Keywords: dehydration, heat wave, kidney injury molecule-1, NephroCheck, neutrophil gelatinase-associated lipocalin
Abstract
The high prevalence of inadequate hydration (e.g., hypohydration and underhydration) is concerning given that extreme heat increases excess hospitalizations for fluid/electrolyte disorders and acute kidney injury (AKI). Inadequate hydration may also be related to renal and cardiometabolic disease development. This study tested the hypothesis that prolonged mild hypohydration increases the urinary AKI biomarker product of insulin-like growth factor-binding protein 7 and tissue inhibitor of metalloproteinase-2 ([IGFBP7·TIMP-2]) compared with euhydration. In addition, we determined the diagnostic accuracy and optimal cutoffs of hydration assessments for discriminating positive AKI risk ([IGFBP·TIMP-2] >0.3 (ng/mL)2/1,000). In a block-randomized crossover design, 22 healthy young adults (11 females and 11 males) completed 24 h of fluid deprivation (hypohydrated group) or 24 h of normal fluid consumption (euhydrated group) separated by ≥72 h. Urinary [IGFBP7·TIMP-2] and other AKI biomarkers were measured following the 24-h protocols. Diagnostic accuracy was assessed via receiver operating characteristic curve analysis. Urinary [IGFBP7·TIMP-2] [1.9 (95% confidence interval: 1.0–2.8) vs. 0.2 (95% confidence interval: 0.1–0.3) (ng/mL)2/1,000, P = 0.0011] was markedly increased in hypohydrated versus euhydrated groups. Urine osmolality (area under the curve: 0.91, P < 0.0001) and urine specific gravity (area under the curve: 0.89, P < 0.0001) had the highest overall performance for discriminating positive AKI risk. Optimal cutoffs with a positive likelihood ratio of 11.8 for both urine osmolality and specific gravity were 952 mosmol/kgH2O and 1.025 arbitrary units. In conclusion, prolonged mild hypohydration increased urinary [IGFBP7·TIMP-2] in males and females. Urinary [IGFBP7·TIMP-2] corrected to urine concentration was elevated in males only. Urine osmolality and urine specific gravity may have clinical utility for discriminating positive AKI risk following prolonged mild hypohydration.
NEW & NOTEWORTHY This study found that prolonged mild hypohydration in healthy young adults increased the Food and Drug Administration approved acute kidney injury (AKI) biomarker urinary insulin-like growth factor-binding protein 7 and tissue inhibitor of metalloproteinase-2 [IGFBP7·TIMP-2]. Urine osmolality and specific gravity demonstrated an excellent ability to discriminate positive AKI risk. These findings emphasize the importance of hydration in protecting renal health and lend early support for hydration assessment as an accessible tool to assess AKI risk.
INTRODUCTION
The devastating consequences of extreme heat were exemplified by the 4-day 2021 western North American heat event that produced temperatures in excess of 46.7°C (1) and caused an estimated 1,400 or more deaths (2, 3). Extreme heat events (e.g., heat waves) are predicted to increase in frequency and severity (4), with increased risk of death associated with several factors, including cardiovascular disease and hypohydration (4). Fluid and/or electrolyte disturbances and acute kidney injury (AKI) are the greatest contributors to excess hospital admissions during extreme heat (5, 6). Evidence is also accumulating for the role of heat and hypohydration in the etiology of chronic kidney disease/kidney injury occurring in agricultural communities and in individuals exposed to frequent occupational heat stress (7, 8).
Understanding the effect of prolonged mild hypohydration on AKI risk is imperative given that 33–74% of United States adults are considered to be inadequately hydrated (9, 10) and that chronic inadequate hydration, independent of heat stress, may be related to renal and cardiometabolic disease development (11). Identifying novel biomarkers that predict the risk of AKI and/or the anatomic location of injury has been an ongoing area of investigation, including the well-studied renal tubular injury biomarkers neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) (12). Currently, the only United States Food and Drug Administration (FDA)-approved biomarker for the clinical screening of AKI risk is urinary [insulin-like growth factor-binding-protein 7 (IGFBP7)·tissue inhibitor of metalloproteinase-2 (TIMP-2)] > 0.3 (ng/mL)2/1,000 (proprietary name NephroCheck, Astute Medical, San Diego, CA), which is the product of the cell cycle arrest markers insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinase-2 (TIMP-2). IGFBP7 and TIMP-2 are preinjury phase biomarkers indicative of renal stress and are secreted by renal tubular epithelial cells to induce G1 phase block and mitigate cell damage to several renal insults, including ischemia (12). Renal ischemia can manifest from severe and prolonged renal hypoperfusion (i.e., reduced renal blood flow) due to hypovolemia (13), including in the pathophysiology of AKI with recurrent heat stress-induced hypohydration (8, 14). Use of urinary [IGFBP7·TIMP-2] is currently limited to clinical settings due to the requirement of expensive equipment and specialized training. Thus, there is an opportunity for the investigation of biomarkers that may discriminate whether [IGFBP7·TIMP-2] likely exceeds the current threshold for positive AKI risk, which is particularly useful information for field studies/work involving individuals at risk of hypohydration (e.g., agricultural workers). Laboratory-controlled studies have used biomarker panels consisting of preinjury phase and injury phase AKI biomarkers. These studies have found that urinary [IGFBP7·TIMP-2] increased within 4 h of simulated extreme heat exposure while seated at rest when male participants were not provided fluids (∼0.6% body mass loss) (15) and that maintaining euhydration during extreme heat exposure protects against increases in AKI biomarkers (16, 17). Collectively, these findings suggest that hydration is an important factor in determining AKI risk.
The effect of prolonged mild hypohydration independent of heat stress on urinary [IGFBP7·TIMP-2] remains unclear. Two studies have used short-term fluid deprivation models in humans with inconsistent findings, where urinary [IGFBP7·TIMP-2] increased with 8 h restriction (18) but did not change from baseline with 12 h restriction (19). Given the high prevalence of adults who are chronically inadequately hydrated and that kidney-related complications of hypohydration account for the top two causes of excess hospital admissions during extreme heat, it is important that the effects of prolonged mild hypohydration on AKI risk is more comprehensively investigated. Furthermore, because the current FDA-approved AKI biomarker is not accessible for use at the population level, there is an area of opportunity to investigate whether other biometrics can be used to provide insights into AKI risk. Specifically, in the context of the public health concerns related to extreme heat, it is also critical to understand whether other metrics, including noninvasive hypohydration measurements (e.g., change in body mass and urine color), have sufficient discriminatory ability to identify individuals at elevated AKI risk compared with urinary [IGFBP7·TIMP-2]. With this background, the primary purpose of the present study was to test the hypothesis that, in the absence of heat stress, prolonged mild hypohydration elevates urinary [IGFBP7·TIMP-2] in healthy young male and female adults compared with euhydration. The secondary purpose of the present study was to determine the diagnostic accuracy of commonly used criterion hypohydration values in predicting a positive AKI risk score of >0.3 urinary [IGFBP7·TIMP-2] (ng/mL)2/1,000. In addition, this study sought to identify the optimal cutoffs for hypohydration measurements in discriminating positive AKI risk.
METHODS
Participants
Based on our previous study involving physical work in the heat, when subjects were deprived of fluids versus when euhydrated, a power analysis determined that a total sample size of 22 participants was needed to achieve 90% power with α = 0.05 and an estimated effect size of d = 0.65 in peak urinary [IGFBP7·TIMP-2] (16). At the time of designing this study, there were no relevant sex-based data on [IGFBP7·TIMP] for us to include in the model for the power analysis. To acknowledge sex as a biological variable in biomedical research, we recruited an equal number of self-identified male and female participants to allow for disaggregation of data by sex and explore the potential for sex differences. Twenty-six volunteers consented, with three participants choosing to leave the study before participating in an experimental visit and one participant withdrawing from the study after completing one experimental visit. Therefore, 22 healthy young adults (11 males and 11 females) completed the study and were included in the final analyses (Table 1). The influence of environmental heat stress in our experimental model was minimized because all visits were conducted in Eugene, OR, a temperate climate, and 97% of visits were completed before the end of May (one visit occurred in June 2022). Of the 11 female participants, seven participants were on hormonal contraceptives (with 4 participants taking oral contraceptive pills and 3 participants with hormone-releasing intrauterine devices) and four participants were not but were normally menstruating. Female participants who did not have intrauterine devices (n = 7) were tested within the first 10 days of their self-identified onset of menses or during the placebo phase of their oral contraceptives (20), which reduced a potential scheduling barrier for females by not requiring experimental visits to be scheduled during consecutive cycles. Written informed consent was obtained from all participants in this study, which was approved by the University of Oregon’s Institutional Review Board in accordance with the Declaration of Helsinki, except for registration in a database. Individuals aged 18–40 yr were deemed eligible for the study but were excluded under the following criteria: 1) systolic blood pressure ≥ 120 mmHg or diastolic blood pressure ≥ 80 mmHg was obtained during the screening visit using the procedure recommended by clinical practice guidelines (21); 2) body mass index > 28 kg/m2; 3) prior diagnosis of any cardiometabolic disease, autonomic disorders, kidney disease, or gastrointestinal disease; 4) milk protein allergy; 5) smoking or nicotine use; 6) ongoing medical therapy (other than birth control); or 7) pregnant or breastfeeding.
Table 1.
Participant characteristics
| Parameter | All Participants (n = 22) | Disaggregated by Sex |
|
|---|---|---|---|
| Males (n = 11) | Females (n = 11) | ||
| Age, yr | 21 (3) | 22 (3) | 20 (3) |
| Height, cm | 172 (8) | 178 (6) | 166 (5) |
| Body mass, kg | 68.7 (10.7) | 73.1 (7.5) | 62.5 (11.0) |
| Body mass index, kg/m2 | 22.9 (2.6) | 23.3 (2.1) | 22.5 (3.1) |
| Systolic blood pressure, mmHg | 114 (5) | 117 (4) | 112 (4) |
| Diastolic blood pressure, mmHg | 70 (5) | 68 (5) | 71 (4) |
| Race and ethnicity, n (%) | |||
| American Indian or Alaskan Native | 1 (4.5) | 0 (0.0) | 1 (9.1) |
| Asian or Asian American | 4 (18.2) | 2 (18.2) | 2 (18.2) |
| Black or African American | 1 (4.5) | 1 (9.1) | 0 (0.0) |
| Hispanic or Latino | 1 (4.5) | 0 (0.0) | 1 (9.1) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White | 15 (68.2) | 8 (72.7) | 7 (63.6) |
Values are represented as means (SD) except for race and ethnicity, which are numbers of participants (n) with percentages of total participants.
