The face of chronic obstructive pulmonary disease (COPD) is rapidly evolving. Rather than a superficial view of a self-inflicted disease of elderly smokers, COPD has now been recognized as a complex condition with multiple etiotypes (1). Ultimately, the development of new therapies, however, relies on the discovery of endotypes that reflect varied pathobiological manifestations. Such COPD endotypes are currently being investigated, leveraging the numerous omics tools that are increasingly available (2). Indeed, the use of omics is driving the effort toward a “personalized,” “precision,” and “individualized” approach to COPD, which relies on genetic, biomarker, phenotypic, and psychosocial characteristics to distinguish between patients who all have airflow obstruction that defines the disease. Combined, this information could potentially help us anticipate the disease course and individual patient responses to therapies. Ultimately, it is hoped that an omics-based approach could help circumvent the current trial and error required to find effective therapies for individual patients living with COPD (3).
One of the major unmet needs in the identification of targetable COPD pheno/endotypes is the lack of blood biomarkers that can function as reliable surrogates of the pathobiological manifestations occurring in the COPD lung. The search for these biomarkers has recently been revolutionized by high-throughput sequencing techniques and multiplex platforms that can measure thousands of gene transcripts, proteins, or metabolites (4). Additionally, the development of several well-phenotyped longitudinal COPD cohorts with blood sampling has facilitated the identification of blood biomarkers of COPD (5).
Although most of the studies of blood biomarkers have focused on large-scale measurement of omics data types, including DNA, RNA, proteins, and metabolites, the circulating immune compartment in COPD has not been well explored, despite evidence that COPD is associated with profound alterations in immune cells (6). Novel, cell-type deconvolution approaches accurately infer the relative proportions of immune cell types from genome-wide blood gene expression data. Thus, cell-type deconvolution is a potentially powerful approach to enable the simultaneous study of many different cell types in large cohorts of participants (7) with available blood gene expression. This link between blood biomarkers and the identification of a circulating immune signature, or what one might term an “immunome,” could be a powerful tool to predict COPD onset, progression, and therapeutic targets.
In this issue of the Journal, Ryu and colleagues (pp. 247–255) looked at the relationship between blood-based molecular and cellular phenotypes with COPD exacerbations using three complementary datasets: blood RNA-sequencing data from the COPDGene study, blood microarray data from the ECLIPSE study for validation, and blood flow cytometry data from SPIROMICS (8–11).
The COPDGene study identified over 3,500 unique genes in the blood transcriptome that were associated with a history of exacerbation, persistent exacerbations, and/or prospective exacerbation rate. These data extend the findings of previous works associating frequent exacerbations to gene expression in the ECLIPSE study (10) and the authors’ previous analysis of the Treatment of Emphysema with a γ-Selective Retinoid Agonist study (12). However, whereas individual genes or even pathways may explain only a minority of the variance in clinical phenotypes, combinations of multiple biomarkers can explain a more extended variance for some COPD phenotypes. Thus, the authors took the next step to deconvolute the circulating cell-type composition to derive a blood immune signature, or “immunome,” associated with the risk of future exacerbations. It is interesting that, in the COPDgene study, the number of prospective exacerbations in subjects with COPD was inversely associated with the numbers of circulating CD8+ T cells, CD4+ T cells, and resting natural killer cells. The inverse association with naive CD4+ T cells was replicated in the ECLIPSE study. In the flow cytometry study, an increase in the immune checkpoint CTLA4 on CD4+ T cells, indicating an inhibition of the CD4+ T cell activity, was positively associated with acute exacerbations of COPD (AECOPDs).
