Abstract
Rationale
Predictors of adverse outcome in pulmonary hypertension (PH) are well established; however, data that inform survival are lacking.
Objectives
We aim to identify clinical markers and therapeutic targets that inform the survival in PH.
Methods
We included data from patients with elevated mean pulmonary artery pressure (mPAP) diagnosed by right heart catheterization in the U.S. Veterans Affairs system (October 1, 2006–September 30, 2018). Network medicine framework was used to subgroup patients when considering an N of 79 variables per patient. The results informed outcome analyses in the discovery cohort and a sex-balanced validation right heart catheterization cohort from Vanderbilt University (September 24, 1998–December 20, 2013).
Measurements and Main Results
From an N of 4,737 complete case patients with mPAP of 19–24 mm Hg, there were 21 distinct subgroups (network modules) (all-cause mortality range = 15.9–61.2% per module). Pulmonary arterial compliance (PAC) drove patient assignment to modules characterized by increased survival. When modeled continuously in patients with mPAP ⩾19 mm Hg (N = 37,744; age, 67.2 yr [range = 61.7–73.8 yr]; 96.7% male; median follow-up time, 1,236 d [range = 570–1,971 d]), the adjusted all-cause mortality hazard ratio was <1.0 beginning at PAC ⩾3.0 ml/mm Hg and decreased progressively to ∼7 ml/mm Hg. A protective association between PAC ⩾3.0 ml/mm Hg and mortality was also observed in the validation cohort (N = 1,514; age, 60.2 yr [range = 49.2–69.1 yr]; 48.0% male; median follow-up time, 2,485 d [range = 671–3,580 d]). The association was strongest in patients with precapillary PH at the time of catheterization, in whom 41% (95% confidence interval, 0.55–0.62; P < 0.001) and 49% (95% confidence interval, 0.38–0.69; P < 0.001) improvements in survival were observed for PAC ⩾3.0 versus <3.0 ml/mm Hg in the discovery and validation cohorts, respectively.
Conclusions
These data identify elevated PAC as an important parameter associated with survival in PH. Prospective studies are warranted that consider PAC ⩾3.0 ml/mm Hg as a therapeutic target to achieve through proven interventions.
Keywords: hypertension, pulmonary, hemodynamics, survival, network medicine
At a Glance Commentary
Current Scientific Knowledge on the Subject
Predictors of adverse outcome in pulmonary hypertension (PH) are well established; however, data on survival are lacking.
What This Study Adds to the Field
We used network medicine and conventional biostatistical approaches to identify pulmonary arterial compliance (PAC) as a key marker of survival in PH. Patient-patient networks and classical analyses identified elevated PAC as a marker of PH survival. These data support prospective studies that consider PAC as a novel PH therapeutic target.
Pulmonary hypertension (PH) is associated with increased morbidity (1) and is identified commonly among patients with cardiopulmonary diseases referred for right heart catheterization (RHC) (2). Among patients with PH, numerous independent predictors of adverse outcome are reported, including elevated right atrial pressure (3), increased pulmonary vascular resistance (PVR) (4), diminished functional status (5), and others. At present, prognosticating patients hinges primarily on the presence or absence of these high-risk findings, is limited mainly to pulmonary arterial hypertension, and does not address patients with mild PH (6–10). By contrast, little data are available that inform factors that are important for survival in PH, despite the potential clinical implications of this information.
It is important to note that PH is a heterogeneous disease often involving interplay between multiple organ systems in patients with comorbidities (11). Therefore, methods that integrate clinical information and focus on relationships between variables may be helpful for subgrouping patients (12, 13). Here, we innovated a strategy that defines an individual patient as a composite of 79 clinical variables (which we term the “whole-patient” phenotype). Network medicine framework was used to identify patients that shared a whole-patient phenotype. This approach was applied to a large RHC referral cohort, and the derivative findings were then validated by classical analytical methods in two patient cohorts using the endpoints of all-cause mortality and hospitalization. We identified elevated pulmonary arterial compliance (PAC) as a novel parameter that was associated with survival in PH. This finding introduces a conceptual shift that emphasizes protective markers when prognosticating at-risk patients.
Methods
Additional information on the methodology used in this study is available online (see Supplemental Methods in the online supplement).
Veterans Affairs Cohorts
We evaluated patients with procedural data recorded in the Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA-CART) Program (14) who underwent RHC as an outpatient or inpatient in the VA system between October 1, 2006, and September 30, 2018. Additional details on development of the VA-CART cohorts are provided online (see Supplemental Methods). In patients undergoing multiple RHCs, the first RHC was considered the index procedure and was the only one included in the analysis. Patients were included in the analyses if data from a complete RHC were available, defined as a recorded value for mean pulmonary artery pressure (mPAP), pulmonary artery wedge pressure (PAWP), height, weight, cardiac output, heart rate, systolic and diastolic PA pressure, and PVR. To calculate PVR, we used the standard equation (mPAP − PAWP)/cardiac output, expressed in Wood units (WU). PAC was calculated as stroke volume/(systolic PAP − diastolic PAP), where stroke volume was the quotient of cardiac output/heart rate (15). The Colorado Multiple Institutional Review Board approved this study with a waiver of informed consent. Censoring date was set at September 30, 2019.
Network cohort
The association between mPAP and mortality is continuous and begins at ∼19 mm Hg in the discovery (14) and validation cohorts (2). However, an overarching objective of this project was to identify novel phenotypes that are associated with survival in PH; therefore, we focused the network analysis on patients with mildly elevated mPAP (19–24 mm Hg), because this subgroup is characterized by significantly greater longitudinal survival after RHC compared with patients with mPAP ⩾25 mm Hg. The network cohort included patients who underwent RHC as an outpatient or inpatient with concomitant coronary or bypass graft angiography in the VA system between October 1, 2006, and September 30, 2018. The remaining inclusion and exclusion criteria were similar to those for the discovery cohort. There were 4,737 patients and 79 variables available for network construction (see Figure E1 and Table E1 in the online supplement). The Pearson correlation between two patients was required to be significant after the Benjamini-Hochberg adjustment for them to have an edge (which is a network term for the link between two nodes). In addition, we put a correlation coefficient threshold on the relationship: If two patients’ correlation coefficient was r ⩾0.5 when considering all 79 patient variables, these two patients were connected with an edge (see Supplemental Methods and Figure E2A). Otherwise, an edge was not formed between the two patients (Figure 1A). In this network, there were 4,715 nodes (patients) and 66,809 edges (patient-patient similarities).
