Abstract
Objectives:
Opioid-related disparities are magnified among Alaska Native and American Indian (ANAI) people. Yet, no outcome studies on medication for addiction treatment, an effective treatment in other populations, among ANAI people exist. The objective of this study was to identify variables associated with buprenorphine/naloxone retention among ANAI people with opioid use disorder (OUD).
Methods:
The sample was 240 ANAI adults in Anchorage, Alaska who received buprenorphine/naloxone treatment for an OUD. We gathered data from the electronic health record from January 1, 2015 to December 31, 2019. We used survival analysis to explore possible predictors (demographic variables, psychiatric comorbidity, medical severity, previous opioid prescriptions, previous injury, alcohol use disorder, and co-occurring substance use of length of treatment retention (in days) while accounting for right censoring.
Results:
We found that 63% of the 240 patients were retained in buprenorphine/naloxone treatment at 90 days, 51% at 6 months, and 40% at 1 year, slightly lower than the general US population. Younger age (hazard ratio 1.69, 95% confidence intervals 1.17–2.45) and co-occurring substance use (hazard ratio 2.95, 95% confidence intervals 1.99–4.38) were associated with increased rate of buprenorphine/naloxone treatment discontinuation.
Conclusions:
Younger patients and those with co-occurring substance use remain at higher risk of discontinuing buprenorphine/naloxone treatment for OUD in this population of ANAI people. Treatment programs serving ANAI people may consider paying special attention to patients with these characteristics to prevent treatment discontinuation. Our study highlights the need to address poly-substance use among ANAI people in treatment.
Keywords: Alaska Native people, American Indian people, buprenorphine/naloxone, opioid use disorder, treatment retention
The US is experiencing an alarming opioid epidemic, which has resulted in increased rates of overdose and death. Since 1999, the number of opioid-related overdose deaths has quadrupled,1 with increases across age groups, racial/ethnic groups, urbanization levels, and in multiple states.2 In 2017 alone, there were 47,600 opioid-related overdose deaths.2 These trends are magnified among Alaska Native and American Indian (ANAI) people compared to most other populations in the US. ANAI people are second to Whites in the rate of overdose mortality (15.7/100,000 vs 19.4/100,000 deaths, respectively),2 and overdose mortality among ANAI people has risen continuously from 1999 to 2016.3 Moreover, 1 study found evidence of racial misclassification impacted true numbers of opioid use disorder (OUD) and overdose.4 Specifically, Washington death certificates that were not corrected for misclassification of ANAI race underestimated drug overdose mortality rates among ANAI people by approximately 40%. Despite this negative disparity, there is a paucity of OUD treatment research within ANAI communities.
One highly effective method of treatment for OUD is medication for addiction treatment (MAT), which is the use of certain medications with counseling and behavioral therapies.5 Three medications are currently FDA-approved for OUD: methadone, buprenorphine/naloxone, and naltrexone.6 Results from clinical trials demonstrate that MAT produces superior abstinence and treatment retention outcomes compared to psychosocial treatments without medication or with placebo.6 For example, in the general population buprenorphine/naloxone is highly efficacious for treating OUD, with 3 to 8 times the abstinence rates compared to placebo or detoxification treatment alone,7–9 and it is most effective when used long-term.10 One study reported 77% of patients remained continuously on buprenorphine/naloxone at follow-up (18–42 months), and patients on continuous buprenorphine/naloxone were more likely to report 12-step affiliation, employment, and less likely to report using heroin and other substances.10 Patients on buprenorphine/naloxone were also less likely to report damaging a close relationship, hurting family, being unhappy, and doing regretful or impulsive things. To date, however, there are no published quantitative studies of MAT among ANAI people in the US.
