Abstract
Muscle fibres are multinuclear cells, and the cytoplasmic territory where a single myonucleus controls transcriptional activity is called the myonuclear domain (MND). MND size shows flexibility during muscle hypertrophy. The MND ceiling hypothesis states that hypertrophy results in the expansion of MND size to an upper limit or MND ceiling, beyond which additional myonuclei via activation of satellite cells are required to support further growth. However, the debate about the MND ceiling hypothesis is far from settled, and various studies show conflicting results about the existence or otherwise of MND ceiling in hypertrophy. The aim of this review is to summarise the literature about the MND ceiling in various settings of hypertrophy and discuss the possible factors contributing to a discrepancy in the literature. We conclude by describing the physiological and clinical significance of the MND ceiling limit in the muscle adaptation process in various physiological and pathological conditions.
Keywords: Muscle hypertrophy, myonuclear domain, satellite cells, skeletal muscle
INTRODUCTION
Skeletal muscle is the largest organ in the body and is primarily composed of post-mitotic muscle fibres.[1] A single muscle fibre can be several centimetres long and possess hundreds of nuclei to control transcription over large cytoplasmic territories. Skeletal muscle fibres are highly flexible in size, and hypertrophic stimuli can result in radial growth of up to two folds or higher. This growth challenges the maximal transcriptional reserves of post-mitotic myonuclei. Skeletal muscle responds to such demands by activating muscle stem cells or satellite cells (SCs), which can divide and donate extra myonuclei to growing muscle fibres. Following the discovery of SCs,[2] their role in muscle fibre growth and regeneration has been well recognised in several hypertrophy conditions. These findings lead to the concept that a single myonucleus can control transcriptional activity in a limited cytoplasmic territory, called the myonuclear domain (MND). This hypothesis was supported by early findings that additional myonuclei are required for hypertrophy in rodents.[3] The first direct evidence of MND was provided in 1989, when it was shown that gene products of a myonucleus stay in its vicinity.[4] Further studies confirmed that the synthesis, processing, and distribution of proteins remain in the localised cytoplasmic region surrounding the myonucleus,[5] although some membrane proteins can diffuse over long distances.[6]
It is speculated that, unlike highly plastic muscle fibre size, MND size is relatively rigid in multiple hypertrophy conditions. In support of this, several studies suggest that muscle hypertrophy is accompanied by the incorporation of myonuclei via activation of SCs so that the expansion of MND size lags behind the expansion of fibre size in hypertrophy.[7,8] It is postulated that there is a limit in the maximal transcriptional capacity beyond which the myonucleus cannot sustain hypertrophy. This limit — or MND ceiling — has been a popular concept in recent literature and supports the necessity of SCs in muscle hypertrophy [Figure 1]. However, other studies show that hypertrophy can occur even when the DNA content of skeletal muscle is unchanged.[9,10,11] This discrepancy in the literature has recently generated debates about the concept and validity of the MND ceiling limit in the muscle hypertrophy process.[9,11,12,13,14] However, the conclusion is far from settled. The purpose of this review is to highlight recent perspectives on the hypothesis of the MND ceiling in the setting of muscle hypertrophy. We first discuss MND size variability in different fibre types and then highlight recent advances in our understanding of the contribution of MND ceiling, myonuclear accretion and SCs to various hypertrophy types.
Figure 1.
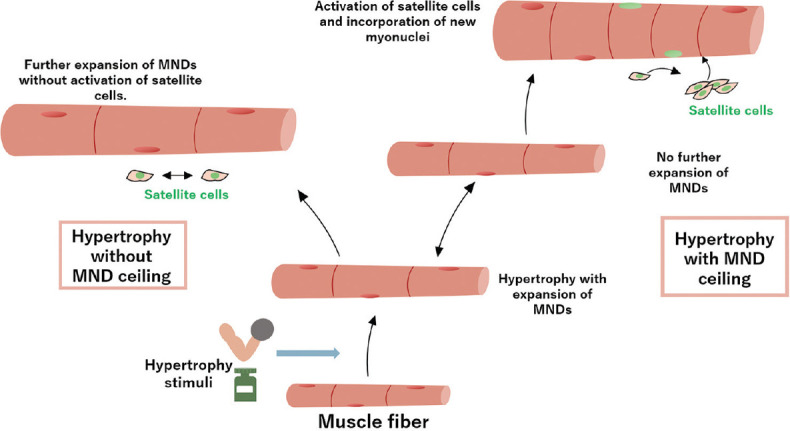
Diagram shows scenarios of muscle hypertrophy with or without the myonuclear domain (MND) ceiling limit.
MYONUCLEAR DOMAIN IN DIFFERENT MUSCLE FIBRE TYPES
The average MND size varies between different muscle fibre types, as Type I slow-twitch muscle fibres have smaller MNDs compared to Type II fast-twitch muscle fibres.[10] Differences in metabolic rates and oxygen usage between Type I and Type II fibres partly account for MND size variability. Higher metabolic activity and larger mitochondrial volume in Type I fibres require more transcriptional activity per myonucleus; hence, MND sizes are smaller compared to Type II fibres.
