Mesenchymal stromal cells (MSCs) have garnered considerable attention as a potential therapeutic option for patients with acute respiratory distress syndrome (ARDS) because of their capacity to modulate inflammation and promote tissue repair (1, 2). In this editorial, we summarize the noteworthy findings of a study published in this issue of the Journal by Gorman and colleagues (pp. 256–269) on patients with coronavirus disease (COVID-19)-related ARDS (3). We discuss the implications of this study for the field and propose a potential strategy to enhance the likelihood of MSCs fulfilling their promise to improve outcomes in patients with ARDS.
The Study by Gorman and Colleagues
Gorman and colleagues conducted a multicenter, randomized, double-blind, placebo-controlled trial to investigate the safety and efficacy of a single dose of 400 × 106 cells (ORBCEL-C), a CD362-enriched, umbilical cord–derived MSC product, in 60 patients with moderate-to-severe COVID-19–related ARDS. The primary outcomes assessed were the incidence of serious adverse events (safety) and the oxygenation index (efficacy) at Day 7. Secondary outcomes included respiratory compliance, driving pressure, PaO2/FiO2 ratio, and Sequential Organ Failure Assessment score, as well as clinical outcomes such as ventilation duration, length of ICU and hospital stays, mortality, and long-term follow-up measures.
The study yielded encouraging results regarding the safety profile of ORBCEL-C, demonstrating no significant difference in the incidence of serious adverse events compared with placebo. In addition, transcriptomic analysis of peripheral blood samples provided evidence that the ORBCEL-C was active in modulating the peripheral blood transcriptome. However, despite the administration of a very large single dose of MSCs, there were no significant differences in the Day 7 oxygenation index, most secondary outcomes (including surrogate markers of pulmonary dysfunction), and mortality rates at various time points up to 2 years.
Dosing, Sex Disparity, and Subgroup Analysis
A key question is why this study was negative, considering the substantial body of basic science evidence suggesting the efficacy of MSCs in acute lung injury. Several potential explanations exist—aside from the possibility that MSCs simply lack efficacy in COVID-19 ARDS. First, it is possible that the specific dose of ORBCEL-C used, although large, was still insufficient to exert significant therapeutic effects in this patient population. The heterogeneity of the baseline characteristics, biology, physiology, and pathology among patients with ARDS may lead to variability in treatment response, necessitating individualized dosing strategies (4, 5). Second, the effectiveness of MSC therapy may be influenced by factors beyond dosage, including the timing of administration and the local microenvironment within the lungs (6). For example, the function of MSCs can be compromised by unfavorable metabolic conditions, such as acidosis, which are commonly observed in injured lungs (7). Third, the observed sex disparity in COVID-19 outcomes, with higher mortality rates in males, has been observed globally (5). In the study by Gorman and colleagues, 80% of participants in the ORBCEL-C group were male (similar to other studies). Considering the observed heightened inflammatory responses (IL-6, IL-8, MCP-1, GRO, sCD40L, and MIP-1β) in males compared with females, even when matched for COVID-19 severity (8), and preclinical research highlighting the crucial role of the overwhelming inflammatory lung environment in determining outcomes during MSC administration (6), it is possible that sex-specific effects of ORBCEL-C therapy may have been present. Further research with a larger sample size would be required to assess this hypothesis.
Limitations
Although the study by Gorman and colleagues was executed meticulously, incorporating blinding, randomization, biological analyses, and long-term follow-up, it is important to acknowledge several limitations. The study did not report cytokine levels before and after MSC treatment, including the pivotal inflammatory cytokine IL-6 implicated in COVID-19 ARDS. The absence of IL-6 blockade might have influenced the observed outcomes (2, 9). In addition, transcriptomic profiles obtained from peripheral blood samples were not extensively described in this publication, leaving a gap in our understanding of potential biomarkers that could guide MSC therapy. Further analysis of the transcriptomic data, including IL-6, fibronectin, and total antioxidant capacity levels (6), could provide valuable insights into patient-specific responses to MSCs and help identify potential responders.
Future Directions
Based on the results, we suggest the following future directions (Figure 1):
-
1.
Pulmonary microenvironment: Understanding the individual patient’s pulmonary microenvironment is likely important to help identify potential responders to MSC therapy in ARDS (6). Factors such as cytokine and growth factor secretion and oxidative stress levels can influence the immunomodulatory effects of MSCs and identify patients likely to respond (6, 9, 10). Modifying the microenvironment, for example, by activating antioxidant enzymes (6) and correcting the acidic conditions in the lungs (7) before administering MSCs may be beneficial in patients in whom the pulmonary microenvironment is considered unfavorable for cell therapy. The use of second-generation bioengineered MSCs incorporating genes targeting specific risk molecules could also confer advantages (6, 10). Detailed transcriptomic analyses, similar to those performed in this study, coupled with statistical tools addressing heterogeneous treatment effects (11), can provide valuable insights into the patients most likely to respond.