Qualitative studies have identified barriers to the acceptability and uptake of MAT for OUD among ANAI people,11–13 suggesting that the results of MAT studies among other populations might not generalize to this group. For instance, qualitative studies among ANAI people who were using opioids and providers on a reservation reported concerns about the use of buprenorphine/naloxone, including diversion and only using it until more prescription opioids were available.12,13 Culturally-centered MAT services have been implemented with Indigenous people in Australia and Canada.14–16 In Australia, success of the MAT program was attributed to the culturally-specific design, integrated care, and a focus on family and community wellness.16 In Canada, Indigenous patients reported positive treatment outcomes, improvements in housing, employment, and family support, and general satisfaction and acceptance of MAT.15 Currently, no outcome studies on MAT among ANAI people exist, highlighting an urgent need for such studies given the negative opioid-related disparities experienced by this population.
We conducted a quantitative secondary data analysis using electronic health record (EHR) data from January 1, 2015 to December 31, 2019 to identify factors associated with buprenorphine/naloxone retention. Data was collected from an urban ANAI population in Alaska. We focused on urban ANAI people because the majority of ANAI people (>70%) reside in urban areas.17 Furthermore, the urban ANAI population is likely to increase as it grew 33.6% from 2000 to 2010, with no sign of slowing.18 This study was the first to quantitatively evaluate buprenorphine/naloxone for OUD treatment in an urban ANAI population.
METHODS
Setting
Data collection for this research project occurred at Southcentral Foundation (SCF). SCF is a tribally-owned and - operated healthcare system in Anchorage, Alaska with a robust EHR database and serves 65,000 ANAI people. SCF provides services to all Indian Health Service beneficiaries. The SCF service population is approximately 85% Alaska Native (alone or in combination) and 15% American Indian (alone or in combination). Anchorage is an urban setting and is the most populous city in Alaska (pop. 291,538) and has a large ANAI community (8.9% ANAI alone)19 representing 229 federally-recognized tribes from Alaska and others across the US. The ANAI population served at SCF is extremely diverse. For instance, there are 20 different Alaska Native languages and roughly 8 broad cultural groups in Alaska.20 The different cultural groups have varied social beliefs and practices. As with the rest of the US, Alaska is being challenged by a rise in deaths due to drug overdoses. During 2010 to 2017, 661 opioid-related deaths occurred in Alaska.21 The total opioid-related mortality increased by 77% from 2010 to 2017 (from 8 per 100,000 to 14 per 100,000). Increases in opioid-related mortality during the same time period were seen among both sexes and across most races, with ANAI people experiencing the highest rates (from 10 to 22 per 100,000), followed by Whites (from 11 to 13 per 100,000).
SCF uses buprenorphine/naloxone, delivered by sublingual film, as the medication component of MAT for OUD. Methadone is not offered at SCF. Naltrexone is offered at SCF for OUD. However, most patients use buprenorphine/naloxone for OUD. Patients are not typically discharged or discontinued from receiving MAT for OUD. However, treatment is adjusted for some patients or they are transitioned to a higher level of care depending on their individual needs. MAT at SCF is delivered through an integrated, systems approach, which entails requiring that patients establish care with their primary care provider, complete screening labs, engage in both substance use treatment and MAT with scheduled urine toxicology and pill counts. The level of treatment intensity and therapy varies among patients and is based on the severity of the disorder. A spectrum of therapy services is available including primary care behavioral health consultants, integrated behavioral health, intensive outpatient treatment, and residential treatment. Buprenorphine/naloxone can be prescribed by most clinical staff in primary care, integrated behavioral health, outpatient substance use treatment, the detoxification unit, and the hospital. Most settings are outpatient but inpatient substance use treatment is offered for pregnant women and individuals in the detoxification unit. During initiation on day 1, patients are given 2 to 4 mg of buprenorphine/naloxone depending on the Clinical Opioid Withdrawal Scale. Maintenance doses and frequency will vary among patients and is based on disorder severity. Given that SCF is tribally-owned and -operated healthcare system, all of the care provided at SCF is delivered in a culturally-centered manner that focuses on individual, family, and community wellness.