Measurements and Instrumentation
Relative body fluid volume losses were estimated via the percent change in nude body mass over 24 h (Sartorius Midrics 2, Goettingen, Germany). Spot urine samples were assessed for urine specific gravity (Atago refractometer, Bellevue, WA) in duplicate and then aliquoted and stored at −80°C for future biomarker analyses. A urinary biomarker panel was used to provide insights into the anatomic location of potential renal injury (6, 15, 16, 22–24). The primary dependent variable was urinary [IGFBP7·TIMP-2]. In addition to being FDA-approved for clinical use, [IGFBP7·TIMP-2] is a renal stress biomarker that is present in urine before renal injury (25). Urinary IGFBP7 and TIMP-2 are presented as both the mathematical product (i.e., [IGFBP7·TIMP-2]) and disaggregated to allow for the identification of the anatomic location of renal tubular stress because IGFBP7 is preferentially secreted in proximal tubules and TIMP-2 is preferentially secreted in distal tubules (6, 26). Urinary KIM-1 is a biomarker for renal proximal tubule injury (6, 27), and urinary NGAL is a biomarker of nonspecific tubular injury (23, 28, 29). Urinary IGFBP7 [dilution: 1:100, intra-assay coefficient of variation (CV): 6.0%, interassay CV: 9.9%], TIMP-2 (dilution: 1:300, intra-assay CV: 2.9%, interassay CV: 7.8%), KIM-1 (dilution: 1:1, intra-assay CV: 4.9%, interassay CV: 1.0%), and NGAL (dilution: 1:20, intra-assay CV: 3.8%, interassay CV: 12.8%) were measured in batch with ELISAs (RayBiotech Life, Peachtree Corners, GA). Urinary [IGFBP7·TIMP-2], KIM-1, and NGAL have excellent stability when stored at −80°C (30, 31). Participants were fully voided into 1-L collection containers, which were well mixed and then aliquoted into 1 mL volumes into 1.7-mL clear graduated microcentrifuge tubes (Part No. C-3262-1, BioExpress, Kaysville, UT). Urine color was assessed independently by three investigators using the 1 mL samples in a well-lit room using a standardized background with the validated 8-point urine color scale [ranging from very pale yellow (score: 1) to brownish green (score: 8)] developed by Armstrong et al. (32).
A venous blood sample was obtained from the antecubital space via an intravenous catheter using serum separator tubes that were left to clot for 30 min at room temperature before centrifugation (10 min at 1,500 g at 4°C). Serum was stored at −80°C. After being thawed, serum and urine were analyzed for osmolality via freezing point depression (Model 3320, Advanced Instruments, Norwood, MA), and Na+ and K+ concentrations were analyzed via flame photometry (Bio Flame Photometer, BWB Technologies, Newbury, UK). Serum and urine samples were analyzed for creatinine via Jaffe reaction (Serum Creatinine or Urine Creatinine Colorimetric Assay Kit, Cayman Chemical, Ann Arbor, MI). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula without race as recommended by the National Kidney Foundation-American Society of Nephrology (NKF-ASN) task force (33). Fractional excretion of Na+ and K+ were determined using standard calculations (34). Blood pressure was measured in duplicate following 20 min of supine rest with 1 min between measurements using an automated ECG-gated auscultatory device (Tango M2, SunTech Medical, Morrisville, NC). A third measurement was taken in the rare occurrence that either of the successive systolic or diastolic pressure measurements were >3 mmHg apart. Mean arterial pressure was calculated as the sum of one-third pulse pressure and diastolic pressure.
Renal hemodynamic measurements were obtained in the supine position via Doppler ultrasound (iE33, Philips, Andover, MA) by triplicate measurements of blood velocity in the distal segment of the right renal artery and the middle portion of a renal segmental artery in the right kidney (35, 36). To avoid large changes in blood pressure and to allow for accurate placement of the sample volume on the renal vasculature, ultrasound measurements were obtained during a non-Valsalva midexhalation following a standard verbal script and familiarization protocol that we have previously detailed (35, 37, 38). Measurements were taken using the coronal approach in the same anatomic location marked by indelible ink with a 5-1 MHz phased-array transducer. The deepness of the renal vasculature precluded adequate resolution for vessel wall diameter measurement. Therefore, blood velocity in the renal and segmental arteries was interpreted to reflect changes in blood flow in these conduit vessels with the assumption that vessel diameter is unchanged (39, 40). The strengths and limitations of using this approach to quantify renal hemodynamics have been extensively discussed elsewhere (6, 35, 41). Mean blood velocity was calculated using time-averaged maximum velocity (35). Vascular resistance in the renal and segmental arteries was calculated as the quotient of mean arterial pressure and blood velocity, with blood pressure measurements obtained immediately before ultrasound measurements. The use of Doppler ultrasonography was deemed to be advantageous in the present study given the ability to obtain quick noninvasive measurements without the need for intravenous infusion (42) or sustained urine output with clearance techniques (43). It is worth noting that we were unable to obtain cross-sectional area for the determination of volumetric renal blood flow due to the depth of the renal vasculature (44) and that the interday reliability of renal and segmental artery blood velocity measurements obtained in the supine position has not been directly investigated. We previously found that the interday reliability of renal artery blood velocity is strong and segmental artery blood velocity is relatively poor when measurements are obtained in the left lateral recumbent position (35). The modest volume loading induced by having participants ingest 3 mL/kg of water before renal measurements was necessary to obtain images. In support of our approach, we have previously found that renal and segmental artery blood velocity are not altered 30 min after ingestion of 500 mL of water when euhydrated (45).
Experimental Protocol
In a block-randomized crossover design, participants completed two 24-h protocols: 1) the “euhydrated” protocol (euhydrated group) involving 24 h of normal fluid and food consumption and 2) the “hypohydrated” protocol (hypohydrated group) involving 24 h deprivation of fluids and high water content-containing foods, specifically fruits, vegetables, soups, broths, stews, oatmeal, yogurt, ice cream, sauces, and condiments (e.g., ketchup and mayonnaise). A 24-h fluid deprivation model was used because it elicits an approximate 2% reduction in body mass (46) that is considered mild hypohydration (47, 48). A minimum 72-h washout occurred between the 24-h protocols to minimize a potential influencing effect between protocols (49, 50). Measurements were obtained at the same morning time for a given participant to control for diurnal effects on kidney function. Prior to each laboratory visit, participants abstained from 1) heat stress (e.g., sauna or hot outdoor environment), moderate and vigorous exercise, alcohol, recreational drugs, nutritional supplements, over-the-counter medications, and prescription drugs (except oral contraceptives) for 24 h; 2) caffeine for at least 12 h; and 3) food for at least 3 h.
Participants provided a spot urine sample to confirm euhydration status via a urine specific gravity of ≤1.020 (51) before commencing each 24-h protocol. With euhydration confirmed, participant nude body mass was measured. Participants were then sent home after instruction from the investigators about the fluid and food intake requirements for their given experimental condition. Compliance to the protocol was confirmed via dietary food and fluid records taken over the 24-h period (Supplemental Table S1). Participants returned to the laboratory 24 h later and provided a second spot urine sample, and nude body mass was remeasured. Participants then ingested 3 mL of water per kilogram of body mass to assist with renal ultrasound measurements. Participants were instrumented and then rested in the supine position for 20 min. Blood pressure was measured, and renal ultrasound measurements were taken. Finally, a venous blood sample was obtained.
Data and Statistical Analyses
All statistical analyses were performed in Prism software (GraphPad Prism 9.5, San Diego, CA) and RStudio (v.2022.12.0.353, Posit Software, Boston, MA). Data normality was confirmed by visual inspection of quantile-quantile plots. Non-normally distributed data were log transformed before analysis (urinary [IGFBP7·TIMP-2]; urinary NGAL; osmolality-corrected [IGFBP7·TIMP-2], IGFBP7, TIMP-2, and NGAL; and creatinine-corrected [IGFBP7·TIMP-2], NGAL, and TIMP-2) and are presented in figures as nontransformed values with statistical analysis following log transformation. Data were analyzed using two-way ANOVA with factors of hydration and sex and post hoc Holm–Šídák tests to account for multiple comparisons. Biological sex was included in our statistical model to acknowledge sex as a variable in biomedical research and to allow for exploration of potential differences between males and females in outcome variables. A single statistical outlier for the primary dependent variable urinary [IGFBP7·TIMP-2], a hypohydrated male, was noted as more than 3 SDs from the mean of all hypohydrated participants [10.2 vs. 1.9 (ng/mL)2/1,000]. However, the statistical findings were the same regardless of whether this participant’s data were included in the analysis. The urinary [IGFBP7·TIMP-2] of 10.2 (ng/mL)2/1,000 remains well within the reported range of other studies (15), and, therefore, this participant’s data were included in the final analysis. Urinary [IGFBP7·TIMP2], IGFBP7, TIMP-2, KIM-1, and NGAL were corrected to urine osmolality and creatinine to account for the effect of urine concentration (6, 18, 19, 51, 52) with [IGFBP7·TIMP-2] correction calculated per the method of Bitker et al (19).
The diagnostic accuracy of established hypohydration thresholds in predicting urinary [IGFBP7·TIMP-2] of >0.3 (ng/mL)2/1,000 (analogous to a NephroCheck AKI risk score of >0.3) was assessed through the following workflow. Criterion values for commonly used hypohydration thresholds of ≥2% loss in body mass (47) and urine specific gravity > 1.020 arbitrary units (au) (53) were used. Due to discrepancy in the literature for criterion values, we also assessed the diagnostic accuracy of urine specific gravity > 1.025 au (54); serum osmolality ≥ 290 mosmol/kgH2O (53), ≥295 mosmol/kgH2O (55), ≥297 mosmol/kgH2O (54), and ≥300 mosmol/kgH2O (56); urine osmolality ≥ 700 mosmol/kgH2O (53), ≥800 mosmol/kgH2O (9), and ≥831 mosmol/kgH2O (54); and urine color ≥ 3 au, ≥4 au, and ≥5 au (32, 54, 57). We also performed this analysis for serum Na+ concentration of 145 mmol/L (i.e., hypernatremia). Data were plotted as a scatterplot with criterion hypohydration values and urinary [IGFBP7·TIMP-2] as the independent and dependent variables. The association between a criterion hypohydration value and urinary [IGFBP7·TIMP-2] > 0.3 (ng/mL)2/1,000 was assessed with a two-tailed Fisher’s exact test, a conservative approach given the relatively small sample size (58). When Fisher’s exact test revealed a significant association, 2 × 2 contingency tables were created and analyzed for sensitivity, specificity, and predictive values (59, 60) with 95% confidence intervals (CIs) computed using the modified Wilson method previously described by Brown et al (61). The Koopman asymptotic score method was used to compute 95% CIs for the relative risk of developing an AKI risk score of >0.3 for a given criterion hypohydration value.
Receiver operating characteristic curves were analyzed to assess the overall diagnostic accuracy of the hypohydration measures and to determine the optimal cutoff for discriminating positive AKI risk as determined by urinary [IGFBP7·TIMP-2] of >0.3 (ng/mL)2/1,000. The overall diagnostic performance of a hypohydration measurement was assessed with area under the curve (AUC) analysis with 95% CIs computed by the Wilson/Brown method (61). The discriminatory ability of the hypohydration measures were interpreted from AUC using the guidelines of Hosmer et al. (62) with AUC = 0.5 as “no discrimination,” 0.5 < AUC < 0.7 as “poor,” 0.7 ≤ AUC < 0.8 as “acceptable,” 0.8 ≤ AUC < 0.9 as “excellent,” and AUC ≥ 0.9 as “outstanding.” The AUC was compared between hypohydration measurements using the method of Delong et al (63). Optimal cutoffs for a given receiver operating characteristic curve were determined using 1) Youden’s index (64), which defines the cutoff by the maximum potential discriminatory ability of a measurement by applying equal weighing between sensitivity and specificity, and 2) positive likelihood ratio, which was calculated as follows: sensitivity/(1 − specificity). A positive likelihood ratio of >10 is considered to be strong and clinically significant evidence that a positive hypohydration test correctly discriminates urinary [IGFBP7·TIMP-2] > 0.3 (ng/mL)2/1,000 (65).
