pTM1-gI-S343A were the templates for PCR mutagenesis. The mutation primer for the sense strand was 5′-CAAAATGCTGCACCAGAATCCGATG-3′, and the mutation primer for the antisense strand was 5′-GATTCTGGTGCAGCATTTTGTATGCC-3′. The nonmutation primer for the sense strand of the beta-lactamase gene was 5′-GGGTGCACGAGTGGGTTACATC-3′, and the nonmutation primer for the antisense strand of the beta-lactamase gene was 5′-GATGTAACCCACTCGTGCACCCAACTGAT-3′. After mutagenesis, the resulting plasmids, pTM1-gI-T322A and pTM1-gI-T322A/S343A, were subjected to DNA sequencing through the mutation; both exhibited a single-amino-acid change from threonine to alanine at position 322.
To transfect HeLa cells with pTM1-based recombinant plasmids, 6.2 × 105 HeLa cells were seeded into each well of a six-well tissue culture plate 16 h before transfection. The cells were then washed twice with 5% fetal bovine serum containing Eagle’s essential medium. The washed cells were subsequently infected with 2.5 × 107 PFU of recombinant vaccinia virus encoding bacterial phage T7 RNA polymerase (12, 36). After a 30-min incubation at 37°C, the virus was removed and the cells were washed once. After the addition of 1 ml of medium into each well, 30 μl of DNA-Lipofectin (Life Technologies) mixture was added to each monolayer. The DNA-Lipofectin mixture was prepared by mixing 4 μg of DNA in 15 μl of water with 15 μl of 30% Lipofectin. The cultures were incubated at 37°C for 6 h, after which the cells were labeled for 16 h with 35S-Promix ([35S]methionine and [35S]cysteine; >1,000 Ci/mmol) (Amersham) or [32P]orthophosphate.
Construction of plasmids pCDNA-gI and pCDNA-gI-S343A.
Recombinant plasmid pCDNA-gI was constructed by inserting the VZV gI gene from plasmid pTM1-gI into a eukaryotic expression vector, pCDNA3 (Invitrogen). Briefly, two primers were synthesized. The primer sequence corresponding to the 5′ end of the gI coding region was 5′-GCCGGATCCACGATGGTTTTAATCCAATGTTTG-3′. A restriction site, BamHI (underlined), was added to the 5′ end of the primer. A minor change was also made in the coding region by replacing nucleotide T with G at the plus-4 position of the open reading frame as well as substituting A for G at position minus 3 of the 5′ noncoding region so that the translation signal was the same as the consensus sequence defined by Kozak (22). The primer sequence corresponding to the 3′ end of the coding region was: 5′-GCGCGCTCGAGCTATTTAACAAACGGGTTTACAAC-3′. A restriction site, XhoI (underlined in the primer sequence), was added to the 5′ end of the primer. The gene coding for VZV gI was amplified from pTM1-gI with the above-mentioned two primers. After the amplified gI gene was inserted into vector pCDNA3 at restriction sites BamHI and XhoI, the resulting recombinant plasmid was designated pCDNA-gI. Recombinant plasmid pCDNA-gI-S343A was similarly constructed, except that the pTM1-gI-S343A plasmid was used as a template from which to amplify the mutant gene gI-S343A.
Transfection of a human embryonic kidney cell line.
293T cells were transfected with pCDNA-gI or pCDNA-gI-S343A DNA by either a CaCl2-HBS or a Lipofectin transfection method (50, 56). Briefly, 293T cells were subcultured the day before transfection into 60-mm-diameter culture dishes. At the time of transfection, the 293T cell monolayers were 30 to 40% confluent. About 30 min before transfection, the DNA-CaCl solution was prepared by first mixing 10 μg of plasmid DNA in 25 μl of water; the 25-μl volume was added to 500 μl of 2× HBS (25 mM Hepes, 140 mM NaCl, 5 mM KCl, 0.75 mM Na2HPO4 [pH 7.05], 6 mM dextrose). Thereafter, 475 μl of 250 mM CaCl2 was added to the 525-μl volume. The 1-ml DNA suspension was added to the monolayer in a dropwise fashion, and the transfected cells were incubated at 37°C. Alternatively, 293T cells were transfected by a Lipofectin-mediated method as suggested by the manufacturer for the study of phosphorylation. At 6 h posttransfection, the cells can be labeled with [32P]orthophosphate or 35S-Promix. At 48 h posttransfection, the transfected cells were examined by confocal microscopy or harvested for preparation of cell lysates for an immunoprecipitation assay.
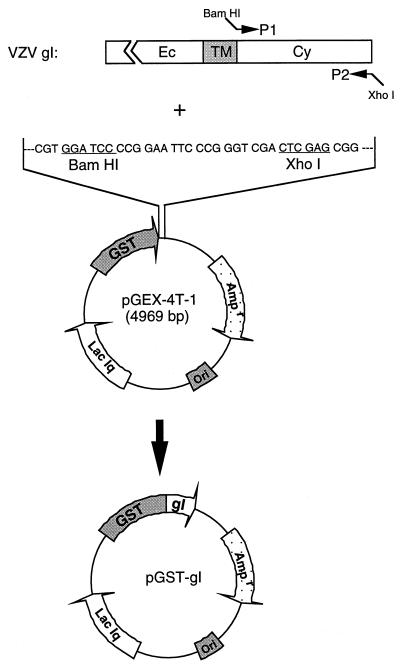
Construction of recombinant plasmid GST-gI fusion protein.
A recombinant plasmid expressing the glutathione-S-transferase (GST)-gI fusion protein was constructed by amplifying the cytoplasmic tail of the gI gene in pTM1-gI and inserting the DNA fragment into vector pGEX-4T-1 (Pharmacia) at BamHI and XhoI sites (see Fig. 6). Briefly, two primers were synthesized to amplify the desired portion of the gI gene. The primer sequence corresponding to the amino end of the cytoplasmic tail of gI protein is 5′-GGCCGGGATCCATAAGCGTTAAGCGACGTAGA-3′. A restriction site, BamHI (underlined in the sequence), was introduced at the 5′ end of the primer. The primer corresponding to the carboxy-terminal end of the gI protein is 5′-GCGCGCTCGAGCTATTTAACAAACGGGTTTACAAC-3′. A restriction site, XhoI (underlined in the sequence), was inserted in the primer. With the above-mentioned two primers, the DNA fragment corresponding to the cytoplasmic tail of the VZV gI gene was amplified and inserted into expression vector pGEX-4T-1 at restriction sites BamHI and XhoI; the recombinant plasmid was designated pGST-gI-wt. Plasmid pGST-gI-S343A was similarly constructed, except that pTM1-gI-S343A was used as a template to amplify the DNA fragment coding for the cytoplasmic tail of the gI protein.
FIG. 6.
Construction of a recombinant plasmid expressing GST-gI fusion protein. The DNA sequence encoding the cytoplasmic tail of VZV gI was amplified by PCR technology with primers P1 and P2. A BamHI site was inserted into the 5′ end of primer P1, and a XhoI site was inserted into the 5′ end of primer P2. The amplified DNA fragment was inserted into vector pGEX-4T-1 at compatible sites: BamHI and XhoI sites (underlined). The resulting recombinant plasmid, pGST-gI, encodes a fusion protein consisting of GST with the gI cytoplasmic tail.
After recombinant plasmids pGST-gI-wt and pGST-gI-S343A were constructed, they were subsequently transformed into Escherichia coli BL21/DE3. Positive clones were verified by a PCR method. To induce the fusion protein, one colony was picked and inoculated into 5 ml of Circlegrow medium (Bio101). The cells were incubated at 30°C overnight. The next day, 2 ml of the liquid culture was transferred to a 100-ml container of Circlegrow medium and incubated for an additional 4 h at 30°C. When the optical density at 600 nm reached 1.0, 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to the cells. The cells were pelleted after 3 h of induction and were subsequently lysed by a sonication method. The fusion protein in the lysate was purified by a glutathione-Sepharose 4B affinity column (Pharmacia) under conditions suggested by the manufacturer. Verification of the authenticity of the gI fusion protein was performed by immunoblotting procedures described in this laboratory (28).
Kinase assay with P-TEFb.