Sample
We identified a cohort of ANAI patients from EHR data. The sampling frame was all SCF patients from Anchorage and the surrounding 50 miles who received buprenorphine/naloxone and had a preexisting OUD diagnosis between January 1, 2015 to December 31, 2019. We excluded individuals from all analyses that were known to receive their first buprenorphine/naloxone dispense before January 1, 2015. We included individuals who received 2 or more prescriptions for buprenorphine/naloxone to account for misclassification or mistaken prescriptions. We used ICD-9 and ICD-10 codes to identify individuals with a preexisting OUD diagnosis: the diagnosis must have occurred within 3 years before the first buprenorphine/naloxone dispense. Our sample consisted of 240 ANAI patients who began buprenorphine/naloxone treatment and had a preexisting OUD during the study period. The study received tribal and institutional review board approvals.
Measures
Inasmuch as buprenorphine/naloxone is most effective when used long-term,10 we used buprenorphine/naloxone retention as the primary outcome in our study. Thus, the dependent variable in the analyses was the number of days from the first buprenorphine/naloxone dispense to the last buprenorphine/naloxone dispense, through December 31, 2019. Subjects were defined as “right-censored,” that is, still continuing buprenorphine/naloxone treatment past the end of the study period, if their last visit dates were within 60 days of December 31, 2019.
The independent variables in the analyses were: age, sex, marital status, psychiatric comorbidity, medical severity as measured by the Charlson Comorbidity Index,22 previous opioid prescriptions, previous injury, alcohol use disorder, and co-occurring substance use. We selected independent variables based on a review of other studies reporting treatment retention variables for OUD.23,24 Medical and psychiatric comorbidity information was obtained by ICD-9 and ICD-10 diagnoses present during the 3 years before the first buprenorphine/naloxone dispense. For this analysis, psychiatric comorbidity was measured using 2 dichotomous variables: “serious mental illness (SMI)” (psychoses and bipolar disorder), and “mood/anxiety disorders” (depressive and anxiety disorders, including posttraumatic stress disorders). Likewise, co-occurring substance use was defined to include prior diagnoses of use, abuse, and dependence for cocaine, cannabis, sedative/hypnotic/anxiolytic, other stimulants, hallucinogens, amphetamines, inhalants, and other psychoactive substances. We followed the same diagnostic query procedure in generating the Charlson Comorbidity Index.22 The Charlson comorbidity index is a single measure of medical severity created by the collection and differential weighting of 22 specific diagnoses (eg, myocardial infarction, diabetes, renal disease, etc). Each diagnosis is given a score of either 0, 1, 2, 3, or 6 depending on the risk of mortality within 1 year, and then totaled for each person. A higher score indicates greater medical severity. The dichotomous variable for previous opioid prescriptions included prior fills in the last 3 years for: APAP/codeine, APAP/hydrocodone, APAP/oxycodone, buprenorphine patch (indicated for pain), hydromorphone, methadone, morphine, or oxycodone. We initially included access point as an independent variable since patients initiate buprenorphine/naloxone at various access points (eg, primary care, outpatient substance use treatment, and the hospital). However, preliminary analysis indicated that access point was correlated with co-occurring substance use. Therefore, we continued with the co-occurring substance use variable since many patients had missing data on initiation access point.
Data Analysis
To examine treatment retention, we conducted a survival analysis using a Cox proportional hazard model.25 Survival analysis accommodates various intake and end points (left and right-censoring) and is superior to basic linear or logistic regression both in ensuring that such censoring does not bias the results and in producing results that take into account not only whether the outcome occurred or not but also that there is value in delaying a negative outcome. A 2-step process was used to develop the model. First, bivariate analyses explored the association between each potential predictor variable and days in treatment. Second, an a priori decision was made to include variables with a P-value <0.25 in this bivariate testing in further multivariable analysis.26 Alpha was set at 0.05, and all data analyses were performed using R software.27
RESULTS
Descriptive statistics about the sample (N=240) are presented in Table 1. Over half of the sample was middle-aged (31–50 years), and the majority of the sample was female. Most patients were unmarried. On average, patients continued on buprenorphine/naloxone for 482 days (median = 194 days, SD=590 days), with a minimum of 1 day and a maximum of 1819 days. Of the 240 patients, 63% were retained in buprenorphine/naloxone treatment at 90 days, 51% at 6 months, and 40% at 1 year. More than half of the sample had previously been prescribed opioids, and about half of the sample had a previous injury. More than half of the sample had a mood/anxiety disorder, and approximately 8% had a SMI. Approximately 59% had co-occurring substance use and less than a quarter of the sample had an alcohol use disorder. The average Charlson Comorbidity Index was 0.8 (SD=1.2), with a range of 0 to 7. Approximately a quarter of the sample-initiated buprenorphine/naloxone treatment in outpatient substance use treatment and less than a quarter-initiated buprenorphine/naloxone treatment in primary care.