Data are presented as means with 95% CIs, with the exception of the descriptive statistics shown in Table 1, which are presented as means with SDs. All data are reported as n = 22 (11 males and 11 females) with the exception of data involving venous blood samples (Table 2 and serum osmolality in Fig. 1D), which are reported as n = 21 (11 males and 10 females) due to participant discomfort with the blood draw procedure. Statistical significance was set to P < 0.05.
Table 2.
Kidney function
| Parameter | Euhydrated Group |
Hypohydrated Group |
P Value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| All Participants | Males | Females | All Participants | Males | Females | Sex | Hydration | Sex × Hydration | |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 117 (111, 123) | 121 (113, 129) | 113 (102, 123) | 118 (112, 124) | 119 (111, 126) | 116 (105, 128) | 0.2990 | 0.8323 | 0.2998 |
| Fractional excretion of Na+, % | 1.1 (0.7, 1.5) | 1.1 (0.7, 1.4) | 1.1 (0.3, 1.9) | 0.2 (0.1, 0.2) | 0.2 (0.1, 0.2)* | 0.2 (0.1, 0.2)* | 0.8299 | <0.0001 | 0.9571 |
| Fractional excretion of K+, % | 14.9 (10.3, 19.5) | 14.2 (9.4, 19.0) | 15.7 (6.3, 25.0) | 1.9 (1.4, 2.3) | 1.5 (1.0, 1.9)* | 2.3 (1.5, 3.2)* | 0.6244 | <0.0001 | 0.8971 |
| Serum | |||||||||
| Creatinine, mg/dL | 0.8 (0.8, 0.9) | 0.9 (0.8, 1.0) | 0.8 (0.7, 0.9) | 0.8 (0.8, 0.9) | 0.9 (0.8, 1.0) | 0.7 (0.6, 0.8)† | 0.0128 | 0.9156 | 0.1174 |
| Na+, mmol/L | 142 (141, 143) | 142 (140, 144) | 142 (140, 143) | 144 (143, 146) | 144 (142, 146)* | 144 (142, 147)* | 0.9782 | <0.0001 | 0.7045 |
| K+, mmol/L | 4.2 (4.1, 4.3) | 4.1 (4.0, 4.3) | 4.2 (4.1, 4.3) | 4.2 (4.1, 4.3) | 4.2 (4.0, 4.4) | 4.2 (4.0, 4.3) | 0.9987 | 0.7610 | 0.4862 |
| Urine | |||||||||
| Creatinine, mg/dL | 85 (62, 108) | 102 (66, 138) | 68 (38, 98) | 259 (220, 297) | 302 (235, 369)* | 215 (187, 243)*† | 0.0039 | <0.0001 | 0.2029 |
| Na+, mmol/L | 29 (21, 37) | 31 (19, 42) | 28 (15, 41) | 88 (76, 99) | 84 (62, 106)* | 91 (79, 103)* | 0.7699 | <0.0001 | 0.4205 |
| K+, mmol/L | 12 (8, 15) | 13 (8, 17) | 10 (6, 15) | 28 (22, 34) | 25 (20, 29)* | 32 (21, 44)* | 0.4017 | <0.0001 | 0.1027 |
Values are represented as means with 95% confidence intervals in parentheses; n = 22 participants (11 males and 11 females) for urine Na+ and K+ and n = 21 participants (11 males and 10 females) for the remaining data. Data were analyzed using two-way ANOVA with factors of sex and hydration and post hoc Holm–Šídák tests for multiple comparisons.
*Different from the euhydrated group for a given sex (P ≤ 0.0087); †different from males for a given condition (P ≤ 0.0057).
Figure 1.
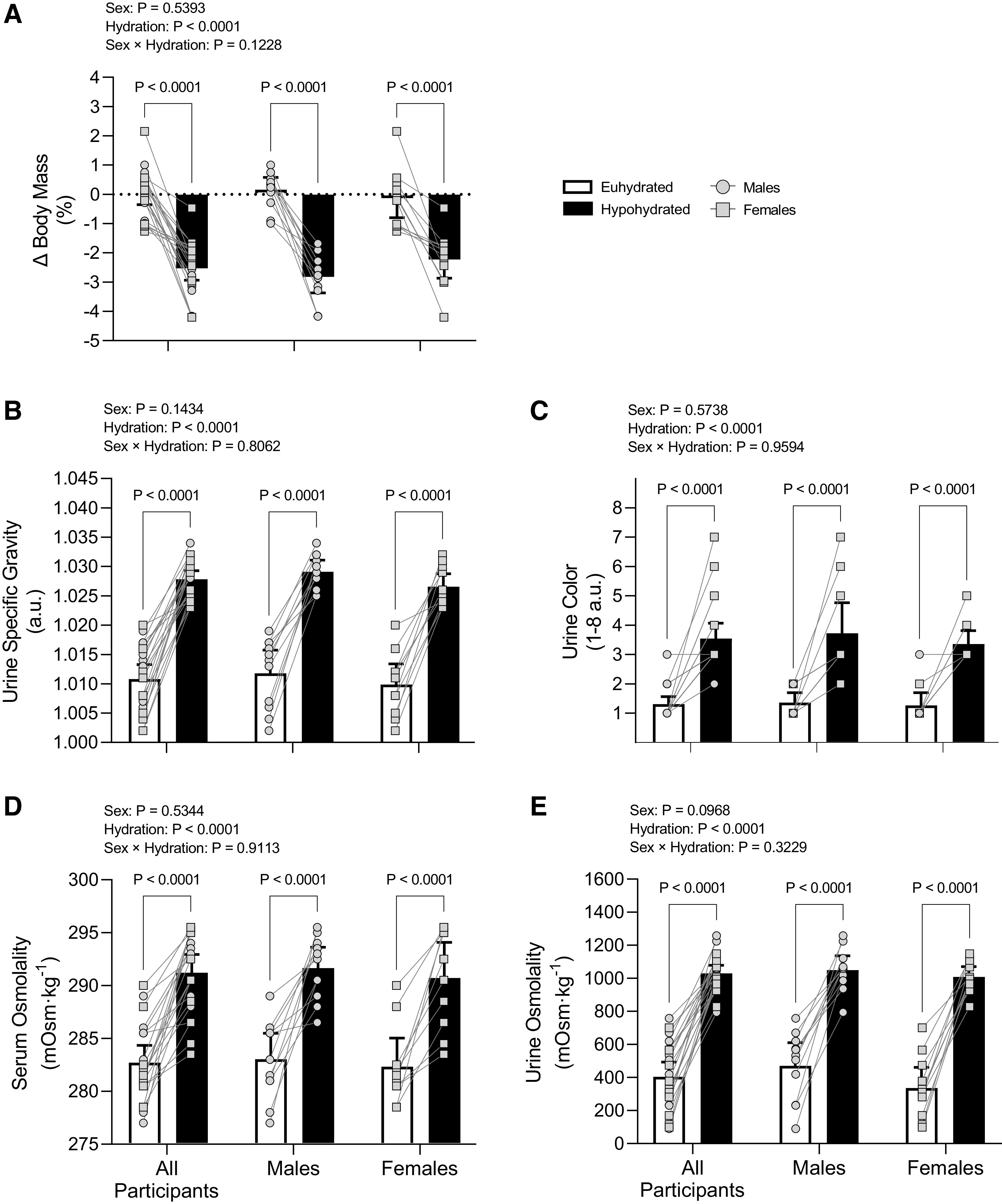
Prolonged mild hypohydration elicited by 24-h fluid deprivation protocol. Healthy males (n = 11) and females (n = 11) underwent 24-h fluid deprivation (hypohydrated group) or 24-h normal fluid consumption (euhydrated group). Change in 24-h nude body mass (A), urine specific gravity (B), urine color (C), serum osmolality (D), and urine osmolality (E) were analyzed using two-way ANOVA with factors of sex and hydration and post hoc Holm–Šídák tests for multiple comparisons. P values from main effects (sex and hydration) and interaction (sex × hydration) reported are shown at the top, and exact P values from multiple-comparison adjustments are shown above a given comparison. Data are presented as means with 95% confidence intervals and individual values. a.u., arbitrary units.
RESULTS
Hydration Status and Kidney Function
Participants were euhydrated before commencing each 24-h protocol [prehypohydrated: 1.011 au (95% CI: 1.008–1.014) and preeuhydrated: 1.009 au (95% CI: 1.007–1.012), P = 0.2408]. The hypohydrated protocol elicited mild hypohydration compared with the euhydrated protocol as demonstrated by reductions in body mass and elevations in urine specific gravity, urine color, serum osmolality, and urine osmolality (Fig. 1). There were no differences between conditions in eGFR, serum creatinine, or serum K+ following the 24-h protocols (Table 2). Fractional excretion of Na+ and K+ were reduced in hypohydrated versus euhydrated groups (Table 2). Serum Na+ and urine creatinine, Na+, and K+ were elevated in hypohydrated versus euhydrated groups (Table 2). Serum and urine creatinine were elevated in hypohydrated males compared with hypohydrated females (Table 2). Mean arterial pressure did not differ between conditions (Table 3). There was a main effect for reduced renal artery blood velocity in hypohydrated versus euhydrated groups with post hoc tests revealing modest reductions occurring in males but not in females (Table 3). There was no effect of hydration status on renal artery vascular resistance or blood velocity and vascular resistance in the segmental artery (Table 3).
Table 3.
Renal hemodynamics
| Parameter | Euhydrated Group |
Hypohydrated Group |
P Value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| All Participants | Males | Females | All Participants | Males | Females | Sex | Hydration | Sex × Hydration | |
| Mean arterial pressure, mmHg | 82 (79, 84) | 83 (79, 87) | 80 (77, 84) | 80 (78, 82) | 81 (78, 84) | 79 (75, 83) | 0.2906 | 0.2070 | 0.9121 |
| Renal artery | |||||||||
| Blood velocity, cm/s | 45.8 (43.2, 48.5) | 45.8 (42.2, 49.3) | 45.9 (41.2, 50.6) | 44.6 (41.8, 47.4) | 43.7 (40.2, 47.1)* | 45.6 (40.5, 50.6) | 0.7034 | 0.0445 | 0.1463 |
| Vascular resistance, mmHg/cm/s | 1.8 (1.7, 1.9) | 1.8 (1.6, 2.0) | 1.8 (1.6, 2.0) | 1.8 (1.7, 1.9) | 1.9 (1.7, 2.0) | 1.8 (1.6, 2.0) | 0.5263 | 0.7876 | 0.2727 |
| Segmental artery | |||||||||
| Blood velocity, cm/s | 36.1 (33.0, 39.1) | 33.8 (30.3, 37.4) | 38.3 (33.1, 43.6) | 36.4 (34.0, 38.7) | 35.0 (32.4, 37.6) | 37.7 (33.6, 41.9) | 0.3530 | 0.6330 | 0.4861 |
| Vascular resistance, mmHg/cm/s | 2.3 (2.1, 2.5) | 2.5 (2.2, 2.7) | 2.2 (1.9, 2.2) | 2.2 (2.1, 2.4) | 2.3 (2.2, 2.5) | 2.1 (1.9, 2.4) | 0.1019 | 0.0623 | 0.3059 |
Values are represented as means with 95% confidence intervals; n = 22 participants (11 males and 11 females). Data were analyzed using two-way ANOVA with factors of sex and hydration and post hoc Holm–Šídák tests for multiple comparisons.
