Abstract
Rationale
Invasive pulmonary aspergillosis has emerged as a frequent coinfection in severe coronavirus disease (COVID-19), similarly to influenza, yet the clinical invasiveness is more debated.
Objectives
We investigated the invasive nature of pulmonary aspergillosis in histology specimens of influenza and COVID-19 ICU fatalities in a tertiary care center.
Methods
In this monocentric, descriptive, retrospective case series, we included adult ICU patients with PCR-proven influenza/COVID-19 respiratory failure who underwent postmortem examination and/or tracheobronchial biopsy during ICU admission from September 2009 until June 2021. Diagnosis of probable/proven viral-associated pulmonary aspergillosis (VAPA) was made based on the Intensive Care Medicine influenza-associated pulmonary aspergillosis and the European Confederation of Medical Mycology (ECMM) and the International Society for Human and Animal Mycology (ISHAM) COVID-19–associated pulmonary aspergillosis consensus criteria. All respiratory tissues were independently reviewed by two experienced pathologists.
Measurements and Main Results
In the 44 patients of the autopsy-verified cohort, 6 proven influenza-associated and 6 proven COVID-19–associated pulmonary aspergillosis diagnoses were identified. Fungal disease was identified as a missed diagnosis upon autopsy in 8% of proven cases (n = 1/12), yet it was most frequently found as confirmation of a probable antemortem diagnosis (n = 11/21, 52%) despite receiving antifungal treatment. Bronchoalveolar lavage galactomannan testing showed the highest sensitivity for VAPA diagnosis. Among both viral entities, an impeded fungal growth was the predominant histologic pattern of pulmonary aspergillosis. Fungal tracheobronchitis was histologically indistinguishable in influenza (n = 3) and COVID-19 (n = 3) cases, yet macroscopically more extensive at bronchoscopy in influenza setting.
Conclusions
A proven invasive pulmonary aspergillosis diagnosis was found regularly and with a similar histological pattern in influenza and in COVID-19 ICU case fatalities. Our findings highlight an important need for VAPA awareness, with an emphasis on mycological bronchoscopic work-up.
Keywords: invasive pulmonary aspergillosis, human influenza, COVID-19, critical illness, histology
At a Glance Commentary
Scientific Knowledge on the Subject
Probable influenza and coronavirus disease (COVID-19)-associated pulmonary aspergillosis is frequently diagnosed in critically ill patients, but data regarding histological proof of disease are lacking.
What This Study Adds to the Field
The autopsy data presented here show for the first time that proof of viral-associated pulmonary aspergillosis can be found regularly and with a similar histological pattern in deceased critically ill patients with influenza and COVID-19. Moreover, bronchoscopy with tracheobronchial tract visualization and galactomannan analysis of bronchoalveolar lavage provide the best nondefinite clues of viral-associated pulmonary aspergillosis disease, which is explained by the impeded, dispersed fungal growth pattern. Fungal tracheobronchitis was microscopically indistinguishable in influenza and COVID-19 cases, yet a more extensive disease severity was identified in influenza-associated pulmonary aspergillosis cases.
Respiratory viruses are known for their important strain on our healthcare system, specifically when epidemic and pandemic outbreaks occur, such as the H1N1pdm influenza outbreak of 2009 and recently the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral outbreak. Importantly, fungal infections have been increasingly recognized in the critically ill viral pneumonia setting, with a myriad of published reports in recent years and with increasing morbidity and mortality rates of superinfected patients. Influenza-associated pulmonary aspergillosis (IAPA) has been established as a life-threatening infection, occurring early after ICU admission in up to 19–25% of critically ill patients with influenza (1, 2). Serum mycological test positivity and histopathological proof are frequently reported, underscoring the invasive nature of the disease (3, 4). Coronavirus disease (COVID-19)-associated pulmonary aspergillosis (CAPA) has similarly emerged as an important coinfection in critically ill patients with COVID-19, although the clinical relevance of the disease has been more debated. The low sensitivity of serum mycological markers, reports on survival despite withholding antifungal treatment, and variable incidences of probable CAPA with discrepantly scarce evidence of proven disease upon histopathological examination have questioned the invasive nature of CAPA (5–8). Indeed, despite reported rates of probable CAPA in 15% of critically ill patients with COVID-19 with adequate mycological workup (8, 9), a review of autopsy studies of 677 COVID-19 decedents up to September 2020 showed evidence of proven invasive mold disease in only 2% of cases (10). Flikweert and colleagues could not find fungal presence in a single-center cohort of six postmortem lung biopsies from patients with antemortem probable CAPA diagnoses (5), whereas other single-center pathology cohorts with routine fungal staining identified proof of mold disease in 20–50% of performed autopsies (6, 11). Whether this discrepancy is the result of sampling or reporting bias or whether the positive mycological criteria represent a spectrum of disease with limited gold-standard fungal proof representing the tip of the iceberg of truly invasive infection is unclear. We, therefore, set out to investigate the invasive nature of pulmonary aspergillosis in the setting of critically ill patients with COVID-19 and influenza disease by examining a cohort of autopsy-verified patients from our tertiary care hospital. A descriptive analysis of mycological antemortem test characteristics with tissue-based proof of diagnosis of viral-associated pulmonary aspergillosis (VAPA) is reported here, showing that the proof lies in the tissue, yet clues can be identified antemortem by bronchoscopy.