TABLE 1.
Sample Characteristics
| Variable | Mean (SD) or Percentage |
|---|---|
| Age (yr) | |
| 18–30 | 33% |
| 31–50 | 55% |
| 50+ | 12% |
| Male | 39% |
| Married | 17% |
| Buprenorphine/naloxone retention (d) | 482 (590) |
| Previous opioid prescriptions | 61% |
| Previous injuries | 53% |
| Mood/anxiety disorder | 59% |
| Anxiety | 47% |
| Major depression | 28% |
| PTSD | 10% |
| Serious mental illness | 8% |
| Psychosis | 5% |
| Bipolar | 5% |
| Co-occurring substance use | 59% |
| Alcohol use disorder | 22% |
| Charlson Comorbidity Index | 0.8 (1.2) |
| Buprenorphine/naloxone initiation access point | |
| Primary care | 21% |
| Outpatient substance use treatment | 25% |
| Other/unknown | 53% |
PTSD, post-traumatic stress disorder.
Results from the bivariate analysis for potential predictors of buprenorphine/naloxone retention identified age, mood/anxiety disorders, co-occurring substance use, and previous injury as meeting the initial cutoff of P < 0.25. Results from the multivariable analysis are shown in Table 2 and Figure 1. Table 2 demonstrates that the significant predictors of buprenorphine/naloxone retention were age, mood/anxiety disorder, and co-occurring substance use. Compared to middle-aged (31–50 years) patients (the reference group), younger patients (18–30 years) were more likely to discontinue buprenorphine/naloxone sooner. Specifically, the hazard of stopping buprenorphine/naloxone treatment for younger patients was 1.69 times the hazard for middle-aged patients (95% confidence interval [CI] 1.17–2.45). Patients with co-occurring substance use were more likely to discontinue treatment sooner. Specifically, the hazard of stopping buprenorphine/naloxone treatment for patients with co-occurring substance use was 2.95 times greater than those without co-occurring substance use (95% CI 1.99–4.38). On the other hand, patients with a mood/anxiety disorder were less likely to discontinue buprenorphine/naloxone treatment sooner: having a mood/anxiety disorder reduced the hazard by a factor of 0.65 (95% CI 0.45–0.93).
TABLE 2.
Nonproportional Hazard Model for Days in Buprenorphine/Naloxone Treatment
| Variable | Coefficient | SE | P | Hazard Ratio | 95% Hazard Ratio CI |
|---|---|---|---|---|---|
| Age (ref: Middle, 31–50 yr) | |||||
| Young (18–30 years) | 0.53 | 0.19 | 0.01 | 1.69 | (1.17, 2.45) |
| Old (50+ yr) | −0.53 | 0.34 | 0.12 | 0.59 | (0.30, 1.15) |
| Mood/Anxiety | −0.44 | 0.18 | 0.02 | 0.65 | (0.45, 0.93) |
| Co-occurring substance use | 1.08 | 0.20 | <0.01 | 2.95 | (1.99, 4.38) |
| Injury | −0.06 | 0.18 | 0.73 | 0.94 | (0.66, 1.33) |
CI, confidence interval.
FIGURE 1.
