To the Editor:
Pulmonary arterial (PA) pressures and right ventricular (RV) afterload increase in response to hypoxia (1). Limited data are available that describe RV adaptations to acute hypoxia and the associated increases in afterload, occurring in response to clinical scenarios such as infections, pulmonary embolism, and hypoxia associated with high-altitude exposure (2).
RV pressure-volume analysis by means of conductance catheterization is a gold-standard method of characterizing RV function using metrics of contractility, lusitropy, energetics, and ventricular-arterial coupling (3). The primary objective of this single-center prospective study (ClinicalTrials.gov ID NCT 05272514) was to characterize RV performance in response to acute progressive hypoxia.
The results of this study have been previously reported in an unpublished abstract (4).
Healthy adults free of cardiovascular, hematologic, and pulmonary disease underwent hemodynamic assessment. Individuals residing at ⩾2,500 m (8,000 ft) for three or more consecutive nights within 30 days of testing were excluded (2). Written informed consent was obtained. The study was approved by the institutional review board of the University of Colorado Anschutz Medical Campus and overseen by an independent data safety and monitoring board. Participants underwent baseline evaluation with Swan-Ganz catheterization. Thereafter, they were randomized 1:1 to undergo hypoxic testing with Swan-Ganz catheterization versus a conductance catheter inserted into the right ventricle. Oxygen saturation (SaO2) and blood pressure were monitored with a radial arterial catheter. Oxygen uptake was continuously recorded (Vyntus, Vyaire Medical) for calculation of direct Fick cardiac output (5, 6). For participants randomized to testing with a conductance catheter, the Swan-Ganz catheter was replaced by a 7F high-fidelity conductance catheter (CD Leycom). Insertion and calibration were performed according to previous protocols (5, 6). Single-beat estimation was used to determine end-systolic elastance, effective arterial elastance, and ventricular-arterial coupling (5, 6). Participants were exposed to staged reductions in FiO2 at 0.21, 0.17, 0.15, and 0.12 every 8–10 minutes by means of an open circuit with Douglas bag reservoirs. Hemodynamic parameters were recorded in the final minute of each stage of hypoxia. Data are reported as mean ± SD or median (and interquartile range) unless otherwise specified.
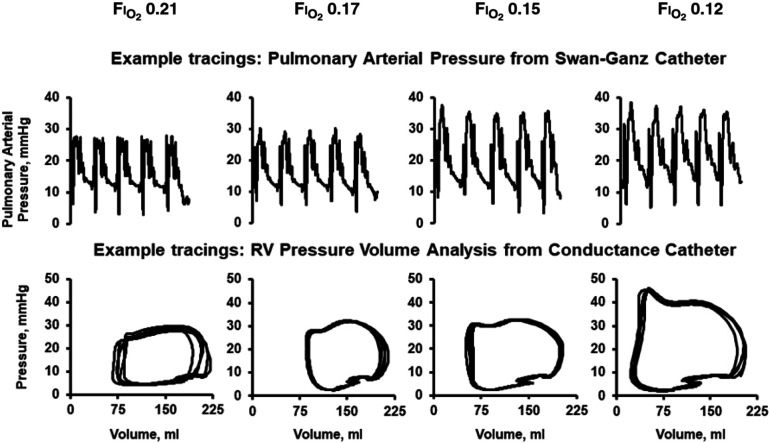
Ten participants completed the study (34 ± 10 years; three women; body mass index, 24.3 ± 2.7 kg/m2). Baseline hemodynamics among all participants demonstrated a right atrial pressure of 3 mm Hg (1, 3), a mean PA pressure of 12 mm Hg (9, 15), PA wedge pressure of 6 mm Hg (6, 6), and direct Fick cardiac output of 8.8 L/min (7.2, 9.2). SaO2 at a FiO2 of 0.21 was 95% (94, 96) and decreased to 71% (70, 77) at a FiO2 of 0.12. Hemodynamics during progressive reduction in FiO2 are demonstrated in Table 1. Example hemodynamic tracings are demonstrated in Figure 1. Progressive hypoxia led to a modest increase in PA pressure from 12 (7, 13) to 14 (13, 19) mm Hg among participants evaluated with a Swan-Ganz catheter (N = 5) and in RV afterload from 0.16 (0.15, 0.17) to 0.18 (0.14, 0.18) mm Hg/ml among those evaluated with a conductance catheter (N = 5). Cardiac output increased because of modest increases in heart rate and stroke volume. Acute hypoxia increased metrics of RV contractility, lusitropy, and myocardial energetics, and RV ventricular-arterial coupling was preserved.
