Abstract
Strain KD21T, isolated from the fecal sample of a healthy female volunteer, is a strictly anaerobic, non-motile, Gram-staining-positive, saccharolytic small rod that does not produce spores. Strain KD21T was able to grow in the range of temperature 28°C–37°C (optimum, 37 °C), pH 6.0–8.0 (optimum, pH 7.0), and with 0–5.0 g/l NaCl (optimum, 0 g/l NaCl). Bacteria cells reduced nitrates to nitrites. Its major fatty acids were C18:1ω9c, C16:0, C18:0, and summed in feature 8 (C18:1ω7c and/or C18:1ω6c). 16S rRNA gene phylogenetic analysis revealed that KD21T is a member of the genus Tractidigestivibacter and is distinct from any species with validly published names. The sequence showed 98.48% similarity with T. scatoligenes SK9K4T. The DNA G + C content of strain KD21T was 62.6 mol%. The DNA–DNA hybridization and OrthoANI values between strain KD21T and T. scatoligenes SK9K4T were 40.2% and 90.2%, respectively. Differences in phenotypic, phylogenetic, chemotaxonomic, and genomic characteristics indicated that strain KD21T represents a novel species within the genus Tractidigestivibacter. The name T. montrealensis sp. nov. is proposed and the type strain is KD21T (= CSUR Q8103T = DSM 115111T).
Keywords: culturomics, human feces, gut microbiota, Tractidigestivibacter montrealensis
We present a polyphasic taxonomic description of Tractidigestivibacter montrealensis sp. nov., a new member of human gut microbiota.
Abbreviations
- CSUR
Collection de souches de l'Unité des Rickettsies
- dDDH
digital DNA-DNA Hybridization
- DSM
Deutsche Sammlung von Mikroorganismen
- MALDI-TOF
Matrix-assisted laser desorption/ionization time-of-flight
- MS
Mass spectrometry
- OrthoANI
Orthologous average nucleoid identity
Introduction
Recently based on phenotypic, 16S rRNA gene- and core genome-based taxonomic classification, Zgheib and her colleagues proposed the reclassification of members of the genus Olsenella and the creation of Tractidigestivibacter scatoligenes (Zgheib et al. 2021) originally described as O. scatoligenes in 2015 by Li et al. (2015). The genus Tractidigestivibacter is classified in the phylum Actinomycetota within the Atopobiaceae family, which contains ten other validly published genera that have been recovered from various samples, including human gut microbiota (https://lpsn.dsmz.de/family/atopobiaceae). At the time of writing, T. scatoligenes represents the only validly published species assigned in this genus (https://lpsn.dsmz.de/species/tractidigestivibacter-scatoligenes). It is a strictly anaerobic non-motile, asporogenous, Gram-stain-positive saccharolytic coccobacillus isolated from pig feces.
In recent decades, the role played by the microbiota in human health has attracted increasing interest (Claesson et al. 2012, Milani et al. 2017, Imhann et al. 2018). Research in this field has led to a better understanding of how human gut microbiota interacts with the gastrointestinal tract and forms a dynamic and complex ecosystem that plays a crucial role in health and diseases (Schuijt et al. 2016, Routy et al. 2018, Tonneau et al. 2022). As part of our ongoing project on human gut microbiota in health and cancer patients using culturomics (Lagier et al. 2015, 2016), many previously unknown bacteria have been isolated. The 16S rRNA gene sequence similarity shows that one of these unknown isolates, designated KD21T, is affiliated with the genus Tractidigestivibacter. This study provides characterization and polyphasic taxonomic description, which combines phylogenetic analysis based on 16S rRNA gene sequence, determination of phenotypic characteristics, and genomic properties, of a new species named T. montrealensis. The type strain is KD21T, isolated from human feces.