Methods
Patients and Methods
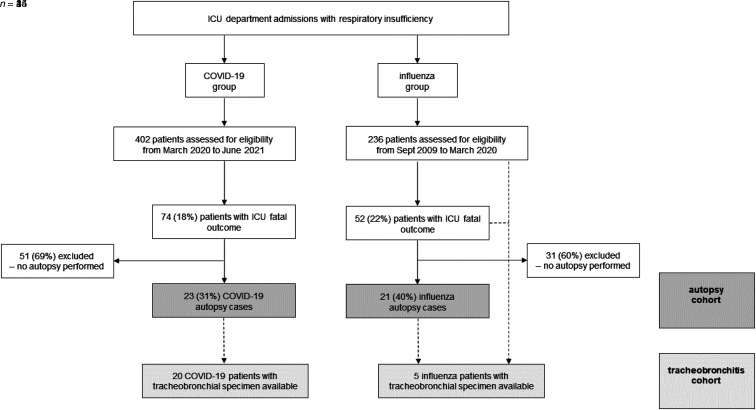
Eligible patients were adult ICU-admitted patients with PCR-proven influenza or COVID-19 respiratory insufficiency and a fatal ICU stay, whose postmortem examination was performed in routine care at our tertiary care hospital. For COVID-19 cases, the inclusion period ranged from March 2020 to June 2021, whereas influenza patients were included from March 2020 reaching backward until a similar autopsy sample size was obtained (until September 2009), as there was no concurrent influenza activity during the COVID-19 study period. This process defined our COVID-19 and influenza autopsy cohort. Limited tracheobronchial specimens were identified from autopsy cases; therefore, we also included patients with viral acute respiratory distress syndrome who underwent a lesion-driven tracheobronchial biopsy during ICU admission within the same period. The tracheobronchial tissue dataset (i.e., patients with antemortem and postmortem tracheobronchial tissue availability) was used to improve our view on viral-associated fungal tracheobronchitis (Figure 1). Demographic, microbiological, treatment, and outcome data were derived from the patient electronic medical records. Patients were first classified based on clinical suspicion of VAPA (probable VAPA), as determined by the Intensive Care Medicine consensus criteria for IAPA diagnosis (12) and on the European Confederation of Medical Mycology (ECMM) and the International Society for Human and Animal Mycology (ISHAM) criteria for CAPA (13). Second, patients were reclassified, based on pathological review, into one of the following categories: proven VAPA, probable unconfirmed VAPA, and no VAPA. Additional details on the construction of the autopsy and tracheobronchial tissue dataset and definitions used are provided in the online supplement (Methods and Figure E1). The retrospective study protocol was approved by the Ethical Committee of the University Hospitals Leuven, Belgium (S66599).
Figure 1.
Patient flowchart. Flowchart representation of the autopsy and tracheobronchial tissue datasets. COVID-19 = coronavirus disease.
Mycological Diagnostic Testing
During the retrospective study period, mycological diagnostic tests were performed according to the treating physician’s request. Galactomannan (GM) testing, Aspergillus PCR, and conventional mycological culture were performed in the clinical routine at our laboratory, the National Reference Center for Mycosis. GM testing was performed using the Platelia Aspergillus enzyme immunoassay (Bio-Rad), and Aspergillus PCR analysis was based on the AsperGenius multiplex real-time PCR assay (PathoNostics), according to the manufacturer’s instructions.
Histology Samples and Examination
The dataset included pulmonary tissue from postmortem examination and tracheobronchial tissue obtained either antemortem at a site of macroscopic tracheobronchitis or postmortem. All available hematoxylin and eosin–stained respiratory tract sections were collected from pathology archives, with additional Grocott-Gomori’s methenamine silver staining. Each sample was analyzed independently by two experienced pathologists (E.V. and G.D.H.). Per patient, a number of microscopic characteristics were evaluated according to presence and distribution: neutrophilic inflammation, inflammatory necrosis, coagulative necrosis, and Aspergillus-like hyphae. Distribution of organisms, morphology, and relation to surrounding tissue were recorded. Histological identification of Aspergillus-like hyphae was based on typical morphological features of hyaline, septate hyphae with acute-angle branching. Additional details on the type of histology samples and Aspergillus morphologic features are provided in the online supplement.
Statistical Analyses
Continuous variables are presented as mean (SD) or median (interquartile range [IQR]) according to distribution; categorical variables are summarized as numbers (percentages). We determined sensitivity and specificity of antemortem serum and BAL mycological tests. IBM SPSS v28.0.1.1 and Graphpad Prism v9 were used for statistical analysis.
Results
Autopsy Cohort: Clinical and Antemortem Mycological Characteristics
During the study period, 236 patients with severe influenza and 402 patients with severe COVID-19 were admitted to our ICU departments. The all-cause mortality rate was 22% (n = 52/236) for influenza-related critical illness and 18% (n = 74/402) for COVID-19–related critical illness. An autopsy was performed in 35% (n = 44/126) of critical viral disease fatalities: 40% (n = 21/52) of influenza and 31% (n = 23/74) of COVID-19 fatalities (Figure 1).
The baseline characteristics, ICU characteristics, and ICU timeline of the 44 autopsied patients who were critically ill with viral pneumonia—further referred to as the autopsy cohort—are summarized in Tables 1, E1, and E2, and Figure E2.
Table 1.
Clinical Characteristics of 44 Patients Critically Ill with Viral Pneumonia in the Autopsy Cohort
| Autopsy Cohort | Total (n = 44) | Antemortem Probable VAPA (n = 21) | Antemortem No Suspicion of VAPA (n = 23) |
|---|---|---|---|
| Baseline characteristics | |||
| Mean age (SD), yr | 65 (12) | 67 (9) | 64 (15) |
| Male sex | 29 (66) | 13 (62) | 16 (70) |
| BMI > 30 kg/m2 | 11 (25) | 3 (14) | 8 (35) |
| Diabetes mellitus | 11 (25) | 6 (29) | 5 (22) |
| Liver cirrhosis | 2 (5) | 2 (10) | 0 (0) |
| COPD | 5 (11) | 5 (24) | 0 (0) |
| Interstitial lung disease | 3 (7) | 2 (10) | 1 (4) |
| Smoking | 6 (14) | 2 (10) | 4 (17) |