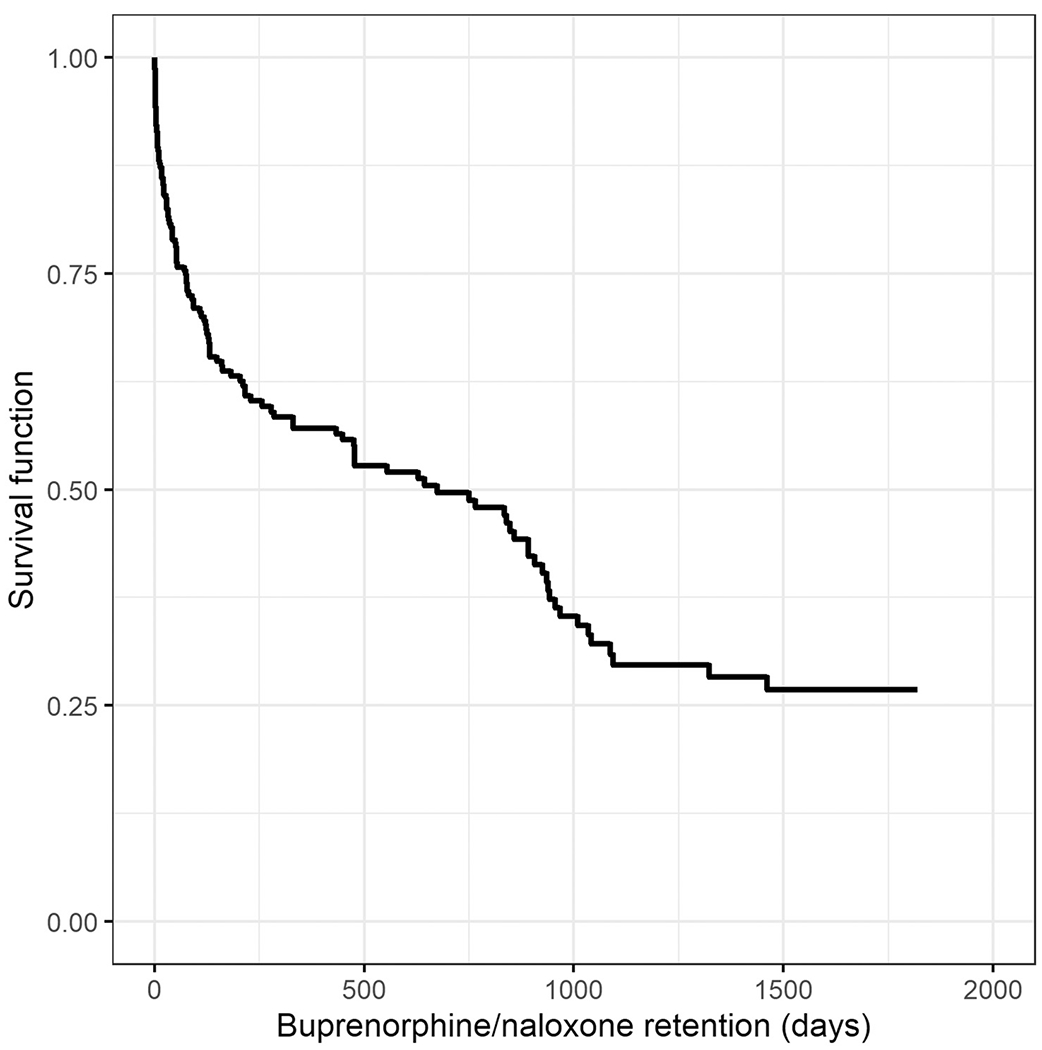
Survival function for buprenorphine/naloxone retention in days.
DISCUSSION
This is the first study to quantitatively evaluate buprenorphine/naloxone for OUD treatment in an urban ANAI population. Our findings indicated that younger age and co-occurring substance use (ie, poly-substance use) were associated with lower rates of buprenorphine/naloxone retention in an urban ANAI population that received services at a tribal healthcare system. Our study did not show any relations between buprenorphine/naloxone retention and sex, marital status, medical comorbidity, previous injury, previous opioid prescriptions, alcohol use disorder, and SMI. We also found that having a mood/anxiety disorder was associated with higher rates of buprenorphine/naloxone retention. The overall retention rate in our sample of 63% at 90 days was slightly lower than previously reported retention rates at 90 days among the general population,24,28 which might be explained by the high rates of co-occurring substance use among patients in our sample. Future research is recommended to understand the lower retention rate in our study and if this is the most effective OUD treatment approach for ANAI populations.