Materials and methods
Sampling and bacterial strain isolation
Strain KD21T was isolated from a fecal sample of a 33 year-old healthy volunteer. The fecal sample was collected in February 2022, and signed informed consent was obtained before collecting the sample. She was not being treated with any medication within 6 months before sampling. The study was approved by the CHUM Research Ethics Committee under agreement number 20.300. To identify bacterial diversity, culturomics was performed as previously described (Lagier et al. 2012, Routy et al. 2022). The stool was pre-incubated at 37°C in an anaerobic culture bottle (BACTEC Lytic/10 Anaerobic/F Culture Vials) supplemented with 4 ml filter-sterilized rumen fluid and 5% sheep blood (Cedarlane Labs, Burlington, Canada). Strain KD21 was isolated after three days of pre-incubation. Bacterial colonies were purified and identified using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) with a Microflex LT spectrometer (Bruker, Daltonics, Germany) as previously described (Seng et al. 2009, 2013). Bacterial isolates were considered correctly identified at the species level for a score >2 and at the genus level for a score between 2 and 1.7. In contrast, for unidentified bacteria (score <1.7), 16S rRNA gene sequencing was performed.
16S rRNA gene sequence and phylogenetic analysis
As MALDI-TOF MS was unable to identify strain KD21T, the identification was carried out by sequencing its 16S rRNA gene as previously reported using fd1 and rp2 primers and a 3730xl DNA Analyzer from Applied BiosystemsTM (Technelysium Pty. Ltd) (Routy et al. 2022). Obtained 16S rRNA gene sequence was assembled and corrected using the ChromasPro software (http://technelysium.com.au/wp/chromaspro/). Phylogenetic neighbors of KD21T were identified using the BLASTn program (Altschul et al. 1997) and the nucleotide collection (nr/nt) of the NCBI database available at https://blast.ncbi.nlm.nih.gov/Blast.cgi. Based on the BLAST results, the 16S rRNA gene sequences of closest relatives with validly published were extracted from the GenBank database and aligned using the CLUSTALW tool (Thompson et al. 1994, Higgins et al. 1996) integrated into the MEGAX program (Kumar et al. 2018). Phylogenetic interferences were reconstructed using the neighbor-joining method (Saitou and Nei 1987) with bootstrap values of 500 replicates using MEGAX software (available at https://www.megasoftware.net/).
Morphologic and phenotypic characteristics
Strain KD21T cell morphology was assessed by transmission electron microscopic as previously reported (Routy et al. 2022). Gram-stain was assessed using the standard protocol. The bacterium motility was investigated using a Leica DM 1000 photonic microscope (Leica Microsystems) at 100 magnification. To test sporulation, a thermal shock at 80°C for 20 minutes of strain KD21T was performed. The growth temperature range was determined by culturing strain KD21T on Columbia agar and was incubated for 2 days at various temperatures (room, 28°C, 37°C, 42°C, and 56°C) under different atmospheres (anaerobic, microaerophilic, and aerobic conditions). The pH range growth was also tested at pH 5, 6, 6.5, 7, 7.5, and 8.5. Tolerance of NaCl was determined for concentrations ranked between 0 and 100 g/l. Catalase and oxidase productions were also detected (bioMérieux). Enzymatic and biochemical properties of strain KD21T were determined in duplicate using the API® ZYM, API® 20A, and Rapid ID 32A identification systems (bioMérieux). Short-chain fatty acids were analyzed using both a gas chromatograph (Hewlett-Packard) and Microbial Identification System software.
Whole-genome sequencing
Genomic DNA was sequenced using MiSeq Illumina. Libraries were generated using the NxSeq® AmpFREE Low DNA Library Kit Library Preparation Kit (Lucigen) according to the manufacturer’s recommendations, with 700 ng of genomic DNA as starting material. Dual-indexed adaptors were purchased from IDT. Libraries were quantified using the Kapa Illumina GA with Revised Primers-SYBR Fast Universal kit (Kapa Biosystems). The average size fragment was determined using a LabChip GX II (PerkinElmer) instrument. The libraries were normalized and pooled, denatured in 0.05 N NaOH, and neutralized using HT1 buffer. The pool was loaded at 225 p.m. on an Illumina NovaSeq S4 lane using the Xp protocol as per the manufacturer’s recommendations. The run was performed for 2 × 150 cycles (paired-end mode). A phiX library was used as a control and mixed with libraries at 1% level. Base calling was performed with RTA v3. Program bcl2fastq2 v2.20 was then used to demultiplex samples and generate fastq reads. Quality control checks on raw sequences were performed with the FastQC software v0.11.9 (Babraham Bioinformatics—FastQC A Quality Control Tool for High Throughput Sequence Data). Trimming and filtering were done through the Trimmomatic software v0.39 (Bolger et al. 2014) using the following settings: HEADCROP:10 ILLUMINACLIP: TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:25 MINLEN:100.