| EORTC/MSGERC host factor | 12 (27) | 7 (33) | 5 (22) |
| CS EORTC/MSGERC criterium | 4 (9) | 3 (14) | 1 (4) |
| Hematological malignancy | 3 (7) | 2 (10) | 1 (4) |
| Immunosuppressive therapy | 12 (27) | 7 (33) | 5 (22) |
| Neutropenia | 1 (2) | 1 (5) | 0 (0) |
| Solid organ transplantation | 4 (9) | 3 (14) | 1 (4) |
| Systemic CS 30 d before ICU admission | 21 (48) | 12 (57) | 9 (39) |
| Median dose systemic CS 30 d before ICU admission* | 0.10 (0.03 to 0.33) (n = 21) | 0.10 (0.04 to 0.56) (n = 12) | 0.10 (0.02 to 0.23) (n = 9) |
| Viral disease | |||
| Influenza | 21 (48) | 9 (43) | 12 (52) |
| COVID-19 | 23 (52) | 12 (57) | 11 (48) |
| ICU stay characteristics | |||
| Mean APACHE II score on admission (SD) | 22 (7) (n = 30) | 22 (8) (n = 16) | 23 (6) (n = 14) |
| Duration between viral symptom onset and ICU admission, d | 6 (3 to 10) (n = 41) | 8 (2 to 10) (n = 19) | 6 (4 to 8) (n = 22) |
| Invasive ventilator support | 42 (95) | 20 (95) | 22 (96) |
| Nitric oxide inhalation | 16 (36) | 8 (38) | 8 (35) |
| Prone ventilation | 23 (52) | 13 (62) | 10 (43) |
| ECMO | 11 (25) | 2 (10) | 9 (39) |
| Duration of noninvasive ventilation,† d | 4 (2 to 7) (n = 35) | 4 (1 to 8) (n = 18) | 4 (3 to 8) (n = 17) |
| Duration of invasive ventilation, d | 20 (9 to 27) (n = 42) | 21 (10 to 31) (n = 21) | 16 (8 to 26) (n = 21) |
| Vasopressor therapy | 40 (91) | 17 (81) | 23 (100) |
| Renal replacement therapy | 19 (43) | 9 (43) | 10 (43) |
| Systemic CS during ICU stay | 43 (98) | 21 (100) | 22 (96) |
| Median dose systemic CS during ICU* | 0.60 (0.40 to 1.00) (n = 43) | 0.60 (0.35 to 0.92) (n = 21) | 0.70 (0.38 to 1.00) (n = 22) |
| Tocilizumab | 1 (2) | 1 (5) | 0 (0) |
| ICU length of stay, d | 22 (11 to 32) | 21 (13 to 44) | 22 (10 to 30) |
| Antemortem suspicion of aspergillosis | |||
| Serum GM testing | |||
| Performed | 28 (64) | 17 (81) | 11 (48) |
| Time between first sampling and ICU admission, d | 5 (1 to 10) (n = 28) | 7 (4 to 12) (n = 17) | 2 (0 to 2) (n = 11) |
| BAL GM testing | |||
| Performed | 40 (91) | 20 (95) | 20 (87) |
| Time between first sampling and ICU admission, d | 5 (2 to 11) (n = 40) | 5 (2 to 10) (n = 20) | 4 (−1 to 11) (n = 20) |
| Fungal culture | |||
| Bronchial aspirate fungal culture performed | 23 (52) | 14 (67) | 9 (39) |
| BAL fungal culture performed | 39 (89) | 20 (95) | 19 (83) |
| CT thorax | |||
| Performed | 36 (82) | 19 (90) | 17 (74) |
| Time between first CT thorax and ICU admission, d | 0 (−2 to 17) (n = 36) | 0 (−1 to 22) (n = 19) | 0 (−3 to 16) (n = 17) |
| Histology | |||
| Time between viral symptom onset and histology, d | 29 (17 to 42) (n = 41) | 30 (19 to 51) (n = 19) | 25 (14 to 41) (n = 22) |
| Histological evidence of IPA (all forms) | 12 (27) | 11 (52) | 1 (4) |
Definition of abbreviations: APACHE II = acute physiology and chronic health evaluation II; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CS = corticosteroids; CT = computed tomography; ECMO = extracorporeal membrane oxygenation; EORTC = European Organization for Research and Treatment of Cancer; GM = galactomannan; IPA = invasive pulmonary aspergillosis; MSGERC = Mycoses Study Group Education and Research Consortium; VAPA = viral-associated pulmonary aspergillosis.
Data are number (percentage) or median (interquartile range) unless otherwise specified.
Corticosteroid dose is expressed as mean dose in mg/kg/d of prednisone equivalent.
Noninvasive ventilation includes high-flow nasal oxygen therapy and noninvasive bilevel positive airway pressure/continuous positive airway pressure via face mask.
In 21 (48%) autopsy cases, a diagnosis of probable VAPA was made antemortem, which was largely based on respiratory tract sampling. Mycological antemortem characteristics are summarized in Table 2, showing limited contribution of serum GM evaluation in the probable VAPA diagnosis.
Table 2.
Mycological Characteristics of 21 Patients Critically Ill with Viral Pneumonia in the Autopsy Cohort with Probable Antemortem Invasive Pulmonary Aspergillosis Diagnosis
| Autopsy Cohort Patients with Antemortem Probable IPA Diagnosis (n = 21) | |
|---|---|
| Timing of antemortem IPA diagnosis | |
| Time between ICU admission and IPA diagnosis, d | 6 (3–17) |
| Time between viral symptom onset and IPA diagnosis, d | 14 (10–21) (n = 19) |
| Serum GM test positive (ODI > 0.5) | 5 (24) |
| Time between first positive GM serum and ICU admission, d | 9 (4–17) (n = 5) |
| Median ODI if positive | 4.6 (2.9–5.1) (n = 5) |
| BAL GM test positive (ODI ⩾ 1.0) | 20 (95) |
| Time between first positive GM BAL and ICU admission, d | 6 (2–21) (n = 20) |
| Median ODI if positive | 5.5 (4.6–6.1) (n = 20) |
| Fungal culture positive | |
| Positive fungal bronchial aspirate culture | 10 (48) |
| Positive fungal BAL fluid culture | 9 (43) |
| Tracheobronchitis* macroscopic diagnosis | 6 (29) |
| CT thorax cavitation | 2 (10) |
| Antifungal therapy | |
| Mold-active antifungal therapy during ICU | 21 (100) |
| Duration of treatment, d | 9 (6–25) |
| Tissue diagnosis of aspergillosis | |
| Histological evidence of IPA (all forms) | 11 (52) |
| Proven invasive Aspergillus tracheobronchitis | 4 (19) |
| Proven invasive pulmonary aspergillosis | 10 (48) |
Definition of abbreviations: CT = computed tomography; GM = galactomannan; IPA = invasive pulmonary aspergillosis; ODI = optical density index.
Data are number (percentage) or median (interquartile range).
Tracheobronchitis: signs of Aspergillus tracheobronchitis upon bronchoscopic evaluation include ulceration(s), nodule(s), pseudomembrane(s), plaque(s), and eschar(s).