A hypertrophy stimulus can demonstrate distinct effects on MND size in Type I and Type II fibres. For example, Type II fibres can undergo more than 30% hypertrophy and a 29% increase in MND size without a notable increase in myonuclei number.[5] Type I fibres, on the other hand, respond to hypertrophy by myonuclear accretion rather than expanding their MND size due to having higher metabolic activity than Type II fibres.[3]
These findings suggest greater MND size flexibility in Type II than Type I fibres in the settings of hypertrophy. The relevance of these findings to the MND ceiling hypothesis is evident in variations in fibre-type compositions in different muscles and species. For example, with the exception of the soleus muscle, mouse hind-limbs muscles are almost exclusively composed of Type II fibres.[15] On the other hand, human quadriceps and rat hind-limb muscles have ~50% Type II fibres.[16,17,18] The proportion of Type I fibres is ~50% in mice and ~95% in rats.[16,19] Since several studies investigating hypertrophy response do not consider fibre-type differences, the discrepancy in the literature about MND ceiling, myonuclear accretion, and SCs activation can at least partly be due to variances in fibre-type composition of the muscle and species under investigation.
CHANGES IN MYONUCLEAR DOMAIN SIZE DURING MUSCLE HYPERTROPHY
A number of studies suggest that muscle fibre hypertrophy is accompanied by myonuclear addition in animals[2,13,14,15,16] and humans,[3,17,20,21] thus maintaining the size of the MND. This suggests that additional myonuclei are required to support muscle fibre hypertrophy [Figure 1]. This notion of mandatory myonuclear accretion in hypertrophy is supported by the finding of reduced hypertrophy in irradiated muscle, which has minimal activation and proliferation of SCs.[12,14,22] However, the literature is not consistent with this notion, as other studies show that the early stages of hypertrophy can precede myonuclear accretion, thus expanding the size of the existing MNDs.[23] In support of this finding, up to sevenfold upregulation in myofibre transcription has been reported in the early stages of overload-induced hypertrophy.[10] This upregulation is due to an increase in transcriptional activity per myonucleus rather than the addition of new myonuclei.[10] Thus, resident myonuclei can support hypertrophy by simply increasing their transcription rates.
In addition to the amount of hypertrophy, many other factors need to be considered to determine the need for myonuclear accretion to support muscle hypertrophy. These include, among other factors, the type of hypertrophic stimulus, its strength and duration of exposure, and age of the skeletal muscle, all of which can have varying effects on SC activation and proliferation. The response of myonuclei and changes in MND size for individual hypertrophy stimuli are detailed in Table 1.
Table 1.
Effects of different stimuli of hypertrophy on skeletal muscle SC contents, myonuclei count, MND size and force-generating capacity.
| Hypertrophic stimulus | SC content | Myonuclei content | MND size | Changes in force |
|---|---|---|---|---|
| Myostatin knockout | Downregulated[24,25] Upregulated[26] | Upregulated[24,27] No change[25] | Upregulated[25,27] | Downregulated[27] |
|
| ||||
| Resistance exercise | Upregulated[21,28,29,30,31,32,33,34,35,36,37,38,39] No change[32,40] | Upregulated[21,28,32,34,35,36,37] No change[29,31,32,33,37,38,39,40] | Upregulated[34,37,38,39] No change[21,31,32,36,37] | Upregulated[29,35,36] |
|
| ||||
| Synergist ablation | Upregulated[41] | Upregulated[11,23,42,43,44] | Upregulated[23] No change[23,43] Downregulated[44] | Upregulated[42] |
|
| ||||
| GH/IGF-1 | Upregulated[27] | No change[27] | Upregulated[27] | |
|
| ||||
| Anabolic steroids | Upregulated[45,46] No change[47] | Upregulated[45,48] | Upregulated[47] | Upregulated[49] |
Downregulated: significantly downregulated, GH/IGF-1: growth hormone/insulin-like growth factor-1, MND: myonuclear domain, no change: no statistically significant change, SC: satellite cell, upregulated: significantly upregulated
Resistant exercise
Resistant exercise (RE) involves the voluntary activation of specific skeletal muscles to ≥80% of maximal muscle force against an external resistance.[50,51] RE results in a unique cascade of signalling events that promote myofibre enlargement and remodelling of the extracellular matrix.[52] This is primarily achieved by mechanical loading of sarcolemma in RE and activation of SCs to promote protein synthesis and incorporate new myonuclei to support hypertrophy. However, the SC response to RE is not uniform in the literature, as increased SC pools with[3,17,20,21,28] or without[4,18,29] myonuclear accretion have been reported in RE. The age and/or gender of the participants as well as the type and duration of RE, can partly account for these differences in SC response. For example, it is well recognised that RE-induced hypertrophy increases SC content in young men.[3,17,21,30,31] On the other hand, reports in older men are inconsistent and show an increase in[32,33,34] or maintenance[17,40] of SCs. These results are similar to findings in young women, which also show expansion of MNDs in hypertrophy.[4,17] Less is known about the response of myonuclei to hypertrophy in older women, but it is apparent that RE increases their SC content.[30,34] In addition to gender, duration of training also affects SC activation, as acute and prolonged periods of RE have distinct effects on SC count, activation, and proliferation.[53]