Altogether, these data point to a blunted adaptive immune response, involving both the T helper and cytotoxic component, contributing to AECOPDs, either directly or indirectly by means of an off-targeted stimulation of the B cell compartment. In fact, the decrease in naive and resting memory CD4+ T cell populations was associated with increases in the plasma cell population in the frequent exacerbators. The involvement of activated antibody-producing B cells was confirmed by KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis and led the authors to put forth the compelling hypothesis that autoimmune-like responses may predispose patients with COPD to future exacerbations. Previous data have shown that patients with COPD—in particular, those with emphysema-predominant COPD—have upregulated B cell responses with autoimmune features (13, 14). To clarify whether this is also occurring in AECOPDs, a logical next step would be to assess whether the circulating plasma cell signature is enriched in frequent exacerbators with a greater extent of emphysema as defined by means of a computed tomography scan.
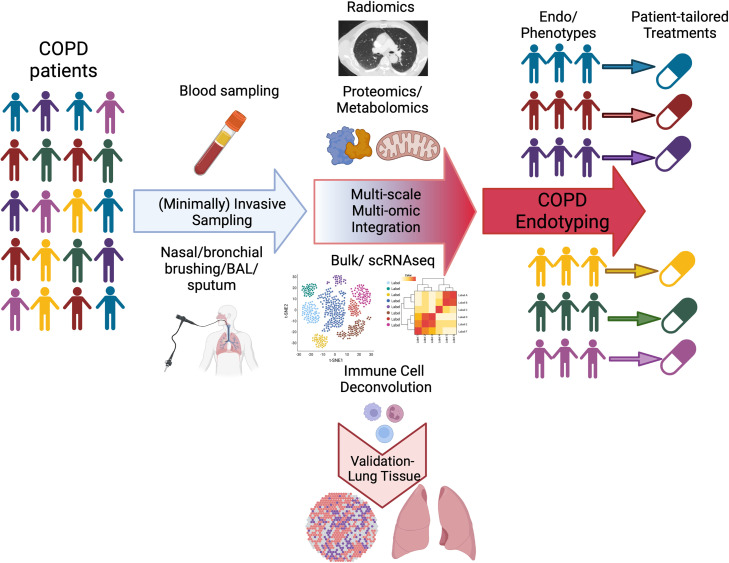
Furthermore, the exact role of the adaptive immune compartment in determining AECOPDs remains unclear, given the limitations of these observational cohorts. Whether these blood-based immunologic signatures reflect specific signatures in the COPD lung is hard to determine and opens interesting research avenues. The systemic inflammation may reflect a “spillover” of inflammatory processes within the lung, primary alterations in the extrapulmonary immune response, or a combination of both (7). It would be compelling to determine whether any of these immune cell populations deconvoluted in the blood can be confirmed longitudinally in the same compartment and/or in airway samples (e.g., bronchial brushing) using bulk and/or single-cell RNA sequencing. Also, it is important to determine whether, in other independent cohorts, the same features of the circulating immunome identified here can be found in lung tissue from frequent exacerbators versus nonexacerbators, although that would require a cross-sectional analysis of the COPD lung because of the obvious limitations in sampling lung tissue longitudinally (Figure 1).
Figure 1.
Leveraging omic endotyping to enable personalized medicine in chronic obstructive pulmonary disease (COPD). Individuals with COPD share many clinical traits that allow a common disease diagnosis, yet this shared phenotype accounts for different types of pathobiology (i.e., endotypes). Important steps in patient endotyping are 1) minimally invasive sampling of patient diseased tissue; 2) molecular and cellular characterization of diseased samples with analyses of bulk and or scRNAseq, metabolomic and proteomic processes, and radiological features to identify pathobiological mechanisms in the disease group (ultimately, this step will require validation of the findings in lung tissue from independent cohorts); 3) integration of multiscale, multi-omic data with machine-learning approaches; and 4) separation of patients into endotype groups on the basis of the presence and/or level of one or more of these pathobiology types. The endotype of the patient with COPD forms the basis for personalized clinical disease management. scRNAseq = single-cell RNA sequencing.
To conclude, this study from Ryu and colleagues highlights the need of shifting toward multi-omics panels as opposed to relying on singular markers in the stratification of patients with COPD. As we navigate this multi-omics era (Figure 1), machine-learning approaches have emerged as effective methods for mining and integrating large-scale, heterogeneous medical data for clinical practice. Rather than oversimplifying subjects into broad categories that are based on clinical phenotyping alone, large-scale, data-driven analyses could integrate select biomarker subsets, thus allowing better identification of patients who are susceptible to COPD exacerbation and enabling more nuanced and effective stratification of patients with COPD.