The kinase P-TEFb was purified as described by Marshall et al. (30, 31). To perform the kinase assay, 100 μl of bacterial cell lysate containing GST-gI fusion protein was incubated with 20 μl of 50% glutathione-Sepharose 4B beads at 4°C for 30 min. The beads were washed three times with 300 μl of STE buffer (100 mM NaCl, 10 mM Tris-HCl [pH 8.0], 1 mM EDTA) and once with kinase buffer (100 mM Tris-acetate [pH 7.5], 10 mM magnesium acetate). The kinase assay was continued by adding 78 μl of kinase buffer, 1 μl of 28 mM P-TEFb, and 10 μCi of [γ-32P]ATP. After the reaction had proceeded at 30°C for 30 min, the beads were washed four times with 500 μl of STE buffer. Then, 100 μl of loading buffer (0.125 M Tris [pH 6.8], 6% SDS, 20% glycerol, 0.5% bromophenol blue) was added to the beads, and the beads were boiled in a water bath for 5 min. The protein was resolved by 12 to 18% gradient SDS-PAGE.
Immunoprecipitation kinase assay with CDKs.
The coupled immunoprecipitation kinase assays were performed similarly to those previously described (20, 46). Briefly, CEM or Jurkat cell lysates containing 300 μg of total protein were immunoprecipitated for 16 h with rabbit serum against CDK1, CDK2, or CDK6 and incubated for 3 h with protein A-Sepharose beads (Sigma) (20). Peptide competition was performed by preincubating the rabbit antiserum for 10 min with the peptide against which it was raised (46). Subsequently, the kinase assays were performed with histone H1 (Boehringer Mannheim) or GST fusion proteins employed as the substrate (46). The phosphorylated substrates were resolved by SDS-PAGE and analyzed with a phosphorimager; subsequently, the gels were exposed to film.
Roscovitine treatment of transfected cells.
HeLa cell monolayers in six-well plates were transfected with 4 μg of pTM1 or pTM1-gI DNA as described above. After transfection had proceeded for 6 h, the cells were either left untreated or treated with 15 or 30 μM roscovitine (Calbiochem) in medium containing [32P]orthophosphate or 35S-Promix. The monolayers were lysed 16 h later with RIPA buffer containing 50 mM NaF. Immunoprecipitation of gI and autoradiography were carried out as previously described.
Confocal microscopy.
Laser scanning confocal microscopy of transfected cells was carried out by methods described in an earlier publication by Duus and Grose (11). The Bio-Rad 1024 confocal microscope is located in the Central Microscopy Research Facility of the University of Iowa.
RESULTS
Phosphorylation of VZV gI in infected and transfected cells.
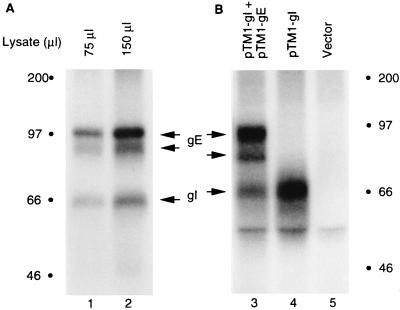
To demonstrate the fact that VZV gI was phosphorylated, MeWo cells were infected with strain VZV-32 and then labeled metabolically with [32P]orthophosphate. After lysis of the infected cells with RIPA buffer, aliquots of the cell lysate were incubated with MAb 6B5 to immunoprecipitate VZV gI. When immunoprecipitated gI was subjected to SDS-PAGE and autoradiography, a radioactive protein corresponding to VZV gI was detected (Fig. 1A, lanes 1 and 2). As has been reported, VZV gE, which migrates as two broad bands between 83 and 98 kDa, coprecipitated with gI (57). Because the VZV genome encodes putative protein kinases, we wished to determine whether gI was phosphorylated in the absence of other VZV proteins. For this experiment, we investigated the extent of VZV gI phosphorylation after subcloning the VZV gI gene into a eukaryotic expression vector, pTM1, resulting in a recombinant plasmid, pTM1-gI (56). Thereafter, the pTM1-gI plasmid was transfected into HeLa cells which were preinfected with a recombinant vaccinia virus expressing bacteriophage T7 RNA polymerase. The transfected cells were labeled with [32P]orthophosphate, and VZV gI was immunoprecipitated with MAb 6B5. As in VZV-infected cells, VZV gI was phosphorylated (Fig. 1B, lane 4). When HeLa cells were cotransfected with pTM1-gI and pTM1-gE, both gI and gE were phosphorylated (Fig. 1B, lane 3). In addition, as in infected cells, gE was coprecipitated with VZV gI, a result which confirmed that VZV gI associated with gE in transfected cells.
FIG. 1.
Phosphorylation of gI in VZV-infected cells and in transfected cells. (A) MeWo cells were infected with VZV and labeled with [32P]orthophosphate. After lysis of the cells with RIPA buffer, the indicated amounts of cell lysate were incubated with MAb 6B5 and subsequently with protein A-Sepharose CL-4B beads. The precipitates were resolved by SDS-PAGE (12% acrylamide) and subjected to autoradiography. (B) HeLa cells were transfected with pTM1 vector alone (lane 5) or pTM1-gI alone (lane 4) or cotransfected with pTM1-gI and pTM1-gE (lane 3) and labeled with [32P]orthophosphate. Immunoprecipitation of gI was performed as described for panel A. Proteins corresponding to VZV gE and gI are indicated with arrows; molecular mass markers are indicated by solid circles.
Phosphorylation of VZV gI by a cellular kinase.
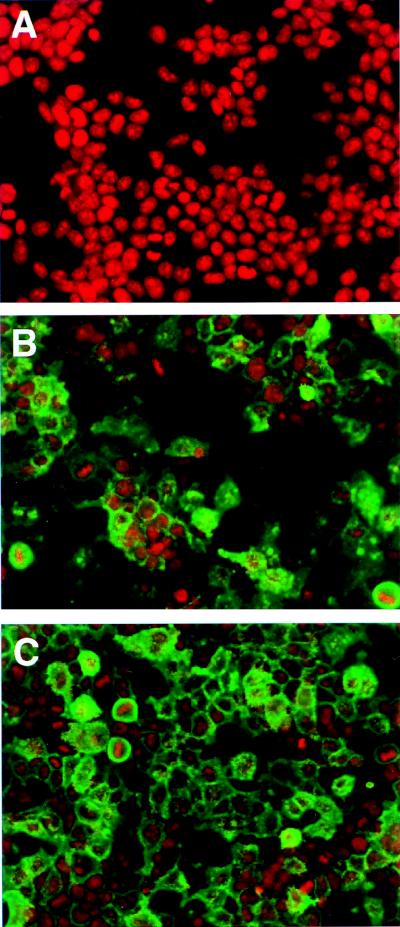
Since the phosphorylation studies of VZV gI shown in Fig. 1 were conducted in either VZV-infected cells or vaccinia virus-infected and transfected cells, it remained to be determined whether gI was phosphorylated by a cellular kinase or possibly by a kinase originating from vaccinia virus (3, 24, 39, 53). To circumvent the obstacles concerning the origin of the kinase, we subcloned the gI gene from the pTM1-gI recombinant plasmid into another eukaryotic expression vector, pCDNA3; the resultant plasmid was called pCDNA-gI. To assure a high expression of gI protein in transfected cells, we made minor modifications in the nucleotide sequence around the initiation methionine codon ATG (underlined). The modification resulted in a change in nucleotide sequence from GCGATGT to ACGATGG, the latter being a consensus sequence required for translation signaling as defined by Kozak (22). This modification led to a change of a phenylalanine at the second position, the amino acid immediately after the first methionine, into a valine. Since this amino acid is located within the deduced signal sequence which will be cleaved in the endoplasmic reticulum, the mature VZV gI protein encoded by the modified gene should not differ in sequence from the product of the wild-type gene (7, 21). We transfected the plasmid pCDNA-gI into human 293T cells and examined gI protein expression by laser scanning confocal microscopy. As shown in Fig. 2, at least 70% of the cells expressed wild-type gI (compare Fig. 2A and B). The majority of the gI-transfected cells had high levels of fluorescence staining, demonstrating that the gI protein was expressed abundantly in 293T cells. At the same time, we compared the expression of wild-type VZV gI in 293T cells with that in HeLa cells, the substrate in which many previous experiments had been carried out (Fig. 3A). The degrees of wild-type gI expression in both transfection systems were similar. This comparison was performed, in part, in order to allay concerns that the pTM1 expression system may not be an accurate indicator of the usual gI trafficking patterns.