In most cases of RE, expansion of existing MNDs precedes SC activation and myonuclear accretion in hypertrophy. Thus, MND size and muscle fibre size undergo a parallel increase in initial hypertrophy. This hypertrophy is regulated by increasing the transcriptional capacity of myonuclei.[52] The basal characteristics of skeletal muscle myonuclei and SCs play a role in the amount of response to the hypertrophic stimulus. For example, an extreme ‘hypertrophy’ response to RE occurs in muscles with greater basal SC content than ‘lower’ and ‘moderate’ responders. Thus, hypertrophy in extreme responder muscles is accompanied by a greater expansion of the SC pool with training and expansion of myonuclear content, as mentioned earlier.[54] Furthermore, as manifested by muscles with SC deletion, some amount of hypertrophy is supported by the resident myonuclei.[10] However, after a certain amount of hypertrophy, SCs get activated and donate new myonuclei, which prevents further expansion of MNDs, while hypertrophy of muscle continues. This notion that the activation of SCs assists in further hypertrophy beyond a certain stage supports the concept of the MND ceiling limit.[14]
However, the flexibility of MND size in RE is challenged by some investigations that show that myonuclear accretion is an absolute requirement to support hypertrophy in RE. There is an age- and duration-specific response of myonuclear addition in RE in young versus old and short- versus long-term RE.[55] Further, myonuclear accretion is also observed in non-hypertrophy stimuli such as endurance exercises,[56] which challenges the notion that resident myonuclei have great transcriptional reserves to meet the increased demand for protein synthesis in the muscle adaptation process.
Certainly, more work is required to establish the notion of MND ceiling in RE-induced hypertrophy. However, it seems that most of the literature supports myonuclear accretion in hypertrophy due to RE, which indirectly indicates an upper MND ceiling limit and is influenced by age, gender, and fibre type.
Synergistic ablation
Synergistic ablation offers an attractive model to investigate the response of myonuclei and SCs in mechanical overload. It involves the removal of the gastrocnemius and soleus muscles, which results in hypertrophy of the plantaris muscle due to mechanical overload. In addition, it induces SC proliferation activation, and fusion into the myofibres. Initial hypertrophy in 3–5 days following synergistic ablation occurs without myonuclear accretion,[12] followed by the addition of new myonuclei to support hypertrophy. After two weeks of functional overload, the myonuclei content increases by ~60%, and SC content increases by ~275% compared to sham control muscle.[2] Since 12–15 days of synergistic ablation results in even greater hypertrophy, this indicates that prolonged postoperative durations of synergistic ablation lead to an increase in the amount of hypertrophy.
Similar to the findings in RE in humans, synergistic ablation results in greater hypertrophy of fast-twitch Type IIa fibres rather than the slow-twitch Type I. In a synergistic ablation rat model, after functional overload of ten weeks, an increase was seen in the size of fibres and myonuclei number in plantaris muscles.[3] MND size is maintained during hypertrophy in both Types I and IIa fibres, but decreases in Type IIx/b fibres. This is explained by a parallel increase in myonuclei count and fibre size in Type I and Type IIa, but not in Type IIx/b, fibres. These findings differ in different muscles based on the duration of the overload. For example, during the early stages of compensatory hypertrophy, muscle fibre structural abnormalities play a significant role in SC activation; however, in later stages, increased levels of muscle activity lead to SC activation.[10]
To further understand the role(s) of SCs in hypertrophy due to synergistic ablation, mice with conditional SC ablation are used.[57] These mice show similar levels of hypertrophy in the presence or absence of SCs along with increased MND size in SC-depleted mice.[57] This showcases the ability of resident myonuclei to increase their transcriptional capacity to support hypertrophy. However, these findings are not consistent in the literature, partly because the amount of hypertrophy may vary due to the magnitude of exercise and presence of regenerating fibres during analysis.[58] To understand the age-specific response of myonuclei to hypertrophic stimulus, Murach et al. performed synergistic ablation in young and mature mice and found that SC depletion prevented hypertrophy in the young mice but not the adult mice.[11] This discrepancy may be explained by the fact that the young mice in the maturation phase were dependent on SCs for growth and hypertrophy, whereas adult mice that have attained maximal growth use the reserve transcriptional capacity of their resident myonuclei to support hypertrophy. These findings highlight the essential role of SCs in supporting hypertrophy in an age-specific manner.
These findings show that the notion of the MND ceiling is partly age- and fibre type-dependent. Young rodents require SCs for muscle hypertrophy, as the resident MNDs hit a ceiling limit during initial hypertrophy before SC activation becomes mandatory to support further hypertrophy. However, mature rodents have myonuclei with greater transcriptional reserves, and the concept of MND ceiling and domain size rigidity is not strictly applied in them. There is a role of fibre type as well, as myonuclei from Type II fibres have greater transcriptional capacity than the myonuclei from Type I fibres. Fibre type transformation during mechanical overload can further complicate the notion of an MND ceiling, as many Type I fibres with limited myonuclear transcriptional reserves are converted to Type II fibres during sustained mechanical overload.
