Keywords: blood pressure, circadian rhythm, clock genes, fetal growth, innate immune system
Abstract
Bacterial infections and impaired circulating mitochondrial DNA dynamics are associated with adverse pregnancy outcomes. Unmethylated cytosine-guanine dinucleotide (CpG) motifs are common in bacterial and mitochondrial DNA and act as potent immunostimulators. We tested the hypothesis that exposure to CpG oligonucleotides (ODN) during pregnancy would disrupt blood pressure circadian rhythms and placental molecular clock network, mediating aberrant fetoplacental growth dynamics. Rats were repeatedly treated with CpG ODN in the third trimester [gestational days (GD) 14, 16, and 18] and euthanized on GD20 (near term) or treated with a single dose of CpG ODN on GD14 and euthanized 4 h after treatment. Hemodynamic circadian rhythms were analyzed via Lomb–Scargle periodogram analysis on 24-h raw data collected continuously via radiotelemetry. A P value ≥ 0.05 indicates the absence of a circadian rhythm. Following the first treatment with CpG ODN, maternal systolic and diastolic blood pressure circadian rhythms were lost (P ≥ 0.05). Blood pressure circadian rhythm was restored by GD16 and remained unaffected after the second treatment with CpG ODN (P < 0.0001). Diastolic blood pressure circadian rhythm was again lost after the last treatment on GD18 (P ≥ 0.05). CpG ODN increased placental expression of Per2, Per3, and Tnfα (P ≤ 0.05) and affected fetoplacental growth dynamics. Reduced fetal and placental weights were disproportionately associated with increases in the number of resorptions in ODN-treated dams compared with controls. In conclusion, gestational exposure to unmethylated CpG ODN dysregulates the placental molecular clock network and fetoplacental growth dynamics and disrupts blood pressure circadian rhythms.
NEW & NOTEWORTHY Gestational exposure to unmethylated CpG ODN dysregulates placental molecular clock network and fetoplacental growth dynamics and disrupts blood pressure circadian rhythms. These findings provide novel insights into the relationship between circadian rhythms and immune responses in pregnancy and propose new mechanisms by which maternal responses to immune triggers could dictate circadian rhythms of cardiovascular processes and placental clock machinery function to determine fetal growth trajectories.
INTRODUCTION
During pregnancy, the maternal immune system undergoes adaptations that ensure tolerance to the fetal allograft while mothers maintain their ability to respond to exogenous pathogens (1). It is postulated that the interaction between endocrine and immune system adaptations during pregnancy often guides an aberrant maternal response to exogenous pathogens (2). As a result, pregnant women show greater susceptibility and worse clinical outcomes in response to bacterial and viral infections, such as Listeria monocytogenes and COVID-19, compared with age-matched nonpregnant women (2–5). Moreover, infections and dysregulation of the immune system during pregnancy are associated with adverse cardio-obstetric outcomes such as gestational hypertension and preeclampsia, as well as other obstetric complications including preterm birth, intrauterine growth restriction, and stillbirth (2, 6–9).
Unmethylated cytosine-phosphodiester-guanine (CpG) motifs are potent triggers of host immune responses via stimulation of Toll-like receptor 9 (TLR9), which is a pattern recognition receptor of the innate immune system (10–13). These unmethylated motifs are enriched in microbial DNA, mitochondrial DNA (mtDNA), and fetal DNA; yet these modified sequences are rare in mammalian nuclear DNA (10). Notably, in humans, concentrations of cell-free mtDNA in maternal sera increase during healthy pregnancy and return to nonpregnant values within 6–8 wk postpartum (14). Elevations in mtDNA and the presence of fetal DNA in maternal blood during pregnancy increase endogenous sources of unmethylated CpG sequences in pregnant individuals and may modulate immune responses and perinatal outcomes. In addition, microbial infections (i.e., exogenous immune triggers), circulating cell-free mtDNA, and fetal DNA (i.e., endogenous damage-associated molecular patterns) have all been implicated in obstetric complications such as preeclampsia and preterm birth (11, 15–17). In addition, synthetic CpG oligodeoxynucleotides (ODN) are increasingly used in preclinical studies and ongoing clinical trials as effective vaccine adjuvants for mucosal vaccines against infectious diseases and allergens (18–24). However, these studies and trials are devoid in examining the effects of CpG ODN on maternal outcomes during pregnancy.
Cardiovascular functions such as blood pressure and heart rate follow a circadian pattern, exhibiting an increase during the wake phase (rodents), or mornings (humans), and a decrease during the sleep phase, or nights, respectively (25). Previous studies have shown that circadian patterns of blood pressure are stronger predictors of cardiovascular events compared with averaged daily blood pressure measurements in young patients with hypertension (26). Importantly, disruption of the circadian rhythm during pregnancy adversely affects fetal development (27) and is associated with pregnancy complications, such as intrauterine growth restriction, preeclampsia, HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, and preterm birth, which have long-lasting effects on maternal and offspring health outcomes (28–30). Others have established an association between blood pressure circadian patterns and markers of immune system activation in nonpregnant patients with autoimmune disorders (31). Circadian rhythms are controlled by “clock” genes, which are regulated by mediators of proinflammatory innate immune system responses (32–34). Clock genes have been identified in the placenta, and circadian disruption is associated with compromised placental function and fetal growth restriction (35, 36). Still, an association between immune system dysregulation during pregnancy, which is a feature of many pregnancy complications, and circadian rhythms of maternal blood pressure remains unclear. Moreover, the effects of maternal immune system dysregulation during pregnancy on placental clock genes have not been established.
In this study, we used the gravid rat as an experimental model to determine the impact of exposure to unmethylated CpG ODN during pregnancy on circadian patterns of maternal cardiovascular parameters, expression of clock genes in the placenta, and the interactive effects of these consequences on perinatal outcomes. We treated rats with CpG ODN as an immune trigger and synthetic stimulant of TLR9. Previous studies have shown that exposure to CpG ODN during pregnancy induced adverse maternal cardiovascular and perinatal outcomes in rats (37–39). We hypothesized that maternal treatment with CpG ODN during rat pregnancy would elicit aberrant inflammatory responses, disrupt maternal blood pressure circadian rhythms, and dysregulate the placental molecular clock machinery, mediating adverse fetoplacental outcomes.
METHODS
All protocols were approved by the Institutional Animal Care and Use Committees (IACUC) of the University of North Texas Health Science Center (IACUC-2020-032) and Loma Linda University (IACUC-22-003) and were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All investigators followed the ethical principles outlined in the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments).
Animals
Male (body weight and age on arrival: 400 g and 12–15 wk) and virgin female (body wt and age on arrival: 200 g and 9–11 wk) Sprague–Dawley rats (Envigo, Indianapolis, IN and Houston, TX) were single housed under 12-h:12-h light/dark cycles (lights on, 07:00 h; lights off, 19:00 h) in a temperature and humidity-controlled environment. Animals were provided standard laboratory chow and water ad libitum. Male rats were only used for breeding purposes. Following 1 wk of acclimatization to the animal facilities, female rats were familiarized with handling and vaginal smears before any experimentation, and a normal estrous cycle was determined using vaginal cytology. For pregnancy studies, female rats were mated in-house (pair mating) overnight, and vaginal smears were assessed the following morning for presence of spermatozoa. Gestational day (GD) 1 (term = 22–23 days) was designated as the morning on which spermatozoa were observed in vaginal smears. Daily body weights were recorded throughout gestation. All experiments were performed when rats were 12–24 wk old. In total, 47 female and 12 male rats were used for these studies.
Experimental Design and Time Line
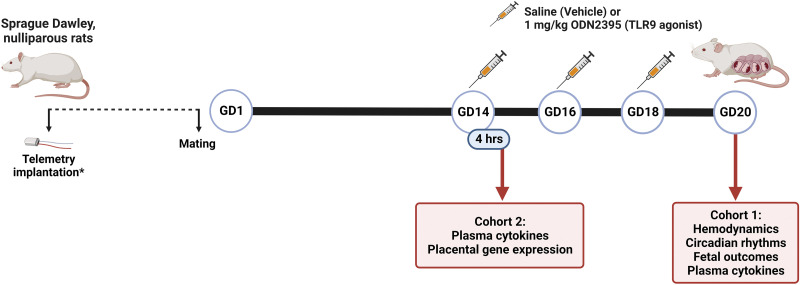
Two studies were conducted and included two separate cohorts of animals (Fig. 1). The purpose of the first study (cohort 1) was to determine the impact of repeated exposure to CpG ODN on maternal hemodynamics, circadian rhythms of maternal blood pressure and heart rate, plasma cytokine concentrations, and fetoplacental biometrics. The purpose of the second study (cohort 2) was to determine acute maternal systemic inflammatory responses to a single dose of CpG ODN and the impact of pregnancy on these responses. Placental inflammation and clock gene expression were measured in placentas from rats exposed to a single dose of CpG ODN to determine placental responses in the absence of chronic systemic responses to this treatment. These studies were conducted only in female rats because the focus of this research is on maternal physiology during pregnancy, which is a female-specific condition.
Figure 1.
Experimental design and time line. Two cohorts were included in the experimental design. Rats assigned to cohort 1 were virgin, nonpregnant females implanted with telemeters before mating, followed by repeated intraperitoneal injections of saline (vehicle) or 1 mg/kg ODN2395 during the third trimester of their pregnancy on gestational day (GD) 14, GD16, and GD18, with euthanasia on GD20 to assess maternal hemodynamics, circadian rhythms of maternal blood pressure and heart rate, fetal outcomes, and maternal plasma cytokines. Cohort 2 included pregnant females at GD14 and age-matched virgin, nonpregnant females. Rats assigned to cohort 2 were administered SALINE (vehicle) or 1 mg/kg ODN2395 via intraperitoneal injection and euthanized 4 h after injections to assess maternal plasma cytokines and placental gene expression. TLR, Toll-like receptor 9. *Telemeters were only implanted in cohort 1. Image was created using a licensed version of BioRender. ODN, oligodeoxynucleotides.
Animal Treatments
Female rats were given intraperitoneal injections of 300 µL of 0.9% saline (vehicle) or CpG ODN (ODN2395, 1 mg/kg body wt; InvivoGen, Cat. No. tlrl-2395; TLR9 agonist). ODN2395 has unmethylated CpG sequences (5′-TCGTCGTTTTCGGCGC:GCGCCG-3′, palindrome is underlined) and is designed with a phosphorothioate-modified backbone for increased resistance to nucleases resulting in a greater half-life of ∼1 h (40). The dose of ODN2395 used in treatments was based on the manufacturer’s recommended range and preliminary studies. Notably, rodent TLR9 has rhythmic expression with greatest expression levels and activity during the wake phase (41). Thus, injections were given in the afternoon between 16:00–18:00 h (just before the wake phase). Cohort 1 included pregnant rats that were treated on GD14, GD16, and GD18 and euthanized on GD20 at 08:00–09:00 h, as previously described (37, 38). Cohort 2 included pregnant rats that were treated on GD14 and age-matched nonpregnant rats. Rats assigned to cohort 2 were euthanized 4 h after treatment (20:00–22:00 h). This euthanasia time point coincides with 2–3 h after the start of the active phase on GD14, when clock genes have been previously shown to have their highest expression during rat gestation (42).