FIG. 2.
Expression of VZV gI in 293T cells. Human 293T cells were transfected with pCDNA3 (A), pCDNA-gI (B), or pCDNA-gI-S343A (C). Immunolabeling was carried out with MAb 6B5 against VZV gI; the secondary antibody was an Oregon green-conjugated sheep anti-mouse reagent. Subsequently the cells were examined with a laser scanning confocal microscope at ×20 magnification. Green represents expressed gI, and red designates nuclei stained with ethidium bromide.
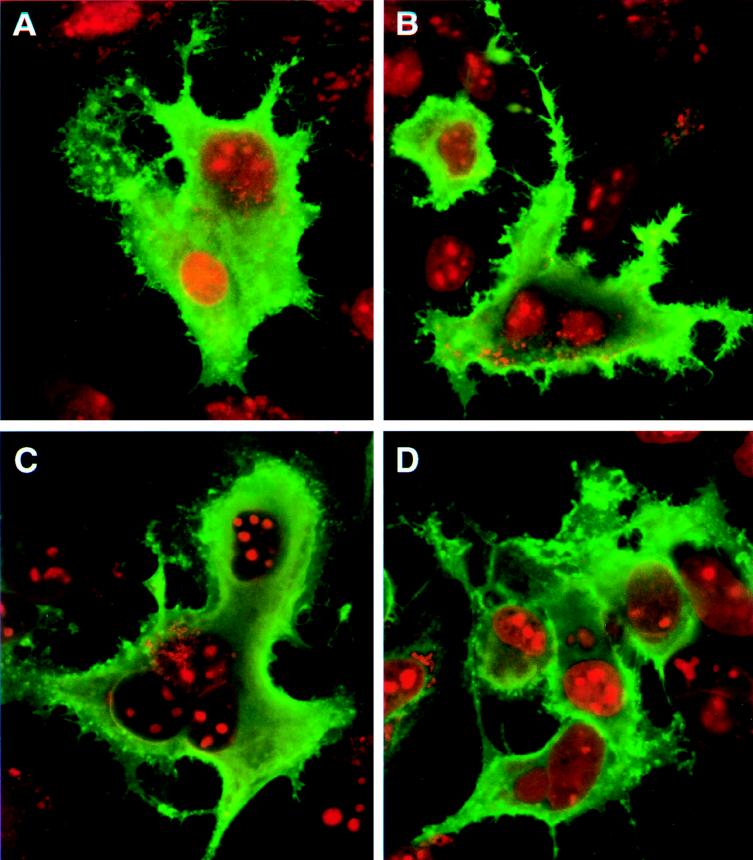
FIG. 3.
Cytoplasmic expression of wild-type and mutant VZV gI proteins. HeLa cells were transfected with plasmids pTM1-gI (A), pTM1-gI-S343A (B), pTM1-gI-P344A (C), and pTM1-gI-P345A (D). Immunolabeling was performed with MAb 6B5 and Oregon green-tagged sheep anti-mouse antibody. The cells were examined by a confocal microscope at ×60 magnification. Green indicates gI, while the nuclei are stained red with ethidium bromide. Cells transfected with vector alone had no green staining (data not shown; see Fig. 2A).
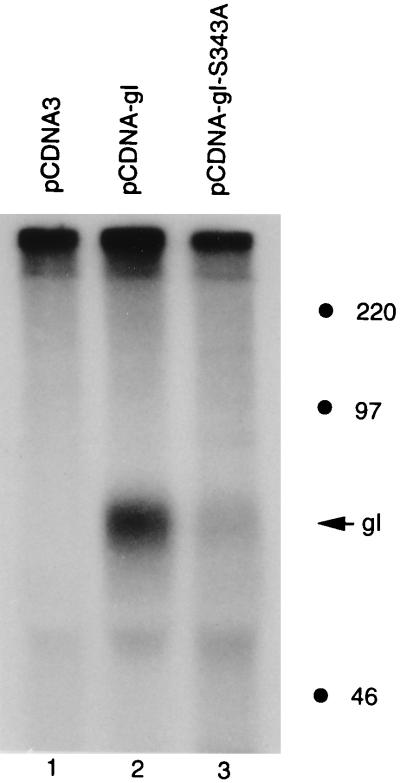
With the success of this alternative transfection system, we then investigated whether gI was phosphorylated in 293T cells. After 293T cells were transfected with pCDNA-gI, they were labeled with [32P]orthophosphate. At 48 h posttransfection, the cultures were harvested and analyzed by an immunoprecipitation assay followed by autoradiography. As shown in Fig. 4, a phosphoprotein band corresponding to VZV gI was detected (lane 2), a result which indicated that VZV gI was phosphorylated in 293T cells. There was no phosphorylation of the vector alone (lane 1). Since VZV gI was phosphorylated in the absence of VZV or vaccinia virus infection, the viral protein was obviously phosphorylated by a cellular kinase in 293T cells.
FIG. 4.
Phosphorylation of VZV gI by a cellular kinase. Human 293T cells were transfected with plasmids pCDNA3 (lane 1), pCDNA-gI (lane 2), or pCDNA-gI-S343A (lane 3). The cells were labeled with [32P]orthophosphate and harvested with RIPA buffer. VZV gI was immunoprecipitated with MAb 6B5 and analyzed by SDS-PAGE (12 to 18% gradient acrylamide) followed by autoradiography. The migration of gI is indicated with an arrow. Molecular mass markers are on the right.
Serine-proline consensus phosphorylation sequence.
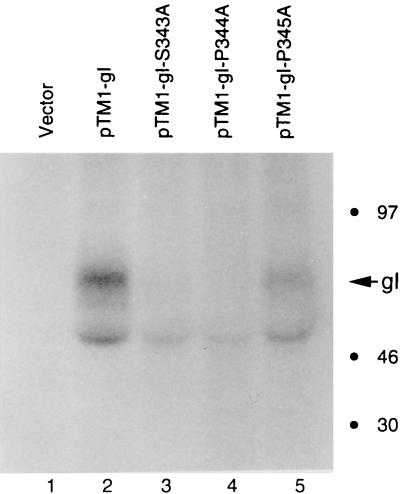
Previously, some mutations were made within the gI gene in the pTM1-gI recombinant plasmid (56). These mutations had never been examined under the sensitive laser scanning confocal microscope to compare expression. Therefore, three mutant gI recombinant plasmids, pTM1-gI-S343A, pTM1-gI-P344A, and pTM1-gI-P345A, were transfected into HeLa cells and observed by confocal microscopy after permeabilization (Fig. 3). Levels of expression appeared comparable for the wild-type gI (Fig. 3A) and all three mutant proteins (gI-S343A [Fig. 3B], gI-P344A [Fig. 3C], and gI-P345A [Fig. 3D]). When examined by optical tomography, all of them were similarly distributed in the cytoplasm and all exhibited similar fluorescence labeling on the membrane. Next, the same transfection experiment was repeated, but the cultures were labeled metabolically with [32P]orthophosphate. After immunoprecipitation and autoradiography, the phosphorylation status of each mutant gI protein was compared with that of wild-type gI. As shown in Fig. 5, the phosphorylation signals of all mutant gI proteins (lanes 3, 4, and 5) were much lower than that of the wild-type (lane 2); there was no phosphorylation of vector alone (lane 1). Furthermore, a difference in signal reduction was calculated based on phosphorimaging analysis; i.e., phosphorylation signals of gI-S343A and gI-P344A were about 10% of that seen with wild-type gI, while that of gI-P345A was about 30% of that of the wild type. These results indicated that both serine residue 343 and proline residue 344 were essential for the phosphorylation of gI. In contrast, proline 345 modulated, but did not abrogate, the phosphorylation of VZV gI. In other words, the phosphorylation site within gI consisted primarily of the serine 343-proline 344 sequence.
FIG. 5.