Our finding that co-occurring substance use was negatively associated with buprenorphine/naloxone retention is similar to other studies.28,29 For example, Hser et al conducted a study with 1267 opioid-dependent patients and found that amphetamine, cannabis, and cocaine were negatively associated with buprenorphine/naloxone treatment retention. Specifically, the hazard of stopping buprenorphine/naloxone treatment for patients who used amphetamine was 3.18 times greater than patients who did not use amphetamine, and the hazard ratio for cannabis was 1.78 and 2.41 for cocaine.29 We similarly found that co-occurring substance use was a strong predictor of discontinuing buprenorphine/naloxone treatment, approximately tripling the risk of discontinuing treatment. Given the magnitude of polysubstance use in other ANAI populations,30 and that co-occurring substance use was found to be a strong predictor of treatment retention in our study, treatment programs may consider paying special attention to patients with these characteristics to prevent treatment discontinuation.
Our finding that younger age was negatively associated with buprenorphine/naloxone treatment retention is consistent with previous studies with the general population24,28,29. We found that the hazard of discontinuing buprenorphine/naloxone treatment for those age 30 and below was nearly double compared to those that were middle-aged (ie, 31–50 years). Other studies found an association between older age and treatment success, including retention29 and reduced return to opioid use.31–33 Although, some studies did not find an association between age and either retention or abstinence,34 when patients were grouped dichotomously by emerging adult status (18–25 years) versus older adult status (>25) in one cohort, younger age was a robust predictor of treatment retention at both 3 and 12 months.28 Thus, targeting emerging adults is increasingly supported by the literature, and our study supports this trend.
In our study, an interesting but difficult to explain finding was the fact that individuals with a mood/anxiety disorder were less likely to discontinue treatment sooner. It is possible that this finding is due to unidentified confounding, or it may be that the treatment these patients receive for their mood or anxiety disorder may help reinforce continued treatment engagement. ANAI people experience disproportionally higher rates of acute, chronic, and intergenerational trauma than the general population,35,36 with adverse impacts on behavioral and physical health.37,38 Emotional trauma and the development of anxiety disorders appears to be linked.39 Therefore, it is plausible that ANAI people receiving continued mental health and counseling for histories of trauma and post-traumatic stress disorder positively impacts MAT retention for OUD. We suggest future research focus on the association between buprenorphine/naloxone retention and mood/anxiety disorder to better understand this relationship within ANAI people.
Several limitations of the study should be noted. First, we did not account for counseling and therapy services that patients received in addition to buprenorphine/naloxone. SCF uses a spectrum of therapy services such as primary care behavioral health consultants, integrated behavioral health, intensive outpatient treatment, and residential treatment. It is likely treatment retention was influenced by these various therapy services. However, we did not have adequate data to account for the type and duration of therapy that patients received. Moreover, we did not have adequate data for initiation access point of buprenorphine/naloxone for many patients, which may influence buprenorphine/naloxone retention. More research is necessary to examine how therapy and initiation access point influences buprenorphine/naloxone retention among ANAI people. Second, these analyses included a modest sample size, which restricts their statistical power. We suggest that future studies examine buprenorphine/naloxone retention with a larger sample of ANAI people to understand the generalizability of our findings. Third, because our data was from 1 urban site, our findings may not generalize to other ANAI populations, particularly rural communities and ANAI communities outside of Alaska. More research is necessary to determine the generalizability of our findings to ANAI people with OUD in other settings. Fourth, SCF providers indicated that a small number of patients switch to other forms of MAT for OUD at SCF or outside of SCF, such as naltrexone or methadone, for various reasons (eg, easier to arrange initiation, less frequent follow-up requirements). We did not account for patients that switched to other forms of MAT due to the difficulty of capturing this information from the EHR, especially for individuals that opted for methadone outside of the SCF system. However, switching to other forms of MAT for OUD is infrequent and most individuals are retained within the SCF system. Thus, switching to other forms of MAT for OUD likely did not influence our findings. Lastly, we were unable to include housing, employment, and certain family variables in our analyses because these variables are not reliably available in the EHR. We suggest future studies include more demographic variables to determine their influence on buprenorphine/naloxone retention for OUD.