Table 1.
Invasive Hemodynamic Response to Acute Hypoxia Derived from Pulmonary Arterial and Conductance Catheterization
| FiO2 = 0.21 | FiO2 = 0.17 | FiO2 = 0.15 | FiO2 = 0.12 | P Value | |
|---|---|---|---|---|---|
| Swan-Ganz catheter (N = 5) | |||||
| HR, bpm | 53 (50, 62) | 54 (52, 62) | 55 (53, 72) | 63 (53, 73) | 0.06 |
| RAP, mm Hg | 3 (1, 3) | 3 (1, 3) | 3 (1, 3) | 1 (0, 2]*†‡ | <0.01 |
| PAS, mm Hg | 18 (14, 20) | 17 (15, 22) | 20 (16, 22)* | 25 (25, 31)*†‡ | <0.01 |
| PAD, mm Hg | 8 (4, 10) | 9 (8, 11) | 9 (8, 11) | 9 (7, 13) | 0.25 |
| mPAP, mm Hg | 12 (7, 13) | 12 (11, 13) | 13 (13, 13) | 14 (13, 19)* | 0.01 |
| PAWP, mm Hg | 6 (6, 6) | 6 (6, 7) | 5 (4, 10) | 6 (3, 10) | 0.92 |
| PA Sat, % | 71 (69, 72) | 64 (64, 67) | 64 (58, 64)* | 53 (49, 61)*†‡ | <0.01 |
| Arterial Sat, % | 96 (94, 97) | 93 (89, 96) | 86 (83, 90)*† | 76 (71, 81)*†‡ | <0.01 |
| SV, ml/beat | 125 (101, 151) | 107 (80, 184) | 114 (97, 174) | 147 (124, 166) | 0.69 |
| Qc, L/min | 7.4 (6.1, 8.7) | 6.2 (5.8, 8.8) | 8.2 (6.8, 9.2) | 9.7 (7.2, 11.3) | 0.40 |
| PVR, WU | 0.9 (0.5, 1.0) | 1.0 (0.8, 1.1) | 1.1 (0.5, 1.1) | 1.0 (1.0, 1.1) | 0.10 |
| Conductance catheter (N = 5) | |||||
| RV contractility | |||||
| dPdtmax, mm Hg/s | 268 (240, 271) | 283 (257, 312) | 315 (296, 340) | 448 (342, 476)*†‡ | <0.01 |
| ESP, mm Hg | 17 (17, 18) | 19 (18, 21) | 23 (19, 23) | 27 (23, 28)*† | <0.01 |
| PRSW, mm Hg | 7 (6, 13) | 14 (12, 14) | 14 (14, 19) | 20 (11, 26)* | 0.02 |
| RV lusitropy | |||||
| dPdtmin, mm Hg/s | −208 (−219, −195) | −243 (−260, −225) | −275 (−297, −232) | −331 (−396, −295)*†‡ | <0.01 |
| RV energetics | |||||
| SW, mm Hg ⋅ ml | 1,750 (1,489, 2,697) | 2,777 (2,697, 2,900) | 3,503 (3,229, 3,536) | 3,578 (2,558, 5,440)* | 0.03 |
| Ventricular-arterial coupling | |||||
| EES, mm Hg/ml | 0.21 (0.19, 0.25) | 0.19 (0.18, 0.25) | 0.23 (0.18, 0.25) | 0.24 (0.20, 0.25) | 0.54 |
| EA, mm Hg/ml | 0.16 (0.15, 0.17) | 0.13 (0.11, 0.15) | 0.14 (0.12, 0.15) | 0.18 (0.14, 0.18)†‡ | 0.02 |
| EES/EA, units | 1.51 (1.35, 1.73) | 1.50 (1.42, 1.62) | 1.43 (1.32, 1.84) | 1.35 (1.33, 1.41) | 0.47 |
Definition of abbreviations: Arterial Sat = arterial oxygen saturation; bpm=beats per minute; dPdtmax = maximum rate of pressure change; dPdtmin = minimum rate of pressure change; EA = effective arterial elastance; EES = end-systolic elastance; EES/EA = ventricular-arterial coupling; ESP = end-systolic pressure; HR = heart rate; mPAP = mean pulmonary arterial pressure; PA Sat = pulmonary arterial oxygen saturation; PAD = pulmonary arterial diastolic; PAS = pulmonary arterial systolic; PAWP = pulmonary artery wedge pressure; PRSW = preload recruitable stroke work; PVR = pulmonary vascular resistance; Qc = cardiac output; RAP = right atrial pressure; RV = right ventricular; SV = stroke volume; SW = stroke work; WU = Wood units.