-
2.
Dose and route of administration: Ascertaining the appropriate dosage of MSCs and the most suitable route of administration (i.e., intravenous infusion, inhalation, or direct lung delivery) based on factors such as target site, safety, and efficacy is vital to achieving optimal responses. Exploring different dosing strategies, including fractionated doses or multiple administrations, and conducting studies involving large patient cohorts, albeit challenging, would be extremely helpful.
-
3.
Patient heterogeneity and characteristics: Assessing heterogeneous treatment effects based on clinical variables (e.g., severity, sex) alongside biomarkers would be helpful, including investigating sex hormone levels, immune response markers, and comorbidities. Evaluating whether baseline transcriptomic profiles differ between sexes and influence treatment response would also be pertinent.
-
4.
Potential benefits of combining MSC therapy: Investigating combination therapies, such as the concurrent administration of ORBCEL-C with other therapeutic agents or interventions (12), could enhance the efficacy of MSC therapy. Combination therapies targeting multiple aspects of ARDS, encompassing inflammation, immune dysregulation, and tissue repair and regeneration, may yield synergistic effects and improve patient outcomes.
Figure 1.
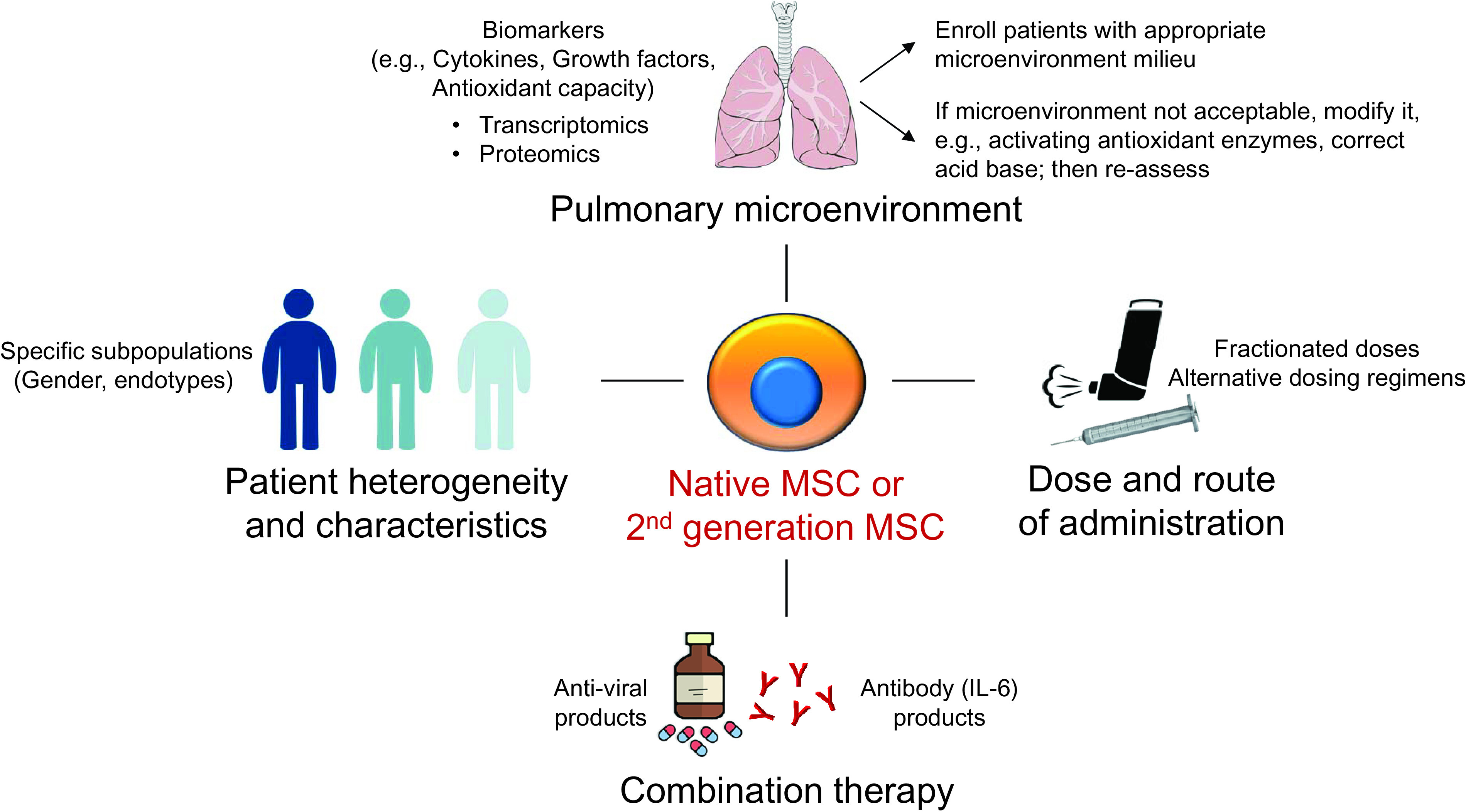
Suggested future lines of research for MSCs in patients with acute respiratory distress syndrome. MSCs = mesenchymal stromal cells.