*Different from the euhydrated group for a given sex (P = 0.0351).
AKI Biomarkers
There were marked increases in urinary [IGFBP7·TIMP-2], IGFBP7, TIMP-2, and KIM-1 in both males and females during hypohydrated versus euhydrated protocols (Fig. 2, A–D). There was no effect of hydration status on urinary NGAL (Fig. 2E). However, there was a main effect of sex on urinary NGAL with post hoc tests revealing elevated urinary NGAL in females versus males during the euhydrated protocol and a trend for this effect between females and males during the hypohydrated protocol (Fig. 2E).
Figure 2.
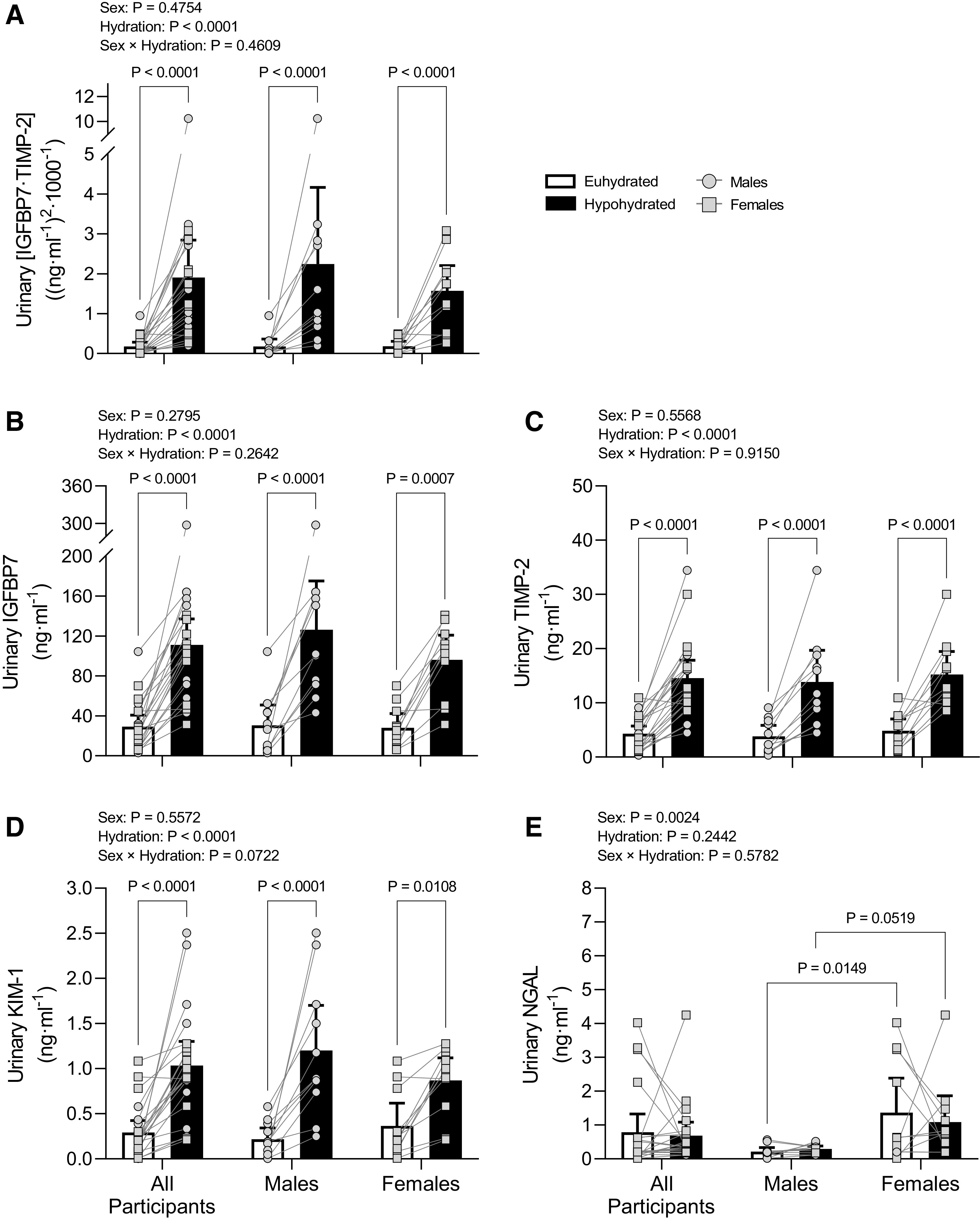
Elevated urinary acute kidney injury biomarkers following prolonged mild hypohydration. Healthy males (n = 11) and females (n = 11) underwent 24-h fluid deprivation (hypohydrated group) or 24-h normal fluid consumption (euhydrated group). A: product of insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinase (TIMP)-2 ([IGFBP7·TIMP-2]). B: IGFBP7. C: TIMP-2. D: kidney injury molecule-1 (KIM-1). E: neutrophil gelatinase-associated lipocalin (NGAL). Data were analyzed with two-way ANOVA with factors of sex and hydration and post hoc Holm–Šídák tests for multiple comparisons. P values from main effects (sex and hydration) and interaction (sex × hydration) are shown at the top, and exact P values from multiple-comparison adjustments are shown above a given comparison. Data are presented as means with 95% confidence intervals and individual values.
There was a sex × hydration interaction effect for osmolality corrected urinary [IGFBP7·TIMP-2], IGFBP7, TIMP-2, and KIM-1 as well as creatinine-corrected urinary KIM-1 whereby all biomarkers increased during the hypohydrated protocol compared with the euhydrated protocol in males but not in females (Fig. 3, A, C, E, G, and H). There was a trend for elevated creatinine-corrected urinary [IGFBP7·TIMP-2] in the hypohydrated group versus the euhydrated group, with increases driven by males (Fig. 3B). Elevations in creatinine-corrected urinary IGFBP7 in the hypohydrated versus euhydrated group were driven by males (Fig. 3D), and there was no effect of hydration on creatinine-corrected urinary TIMP-2 (Fig. 3F). Osmolality- and creatinine-corrected urinary NGAL were reduced in females in hypohydrated versus euhydrated groups but did not change in males, and females had elevated values compared with males under both conditions (Fig. 3, I and J).
Figure 3.
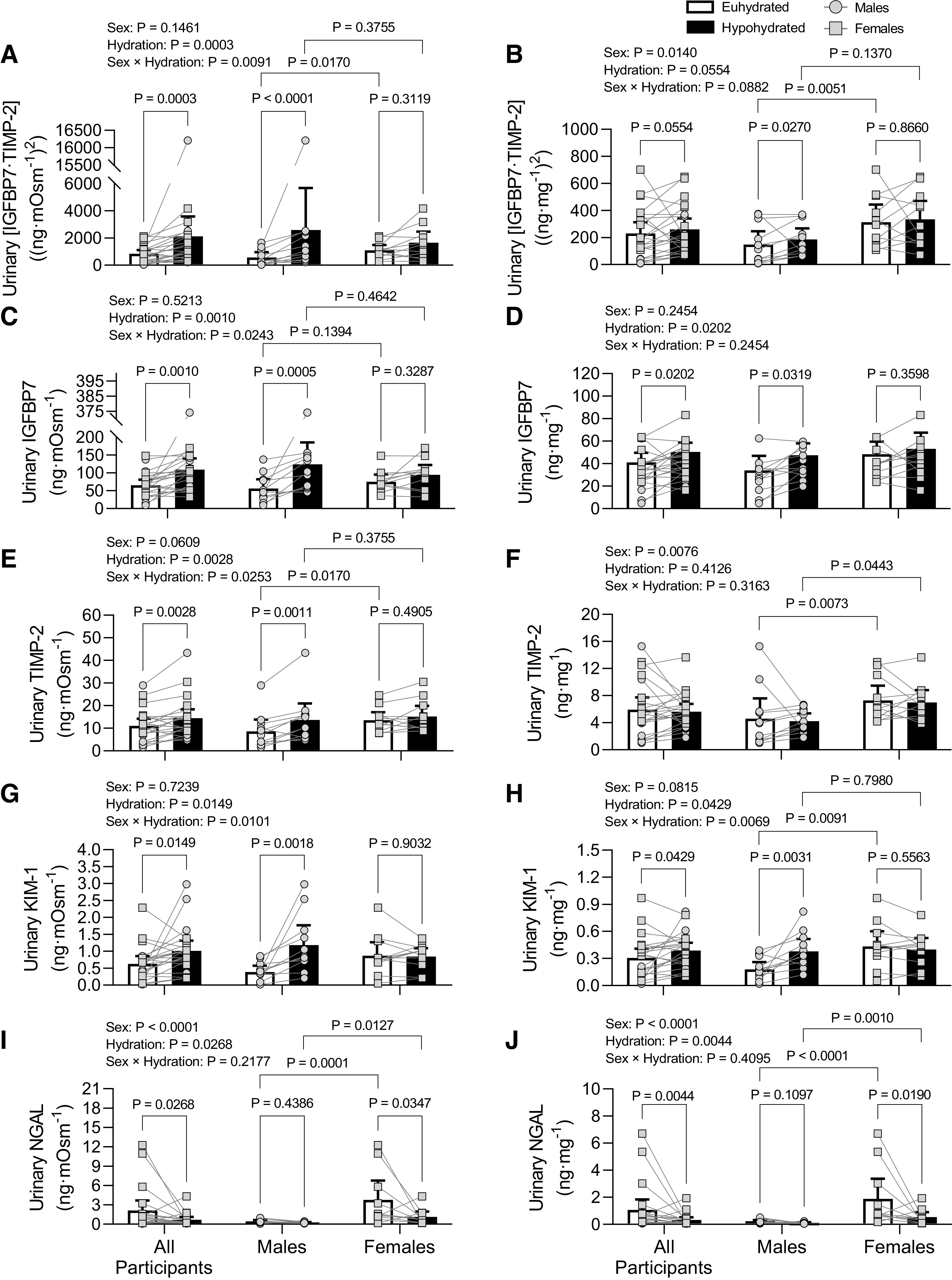
Urine osmolality-corrected (A, C, E, G, and I) and urine creatinine-corrected (B, D, F, H, and J) corrected acute kidney injury biomarker responses to prolonged mild hypohydration. Healthy males (n = 11) and females (n = 11) underwent 24-h fluid deprivation (hypohydrated group) or 24-h normal fluid consumption (euhydrated group). A and B: product of insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinase (TIMP)-2 ([IGFBP7·TIMP-2]). C and D: IGFBP7. E and F: TIMP-2. G and H: kidney injury molecule-1 (KIM-1). I and J: neutrophil gelatinase-associated lipocalin (NGAL). Data were analyzed with two-way ANOVA with factors of sex and hydration and post hoc Holm–Šídák tests for multiple comparisons. P values from main effects (sex and hydration) and interaction (sex × hydration) are shown at the top, and exact P values from multiple-comparison adjustments are shown above a given comparison. Data are presented as means with 95% confidence intervals and individual values.