Anabolic hormones
Several hormones, including growth hormone (GH), testosterone, and thyroid hormones, can induce skeletal muscle growth and strength. Among them, GH appears to act as a biochemical amplifying system for the cell’s anabolic machinery and determines the absolute changes in muscle size that result from changes in muscular work.[59]
Growth hormone signalling controls the size of the differentiated myotubes in a cell-autonomous manner while having no effect on the size, proliferation, and differentiation of the myoblast precursor cells. The GH hypertrophic action leads to an increased myonuclear number, indicating that GH facilitates the fusion of myoblasts with nascent myotubes.[60] Insulin-like growth factor-1 (IGF-I) is the major downstream-signalling molecule of GH, and its circulating levels are positively associated with muscle mass and aerobic fitness. The anabolic effects of the GH/IGF-1 axis are well recognised. Mice with IGF-1 overexpression show hypertrophy with SC activation, which is considered the source of new myonuclei.[61] We have previously confirmed and extended these findings by showing that the IGF-1 overexpression results in relative maintenance of MND size in single muscle fibres.[27] The slight increase of ~10% in MND size in Type II fibres probably reflected the maximal synthetic capacity of resident myonuclei in IGF-1-induced hypertrophy. While most studies do not investigate muscle functional performance, we showed that muscle force is maintained with a slight expansion of MND size in Type II fibres. We also proposed an MND ceiling size of ~31,000 um3, which reflects the maximal transcriptional capacity of myonuclei.[27] On the other hand, the Type I fibres showed a greater reserve for synthetic capacity in hypertrophy.
Similar to IGF-1, anabolic steroids also induce muscle hypertrophy. Testosterone has been linked with an increase in the number of myonuclei and SCs in healthy young men.[9,10] Similarly, athletes who use anabolic steroids to boost muscle mass and/or performance have more myonuclei than athletes who do not.[47] In older men, testosterone administration results in an increase in SCs and myonuclear numbers compared to baseline.[11] However, testosterone has also been shown to induce hypertrophy in SC mice.[62] While MND size and the concept of the MND ceiling were not rigorously characterised in these studies, it seems that the prerequisite of additional myonuclei in hypertrophy is conditional and partly determined by the type of hormone and physical status of skeletal muscle.
ADDITIONAL MYONUCLEI RELEVANT DURING REGENERATION BUT NOT HYPERTROPHY?
Satellite cells, which are the donors of myonuclei during regeneration, are activated by muscle damage, which elicits an inflammatory response.[63] Their importance in muscle regeneration is evident by the fact that ablation of these cells, or the regulatory cell types, or even their residence in the pathogenic environment results in a significant loss of muscle regeneration following injury.[1,2] Contrary to the well-recognised role of SCs in muscle regeneration, their contribution to hypertrophy is debated. It has been postulated that myonuclear accretion and SC proliferation accompany hypertrophic growth at some stage. Initially, hypertrophy leads to an increase in the size of the existing MND without the addition of extra myonuclei. However, beyond a certain degree of hypertrophy, SCs are activated and donate myonuclei to the growing muscle fibre, which restores the MND size to its baseline levels.
Conversely, it is claimed that the type of hypertrophic stimulus is the prime determinant of activation or otherwise of SCs and the amount of hypertrophy. During RE and high-to-moderate endurance exercises, myotrauma and micro-injuries stimulate the release of cytokines by the immune system, which activates SCs. Thus, it seems that SC activation in RE is at least partly due to the micro-injuries and the resultant inflammatory response, which initiate the process of regeneration. The repeating cycle of damage and repair, along with the activation of myogenic regulatory genes and increased plasma levels of anabolic hormones such as testosterone and IGF, stimulate SCs to support hypertrophy. According to the nuclear domain theory, the donation of additional myonuclei by SCs occurs when the MND reaches a maximum threshold size.[4] Among the anabolic hormones triggering hypertrophy, increased levels of IGF-1, which is stimulated by mechanical overload, contribute to hypertrophy by inducing SC proliferation and differentiation.[64] By increasing the expression of cell-cycle progression factors, IGF-1 triggers the proliferation of myoblasts, which is then followed by triggering the expression of myogenic regulatory factors, resulting in differentiated myoblasts. Through its role in SC activation, IGF-1 rescues atrophied muscles in age-related muscle loss, thus further accentuating its role in muscle growth and hypertrophy.[65]
On the contrary, several studies have shown that hypertrophy requires little or no SC involvement. For example, the absence or blockage of myostatin, a TGF-beta (transforming growth factor beta) cytokine, does not activate SCs.[24] This mechanism is proposed for treating postnatal congenital or acquired myopathies. These findings are in agreement with recent data in which hypertrophied muscle fibres showed no additional myonuclei or SCs after blockage of myostatin.[25] Additionally, mice with dysfunctional myostatin demonstrate reduced strength despite muscle hypertrophy, showing that force generation does not increase in parallel with an increase in muscle size in the absence of myostatin.[66]
Supporting these findings of muscle hypertrophy in myostatin-deficient mice, when SCs are ablated in muscles of adult mice by tamoxifen injections, two weeks of mechanical overload can still cause significant hypertrophy.[57] This leads to expansion of the existing MND as a compensatory mechanism to support hypertrophy in the absence of SCs. Such results may be attributed to the higher threshold of the transcriptional capacity per myonuclei, which was not challenged by two weeks of muscle overload, thus enabling hypertrophy without SC involvement. However, similar hypertrophy is not maintained with a prolonged functional overload of 4–8 weeks in the SC-depleted muscle, which shows that muscle growth plateaued and that perhaps the MND ceiling limit was reached before four weeks of functional overload.[67]
Taken together, our findings show that while the contribution of SCs to regeneration is well established, their contribution to muscle hypertrophy is evident only when the hypertrophy stimulus is consistent and strong enough to challenge the maximal transcription capacity of individual myonuclei. These findings are consistent with the presence of an MND ceiling limit when the muscle is exposed to hypertrophy stimulus.