Effect of mutagenesis of serine 343 and proline 344 on the phosphorylation of VZV gI. HeLa cells were transfected with plasmids pTM1 (lane 1), pTM1-gI (lane 2), pTM1-gI-S343A (lane 3), pTM1-gI-P344A (lane 4), and pTM1-gI-P345A (lane 5). The transfected cells were labeled with [32P]orthophosphate. VZV gI was immunoprecipitated with MAb 6B5 and analyzed by SDS-PAGE (12% acrylamide) followed by autoradiography. VZV gI is designated with an arrow. Molecular mass markers are on the right.
Even though VZV gI was phosphorylated both in vaccinia virus-infected HeLa cells and in transfected 293T cells, there was a possibility that gI was phosphorylated at one site in HeLa cells and at a second site in non-vaccinia virus-infected 293T cells. To exclude this possibility, we subcloned the gI-S343A mutant gene into the pCDNA3 vector; the new construct was called pCDNA-gI-S343A. As with each new construct described previously, expression of the mutant gI protein was analyzed first by confocal microscopy (Fig. 2C). We next transfected both wild-type pCDNA-gI and mutant pCDNA-gI-S343A into 293T cells and metabolically labeled the cultures with either [32P]orthophosphate or 35S-Promix. When serine 343 was replaced with an alanine, the phosphorylation signal was greatly reduced compared with that of wild-type gI. This reduction of phosphorylation was not the result of decreased protein production, because the expression levels in wild-type gI and mutant gI-S343A were similar, as shown by optical tomography (Fig. 2B and C) and in 35S-Promix-labeled protein gels (data not shown). These results were most consistent with the interpretation that the serine 343-proline 344 sequence was essential for the phosphorylation of VZV gI.
Phosphorylation of VZV gI-GST fusion proteins.
After the phosphorylation site was localized and the cellular origin of the kinase was determined, our next aim was to identify the nature of the cellular kinase phosphorylating VZV gI protein at serine 343-proline 344. An in vitro kinase assay was required for this purpose. To obtain the large amount of substrate required for individual protein kinase assays, we constructed GST-gI fusion proteins. Since the phosphorylation site within gI is located at the cytoplasmic tail, we amplified the DNA fragment coding for the cytoplasmic tail of gI. The resulting DNA fragment was inserted into an expression vector, pGEX-4T-1, at compatible sites (Fig. 6). Two fusion proteins were constructed, GST-gI-wt and GST-gI-S343A. GST-gI-wt is a fusion protein of GST and the cytoplasmic tail of wild-type gI, and GST-gI-S343A is a fusion protein of GST and the cytoplasmic tail of mutant gI-S343A.
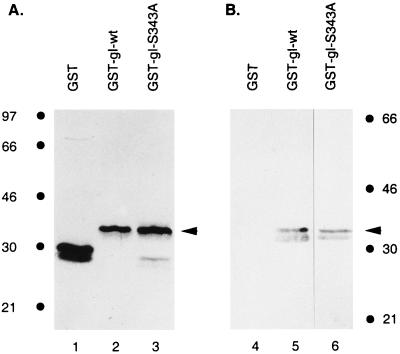
Coomassie brilliant blue staining of an acrylamide gel demonstrated that the purified proteins were the correct size: both GST-gI and GST-gI-S343A migrated at a molecular mass of 33 kDa (Fig. 7A, lanes 2 and 3). To verify that GST and the cytoplasmic tail of gI were fused in frame, we performed Western blot analysis with rabbit antiserum against gI. Protein bands corresponding to GST-gI-wt in a Coomassie blue-stained gel reacted with the anti-gI antibody (Fig. 7B, lane 5). In a separate experiment, immunoblotting was also performed with GST-gI-S343A and the result was similarly positive (Fig. 7B, lane 6). These findings indicated that both the wild-type fusion protein GST-gI-wt and the mutant fusion protein GST-gI-S343A were correctly constructed.
FIG. 7.
Analysis of the fusion proteins GST-gI-wt and GST-gI-S343A. Purified GST (lanes 1 and 4), GST-gI-wt (lanes 2 and 5), and pGST-gI-S343A (lanes 3 and 6) were stained with Coomassie brilliant blue (A) or analyzed by immunoblotting with a rabbit serum against gI (B). The arrowheads designate the GST-gI fusion proteins. Molecular mass markers are on the right and left.
Phosphorylation of VZV gI by a Drosophila kinase homologous to CDK1.
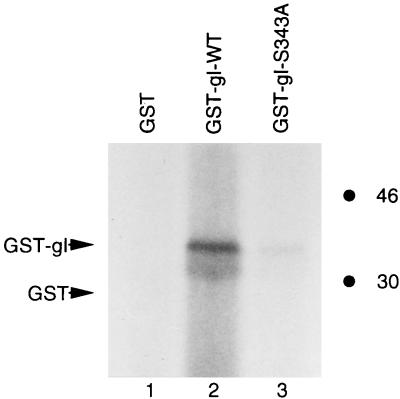
After fusion proteins containing GST and the cytoplasmic tail of gI were successfully expressed, we determined whether they were phosphorylated by a cellular kinase. Since the putative phosphorylation site within VZV gI is identical to a consensus sequence phosphorylated by CDKs, we examined the ability of a CDK to phosphorylate the target site within gI when it is provided in the form of GST-gI-wt fusion protein. Recently, genetically similar Drosophila and human kinases homologous to CDK1 (cdc2) were identified and partially characterized (30, 31, 45, 60). They were initially named positive transcription elongation factor b (P-TEFb) but are now called CDK9 and cyclin T complex. Since this kinase is highly homologous to other CDKs (see Discussion), it was a potentially valuable reagent to examine phosphorylation of the target site in the GST-gI-wt fusion protein. When a kinase assay was performed with purified P-TEFb, the results demonstrated that GST-gI-wt was phosphorylated (Fig. 8, lane 2) while the vector alone was not phosphorylated (Fig. 8, lane 1). When the mutant fusion protein GST-gI-S343A was similarly tested, the phosphorylation signal was 30% that of wild-type GST-gI-wt fusion protein (Fig. 8, compare lane 2 with lane 3). These results indicated that the serine 343 residue within GST-gI-wt strongly influenced phosphorylation by the P-TEFb kinase.
FIG. 8.
Phosphorylation of GST-gI-wt by a Drosophila CDK homologue. Drosophila P-TEFb is a homologue of the mammalian CDK which has been recently designated CDK9. GST (lane 1), GST-gI-wt (lane 2), and GST-gI-S343A (lane 3) were purified with glutathione-Sepharose 4B beads. The kinase assay was performed as described in Materials and Methods, and the phosphorylated proteins were identified by autoradiography. The positions of GST and GST-gI fusion proteins are indicated with arrows. The biological substrate of CDK9 is the carboxy-terminal domain of the largest subunit of RNA polymerase II; its phosphorylation is shown in reference 45. Molecular mass markers are on the right.
Phosphorylation of fusion protein GST-gI-wt by CDK1 and CDK2.
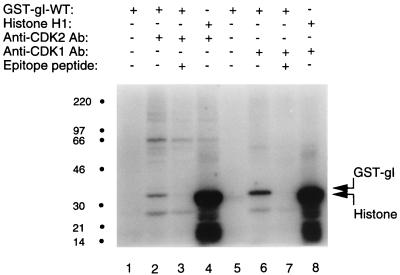
Since the catalytic domain of Drosophila P-TEFb has the highest sequence homology with CDK1 and CDK2, we next investigated whether GST-gI-wt was phosphorylated by these two human CDKs. To perform this experiment, CDK1 and CDK2 were individually precipitated from a lysed leukemia cell line, CEM, with rabbit serum directed against an epitope of CDK1 or CDK2. These kinase preparations were then added to in vitro kinase assays. Figure 9 illustrates the results of the kinase assays for both CDK2 (lanes 1 to 4) and CDK1 (lanes 5 to 8). As shown in lanes 2 and 6, respectively, the GST-gI-wt protein was phosphorylated by both CDK2 and CDK1. In the negative controls lacking kinases in the reaction mixtures, no phosphorylation signals were detected (lanes 1 and 5). In the positive controls in lanes 4 and 8, respectively, histone H1 was strongly phosphorylated by both CDK2 and CDK1.
FIG. 9.