Genome annotation and comparison
The de novo genome assembly was made using the software SPAdes v3.15.2 (Bankevich et al. 2012) with the option –isolate. Final polishing of the draft genome assembly was performed with the Redundans software v0.13c (Pryszcz and Gabaldón 2016) for gap closing and overlapped scaffolds merging with the options –identity 1 –noreduction. Quality and completeness assessments of the genome assembly were made through BlobTools2 (Challis et al. 2020) and BUSCO v4 (Seppey et al. 2019). The bacterial proteome was predicted with the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) v5.2 (Li et al. 2021) and the Best-placed reference protein set (GeneMarkS-2+) annotation method. Predicted protein was compared to the clusters of orthologous groups (COG) database (Galperin et al. 2021) using the basic local alignment search tool for protein (BlastP) v2.11.0+ (Camacho et al. 2009) (E-value 1e-03, coverage 0.7, and identity percent 30). Then, the genome of strain KD21T was compared to those of type strains of phylogenetically related species by calculating two parameters: digital DNA–DNA hybridization (dDDH) and orthologous average nucleotide identity (OrthoANI) values using genome-to-genome distance calculator (GGDC; Auch et al. 2010) and the Orthologous ANI tool (OAT) software (Lee et al. 2016), respectively.
Results and discussion
Strain identification and phylogenetic analysis
Strain KD21T was isolated after three days of pre-incubation of the stool at 37°C in an anaerobic culture bottle supplemented with 4 ml filter-sterilized rumen fluid and 5% sheep blood. The MALDI-TOF MS assay showed that KD21T was not identified by any spectrum according to the Bruker database. However, the 16S rRNA gene sequence analysis revealed that strain KD21T (1449 bp, accession number OP557700) is a member of the family Atopobiaceae. A phylogenetic analysis based on partial 16S rRNA gene sequences using the neighbor-joining method shows that KD21T forms a cluster with T. scatoligenes strain SK9K4T, and this clade was sustained by a high bootstrap value of 100 % (Fig. 1). In addition, the NCBI nucleotide BLAST of this 16S rRNA gene sequence showed that this sequence was most closely related to T. scatoligenes strain SK9K4T (accession number JX905358.1), the type strain of phylogenetically closest species standing in nomenclature, with 98.48% similarity. As 16S rRNA sequence similarity is below the recommended threshold of 98.65% for species demarcation (Stackebrandt and Ebers 2006, Kim et al. 2014, Yarza et al. 2014), strain KD21T can be classified as a novel species of the genus Tractidigestivibacter.
Figure 1.
Neighbor-joining phylogenetic tree reconstructed using partial 16S rRNA gene sequences showing the phylogenetic relationship of strain KD21T and some related taxa. GenBank accession numbers are shown in parentheses. Sequences were aligned using Muscle v3.8.31 with default parameters, and the tree is the unrooted consensus of 500 bootstrap replicates using MEGAX software. The scale bar represents a 2% nucleotide sequence divergence.