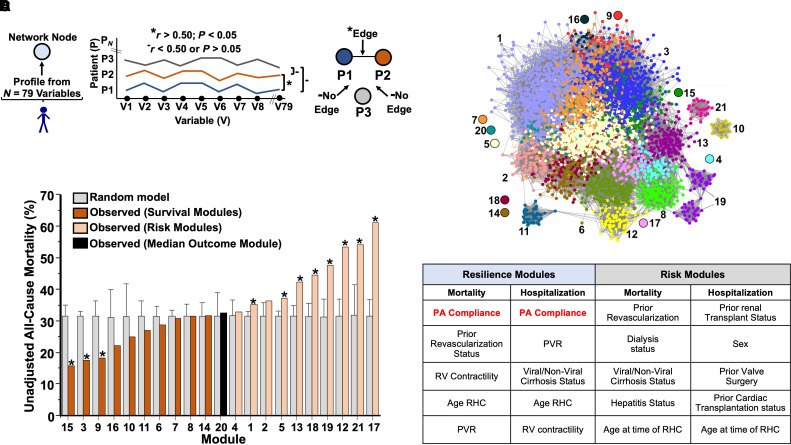
Figure 1.
Network medicine framework identifies PA compliance as a determinant of increased survival in pulmonary hypertension in silico. (A) A network was constructed in which each node (circle) represents one patient, defined by using N = 79 clinical variables, and each edge (known as a link or edge) represents patients who are similar to one another on the basis of their 79-variable clinical profile. To determine which patients were connected by an edge in the network, we performed a Pearson correlation between patients’ clinical profiles. If two patients’ correlation is larger than 0.5, we connect the patients with an edge. Otherwise, there is no edge between the two patients. Asterisks represent the criterion for having an edge. Solid dashes represent the criterion for not having an edge. (B) To stratify the patients into subgroups, a modularity optimization approach was used, resulting in 21 distinct network modules, which represent patient subgroups. (C) All-cause mortality in each module and the corresponding randomized module. The error bars represent the standard deviation of mortality in 1000 randomized modules. The modules that have a significantly different mortality compared to the randomized modules are marked with an asterisk. (D) A random forest-based variable selection method was adopted to rank variable importance for each module. A rank aggregation method was used to aggregate the variable rankings of the top 10 modules with highest survival and the variable rankings of the bottom 10 modules with highest mortalities (risk modules). PA = pulmonary arterial; PVR = pulmonary vascular resistance; RHC = right heart catheterization; RV = right ventricle; V = variable.
Stratification of patients by network modules
A modularity optimization approach, implemented in a Python package, NetworkX (16–18), was used to identify distinct network modules (patient subgroups) (see the online supplement).
Use of the term “survival” in this study
Survival was defined in the network analysis as modules for which there was a lower unadjusted all-cause mortality rate compared with the median module. In the spline curve analyses, survival refers to patients with a hazard ratio (HR) for the clinical endpoint that was significantly below the reference point.
Discovery cohort
On the basis of results from the network analyses, we examined the clinical characteristics and prognostic value of PAC using traditional analytic methods in a discovery cohort. The discovery cohort comprised patients in the VA-CART program who had been referred for right heart catheterization between October 1, 2007, and September 30, 2018, with mPAP ⩾19 (see the online supplement for additional details). Therefore, the discovery cohort included all patients in the network analysis cohort (i.e., with mPAP 19–24 mm Hg) and added those patients with mPAP ⩾25 mm Hg.
Validation cohort
Analysis of the Vanderbilt cohort was approved by the local institutional review board under a waiver of consent because of the deidentified nature of the data. The methods for extracting clinical, RHC, and echocardiographic data in the validation cohort have been published previously (2, 4). Briefly, we used the Synthetic Derivative database, a deidentified mirror of Vanderbilt’s electronic health record, to find all RHC reports between September 24, 1998, and December 20, 2013. Use of the Synthetic Derivative is considered research on nonhuman subjects because all data are deidentified. In concert with analyses involving the primary cohort, the follow-up time was calculated from the date of index RHC, and outcome data included only patients with at least 1-year follow-up (unless a patient died within 1 year) (2, 4). If death from any cause was reported to the Social Security Death Index, the reported date of death was recorded. Otherwise, patients were considered alive and were censored on the last search date of June 1, 2016.
Statistical Analysis
Differences in baseline clinical and hemodynamic characteristics between groups were analyzed using the Wilcoxon or Kruskal-Wallis test for continuous variables or a chi-square test for categorical variables. Details of the adjustment models for outcome analyses are provided (see the Statistical Methods section of the online supplement). Data preparation and analyses were conducted using SAS, Version 9.4 (SAS Institute), and R, Version 4.0.3.
Results
Network Analysis
There were 21 distinct modules (patient subgroups) (median 100 [IQR 73, 184] patients per module) (Figure 1B), which were characterized by variable clinical profiles (see Supplemental Results, Figure E2B, and Tables E2 and E3). The ranges for all-cause mortality (see Table E4) and all-cause hospitalization (see Table E5) across the 21 modules were 15.9–61.2% and 14.0–54.2%, respectively. Compared with randomly generated modules, there were 11 modules (52%) with significantly different all-cause mortality (Figure 1C), including 3 of the 10 (30%) survival modules (15, 3, and 9) that also had the lowest mortality rates of any modules in the network.
To determine which variables were important for driving patient assignment to a particular module, a binary classification problem (i.e., a patient is in the module vs. not in the module) was created for each module, and a random forest-based variable selection method was used to rank the variables on the basis of their roles in accurately predicting the class assignment (see Figure E3 and Table E6). We next analyzed survival or risk modules as a collective. From this approach, PAC emerged as the strongest determinant for patient assignment to survival modules (Figures 1D and E4A and E4B); however, PAC was not identified among the top 10 variables driving patient assignment to risk modules (Figures E4C and E4D). These data informed the additional analyses detailed in the following text profiling the importance of PAC to survival in two large cohorts with PH using classical analytical methodologies.