PHYSIOLOGICAL AND CLINICAL SIGNIFICANCE OF MYONUCLEAR DOMAIN CEILING
Pushing the myonuclear transcriptional capacity to the maximal limit with hypertrophic stimuli such as RE seems an attractive intervention to boost muscle mass. This is mainly because SC activation and myonuclear incorporation follow the MND ceiling limit during hypertrophy. The newly added myonuclei become a part of skeletal muscle memory and are retained after the hypertrophy stimulus is discontinued. In this way, hypertrophy is ‘remembered’ by the skeletal muscle despite the subsequent loss of muscle mass. Such muscle can regain mass faster than untrained fibres when a hypertrophic stimulus is applied in the future. The duration of retention of new myonuclei is still a subject of debate; however, they reside in skeletal muscle for long periods of time and possibly permanently.[68] In doing so, cellular memory develops where skeletal myofibres, through the retention of the acquired nuclei, seem to be more inclined to respond to a future hypertrophic stimulus, in contrast to untrained muscles. When a new hypertrophic stimulus is applied, the muscle cells that previously acquired a higher number of myonuclei are the ones that expand and mature faster than naive fibres. Studies show that periods of overload exercise, specifically at a younger age, significantly increase myonuclei density. The newly acquired myonuclei stabilise cellular metabolism during periods of physical inactivity and muscle atrophy. However, contrasting results show that after 12 weeks of detraining, the number of myonuclei in myofibres returns to its original pre-training status.[20] Nevertheless, muscle memory presents a model that supports the theory of the essential accretion of myonuclei for support of hypertrophy.[12,68]
While the concept of the MND ceiling limit might not have a global application for all fibre types, different muscles, and species, it still offers SC transplantation as an attractive intervention to boost muscle mass beyond the MND ceiling threshold. In an immunodeficient mouse model, transplant of human SCs showed accelerated muscle repair and regeneration following injury by establishing a heterogenic SCs pool.[69,70] However, SC transplantation presents different challenges that hinder the possible use of this therapy in clinical trials. Transplantation efficacy depends on several limiting factors, including hormonal status, the gender of the recipient, and the characteristic of transplanted SCs.[71] A detailed description of these factors in the settings of SC transplantation is beyond the scope of this review. However, myoblast transplant offers a unique substitute for SC transplantation. When transplanted, these cells fuse with the endogenous myofibres, adding to the host’s myocytes pool. Donor nuclei can be traced through different methods, including β-galactosidase-labeled nuclei or through fluorescence in situ hybridisation.[5,6,11] The transplanted myoblasts are also effective in regeneration after re-implantation in a new host, where they resume their transcriptional activity and increase muscle strength or remain in a dormant state for future needs. Accordingly, injecting myoblast cells into rejuvenating tissues enhances muscle mass and function.[3,9] This procedure yields no side effects or long-term complications, although the host’s muscle microenvironment and immune reactions challenge the efficacy of this procedure.[6,10,72]
It must be noted that despite its potential clinical applications, the MND ceiling limit is mainly conceptual, with pre-clinical data. As such, rigorous characterisation of MND size in muscle adaptation process is required before it can be applied in clinics.
IS MYONUCLEAR DOMAIN CEILING STILL RELEVANT?