Footnotes
Supported by NHLBI Division of Intramural Research grant HL149744.
Originally Published in Press as DOI: 10.1164/rccm.202306-0978ED on June 23, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 report: GOLD executive summary. Am J Respir Crit Care Med . 2023;207:819–837. doi: 10.1164/rccm.202301-0106PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stolz D, Mkorombindo T, Schumann DM, Agusti A, Ash SY, Bafadhel M, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet . 2022;400:921–972. doi: 10.1016/S0140-6736(22)01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sidhaye VK, Nishida K, Martinez FJ. Precision medicine in COPD: where are we and where do we need to go? Eur Respir Rev . 2018;27:180022. doi: 10.1183/16000617.0022-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Regan EA, Hersh CP, Castaldi PJ, DeMeo DL, Silverman EK, Crapo JD, et al. Omics and the search for blood biomarkers in chronic obstructive pulmonary disease. Insights from COPDGene. Am J Respir Cell Mol Biol . 2019;61:143–149. doi: 10.1165/rcmb.2018-0245PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stockley RA, Halpin DMG, Celli BR, Singh D. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med . 2019;199:1195–1204. doi: 10.1164/rccm.201810-1860SO. [DOI] [PubMed] [Google Scholar]
- 6. Bhat TA, Panzica L, Kalathil SG, Thanavala Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc . 2015;12(Suppl 2):S169–S175. doi: 10.1513/AnnalsATS.201503-126AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halper-Stromberg E, Yun JH, Parker MM, Singer RT, Gaggar A, Silverman EK, et al. Systemic markers of adaptive and innate immunity are associated with chronic obstructive pulmonary disease severity and spirometric disease progression. Am J Respir Cell Mol Biol . 2018;58:500–509. doi: 10.1165/rcmb.2017-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ryu MH, Yun JH, Morrow JD, Saferali A, Castaldi P, Chase R, et al. Blood gene expression and immune cell subtypes associated with COPD exacerbations. Am J Respir Crit Care Med . 2023;208:247–255. doi: 10.1164/rccm.202301-0085OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghosh AJ, Saferali A, Lee S, Chase R, Moll M, Morrow J, et al. Blood RNA sequencing shows overlapping gene expression across COPD phenotype domains. Thorax . 2022;77:115–122. doi: 10.1136/thoraxjnl-2020-216401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh D, Fox SM, Tal-Singer R, Bates S, Riley JH, Celli B. Altered gene expression in blood and sputum in COPD frequent exacerbators in the ECLIPSE cohort. PLoS One . 2014;9:e107381. doi: 10.1371/journal.pone.0107381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freeman CM, Crudgington S, Stolberg VR, Brown JP, Sonstein J, Alexis NE, et al. Design of a multi-center immunophenotyping analysis of peripheral blood, sputum and bronchoalveolar lavage fluid in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) J Transl Med . 2015;13:19. doi: 10.1186/s12967-014-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morrow JD, Qiu W, Chhabra D, Rennard SI, Belloni P, Belousov A, et al. Identifying a gene expression signature of frequent COPD exacerbations in peripheral blood using network methods. BMC Med Genomics . 2015;8:1. doi: 10.1186/s12920-014-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sullivan JL, Bagevalu B, Glass C, Sholl L, Kraft M, Martinez FD, et al. B cell–adaptive immune profile in emphysema-predominant COPD. Am J Respir Crit Care Med . 2019;200:1434–1439. doi: 10.1164/rccm.201903-0632LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polverino F, Cosio BG, Pons J, Laucho-Contreras M, Tejera P, Iglesias A, et al. B cell-activating factor. An orchestrator of lymphoid follicles in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2015;192:695–705. doi: 10.1164/rccm.201501-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]