CONCLUSIONS
Our study suggests that within an urban tribal healthcare system, younger patients and those with co-occurring substance use remain at substantially higher risk of discontinuing buprenorphine/naloxone treatment for OUD. We recommend treatment programs serving ANAI people consider paying special attention to patients with these characteristics to prevent treatment discontinuation. Our sample had slightly lower overall treatment retention than previously reported retention rates among the general population, which might be explained by the relatively high rates of co-occurring substance use in our sample. Our findings highlight a need to address poly-substance use among ANAI people in treatment in efforts to improve outcomes and retention in MAT.
ACKNOWLEDGMENTS
The authors thank the tribal leaders for supporting our research and SCF data analysts for assisting with electronic health record data abstraction.
This project was supported by grant number R25HS023207 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Kate M. Lillie, Southcentral Foundation, 4085 Tudor Centre Drive, Anchorage, AK.
Jennifer Shaw, Southcentral Foundation, 4085 Tudor Centre Drive, Anchorage, AK.
Kelley J. Jansen, Southcentral Foundation, 4085 Tudor Centre Drive, Anchorage, AK.
Michelle M. Garrison, University of Washington, 4333 Brooklyn Ave NE, Seattle, WA.
REFERENCES
- 1.Wide-ranging online data for epidemiologic research (WONDER). National Center for Health Statistics; 2016. [Google Scholar]
- 2.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tipps RT, Buzzard GT, McDougall JA. The opioid epidemic in Indian country. J Law Med Ethics. 2018;46(2):422–436. [DOI] [PubMed] [Google Scholar]
- 4.Joshi S, Weiser T, Warren-Mears V. Drug, opioid-involved, and heroin-involved overdose deaths among American Indians and Alaska Natives - Washington, 1999–2015. MMWR Morb Mortal Wkly Rep. 2018;67(50):1384–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. Medication and Counseling Treatment. 2018. Available at: https://www.samhsa.gov/medication-assisted-treatment/treatment. Accessed January 15, 2020.
- 6.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63–75. [DOI] [PubMed] [Google Scholar]
- 7.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949–958. [DOI] [PubMed] [Google Scholar]
- 8.Woody GE. Advances in the treatment of opioid use disorders. F1000Res. 2017;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parran TV, Adelman CA, Merkin B, et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106(1):56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venner KL, Donovan DM, Campbell ANC, et al. Future directions for medication assisted treatment for opioid use disorder with American Indian/Alaska Natives. Addict Behav. 2018;86:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momper S, Dennis M, Mueller-Williams A. Service provider views of OxyContin use on an Indian reservation: traumatic effects on the tribal community. Fam Soc. 2012;93(4):312–318. [Google Scholar]
- 13.Momper SL, Delva J, Reed BG. OxyContin misuse on a reservation: qualitative reports by American Indians in talking circles. Subst Use Misuse. 2011;46(11):1372–1379. [DOI] [PubMed] [Google Scholar]
- 14.Black A, Khan S, Brown R, Sharp P, Chatfield H, McGuiness C. An evaluation of opioid replacement pharmacotherapy in an urban Aboriginal Health Service. Aust N Z J Public Health. 2007;31(5):428–432. [DOI] [PubMed] [Google Scholar]
- 15.Poirier NB. Evaluating an On-reserve Methadone Maintenance Therapy Program for First Nations Peoples [Master’s thesis]. Thunder Bay, Canada: Lakehead University; 2015. [Google Scholar]
- 16.Williams N, Nasir R, Smither G, Troon S. Providing opioid substitution treatment to Indigenous heroin users within a community health service setting in Adelaide. Drug Alcohol Rev. 2006;25(3):227–232. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Census Bureau. American Indian and Alaska Native Summary File. 2012. Available at: https://www.census.gov/prod/cen2010/doc/aiansf.pdf. Accessed January 15, 2020.