Association of PAC with Risk and Survival
There were 37,744 patients with mPAP ⩾19 mm Hg available for analysis in the discovery cohort, including 10,421 (27.6%) and 27,323 with mPAP 19–24 mm Hg and mPAP ⩾25 mm Hg, respectively. The distribution of PAC for the entire cohort is shown in Figures E5A and E5B. Compared with PAC quartile (Quartile 1; 0.30–2.0 ml/mm Hg), patients in Quartile 4 (4.18–14.9 ml/mm Hg) were characterized by younger age, higher BMI, and less enrichment for cardiopulmonary and renal diseases (Table 1). We observed an inverse relationship between mPAP and PAC (Figure E5C); consistent with this finding, patients in Quartile 1 also had the highest mPAP as well as higher PAWP and lower cardiac index compared with patients in Quartiles 2–4.
Table 1.
Discovery Cohort Characteristics and Hemodynamics Stratified by PAC Quartile
| Variable | PAC Quartile (ml/mm Hg) |
All | P | |||
|---|---|---|---|---|---|---|
| 1 (0.30–2.00) | 2 (2.00–2.94) | 3 (2.94–4.18) | 4 (4.18–14.93) | |||
| N | 9,437 | 9,435 | 9,436 | 9,436 | 37,744 | — |
| Age, yr | 68.1 (62.0–75.7) | 68.1 (62.3–75.3) | 67.4 (62.1–73.8) | 65.6 (60.5–70.8) | 67.2 (61.7–73.8) | <0.001 |
| Male | 96.1 (9,068) | 96.7 (9,120) | 96.6 (9,117) | 97.2 (9,176) | 96.7 (36,481) | <0.001 |
| BMI | 27.9 (24.4–32.3) | 29.4 (25.7–34.3) | 30.8 (27.0–35.6) | 32.6 (28.5–37.5) | 30.2 (26.2–35.1) | <0.001 |
| Inpatient RHC | 59.1 (5,578) | 48.1 (4,538) | 36.8 (3,472) | 28.6 (2,696) | 43.1 (16,284) | <0.001 |
| Race | ||||||
| White | 71.6 (6,758) | 77.6 (7,326) | 81.7 (7,707) | 83.7 (7,900) | 78.7 (29,691) | <0.001 |
| Black | 26.5 (2,501) | 20.5 (1,931) | 16.6 (1,562) | 14.6 (1,377) | 19.5 (7,371) | |
| Other | 1.9 (178) | 1.9 (178) | 1.8 (167) | 1.7 (159) | 1.8 (682) | |
| Systemic hypertension | 90.7 (8,562) | 90.6 (8,552) | 90.1 (8,499) | 89.4 (8,435) | 90.2 (34,048) | 0.007 |
| Congestive heart failure | 81.7 (7,706) | 70.2 (6,628) | 56.2 (5,306) | 45.5 (4,290) | 63.4 (23,930) | <0.001 |
| Atrial arrhythmia | 40.6 (3,828) | 37.9 (3,580) | 28.6 (2,698) | 20.8 (1,958) | 32.0 (12,064) | <0.001 |
| Peripheral arterial disease | 24.4 (2,306) | 23.6 (2,228) | 21.2 (1,999) | 18.1 (1,706) | 21.8 (8,239) | <0.001 |
| Diabetes | 52.2 (4,923) | 51.5 (4,863) | 50.4 (4,755) | 50.5 (4,766) | 51.2 (19,307) | 0.042 |
| Previous CABG | 24.2 (2,282) | 23.3 (2,195) | 20.5 (1,932) | 16.1 (1,516) | 21.0 (7,925) | <0.001 |
| PCI | 23.0 (2,175) | 21.9 (2,069) | 20.9 (1,976) | 20.1 (1,895) | 21.5 (8,115) | <0.001 |
| Coronary heart disease | 60.5 (5,713) | 59.1 (5,579) | 57.0 (5,375) | 51.9 (4,900) | 57.1 (21,567) | <0.001 |
| Previous valvular disease | 43.0 (4,057) | 41.8 (3,946) | 42.1 (3,970) | 36.9 (3,481) | 40.9 (15,454) | <0.001 |
| Previous stroke or TIA | 11.3 (1,066) | 9.4 (889) | 8.6 (816) | 7.6 (718) | 9.2 (3,489) | <0.001 |
| Pulmonary embolism | 6.4 (602) | 4.6 (438) | 4.0 (380) | 4.0 (377) | 4.8 (1,797) | <0.001 |
| Tobacco use | 63.8 (6,024) | 63.7 (6,011) | 65.1 (6,142) | 63.3 (5,969) | 64.0 (24,146) | 0.056 |
| COPD | 41.3 (3,899) | 39.1 (3,689) | 35.4 (3,337) | 31.0 (2,921) | 36.7 (13,846) | <0.001 |
| Interstitial lung disease | 1.1 (101) | 1.0 (97) | 0.8 (73) | 0.7 (63) | 0.9 (334) | 0.006 |
| Obstructive sleep apnea | 13.8 (1,304) | 15.2 (1,434) | 15.2 (1,437) | 17.4 (1,642) | 15.4 (5,817) | <0.001 |
| Portal hypertension | 0.9 (88) | 0.8 (77) | 0.9 (85) | 1.8 (169) | 1.1 (419) | <0.001 |
| Chronic kidney disease | 43.2 (4,076) | 36.7 (3,458) | 31.9 (3,009) | 27.4 (2,585) | 34.8 (13,128) | <0.001 |
| Connective tissue disease | 2.8 (264) | 2.8 (263) | 3.1 (294) | 2.6 (244) | 2.8 (1,065) | 0.176 |
| Renal replacement therapy | 5.9 (554) | 5.5 (515) | 5.3 (496) | 4.7 (439) | 5.3 (2,004) | 0.002 |
| Cancer | 13.6 (1,279) | 13.7 (1,296) | 13.2 (1,245) | 11.8 (1,109) | 13.1 (4,929) | <0.001 |
| Psychiatric disease | 5.4 (506) | 6.0 (566) | 6.6 (625) | 7.5 (704) | 6.4 (2,401) | <0.001 |
| Cardiopulmonary hemodynamics | ||||||
| mPAP, mm Hg | 40.0 (34.0–47.0) | 32.0 (26.0–37.5) | 27.0 (23.0–32.0) | 24.0 (21.0–29.0) | 30.0 (24.0–38.0) | <0.001 |
| PASP, mm Hg | 62.0 (52.0–72.0) | 48.0 (41.0–56.0) | 40.0 (35.0–47.0) | 35.0 (31.0–40.0) | 45.0 (36.0–56.0) | <0.001 |