Morphological, physiological, and biochemical features
Cells of strain KD21T were obligate anaerobic, Gram-stain-positive, shaped like coccobacilli, or small rods arranged singly, in pairs or chains. Single cells are 0.7–1.3 µm in length, and chains of cells are up to 2–5.5 µm long (Fig. S1). Colonies on Columbia plates, after 2 days of incubation at 37°C under anaerobic conditions, were 1.2–2.5 mm in diameter, circular and entire, convex, watery, opaque, and gray to off-white/gray. Strain KD21T grew at 28°C–37°C, with optimal growth at 37°C. No growth was observed at 25°C, 42°C, and 56°C. Strain KD21T grew at pH 6.0–8.0. The optimum pH was 7.0. The NaCl concentration request for growth was <5.0 g/l Strain KD21T was non-spore-forming, non-motile, and could produce oxidase but not catalase, indole, and urease. In addition, it hydrolyzed aesculin and did not liquefy gelatin. The other biochemical characteristics of KD21T are given in the description section, and features that differentiate it from its neighbors are presented in Table 1. The detailed cell membrane fatty acids profile of strain KD21T with those of its closest related species is shown in Table 2. The predominant cellular fatty acids (>10%) of strain KD21T were C18:1ω9c (30.38%), C16:0 (29.11%), C18:0 (13.72%), and summed in feature 8 (C18:1ω7c and/or C18:1ω6c; 10.01%). In contrast, C14 : 0 (25.9%), C18 : 1ω9c (25.70%), and summed feature 1 (C13 : 0 3OH and/or C15 : 1 iso H; 20.70%) were reported as main cellular fatty acids in T. scatoligenes (Li et al. 2015). However, the differences noted in the cellular fatty acid profile between studies may be due to differences in the bacterial culture conditions and the extraction method used.
Table 1.
Differential phenotypic features between strain KD21T and closest related species.
| Characteristic | 1 | 2* | 3* | 4* | 5* | 6* | 7* | 8* |
|---|---|---|---|---|---|---|---|---|
| Nitrate reduction | + | - | - | - | - | - | - | - |
| Gelatin digestion | - | - | - | + | - | + | - | - |
| Aesculin hydrolysis | + | + | + | + | + | - | v | + |
| Acid production from (API 20A) | ||||||||
| Glucose | + | + | - | + | + | + | - | + |
| Lactose | + | + | - | − | - | - | - | - |
| Mannose | + | + | + | + | + | + | - | + |
| Raffinose | - | - | - | - | - | - | - | + |
| Rhamnose | + | + | - | - | - | - | - | - |
| Sucrose | - | + | - | + | + | - | - | na |
| Salicin | + | + | - | + | - | - | - | + |
| Cellobiose | + | + | - | - | - | - | - | + |
| Trehalose | - | - | - | + | - | - | - | - |
| Enzyme activity (Rapid ID 32A) | ||||||||
| Arginine dihydrolase | - | - | - | + | + | - | - | + |
| Arginine arylamidase | - | + | - | + | + | + | + | + |
| α-Galactosidase | w | - | - | - | - | - | - | + |
| β-Galactosidase | - | + | - | - | - | - | - | - |
| Enzyme activity (API ZYM) | ||||||||
| Alkaline phosphatase | + | + | + | - | - | - | w | + |
| Esterase (C4) | - | - | - | - | - | - | + | - |
| Esterase lipase (C8) | + | + | - | - | - | - | + | - |
| α -Glucosidase | - | + | - | - | - | - | - | + |
| β-Glucosidase | + | + | + | + | - | - | - | + |
| Naphthol-AS-BI-phosphohydrolase | + | - | + | - | + | + | + | + |
1. T. montrealensis KD21T; 2. T. scatoligenes SK9K4T (Li et al. 2015); 3. Paratractidigestivibacter faecalis KCTC 15699T (Han et al. 2019); 4. O. uli DSM 7084T (Olsen et al. 1991, Dewhirst et al. 2001); 5. O. phocaeensis Marseille-P2936T (Zgheib et al. 2021); 6. P. catena JCM 31932T (Sakamoto et al. 2018); 7. P. massiliensis JCM 33000T (Sakamoto et al. 2019); and 8. Thermophilibacter provencensis Marseille-P2912T (Zgheib et al. 2021).
+, Positive; -, negative; na, no available data; v, variable; and w, weakly. *Data were obtained from the original descriptions of species.
Table 2.