Diagnostic Accuracy of Hypohydration Criterion Values in Predicting AKI Risk Score of >0.3
Urinary [IGFBP7·TIMP-2] as a function of criterion hypohydration values was plotted against urinary [IGFBP7·TIMP-2] for visual representation of data distribution (Fig. 4). Contingency analyses revealed a significant Fisher’s exact test for all criterion values shown in Table 4. All criterion hypohydration values were determined to have high positive predictive values (≥0.88). However, there was less certainty for the positive predictive values of serum osmolality given the width of the 95% CI (Table 4). The highest positive predictive values were noted with change in body mass loss ≥ 2%, urine specific gravity ≥ 1.025 au, and serum osmolality ≥ 295 mosmol/kgH2O. In general, the negative predictive value of all criterion hypohydration values were comparatively lower than the positive predictive values or had wide 95% CIs thereby reducing the certainty of the mean (Table 4). The mean relative risk values for urinary [IGFBP7·TIMP-2] > 0.3 (ng/mL)2/1,000 for criterion hypohydration values ranged between 2.0 and 3.5, with 95% confidence of the mean between 1.2 and 7.9 for all criterion values (Table 4).
Figure 4.
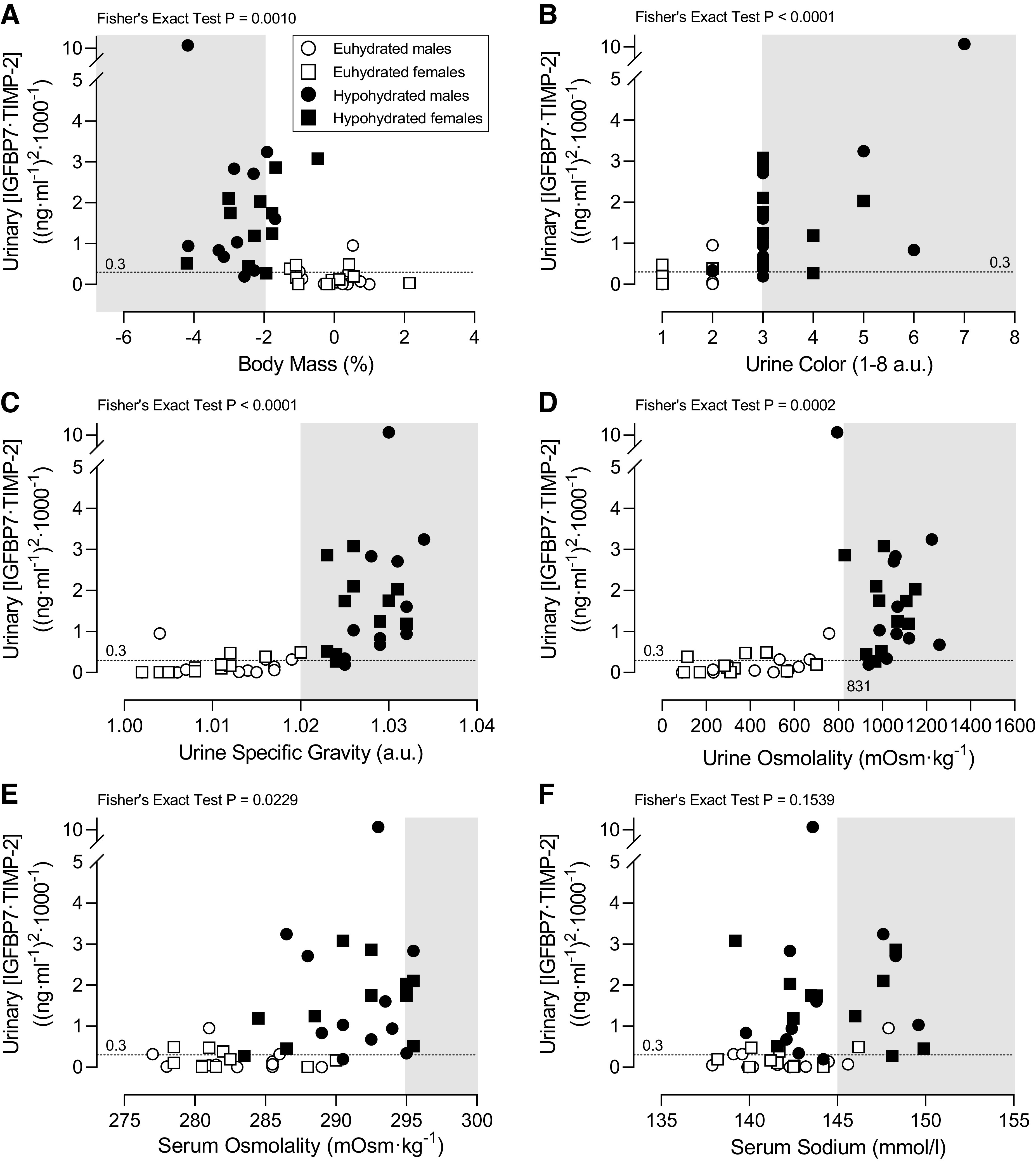
Criterion hypohydration values vs. urinary product of insulin-like growth factor-binding protein 7 and tissue inhibitor of metalloproteinase-2 ([IGFBP7·TIMP-2]) > 0.3 (ng/mL)2/1,000. Individual values of urinary [IGFBP7·TIMP-2] plotted as a function of the percent change in body mass over 24 h (A), urine color (B), urine specific gravity (C), urine osmolality (D), serum osmolality (E), and serum Na+ (F). Healthy males and females underwent 24-h fluid deprivation (hypohydrated group) or 24-h normal fluid consumption (euhydrated group). A positive acute kidney injury risk score was identified as urinary [IGFBP7·TIMP-2] > 0.3 (ng/mL)2/1,000. Areas in the shaded zone represent the criterion range for hypohydration. Fisher’s exact test was used as a conservated method to determine an association of the data. Body mass loss over 24 h (Δbody mass) and all urine data are n = 44 (11 males and 11 females in two conditions; A–D), whereas serum data are n = 42 (11 males and 10 females in two conditions; E and F). a.u., arbitrary units.
Table 4.
Diagnostic accuracy and relative risk of criterion hypohydration values in predicting urinary [IGFBP7·TIMP-2] > 0.3 (ng/mL)2/1,000
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Relative Risk | |
|---|---|---|---|---|---|
| Change in body mass loss ≥ 2% | 0.54 (0.35, 0.71) | 0.94 (0.74, 1.00) | 0.93 (0.70, 1.00) | 0.59 (0.41, 0.74) | 2.3 (1.5, 3.7) |
| Urine specific gravity > 1.020 au | 0.77 (0.58, 0.89) | 0.89 (0.67, 0.98) | 0.91 (0.72, 0.98) | 0.73 (0.52, 0.87) | 3.3 (1.8, 7.0) |
| Urine specific gravity ≥ 1.025 au | 0.67 (0.48, 0.81) | 0.94 (0.74, 0.99) | 0.94 (0.75, 0.99) | 0.65 (0.46, 0.81) | 2.7 (1.7, 4.9) |
| Urine osmolality ≥ 700 mosmol/kgH2O | 0.81 (0.62, 0.91) | 0.83 (0.61, 0.94) | 0.88 (0.69, 0.96) | 0.75 (0.53, 0.89) | 3.5 (1.8, 7.9) |
| Urine osmolality ≥ 800 mosmol/kgH2O | 0.73 (0.54, 0.86) | 0.89 (0.67, 0.98) | 0.90 (0.71, 0.98) | 0.70 (0.49, 0.84) | 3.0 (1.7, 5.9) |
| Urine osmolality ≥ 831 mosmol/kgH2O | 0.69 (0.50, 0.84) | 0.89 (0.67, 0.98) | 0.90 (0.70, 0.98) | 0.67 (0.47, 0.82) | 2.7 (1.6, 5.1) |
au, arbitrary units; [IGFBP7·TIMP-2], product of insulin-like growth factor-binding protein 7 and tissue inhibitor of metalloproteinase-2.
Optimal Cutoff for Hypohydration Measures
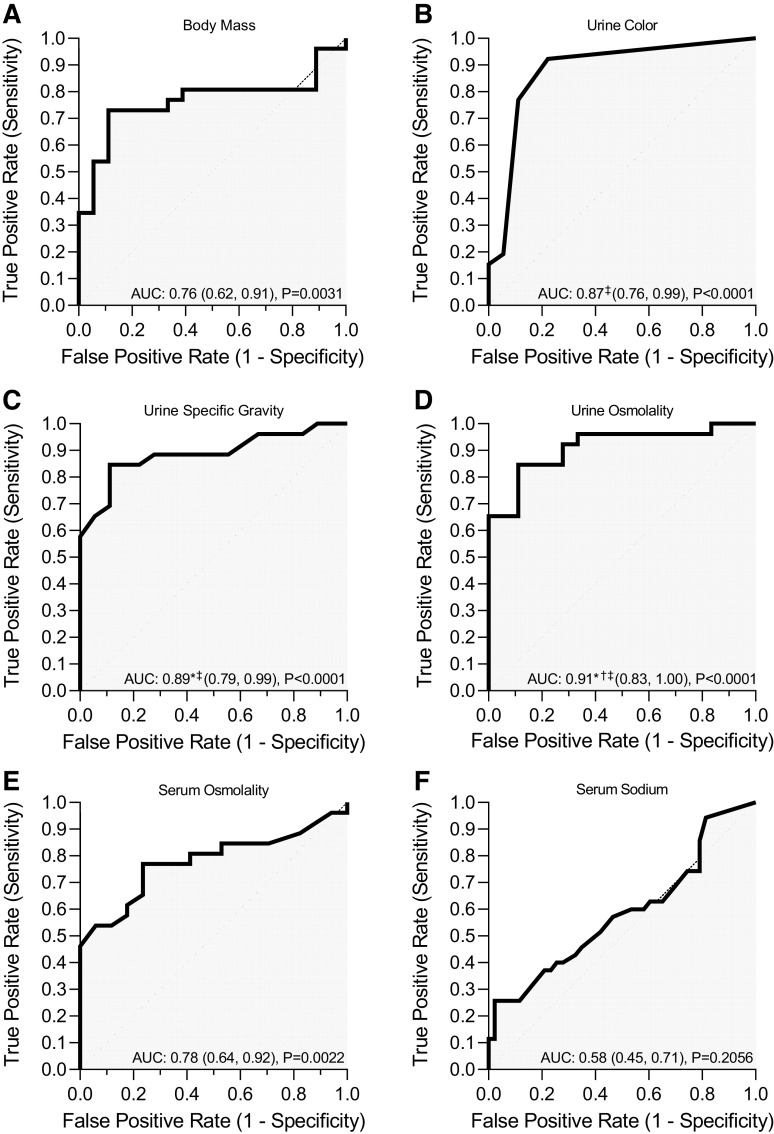
Change in body mass, urine color, urine specific gravity, urine osmolality, and serum osmolality all performed better than random chance in discriminating when urinary [IGFBP7·TIMP-2] was >0.3 (ng/mL)2/1,000 (Fig. 5, A–E). Serum Na+ did not perform better than random chance (Fig. 5F). Urine osmolality (Fig. 5D) had outstanding discrimination for assessing urinary [IGFBP7·TIMP-2] > 0.3 (ng/mL)2/1,000 with a higher AUC compared with change in body mass (Fig. 5A), serum osmolality (Fig. 5E), and serum Na+ (Fig. 5F). Urine specific gravity (Fig. 5C) and urine color (Fig. 5B) were considered to have excellent discrimination, with both performing better than serum Na+ (Fig. 5F) and urine specific gravity performing better than change in body mass (Fig. 5A). Serum osmolality (Fig. 5E) and change in body mass (Fig. 5A) were considered to have acceptable discrimination.