Radiotelemetry Implantation and Maternal Hemodynamic Measurements
To assess blood pressure and heart rate in freely moving conscious rats, nonpregnant female rats (cohort 1) were implanted with a catheter attached to a HD-S10 radiotelemeter transmitter (Data Sciences International, St. Paul, MN). Catheters were implanted into animals’ descending aorta just caudal to the renal arteries and rostral to the iliac bifurcation. The transmitter’s catheter tip was inserted into the abdominal aorta about 4–5 mm and secured, while the transmitter body was secured to the muscle wall in the abdominal cavity before stitching up the muscle and skin. Rats were allowed 7 days to recover from the surgery, and this period was followed by the determination of a normal estrous cycle and mating procedures. Overall, rats recovered approximately 15–20 days after surgical implantation of aortic catheters before data collection commenced. Three days of baseline hemodynamics were recorded before mating, and telemetry measurements continued throughout gestation. Maternal systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) were measured during a 10-s sampling period (500 Hz) and were averaged and recorded every 5 min using Ponemah software (Data Sciences International, St. Paul, MN). Data are first summarized as either a 12-h average of dark cycle (wake phase) or 12-h average of light cycle (sleep phase) and are presented as absolute values (i.e., mmHg or beats/min). Blood pressure and heart rate tracings were used for circadian rhythm analysis as described in Sample Size Determination, Data, and Statistical Analyses.
Euthanasia and Tissue Harvest
Rats were anesthetized with isoflurane (5% for induction, 3% for maintenance) and euthanized by isoflurane overdose followed by bilateral thoracotomy and removal of their hearts. Whole blood was collected from the inferior vena cava when rats were under a deep plane of anesthesia. Following euthanasia, plasma was isolated from whole blood using EDTA-coated collection tubes (BD, Cat. No. 367856) centrifuged at 1,700 g for 15 min at 4°C. Freshly isolated plasma was snap-frozen in liquid nitrogen and stored at −80°C until further analysis. The left and right uterine horns containing corresponding fetuses and placentas were excised, and fetoplacental biometrics were recorded before fetal euthanasia via decapitation. Maternal deciduae were removed from placentas before snap freezing in liquid nitrogen and stored at −80°C for subsequent analysis.
Plasma Cytokine and Chemokine Analyses
Plasma cytokines and chemokines were assessed using a magnetic bead panel (Bio-Rad, Cat. No. 171K1001M). Plasma samples were diluted 1:4 in assay buffer before running the assay according to the manufacturer’s instructions. All samples and standards were run in duplicate, and plates were read on the Bio-Plex MAGPIX Multiplex Reader (Bio-Rad, Hercules, CA) using xPONENT software version 4.3 (Luminex, Austin, TX). The following analytes were measured: proinflammatory cytokines (TNF-α, IL-6, IL-1β, IL-1α, IL-18, IL-12p70, IL-17A, IFNγ, IL-7), anti-inflammatory cytokines (IL-4, IL-5, IL-13, IL-10, IL-2), and chemoattractants (GRO/KC, MCP-1, MIP-1α, MIP-3α, and RANTES).
Extraction of RNA from Placentas
RNA was isolated from GD14 placentas after the removal of decidua. To extract RNA, placentas were digested in 1 mL of TRIzol Reagent (Invitrogen, Cat. No. 15596018) per 100 mg tissue and homogenized on ice using a Qsonica sonicator (three sets of 3-s pulses with 10 min rest on ice between pulsing). Homogenized placentas were treated with 200 µL chloroform (Sigma, Cat. No. C2432) per 1 mL TRIzol used during digestion, vortexed, and allowed to rest at room temperature for 2 min before centrifuging at 12,000 g for 15 min at 4°C. RNA was precipitated from the aqueous phase using 500 µL isopropyl alcohol (Sigma, Cat. No. I9516) per 1 mL TRIzol used during homogenization. After allowing RNA to precipitate for 10 min at room temperature, samples were centrifuged at 12,000 g for 10 min at 4°C. Following centrifugation, the supernatant was removed, and the RNA pellet was washed with 75% ethanol, dried, and suspended in RNase-free water. RNA was dissolved by incubating samples at 60°C for 10 min. The purity and quantity of total RNA isolated from each sample were determined using a Nanodrop spectrophotometer (Thermo Scientific). All samples had a 260/280 ratio of 1.98–2.07 and were diluted to 50 ng/µL RNA using RNase-free water.
Synthesis of cDNA
RNA was reverse transcribed to cDNA using Sensiscript RT Kit reagents (Qiagen, Cat. No. 205213) with RiboGuard RNase inhibitor (Lucigen, Cat. No. E0126-40D7) and oligo-dT primers (Qiagen, Cat. No. 79237). Each 20-µL reaction included 2 µL 10× RT buffer, 2 µL dNTP mix, 2 µL oligo-dT primers, 0.25 µL RNase inhibitor (40 U/µL), 1 µL Sensiscript RT, 8.75 µL RNase-free water, 4 µL of 50 ng/µL RNA. cDNA was synthesized using a T100 thermocycler (Bio-RAD) set at 37°C for 1 h. Synthesized cDNA was stored at −20°C until subsequent analysis.
Quantitative Real-Time Polymerase Chain Reaction
The expression of rat placenta proinflammatory cytokine (Tnfα, Il6, Il1β), clock (Clock, Bmal1, Cry1, Per1, Per2, Per3), and reference (Ppia and Sdha) genes was determined using qRT-PCR. Primer sequences were identified from previous studies (Supplemental Table S1: https://doi.org/10.6084/m9.figshare.22229488) and purchased from Integrated DNA Technologies (IDT). Reactions consisted of 7.5 µL iQ SYBR Green Supermix (Bio-Rad, Cat. No. 1708882), 1.2 µL primer mix (50 µL of 100 mM forward primer, 50 µL of 100 mM reverse primer, 100 µL RNase-free water), 1.8 µL cDNA, and 4.5 µL RNase-free water. Nontemplate control (NTC) reactions were included for each primer set. Each sample and NTC were run in duplicate for target and reference genes. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed on a CFX96 Real-Time PCR Detection System with CFX Maestro Software v.2.3 (Bio-Rad). Cycling parameters were initial denaturation at 95°C for 3 min followed by 40 cycles of 95°C for 10 s and 60°C for 1 min. At the end of the cycling, melt curves were generated by 5-s interval increases in 5°C from 65°C–95°C. Primer specificity was determined by the presence of a single melt peak in samples and no generated melt peaks in NTC. Gene expression was analyzed using the 2−ΔΔCT method. Ppia was chosen for normalization based on its stable expression across all samples.
Sample Size Determination, Data, and Statistical Analyses
The smallest sample size needed to determine differences in blood pressure responses between rats treated with ODN2395 or vehicle was estimated using power analysis based on our previous and preliminary observations. The sample size was selected with a goal to achieve a power of 0.80 to 0.85 with a probability of a type I error of 0.05.
Unpaired t tests were used to determine group differences in plasma cytokine concentrations and fetoplacental outcomes. Hemodynamic responses over the treatment window (GD14–GD19) in saline and ODN2395-treated rats were analyzed by fitting a mixed model with Geisser–Greenhouse correction as implemented in GraphPrism (version 9.2, GraphPad Software). This mixed model uses a compound symmetry covariance matrix and is fit using restricted maximum likelihood (REML). We used this analysis instead of ANOVA with repeated measures because ANOVA cannot handle missing values. In our data set, values were missing completely at random because of occasional connectivity issues with the radiotelemeters. To evaluate the effect of advancing gestational age on hemodynamic responses within the treatment group (GD15, GD16, GD17, GD18, GD19 vs. GD14), we performed multiple comparisons (Dunnett’s test) and calculated multiplicity-adjusted P values for each comparison. Outliers were determined using ROUT (robust regression and outlier removal, ROUT coefficient Q = 1%), and normal distributions were tested using Shapiro–Wilk test (GraphPrism). Data determined to have non-Gaussian distributions were normalized using log transformation before applying parametric statistics. For clarity, raw data are presented in figures. The relationship between placental clock gene expression and inflammatory gene expression was determined using Spearman correlation analysis. The significance level was set to α = 0.05 and P ≤ 0.05 was considered significant. Values are expressed as means ± SD unless otherwise noted.
Multiple linear regression analysis of fetoplacental outcomes was incorporated to examine the effect of covariates (number of resorptions, average placental weight, and litter size) on the main outcome of fetal weight (R statistical software version 4.0.2). Multivariate outliers were detected and removed by median absolute distance (MAD) using principal component analysis (PCA) on fetal biometrics. Sample measures with PC scores ≥ 2 SDs from median PC score were deemed outliers. After outlier removal, multicollinearity was detected between the independent variables: number of resorptions and litter size. To remove multicollinearity, we assessed three multiple linear models and determined the best fit to our data using the Akaike’s information criterion (AIC): 1) average fetal weight as a function of treatment, litter size, and average placental weight; 2) average fetal weight as a function of treatment, average placental weight, and number of resorptions; and 3) average fetal weight as a function of treatment, average placental weight, and ratio of resorptions within a litter. Model 2 consists of average fetal weight as a function of treatment, average placental weight, and number of resorptions, displayed the best fit (AIC = −50.3, Supplemental Table S2: https://doi.org/10.6084/m9.figshare.22229941) and was therefore used to draw conclusions.
We used Lomb–Scargle periodogram to detect and analyze circadian rhythmicity and characteristics of the identified rhythms in SBP, DBP, and HR time series data. Lomb–Scargle periodogram analysis was performed on continuous 24-h raw data with a resolution of 5-min bins for each gestational day using R [DiscoRhythm package (43), R statistical software version 4.0.2, R Core Team 2020] after outlier detection and removal by anomaly detection [Anomalize package for R (44)]. DiscoRhythm determines a common period across multiple parallel time series (i.e., able to find a common period given multiple subjects monitored at the same time) (43). Rhythmicity was determined for each treatment window, which was defined as point of injectionn to point of injectionn + 1, per rat per treatment on 24-h cycle. A P value ≤ 0.05 indicates significant period (i.e., circadian rhythm detected) detected. The following rhythmic parameters were computed: acrophase and amplitude. Acrophase denotes the time of maximum value (peak) and is expressed in Zeitgeber time (0–12 = sleep phase, 07:00–19:00 h; 12–24 = wake phase, 19:00–07:00 h), while amplitude denotes the magnitude of the peak at given acrophase and is expressed in native units (mmHg for blood pressure and BPM for heart rate). We selected the Lomb–Scargle periodogram for rhythmicity detection because it allows the analysis of data sets with missing values (e.g., because of transient signal loss) and is considered to have a better detection efficiency and accuracy in the presence of noise, while it avoids bias that could arise from replacement of missing data by interpolation techniques (45). Specifically, Lomb–Scargle periodogram has been suggested as a suitable method to study hemodynamic variable rhythms in telemetrical time series recorded from living animals (46).