Autopsy Cohort: Rate of Proven Fungal Disease
More than half of patients (n = 11/21, 52%) with an antemortem probable VAPA diagnosis had evidence of invasive pulmonary fungal disease upon autopsy, despite all receiving antifungal treatment for a median of 9 (IQR, 6–25) days. In addition, we identified one proven diagnosis upon autopsy, labeled as IAPA-negative antemortem given multiple negative mycological test results while alive. In total, a proven VAPA diagnosis was established in 27% of autopsy cases (n = 12/44), consisting of 29% (n = 6/21) of influenza autopsies and 26% (n = 6/23) of COVID-19 autopsy cases. Comparing proven (n = 12) and probable unconfirmed (n = 10) VAPA autopsy cases, we found a shorter median ICU stay and duration of mechanical ventilation before expiration in cases with a proven tissue diagnosis. An antemortem VAPA diagnosis tended to have taken place at a point in time closer to the autopsy (median, 9 vs. 21 d) in proven compared with probable unconfirmed cases, and, consequently, proven cases were treated with antifungals for a shorter length of time (proven vs. probable unconfirmed: median, 9 vs. 15 d) (Table E1).
Autopsy Cohort: Mycological Test Characteristics
Within the autopsy cohort, the sensitivity of BAL GM testing, BAL culture, and serum GM for VAPA diagnosis were 92%, 58%, and 33%, respectively. The sensitivity of serum GM for angioinvasion was 57%. Specificity was calculated from the test results of the VAPA histologically absent cases, irrespective of prior mold-active antifungal therapy, and was 64% for BAL GM, 93% for BAL culture, and 94% for serum GM. False-negative serum GM was seen in three patients with angioinvasion on histology, likely related to timing: the last serum measurement was 8 (IQR, 4–31) days before death if the serum GM was negative yet angioinvasion was found versus 1 (IQR, 0–2) day if the serum GM was positive and angioinvasion was found. Positive BAL GM results were comparable in optical density index (ODI) yet positive a shorter time before autopsy among proven cases: 4 (IQR, 3–8) days for proven cases versus 13 (IQR, 5–28) days in probable unconfirmed cases. Among the VAPA histologically absent cases without prior mold-active antifungal therapy, BAL GM specificity was 94%.
Autopsy Cohort: VAPA Histopathological Disease Pattern
Investigating the histopathological evidence of VAPA of the autopsy cohort, we found invasive fungal tracheobronchitis as the only manifestation in one patient and invasive fungal bronchopneumonia, either with (n = 3) or without (n = 8) associated proven tracheobronchitis, in 11 patients. Two distinct patterns of pulmonary fungal growth were identified within the autopsy cohort, namely unimpeded or impeded fungal growth.
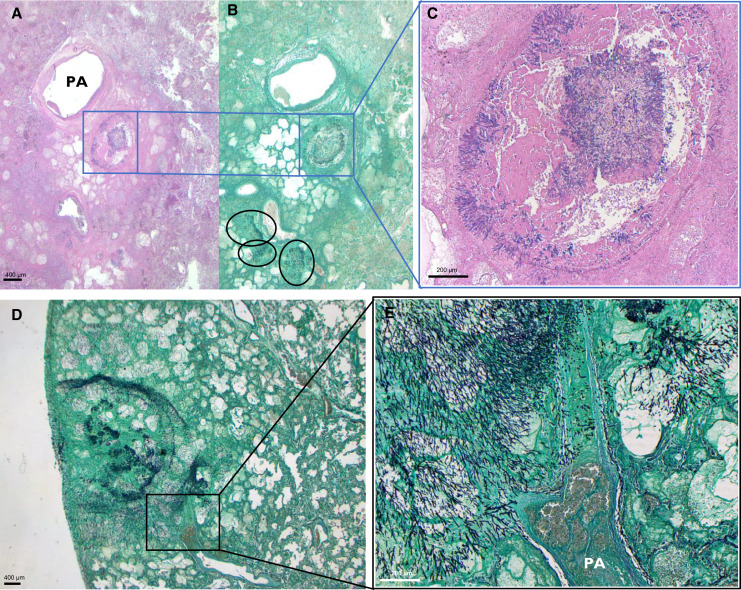
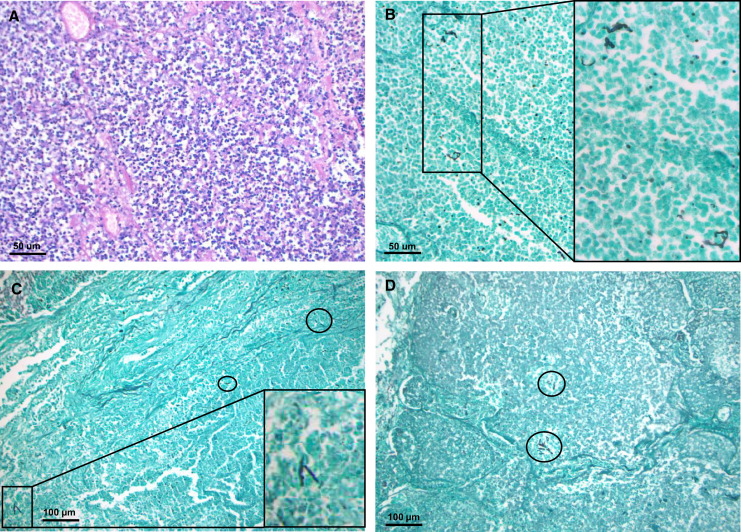
The unimpeded growth pattern (n = 2/11) was characterized by a high fungal load, manifesting as isometric centrifugal growing hyphae within large areas of coagulative necrosis (Figure 2). Alternatively, the impeded fungal growth pattern, most frequent within autopsy cases (n = 9/11), consisted of sparsely dispersed, fragmented hyphae found in acutely inflamed and/or necrotic lung tissue (Figure 3). Fungal burden was limited and was surmounted by the host inflammatory response within this second pattern, yet it was frequently found as multifocal (n = 9/11) and even multilobular (n = 6/11) disease.
Figure 2.