Phosphorylation of the fusion protein GST-gI by CDK1 and CDK2. The coupled immunoprecipitation kinase assay was performed as described in Materials and Methods. The constituents in each assay are listed in tabular format above each lane at the top of the figure (+, present; −, absent). CDK2 was present in lanes 2 to 4; CDK1 was present in lanes 6 to 8. Histone H1 was the artificial substrate for the positive-control assays in lanes 4 and 8. The negative-control lanes 1 and 5 contain only GST-gI protein. For the competition assay, antibodies against CDK2 or CDK1 were preincubated for 10 min with an epitope peptide of CDK2 (lane 3) or CDK1 (lane 7). The locations of GST-gI and histone are indicated in the right margin. Molecular mass markers are on the left.
There was a possibility that a kinase other than a CDK was present in the immunoprecipitate and phosphorylated GST-gI-wt. To exclude this possibility, a competition assay was performed, in which the anti-CDK2 or anti-CDK1 antibody was incubated with CDK2 or CDK1 epitope peptides for 10 min. These antigen-antibody mixtures were subsequently incubated with CEM cell lysate as before. Each resulting immunoprecipitate was tested for its ability to phosphorylate GST-gI-wt. As shown in Fig. 9, lane 3, preincubation of the anti-CDK2 antibody with the epitope peptide used to generate the antibody led to suppression in the competition assay and eliminated the phosphorylation of GST-gI-wt. Similarly, as shown in Fig. 9, lane 7, preincubation of the anti-CDK1 antibody with the epitope peptide used to generate the antibody led to suppression in the competition assay and abrogated the phosphorylation of GST-gI-wt. Thus, the competition assays reinforced the specificity of the CDK in vitro phosphorylation assays.
Preferential phosphorylation of the serine 343-proline 344 sequence.
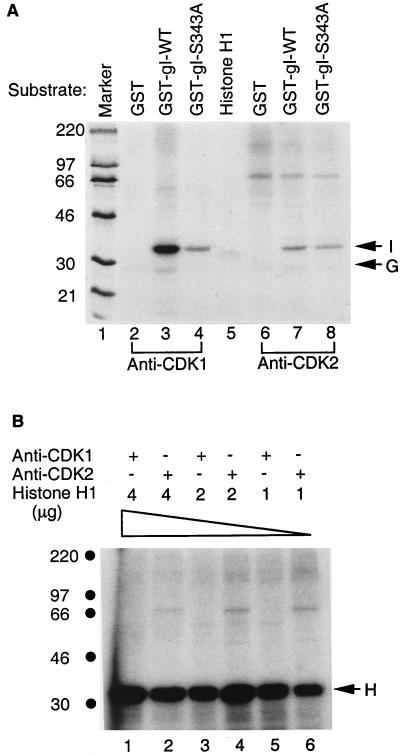
In vitro kinase assays demonstrated that the serine 343 residue strongly influenced phosphorylation by the P-TEFb kinase. To investigate whether the same serine residue affected phosphorylation by CDK1, kinase assays with CDK1 were performed with substrates GST, GST-gI-wt, and GST-gI-S343A. Similarly to transfected cells, GST-gI-wt was phosphorylated by CDK1, while GST itself was not phosphorylated (Fig. 10A, lanes 2 and 3). Although GST-gI-S343A was also phosphorylated, the phosphorylation signal of GST-gI-wt was fivefold higher than that of GST-gI-S343A when the radioactivities of the two proteins were measured quantitatively (Fig. 10A, lanes 3 and 4). Again, this result suggested that GST-gI-wt was phosphorylated by CDK1 mainly at the serine 343-proline 344 sequence. To determine whether wild-type gI was also phosphorylated by CDK2, we performed a similar kinase assay with GST, GST-gI-wt, and GST-gI-S343A (Fig. 10A, lanes 6 to 8). As can be seen, the phosphorylation signal of GST-gI-wt was about twofold higher than that of GST-gI-S343A (Fig. 10A, lanes 7 and 8).
FIG. 10.
Phosphorylation of GST-gI-wt and mutant proteins. (A) Kinase assays were performed as described for Fig. 9. CDK1 is the kinase in lanes 2 to 4, and CDK2 is the kinase in lanes 6 to 8; the substrates include GST in lanes 2 and 6, GST-gI-wt in lanes 3 and 7, and GST-gI-S343A in lanes 4 and 8. Histone H1 was incubated with a mock kinase preparation lacking both CDK1 and CDK2 in lane 5. Lane 1 includes markers. (B) CDK phosphorylation of histone H1 substrate. Decreasing amounts of substrate were added to the kinase assays illustrated in lanes 1 to 6. The arrows in the right margin are labeled as follows: I, VZV gI; G, GST; and H, histone substrate. Molecular mass markers are on the left.
When the phosphorylation signals of GST-gI-wt by CDK1 and CDK2 were compared, a large difference was apparent (Fig. 9 and 10A). A fourfold-higher phosphorylation signal accompanied CDK1 in the protein kinase assay. Two factors could account for the observed differences. The first is that GST-gI-wt is the preferred substrate for CDK1 rather than CDK2. The second is that more CDK1 activity than CDK2 activity was present during the immunoprecipitation kinase assay. To rule out the second possibility, we examined the relative phosphorylation of histone H1 by CDK1 and CDK2 isolated by the above-described immunoprecipitation method. After CDK1 and CDK2 kinases were prepared, various amounts of substrate histone H1 were tested, as shown in Fig. 10B. If there is a difference in activity between CDK1 and CDK2, there will be a constant difference in the phosphorylation signals at each concentration of histone H1. The result of the kinase assay indicated that even 1 μg of histone H1 provided sufficient substrate for CDK1 and CDK2 under the conditions of the kinase assay (Fig. 10B, lanes 5 and 6). An increase in the amount of substrate histone H1 did not increase the phosphorylation signals generated by CDK1 and CDK2. With all three concentrations of substrate, both CDKs generated similar phosphorylation signals. Therefore, we concluded that the CDK1 and CDK2 preparations contained similar histone H1 kinase activity, and consequently the different phosphorylation signals detected with the GST-gI-wt substrate were most likely the result of phosphorylation site preferences.
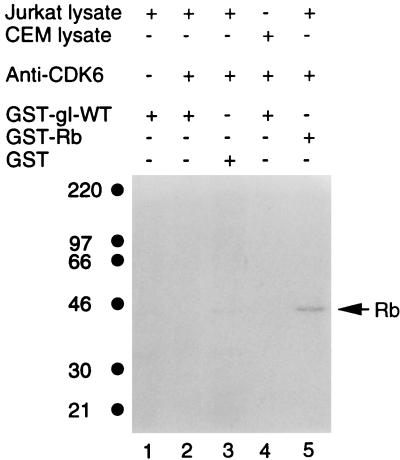
To further investigate CDK substrate specificity, we tested whether gI was phosphorylated by another CDK less homologous to CDK1 or CDK2. In the same manner as described for CDK1 and CDK2, CDK6 was isolated by immunoprecipitation from either Jurkat cells or CEM cells and placed in an in vitro protein kinase assay. The results of this kinase assay are shown in Fig. 11: there was no phosphorylation of GST-gI-wt by Jurkat-derived CDK6 (lane 2), while CEM-derived CDK6 showed a similar negative result (lane 4). There was also no GST phosphorylation by CDK6 (lane 3). Therefore, CDK specificity was further documented when GST-gI-wt was a substrate in the coupled immunoprecipitation protein kinase assay.
FIG. 11.
Lack of phosphorylation of GST-gI-wt by CDK6. CDK6 was immunoprecipitated with an antibody raised against a peptide of CDK6. The reagents in the coupled immunoprecipitation kinase assay are listed in tabular format above each lane (+, present; −, absent). CDK6 was present in lanes 2 to 5, while lane 1 is the negative control lacking CDK6. Rb designates the fusion protein of GST and retinoblastoma protein (GST-Rb); Rb is a positive control substrate for CDK6. Molecular mass markers are on the left.
Investigation of a threonine-proline sequence within VZV gI.