Cellular fatty acid composition (%) of strain KD21T compared to those of related species.
| Fatty acid (%) | 1 | 2* | 3* | 4* | 5* | 6* | 7* | 8* | 9* |
|---|---|---|---|---|---|---|---|---|---|
| Saturated straight-chain | |||||||||
| C9:0 | TR | - | 1.4 | - | - | - | - | - | - |
| C10:0 | TR | - | - | - | 1.2 | - | - | - | - |
| C10:0 FAME | - | - | - | 10.1 | - | - | - | - | - |
| C12:0 | TR | TR | - | - | 1.2 | 3.4 | - | 6.5 | 4.7 |
| C12:0 FAME | - | - | - | 2.6 | - | - | - | - | - |
| C12:0 DMA | - | - | - | - | - | - | - | - | 2.6 |
| C14:0 | 3.1 | 25.9 | 5.2 | - | 7.0 | 7.3 | 9.7 | 54.8 | 33.8 |
| C14:0 DMA | - | - | - | - | - | - | - | - | 30.3 |
| C14:0 ALDEHYDE | - | - | - | - | - | - | - | - | 11.6 |
| C14:0 ALCOHOL | - | - | - | - | - | - | - | - | 2.5 |
| C14:0 FAME | - | - | - | 3.1 | - | - | - | - | - |
| C16:0 | 29.1 | 2.7 | 16.2 | - | 23.0 | 6.8 | 9.4 | 19.8 | - |
| C16:0 ALDEHYDE | - | - | 1.6 | 1.3 | - | - | - | - | - |
| C16:0 DMA | - | - | 6.0 | 4.0 | - | 12.0 | 9.2 | - | 4.3 |
| C16:0 FAME | - | - | - | 4.8 | - | - | - | - | - |
| C17:0 | 1.2 | - | - | - | TR | - | - | - | - |
| C18:0 | 13.7 | TR | 5.0 | - | 14.2 | 5.7 | - | 7.2 | - |
| C18:0 DMA | - | - | 1.2 | TR | - | 3.7 | - | - | - |
| C18:0 FAME | - | - | 1.0 | - | - | - | - | - | |
| C17:2 | - | - | 5.4 | - | - | - | - | - | - |
| Unsaturated straight-chain | |||||||||
| C13:1 AT12-13 | - | 6.6 | - | - | - | - | - | - | - |
| C16:1ω9C | TR | - | 1.3 | - | - | - | - | - | - |
| C16:1ω9C DMA | - | - | - | 2.8 | - | - | - | - | - |
| C16:1ω9C FAME | - | - | - | 1.3 | - | - | - | - | - |
| C17:1ω8C | - | - | - | - | - | 3.9 | - | - | - |
| C17:1ω8C FAME | - | - | - | 6.9 | - | - | - | - | - |
| C18:1ω7 | - | - | - | - | 1.1 | - | - | - | |
| C18:2ω6C | - | - | - | - | 14.9 | - | - | 3.7 | - |
| C18:1ω9C | 30.3 | 25.7 | 23.3 | - | 35.3 | 39.3 | 50.0 | 7.7 | - |
| C18:1ω9C DMA | - | - | 21.0 | 19.9 | - | 26.7 | 20.3 | - | - |
| C18:1ω9C FAME | - | - | - | 32.1 | - | - | - | - | - |
| C18:1ω11C DMA | - | - | 3.4 | 3.6 | - | - | - | - | - |
| C18:1ω11C | - | - | 4.9 | - | - | - | - | - | - |
| C20:1ω11C | - | - | 4.3 | - | - | - | - | - | - |
| C18:2ω7C | - | 3.1 | - | - | - | - | - | - | - |
| C17:1 iso | - | 7.7 | - | - | - | - | - | - | - |
| Hydroxy acids | |||||||||
| C17:0 2-OH | 3.4 | - | - | - | - | - | - | - | - |