RESULTS
Maternal Blood Pressure and Heart Rate Responses during Sleep and Wake Cycles and Their Circadian Rhythmicity in Rats Exposed to CpG ODN during Pregnancy
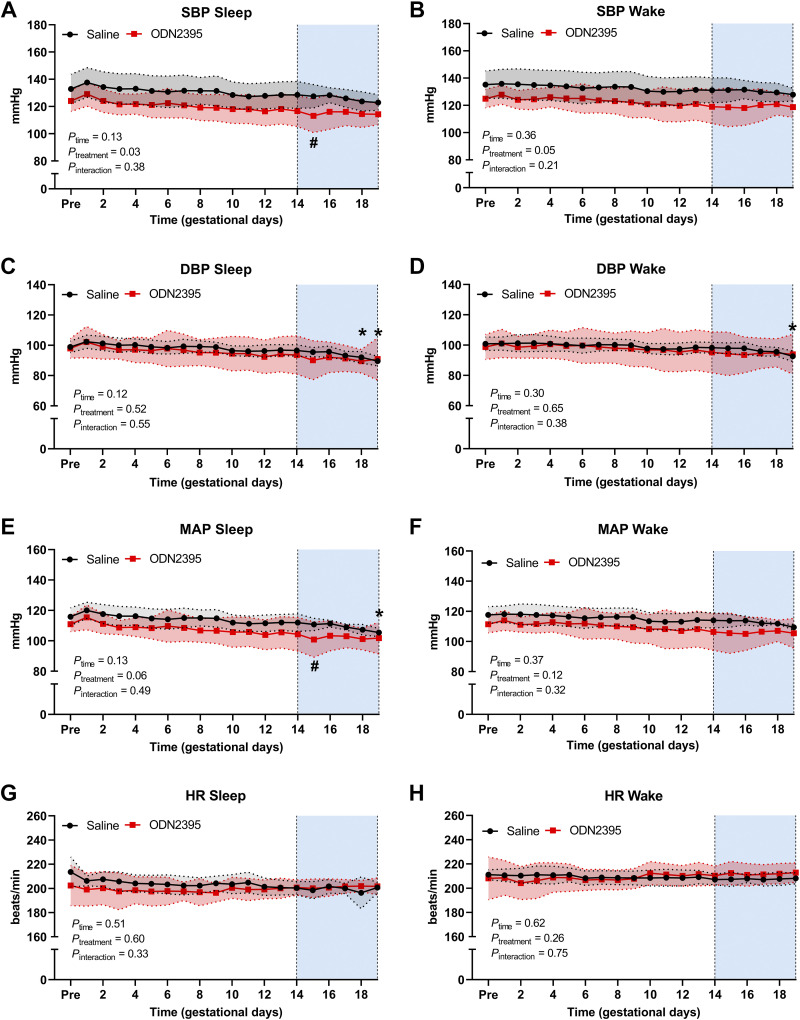
There were no differences in sleep and wake SBP, DBP, MAP, or HR before pregnancy between rats assigned to saline versus ODN2395 groups (P > 0.05, Supplemental Table S3: https://doi.org/10.6084/m9.figshare.22229962). To determine the effects of repeated exposure to treatment with ODN2395 on maternal hemodynamics, we used mixed-effect analysis on data collected using radiotelemetry during the treatment window (GD14–19, GD14: before treatment started, GD19: after the last treatment) in saline-treated and ODN2395-treated groups. We found that the effect of treatment (responses in saline-treated rats minus responses to ODN2395) was consistent across the treatment window (GD14–19) for SBP (Fig. 2, A and B), DBP (Fig. 2, C and D), MAP (Fig. 2, E and F) or HR (Fig. 2, G and H) in the sleep phase (time × treatment interaction, P ≥ 0.33) or wake phase (time × treatment interaction, P ≥ 0.21). Multiple comparison tests with adjustments for multiplicity were used to evaluate the effect of advancing gestational age on hemodynamic responses within each treatment group. Sleep DBP was lower on GD18 (P = 0.04, mean difference ± SE: 4.5 ± 1.2 mmHg) and GD19 (P = 0.003, mean difference ± SE: 7.0 ± 1.0 mmHg) compared with GD14 in saline-treated rats. In a similar manner, wake DBP was also lower on GD19 (P = 0.04, mean difference ± SE: 5.6 ± 1.1 mmHg) compared with GD14 in saline-treated rats. Moreover, sleep MAP was lower on GD19 (P = 0.02, mean difference ± SE: 6.5 ± 1.5 mmHg) compared with GD14 in saline-treated rats. However, reductions in sleep and wake maternal DBP at the end of pregnancy were not seen in ODN2395-treated rats (P > 0.05; Fig. 2 and Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.23154716). These data suggest that DBP recorded during the sleep and wake phases decreases with advancing gestational age in healthy pregnant rats but not in rats treated with ODN2395.
Figure 2.
Temporal changes in maternal blood pressure and heart rate (HR) responses during sleep and wake cycles before and throughout gestation in rats treated with ODN2395 or saline vehicle. A–H: systolic blood pressure (SBP; A and B), diastolic blood pressure (DBP; C and D), mean arterial pressure (MAP; E and F), and HR (G and H) during sleep (A, C, E, and G) and wake (B, D, F, and H) cycles. Intraperitoneal injections of ODN2395 or saline (vehicle) were given on gestational days (GD) 14, 16, and 18. Shaded vertical region represents treatment window (GD14–GD19). Data within the treatment window were analyzed by fitting a mixed model with Geisser–Greenhouse correction. Multiple comparisons to assess simple effects were performed using Dunnett’s test (responses on GD15, GD16, GD17, GD18, GD19 vs. GD14 within each treatment group), and multiplicity adjusted P values for each comparison were calculated. Simple effects: *P < 0.05 vs. GD14 in saline group; #P < 0.05 vs. GD14 in ODN2395-treated group. Values are presented as means ± SD; n = 7/group. Pre, 3-day average of values before mating. ODN, oligonucleotides.
Sleep SBP measured after the first treatment of ODN2395 (GD15) was lower compared with SBP before treatment, on GD14 (P = 0.009, mean difference ± SE: 3.5 ± 0.69 mmHg). This reduction in SBP contributed to a decrease in sleep MAP on GD15 compared with GD14 in the ODN2395 group (P = 0.008, mean difference ± SE: 3.5 ± 0.70 mmHg). After GD15, sleep blood pressure of ODN2395-treated rats returned to pretreatment values and remained unchanged throughout the rest of the treatment window (GD16, GD17, GD18, GD19 vs. GD14, P > 0.05). These data suggest that SBP and MAP recorded during the sleep phase were reduced in response to the first dose of ODN2395 and returned to pretreatment values despite subsequent repeated exposure to ODN2395.
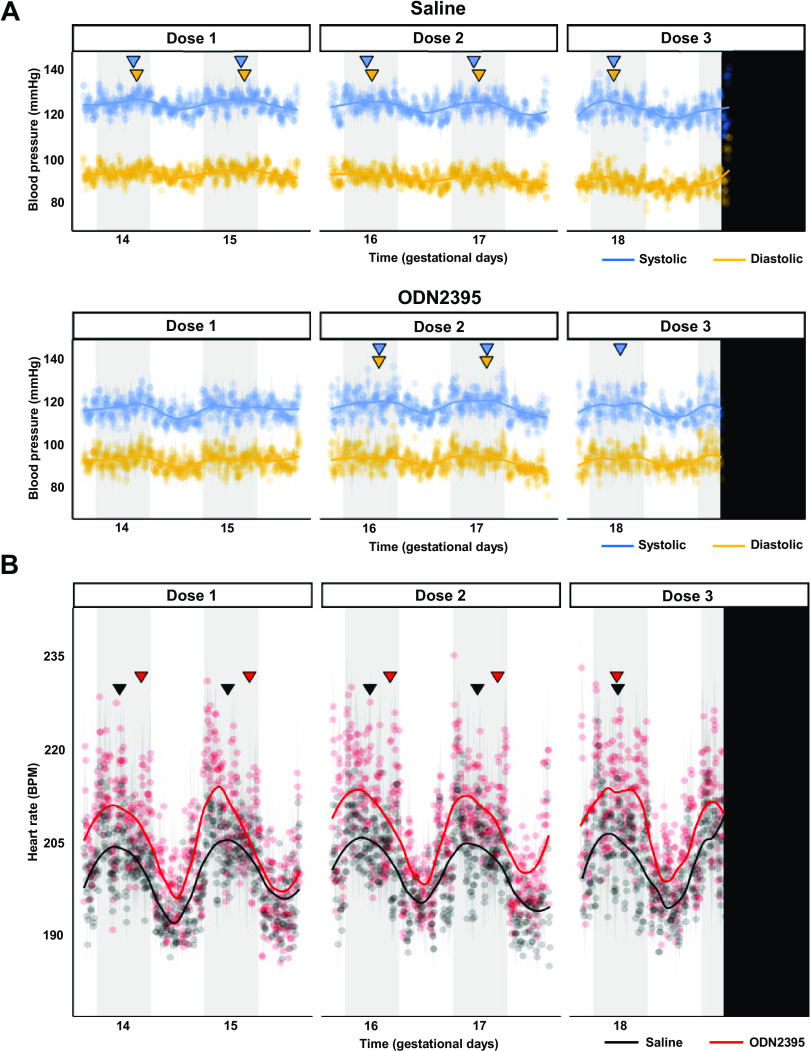
A significant circadian rhythm was detected for all hemodynamic variables over the treatment window (Fig. 3). Circadian rhythm was lost for SBP and DBP after the first ODN2395 treatment on GD14 but recovered and was unaffected after the second treatment with ODN2395 on GD16 (Fig. 3A, Table 1). After the last treatment on GD18, DBP lost circadian rhythmicity (Fig. 3A, Table 1). The amplitude of HR was magnified in the ODN2395 group, and the acrophase of HR was slightly later, but otherwise HR was unaffected (Fig. 3B, Table 1).
Figure 3.