| PADP, mm Hg | 26.0 (20.0–32.0) | 21.0 (16.0–26.0) | 18.0 (14.0–23.0) | 18.0 (15.0–22.0) | 20.0 (15.0–26.0) | <0.001 |
| PAWP, mm Hg | 23.0 (17.0–30.0) | 19.0 (14.0–25.0) | 16.0 (13.0–22.0) | 15.0 (12.0–20.0) | 18.0 (14.0–24.0) | <0.001 |
| PVR, WU | 3.9 (2.7–5.6) | 2.5 (1.8–3.3) | 1.9 (1.4–2.4) | 1.4 (1.0–1.9) | 2.1 (1.4–3.2) | <0.001 |
| eFick CO, L/min | 4.0 (3.3–4.8) | 4.8 (4.1–5.7) | 5.3 (4.5–6.3) | 6.0 (5.1–7.0) | 5.0 (4.1–6.1) | <0.001 |
| Td CO, L/min | 3.9 (3.2–4.7) | 4.8 (4.1–5.7) | 5.4 (4.6–6.4) | 6.3 (5.4–7.5) | 5.1 (4.2–6.3) | <0.001 |
| mVO2, % | 58.0 (51.0–64.0) | 63.0 (58.0–68.0) | 66.0 (62.0–70.0) | 69.0 (65.0–73.0) | 65.0 (58.0–69.0) | <0.001 |
| SBP, mm Hg | 128.0 (117.0–139.0) | 130.0 (120.0–141.0) | 132.0 (123.0–142.0) | 132.0 (123.0–141.0) | 131.0 (121.0–141.0) | <0.001 |
| DBP, mm Hg | 73.0 (67.0–80.0) | 73.0 (67.0–80.0) | 74.0 (68.0–80.0) | 74.0 (68.0–80.0) | 73.0 (67.0–80.0) | <0.001 |
| mPAP category | ||||||
| 19–24 mm Hg | 4.2 (399) | 17.8 (1,676) | 36.4 (3,430) | 52.1 (4,916) | 27.6 (10,421) | — |
| ⩾25 mm Hg | 95.8 (9,038) | 82.2 (7,759) | 63.6 (6,006) | 47.9 (4,520) | 72.4 (27,323) | — |
| PVR ⩾2.2 WU | 84.5 (7,976) | 60.4 (5,696) | 34.9 (3,292) | 12.7 (1,202) | 48.1 (18,166) | <0.001 |
Definition of abbreviations: BMI = body mass index; CABG = coronary artery bypass graft surgery; COPD = chronic obstructive pulmonary disease; DBP = diastolic blood pressure; eFick CO = estimated Fick cardiac output; mPAP = mean pulmonary artery pressure; mVO2 = mixed venous oxygen saturation; PAC = pulmonary arterial compliance; PADP = pulmonary artery diastolic pressure; PASP = pulmonary artery systolic pressure; PVR = pulmonary vascular resistance; PAWP = pulmonary artery wedge pressure; PCI = percutaneous coronary intervention; RHC = right heart catheterization; SBP = systolic blood pressure; Td = thermodilution; TIA = transient ischemic attack; WU = Wood units.
Categorical data are presented as percentage (n). Continuous data are presented as median (interquartile range). Data for the following variables were missing for the number of patients indicated (the remaining variables had no missing data): systolic blood pressure, 912; diastolic blood pressure, 912; hemoglobin, 2,407; arterial saturation, 4,525; and mixed venous saturation, 4,812.
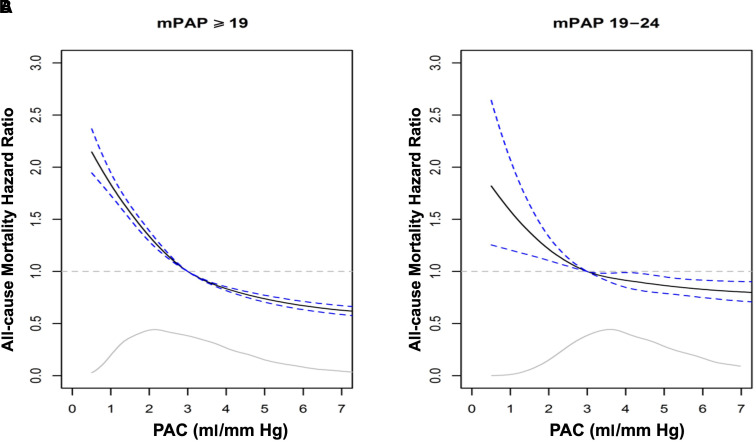
When using the PAC value of 3.0 ml/mm Hg (derived from the median among patients in the cohort with mPAP ⩾19 mm Hg) as a reference point, we observed a semiparabolic relationship between PAC and adjusted all-cause mortality (Figure 2A) that was maintained after restricting mPAP to 19–24 mm Hg (Figure 2B). Notably, PAC ⩾3.0 ml/mm Hg was associated with an adjusted all-cause mortality HR of <1.0 that decreased progressively to PAC of ∼7 ml/mm Hg. The slope of this relationship indicated that subtle elevation in PAC may be protective: For example, decreases in mortality of 11% (HR, 0.89; 95% confidence interval [CI], 0.88–0.90) and 16% (HR, 0.84; 95% CI, 0.82–0.85) were observed with PAC of 3.6 ml/mm Hg and 4.0 ml/mm Hg, respectively, compared with 3.0 ml/mm Hg (see Table E7). Directionally similar findings were observed when using a higher (i.e., more conservative) PAC threshold of 5.3 ml/mm Hg (for details, see Figure E6 and Table E8) extrapolated retrospectively from a historical cohort of normal volunteers.
Figure 2.
Adjusted hazard ratio for all-cause mortality by varying mean pulmonary artery pressure (mPAP). (A and B) From the primary cohort, the hazard ratio (95% confidence interval) for all-cause mortality is plotted for pulmonary arterial compliance (PAC) 0–7 ml/mm Hg relative to a reference value of 3.0 ml/mm Hg in patients with mPAP ⩾19 mm Hg (A) and restricted to mPAP 19–24 mm Hg (B). The gray line inset indicates the kernel density estimate, representing the relative proportion of patients at difference values of PAC. In the Cox regression models, the effects of PAC and continuous covariates were assumed to be smooth, but not linear, by using a natural spline with 3 degrees of freedom. Solid lines represent kernel density. Dotted lines represent hazard ratio of 1.