Viral-associated pulmonary aspergillosis: unimpeded fungal growth pattern. (A–C) Histological images of a patient with influenza-associated pulmonary aspergillosis (IAPA). Histological image derived from left lower lobe, at a magnification of ×8.75; hematoxylin and eosin stain (A) and Grocott-Gomori’s methenamine silver stain (Grocott) (B). Multifocal unimpeded fungal growth within an area of coagulative necrosis is visualized, indicated by black circles and blue box. (C) Magnification of ×50 of the blue-boxed area, showing intrabronchial hyphal growth. (D and E) Lung slide Grocott staining of another patient with IAPA at ×10 (D) and ×50 (E), showing isometric centrifugal growth, invading into a bifurcating artery. PA = pulmonary artery.
Figure 3.
Viral-associated pulmonary aspergillosis: impeded fungal growth pattern. (A and B) Images derived from a case of coronavirus disease (COVID-19)-associated pulmonary aspergillosis, showing acute inflammation of alveolar tissue visualized with hematoxylin and eosin staining (A) and Grocott-Gomori’s methenamine silver (Grocott) staining (B) at ×200 magnification. Dispersed presence of hyphal structures within pneumonia, only apparent upon Grocott stain, is indicated with black box (with magnification). (C and D) Patient with influenza-associated pulmonary aspergillosis, type impeded fungal growth. Grocott staining visualizes bronchopneumonia with fragmented hyphae (black circles/box for magnification), overall low fungal burden, and important neutrophilic infiltration, ×100 magnification.
The VAPA pattern was unrelated to underlying viral disease or the duration of antifungal therapy. However, neutropenia was identified in the only case of pure, multilobular, unimpeded growth, and higher total doses of corticosteroid therapy were given during ICU stay to patients with exclusively impeded growth (0.92 vs. 0.40 mg/kg [mixed/unimpeded growth only] median daily dose of prednisone equivalent), evidently linking host response and fungal burden. The most frequent host response to hyphal presence was a neutrophilic necrotizing inflammation, yet granuloma formation was identified in a single case of proven VAPA (Figure E3A). Sporulating heads of Aspergillus without evident inflammatory reaction were visualized within the pleural cavity of a patient with COVID-19 acute respiratory distress syndrome and a long-standing CAPA diagnosis (Figure E3B). Together, these fungal histological manifestations underscore the interplay between the fungus and the host.
Within the impeded fungal growth pattern, the characteristic appearance of hyphal structures was frequently disturbed. Aspergillus fumigatus was identified, however, in the majority of all proven cases through culture and/or PCR (supplementary data available in the online supplement).
Tracheobronchial Tissue Cohort
In a limited subset of autopsy cases (n = 21/44; 48%), tracheobronchial tissue was available for review, yet we identified four additional critically ill patients with viral disease who underwent lesion-driven tracheobronchial sampling (without autopsy) within the study period. An overview of the tracheobronchial tissue dataset can be found in Figures 1 and E4, showing bronchoscopic fungal tracheobronchitis in 32% (n = 8/25) of patients and a histologically proven diagnosis in 24% (n = 6/25).
Within the tracheobronchial tissue–verified group of patients, irrespective of prior mold-active antifungal therapy, sensitivity and specificity of macroscopic identification of ulceration(s), plaque(s), pseudomembrane(s), or nodule(s) upon bronchoscopy were both 83%.
Fungal disease presence could not be verified by pathology in cases of atypical, limited macroscopic findings with negative further mycological test results (additional details provided in the online supplement).
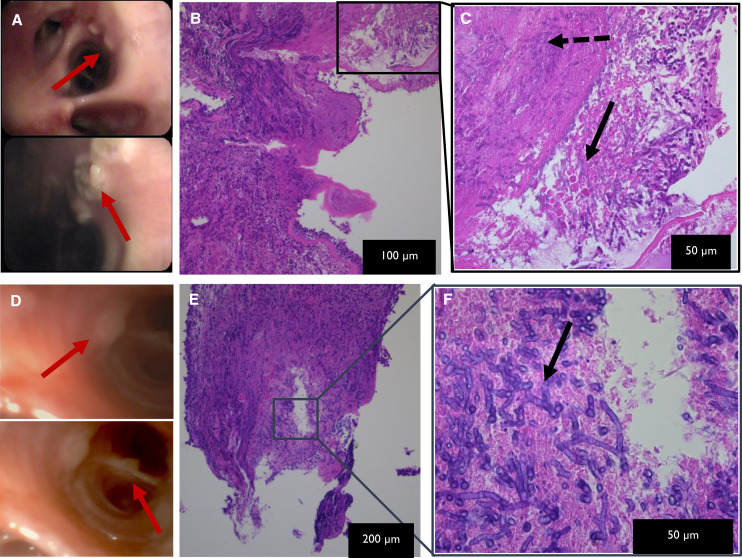
Interestingly, the three cases of proven influenza-associated invasive Aspergillus tracheobronchitis (IATB) showed extensive bilateral lesions upon bronchoscopy and were all serum GM positive, in contrast to the three less abundant and serum GM–negative proven COVID-19–associated IATB cases (Table E3), although microscopically the images were similar (Figure 4).
Figure 4.
Viral-associated invasive Aspergillus tracheobronchitis. Influenza-associated invasive Aspergillus tracheobronchitis: (A) Macroscopic image from bronchoscopy, showing extensive white nodular (red arrows) tracheobronchitis with central ulceration of noduli. (B and C) Microscopic hematoxylin and eosin–stained images at different magnifications, as indicated on the pictures of endobronchial biopsy, showing ulcerated epithelium with neutrophilic debris and acute-angle branching hyphae (black solid arrow) and hyphal invasion into tissue (black dashed arrow). Similar combination of macroscopic (D) and microscopic (E and F) evaluations from a case of coronavirus disease (COVID-19)–associated invasive Aspergillus tracheobronchitis. More extensive macroscopic inflammation visualized in influenza setting, yet comparable microscopic image in influenza and COVID-19 viral background.
Discussion
A proven diagnosis of invasive pulmonary aspergillosis (IPA) requires tissue analysis (14). Here, we identified six proven IAPA and six proven CAPA cases, corresponding to a prevalence of proven VAPA of at least 12% (n = 6/52) and 8% (n = 6/74) among all ICU influenza and COVID-19 case fatalities within the study period. Histopathological proof of fungal disease was identified as a missed diagnosis upon autopsy in 8% of proven cases (n = 1/12) and was frequently found as confirmation of a probable VAPA antemortem diagnosis (n = 11/21, 52%). Our dataset of patients with a fatal outcome, therefore, corroborates the ICM IAPA consensus expert (12) and ECMM/ISHAM CAPA (13) probable diagnostic criteria. Moreover, our data mark the invasive nature of IPA in the context of severe viral infection, irrespective of the underlying viral disease entity and antifungal therapy.