Circadian rhythms of maternal blood pressure and heart rate (HR) during late gestation in rats treated with ODN2395 or saline vehicle. A: mean systolic (blue) and diastolic (gold) blood pressures during treatment window [gestational days (GD) 14–19] for saline (top) and ODN2395 (bottom) cohorts. B: mean heart rate during treatment window (GD14–19) for saline (black) and ODN2395 (red) cohorts. Blue (systolic) and gold (diastolic) inverted triangles (A) represent significant acrophases of respective blood pressures; while black (saline) and red (ODN2395) inverted triangles represent significant acrophases of HR circadian rhythm. Acrophases denote the time of maximum value (peak) and were calculated by Lomb–Scargle periodogram analysis performed on continuous 24-h raw data with a resolution of 5-min bins. Lack of inverted triangle indicates no significant circadian rhythm detected. Vertical black bar denotes end of measurement window (midnight, GD20). Gray vertical bars represent dark hours (active phase). Shaded area flanking data points represent standard error; n = 7/group. Trend lines were estimated by loess smoothing specifying an α of 0.4. BPM, beats/min; ODN, oligodeoxynucleotides.
Table 1.
Lomb–Scargle periodogram analysis of maternal blood pressure and heart rate circadian rhythms
| Saline |
ODN2395 |
|||||
|---|---|---|---|---|---|---|
| SBP | DBP | HR | SBP | DBP | HR | |
| n | 9 | 7 | ||||
| Acrophase (ZT) | ||||||
| GD14–16 | 20:15* | 21:00* | 16:55* | 20:12 | 20:28 | 21:44* |
| GD16–18 | 17:00* | 18:11* | 17:13* | 20:05* | 19:58* | 21:44* |
| GD18–20 | 17:00* | 17:00* | 17:14* | 18:44* | 17:00 | 17:00* |
| Amplitude, mmHg/(beats/min) | ||||||
| GD14–16 | 130.12* | 97.23* | 206.06* | 117.93 | 93.68 | 213.31* |
| GD16–18 | 129.48* | 95.36* | 205.83* | 119.61* | 93.79* | 211.93* |
| GD18–20 | 130.44* | 94.42* | 207.84* | 118.43* | 93.22 | 216.69* |
Acrophase in Zeitgeber time (0–12 = sleep phase, 07:00–19:00 h; 12–24 = wake phase, 19:00–07:00 h) and denotes the time of maximum value (peak). Amplitude expressed in native units [in mmHg for blood pressure and in beats/min for heart rate (HR)] and denotes the magnitude of the peak at given acrophase. *P < 0.05, significant periodicity detected. Boldface indicates no significant periodicity detected, representing a loss of circadian rhythmicity. GD, gestational days; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Fetoplacental Biometrics in Response to Gestational Exposure to CpG ODN
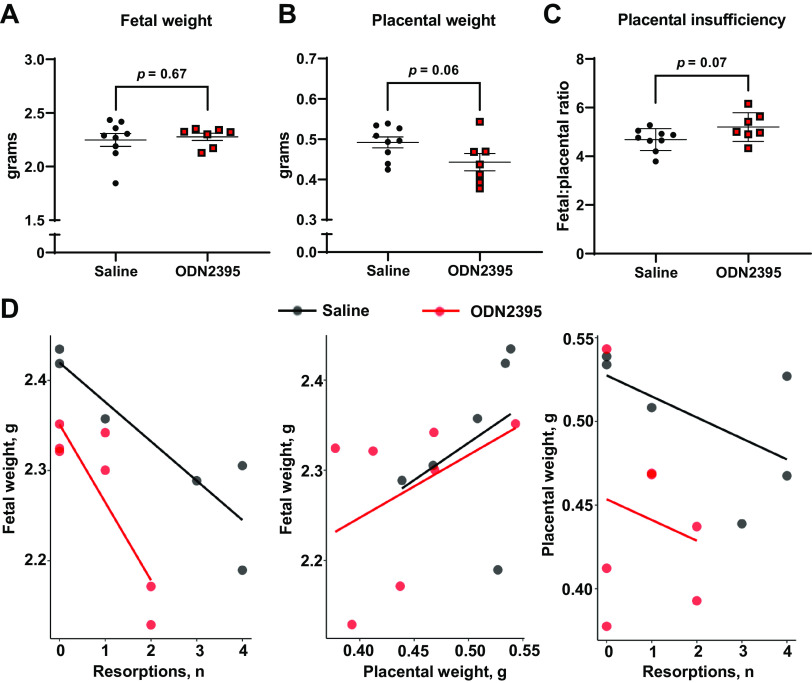
Univariate analysis showed no differences in mean fetal weights (Saline: 2.25 ± 0.18 g, ODN2395: 2.28 ± 0.09 g, P = 0.67, Fig. 4A), placental weights (Saline: 0.49 ± 0.04 g, ODN2395: 0.44 ± 0.06 g, P = 0.06, Fig. 4B), fetoplacental ratio (Saline: 4.69 ± 0.45, ODN2395: 5.20 ± 0.59, P = 0.07, Fig. 4C), litter size (Saline: 14.78 ± 1.56, ODN2395: 14.86 ± 2.41; P = 0.94, unpaired t test) or number of resorptions per litter (Saline: 2.22 ± 2.17, ODN2395: 0.86 ± 90; P = 0.14, unpaired t test) on GD20. Fetal weights vary with litter size and thus, toxicology studies suggest that litter size and number of resorptions should be considered when the effect of a test substance on fetal weights is measured (47). Therefore, in addition to univariate analysis for mean comparisons between groups, we assessed the effects of ODN2395 treatment on the relationship between perinatal outcomes, namely fetal weights, placental weights, and number of resorptions. Notably, we found increases in the number of resorptions were disproportionately associated with reduced fetal weights and placental weights in ODN2395-administered dams compared with saline-administered dams (Fig. 4D). Treatment with ODN2395 amplified the relationship of reduced fetal weights with increased resorptions in the litter, promoted overall lower placental weight when resorptions are present, and reduced the association of larger placentas and increased fetal weight (Fig. 4D; weighted multiple linear regression, treatment × resorption and treatment × placental weight × resorptions, P = 0.02, Supplemental Table S4: https://doi.org/10.6084/m9.figshare.22229971). Collectively, these data suggest that repeated exposure to CpG ODN resulted in interactive effects on pregnancy dynamics that contributed to reduced fetal weight (Fig. 4D, Supplemental Table S4). Of note, treatment with CpG ODN did not impact maternal weight gain during pregnancy (P = 0.54, Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.23154896).
Figure 4.
Effects of ODN2395 on fetal and placental biometrics and fetoplacental dynamics on gestational day 20. Individual and mean fetal weights (A; g) per litter, placental weights (B; g) per litter, fetal to placental weight ratio per litter (C), and scatterplots representing correlations of average fetal weight (D; g) with number of resorptions (left), average fetal weight (g) with average placental weight (g, middle), and average placental weight (g) with number of resorptions (right). Each dot represents the average from a single litter (n = 7–9/group). A–C: values are presented as means ± SD, unpaired t test. D: trend lines (saline = black, ODN2395 = red) represent the linear relationships within the data determined by multiple linear regression. ODN, oligodeoxynucleotides.
Inflammatory Cytokine and Chemoattractant Concentrations in Maternal Circulation after Exposure to CpG ODN
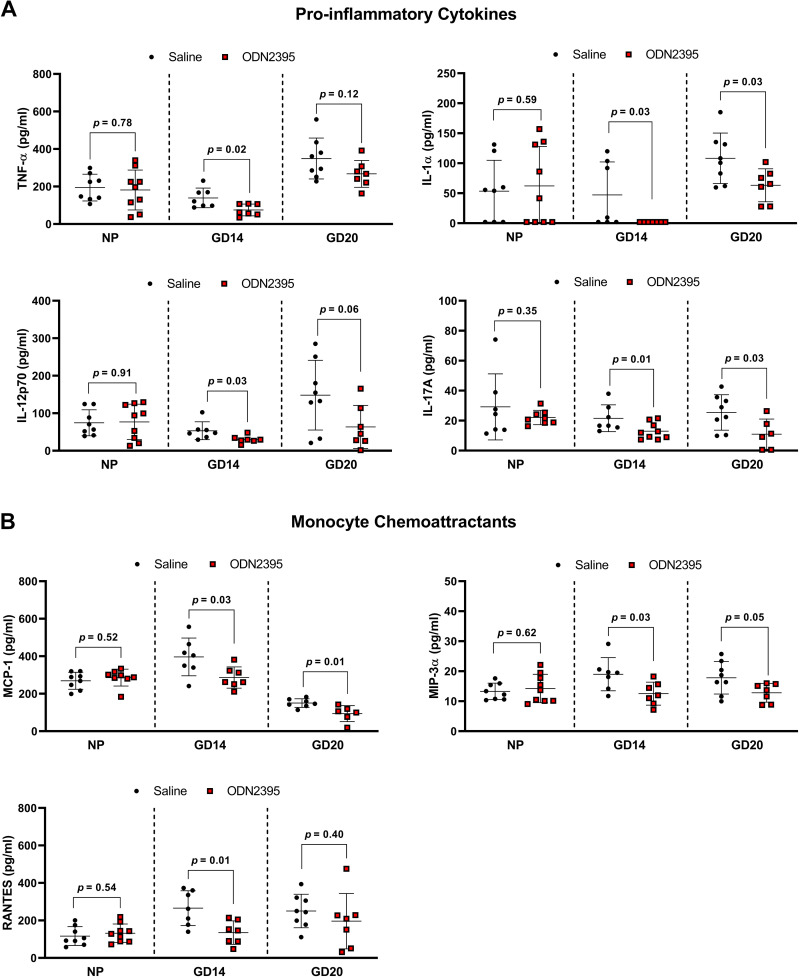
Pregnant rats treated with ODN2395 in late pregnancy (GD14, 16, 18) had reduced plasma concentrations of proinflammatory cytokines and monocyte chemoattractants at GD20 (Fig. 5, Supplemental Table S5: https://doi.org/10.6084/m9.figshare.22229977). Remarkably, maternal plasma concentrations of IL-1α and IL-17A were reduced in ODN2395-treated dams compared with controls at GD20 (P = 0.03, Fig. 5A, Supplemental Table S5). In addition, maternal plasma concentrations of chemoattractants MCP-1 and MIP-3α were reduced in the ODN2395 group compared with control at GD20 (P ≤ 0.05, Fig. 5B, Supplemental Table S5).
Figure 5.
Effects of a single and repeated exposures to ODN2395 on circulating proinflammatory cytokines and monocyte chemoattractants in nonpregnant (NP) and pregnant rats. A–B: proinflammatory cytokines (A) and monocyte chemoattractants (B) in plasma of nonpregnant, gestational day (GD) 14 dams (cohort 1), and GD20 dams (cohort 2) intraperitoneally administered ODN2395 or saline vehicle. Nonpregnant and GD14 dams received a single intraperitoneal injection while GD20 dams received three sequential intraperitoneal injections of ODN2395 or saline vehicle. Values are presented as means ± SD (n = 7–9/group) and were analyzed by unpaired t test. Black circles, saline; red boxes, ODN2395; TNF-α, tumor necrosis factor-α, IL-1 α, interleukin-1α; IL-12p70, bioactive dimer of interleukin-12 p35 and p40 subunits; IL-17A, interleukin-17A; MCP-1, monocyte chemoattractant protein-1; MIP-3α, macrophage inflammatory protein-3α; RANTES, regulated on activation, normal T cell expressed and secreted. ODN, oligodeoxynucleotides.