Higher pulmonary venous pressure is associated with lower PAC (19), and the discovery cohort is enriched with patients with left heart disease. Therefore, to determine whether elevated PAWP affects the relationship between PAC and outcome, we next dichotomized PAWP by ⩽15 mm Hg versus >15 mm Hg (Figure E7A) and ⩽12 mm Hg versus >12 mm Hg (Figure E7B). These data show that the distribution of PAC varies substantially by PAWP category, and optimal clinical risk reduction in association with elevated PAC is observed among patients without pulmonary venous hypertension at the time of RHC. We report additional analyses examining the predictive performance of the individual components of PAC (stroke volume and pulse pressure; see Supplemental Results).
A summary of the adjusted all-cause mortality HRs after dichotomizing by PAC <3.0 versus ⩾3.0 ml/mm Hg, PAWP <15 versus ⩾15 mm Hg, and PVR <2.2 versus ⩾2.2 WU is presented in Table 2, with corresponding 1-yr and 5-yr all-cause mortality rates in Table E9 (see Table E9). In all subgroup analyses, PAC ⩾3.0 ml/mm Hg was protective compared with the corresponding referent group. Of note, the protective association with elevated PAC persisted even in the setting of elevated PVR (HR, 0.74; 95% CI, 0.70–0.78; P < 0.001). Adjusted HRs for mortality when combining all three hemodynamic variables in patients with PH using a common reference group of high PAC (⩾3.0 ml/mm Hg), low PAWP (⩽15 mm Hg), and low PVR (<2.2 WU) are shown in Table E10. This analysis tests the effect of adding PAC to hemodynamic combinations used to diagnose and direct treatment in patients with PH. Low PAC is associated with increased risk among those subgroups defined by otherwise normal hemodynamics (HR, 1.27; 95% CI, 1.12–1.45; P < 0.001), isolated elevation in PAWP (i.e., postcapillary PH) (HR, 1.27; 95% CI, 1.10–1.46; P = 0.001), and isolated elevation in PVR (i.e., precapillary PH) (HR, 1.34; 95% CI, 1.21–1.48; P < 0.001).
Table 2.
Hazard Ratios for Outcome among Patients with Elevated Mean Pulmonary Artery Pressure Dichotomized by Pulmonary Arterial Compliance and Stratified by Pulmonary Vascular Resistance or Pulmonary Artery Wedge Pressure
| Variable | Adjusted All-Cause Mortality |
|||
|---|---|---|---|---|
| VA-CART (Discovery) Cohort (N = 37,744) |
VUMC (Validation) Cohort (N = 1,514) |
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| PAC <3 ml/mm Hg | 1.0 | — | 1.0 | — |
| PAC ⩾3 ml/mm Hg | 0.67 (0.64–0.69) | <0.001 | 0.58 (0.45–0.71) | <0.001 |
| PAC <3 ml/mm Hg + PAWP ⩽15 mm Hg | 1.0 | — | 1.0 | — |
| PAC ⩾3 ml/mm Hg + PAWP ⩽15 mm Hg | 0.59 (0.55–0.62) | <0.001 | 0.51 (0.38–0.69) | <0.001 |
| PAC <3 ml/mm Hg + PAWP >15 mm Hg | 1.0 | — | 1.0 | — |
| PAC ⩾3 ml/mm Hg + PAWP >15 mm Hg | 0.72 (0.69–0.75) | <0.001 | 0.65 (0.49–0.87) | 0.003 |
| PAC <3 ml/mm Hg + PVR ⩾2.2 WU | 1.0 | — | 1.0 | — |
| PAC ⩾3 ml/mm Hg + PVR ⩾2.2 WU | 0.74 (0.70–0.78) | <0.001 | 0.66 (0.50–0.87) | 0.004 |
| PAC <3 ml/mm Hg + PVR <2.2 WU | 1.0 | — | 1.0 | — |
| PAC ⩾3 ml/mm Hg + PVR <2.2 WU | 0.72 (0.69–0.76) | <0.001 | 0.65 (0.44–0.98) | 0.039 |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; PAC = pulmonary arterial compliance; PAWP = pulmonary artery wedge pressure; VA-CART = Veterans Affairs Clinical Assessment, Reporting, and Tracking program; VUMC = Vanderbilt University Medical Center; WU = Wood units.
Estimates for the effect of PAC level in patients with mean pulmonary arterial pressure ⩾19 mm Hg and then further subgrouped by PAWP and pulmonary vascular resistance (PVR) are presented for the primary cohort (VA-CART) and validation cohort (VUMC). The adjustment model included the following clinical variables: categorical age, sex, race, categorical body mass index, and history of systemic hypertension, congestive heart failure, left heart failure, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, liver cirrhosis, chronic kidney disease that included the patient receiving renal replacement therapy, portal hypertension, connective tissue disease, atrial arrhythmia, interstitial lung disease, pulmonary embolism, valvular disease, tobacco use, psychiatric disease, stroke, obstructive sleep apnea, and inpatient hospital status at the time of right heart catheterization. For the validation cohort, the following variables from the adjustment used in the primary cohort analysis were available: age, sex, race, diabetes mellitus, body mass index, coronary artery disease, valvular heart disease, chronic obstructive pulmonary disease, atrial arrhythmia, interstitial lung disease, connective tissue disease, systemic hypertension, chronic kidney disease, obstructive sleep apnea, and congestive heart failure. For both the primary and validation cohorts, the analyses with PAWP or PVR were based on the models including an interaction between PAC and PAWP or PAC and PVR. Data are presented as HR (95% CI). Reference groups are signified by an HR of 1.
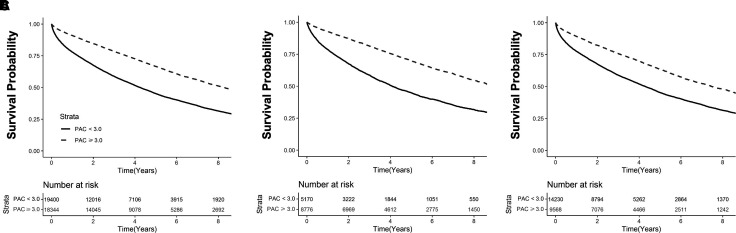
The median follow-up time for the discovery cohort was 1,236 days (range = 591–2,160 d). The estimated 1-year mortality was 22.2% in patients with PAC <3.0 ml/mm Hg and 9.1% in patients with PAC ⩾3.0 ml/mm Hg. Kaplan-Meier curves stratified by dichotomous PAC (⩾3.0 ml/mm Hg vs. <3.0 ml/mm Hg) for patients with mPAP ⩾19 mm Hg, mPAP ⩾19 mm Hg + PAWP ⩽15 mm Hg, and mPAP ⩾19 + PAWP >15 mm Hg are shown in Figures 3A, 3B, and 3C, respectively. Results were similar when restricting to cases where there was at least 1 year of survival after the index catheterization (Figures E8A–E8C). This approach removes early events that occurred in individuals who were referred for hemodynamic assessment while critically ill, thereby decreasing referral bias.