The prevalence of proven VAPA described here is high, although it is still an underestimation given the overall 35% rate of postmortem examination. This is in line with data from the largest ICU retrospective study of more than 400 patients with severe influenza, in which a proven IAPA diagnosis was made in 15% of all fatal influenza ICU admissions (1). Earlier evidence from IAPA case reports and case series report an even higher frequency of proven IAPA, in up to 31% or 61%, when evaluating only immunocompetent IAPA cases (3, 15), likely due to a publication bias with frequent histological examination. More recent retrospective cohort studies rarely indicate proven IAPA (13% of IAPA cases or 3% of fatal influenza ICU admissions in a cohort from Canada [16]; 0 cases in a Swiss cohort of 81 patients with influenza with 11% probable diagnosis [17]); importantly, none of these studies report the rate of postmortem examination. Published rates of proven CAPA are similarly low, as demonstrated by a review of histology reports from 677 COVID-19 casualties showing evidence of IPA in merely 1% of cases, although not all autopsies were ICU cases, nor was fungal staining routinely performed (10). Large single-center autopsy studies from the first waves of the COVID-19 pandemic report proven CAPA incidence varying between 4% and 38% (6, 7, 11). A recent Italian study showed a 12% incidence of probable CAPA among 167 ICU-admitted patients with COVID-19. The COVID-19 ICU mortality rate was not specified, yet 20% of CAPA cases (n = 4/20) were proven upon autopsy (18). These contradictory pathology results and overall low recent published rates of proven CAPA (and IAPA) need to be interpreted with caution.
First, the frequency of postmortem examination has declined considerably in the past decades, thereby limiting our chances to correctly identify the prevalence of proven fungal disease. The latest prepandemic data of the World Health Organization–European Health Information Gateway demonstrate an overall hospital autopsy rate within the European Union of <10% (19). During the first waves of the COVID-19 pandemic, pathologic examination was rarely performed, as evidenced by the discrepantly low number of only 677 published autopsy reports available for review up to September 2020 and the cumulative reported COVID-19 death rate of 1 million in the same period (10, 20). These low necropsy rates lead to a reporting bias that simply does not allow reliable assessment (21).
Second, our study findings warn for sampling error, given hyphal presence was sparsely dispersed in the majority of patients with VAPA, and given the exemplary absence of fungal detection upon postmortem bedside biopsy in a patient notwithstanding that definite VAPA proof was found upon additional extensive autopsy review. Sampling error could explain why none of the postmortem lung biopsies of six patients with probable CAPA in a case series from the Netherlands verified fungal presence (5).
Third, it is important to note that aspergillosis still ranks among the top major missed diagnoses upon autopsy, specifically in critically ill patients (22–24). Indeed, a missed diagnosis of VAPA was still identified upon autopsy in one patient with proven VAPA in this retrospective dataset. Moreover, in a recent Spanish publication on the value of Aspergillus recovery in respiratory samples, a total of 3 cases of unsuspected CAPA were identified at autopsy among 31 hospitalized patients with COVID-19 undergoing autopsy analysis (25).
Our data argue for high awareness of fungal infection in patients with influenza or COVID-19 in the ICU, with the need for bronchoscopic diagnostic evaluation to reduce the risk of a missed diagnosis. Importantly, BAL GM was the best predictor for definite VAPA diagnosis, with a sensitivity at 92%. This high sensitivity confirms that identified by the prospective study of Meersseman and colleagues in a general medical ICU setting with a high autopsy rate (26) and endorses the probable IAPA and CAPA consensus definitions, which rely heavily on BAL GM positivity (12, 13). Evaluating all patients without fungal presence upon autopsy, irrespective of antifungal treatment, our BAL GM–calculated specificity was only 64%, with a false-positivity rate of 36%. One possible explanation is remission of fungal disease at expiration, as proven cases received shorter duration of antifungal treatment and were found BAL GM positive until a point in time closer to the autopsy. Only one patient with a “false-positive” BAL GM did not receive mold-active treatment. This patient had a singular positive BAL GM value (with repeated testing yielding negative results) coinciding with a high-quantity culture of Candida species from the BAL fluid sample, a finding known to cause false positivity of BAL GM (27). Although a cut-off ODI of 1.0 or more was used, as per current IAPA and CAPA diagnostic criteria (12, 13), we noticed high ODI values of BAL GM in positive cases. We, therefore, argue that, given the right clinical context, a high positive BAL GM in a critically ill patient with influenza or COVID-19 should not be discarded as colonization. When in doubt, specifically if a low positive ODI is reported, repeat bronchoscopy with sampling should be performed or additional PCR testing on the BAL sample could be ordered (28).
Our study is the first to identify different histologic patterns of IPA in the context of viral respiratory failure. The sole case of multifocal, unimpeded fungal growth in respiratory tissue and vasculature was identified in the only patient with neutropenia within our cohort, comparable to the histology of neutropenic IPA animal models (29, 30). Importantly, the predominant fungal pattern found in our VAPA cohort, so-called impeded fungal growth, was characterized by neutrophilic inflammatory tissue damage and limited fungal burden. This difference in pattern, also seen in hematopoietic stem cell transplant recipients with graft-versus-host disease and nonneutropenic immunocompromised patients (31, 32), explains the importance of BAL mycological testing in the viral pneumonia setting. Angioinvasion was, however, more frequently identified than anticipated based on serum GM measurements, both in influenza and in COVID-19 cases, probably because of the antemortem timing of serum measurements. Although only influenza-associated IATB was associated with serum GM positivity, angioinvasion could not be verified within the available tracheobronchial tissue samples because of lack of depth. Our data show that typical bronchoscopic tracheobronchial lesions, especially when extensive and combined with other positive mycological test results, do not require biopsy verification. Immediate antifungal treatment is required, given the reported high mortality rates of IATB (4, 33).