Figure 5.
Receiver operating characteristic curves for hypohydration measures in discriminating positive acute kidney injury (AKI) risk. Positive AKI risk was determined as urinary product of insulin-like growth factor-binding protein 7 and tissue inhibitor of metalloproteinase-2 ([IGFBP7·TIMP-2]) > 0.3 (ng/mL)2/1,000. The line of identify represents random chance. Area under the curve (AUC) values are reported as means with 95% confidence intervals and P values from statistical comparison with random chance. AUC was compared using the nonparametric method of Delong et al. (63). AUCs for all hypohydration measures were statistically different from random chance (P ≤ 0.0240). *AUC different from the change in body mass (Δbody mass) (P ≤ 0.0097); †AUC different from serum osmolality (P = 0.0240); ‡AUC different from serum Na+ (P ≤ 0.0193). A–F: body mass (A), urine color (B), urine specific gravity (C), urine osmolality (D), serum osmolality (E), and serum sodium (F).
The optimal cutoffs for hypohydration measurements determined by Youden’s index and positive likelihood ratio are shown in Table 5. Urine osmolality of 670 mosmol/kgH2O and specific gravity of 1.019 demonstrated the highest Youden’s index. Urine osmolality of 952 mosmol/kgH2O and specific gravity of 1.025 had clinically significant positive likelihood ratios. Despite the overall diagnostic accuracy of serum Na+ having “no discrimination” (Fig. 5F), a clinically significant cutoff was found for a serum Na+ concentration of 145.8 mmol/L (Table 5). The optimal cutoffs for change in body mass, urine color, and serum osmolality did not have clinically significant positive likelihood ratios.
Table 5.
Optimal cutoff for hypohydration values in predicting urinary [IGFPB7·TIMP-2] > 0.3 (ng/mL)2/1,000
| Parameter | Criterion Value | Sensitivity | Specificity | Youden’s Index | Positive Likelihood Ratio |
|---|---|---|---|---|---|
| Youden’s index | |||||
| Change in body mass loss, % | −1.3 | 0.89 | 0.73 | 0.62 | |
| Urine specific gravity, au | 1.019 | 0.85 | 0.89 | 0.74 | |
| Urine osmolality, mosmol/kgH2O | 670 | 0.85 | 0.89 | 0.74 | |
| Urine color, au | 2 | 0.92 | 0.78 | 0.70 | |
| Serum osmolality, mosmol/kgH2O | 286 | 0.77 | 0.76 | 0.53 | |
| Serum Na+, mmol/L | 146.0 | 0.35 | 0.94 | 0.28 | |
| Positive likelihood ratio | |||||
| Change in body mass loss, % | −2.0 | 0.54 | 0.94 | 9.7 | |
| Urine specific gravity, au | 1.025 | 0.65 | 0.94 | 11.8 | |
| Urine osmolality, mosmol/kgH2O | 952 | 0.65 | 0.94 | 11.8 | |
| Urine color, au | 2.5 | 0.77 | 0.89 | 6.9 | |
| Serum osmolality, mosmol/kgH2O | 290 | 0.54 | 0.94 | 9.2 | |
| Serum sodium, mmol/L | 145.8 | 0.26 | 0.98 | 11.1 | |
Data were analyzed using receiver operating characteristic curves to determine the optimal cutoff value using Youden’s index and positive likelihood ratio. n = 44 (11 males and 11 females in two conditions) for body mass loss over 24 h (change in body mass loss) and all urine data and n = 42 (11 males and 10 females in two conditions) for serum data. au, arbitrary units.
DISCUSSION
The purpose of this study was twofold: 1) to test the hypothesis that prolonged mild hypohydration elevates biomarkers of AKI in healthy young males and females and 2) to determine the diagnostic accuracy and optimal cutoffs of criterion hypohydration values in predicting urinary [IGFBP7·TIMP-2] > 0.3 (ng/mL)2/1,000. We sought to induce prolonged mild hypohydration because of the ecological validity with the high prevalence of inadequate fluid consumption among the United States adult population (9, 10). Indeed, participants in the present study experienced prolonged mild hypohydration as characterized by relative hyperosmotic hypovolemia with reductions in body mass of −2.5% (95% CI: −2.9 to −2.1) and elevated serum osmolality in the hypohydrated versus euhydrated group [291 mosmol/kgH2O (95% CI: 290–293) vs. 283 mosmol/kgH2O (95% CI: 281–284)].
The first major finding of the present study was that prolonged mild hypohydration, in the absence of heat stress, caused marked elevations in urinary [IGFBP7·TIMP-2] in adults. Moreover, we observed elevated urinary KIM-1 in the hypohydrated group compared with the euhydrated group but did not observe differential responses in urinary NGAL between conditions. It has been recommended by some that correction to urine concentration be considered when assessing AKI risk (18, 19, 51, 52). We found that increased urinary [IGFBP7·TIMP-2] and KIM-1 persisted with correction to osmolality and creatinine, but post hoc analysis revealed that these findings were driven by the male participants. Our methodology was consistent with the current clinical practice of using an uncorrected value of [IGFBP7·TIMP-2] for screening AKI risk (66). The contrasting findings between males and females in the urine concentration-corrected biomarker data suggest that, when feasible, it is likely beneficial to obtain serial measurements to track relative changes from baseline in concentration-corrected AKI biomarkers and to allow for adjustment to urine output (i.e., urine flow rate). Notably, however, there is currently no FDA-approval threshold for concentration-corrected urinary [IGFBP7·TIMP-2]. The mechanism underlying the increased osmolality- and creatinine-corrected urinary [IGFBP7·TIMP-2] and KIM-1 in males but not in females is unclear. Although we observed lower renal artery blood velocity during the hypohydrated protocol versus the euhydrated protocol in males (−4.6%) but not females, it appears unlikely that differences in renal blood flow explain sex differences in urine concentration-corrected biomarker responses given that there were no sex differences in segmental artery blood velocity or vascular resistance.
Previous studies have observed similar volume of body fluid losses between males and females following 24-h fluid deprivation (50). However, unlike females, males exhibit increased circulating copeptin (a stable surrogate measure for arginine vasopressin) with a maintenance of plasma osmolality (50) that is believed to be due to a comparatively less sensitive vasopressin response in females (67). Increased circulating vasopressin has been proposed as a potential pathophysiological mediator for adverse renal effects with chronic hypohydration, including increased intrarenal vascular tone, hyperfiltration, and exacerbation of the polyol-fructokinase pathway (68–70). In the present study, there were no sex differences in increased serum osmolality, and, thus, it is unclear whether vasopressin sensitivity was a mechanism for the differential urine-corrected AKI biomarker response between males and females. Nonetheless, the interaction of sex hormones and body fluid balance is complicated and not fully understood. Changes in estrogen and progesterone concentrations across the menstrual cycle alter fluid regulatory hormones, and these responses may vary depending on the preparation and use of hormonal contraceptives (71, 72). A strength of the present study was accounting for sex as a biological variable by including an equal number of male and female participants. Studies using mouse models of cisplatin-induced AKI and ischemia-reperfusion injury have reported that young female mice are more resistant to renal injury as determined by attenuated NGAL compared with male mice (73, 74). Although the mechanism remains to be identified for sex-based differences for the increased osmolality- and creatinine-corrected urinary [IGFBP7·TIMP-2] and KIM-1 following prolonged mild hypohydration in the present study, our findings indicate that AKI biomarkers suggestive of increased AKI risk are increased in males irrespective of how data are processed, whereas caution is warranted in interpreting this response in females.
The second major finding of the present study was that some of the criterion hypohydration measurement values have high diagnostic accuracy for discriminating positive AKI risk as determined by urinary [IGFBP7·TIMP-2] was >0.3 (ng/mL)2/1,000. Receiver operating characteristic curve analysis determined that urine osmolality and specific gravity had the highest overall performance. The cutoff for the best balance of sensitivity and specificity as determined by Youden’s index was urine osmolality of 670 mosmol/kgH2O and urine specific gravity of 1.019 au. However, clinically significant optimal cutoffs (i.e., positive likelihood ratio > 10) were determined to be at higher values with urine osmolality of 952 mosmol/kgH2O and urine specific gravity of 1.025 au. Given these findings, using urine specific gravity with a cutoff of 1.025 au is likely the most practical method to discriminate positive AKI risk in real-life settings because it is more cost effective and requires less specialized training than osmolality. An interesting finding of the present study was that, although the overall diagnostic accuracy of serum Na+ was considered to have no discriminatory ability, a clinically significant positive likelihood ratio was found for concentrations of 145.8 mmol/L. This suggests that serum Na+ at this cutoff, but not others, may also have utility in this setting. Overall, we demonstrated the optimal hypohydration cutoffs for positive AKI risk using a highly controlled study that builds on the decades of rigorous work by others that investigated methods of assessing hydration status and hydration physiology (32, 47, 54, 75, 76). Our findings demonstrate a clear relation between individuals below or above these cutoffs and urinary [IGFBP7·TIMP-2], which offers early support for the utility of these criterion values in signaling at what magnitude hypohydration may increase AKI risk. These findings also emphasize the importance of adequate hydration to protect renal health. These data suggest that inadequate hydration increases the susceptibility of the kidneys, which is relevant in the context of a warming climate. In support of this, there are experimental models involving extreme heat exposure where adequate hydration reduced renal damage in mice (17) and reduced AKI biomarkers in humans (16). In addition, and while not the primary context for conducting the present study, our findings may also have implications for athletes at risk of severe or prolonged hypohydration, including those who acutely restrict fluids in an effort to rapidly reduce body mass due to competing in a weight-class sport (e.g., wrestling, boxing, and mixed martial arts) (77). Future investigations are also warranted into whether prolonged mild hypohydration may increase the susceptibility of the kidneys to subsequent dietary stress, such as that imposed by high-fructose drinks (45, 78) and high-dietary Na+ (79).
There are a few considerations of the present study worth discussing. First, caution is warranted in generalizing from data obtained in healthy young adults. Further investigation is needed to determine whether pre-extreme heat exposure hypohydration further increases kidney complications in individuals already at heightened risks during extreme heat, including older adults and those with cardiovascular disease. Second, it is important to note that in our effort to enhance scientific rigor and reproducibility, participants in the present study underwent several dietary and lifestyle restrictions that are known to influence these criterion hypohydration values (e.g., abstaining from exercise, heat stress, and nutritional supplements such as B vitamins). Thus, although the present study was designed to induce an externally valid and physiologically relevant mild hypohydration, the results of the diagnostic accuracy analyses are confined to the experimental paradigm used. Third, our present data set is unable to provide the pathophysiological mechanism of the AKI biomarker responses despite evidence indicating the renal proximal tubule as the anatomic location of potential injury in the kidneys. Fourth, urinary [IGFBP7·TIMP-2] was assessed using commercially available ELISAs and not the NephroCheck system. Notably, however, both approaches use a sandwich immunoassay technique. Finally, the diagnostic accuracy analyses were performed on uncorrected urinary [IGFBP7·TIMP-2], which is consistent with the current FDA-approved threshold and clinical practice. Future studies will be needed to determine the threshold values for creatinine or osmolality-corrected urinary [IGFBP7·TIMP-2] for screening AKI in a clinical setting as the current guidelines only include the uncorrected value.