We have described some of the variations in results concerning muscle hypertrophy and the MND ceiling. Several reasons can account for this discrepancy. Among those factors, the exact role of SCs during muscle hypertrophy is poorly understood. For example, the deprivation of SCs in a synergistic ablation model still produces muscle hypertrophy along with the expansion of resident MNDs; however, this hypertrophy is accompanied by functional compromise with reduced muscle strength. These effects are partly age-dependent, as adult mice show greater MND size flexibility than young mice during hypertrophy. Additionally, fibre type also plays a part, with Type II fibres having greater transcriptional reserves than Type I fibres. Further, the duration of an RE programme can induce a degree of varying hypertrophy and adaptation response by the skeletal muscle. For example, long-term RE is associated with the recruitment of SCs, whereas hypertrophy due to brief short-term RE is supported by existing myonuclei. Different methodologies of measuring myonuclear content or MND size can also account for some of the discrepancies in the literature. For instance, some studies use analysis of muscle cross-section areas, while others use captured three-dimensional images of single muscle fibres. The type of hypertrophic stimuli also plays a crucial role in modulating hypertrophy mechanisms at a cellular level. For instance, anabolic hormones such as testosterone stimulate myonuclear accretion during early hypertrophy, while lack of myostatin does not induce myonuclear accretion.
In conclusion, it seems that the concept of the MND ceiling during hypertrophy is conditional and not universal, as various factors including SCs activation, age, fibre type, duration, and type of hypertrophic stimulus can determine the presence and/or extent of the MND ceiling during hypertrophy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We are thankful to Dr Anu Ranade and Dr Asima Karim from the Basic Medical Sciences Department of College of Medicine, University of Sharjah, United Arab Emirates, for their support.
REFERENCES
- 1.Liu JX, Höglund AS, Karlsson P, Lindblad J, Qaisar R, Aare S, et al. Myonuclear domain size and myosin isoform expression in muscle fibres from mammals representing a 100,000-fold difference in body size. Exp Physiol. 2009;94:117–29. doi: 10.1113/expphysiol.2008.043877. [DOI] [PubMed] [Google Scholar]
- 2.Robertson JD. Electron microscopy of the motor end-plate and the neuromuscular spindle. Am J Phys Med. 1960;39:1–43. [PubMed] [Google Scholar]
- 3.Schiaffino S, Bormioli SP, Aloisi M. The fate of newly formed satellite cells during compensatory muscle hypertrophy. Virchows Arch B Cell Pathol. 1976;21:113–8. doi: 10.1007/BF02899148. [DOI] [PubMed] [Google Scholar]
- 4.Pavlath GK, Rich K, Webster SG, Blau HM. Localization of muscle gene products in nuclear domains. Nature. 1989;337:570–3. doi: 10.1038/337570a0. [DOI] [PubMed] [Google Scholar]
- 5.Hall ZW, Ralston E. Nuclear domains in muscle cells. Cell. 1989;59:771–2. doi: 10.1016/0092-8674(89)90597-7. [DOI] [PubMed] [Google Scholar]
- 6.Dupont-Versteegden EE. Apoptosis in muscle atrophy:Relevance to sarcopenia. Exp Gerontol. 2005;40:473–81. doi: 10.1016/j.exger.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo VC, McCarthy JJ. Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology (Bethesda) 2019;34:30–42. doi: 10.1152/physiol.00034.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz LM. Skeletal muscles do not undergo apoptosis during either atrophy or programmed cell death-revisiting the myonuclear domain hypothesis. Front Physiol. 2019;9:1887. doi: 10.3389/fphys.2018.01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murach KA, Englund DA, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Myonuclear domain flexibility challenges rigid assumptions on satellite cell contribution to skeletal muscle fiber hypertrophy. Front Physiol. 2018;9:635. doi: 10.3389/fphys.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirby TJ, Patel RM, McClintock TS, Dupont-Versteegden EE, Peterson CA, McCarthy JJ. Myonuclear transcription is responsive to mechanical load and DNA content but uncoupled from cell size during hypertrophy. Mol Biol Cell. 2016;27:788–98. doi: 10.1091/mbc.E15-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murach KA, White SH, Wen Y, Ho A, Dupont-Versteegden EE, McCarthy JJ, et al. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet Muscle. 2017;7:14. doi: 10.1186/s13395-017-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadi F. In response to Point:Counterpoint: “Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy”. J Appl Physiol (1985) 2007;103:1105. doi: 10.1152/japplphysiol.00466.2007. [DOI] [PubMed] [Google Scholar]
- 13.Lowe DA. In response to Point:Counterpoint: “Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy”. J Appl Physiol (1985) 2007;103:1106. doi: 10.1152/japplphysiol.00466.2007. [DOI] [PubMed] [Google Scholar]
- 14.Qaisar R, Larsson L. What determines myonuclear domain size? Indian J Physiol Pharmacol. 2014;58:1–12. [PubMed] [Google Scholar]