Our study has several limitations. First, because of the retrospective nature of the study, patient selection bias cannot be ruled out. Autopsy is routinely requested in patients who die in our ICU departments, yet the overall autopsy rate was only 35% because of capacity limitations of the pathology department. Even so, to reduce the risk of additional bias, such as sampling bias, we extensively reviewed all available pulmonary tissue samples acquired during ICU admission. Second, no statistical comparisons between patients with influenza and patients with COVID-19, or between antemortem diagnostics and autopsy findings, are reported because of sample size limitations and the associated potential for irreproducibility of odds ratios for VAPA prediction. Third, as this study was based predominantly on autopsy material, association of the diagnostic criteria with outcome is not possible. VAPA likely represents a spectrum of disease, varying from tissue- to angioinvasion, with associated gradational mortality (28, 34). Our study can only shed light on the characteristics of the most severe course of VAPA disease. Finally, Aspergillus species identification was available for the majority, although not all proven cases. Lack of tissue-based PCR verification could be explained, however, by technical issues due to formalin fixation (35). All cases without species identification were BAL and/or serum GM positive, a finding that has infrequently been described in cases of fusariosis (36, 37), rendering it very likely that all proven invasive mold infections presented here represent invasive aspergillosis cases.
Overall, our retrospective case series highlights for the first time that histological proof of invasive fungal disease can be found regularly and with a similar histological pattern among critically ill influenza and COVID-19 cases, indicating a remarkable resemblance in pathophysiology, at least for the most severe disease courses. Although proof of VAPA requires tissue, bronchoscopy with tracheobronchial tract visualization and GM analysis of BAL provides the best nondefinite clues of VAPA disease, which is explained by the impeded, dispersed fungal growth pattern. Given the frequent presence of tissue-invasive fungal disease despite antifungal treatment administration in all probable VAPA cases, there is a high need for VAPA awareness with early mycological bronchoscopic evaluation, host-derived biomarkers, and improved immunomodulatory therapeutic strategies in critically ill patients with influenza and COVID-19.
Acknowledgments
Acknowledgment
The authors thank Geert Van der Borght (Pathology Laboratory, University Hospitals Leuven) and Bo Corbeels and Kathleen Van den Eynde (Department of Imaging and Pathology, KU Leuven), for their assistance in sample collection and preparation. They also thank Els Tuerlinckx (Forensic Medicine, University Hospitals Leuven) and Lauren Vandersloten (Medical Intensive Care Unit, University Hospitals Leuven) for their assistance in data collection, and Michel Feron (information technology department, University Hospitals Leuven) for his assistance in bronchoscopic image processing.
Footnotes
Supported by Fonds Wetenschappelijk Onderzoek (FWO) grant G053121N (S.H.-B., K.L., G.V.V., and J.W.); FWO Ph.D. fellowship grants 11E9819N (L.V.), 11M6922N (S.F.), and 1186121N (L.S.); and FWO Fundamental Clinical Research fellowship grant 1805116N (G.H.).
Author Contributions: Conceptualization and study design: L.V., E.V., G.D.H., and J.W. Investigation: L.V., C.J., S.F., A.R.-S., Y.D., G.H., S.H.-B., K.L., P.M., M.P., L.S., A.V., G.V.V., E.V.W., A.W., E.V., G.D.H., and J.W. Writing – original draft: L.V. Writing – review & editing: G.D.H., E.V., and J.W. Supervision: E.V., G.D.H., and J.W. All authors read and approved the final manuscript.
Data sharing: Individual patient data that underlie the results reported in this article are available from the corresponding author after publication upon reasonable and ethically approved request.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202208-1570OC on June 13, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Dutch-Belgian Mycosis study group Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med . 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 2. Vanderbeke L, Janssen NAF, Bergmans DCJJ, Bourgeois M, Buil JB, Debaveye Y, et al. Dutch-Belgian Mycosis Study Group Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial. Intensive Care Med . 2021;47:674–686. doi: 10.1007/s00134-021-06431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis . 2018;31:471–480. doi: 10.1097/QCO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 4. Nyga R, Maizel J, Nseir S, Chouaki T, Milic I, Roger PA, et al. Invasive tracheobronchial aspergillosis in critically ill patients with severe influenza: a clinical trial. Am J Respir Crit Care Med . 2020;202:708–716. doi: 10.1164/rccm.201910-1931OC. [DOI] [PubMed] [Google Scholar]
- 5. Flikweert AW, Grootenboers MJJH, Yick DCY, du Mée AWF, van der Meer NJM, Rettig TCD, et al. Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care . 2020;59:149–155. doi: 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fortarezza F, Boscolo A, Pezzuto F, Lunardi F, Jesús Acosta M, Giraudo C, et al. Proven COVID-19-associated pulmonary aspergillosis in patients with severe respiratory failure. Mycoses . 2021;64:1223–1229. doi: 10.1111/myc.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farias ME, Santana MF, Ferreira L, Borba M, Silva-Neto J, Brito-Sousa JD, et al. COVID-19-associated pulmonary aspergillosis in a series of complete autopsies from the Brazilian Amazon. Am J Trop Med Hyg . 2022;106:571–573. doi: 10.4269/ajtmh.21-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prattes J, Wauters J, Giacobbe DR, Salmanton-García J, Maertens J, Bourgeois M, et al. ECMM-CAPA Study Group Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect . 2022;28:580–587. doi: 10.1016/j.cmi.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gangneux JP, Dannaoui E, Fekkar A, Luyt CE, Botterel F, De Prost N, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med . 2022;10:180–190. doi: 10.1016/S2213-2600(21)00442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kula BE, Clancy CJ, Hong Nguyen M, Schwartz IS. Invasive mould disease in fatal COVID-19: a systematic review of autopsies. Lancet Microbe . 2021;2:e405–e414. doi: 10.1016/S2666-5247(21)00091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evert K, Dienemann T, Brochhausen C, Lunz D, Lubnow M, Ritzka M, et al. Autopsy findings after long-term treatment of COVID-19 patients with microbiological correlation. Virchows Arch . 2021;479:97–108. doi: 10.1007/s00428-020-03014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verweij PE, Rijnders BJA, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med . 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. European Confederation of Medical Mycology; International Society for Human Animal Mycology; Asia Fungal Working Group; INFOCUS LATAM/ISHAM Working Group; ISHAM Pan Africa Mycology Working Group; European Society for Clinical Microbiology; Infectious Diseases Fungal Infection Study Group; ESCMID Study Group for Infections in Critically Ill Patients; Interregional Association of Clinical Microbiology and Antimicrobial Chemotherapy; Medical Mycology Society of Nigeria; Medical Mycology Society of China Medicine Education Association; Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology; Association of Medical Microbiology; Infectious Disease Canada Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance Lancet Infect Dis 2021. 21 e149 e162 33333012 [Google Scholar]