Figure 3.
Kaplan-Meier curves for mortality by pulmonary arterial compliance (PAC) and pulmonary artery wedge pressure (PAWP) in patients with elevated mean pulmonary artery pressure in the primary cohort. (A) From the primary cohort, a Kaplan-Meier analysis was performed to determine the probability of all-cause mortality stratified by dichotomous PAC (PAC ⩾3.0 ml/mm Hg vs. <3.0 ml/mm Hg) in patients with mPAP ⩾19 mm Hg. (B and C) This population was then subgrouped by PAWP ⩽15 mm Hg (B) and PAWP >15 mm Hg (C). Results from a log-rank test comparing strata are provided for each analysis. Censoring begins at and beyond 1 year after the index right heart catheterization, represented here as Day 0. Number of patients at risk are provided at every 2-year interval after right heart catheterization. Solid lines represent kernel density. Dotted lines represent hazard ratio of 1.
Validation Cohort
Compared with the discovery cohort, the validation cohort from VUMC had similar distributions of age (60.2 yr; range = 49.2–69.1 yr) and body mass index (29.0 kg/m2; range = 25.0–34.9 kg/m2) but was sex balanced (N = 1,514 [48%] male). Median follow-up time in the validation cohort was 2,485 days (range = 671–3,580 d). The clinical and hemodynamic profiles of the validation cohort stratified by PAC quartiles are reported in Table E11. Among the 1,514 patients in the validation cohort, 567 (37%) had PAC ⩾3 ml/mm Hg. Results from the validation cohort confirmed a protective association with higher PAC when referenced to 3 ml/mm Hg (Figure E9). Adjusted HRs for all-cause mortality across various hemodynamic subgroups were similar in the primary and validation cohorts (Table 2), as were raw estimates of 1- and 5-yr mortality (Table E9). We conducted an additional analysis using a PAWP of 12 mm Hg to discriminate pre- and postcapillary PH. The interaction term between PAC and dichotomized PAWP (i.e., >12 and ⩽12) yielded a P value of 0.08. We found no interaction between PAC and sex with respect to the association between PAC and mortality.
Discussion
A patient–patient network was assembled from a large population defined solely by mildly elevated mPAP, which identified 21 unique subgroups with variable clinical and outcome profiles. Among 79 clinical variables, PAC was the most important driver of patient assignment to subgroups characterized by survival. When considering all patients with elevated mPAP in a national referral cohort, a protective association between PAC and all-cause mortality beginning at ∼3.1 ml/mm Hg was observed that was progressive through ∼7 ml/mm Hg. The association between elevated PAC and increased survival was maintained after adjusting for clinical covariates and validated in a separate sex-balanced referral population. The protective effect of higher PAC was also consistent across the PAWP and PVR spectra and particularly evident in patients with precapillary PH. Overall, these findings suggest that elevated PAC may be important for survival in PH (20).
PH is recognized increasingly as a systemic disease in which integrated hemodynamics (21), comorbidities (22), and other patient-level characteristics (10, 13) influence outcome. In line with this, we used a wide gamut of clinical data to summarize the whole phenotype of an individual patient with PH and then used a network medicine approach to ultimately identify novel subgroups. This approach is consistent with efforts to apply unbiased methods to reduce the complexity of large cohorts, such as digital twinning (23), cluster analyses (24), and others (12), but it advances contemporary phenotyping in several ways. First, correlations across clinical variables were used to establish patient-patient relationships; thus, in this analysis, the network topology itself informed patient subgroups. This diverges from prior work in which subgroups were determined by data variance within a population (25) and visualized in a network form (26). Second, the importance of variables for determining which patient subgroups emerged in the network was analyzed using a variable selection model. Thus, it was the convergence of two rigorous but orthogonal computational analyses that led to our focus on PAC. Third, data informed by the networks were validated in two robust cohorts linked to clinical events.
The network findings exposed profound clinical heterogeneity in the study cohort, manifest by the discovery of 21 distinct subgroups and wide variability in outcome despite an mPAP range of only 5 mm Hg for all patients. In selected instances, modules were populated on the basis of a comorbidity or by patients sharing extreme hemodynamic results, but this was rare. Instead, differences across modules were often subtle, discovered only after a review of the results from the variable selection model. Our finding that PAC was the most important determinant of patient assignment across the aggregate of all survival modules was novel (15) but, nonetheless, internally consistent with the observations that PAC was particularly important for specific modules with the lowest mortality rates (3, 15) and that PAC was not important for patient assignment to modules characterized by high mortality relative to other patients within the same cohort. Thus, findings from the network unexpectedly directed a shift in focus away from traditional outcome studies that emphasize independent predictors of adverse outcome (27) toward discovering patient profiles that may be important for PH survivability.
Compliance describes the change in arterial blood volume that is due to a given change in arterial blood pressure; in the lung, PAC regulates perfusion and, thereby, oxygenation (28, 29). The ratio of stroke volume to pulmonary pulse pressure is a validated PAC surrogate in clinical studies (30) and associates with adverse outcome in patients with PH or heart failure (31–34). Nonetheless, prior reports were not powered sufficiently to profile the PAC range associated with hard clinical events. Here, when modeling PAC as a continuous variable, we observed a distribution pattern in which levels below and above 3.0 ml/mm Hg inform clinical risk and survival patterns, respectively. Higher PAC remained protective after focusing on hemodynamic combinations that capture PH profiles encountered regularly in clinical practice, such as precapillary and postcapillary PH (35). Indeed, many have advanced PVR as a helpful measure to separate functional (i.e., postcapillary) PH from pulmonary vascular disease per se (36). Therefore, it is notable that elevated PAC in this study was linked to increased survival among patients with PVR <2.2 WU (the level independently associated with adverse outcome in the study population). The hemodynamic relationship between PVR and PAC follows an inverse hyperbolic relationship (37, 38), and this relation is particularly steep when PVR is <3 WU (39). In this situation, subtle increases in resistance will lead to much larger reductions in compliance, suggesting that PAC itself may be a particularly sensitive indicator of pulmonary vascular remodeling. Our findings are also consistent with reports proposing that PAC represents pulsatile afterload left unaccounted for by PVR and thereby outperforms PVR in clinical prediction models of patients with PH (34, 36, 40).