| Summed features* | |||||||||
| 1 | TR | 20.7 | - | - | - | - | - | - | - |
| 2 | - | 2.2 | - | - | - | - | - | - | - |
| 3 | 2.6 | 1.0 | - | - | - | - | - | - | - |
| 4 | - | 7.7 | - | - | - | - | - | - | - |
| 6 | 0.2 | - | - | - | - | - | - | - | - |
| 7 | - | - | 5.4 | 6.9 | - | 3.9 | - | - | - |
| 8 | 10.0 | - | - | - | - | - | - | - | - |
| 10 | - | - | 4.9 | 5.3 | - | - | - | - | - |
1. T. montrealensis KD21T; 2. T. scatoligenes SK9K4T (Li et al. 2015); 3. Paratractidigestivibacter faecalis KCTC 15699T (Han et al. 2019); 4. O. uli DSM 7084T (Olsen et al. 1991, Dewhirst et al. 2001); 5. O. phocaeensis Marseille-P2936T (Zgheib et al. 2021); 6. P. catena JCM 31932T (Sakamoto et al. 2018); 7. P. massiliensis Marseille-P3237T (Sakamoto et al. 2019); 8. Thermophilibacter provencensis Marseille-P2912T (Zgheib et al. 2021); and 9, Thermophilibacter gallinarum DSM 107455T (Zenner et al. 2021).
Major components (>10%) are highlighted in bold; TR, trace amounts <1%; and -, not detected. *Data were obtained from the original descriptions of species.
*Summed features represent groups of two or three fatty acids that could not be separated by the microbial identification system. Summed feature 1 comprises C13 : 0 3OH and/or C15 : 1 iso H; summed feature 2 comprises C14 : 03OH and/or C16 : 1iso I and/or unknown ECL 10.928; summed feature 3 comprises C16 : 1ω6c and/or C16 : 1ω7c; summed feature 4 comprises iso-C17 : 1 and/or anteiso-C17 : 1; summed feature 6 comprises C19:1ω9c/C19:1ω11c; summed feature 7 comprised C17 : 2 and/or C17 : 1ω8; summed feature 8 comprises C18:1ω6c and/or C18:1ω7c; and summed feature 10 comprised C18 : 1 cis11/trans9/tran s6 and/or unknown 17.834.
Genome characteristic and comparison
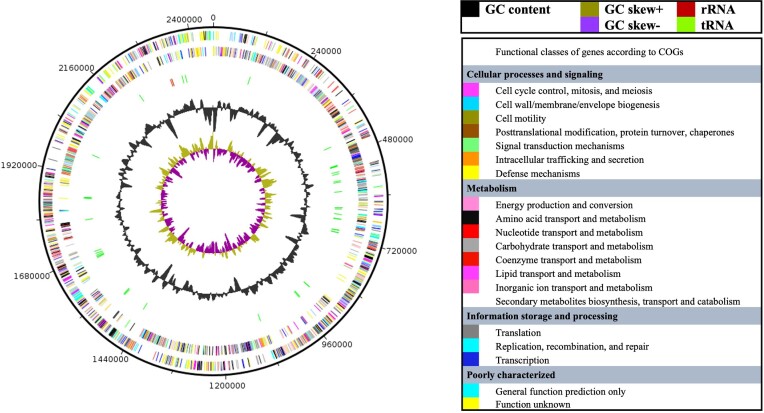
The draft genome of strain KD21T (accession number JANSKA000000000.1) resulted in 12 scaffolds distributed into 12 contigs. Its size was 2456 124 bp long (Table S1, Fig. 2). A total of 2171 genes was predicted with 2099 protein-coding genes. Fifty-two RNAs genes were identified, including one 5S rRNA, one 16S rRNA, one 23S rRNA, 46 tRNAs, and 3 ncRNAs genes (Table S1). The repartition of genes into the 25 general COG categories was summarized in Table S2. The genome of strain KD21T was compared to those of its closest neighbors in Fig. 3, Fig. S2, and Table 3.
Figure 2.