In conclusion, we found increased urinary [IGFBP7·TIMP-2] and KIM-1, with no changes in urinary NGAL, following prolonged mild hypohydration in healthy young adults. Males demonstrated the same effect with urinary [IGFBP7·TIMP-2] and KIM-1 irrespective of whether data were corrected to urine concentration, but increases in these biomarkers were abolished in females with urine concentration correction. Finally, we found that urine osmolality of 952 mosmol/kgH2O and urine specific gravity of 1.025 au were the optimal cutoffs for discriminating positive AKI risk in healthy young males and females following prolonged mild hypohydration.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.5281/zenodo.7931178.
GRANTS
This work was supported by National Institutes of Health Grants R01HL144128 (to C.T.M. and J.R.H.) and F32HL164021 (to C.L.C.), the University of Oregon Knight Campus Undergraduate Scholar Program (to S.M.H.), and National Institutes of Health Summer Research Program Grant R25HD070817 (to H.N.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L.C., J.R.H., and C.T.M. and conceived and designed research; C.L.C., S.M.H., C.T.O., S.C.B., W.A.B.H., H.N.M., and E.L.R. performed experiments; C.L.C., S.M.H., C.T.O., S.C.B., W.A.B.H., H.N.M., and K.W.N. analyzed data; C.L.C., J.R.H., and C.T.M. interpreted results of experiments; C.L.C. prepared figures; C.L.C. drafted manuscript; C.L.C., S.M.H., C.T.O., S.C.B., W.A.B.H., H.N.M., E.L.R., K.W.N., J.R.H., and C.T.M. edited and revised manuscript; C.L.C., S.M.H., C.T.O., S.C.B., W.A.B.H., H.N.M., E.L.R., K.W.N., J.R.H., and C.T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Alara Wayne, Colin Thurston, Jadelyn Yep, Jordan Downing, Mallory Gradow, and Saad Mirza for assistance. We thank Dr. Zachary Schlader for sharing equipment.
REFERENCES
- 1. Schramm PJ, Vaidyanathan A, Radhakrishnan L, Gates A, Hartnett K, Breysse P. Heat-related emergency department visits during the northwestern heat wave—United States, June 2021. MMWR Morb Mortal Wkly Rep 70: 1020–1021, 2021. [Erratum in MMWR Morb Mortal Wkly Rep 70: 1103, 2021]. doi: 10.15585/mmwr.mm7029e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin H, Mo R, Vitart F. The 2021 western North American heatwave and its subseasonal predictions. Geophys Res Lett 49: e2021GL097036, 2022. doi: 10.1029/2021GL097036. [DOI] [Google Scholar]
- 3. Thompson V, Kennedy-Asser AT, Vosper E, Lo YTE, Huntingford C, Andrews O, Collins M, Hegerl GC, Mitchell D. The 2021 western North America heat wave among the most extreme events ever recorded globally. Sci Adv 8: eabm6860, 2022. doi: 10.1126/sciadv.abm6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebi KL, Capon A, Berry P, Broderick C, de Dear R, Havenith G, Honda Y, Kovats RS, Ma W, Malik A, Morris NB, Nybo L, Seneviratne SI, Vanos J, Jay O. Hot weather and heat extremes: health risks. Lancet 398: 698–708, 2021. doi: 10.1016/S0140-6736(21)01208-3. [DOI] [PubMed] [Google Scholar]
- 5. Bobb JF, Obermeyer Z, Wang Y, Dominici F. Cause-specific risk of hospital admission related to extreme heat in older adults. JAMA 312: 2659–2667, 2014. doi: 10.1001/jama.2014.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chapman CL, Johnson BD, Parker MD, Hostler D, Pryor RR, Schlader Z. Kidney physiology and pathophysiology during heat stress and the modification by exercise, dehydration, heat acclimation and aging. Temperature 8: 108–159, 2021. doi: 10.1080/23328940.2020.1826841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson RJ, Wesseling C, Newman LS. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med 380: 1843–1852, 2019. doi: 10.1056/NEJMra1813869. [DOI] [PubMed] [Google Scholar]
- 8. Chapman CL, Hess HW, Lucas RAI, Glaser J, Saran R, Bragg-Gresham J, Wegman DH, Hansson E, Minson CT, Schlader ZJ. Occupational heat exposure and the risk of chronic kidney disease of nontraditional origin in the United States. Am J Physiol Regul Integr Comp Physiol 321: R141–R151, 2021. doi: 10.1152/ajpregu.00103.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang T, Ravi N, Plegue MA, Sonneville KR, Davis MM. Inadequate hydration, BMI, and obesity among US adults: NHANES 2009–2012. Ann Fam Med 14: 320–324, 2016. [Erratum in Ann Fam Med 18: 485, 2020]. doi: 10.1370/afm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stookey JD. Analysis of 2009–2012 Nutrition Health and Examination Survey (NHANES) data to estimate the median water intake associated with meeting hydration criteria for individuals aged 12–80 in the US population. Nutrients 11: 657, 2019. doi: 10.3390/nu11030657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson RJ, García-Arroyo FE, Gonzaga-Sánchez G, Vélez-Orozco KA, Álvarez-Álvarez YQ, Aparicio-Trejo OE, Tapia E, Osorio-Alonso H, Andrés-Hernando A, Nakagawa T, Kuwabara M, Kanbay M, Lanaspa MA, Sánchez-Lozada LG. Current hydration habits: the disregarded factor for the development of renal and cardiometabolic diseases. Nutrients 14: 2070, 2022. doi: 10.3390/nu14102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu X, Guan Y, Xu S, Li Q, Sun Y, Han R, Jiang C. Early predictors of acute kidney injury: a narrative review. Kidney Blood Press Res 41: 680–700, 2016. doi: 10.1159/000447937. [DOI] [PubMed] [Google Scholar]
- 13. Okusa MD, Portilla D. Pathophysiology of acute kidney injury. In: Brenner and Rector’s The Kidney edited by Marsden PA, Skorecki K, Taal MW, Yu ASL, Chertow GM, Luyckx V. Philadelphia, PA: Elsevier, 2019, p. 906–939. [Google Scholar]
- 14. Sasai F, Roncal-Jimenez C, Rogers K, Sato Y, Brown JM, Glaser J, Garcia G, Sanchez-Lozada LG, Rodriguez-Iturbe B, Dawson JB, Sorensen C, Hernando AA, Gonzalez-Quiroz M, Lanaspa M, Newman LS, Johnson RJ. Climate change and nephrology. Nephrol Dial Transplant 38: 41–48, 2023. doi: 10.1093/ndt/gfab258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hess HW, Stooks JJ, Baker TB, Chapman CL, Johnson BD, Pryor RR, Basile DP, Monroe JC, Hostler D, Schlader ZJ. Kidney injury risk during prolonged exposure to current and projected wet bulb temperatures occurring during extreme heat events in healthy young men. J Appl Physiol (1985) 133: 27–40, 2022. doi: 10.1152/japplphysiol.00601.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chapman CL, Johnson BD, Vargas NT, Hostler D, Parker MD, Schlader ZJ. Both hyperthermia and dehydration during physical work in the heat contribute to the risk of acute kidney injury. J Appl Physiol (1985) 128: 715–728, 2020. doi: 10.1152/japplphysiol.00787.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, Cicerchi C, Inaba S, Le M, Miyazaki M, Glaser J, Correa-Rotter R, González MA, Aragón A, Wesseling C, Sánchez-Lozada LG, Johnson RJ. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 86: 294–302, 2014. doi: 10.1038/ki.2013.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noto A, Cortegiani A, David A. NephroCheck: should we consider urine osmolality? Crit Care 23: 48, 2019. doi: 10.1186/s13054-019-2341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bitker L, Toh L, Bittar I, Eastwood GM, Bellomo R. Effects of hydration status and urine concentration on the quantification of cell-cycle arrest biomarkers in the urine of healthy volunteers: a randomized crossover trial. Nephrol Dial Transplant 36: 1548–1551, 2021. doi: 10.1093/ndt/gfab069. [DOI] [PubMed] [Google Scholar]
- 20. Vargas NT, Chapman CL, Sackett JR, Johnson BD, Gathercole R, Schlader ZJ. Thermal behavior differs between males and females during exercise and recovery. Med Sci Sports Exerc 51: 141–152, 2019. doi: 10.1249/MSS.0000000000001756. [DOI] [PubMed] [Google Scholar]
- 21. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 71: e127–e248, 2018. [Erratum in J Am Coll Cardiol 71: 2275–2279, 2018]. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 22. Chapman CL, Johnson BD, Sackett JR, Parker MD, Schlader ZJ. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am J Physiol Regul Integr Comp Physiol 316: R189–R198, 2019. doi: 10.1152/ajpregu.00351.2018. [DOI] [PubMed] [Google Scholar]
- 23. Schlader ZJ, Hostler D, Parker MD, Pryor RR, Lohr JW, Johnson BD, Chapman CL. The potential for renal injury elicited by physical work in the heat. Nutrients 11: 2087, 2019. doi: 10.3390/nu11092087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hansson E, Glaser J, Jakobsson K, Weiss I, Wesseling C, Lucas RAI, Wei JLK, Ekström U, Wijkström J, Bodin T, Johnson RJ, Wegman DH. Pathophysiological mechanisms by which heat stress potentially induces kidney inflammation and chronic kidney disease in sugarcane workers. Nutrients 12: 1639, 2020. doi: 10.3390/nu12061639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wołyniec W, Ratkowski W, Renke J, Renke M. Changes in novel AKI biomarkers after exercise. A systematic review. Int J Mol Sci 21: 5673, 2020. doi: 10.3390/ijms21165673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Emlet DR, Pastor-Soler N, Marciszyn A, Wen X, Gomez H, Humphries WH 4th, Morrisroe S, Volpe JK, Kellum JA. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol 312: F284–F296, 2017. doi: 10.1152/ajprenal.00271.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 28. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 29. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 30. Naorungroj T, Serpa Neto A, Yanase F, Bittar I, Eastwood GM, Bellomo R. NephroCheck® quality test. Blood Purif 50: 489–491, 2021. doi: 10.1159/000511727. [DOI] [PubMed] [Google Scholar]
- 31. Chang C, Obeid W, Thiessen-Philbrook H, Parikh CR. Sample processing and stability for urine biomarker studies. J Appl Lab Med 6: 1628–1634, 2021. doi: 10.1093/jalm/jfab082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armstrong LE, Maresh CM, Castellani JW, Bergeron MF, Kenefick RW, LaGasse KE, Riebe D. Urinary indices of hydration status. Int J Sport Nutr 4: 265–279, 1994. doi: 10.1123/ijsn.4.3.265. [DOI] [PubMed] [Google Scholar]
- 33. Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, Miller WG, Moxey-Mims MM, Roberts GV, St Peter WL, Warfield C, Powe NR. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol 32: 2994–3015, 2021. doi: 10.1681/ASN.2021070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steiner RW. Interpreting the fractional excretion of sodium. Am J Med 77: 699–702, 1984. doi: 10.1016/0002-9343(84)90368-1. [DOI] [PubMed] [Google Scholar]