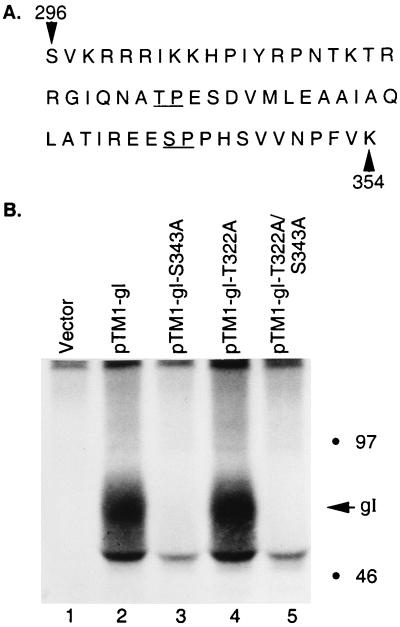
In the kinase assays shown in the preceding figures, a weak phosphorylation signal was occasionally present when GST-gI-S343A was tested as a substrate. To investigate whether an alternative CDK phosphorylation site was present in gI, we reexamined the amino acid sequence of the cytoplasmic tail. A threonine-proline sequence at positions 322 and 323 was identified (Fig. 12A). To determine whether the threonine-proline sequence was a utilized phosphorylation site, we performed site-directed mutagenesis in genes coding for both wild-type gI and mutant gI-S343A. The resulting mutants were named VZV gI-T322A and gI-T322A/S343A, where threonine-322 was replaced by an alanine in both wild-type gI and mutant gI-S343A. When the wild-type and mutant genes were subsequently transfected into HeLa cells and labeled with [32P]orthophosphate, the phosphorylation signals of wild-type gI and the gI-T322A mutant were equally strong (Fig. 12B, lanes 2 and 4); in other words, deletion of the threonine-proline sequence exerted no effect on in vivo phosphorylation. As previously shown (Fig. 5), mutation of serine 343 within the gI-S343A construct resulted in abrogation of phosphorylation (Fig. 12B, lane 3). Similarly, no phosphorylation occurred in the double mutant gI-T322A/S343A (Fig. 12, lane 5).
FIG. 12.
Effects of mutagenesis of threonine 322 on the phosphorylation of VZV gI. (A) Amino acid sequence of the cytoplasmic tail of gI (7). The underlined Ser-343–Pro-344 is the previously described CDK consensus phosphorylation site; the underlined Thr-322–Pro-323 represents a second potential CDK phosphorylation site. (B) Phosphorylation results. HeLa cells were transfected with pTM1 vector only (lane 1), pTM1-gI (lane 2), pTM1-gI-S343A (lane 3), pTM1-gI-T322A (lane 4), or the dual mutant pTM1-gI-T322A/S343A (lane 5). The transfected cells were subsequently labeled with [32P]orthophosphate and harvested as described in the legend to Fig. 5. The gI product was immunoprecipitated with MAb 6B5 and analyzed by SDS-PAGE (12 to 18% acrylamide). The location of gI is indicated in the right margin, along with molecular mass markers.
In addition, previously published data have described two independent single mutations which involved the replacement of either serine 296 or threonine 338 with an alanine residue within the gI tail; neither substitution affected the in vivo phosphorylation of gI (56). In further experiments, serine 347 was replaced with an alanine; again, no decrease of the in vivo phosphorylation signal in this mutant gI was observed compared with that of wild-type gI (data not shown). Therefore, the in vivo phosphorylation experiments failed to detect additional potential phosphorylation sites in the cytoplasmic tail of VZV gI. The same in vivo phosphorylation data suggested that the minimal phosphorylation of the mutant gI construct seen in the in vitro kinase assays was not related to an authentic phosphorylation site.
Inhibition of gI phosphorylation by roscovitine.
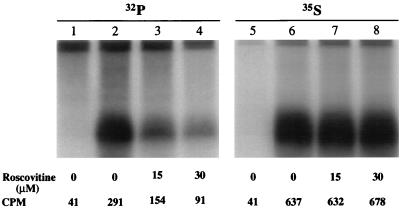
In order to confirm the above results, which suggested that VZV gI was a physiological substrate of a CDK, experiments with a specific CDK inhibitor were next performed. Roscovitine, a purine analog, has been identified as a potent selective inhibitor of the kinase activities of CDK1, CDK2, and CDK5 but much less so for those of CDK4 or CDK6 (9, 33). Treatment of cells with roscovitine leads to cell cycle arrest at G2 or G1 phase as well as inhibition of in vivo phosphorylation of vimentin by CDK1. The 50% inhibitory concentration (IC50) for cell cycle arrest at prophase is 16 μM. The mechanism of inhibition is mediated by competitive binding of roscovitine to the ATP binding site within the catalytic domains of these kinases (9). To investigate whether roscovitine was able to affect the phosphorylation signals of gI in transfected cells, we transfected HeLa cells with the gI gene and treated some of the transfected cells with varying concentrations of roscovitine 5 h posttransfection. At the same time, we labeled another set of transfected cells with [35S]methionine and [35S]cysteine to monitor gI protein expression. In the presence of 15 and 30 μM roscovitine, the phosphorylation signal of gI was reduced by 50 and 70%, respectively (Fig. 13, lanes 1 to 4). These reductions in gI phosphorylation were not a consequence of decreased expression of gI in roscovitine-treated cells, because roscovitine-treated and untreated cells expressed gI at nearly identical levels (Fig. 13, lanes 5 to 8). In other words, the vaccinia-pTM1 infection-transfection system was relatively unaffected by roscovitine treatment at the indicated concentrations. Because of the documented specificity of roscovitine, the results obtained from the inhibition assays described above strongly supported the conclusion that VZV gI was phosphorylated by a CDK in human cells. We attempted to confirm the above-mentioned transfection data by a similar analysis performed in infected cells. However, the roscovitine treatment markedly inhibited VZV replication generally, because of the very low inoculum (about 1 PFU per 1,000 cells).
FIG. 13.
Effects of roscovitine on VZV gI phosphorylation in transfected cells. HeLa cells were transfected with pTM1 vector (lanes 1 and 5) or pTM1-gI (lanes 2 to 4 and 6 to 8) and metabolically labeled with either [32P]orthophosphate (32P) (lanes 1 to 4) or [35S]methionine and [35S]cysteine (35S) (lanes 5 to 8). During the labeling period, the cells were treated with 0 (lanes 1 to 2 and 5 to 6), 15 (lanes 3 and 7), or 30 (lanes 4 and 8) μM roscovitine. After a 16-h incubation, the cells were lysed; immunoprecipitated gI was subjected to electrophoresis and analyzed by an Instantimager (Packard Bell). The counts per minute (CPM) were measured for gI (lanes 2 to 4 and 6 to 8) as well for as an area corresponding to the location of gI in lanes 1 and 5.
DISCUSSION
VZV gI contains 354 residues, while gE contains 623 amino acids. Like its partner, gE, in the VZV gE:gI Fc receptor complex, gI resembles nonviral cell surface molecules. For example, VZV gI undergoes endocytosis in clathrin-coated pits; endocytosis is mediated via a methionine-leucine internalization motif within the cytoplasmic tail (42). As a second example, VZV gI is phosphorylated on its cytoplasmic tail, but the protein kinase catalyzing this event has never been characterized (56). Since VZV gI is often poorly expressed in infected or transfected cells, it has been less well characterized (8, 28). Previously, our laboratory mainly used a vaccinia virus-based transient-transfection system to study VZV glycoproteins in HeLa cells (56, 57). The pTM1 plasmid system relies on a recombinant vaccinia virus expressing bacteriophage T7 RNA polymerase. Since vaccinia virus also encodes protein kinases, those phosphotransferases may cause complications in interpreting phosphorylation data obtained from the transient-transfection system (3, 24). With the aid of an alternative, nonvaccinia expression system in the present study, we were able to express VZV gI protein at a similarly high level in human 293T cells. Our experiments demonstrated that VZV gI was phosphorylated in the absence of VZV or vaccinia virus proteins, a result which strongly suggested that a cellular kinase phosphorylated the VZV receptor protein.