Data are presented as median (interquartile range).
P < 0.05 for comparison with an FiO2 of 0.21.
P < 0.05 for comparison with an FiO2 of 0.17.
P < 0.05 for comparison with an FiO2 of 0.15.
Figure 1.
Example hemodynamic tracings demonstrating the response to acute hypoxia. RV = right ventricular.
The primary findings from this study are as follows: 1) Mean PA pressure increased by ∼17% in response to an acute reduction in FiO2 from 0.21 to 0.12; 2) despite the associated increase in RV afterload, there were concomitant increases in RV contractility, lusitropy, and energetics; consequently, 3) RV ventricular-arterial coupling was preserved.
Limited studies have characterized RV performance in response to hypoxia. Operation Everest II demonstrated that RV afterload increased during a gradual transition from sea level (760 mm Hg) to a simulated Everest summit (240 mm Hg), and RV function, as determined by right atrial pressure, was preserved (1). Among healthy individuals (N = 35), echocardiographic assessment of RV systolic pressure increased after 150 minutes of exposure to an FiO2 of 0.11 (FiO2, 0.21 vs. 0.11: 17.4 ± 3.3 mm Hg vs. 24.9 ± 4.8 mm Hg) with an associated reduction in tricuspid annular plane systolic excursion (FiO2, 0.21 vs. 0.11: 21 ± 1.4 mm vs. 17 ± 1.5 mm; P < 0.05), suggesting decreased RV systolic function in response to increased afterload (7).
This study assessed RV performance using complementary gold-standard methodologies of PA hemodynamics and pressure-volume analysis. Noninvasive methodologies are inherently limited when characterizing PA and RV hemodynamics. Isolated metrics from invasive PA assessments such as right atrial pressure do not adequately characterize RV contractility, lusitropy, energetics, or ventricular-arterial coupling. Thus, data provided in this analysis provide unique insight into RV performance in response to acute increases in afterload, such as that which occurs during acute hypoxia.
These findings can be contextualized among pressure-volume analyses of RV function in other clinical states. For example, metrics of contractility and myocardial energetics during supine rest at FiO2 of 0.15 and 0.12 were similar to values among healthy individuals performing normoxic submaximal exercise (5). Contractility, as assessed by maximum rate of pressure change, was similar, at a FiO2 of 0.12, to that observed in an animal model of acute intermediate–high-risk pulmonary embolism, though in contrast to the pulmonary embolism model, healthy individuals at a FiO2 of 0.12 demonstrated lower afterload and preserved ventricular-arterial coupling (8). Finally, preload recruitable stroke work increased in response to progressive hypoxia to levels comparable with those in patients with PA hypertension associated with systemic sclerosis (9).
Limitations to our study include the small sample size, albeit a size similar to that in prior invasive studies (1). The majority of the participants were males, and gender-specific differences in RV function could not be determined. Second, we evaluated RV performance in response to acute (<1 h) hypoxia among healthy participants and did not include an analysis of RV changes that occur during prolonged exposure to hypoxia.