Graphical circular map of the genome of strain KD21T. From the outside to the center: Genes on the forward strand colored by COG of proteins categories (only genes assigned to COG), genes on the reverse strand colored by COG categories (only genes assigned to COG), RNA genes (tRNAs green and rRNAs red), GC content (dark), and GC skew (negative values purple and positive values olive green).
Figure 3.
Heatmap generated with OAT software indicating the OrthoANI values between Tactidigestivibacter montrealensis and other closely related species with standing in nomenclature. Values bigger than 96% indicate that strains belong to the same species. The results between the two strains are given in the junction point of the diagonals departing from each strain, i.e. the OrthoANI value between T. montrealensis KD21T and T. scatoligenes SK9K4T is 90.1%. (1-column fitting image).
Table 3.
dDDH values obtained by comparison of all studied genomes Using GGDC, Formula 2 (DDH Estimates Based on Identities/HSP length).
| T. montrealensis | T. scatoligenes | P. faecalis | O. uli | O. phocaeensis | P. catena | P. massiliensis | T. provencensis | T. gallinarum | |
|---|---|---|---|---|---|---|---|---|---|
| T. montrealensis | 100.0 ± 00 | 40.2 ± 2.5 | 21.8 ± 2.3 | 20.6 ± 2.3 | 21.3 ± 2.3 | 20.7 ± 2.3 | 20.3 ± 2.3 | 21.1 ± 2.3 | 21.3 ± 2.3 |
| T. scatoligenes | 100.0 ± 00 | 21.4 ± 2.3 | 21.0 ± 2.3 | 21.6 ± 2.3 | 20.3 ± 2.3 | 20.3 ± 2.3 | 20.9 ± 2.3 | 21.2 ± 2.3 | |
| Paratractidigestivibacter faecalis | 100.0 ± 00 | 20.4 ± 2.3 | 21.8 ± 2.3 | 23.3 ± 2.3 | 22.5 ± 2.3 | 22.60 ± 2.3 | 22.4 ± 2.3 | ||
| O. uli | 100.0 ± 00 | 23.1 ± 2.3 | 20.2 ± 2.3 | 19.9 ± 2.3 | 21.3 ± 2.3 | 20.7 ± 2.3 | |||
| O. phocaeensis | 100.0 ± 00 | 21.1 ± 2.3 | 21.4 ± 2.3 | 21.7 ± 2.3 | 21.5 ± 2.3 | ||||
| P. catena | 100.0 ± 00 | 32.6 ± 2.5 | 22.3 ± 2.4 | 21.5 ± 2.3 | |||||
| P. massiliensis | 100.0 ± 00 | 22.4 ± 2.4 | 20.8 ± 2.3 | ||||||
| Thermophilibacter provencensis | 100.0 ± 00 | 28.4 ± 2.4 | |||||||
| Thermophilibacter gallinarum | 100.0 ± 00 |
The calculated genomic DNA G + C content (62.6 mol%) was almost the same as that reported for T. scatoligenes (62.4 mol%) and in the same range (64.7–68 mol%) of those of other closest related species (Table S1). The digital DDH between compared species showed very low levels of genome-sequence-based similarity ranging from 20.3% between KD21T and Parolsenella massiliensis to 40.2% between KD21T and T. scatoligenes (Table 3). In addition, OrthoANI analysis of strain KD21T also exhibited low values of overall genome relatedness ranking from 72.9% with P. massiliensis to 90.2% with T. scatoligenes (Fig. 3). These dDDH and OrthoANI values were much lower than the limit set of 70% (Auch et al. 2010, Meier-Kolthoff et al. 2013) and 95% –96% (Lee et al. 2016), respectively, proposed for the definition of distinct species support that strain KD21T is sufficiently different to T. scatoligenes and its other closest related species.
Conclusion
The low similarity values in 16S rRNA gene sequences (98.5 %), DDH values (≤40.2%), and OrthoANI levels (≤90.2%) coupled with differences in biochemical, physiological as well as chemotaxonomic features clearly prove that strain KD21T deserves to be recognized as the type strain of a novel species within the genus Tractidigestivibacter, for which the name T. montrealensis sp. nov., is proposed.