Previous mutagenesis studies have eliminated some of the other serines in the gI endodomain as potential phosphoacceptors (56). This study further documented the location of the phosphorylation site within VZV gI by investigations with two different transfection vectors and two different cell substrates: HeLa cells and 293T cells. In both systems, the serine 343-proline 344 sequence was essential for gI phosphorylation. The analysis indicated that a proline residue at position 345 was not essential for the phosphorylation of VZV gI; however, repeated transfection assays showed that the phosphorylation signal in gI-P345A decreased by 60 to 70% compared with that of wild-type gI. Thus, proline residue 345 clearly acted as a secondary structural determinant. When the results of this phosphorylation study as well as earlier reports were assessed, the obvious candidate kinase was a proline-directed protein kinase (35).
To identify the nature of the CDK phosphorylating VZV gI, we constructed a fusion protein consisting of GST and the cytoplasmic tail of gI (GST-gI-wt). The in vitro kinase assays demonstrated that the GST-gI-wt fusion protein was phosphorylated by CDK1 (cdc2) and CDK2. Interestingly, GST-gI-wt was also phosphorylated by a protein kinase called P-TEFb, a newly discovered CDK (30, 31). The components of P-TEFb include one subunit which is homologous to cyclins and is named cyclin T and a second subunit which is now known to be PITALRE and was renamed CDK9 (13, 45). Human and Drosophila P-TEFbs exhibit 72% identity and 83% similarity at an amino acid level.
CDKs are a family of serine-threonine protein kinases, some of which are critical in triggering cell cycle progression to successive phases (G1 to S to G2 to M) (14, 38, 52). Nine human CDKs have been identified and named: they are CDK1 (cdc2) to CDK9 (14, 45, 52). When other CDK members are compared with the prototype kinase, CDK1 (cdc2), identical amino acids range from 36 to 66% (10, 13, 34). CDK1 has a high sequence homology with CDK2 (65%). The percent amino acid identities and similarities between CDK1 and CDK9 are about 47 and 63, respectively (13). Some CDKs also are restricted in their locations within human tissues; for example, CDK1 and CDK2 are not found in neurons whereas CDK5 is a prominent kinase within neurons (23).
Only a limited number of substrates for CDKs have been identified. All of the substrates phosphorylated by CDK2, CDK6, or CDK9 are nuclear proteins, while those phosphorylated by CDK1 include both nuclear and cytoplasmic proteins (37, 40, 47, 48, 60). Since VZV gI is a cytoplasmic glycoprotein, the preferential phosphorylation of GST-gI-wt by CDK1 rather than CDK2 is consistent with the intracellular location of viral glycoprotein biosynthesis. CDK2 is considered to be active only inside the nucleus, where it associates with either cyclin E or cyclin A (4, 47). In contrast, CDK1 is active in both cytoplasmic and nuclear compartments. It has long been documented that the CDK1-cyclin B1 complex is first formed in the cytoplasm and then translocated into the nucleus before the nuclear laminae are disassembled, indicating that it is activated in the cytoplasm (47). In addition, a second kinase-cyclin complex, CDK1-cyclin A, has been purified from a cytoplasmic compartment, where it is able to phosphorylate the tyrosine hydroxylase protein (19). When the VZV in vitro and in vivo experiments are considered together, including the roscovitine data, the conclusion is that CDK1 is the kinase most likely to phosphorylate VZV gI. Finally, our results complement to a remarkable degree the recent paper by Schang et al., which found that CDK1 or CDK2 but not CDK4 or CDK6 was required for accumulation of HSV transcripts, viral DNA replication, and production of infectious HSV (51). The last-mentioned paper relied heavily on detailed experimental data obtained with roscovitine and other inhibitors of CDK activity. The investigators determined that 50 and 100 μM roscovitine were sufficient to inhibit HSV replication in human embryonic cells and simian Vero cells, respectively. Specific HSV substrates were not yet identified, although candidate proteins were discussed.
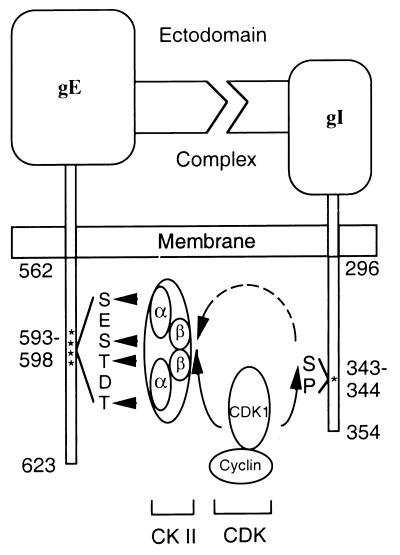
Based on the results in this report, we postulate an intriguing network of phosphorylation interactions between the two components of the VZV gE:gI complex. The cytoplasmic tail of the gE constituent of this complex is phosphorylated by CKII at threonines and serines between amino acid residues 593 and 598; in addition, CKII binds to and coprecipitates with gE (17, 41, 58). CKII is normally active in mammalian cells, and the phosphorylation sites are not susceptible to dephosphorylation activity (54). Further, Litchfield et al. and Bosc et al. have demonstrated that both the alpha and beta subunits of CKII are phosphorylated by CDK1 (5, 25–27). The phosphorylation signals of CKII peak at the G2-M phase of a cell cycle. Therefore, we have constructed a model to depict a plausible interrelationship among CDK1, CKII, and the gE endodomain, as well as the interaction between CDK1 and the gI endodomain (Fig. 14). The likelihood that this series of phosphorylation and dephosphorylation reactions in the VZV gE:gI endodomains will provide signals for internalization and trafficking of the viral glycoproteins is supported by the recent observation that a phosphorylation site in the cytomegalovirus gB endodomain influences its targeting in polarized cells (55).
FIG. 14.
Network of interactions between the phosphorylation motifs in the endomains of the VZV gE:gI complex. VZV gE and gI form a receptor complex through the binding of their ectodomains. Like many other receptors, both components are phosphorylated on their endodomains. As shown in this report, a serine 343-proline 344 sequence in the gI endodomain is essential for phosphorylation. Prior reports have demonstrated that the cytoplasmic tail of gE is phosphorylated by CKII at serine and threonine residues between amino acids 593 and 598. Other reports cited in Discussion have documented that both subunits of CKII are in turn phosphorylated by CDK1. Thus, CDK1 can modify both components of the VZV Fc receptor complex as part of a network of phosphorylation events.
ACKNOWLEDGMENTS
We thank Lishan Su (Chapel Hill) for advice about the in vitro kinase assays.
This research was supported by NIH RO1 grants AI22795, AI36884, and GM35500 as well as T32 grant CA09156.