Findings from this study have several potential implications on clinical practice. First, our data show that PAC is additive, not redundant, to traditional hemodynamic parameters that diagnose and prognosticate patients with PH. This suggests that opportunity exists to refine the prognostic significance of specific hemodynamic measures and their ranges, possibly by including PAC into this framework. Our intent was not to directly compare the prognostic value of PAC and PVR but rather to test their complementarity, in part because it is unlikely that clinicians will ever stop using PVR to prognosticate patient risk. Second, the sizeable reduction in mortality hazard observed for PAC is generally unique for PH hemodynamic studies, introducing the possibility that levels ⩾3.0 ml/mm Hg may be an unrecognized treatment goal for at-risk patients. Future studies are warranted to examine longitudinal measures of PAC in patients with PH being treated for the underlying etiology to answer these questions and determine whether a clinically meaningful change in PAC corresponds with alteration in individual patient-level risk. Finally, we found that the protective effects of elevated PAC extend to patients with mild PH. These results may be important to consider when designing clinical trials to enrich for patients with mild PH who are most likely to benefit from targeted PH therapy (41).
Limitations
Our network analysis method required a complete dataset per patient, and we aimed to capture as many variables as possible. As a result, the unavailability of one or more variables led to the exclusion of a patient and, ultimately, a sizeable proportion of the cohort with mPAP of 19–24 mm Hg. This was unavoidable but could have biased the network results. Further, information on some established independent predictors of outcome in PH, such as renal function (42), was not available for inclusion in the network analyses, which must be considered when interpreting data from this study. Using alternative thresholds to define network modularity or a significant correlation may have affected the number and distinctness of subgroups, altering the variable selection model findings (including the relevance of PAC). Alternative approaches (e.g., propensity matching in the adjustment model) to the one used in this study for defining survival could have been considered, although the network itself was designed specifically to group similar patients. The formula we used for PAC is valid but is, nonetheless, a surrogate for compliance. Our analyses do not clarify the relative contributions to survival of the vasculature versus the right ventricle (43). For example, elevated PAC may represent a resilient right ventricle phenotype in some patients and a resilient vascular phenotype in others, and this requires further investigation.
Our findings may be less relevant to patients with high PVR (such as those with PAH) in whom the range of PAC is relatively narrow. To add rigor and enhance generalizability, we tested the association of PAC with survival using a higher reference value of 5.3 ml/mm Hg, derived from a healthy population, and our findings were similar. However, the optimal normal PAC reference level was not derived from a primary analysis, and, therefore, other thresholds that define a healthy pulmonary vasculature may emerge in the future. An important limitation of this work is the lack of information on the cause of death in both cohorts. As a result, we were unable to ascertain the direct influence of PH per se on mortality. Therefore, this work does not clarify whether PAC is a biomarker of cardiovascular mortality in patients at risk or a direct contributor to mortality in patients with PH. Differences in observation periods between the discovery and validation that cohorts made have confounded our results with respect to therapeutic advances for PH, although new therapies during this period were only introduced for PAH, which is rare in the primary cohort.
Conclusions
These data demonstrate the utility of network medicine to nuance patient subgrouping and outcome estimates, which, in this study, shifted emphasis toward the discovery of novel markers associated with survival. Elevated PAC ⩾3.0 ml/mm Hg was protective against all-cause mortality in a large national referral PH cohort, which was maintained in various subgroup analyses and validated in a second sex-balanced cohort. Additional prospective studies are needed to generalize these findings and clarify the relevance of PAC for optimizing PH hemodynamic classifications and patient risk stratification. Data from this study also suggest that elevated PAC may be an important potential therapeutic target in PH, which must be tested in further studies focused on validating this observation.
Footnotes
Supported by NIH grants R21-HL1343201, R01-HL139613-01, R01-HL153502, and R01-HL155096-01; 2021A007243 BWH/MIT-Broad Institute; and the McKenzie Family Charitable Trust (to B.A.M.); and by NIH grants R01-HL146588-03, 3-R01-HL146588-01S1, R01-HL155278, R01-HL163960, R01-FD007627, and R61-HL158941 (to E.L.B.). The dataset(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU, which is supported by numerous sources: institutional funding, private agencies, and federal grants. These include the NIH-funded Shared Instrumentation Grant S10RR025141 and Clinical and Translational Science Award Program grants UL1TR002243, UL1TR000445, and UL1RR024975. This material is the result of work supported with resources and the use of facilities at the Rocky Mountain Regional VA Medical Center, Aurora, Colorado; the views expressed are those of the authors alone and do not represent the views of the Veterans Affairs or other federal government agencies.