Since we observed a suppressed inflammatory response to repeated treatments of ODN2395 during late pregnancy, we assessed the impact of a single dose of ODN2395 on the maternal proinflammatory profile at GD14. We quantified plasma levels of maternal cytokines and monocyte chemoattractants 4 h after a single dose of ODN2395. Similar to our results in GD20 dams, our data demonstrated ODN2395 exposure during pregnancy reduced plasma proinflammatory cytokine and monocyte chemoattractant concentrations at GD14 (Fig. 5, Supplemental Table S5). Specifically, a single dose of ODN2395 during pregnancy suppressed plasma concentrations of proinflammatory cytokines TNF-α, IL-1β, IL-1α, IL-12p70, IL-17A, and IL-7 (P ≤ 0.03, Fig. 5A, Supplemental Table S5) and monocyte chemoattractants MCP-1, MIP-3α, and RANTES (P ≤ 0.03, Fig. 5B, Supplemental Table S5). There was no effect of repeated ODN2395 exposure on circulating anti-inflammatory cytokines at GD20 (Supplemental Table S5). However, a single dose of ODN2395 at GD14 reduced anti-inflammatory cytokines IL-5 and IL-2 (P ≤ 0.04, Supplemental Table S5). Similar reductions in circulating maternal anti-inflammatory cytokines IL-4 and IL-10 were also observed in the ODN2395-treated rats compared with controls, but these differences did not reach statistical significance (P = 0.06 and 0.08, respectively; Supplemental Table S5). Of note, ODN2395-mediated reductions in circulating cytokines and monocyte chemoattractants were pregnancy specific, as evidenced by no effect of ODN2395 exposure on the systemic immune profile in virgin, nonpregnant females (P ≥ 0.34, Fig. 5 and Supplemental Table S5).
Placental Inflammation and Clock Gene Expression after Exposure to CpG ODN
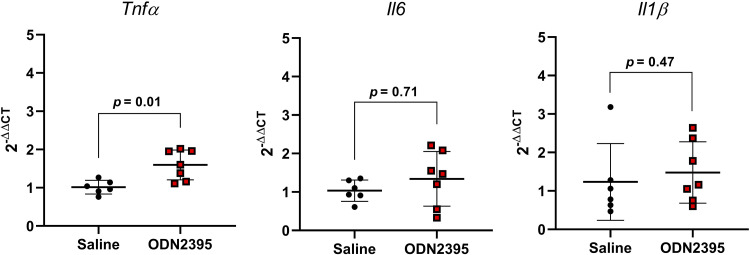
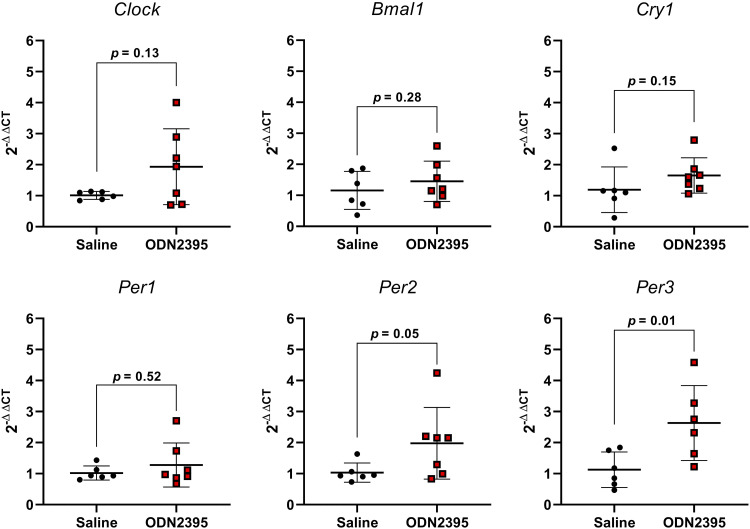
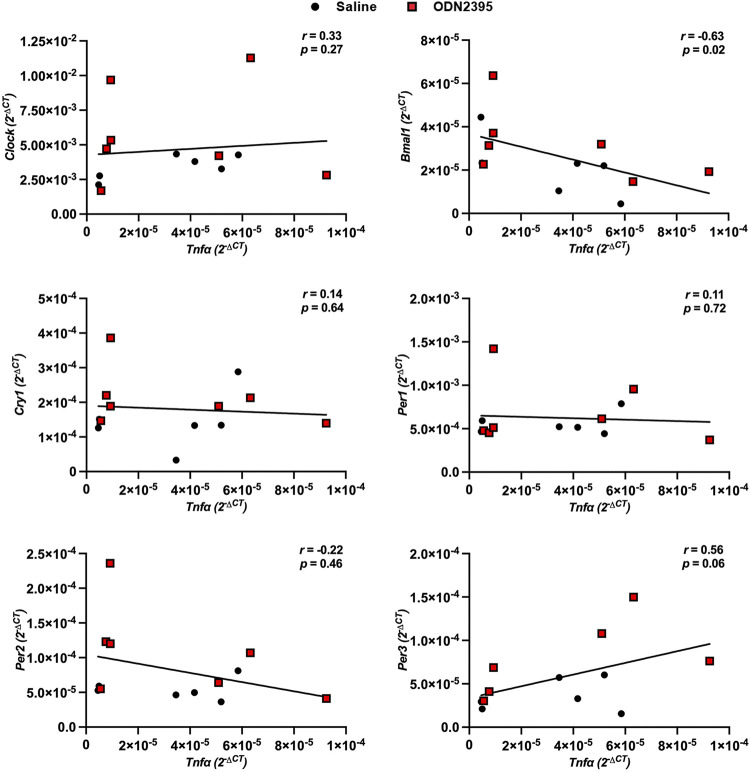
Placentas from dams treated with a single dose of ODN2395 on GD14 had greater expression of proinflammatory Tnfα compared with placentas from saline-treated dams, but there were no group differences in expression of proinflammatory Il6 or Il1β (Fig. 6). In addition, placental expression of CLOCK and BMAL-1 target genes Per2 and Per3 were increased after a single treatment with ODN2395 (P ≤ 0.05, Fig. 7). No effects of ODN2395 exposure were observed in placental gene expression of Clock or Bmal1 (P ≥ 0.13, Fig. 7) or on CLOCK and BMAL-1 target genes Cry1 or Per1 (P ≥ 0.15, Fig. 7). There was a negative correlation between placental Tnfα and Bmal1 (r = −0.63, P = 0.02, Fig. 8) and a positive correlation of placental Tnfα and Per3 (r = 0.56, P = 0.06, Fig. 8). There were no significant correlations between placental Tnfα expression and the expression of Clock, Bmal1, Cry1, or Per2 (P ≥ 0.27, Fig. 8).
Figure 6.
Effect of single exposure to ODN2395 on proinflammatory cytokine gene expression in rat placentas on gestational day 14. Placental gene expression of tumor necrosis factor-α (Tnfα), interleukin-6 (Il6), and interleukin-1β (Il1β) 4 h after administration of ODN2395 or saline vehicle. Values are presented as means ± SD (n = 6–7/group) and were analyzed by unpaired t test. ODN, oligodeoxynucleotides.
Figure 7.
Effect of a single exposure to ODN2395 on clock gene expression in rat placentas on gestational day 14. Placental gene expression of circadian rhythm regulators circadian locomotor output cycles protein kaput (Clock), brain and muscle ARNT [Aryl hydrocarbon receptor nuclear translocator-like protein]-like protein-1 (Bmal1), cryptochrome circadian regulator 1 (Cry1), and period circadian protein homologs 1, 2, and 3 (Per1, Per2, and Per3, respectively) 4 h after administration of ODN2395 or saline vehicle. Values are presented as means ± SD (n = 6–7/group) and were analyzed by unpaired t test. ODN, oligodeoxynucleotides.
Figure 8.
Relationship between placental proinflammatory and clock gene expression in rats administered ODN2395 or saline vehicle on gestational day 14. Spearman correlations of placental proinflammatory cytokine, tumor necrosis factor-α (Tnfa), gene expression correlated to placental clock (Clock, Bmal1, Cry1, Per1, Per2, Per3) gene expression 4 h after administration of ODN2395 or saline vehicle on gestational day 14. Data normalized to Ppia reference gene were used for correlations (2−ΔCT, n = 6–7/group). Black circles, saline-administered pregnant rats; red squares, ODN2395-administered rats. CLOCK, circadian locomotor output cycles protein kaput; Bmal1, brain and muscle ARNT-like protein; Cry1, cryptochrome circadian regulator 1; Per1, Per2, and Per3. period circadian protein homologs 1, 2 and 3, respectively. ODN, oligodeoxynucleotides.
DISCUSSION
In this study, we determined the impact of gestational exposure to CpG ODN on maternal circulating immune profiles, blood pressure circadian patterns, placental expression of clock network genes, and their interactive effects on perinatal outcomes in rats. We found that perinatal exposure to CpG ODN disrupts maternal circadian rhythms of blood pressure and transiently affects maternal blood pressure. In addition, exposure to CpG ODN induces a pregnancy-specific inflammatory profile and dysregulates the expression of placental molecular clock gene expression. This dysregulation was associated with placental inflammation and might contribute to adverse effects on fetoplacental growth dynamics.
Perinatal exposure to CpG ODN disrupted systolic and diastolic blood pressure circadian rhythmicity. Notably, disruptions of circadian rhythms of blood pressure with or without hypertension are associated with elevated risk of cardiovascular disease (25). Indeed, studies in humans indicate that cardiovascular events are associated with disruptions in blood pressure circadian patterns, even in normotensive individuals (26, 48, 49). Moreover, circadian rhythm disruptions of blood pressure during pregnancy are associated with pregnancy complications, such as preeclampsia, and long-term health consequences for mother and child (1, 50).
Even though circadian rhythms of blood pressure were disrupted by CpG ODN treatment, CpG ODN had only transient effects on average maternal blood pressure during the treatment window. We observed a decrease in maternal systolic blood pressure following the first treatment of CpG ODN; however, this effect was transient, which may indicate the efficiency of blood pressure regulatory mechanisms. We previously reported an increase in maternal systolic blood pressure in CpG ODN-treated pregnant rats compared with controls. In this previous study, we used the tail-cuff method to measure blood pressure on GD19, 1 day after the last CpG ODN injection (37, 38). The reproducibility of these results was subsequently confirmed by others, who also used tail-cuff methodology (39). Since there are substantial differences in tail-cuff and telemetry methods (51), the discrepancies between previously published work and our current findings are not surprising. Particularly, the tail-cuff method requires restraint of the animal, which induces stress-associated responses that may contribute to stress-mediated elevations in blood pressure (52). It is possible that CpG ODN exposure renders the maternal cardiovascular system vulnerable to a “second hit,” such as prenatal stress, which is associated with pregnancy complications and adverse fetal outcomes (53). Although in the present study, we did not observe an increase in maternal blood pressure, we noted that the reduction in maternal blood pressure with advancing gestational age recorded in saline-treated rats did not occur in ODN2395-treated rats. A blunted fall in maternal blood pressure has been previously observed in response to gestational stressors such as hypoxia (54, 55), high altitude (56), and exposure to inflammatory triggers (57) in various species. Thus, our data indicate that exposure to CpG ODN is a prenatal stressor that also prevents the normal reduction in blood pressure seen at the end of rodent pregnancy.