REFERENCES
- 1.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Assouline J G, Levin J M, Major E O, Forghani B, Straus S E, Ostrove J M. Varicella-zoster virus infection of human astrocytes, schwann cells, and neurons. Virology. 1990;179:834–844. doi: 10.1016/0042-6822(90)90152-h. [DOI] [PubMed] [Google Scholar]
- 3.Banham A, Smith G L. Vaccinia virus gene B1R encodes a 34 Kdal serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology. 1992;191:803–812. doi: 10.1016/0042-6822(92)90256-o. [DOI] [PubMed] [Google Scholar]
- 4.Baptist M, Lamy F, Gannon J, Hunt T, Dumont J E, Roger P P. Expression and subcellular localization of CDK2 and cdc2 kinases and their common partner cyclin A in thyroid epithelial cells: comparison of cyclic AMP-dependent and -independent cell cycles. J Cell Physiol. 1996;166:256–273. doi: 10.1002/(SICI)1097-4652(199602)166:2<256::AID-JCP3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Bosc D G, Slominski E, Sichler C, Litchfield D W. Phosphorylation of casein kinase II by p34cdc2. J Biol Chem. 1995;270:25872–25878. doi: 10.1074/jbc.270.43.25872. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J I, Nguyen H. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J Virol. 1997;71:6913–6920. doi: 10.1128/jvi.71.9.6913-6920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 8.Davison A J, Waters D J, Edson C M. Identification of the products of a varicella-zoster virus glycoprotein gene. J Gen Virol. 1985;66:2237–2242. doi: 10.1099/0022-1317-66-10-2237. [DOI] [PubMed] [Google Scholar]
- 9.De Azevedo W F, Leclerc S, Meijer L, Havlicek L, Strand M, Kim S. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human CDK2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 10.Doolittle R F. A primer on how to analyze derived amino acid sequences. Sausalito, Calif: University Science Books; 1986. [Google Scholar]
- 11.Duus K M, Grose C. Multiple regulatory effects of varicella-zoster virus (VZV) gL on trafficking patterns and fusogenic properties of VZV gH. J Virol. 1996;70:8961–8971. doi: 10.1128/jvi.70.12.8961-8971.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuerst F R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grana X, Luca A D, Sang N, Fu Y, Claudio P P, Rosenblatt J, Morgan D O, Giordano A. PITALRE, a nuclear CDC2-related protein kinase that phosphorylates the retinoblastoma protein in vitro. Proc Natl Acad Sci USA. 1994;91:3834–3838. doi: 10.1073/pnas.91.9.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grana X, Reddy E P. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 15.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 16.Grose C, Brunell P A. Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32°C. Infect Immun. 1978;19:199–203. doi: 10.1128/iai.19.1.199-203.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grose C, Jackson W, Traugh J A. Phosphorylation of varicella-zoster virus gpI by mammalian casein kinase II and casein kinase I. J Virol. 1989;63:3912–3918. doi: 10.1128/jvi.63.9.3912-3918.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan K, Jenkins C W, Li Y, Nichols M A, Wu X, O’Keefe C L, Matera A G, Xiong Y. Growth suppression by p18, a p16INK4/MTS1 and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 19.Hall F L, Braun R K, Mihara K, Fung Y T, Berndt N, Carbonaro-Hall D A, Vulliet P R. Characterization of the cytoplasmic proline-directed protein kinase in proliferative cells and tissues as a heterodimer comprised of p34cdc2 and p58cyclin A. J Biol Chem. 1991;266:17430–17440. [PubMed] [Google Scholar]
- 20.Jenkins C W, Xiong Y. Immunoprecipitation and immunoblotting in cell cycle studies. In: Pagano M, editor. Cell cycle: materials and methods. New York, N.Y: Springer-Verlag; 1995. pp. 250–263. [Google Scholar]
- 21.Kimura H, Straus S E, Williams R K. Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology. 1997;233:382–391. doi: 10.1006/viro.1997.8625. [DOI] [PubMed] [Google Scholar]
- 22.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 23.Lew J, Qi Z, Huang Q, Paudel H, Matsuura I, Matsushita M, Zhu X, Wang J H. Structure, function, and regulation of neuronal cdc2-like protein kinase. Neurobiol Aging. 1995;16:263–270. doi: 10.1016/0197-4580(95)00014-6. [DOI] [PubMed] [Google Scholar]
- 24.Lin S, Broyles S S. Vaccinia protein kinase 2: a second essential serine/threonine protein kinase encoded by vaccinia virus. Proc Natl Acad Sci USA. 1994;91:7653–7657. doi: 10.1073/pnas.91.16.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litchfield D W, Bosc D G, Slominski E. The protein kinase from mitotic human cells that phosphorylates Ser-209 on the casein kinase II β-subunits is p34cdc2. Biochim Biophys Acta. 1995;1269:69–78. doi: 10.1016/0167-4889(95)00100-7. [DOI] [PubMed] [Google Scholar]
- 26.Litchfield D W, Lozeman F J, Cicirelli M F, Harrylock M, Ericsson L H, Piening C J, Krebs E G. Phosphorylation of the β subunit of casein kinase II in human A431 cells: identification of the autophosphorylation site and a site phosphorylated by p34cdc2. J Biol Chem. 1991;266:20380–20389. [PubMed] [Google Scholar]
- 27.Litchfield D W, Luscher B, Lozeman F J, Eisenman R N, Krebs E G. Phosphorylation of casein kinase II by p34cdc2 in vitro and at mitosis. J Biol Chem. 1992;267:13943–13951. [PubMed] [Google Scholar]
- 28.Litwin V, Jackson W, Grose C. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J Virol. 1992;66:3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallory S, Sommer M, Arvin A M. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall N F, Peng J, Xie Z, Price D H. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J Biol Chem. 1996;271:27176–27183. doi: 10.1074/jbc.271.43.27176. [DOI] [PubMed] [Google Scholar]
- 31.Marshall N F, Price D H. Purification of p-TEFb, a transcription factor required for the transition into productive elongation. J Biol Chem. 1995;270:12335–12338. doi: 10.1074/jbc.270.21.12335. [DOI] [PubMed] [Google Scholar]
- 32.McGeoch D J, Cook S. Molecular phylogeny of the alphaherpesvirinae subfamily and a proposed evolutionary timescale. J Mol Biol. 1994;238:9–22. doi: 10.1006/jmbi.1994.1264. [DOI] [PubMed] [Google Scholar]
- 33.Meijer L, Borgne A, Mulner O, Chong J P J, Blow J J, Inagaki N, Inagaki M, Delcros J, Moulinoux J. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 34.Meyerson M, Enders G H, Wu C, Su L, Gorka C, Nelson C, Harlow E, Tsai L. A family of human cdc2-related protein kinase. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno S, Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990;61:549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- 36.Moss B, Elroy-Stein O, Mijukani T, Alexander W A, Fuerst T R. New mammalian expression vectors. Nature (London) 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 37.Nagasawa M, Melamed I, Kupfer A, Gelfand E W, Lucas J J. Rapid nuclear translocation and increased activity of cyclin-dependent kinase 6 after T cell activation. J Immunol. 1997;158:5146–5154. [PubMed] [Google Scholar]
- 38.Nasmyth K. Viewpoint: putting the cell cycle in order. Science. 1996;74:1643–1645. doi: 10.1126/science.274.5293.1643. [DOI] [PubMed] [Google Scholar]
- 39.Ng T I, Grose C. Serine protein kinase associated with varicella-zoster virus ORF 47. Virology. 1992;191:9–18. doi: 10.1016/0042-6822(92)90161-h. [DOI] [PubMed] [Google Scholar]
- 40.Nigg E A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 41.Olson J K, Bishop G A, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson J K, Grose C. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng J, Zhu Y, Milton J T, Price D H. Identification of multiple cyclin subunits of human p-TEFb. Genes Dev. 1998;12:755–762. doi: 10.1101/gad.12.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phelps D E, Xiong Y. Assay for activity of mammalian cyclin D-dependent kinases CDK4 and CDK6. Methods Enzymol. 1997;283:194–205. doi: 10.1016/s0076-6879(97)83016-9. [DOI] [PubMed] [Google Scholar]
- 47.Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem J. 1995;308:697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynisdottir I, Massague J. The subcellular locations of p15Ink4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 49.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 50.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 16.33–16.37. [Google Scholar]
- 51.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 53.Smith R F, Smith T F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989;63:450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Traugh J. Approaches to examine the role of multiple serine protein kinases in the coordinate regulation of cell growth. In: Mond J J, Cambier J C, Weiss A, editors. Advances in regulation of cell growth. 1. Regulation of cell growth and activation. New York, N.Y: Raven Press; 1989. pp. 173–202. [Google Scholar]
- 55.Tugizov S, Maidji E, Xiao J, Zheng Z, Pereira L. Human cytomegalovirus glycoprotein B contains autonomous determinants for vectorial targeting to apical membranes of polarized epithelial cells. J Virol. 1998;72:7374–7386. doi: 10.1128/jvi.72.9.7374-7386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao Z, Grose C. Unusual phosphorylation sequence in the gpIV (gI) component of the varicella-zoster virus gpI-gpIV glycoprotein complex (VZV gE-gI complex) J Virol. 1994;68:4204–4211. doi: 10.1128/jvi.68.7.4204-4211.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao Z, Jackson W, Forghani B, Grose C. Varicella-zoster virus glycoprotein gpI/gpIV receptor: expression, complex formation, and antigenicity within the vaccinia virus-T7 RNA polymerase transfection system. J Virol. 1993;67:305–314. doi: 10.1128/jvi.67.1.305-314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao Z, Jackson W, Grose C. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J Virol. 1993;67:4464–4473. doi: 10.1128/jvi.67.8.4464-4473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao Z, Jones D H, Grose C. Site-directed mutagenesis of herpesvirus glycoprotein phosphorylation sites by recombination polymerase chain reaction. PCR Methods Appl. 1992;1:205–207. doi: 10.1101/gr.1.3.205. [DOI] [PubMed] [Google Scholar]
- 60.Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews M B, Price D H. Transcription elongation factor p-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]