- 15.Mänttäri S, Järvilehto M. Comparative analysis of mouse skeletal muscle fibre type composition and contractile responses to calcium channel blocker. BMC Physiol. 2005;5:4. doi: 10.1186/1472-6793-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–72. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- 17.Alamdari N, Toraldo G, Aversa Z, Smith I, Castillero E, Renaud G, et al. Loss of muscle strength during sepsis is in part regulated by glucocorticoids and is associated with reduced muscle fiber stiffness. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1090–9. doi: 10.1152/ajpregu.00636.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cristea A, Qaisar R, Edlund PK, Lindblad J, Bengtsson E, Larsson L. Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell. 2010;9:685–97. doi: 10.1111/j.1474-9726.2010.00594.x. [DOI] [PubMed] [Google Scholar]
- 19.Maffei M, Longa E, Qaisar R, Agoni V, Desaphy JF, Camerino DC, et al. Actin sliding velocity on pure myosin isoforms from hindlimb unloaded mice. Acta Physiol (Oxf) 2014;212:316–29. doi: 10.1111/apha.12320. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy JJ, Esser KA. Counterpoint:Satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl Physiol (1985) 2007;103:1100–2. doi: 10.1152/japplphysiol.00101.2007a. discussion 2-3. [DOI] [PubMed] [Google Scholar]
- 21.Snijders T, Smeets JS, van Kranenburg J, Kies AK, van Loon LJ, Verdijk LB. Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance-type exercise training. Acta Physiol (Oxf) 2016;216:231–9. doi: 10.1111/apha.12609. [DOI] [PubMed] [Google Scholar]
- 22.Jurdana M. Radiation effects on skeletal muscle. Radiol Oncol. 2008;42:15–22. [Google Scholar]
- 23.van der Meer SF, Jaspers RT, Jones DA, Degens H. The time course of myonuclear accretion during hypertrophy in young adult and older rat plantaris muscle. Ann Anat. 2011;193:56–63. doi: 10.1016/j.aanat.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 24.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–47. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, et al. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci U S A. 2009;106:7479–84. doi: 10.1073/pnas.0811129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab. 2009;297:E157–64. doi: 10.1152/ajpendo.00193.2009. [DOI] [PubMed] [Google Scholar]
- 27.Qaisar R, Renaud G, Morine K, Barton ER, Sweeney HL, Larsson L. Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level? FASEB J. 2012;26:1077–85. doi: 10.1096/fj.11-192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113:99–103. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- 29.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64:332–9. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker DK, Fry CS, Drummond MJ, Dickinson JM, Timmerman KL, Gundermann DM, et al. PAX7+satellite cells in young and older adults following resistance exercise. Muscle Nerve. 2012;46:51–9. doi: 10.1002/mus.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damas F, Libardi CA, Ugrinowitsch C, Vechin FC, Lixandrão ME, Snijders T, et al. Early- and later-phases satellite cell responses and myonuclear content with resistance training in young men. PLoS One. 2018;13:e0191039. doi: 10.1371/journal.pone.0191039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle;from birth to old age. Age (Dordr) 2014;36:545–7. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blocquiaux S, Gorski T, Van Roie E, Ramaekers M, Van Thienen R, Nielens H, et al. The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp Gerontol. 2020;133:110860. doi: 10.1016/j.exger.2020.110860. [DOI] [PubMed] [Google Scholar]
- 34.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci. 2013;68:769–79. doi: 10.1093/gerona/gls241. [DOI] [PubMed] [Google Scholar]
- 35.Kadi F, Eriksson A, Holmner S, Butler-Browne GS, Thornell LE. Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol. 1999;111:189–95. doi: 10.1007/s004180050348. [DOI] [PubMed] [Google Scholar]
- 36.Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, et al. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One. 2014;9:e109739. doi: 10.1371/journal.pone.0109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291:E937–46. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 38.Herman-Montemayor JR, Hikida RS, Staron RS. Early-phase satellite cell and myonuclear domain adaptations to slow-speed vs. traditional resistance training programs. J Strength Cond Res. 2015;29:3105–14. doi: 10.1519/JSC.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 39.Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, et al. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004;558:1005–12. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dirks ML, Tieland M, Verdijk LB, Losen M, Nilwik R, Mensink M, et al. Protein supplementation augments muscle fiber hypertrophy but does not modulate satellite cell content during prolonged resistance-type exercise training in frail elderly. J Am Med Dir Assoc. 2017;18:608–15. doi: 10.1016/j.jamda.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Snow MH. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat Rec. 1990;227:437–46. doi: 10.1002/ar.1092270407. [DOI] [PubMed] [Google Scholar]
- 42.Roy RR, Monke SR, Allen DL, Edgerton VR. Modulation of myonuclear number in functionally overloaded and exercised rat plantaris fibers. J Appl Physiol (1985) 1999;87:634–42. doi: 10.1152/jappl.1999.87.2.634. [DOI] [PubMed] [Google Scholar]
- 43.McCall GE, Allen DL, Linderman JK, Grindeland RE, Roy RR, Mukku VR, et al. Maintenance of myonuclear domain size in rat soleus after overload and growth hormone/IGF-I treatment. J Appl Physiol (1985) 1998;84:1407–12. doi: 10.1152/jappl.1998.84.4.1407. [DOI] [PubMed] [Google Scholar]
- 44.Egner IM, Bruusgaard JC, Gundersen K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development. 2016;143:2898–906. doi: 10.1242/dev.134411. [DOI] [PubMed] [Google Scholar]