Daily (circadian) rhythms are natural internal responses finely controlled by a network of “clock” genes that respond to predictable environmental cues such as light and dark cycles that align with daily tasks, namely, physical activity and sleep. Timing of the circadian rhythm is driven by the suprachiasmatic nucleus of the hypothalamus, which is a master central clock located within the brain that influences peripheral tissue clocks located in many organs, including the placenta, via hormonal or neuronal signals (35, 58, 59). The key molecular machinery of the circadian clock comprises a network of transcription factors termed “clock” genes that function in autoregulatory transcription-translation feedback loops to drive gene expression of target genes in a tissue-specific manner (60). Specifically, the transcriptional activators, circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like protein 1 (BMAL1), induce expression of Period (Per1, Per2, Per3) and Cryptochrome (Cry1 and Cry2) genes that act as transcriptional repressors of the clock by opposing actions of BMAL1 and CLOCK (60). In this study, we observed that a single exposure to CpG ODN at midpregnancy increased placental expression of clock transcriptional repressors Per2 and Per3, suggesting CpG ODN influences the molecular clock network of the placental tissue.
There is growing evidence in the reciprocal relationship between clock and inflammatory gene expression and function (34, 61, 62). Thus, we assessed the impact of CpG ODN exposure on placental inflammatory gene expression. We found that CpG ODN exposure induced a proinflammatory response characterized by elevated placental Tnfα expression. This finding agrees with previous studies that demonstrate CpG ODN exposure during pregnancy increased placental TNF-α levels, and CpG ODN-mediated fetal demise could be rescued with blockage of TNF-α (63, 64). We then determined if CpG ODN-mediated increases in placental Tnfα were correlated with clock gene expression. Our data reveal increases in placental Tnfα are positively correlated with Per3 expression and negatively correlated with Bmal1 expression, suggesting a relationship between CpG ODN-mediated inflammation and clock gene expression within the placenta. Indeed, direct relationships between clock gene expression and inflammation have been identified in other tissues (62, 65). Particularly, clock genes have been shown to be negatively transcriptionally regulated by TNF-α through binding to transcriptional direct response elements (34). Our observation of negative correlations of placental Bmal1 and Tnfα gene expression provides evidence that CpG ODN-mediated placental inflammation may be driving disruptions in placental clock gene expression and circadian rhythmicity. However, because of the reciprocal relationship between inflammation and clock gene expression, it is also plausible that CpG ODN-mediated disruptions in clock gene expression could be driving enhanced placental inflammation and expression of TNF-α.
Notably, placental inflammation and dysfunction are central to maternal cardiovascular disease in preeclampsia, a hypertensive disorder of pregnancy associated with disruptions in circadian rhythmicity of blood pressure and dysregulation of placental clock gene expression (66). Moreover, blood pressure is regulated by many factors produced and released by the placenta, such as hormones and vasoactive factors whose expression follows circadian patterns (35, 67). Thus, we suggest that the placenta may function as a peripheral clock that contributes to the maternal circadian rhythm of blood pressure through secretory functions acting on central and peripheral oscillators.
Contrary to placental inflammation, our data demonstrate both a single dose of CpG ODN at the beginning of the rat third trimester and repetitive doses throughout the third trimester dampen proinflammatory responses in the circulation, and these immunomodulatory effects are pregnancy-specific given there were no differences in nonpregnant animals. Moreover, a single dose of CpG ODN had greater effects on dampening proinflammatory and anti-inflammatory cytokine and chemokine responses compared with repetitive doses. This finding suggests repetitive CpG ODN exposure during pregnancy, such as occurs during rapid fetal growth and cell turnover in the third trimester, may elicit tolerance to TLR9 stimulation during late gestation. In fact, repetitive TLR stimulation has been shown to induce tolerance to stimuli (68). Even so, alterations in the expression and activity of TLR9 have been demonstrated in placentas and circulation of pregnant individuals diagnosed with pregnancy complications such as preeclampsia (11, 69–71), and this TLR9 dysregulation may alter the impact of CpG ODN exposure on immune modulation during pregnancy. Importantly, we and others have previously demonstrated pregnancy-specific effects of CpG ODN exposure on perinatal outcomes (37, 39). Overall, differential immunomodulatory effects of CpG ODN exposure based on reproductive status (nonpregnant vs. pregnant), tissue (placenta vs. circulation), or disease status (healthy vs. preeclampsia) may contribute to divergent responses to CpG ODN exposure, such as enhanced susceptibility to infection and reduced safety and efficacy of CpG ODN-containing vaccines administered to pregnant individuals. Furthermore, our findings reveal that circulating inflammatory status did not reflect placental inflammatory status, highlighting the limitation of plasma sampling of inflammatory cytokines as a proxy of placental health status.
Similar to our previous studies, there were no group differences in mean absolute placental and fetal biometrics (37, 38). Others have shown that exposure to CpG ODN during the first trimester of pregnancy in mice is teratogenic, resulting in more than 50% resorptions, fetal craniofacial malformations, and polydactyly (63, 72). These studies, however, exposed mice to much greater doses of CpG ODN (300–400 µg/dam, ∼10–20 mg/kg) and started the treatments before placentation. In addition, CpG ODN exposure in the second trimester of mouse pregnancy resulted in increased rate of spontaneous abortions and fetal growth restriction (39). Previous toxicology studies suggest that litter size and resorption numbers should be taken into consideration when fetal growth is assessed in response to a chemical substance. Our multivariate analyses revealed CpG ODN reduced fetal weights in remaining viable pups when resorptions were increased within a litter, suggesting that CpG ODN exposure impairs placental function and disrupts fetoplacental growth dynamics. Given the CpG ODN exposure occurred late in pregnancy, it is likely that CpG ODN exposure in our study impacted placental function rather than inducing anatomical defects.
Advances, Limitations, and Future Studies
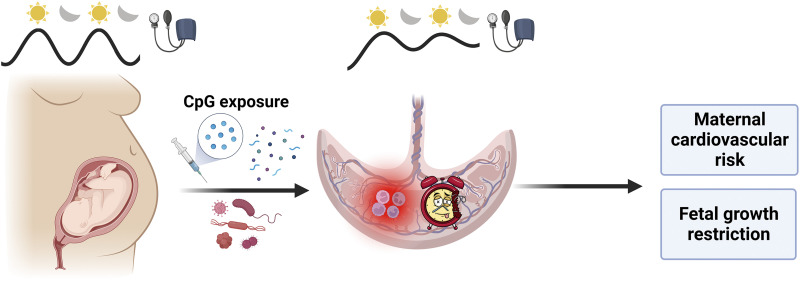
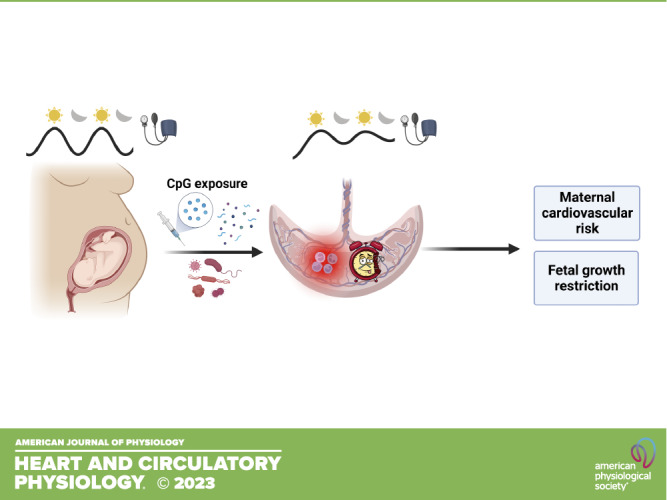
In conclusion, our novel findings elucidate the adverse impact of CpG ODN on maternal circadian rhythms of blood pressure, placental clock gene expression, and fetal growth. Gestational exposure to unmethylated CpG DNA, such as exposure to bacterial or viral infections, tissue turnover from rapid fetoplacental growth, or vaccination using CpG ODN adjuvants, may contribute to adverse perinatal outcomes including maternal cardiovascular complications, aberrant fetal growth, and heightened maternal and offspring cardiovascular risk (Fig. 9).
Figure 9.
Gestational exposure to unmethylated CpG oligonucleotides dysregulates placental molecular clock network and fetoplacental growth dynamics and disrupts maternal blood pressure circadian rhythms in rats. These unmethylated CpG ODN-driven outcomes may contribute to maternal cardiovascular risk and fetal growth restriction. Image was created using a licensed version of BioRender. CpG, cytosine-guanine dinucleotide; ODN, oligodeoxynucleotides.
Here, we characterized the placental clock gene network in response to a gestational stressor and reported an association between inflammatory and clock gene expression. These data set the foundation for future investigations to determine how immune system stimulation during pregnancy may affect circadian patterns in placental clock network gene expression and secretory function, which was not assessed in the current investigation and could have a significant impact on both the mother and the developing fetus. In addition, our data demonstrated that maternal blood pressure circadian rhythms were disrupted in response to immune system stimulation. This finding is novel and lends a framework to future studies to determine the role and contribution of central versus peripheral oscillators in maternal blood pressure regulation during pregnancy. Specifically, future studies examining the cross talk between placenta, kidney, vascular, and central clock machinery are needed to elucidate circadian-driven physiological and pathophysiological adaptations to pregnancy and regulation of maternal blood pressure.
The presented studies defined cytokine and chemokine profiles in maternal circulation and the placenta of pregnant dams in response to CpG ODN exposure. Yet, we did not investigate the source of cytokines or chemokines. Since unmethylated CpG sequences are recognized by TLR9, which is an innate receptor expressed by many cells and not just classical immune cells, the source of CpG-mediated immunomodulation is likely a cooperative response from a vast array of cell types. Future studies examining the impact of CpG exposure on the proliferation and function of specific cell subsets, such as classical immune cells, epithelial cells, and endothelial cells, are warranted. It is noteworthy that in this study, we stimulated the immune system pharmacologically during an otherwise healthy rat pregnancy. Future studies could interrogate the association of the maternal immune system and circadian rhythms to discover potential targets for intervention in experimental models of pregnancy complications, such as preeclampsia.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.22229488.