- 35. Chapman CL, Johnson BD, Hostler D, Lema PC, Schlader ZJ. Reliability and agreement of human renal and segmental artery hemodynamics measured using Doppler ultrasound. J Appl Physiol (1985) 128: 627–636, 2020. doi: 10.1152/japplphysiol.00813.2019. [DOI] [PubMed] [Google Scholar]
- 36. Chapman CL, Schlader ZJ, Reed EL, Worley ML, Johnson BD. Renal and segmental artery hemodynamic response to acute, mild hypercapnia. Am J Physiol Regul Integr Comp Physiol 318: R822–R827, 2020. doi: 10.1152/ajpregu.00035.2020. [DOI] [PubMed] [Google Scholar]
- 37. Chapman CL, Schlader ZJ, Reed EL, Worley ML, Johnson BD. Acute beetroot juice ingestion does not alter renal hemodynamics during normoxia and mild hypercapnia in healthy young adults. Nutrients 13: 1986, 2021. doi: 10.3390/nu13061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chapman CL, Benati JM, Johnson BD, Vargas NT, Lema PC, Schlader ZJ. Renal and segmental artery hemodynamics during whole body passive heating and cooling recovery. J Appl Physiol (1985) 127: 974–983, 2019. doi: 10.1152/japplphysiol.00403.2019. [DOI] [PubMed] [Google Scholar]
- 39. Momen A, Bower D, Leuenberger UA, Boehmer J, Lerner S, Alfrey EJ, Handly B, Sinoway LI. Renal vascular response to static handgrip exercise: sympathetic vs. autoregulatory control. Am J Physiol Heart Circ Physiol 289: H1770–H1776, 2005. doi: 10.1152/ajpheart.01213.2004. [DOI] [PubMed] [Google Scholar]
- 40. Momen A, Thomas K, Blaha C, Gahremanpour A, Mansoor A, Leuenberger UA, Sinoway LI. Renal vasoconstrictor responses to static exercise during orthostatic stress in humans: effects of the muscle mechano- and the baroreflexes. J Physiol 573: 819–825, 2006. doi: 10.1113/jphysiol.2005.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beierwaltes WH, Harrison-Bernard LM, Sullivan JC, Mattson DL. Assessment of renal function; clearance, the renal microcirculation, renal blood flow, and metabolic balance. Compr Physiol 3: 165–200, 2013. doi: 10.1002/cphy.c120008. [DOI] [PubMed] [Google Scholar]
- 42. Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol (1985) 84: 1323–1332, 1998. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- 43. Chasis H, Redish J, Goldring W, Ranges HA, Smith HW. The use of sodium p-aminohippurate for the functional evaluation of the human kidney. J Clin Invest 24: 583–588, 1945. doi: 10.1172/JCI101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rocha MP, Mentetzides SH, Drew RC. Renal blood flow during exercise: understanding its measurement with Doppler ultrasound. J Appl Physiol (1985) 34: 1004–1010, 2023. doi: 10.1152/japplphysiol.00392.2022. [DOI] [PubMed] [Google Scholar]
- 45. Chapman CL, Grigoryan T, Vargas NT, Reed EL, Kueck PJ, Pietrafesa LD, Bloomfield AC, Johnson BD, Schlader ZJ. High-fructose corn syrup-sweetened soft drink consumption increases vascular resistance in the kidneys at rest and during sympathetic activation. Am J Physiol Renal Physiol 318: F1053–F1065, 2020. doi: 10.1152/ajprenal.00374.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Armstrong LE, Giersch GEW, Dunn L, Fiol A, Muñoz CX, Lee EC. Inputs to thirst and drinking during water restriction and rehydration. Nutrients 12: 2554, 2020. doi: 10.3390/nu12092554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cheuvront SN, Kenefick RW. Dehydration: physiology, assessment, and performance effects. Compr Physiol 4: 257–285, 2014. doi: 10.1002/cphy.c130017. [DOI] [PubMed] [Google Scholar]
- 48. Watso JC, Farquhar WB. Hydration status and cardiovascular function. Nutrients 11: 1866, 2019. doi: 10.3390/nu11081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnson EC, Muñoz CX, Le Bellego L, Klein A, Casa DJ, Maresh CM, Armstrong LE. Markers of the hydration process during fluid volume modification in women with habitual high or low daily fluid intakes. Eur J Appl Physiol 115: 1067–1074, 2015. doi: 10.1007/s00421-014-3088-2. [DOI] [PubMed] [Google Scholar]
- 50. Giersch GEW, Colburn AT, Morrissey MC, Butler CR, Pruchnicki ML, Kavouras SA, Charkoudian N, Casa DJ. Effects of sex and menstrual cycle on volume-regulatory responses to 24-h fluid restriction. Am J Physiol Regul Integr Comp Physiol 319: R560–R565, 2020. doi: 10.1152/ajpregu.00173.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hahn RG, Yanase F, Zdolsek JH, Tosif SH, Bellomo R, Weinberg L. Serum creatinine levels and Nephrocheck® values with and without correction for urine dilution—a multicenter observational study. Front Med (Lausanne) 9: 847129, 2022. doi: 10.3389/fmed.2022.847129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hahn RG, Zdolsek J. Nephrocheck® results should be corrected for dilution. Acta Anaesthesiol Scand 61: 261–262, 2017. doi: 10.1111/aas.12836. [DOI] [PubMed] [Google Scholar]
- 53. Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39: 377–390, 2007. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 54. Cheuvront SN, Ely BR, Kenefick RW, Sawka MN. Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr 92: 565–573, 2010. doi: 10.3945/ajcn.2010.29490. [DOI] [PubMed] [Google Scholar]
- 55. Stookey JD. High prevalence of plasma hypertonicity among community-dwelling older adults: results from NHANES III. J Am Diet Assoc 105: 1231–1239, 2005. doi: 10.1016/j.jada.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 56. Thomas DR, Cote TR, Lawhorne L, Levenson SA, Rubenstein LZ, Smith DA, Stefanacci RG, Tangalos EG, Morley JE; Dehydration Council. Understanding clinical dehydration and its treatment. J Am Med Dir Assoc 9: 292–301, 2008. doi: 10.1016/j.jamda.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 57. Perrier ET, Bottin JH, Vecchio M, Lemetais G. Criterion values for urine-specific gravity and urine color representing adequate water intake in healthy adults. Eur J Clin Nutr 71: 561–563, 2017. doi: 10.1038/ejcn.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen YP. Do the chi-square test and Fisher’s exact test agree in determining extreme for 2× 2 tables? Am Stat 65: 239–245, 2011. doi: 10.1198/tas.2011.10115. [DOI] [Google Scholar]
- 59. Altman DG, Bland JM. Diagnostic tests 2: predictive values. BMJ 309: 102, 1994. doi: 10.1136/bmj.309.6947.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Altman DG, Bland JM. Diagnostic tests. 1: sensitivity and specificity. BMJ 308: 1552, 1994. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Statist Sci 16: 101–133, 2001. doi: 10.1214/ss/1009213286. [DOI] [Google Scholar]
- 62. Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression (3rd ed.). Wiley, 2013. [Google Scholar]
- 63. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44: 837–845, 1988. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 64. Youden WJ. Index for rating diagnostic tests. Cancer 3: 32–35, 1950. doi:. [DOI] [PubMed] [Google Scholar]
- 65. Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 329: 168–169, 2004. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, Humphreys BD, Koyner JL, Liu KD, Mour G, Nolin TD, Bihorac A; American Society of Nephrology Acute Kidney Injury Advisory Group. Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis 68: 19–28, 2016. doi: 10.1053/j.ajkd.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol (1985) 91: 1893–1901, 2001. doi: 10.1152/jappl.2001.91.4.1893. [DOI] [PubMed] [Google Scholar]
- 68. Feehally J, Khosravi M. Effects of acute and chronic hypohydration on kidney health and function. Nutr Rev 73: 110–119, 2015. doi: 10.1093/nutrit/nuv046. [DOI] [PubMed] [Google Scholar]
- 69. Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 9: 223–239, 2013. doi: 10.1038/nrneph.2013.22. [DOI] [PubMed] [Google Scholar]
- 70. Roncal-Jimenez C, Lanaspa MA, Jensen T, Sanchez-Lozada LG, Johnson RJ. Mechanisms by which dehydration may lead to chronic kidney disease. Ann Nutr Metab 66, Suppl 3: 10–13, 2015. doi: 10.1159/000381239. [DOI] [PubMed] [Google Scholar]
- 71. Giersch GEW, Charkoudian N, Stearns RL, Casa DJ. Fluid balance and hydration considerations for women: review and future directions. Sports Med 50: 253–261, 2020. doi: 10.1007/s40279-019-01206-6. [DOI] [PubMed] [Google Scholar]
- 72. Stachenfeld NS. Sex hormone effects on body fluid regulation. Exerc Sport Sci Rev 36: 152–159, 2008. doi: 10.1097/JES.0b013e31817be928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Viñas JL, Porter CJ, Douvris A, Spence M, Gutsol A, Zimpelmann JA, Tailor K, Campbell PA, Burns KD. Sex diversity in proximal tubule and endothelial gene expression in mice with ischemic acute kidney injury. Clin Sci (Lond) 134: 1887–1909, 2020. doi: 10.1042/CS20200168. [DOI] [PubMed] [Google Scholar]
- 74. Boddu R, Fan C, Rangarajan S, Sunil B, Bolisetty S, Curtis LM. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol Renal Physiol 313: F740–F755, 2017. doi: 10.1152/ajprenal.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oppliger RA, Magnes SA, Popowski LA, Gisolfi CV. Accuracy of urine specific gravity and osmolality as indicators of hydration status. Int J Sport Nutr Exerc Metab 15: 236–251, 2005. doi: 10.1123/ijsnem.15.3.236. [DOI] [PubMed] [Google Scholar]
- 76. Verney EB. The antidiuretic hormone and the factors which determine its release. Proc R Soc Lond B Biol Sci 135: 25–106, 1947. [PubMed] [Google Scholar]
- 77. Kasper AM, Crighton B, Langan-Evans C, Riley P, Sharma A, Close GL, Morton JP. Case study: extreme weight making causes relative energy deficiency, dehydration, and acute kidney injury in a male mixed martial arts athlete. Int J Sport Nutr Exerc Metab 29: 331–338, 2019. doi: 10.1123/ijsnem.2018-0029. [DOI] [PubMed] [Google Scholar]
- 78. García-Arroyo FE, Cristóbal M, Arellano-Buendía AS, Osorio H, Tapia E, Soto V, Madero M, Lanaspa MA, Roncal-Jiménez C, Bankir L, Johnson RJ, Sánchez-Lozada LG. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am J Physiol Regul Integr Comp Physiol 311: R57–R65, 2016. doi: 10.1152/ajpregu.00354.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Barnett AM, Babcock MC, Watso JC, Migdal KU, Gutiérrez OM, Farquhar WB, Robinson AT. High dietary salt intake increases urinary NGAL excretion and creatinine clearance in healthy young adults. Am J Physiol Renal Physiol 322: F392–F402, 2022. doi: 10.1152/ajprenal.00240.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: https://doi.org/10.5281/zenodo.7931178.
Data Availability Statement
Data will be made available upon reasonable request.