Author Contributions: Conception or design of the work: R.-S.W., M.S.F., R.J.T., G.C., J.A.L., W.M.O., B.A.M., and E.L.B. Acquisition, analysis, or interpretation of data for the work: S.H., S.W.W., E.H., M.G., S.W.J., K.Z., G.K., R.J.T., B.A.M., and E.L.B. Drafting the work or revising it critically for important intellectual content: all authors. Final approval of the version submitted for publication: all authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: B.A.M. and E.L.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202211-2097OC on June 5, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Maron BA, Abman SH, Elliott CG, Frantz RP, Hopper RK, Horn EM, et al. Pulmonary arterial hypertension: diagnosis, treatment, and novel advances. Am J Respir Crit Care Med . 2021;203:1472–1487. doi: 10.1164/rccm.202012-4317SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Assad TR, Maron BA, Robbins IM, Xu M, Huang S, Harrell FE, et al. Prognostic effect and longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol . 2017;2:1361–1368. doi: 10.1001/jamacardio.2017.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med . 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 4. Maron BA, Brittain EL, Hess E, Waldo SW, Barón AE, Huang S, et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med . 2020;8:873–884. doi: 10.1016/S2213-2600(20)30317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Souza R, Channick RN, Delcroix M, Galiè N, Ghofrani HA, Jansa P, et al. Association between six-minute walk distance and long-term outcomes in patients with pulmonary arterial hypertension: data from the randomized SERAPHIN trial. PLoS One . 2018;13:e0193226. doi: 10.1371/journal.pone.0193226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benza RL, Kanwar MK, Raina A, Scott JV, Zhao CL, Selej M, et al. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest . 2021;159:337–346. doi: 10.1016/j.chest.2020.08.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boucly A, Weatherald J, Savale L, de Groote P, Cottin V, Prévot G, et al. External validation of a refined four-stratum risk assessment score from the French pulmonary hypertension registry. Eur Respir J . 2022;59:2102419. doi: 10.1183/13993003.02419-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoeper MM, Pausch C, Olsson KM, Huscher D, Pittrow D, Grünig E, et al. COMPERA 2.0: a refined four-stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J . 2022;60:2102311. doi: 10.1183/13993003.02311-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest . 2012;141:354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 10. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. ESC/ERS Scientific Document Group 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J . 2023;61:61. doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 11. Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation . 2020;141:678–693. doi: 10.1161/CIRCULATIONAHA.116.022362. [DOI] [PubMed] [Google Scholar]
- 12. Oldham WM, Oliveira RKF, Wang RS, Opotowsky AR, Rubins DM, Hainer J, et al. Network analysis to risk stratify patients with exercise intolerance. Circ Res . 2018;122:864–876. doi: 10.1161/CIRCRESAHA.117.312482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kanwar MK, Gomberg-Maitland M, Hoeper M, Pausch C, Pittrow D, Strange G, et al. Risk stratification in pulmonary arterial hypertension using Bayesian analysis. Eur Respir J . 2020;56:2000008. doi: 10.1183/13993003.00008-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation . 2016;133:1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, et al. Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail . 2018;11:e004436. doi: 10.1161/CIRCHEARTFAILURE.117.004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech . 2008;2008:P10008. [Google Scholar]
- 17.Hagberg AA, Schult DA, Swart PJ.2008. pp. 11–15.
- 18. Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci USA . 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation . 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.2018. https://www.nhlbi.nih.gov/events/2018/enhancing-resilience-cardiovascular-health-and-wellness
- 21. Oldham WM, Hess E, Waldo SW, Humbert M, Choudhary G, Maron BA. Integrating haemodynamics identifies an extreme pulmonary hypertension phenotype. Eur Respir J . 2021;58:2004625. doi: 10.1183/13993003.04625-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail . 2011;4:257–265. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 23. Masison J, Beezley J, Mei Y, Ribeiro H, Knapp AC, Sordo Vieira L, et al. A modular computational framework for medical digital twins. Proc Natl Acad Sci USA . 2021;118:e2024287118. doi: 10.1073/pnas.2024287118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Launay D, Montani D, Hassoun PM, Cottin V, Le Pavec J, Clerson P, et al. Clinical phenotypes and survival of pre-capillary pulmonary hypertension in systemic sclerosis. PLoS One . 2018;13:e0197112. doi: 10.1371/journal.pone.0197112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haddad F, Contrepois K, Amsallem M, Denault AY, Bernardo RJ, Jha A, et al. The right heart network and risk stratification in pulmonary arterial hypertension. Chest . 2022;161:1347–1359. doi: 10.1016/j.chest.2021.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hou Y, Zhou Y, Hussain M, Budd GT, Tang WHW, Abraham J, et al. Cardiac risk stratification in cancer patients: a longitudinal patient-patient network analysis. PLoS Med . 2021;18:e1003736. doi: 10.1371/journal.pmed.1003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benza RL, Boucly A, Farber HW, Frost AE, Ghofrani HA, Hoeper MM, et al. Change in REVEAL Lite 2 risk score predicts outcomes in patients with pulmonary arterial hypertension in the PATENT study. J Heart Lung Transplant . 2022;41:411–420. doi: 10.1016/j.healun.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 28. Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, Weir EK. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc . 2016;13:276–284. doi: 10.1513/AnnalsATS.201509-599FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chemla D, Lau EM, Papelier Y, Attal P, Hervé P. Pulmonary vascular resistance and compliance relationship in pulmonary hypertension. Eur Respir J . 2015;46:1178–1189. doi: 10.1183/13993003.00741-2015. [DOI] [PubMed] [Google Scholar]
- 30. Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol . 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 31. Dragu R, Rispler S, Habib M, Sholy H, Hammerman H, Galie N, et al. Pulmonary arterial capacitance in patients with heart failure and reactive pulmonary hypertension. Eur J Heart Fail . 2015;17:74–80. doi: 10.1002/ejhf.192. [DOI] [PubMed] [Google Scholar]
- 32. Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med . 2010;182:252–260. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dupont M, Mullens W, Skouri HN, Abrahams Z, Wu Y, Taylor DO, et al. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ Heart Fail . 2012;5:778–785. doi: 10.1161/CIRCHEARTFAILURE.112.968511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail . 2015;3:467–474. doi: 10.1016/j.jchf.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J . 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, et al. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest . 2014;145:1064–1070. doi: 10.1378/chest.13-1510. [DOI] [PubMed] [Google Scholar]
- 37. Lankhaar JW, Westerhof N, Faes TJ, Gan CT, Marques KM, Boonstra A, et al. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J . 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 38. Khan A, White RJ, Meyer G, Pulido Zamudio TR, Jerjes-Sanchez C, Johnson D, et al. Oral treprostinil improves pulmonary vascular compliance in pulmonary arterial hypertension. Respir Med . 2022;193:106744. doi: 10.1016/j.rmed.2022.106744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tedford RJ. Determinants of right ventricular afterload (2013 Grover Conference series) Pulm Circ . 2014;4:211–219. doi: 10.1086/676020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saouti N, Westerhof N, Postmus PE, Vonk-Noordegraaf A. The arterial load in pulmonary hypertension. Eur Respir Rev . 2010;19:197–203. doi: 10.1183/09059180.00002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huston JH, Frantz RP, Brittain EL. Early intervention: should we conduct therapeutic trials for mild pulmonary hypertension before onset of symptoms? Pulm Circ . 2019;9:2045894019845615. doi: 10.1177/2045894019844994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah SJ, Thenappan T, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation . 2008;117:2475–2483. doi: 10.1161/CIRCULATIONAHA.107.719500. [DOI] [PubMed] [Google Scholar]
- 43. Monzo L, Reichenbach A, Al-Hiti H, Borlaug BA, Havlenova T, Solar N, et al. Acute unloading effects of sildenafil enhance right ventricular-pulmonary artery coupling in heart failure. J Card Fail . 2021;27:224–232. doi: 10.1016/j.cardfail.2020.11.007. [DOI] [PubMed] [Google Scholar]