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.22229941.
Supplemental Table S3: https://doi.org/10.6084/m9.figshare.22229962.
Supplemental Table S4: https://doi.org/10.6084/m9.figshare.22229971.
Supplemental Table S5: https://doi.org/10.6084/m9.figshare.22229977.
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.23154716.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.23154896.
GRANTS
This study was supported by National Institutes of Health Grants R01 HL146562 and HL146562-04S1 (to S.G.) and T32 AG020494 (to S.C.C.) and American Heart Association Grants 19TPA-34850131 (to S.G.), 22POST-903250 (to J.L.B.), and 22PRE-900431 (to J.J.G.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.B., S.C.C., and S.G. conceived and designed research; J.L.B., S.C.C., C.A.R., S.M.T., J.J.G., J.T.L., and O.O. performed experiments; J.L.B., C.A.R., and S.G. analyzed data; J.L.B., C.A.R., and S.G. interpreted results of experiments; J.L.B., C.A.R., and S.G. prepared figures; J.L.B. and S.G. drafted manuscript; J.L.B., S.C.C., C.A.R., S.M.T., J.J.G., J.T.L., O.O., and S.G. edited and revised manuscript; J.L.B., S.C.C., C.A.R., S.M.T., J.J.G., J.T.L., O.O., and S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract was created using a licensed version of BioRender.
Present address of C. A. Ricci: Dept. of Animal Sciences, Washington State University, Pullman, Pullman, WA, United States.
REFERENCES
- 1. Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM. Maternal immunological adaptation during normal pregnancy. Front Immunol 11: 575197, 2020. doi: 10.3389/fimmu.2020.575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol 73: 199–213, 2015. doi: 10.1111/aji.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alberca RW, Pereira NZ, Oliveira LMDS, Gozzi-Silva SC, Sato MN. Pregnancy, viral infection, and COVID-19. Front Immunol 11: 1672, 2020. doi: 10.3389/fimmu.2020.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pohl AM, Pouillot R, Bazaco MC, Wolpert BJ, Healy JM, Bruce BB, Laughlin ME, Hunter JC, Dunn JR, Hurd S, Rowlands JV, Saupe A, Vugia DJ, Van Doren JM. Differences among incidence rates of invasive listeriosis in the U.S. FoodNet population by age, sex, race/ethnicity, and pregnancy status, 2008-2016. Foodborne Pathog Dis 16: 290–297, 2019. doi: 10.1089/fpd.2018.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deutscher M, Lewis M, Zell ER, Taylor TH Jr, Van Beneden C, Schrag S; Active Bacterial Core Surveillance Team. Incidence and severity of invasive Streptococcus pneumoniae, group A Streptococcus, and group B Streptococcus infections among pregnant and postpartum women. Clin Infect Dis 53: 114–123, 2011. doi: 10.1093/cid/cir325. [DOI] [PubMed] [Google Scholar]
- 6. Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol 226: 68–89.e3, 2022. doi: 10.1016/j.ajog.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aleem S, Bhutta ZA. Infection-related stillbirth: an update on current knowledge and strategies for prevention. Expert Rev Anti Infect Ther 19: 1117–1124, 2021. doi: 10.1080/14787210.2021.1882849. [DOI] [PubMed] [Google Scholar]
- 8. Bortolotti D, Gentili V, Santi E, Taliento C, Vitagliano A, Schiuma G, Beltrami S, Rizzo S, Lanza G, Rizzo R, Gafà R, Greco P. Late-onset intrauterine growth restriction and HHV-6 infection: a pilot study. J Med Virol 93: 6317–6322, 2021. doi: 10.1002/jmv.27138. [DOI] [PubMed] [Google Scholar]
- 9. Helmo FR, Alves EAR, Moreira RAA, Severino VO, Rocha LP, Monteiro MLGDR, Reis MAD, Etchebehere RM, Machado JR, Corrêa RRM. Intrauterine infection, immune system and premature birth. J Matern Fetal Neonatal Med 31: 1227–1233, 2018. doi: 10.1080/14767058.2017.1311318. [DOI] [PubMed] [Google Scholar]
- 10. Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20: 709–760, 2002. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 11. Scharfe-Nugent A, Corr SC, Carpenter SB, Keogh L, Doyle B, Martin C, Fitzgerald KA, Daly S, O'Leary JJ, O'Neill LA. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol 188: 5706–5712, 2012. doi: 10.4049/jimmunol.1103454. [DOI] [PubMed] [Google Scholar]
- 12. Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374: 546–549, 1995. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 13. Yi AK, Chace JH, Cowdery JS, Krieg AM. IFN-gamma promotes IL-6 and IgM secretion in response to CpG motifs in bacterial DNA and oligodeoxynucleotides. J Immunol 156: 558–564, 1996. [PubMed] [Google Scholar]
- 14. Cushen SC, Sprouse ML, Blessing A, Sun J, Jarvis SS, Okada Y, Fu Q, Romero SA, Phillips NR, Goulopoulou S. Cell-free mitochondrial DNA increases in maternal circulation during healthy pregnancy: a prospective, longitudinal study. Am J Physiol Regul Integr Comp Physiol 318: R445–R452, 2020. doi: 10.1152/ajpregu.00324.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hahn S, Rusterholz C, Hösli I, Lapaire O. Cell-free nucleic acids as potential markers for preeclampsia. Placenta 32, Suppl: S17–S20, 2011. doi: 10.1016/j.placenta.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 16. Bradshaw JL, Cushen SC, Phillips NR, Goulopoulou S. Circulating cell-free mitochondrial DNA in pregnancy. Physiology (Bethesda) 37: 187–196, 2022. doi: 10.1152/physiol.00037.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Boeckel SR, Davidson DJ, Norman JE, Stock SJ. Cell-free fetal DNA and spontaneous preterm birth. Reproduction 155: R137–R145, 2018. doi: 10.1530/REP-17-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valencia-Hernandez AM, Zillinger T, Ge Z, Tan PS, Cozijnsen A, I McFadden G, Lahoud MH, Caminschi I, Barchet W, Heath WR, Fernandez-Ruiz D. Complexing CpG adjuvants with cationic liposomes enhances vaccine-induced formation of liver TRM cells. Vaccine 41: 1094–1107, 2023. doi: 10.1016/j.vaccine.2022.12.047. [DOI] [PubMed] [Google Scholar]
- 19. Nafar M, Mostafaloo N, Firouzan A, Poorrezagholi F, Samadian F, Dalili N, Barati S, Anjidani N, Kafi H, Shahpari R, Bayat M, Kianipour S, Samavat S. Immunogenicity and safety of SpikoGen, an adjuvanted recombinant SARS-CoV-2 spike protein, as a heterologous third booster dose in kidney transplant patients: a single-arm clinical trial. Clin Ther 44: 1566–1576, 2022. doi: 10.1016/j.clinthera.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren H, Li H, Cao L, Wang Z, Zhou Y, Guo J, Zhang Y, Liu H, Xu W. Intranasal immunization with HRSV prefusion F protein and CpG adjuvant elicits robust protective effects in mice. Vaccine 40: 6830–6838, 2022. doi: 10.1016/j.vaccine.2022.09.071. [DOI] [PubMed] [Google Scholar]
- 21. Babayeva M, Tabynov K, Nurpeisov T, Fomin G, Renukaradhya GJ, Petrovsky N, Tabynov K. A recombinant Artemisia vulgaris pollen adjuvanted Art v 1 protein-based vaccine treats allergic rhinitis and bronchial asthma using pre- and co-seasonal ultrashort immunotherapy regimens in sensitized mice. Front Immunol 13: 983621, 2022. doi: 10.3389/fimmu.2022.983621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torales J, Cuenca-Torres O, Barrios L, Armoa-Garcia L, Estigarribia G, Sanabria G, Lin MY, Antonio Estrada J, Estephan L, Cheng HY, Chen C, Janssen R, Lien CE. An evaluation of the safety and immunogenicity of MVC-COV1901: results of an interim analysis of a phase III, parallel group, randomized, double-blind, active-controlled immunobridging study in Paraguay. Vaccine 41: 109–118, 2023. doi: 10.1016/j.vaccine.2022.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girndt M, Plüer M, Dellanna F, Michelsen AK, Beige J, Toussaint K, Wehweck HJ, Koch M, Hafezi Rachti S, Faust J, Bosselmann HP, Witzke O, Janssen RS; HBV-18 Study Investigators. Immunogenicity and safety of a booster dose of the hepatitis B vaccine HepB-CpG (HEPLISAV-B®) compared with HepB-Eng (Engerix-B®) and HepB-AS04 (Fendrix®) in adults receiving hemodialysis who previously received hepatitis B vaccination and are not seroprotected: Results of a randomized, multicenter phase 3 study. Hum Vaccin Immunother 18: 2136912, 2022. doi: 10.1080/21645515.2022.2136912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kayraklioglu N, Horuluoglu B, Klinman DM. CpG oligonucleotides as vaccine adjuvants. Methods Mol Biol 2197: 51–85, 2021. doi: 10.1007/978-1-0716-0872-2_4. [DOI] [PubMed] [Google Scholar]
- 25. Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med 119: 108–114, 2018. doi: 10.1016/j.freeradbiomed.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palatini P, Reboldi G, Saladini F, Angeli F, Mos L, Rattazzi M, Vriz O, Verdecchia P. Dipping pattern and short-term blood pressure variability are stronger predictors of cardiovascular events than average 24-h blood pressure in young hypertensive subjects. Eur J Prev Cardiol 29: 1377–1386, 2022. doi: 10.1093/eurjpc/zwac020. [DOI] [PubMed] [Google Scholar]
- 27. Gozeri E, Celik H, Ozercan I, Gurates B, Polat SA, Hanay F. The effect of circadian rhythm changes on fetal and placental development (experimental study). Neuro Endocrinol Lett 29: 87–90, 2008. [PubMed] [Google Scholar]
- 28. Ditisheim AJ, Dibner C, Philippe J, Pechère-Bertschi A. Biological rhythms and preeclampsia. Front Endocrinol (Lausanne) 4: 47, 2013. doi: 10.3389/fendo.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. Am J Physiol Renal Physiol 318: F1315–F1326, 2020. doi: 10.1152/ajprenal.00071.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhong L, Deng W, Zheng W, Yu S, Huang X, Wen Y, Chiu PCN, Lee CL. The relationship between circadian blood pressure variability and maternal/perinatal outcomes in women with preeclampsia with severe features. Hypertens Pregnancy 39: 405–410, 2020. doi: 10.1080/10641955.2020.1797777. [DOI] [PubMed] [Google Scholar]