- 14. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis . 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah MM, Hsiao EI, Kirsch CM, Gohil A, Narasimhan S, Stevens DA. Invasive pulmonary aspergillosis and influenza co-infection in immunocompetent hosts: case reports and review of the literature. Diagn Microbiol Infect Dis . 2018;91:147–152. doi: 10.1016/j.diagmicrobio.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwartz IS, Friedman DZP, Zapernick L, Dingle TC, Lee N, Sligl W, et al. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: a retrospective cohort study from Alberta, Canada. Clin Infect Dis . 2020;71:1760–1763. doi: 10.1093/cid/ciaa007. [DOI] [PubMed] [Google Scholar]
- 17. Waldeck F, Boroli F, Suh N, Wendel Garcia PD, Flury D, Notter J, et al. Influenza-associated aspergillosis in critically-ill patients-a retrospective bicentric cohort study. Eur J Clin Microbiol Infect Dis . 2020;39:1915–1923. doi: 10.1007/s10096-020-03923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casalini G, Giacomelli A, Galimberti L, Colombo R, Ballone E, Pozza G, et al. Challenges in diagnosing COVID-19-associated pulmonary aspergillosis in critically ill patients: the relationship between case definitions and autoptic data. J Fungi (Basel) . 2022;8:894. doi: 10.3390/jof8090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization, European Health Information Gateway. 2021. https://gateway.euro.who.int/en/indicators/hfa_544-6400-autopsy-rate-for-hospital-deaths/visualizations/#id=19639&tab=table
- 20.World Health Organization. 2020. https://covid19.who.int/
- 21. Shojania KG, Burton EC, McDonald KM, Goldman L. Overestimation of clinical diagnostic performance caused by low necropsy rates. Qual Saf Health Care . 2005;14:408–413. doi: 10.1136/qshc.2004.011973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tejerina EE, Abril E, Padilla R, Rodríguez Ruíz C, Ballen A, Frutos-Vivar F, et al. Invasive aspergillosis in critically ill patients: an autopsy study. Mycoses . 2019;62:673–679. doi: 10.1111/myc.12927. [DOI] [PubMed] [Google Scholar]
- 23. Caudron de Coquereaumont G, Couchepin J, Perentes JY, Krueger T, Lovis A, Rotman S, et al. Limited index of clinical suspicion and underdiagnosis of histopathologically documented invasive mold infections. Open Forum Infect Dis . 2021;8:ofab174. doi: 10.1093/ofid/ofab174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rusu S, Lavis P, Domingues Salgado V, Van Craynest MP, Creteur J, Salmon I, et al. Comparison of antemortem clinical diagnosis and post-mortem findings in intensive care unit patients. Virchows Arch . 2021;479:385–392. doi: 10.1007/s00428-020-03016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fortún J, Mateos M, de la Pedrosa EG, Soriano C, Pestaña D, Palacios J, et al. Invasive pulmonary aspergillosis in patients with and without SARS-CoV-2 infection. J Fungi (Basel) . 2023;9:130. doi: 10.3390/jof9020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med . 2008;177:27–34. doi: 10.1164/rccm.200704-606OC. [DOI] [PubMed] [Google Scholar]
- 27. Aigner M, Wanner M, Kreidl P, Lass-Flörl C, Lackner M. Candida in the respiratory tract potentially triggers galactomannan positivity in nonhematological patients. Antimicrob Agents Chemother . 2019;63:e00138-19. doi: 10.1128/AAC.00138-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dellière S, Dudoignon E, Voicu S, Collet M, Fodil S, Plaud B, et al. Combination of mycological criteria: a better surrogate to identify COVID-19-associated pulmonary aspergillosis patients and evaluate prognosis? J Clin Microbiol . 2022;60:e02169-21. doi: 10.1128/jcm.02169-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balloy V, Huerre M, Latgé JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun . 2005;73:494–503. doi: 10.1128/IAI.73.1.494-503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poelmans J, Himmelreich U, Vanherp L, Zhai L, Hillen A, Holvoet B, et al. A multimodal imaging approach enables in vivo assessment of antifungal treatment in a mouse model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother . 2018;62:e00240-18. doi: 10.1128/AAC.00240-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chamilos G, Luna M, Lewis RE, Bodey GP, Chemaly R, Tarrand JJ, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989-2003) Haematologica . 2006;91:986–989. [PubMed] [Google Scholar]
- 32. Stergiopoulou T, Meletiadis J, Roilides E, Kleiner DE, Schaufele R, Roden M, et al. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol . 2007;127:349–355. doi: 10.1309/UJRV9DLC11RM3G8R. [DOI] [PubMed] [Google Scholar]
- 33. Koehler P, von Stillfried S, Garcia Borrega J, Fuchs F, Salmanton-García J, Pult F, et al. Aspergillus tracheobronchitis in COVID-19 patients with acute respiratory distress syndrome: a cohort study. Eur Respir J . 2022;59:2103142. doi: 10.1183/13993003.03142-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ergün M, Brüggemann RJM, Alanio A, Dellière S, van Arkel A, Bentvelsen RG, et al. Aspergillus test profiles and mortality in critically ill COVID-19 patients. J Clin Microbiol . 2021;59:e0122921. doi: 10.1128/JCM.01229-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sadamoto S, Mitsui Y, Nihonyanagi Y, Amemiya K, Shinozaki M, Murayama SY, et al. Comparison approach for identifying missed invasive fungal infections in formalin-fixed, paraffin-embedded autopsy specimens. J Fungi (Basel) . 2022;8:337. doi: 10.3390/jof8040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, Munoz P, et al. European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect . 2014;20:27–46. doi: 10.1111/1469-0691.12465. [DOI] [PubMed] [Google Scholar]
- 37. Hoenigl M, Salmanton-García J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis . 2021;21:e246–e257. doi: 10.1016/S1473-3099(20)30784-2. [DOI] [PubMed] [Google Scholar]