- 18.Urban Indian Health Institute. U.S. Census Marks Increase in Urban American Indians and Alaska Natives. 2013. Available at: http://www.uihi.org/wp-content/uploads/2013/09/Broadcast_Census-Number_FINAL_v2.pdf. Accessed January 15, 2020.
- 19.United States Census Bureau. QuickFacts. 2017. Available at: https://www.census.gov/quickfacts/fact/table/anchoragemunicipalityalaska,ak/PST045217. Accessed January 15, 2020.
- 20.Kirk R, Starn O. The Alaska Native Reader: History, Culture, Politics. Durham and London, United Kingdom: Duke University Press; 2009. [Google Scholar]
- 21.State of Alaska Epidemiology. Health Impacts of Opioid Misuse in Alaska. 2018. [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 23.Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11(5):641–653. [DOI] [PubMed] [Google Scholar]
- 24.Marcovitz DE, McHugh RK, Volpe J, Votaw V, Connery HS. Predictors of early dropout in outpatient buprenorphine/naloxone treatment. Am J Addict. 2016;25(6):472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinbaum DG, Klein M. Survival Analysis: A Self-learning Text. 2nd ed. New York, NY: Singer Science + Business Media, Inc; 2005. [Google Scholar]
- 26.Dillard DA, Avey JP, Robinson RF, et al. Demographic, clinical, and service utilization factors associated with suicide-related visits among Alaska native and American Indian adults. Suicide Life Threat Behav. 2017;47(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 28.Schuman-Olivier Z, Weiss RD, Hoeppner BB, Borodovsky J, Albanese MJ. Emerging adult age status predicts poor buprenorphine treatment retention. J Subst Abuse Treat. 2014;47(3):202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeledon I, West A, Antony V, et al. Statewide collaborative partnerships among American Indian and Alaska Native (AI/AN) communities in California to target the opioid epidemic: preliminary results of the Tribal Medication Assisted Treatment (MAT) key informant needs assessment. J Subst Abuse Treat. 2020;108:9–19. [DOI] [PubMed] [Google Scholar]
- 31.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171(5):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreifuss JA, Griffin ML, Frost K, et al. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug Alcohol Depend. 2013;131(1–2):112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann Fam Med. 2007;5(2):146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, Rastegar DA. Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J Subst Abuse Treat. 2009;37(4):426–430. [DOI] [PubMed] [Google Scholar]
- 35.Beals J, Manson SM, Whitesell NR, Spicer P, Novins DK, Mitchell CM. Prevalence of DSM-IV disorders and attendant help-seeking in 2 American Indian reservation populations. Arch Gen Psychiatry. 2005;62(1):99–108. [DOI] [PubMed] [Google Scholar]
- 36.Manson SM, Beals J, Klein SA, Croy CD, Team A-S. Social epidemiology of trauma among 2 American Indian reservation populations. Am J Public Health. 2005;95(5):851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beals J, Novins DK, Whitesell NR, Spicer P, Mitchell CM, Manson SM. Prevalence of mental disorders and utilization of mental health services in two American Indian reservation populations: mental health disparities in a national context. Am J Psychiatry. 2005;162(9):1723–1732. [DOI] [PubMed] [Google Scholar]
- 38.Boyd-Ball AJ, Manson SM, Noonan C, Beals J. Traumatic events and alcohol use disorders among American Indian adolescents and young adults. J Trauma Stress. 2006;19(6):937–947. [DOI] [PubMed] [Google Scholar]
- 39.Fernandes V, Osorio FL. Are there associations between early emotional trauma and anxiety disorders? Evidence from a systematic literature review and meta-analysis. Eur Psychiatry. 2015;30(6):756–764. [DOI] [PubMed] [Google Scholar]