In conclusion, the study by Gorman and colleagues provides valuable insights into the safety and efficacy of ORBCEL-C in COVID-19–related ARDS. Although the majority of clinical studies using MSCs thus far have yielded somewhat disappointing results, further research focused on the importance of the lung microenvironment, the identification of potential responders, and the exploration of combination therapies may help fulfill the promise generated by groundbreaking basic studies on MSCs in acute lung injury.
Footnotes
Supported by the Canadian Institutes of Health Research (OV3-170344, SBC-171482, and VS1-175560 to H.Z. and A.S.).
Originally Published in Press as DOI: 10.1164/rccm.202306-0969ED on June 15, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med . 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med . 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gorman EA, Rynne J, Gardiner HJ, Rostron AJ, Bannard-Smith J, Bentley AM, et al. Repair of acute respiratory distress syndrome in COVID-19 by stromal cells (REALIST-COVID trial): a multicentre, randomised, controlled trial. Am J Respir Crit Care Med . 2023;208:256–269. doi: 10.1164/rccm.202302-0297OC. [DOI] [PubMed] [Google Scholar]
- 4. Adamos G, Gavrielatou E, Sarri K, Kokkoris S. Heterogeneity of acute respiratory distress syndrome. Am J Respir Crit Care Med . 2020;201:728–730. doi: 10.1164/rccm.201906-1110RR. [DOI] [PubMed] [Google Scholar]
- 5. Juschten J, Tuinman PR, Guo T, Juffermans NP, Schultz MJ, Loer SA, et al. Between-trial heterogeneity in ARDS research. Intensive Care Med . 2021;47:422–434. doi: 10.1007/s00134-021-06370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Islam D, Huang Y, Fanelli V, Delsedime L, Wu S, Khang J, et al. Identification and modulation of microenvironment is crucial for effective mesenchymal stromal cell therapy in acute lung injury. Am J Respir Crit Care Med . 2019;199:1214–1224. doi: 10.1164/rccm.201802-0356OC. [DOI] [PubMed] [Google Scholar]
- 7. Nykänen AI, Mariscal A, Duong A, Estrada C, Ali A, Hough O, et al. Engineered mesenchymal stromal cell therapy during human lung ex vivo lung perfusion is compromised by acidic lung microenvironment. Mol Ther Methods Clin Dev . 2021;23:184–197. doi: 10.1016/j.omtm.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qi S, Ngwa C, Morales Scheihing DA, Al Mamun A, Ahnstedt HW, Finger CE, et al. Sex differences in the immune response to acute COVID-19 respiratory tract infection. Biol Sex Differ . 2021;12:66. doi: 10.1186/s13293-021-00410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H, Li Y, Slutsky AS. Precision medicine for cell therapy in acute respiratory distress syndrome. Lancet Respir Med . 2019;7:e13. doi: 10.1016/S2213-2600(19)30089-X. [DOI] [PubMed] [Google Scholar]
- 10. Faner R, Rojas M. Building strong neighborhoods in the lung with a little help from my mesenchymal stem cells. Am J Respir Crit Care Med . 2019;199:1176–1178. doi: 10.1164/rccm.201811-2153ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goligher EC, Lawler PR, Jensen TP, Talisa V, Berry LR, Lorenzi E, et al. REMAP-CAP, ATTACC, and ACTIV-4a Investigators Heterogeneous treatment effects of therapeutic-dose heparin in patients hospitalized for COVID-19. JAMA . 2023;329:1066–1077. doi: 10.1001/jama.2023.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castelnovo L, Tamburello A, Lurati A, Zaccara E, Marrazza MG, Olivetti M, et al. Anti-IL6 treatment of serious COVID-19 disease: a monocentric retrospective experience. Medicine (Baltimore) . 2021;100:e23582. doi: 10.1097/MD.0000000000023582. [DOI] [PMC free article] [PubMed] [Google Scholar]