- 31. Carranza-Leon DA, Oeser A, Wu Q, Stein CM, Ormseth MJ, Chung CP. Ambulatory blood pressure in patients with systemic lupus erythematosus: association with markers of immune activation. Lupus 29: 1683–1690, 2020. doi: 10.1177/0961203320951274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen Y, Endale M, Wang W, Morris AR, Francey LJ, Harold RL, Hammers DW, Huo Z, Partch CL, Hogenesch JB, Wu ZH, Liu AC. NF-κB modifies the mammalian circadian clock through interaction with the core clock protein BMAL1. PLoS Genet 17: e1009933, 2021. doi: 10.1371/journal.pgen.1009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernández-Ortiz M, Sayed RKA, Román-Montoya Y, de Lama MÁR, Fernández-Martínez J, Ramírez-Casas Y, Florido-Ruiz J, Rusanova I, Escames G, Acuña-Castroviejo D. Age and chronodisruption in mouse heart: effect of the NLRP3 inflammasome and melatonin therapy. Int J Mol Sci 23: 6846, 2022. doi: 10.3390/ijms23126846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA 104: 12843–12848, 2007. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waddell BJ, Wharfe MD, Crew RC, Mark PJ. A rhythmic placenta? Circadian variation, clock genes and placental function. Placenta 33: 533–539, 2012. doi: 10.1016/j.placenta.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 36. Pérez S, Murias L, Fernández-Plaza C, Díaz I, González C, Otero J, Díaz E. Evidence for clock genes circadian rhythms in human full-term placenta. Syst Biol Reprod Med 61: 360–366, 2015. doi: 10.3109/19396368.2015.1069420. [DOI] [PubMed] [Google Scholar]
- 37. Goulopoulou S, Wenceslau CF, McCarthy CG, Matsumoto T, Webb RC. Exposure to stimulatory CpG oligonucleotides during gestation induces maternal hypertension and excess vasoconstriction in pregnant rats. Am J Physiol Heart Circ Physiol 310: H1015–H1025, 2016. doi: 10.1152/ajpheart.00834.2015. [DOI] [PubMed] [Google Scholar]
- 38. Osikoya O, Jaini PA, Nguyen A, Valdes M, Goulopoulou S. Effects of low-dose aspirin on maternal blood pressure and vascular function in an experimental model of gestational hypertension. Pharmacol Res 120: 267–278, 2017. doi: 10.1016/j.phrs.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 39. He B, Yang X, Li Y, Huang D, Xu X, Yang W, Dai Y, Zhang H, Chen Z, Cheng W. TLR9 (toll-like receptor 9) agonist suppresses angiogenesis by differentially regulating VEGFA (vascular endothelial growth factor A) and sFLT1 (soluble vascular endothelial growth factor receptor 1) in preeclampsia. Hypertension 71: 671–680, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10510. [DOI] [PubMed] [Google Scholar]
- 40. Zhang Z, Kuo JC, Yao S, Zhang C, Khan H, Lee RJ. CpG oligodeoxynucleotides for anticancer monotherapy from preclinical stages to clinical trials. Pharmaceutics 14: 73, 2021. doi: 10.3390/pharmaceutics14010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol 13: 190–198, 2013. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wharfe MD, Mark PJ, Wyrwoll CS, Smith JT, Yap C, Clarke MW, Waddell BJ. Pregnancy-induced adaptations of the central circadian clock and maternal glucocorticoids. J Endocrinol 228: 135–147, 2016. doi: 10.1530/JOE-15-0405. [DOI] [PubMed] [Google Scholar]
- 43. Carlucci M, Kriščiūnas A, Li H, Gibas P, Koncevičius K, Petronis A, Oh G. DiscoRhythm: an easy-to-use web application and R package for discovering rhythmicity. Bioinformatics 36: 1952–1954, 2019. [Erratum in Bioinformatics 38: 882, 2022]. doi: 10.1093/bioinformatics/btz834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dancho M, Vaughan D. Anomalize: Tidy Anomaly Detection (Online). R package version 0.2.2. https://CRAN.R-project.org/package=anomalize [2022].
- 45. Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 38: 275–325, 2007. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ruf T. The Lomb-Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol Rhythm Res 30: 178–201, 1999. doi: 10.1076/brhm.30.2.178.1422. [DOI] [Google Scholar]
- 47. Romero A, Villamayor F, Grau MT, Sacristán A, Ortiz JA. Relationship between fetal weight and litter size in rats: application to reproductive toxicology studies. Reprod Toxicol 6: 453–456, 1992. doi: 10.1016/0890-6238(92)90009-i. [DOI] [PubMed] [Google Scholar]
- 48. Smolensky MH, Hermida RC, Castriotta RJ, Portaluppi F. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med 8: 668–680, 2007. doi: 10.1016/j.sleep.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 49. Amici A, Cicconetti P, Sagrafoli C, Baratta A, Passador P, Pecci T, Tassan G, Verrusio W, Marigliano V, Cacciafesta M. Exaggerated morning blood pressure surge and cardiovascular events. A 5-year longitudinal study in normotensive and well-controlled hypertensive elderly. Arch Gerontol Geriatr 49: e105–e109, 2009. doi: 10.1016/j.archger.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 50. Larry CD, Yeo S. The circadian rhythm of blood pressure during pregnancy. J Obstet Gynecol Neonatal Nurs 29: 500–508, 2000. doi: 10.1111/j.1552-6909.2000.tb02771.x. [DOI] [PubMed] [Google Scholar]
- 51. Whitesall SE, Hoff JB, Vollmer AP, D'Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol 286: H2408–H2415, 2004. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- 52. Wang T, Gao L, Yang Z, Wang F, Guo Y, Wang B, Hua R, Shang H, Xu J. Restraint stress in hypertensive rats activates the intestinal macrophages and reduces intestinal barrier accompanied by intestinal flora dysbiosis. J Inflamm Res 14: 1085–1110, 2021. doi: 10.2147/JIR.S294630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Coussons-Read ME. Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet Med 6: 52–57, 2013. doi: 10.1177/1753495X12473751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tong W, Allison BJ, Brain KL, Patey OV, Niu Y, Botting KJ, Ford SG, Garrud TA, Wooding PFB, Shaw CJ, Lyu Q, Zhang L, Ma J, Cindrova-Davies T, Yung HW, Burton GJ, Giussani DA. Chronic hypoxia in ovine pregnancy recapitulates physiological and molecular markers of preeclampsia in the mother, placenta, and offspring. Hypertension 79: 1525–1535, 2022. doi: 10.1161/HYPERTENSIONAHA.122.19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol 180: 1161–1168, 1999. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 56. Hu XQ, Chen M, Dasgupta C, Xiao D, Huang X, Yang S, Zhang L. Chronic hypoxia upregulates DNA methyltransferase and represses large conductance Ca2+-activated K+ channel function in ovine uterine arteries. Biol Reprod 96: 424–434, 2017. doi: 10.1095/biolreprod.116.145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lau SY, Barrett CJ, Guild SJ, Chamley LW. Necrotic trophoblast debris increases blood pressure during pregnancy. J Reprod Immunol 97: 175–182, 2013. doi: 10.1016/j.jri.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 58. Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev 59: 449–526, 1979. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- 59. Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21: 67–84, 2020. doi: 10.1038/s41580-019-0179-2. [DOI] [PubMed] [Google Scholar]
- 60. Cox KH, Takahashi JS. Circadian clock genes and the transcriptional architecture of the clock mechanism. J Mol Endocrinol 63: R93–R102, 2019. doi: 10.1530/JME-19-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol 192: 407–417, 2014. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 62. Vieira E, Mirizio GG, Barin GR, de Andrade RV, Nimer NFS, La Sala L. Clock genes, inflammation and the immune system-implications for diabetes, obesity and neurodegenerative diseases. Int J Mol Sci 21: 9743, 2020. doi: 10.3390/ijms21249743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol 183: 1144–1154, 2009. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kang X, Zhang X, Zhao A. Macrophage depletion and TNF-α inhibition prevent resorption in CBA/J × DBA/2 model of CpG-induced abortion. Biochem Biophys Res Commun 469: 704–710, 2016. doi: 10.1016/j.bbrc.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 65. Crespo M, Gonzalez-Teran B, Nikolic I, Mora A, Folgueira C, Rodríguez E, Leiva-Vega L, Pintor-Chocano A, Fernández-Chacón M, Ruiz-Garrido I, Cicuéndez B, Tomás-Loba A, A-Gonzalez N, Caballero-Molano A, Beiroa D, Hernández-Cosido L, Torres JL, Kennedy NJ, Davis RJ, Benedito R, Marcos M, Nogueiras R, Hidalgo A, Matesanz N, Leiva M, Sabio G. Neutrophil infiltration regulates clock-gene expression to organize daily hepatic metabolism. Elife 9: e59258, 2020. doi: 10.7554/eLife.59258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou G, Winn E, Nguyen D, Kasten EP, Petroff MG, Hoffmann HM. Co-alterations of circadian clock gene transcripts in human placenta in preeclampsia. Sci Rep 12: 17856, 2022. doi: 10.1038/s41598-022-22507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med 17: 1402–1409, 2011. doi: 10.1038/nm.2541. [DOI] [PubMed] [Google Scholar]
- 68. Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, , et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol 22: 2–6, 2021. [Erratum in Nat Immunol 22: 928, 2021]. doi: 10.1038/s41590-020-00845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cushen SC, Ricci CA, Bradshaw JL, Silzer T, Blessing A, Sun J, Zhou Z, Scroggins SM, Santillan MK, Santillan DA, Phillips NR, Goulopoulou S. Reduced maternal circulating cell-free mitochondrial DNA is associated with the development of preeclampsia. J Am Heart Assoc 11: e021726, 2022. doi: 10.1161/JAHA.121.021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Williamson RD, McCarthy FP, Kenny LC, McCarthy CM. Activation of a TLR9 mediated innate immune response in preeclampsia. Sci Rep 9: 5920, 2019. doi: 10.1038/s41598-019-42551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Panda B, Panda A, Ueda I, Abrahams VM, Norwitz ER, Stanic AK, Young BC, Ecker JL, Altfeld M, Shaw AC, Rueda BR. Dendritic cells in the circulation of women with preeclampsia demonstrate a pro-inflammatory bias secondary to dysregulation of TLR receptors. J Reprod Immunol 94: 210–215, 2012. doi: 10.1016/j.jri.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 72. Prater MR, Johnson VJ, Germolec DR, Luster MI, Holladay SD. Maternal treatment with a high dose of CpG ODN during gestation alters fetal craniofacial and distal limb development in C57BL/6 mice. Vaccine 24: 263–271, 2006. doi: 10.1016/j.vaccine.2005.07.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.22229488.
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.22229941.
Supplemental Table S3: https://doi.org/10.6084/m9.figshare.22229962.
Supplemental Table S4: https://doi.org/10.6084/m9.figshare.22229971.
Supplemental Table S5: https://doi.org/10.6084/m9.figshare.22229977.
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.23154716.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.23154896.
Data Availability Statement
Data will be made available upon reasonable request.