- 45.Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285:E197–205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- 46.Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006;91:3024–33. doi: 10.1210/jc.2006-0357. [DOI] [PubMed] [Google Scholar]
- 47.Kadi F, Eriksson A, Holmner S, Thornell LE. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999;31:1528–34. doi: 10.1097/00005768-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–64. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 49.Liu H, Bravata DM, Olkin I, Friedlander A, Liu V, Roberts B, et al. Systematic review:The effects of growth hormone on athletic performance. Ann Intern Med. 2008;148:747–58. doi: 10.7326/0003-4819-148-10-200805200-00215. [DOI] [PubMed] [Google Scholar]
- 50.Winett RA, Carpinelli RN. Potential health-related benefits of resistance training. Prev Med. 2001;33:503–13. doi: 10.1006/pmed.2001.0909. [DOI] [PubMed] [Google Scholar]
- 51.Vianna JM, Lima JP, Saavedra FJ, Reis VM. Aerobic and anaerobic energy during resistance exercise at 80% 1RM. J Hum Kinet. 2011;29A:69–74. doi: 10.2478/v10078-011-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bamman MM, Roberts BM, Adams GR. Molecular regulation of exercise-induced muscle fiber hypertrophy. Cold Spring Harb Perspect Med. 2018;8:a029751. doi: 10.1101/cshperspect.a029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin NRW, Lewis MP. Satellite cell activation and number following acute and chronic exercise:A mini review. Cell Mol Exerc Physiol. 2012;1:e3. [Google Scholar]
- 54.Joanisse S, Lim C, McKendry J, Mcleod JC, Stokes T, Phillips SM. Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans. F1000Res. 2020;9:F1000. doi: 10.12688/f1000research.21588.1. Faculty Rev-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 2006;101:531–44. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 56.McKenzie AI, D’Lugos AC, Saunders MJ, Gworek KD, Luden ND. Fiber type-specific satellite cell content in cyclists following heavy training with carbohydrate and carbohydrate-protein supplementation. Front Physiol. 2016;7:550. doi: 10.3389/fphys.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–66. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nascimento de Oliveira-Júnior G, de Sousa JFR, Carneiro MADS, et al. Resistance training volume enhances muscle hypertrophy, but not strength in postmenopausal women:A randomized controlled trial. J Strength Cond Res. 2020 doi: 10.1519/JSC.0000000000003601. doi:10.1519/JSC.0000000000003601. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg AL, Goodman HM. Relationship between growth hormone and muscular work in determining muscle size. J Physiol. 1969;200:655–66. doi: 10.1113/jphysiol.1969.sp008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sotiropoulos A, Ohanna M, Kedzia C, Menon RK, Kopchick JJ, Kelly PA, et al. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc Natl Acad Sci U S A. 2006;103:7315–20. doi: 10.1073/pnas.0510033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–5. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 62.Englund DA, Peck BD, Murach KA, Neal AC, Caldwell HA, McCarthy JJ, et al. Resident muscle stem cells are not required for testosterone-induced skeletal muscle hypertrophy. Am J Physiol Cell Physiol. 2019;317:C719–24. doi: 10.1152/ajpcell.00260.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yablonka-Reuveni Z. The skeletal muscle satellite cell:Still young and fascinating at 50. J Histochem Cytochem. 2011;59:1041–59. doi: 10.1369/0022155411426780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Machida S, Booth FW. Insulin-like growth factor 1 and muscle growth:Implication for satellite cell proliferation. Proc Nutr Soc. 2004;63:337–40. doi: 10.1079/PNS2004354. [DOI] [PubMed] [Google Scholar]
- 65.Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C, Rizzuto E, et al. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell. 2019;18:e12954. doi: 10.1111/acel.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci U S A. 2007;104:1835–40. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Englund DA, Figueiredo V, Dungan C, Murach K, Peck B, Dupont A, et al. Transcriptional profiling of skeletal muscle during hypertrophy in the absence of satellite cell participation reveals muscle-specific diversity and satellite cell dependent signaling networks. FASEB J. 2020;34(Suppl 1):1. [Google Scholar]
- 68.Gundersen K. Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J Exp Biol. 2016;219:235–42. doi: 10.1242/jeb.124495. [DOI] [PubMed] [Google Scholar]
- 69.Meng J, Chun S, Asfahani R, Lochmüller H, Muntoni F, Morgan J. Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol Ther. 2014;22:1008–17. doi: 10.1038/mt.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin J, Yang L, Xie Y, Liu Y, Li S, Yang W, et al. Dkk3 dependent transcriptional regulation controls age related skeletal muscle atrophy. Nat Commun. 2018;9:1752. doi: 10.1038/s41467-018-04038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hall MN, Hall JK, Cadwallader AB, Pawlikowski BT, Doles JD, Elston TL, et al. Transplantation of skeletal muscle stem cells. Methods Mol Biol. 2017;1556:237–44. doi: 10.1007/978-1-4939-6771-1_12. [DOI] [PubMed] [Google Scholar]
- 72.Han WM, Jang YC, García AJ. Engineered matrices for skeletal muscle satellite cell engraftment and function. Matrix Biol. 2017;60-61:96–109. doi: 10.1016/j.matbio.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