In conclusion, the healthy RV demonstrates significant contractile reserve such that ventricular-arterial coupling is preserved during acute hypoxia. Additional studies are necessary to characterize longitudinal changes in RV function during chronic hypoxia as well as RV response to hypoxia among individuals with cardiopulmonary disease (2, 10).
Footnotes
Supported by NHLBI grant T32HL007085-48, NIH/NCATS Colorado CTSA grant UL1 TR002535, an International Society of Travel Medicine research award, and a University of Colorado Pulmonary Vascular Disease research award (L.M.F.).
Author Contributions: Conception and design: L.M.F., T.M.B., T.L., B.D.L., R.C.R., A.W.S., and W.K.C. Data acquisition: L.M.F. and W.K.C. Data analysis: L.M.F., K.H., and W.K.C. Data interpretation: L.M.F., T.M.B., T.L., J.S.L., K.H., B.D.L., A.L., R.C.R., A.W.S., and W.K.C. Drafting the manuscript: L.M.F. and W.K.C. Revision and final approval of the manuscript: All authors.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Groves BM, Reeves JT, Sutton JR, Wagner PD, Cymerman A, Malconian MK, et al. Operation Everest II: elevated high-altitude pulmonary resistance unresponsive to oxygen. J Appl Physiol (1985) . 1987;63:521–530. doi: 10.1152/jappl.1987.63.2.521. [DOI] [PubMed] [Google Scholar]
- 2. Cornwell WK, III, Baggish AL, Bhatta YKD, Brosnan MJ, Dehnert C, Guseh JS, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; and Council on Arteriosclerosis, Thrombosis and Vascular Biology Clinical implications for exercise at altitude among individuals with cardiovascular disease: a scientific statement from the American Heart Association. J Am Heart Assoc . 2021;10:e023225. doi: 10.1161/JAHA.121.023225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forbes LM, Bull TM, Lahm T, Make BJ, Cornwell WK., III Exercise testing in the risk assessment of pulmonary hypertension. Chest . 2023:S0012-3692(23)00509-3. doi: 10.1016/j.chest.2023.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornwell WK., III2023.
- 5. Cornwell WK, Tran T, Cerbin L, Coe G, Muralidhar A, Hunter K, et al. New insights into resting and exertional right ventricular performance in the healthy heart through real-time pressure-volume analysis. J Physiol . 2020;598:2575–2587. doi: 10.1113/JP279759. [DOI] [PubMed] [Google Scholar]
- 6. Tran T, Muralidhar A, Hunter K, Buchanan C, Coe G, Hieda M, et al. Right ventricular function and cardiopulmonary performance among patients with heart failure supported by durable mechanical circulatory support devices. J Heart Lung Transplant . 2021;40:128–137. doi: 10.1016/j.healun.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 7. Netzer NC, Strohl KP, Högel J, Gatterer H, Schilz R. Right ventricle dimensions and function in response to acute hypoxia in healthy human subjects. Acta Physiol (Oxf) . 2017;219:478–485. doi: 10.1111/apha.12740. [DOI] [PubMed] [Google Scholar]
- 8. Lyhne MD, Hansen JV, Dragsbæk SJ, Mortensen CS, Nielsen-Kudsk JE, Andersen A. Oxygen therapy lowers right ventricular afterload in experimental acute pulmonary embolism. Crit Care Med . 2021;49:e891–e901. doi: 10.1097/CCM.0000000000005057. [DOI] [PubMed] [Google Scholar]
- 9. Tedford RJ, Mudd JO, Girgis RE, Mathai SC, Zaiman AL, Housten-Harris T, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail . 2013;6:953–963. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, et al. American Thoracic Society Assembly on Pulmonary Circulation Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An official American Thoracic Society research statement. Am J Respir Crit Care Med . 2018;198:e15–e43. doi: 10.1164/rccm.201806-1160ST. [DOI] [PMC free article] [PubMed] [Google Scholar]