Description of Tractidigestivibacter montrealensis sp. nov.
T. montrealensis (mont.re.al.en'sis. N.L. masc. adj. montrealensis, pertaining to Montreal, the city where the type of strain was first isolated).
Cells are strictly anaerobic, non-motile, non-spore-forming, and Gram-staining-positive. Cell morphology varies from spherical with ∼0.7 µm in diameter to small rods that are ∼0.7 µm in wide and 1.3 µm long. Cells are arranged singly, in pairs, or in chains. Growth occurs at 28°C–37°C (optimum 37°C), at pH 6–8 (optimum pH 7.0), and in the presence of NaCl concentration <5 g/l. After anaerobic incubation on 5% sheep-blood enriched Columbia agar for 2 days, colonies are 1.2–2.5 mm in diameter, circular and entire, convex, watery, opaque, and gray to off-white/gray. Oxidase, but not catalase is detected. In API 20A, indole is not formed. Urease is not produced. Aesculin is hydrolyzed, and gelatin is not digested. Acid is formed from cellobiose, glucose, lactose, maltose, mannose, rhamnose, and salicin but not from arabinose, glycerol, mannitol, melezitose, raffinose, sucrose, sorbitol, trehalose, and xylose. Using rapid ID 32A, mannose and raffinose are fermented, indole is not formed, and nitrate is reduced to nitrites. β-glucosidase, alkaline phosphatase, proline-, phenylalanine-, leucine-, tyrosine-, alanine-, glycine-, histidine-, glutamyl acid glutamic- and serine arylamidase are detected but β-galactosidase and α-glucosidase are weakly positive. Urease, arginine dihydrolase, α-galactosidase, α-galactosidase-6-phosphatase, β-glucosidase, α-arabinosidase, β-glucuronidase, N-acetyl-β-glucosaminidase, α-fucosidase, arginine-, and leucyl glycine arylamidase are not detected. In Api Zym, activity is detected for alkaline phosphatase, esterase lipase, leucine arylamidase, valine arylamidase, cysteine arylamidase, α-chymotrypsin, acid phosphatase, naphthol-AS-BI-phosphohydrolase, β-galactosidase, and glucosidase (α and β). No activity is detected for esterase, lipase, trypsin, α-galactosidase, β-glucuronidase, α-fucosidase and α-mannosidase. Major cell membrane fatty acids are C18:1ω9c, C16:0, and C18:0. Summed in feature 8 composed with C18:1ω7c and/or C18:1ω6c are also detected in high quantity.
The type strain is KD21T (= CSUR Q8103T = DSM 115111T), which was isolated from the feces of a healthy volunteer. The G + C content is 62.6 mol% as calculated from the assembly of the draft genome. The whole genome of T. montrealensis KD21T has been deposited in the NCBI GenBank database under the accession number JANSKA000000000.1. The GenBank accession number of the 16S rRNA gene sequence of strain KD21T is OP557700.
Supplementary Material
Acknowledgements
The authors are grateful to CRCHUM, Montreal Cancer Institute (ICM), and Terry Fox Research Institute (TFRI) Montreal Cancer Consortium pilot project (TFRI—Grant #1084).
Contributor Information
Myriam Benlaïfaoui, Laboratory of Immunotherapy and Onco-microbiome, University of Montreal Research Center (CRCHUM), Montreal, H2X 0A9, QC, Canada.
Corentin Richard, Laboratory of Immunotherapy and Onco-microbiome, University of Montreal Research Center (CRCHUM), Montreal, H2X 0A9, QC, Canada.
Awa Diop, Department of Biology, University of North Carolina Greensboro, 321 Mclver Street, Greensboro, NC, United States.
Sabrine Naimi, Laboratory of Immunotherapy and Onco-microbiome, University of Montreal Research Center (CRCHUM), Montreal, H2X 0A9, QC, Canada.
Wiam Belkaid, Laboratory of Immunotherapy and Onco-microbiome, University of Montreal Research Center (CRCHUM), Montreal, H2X 0A9, QC, Canada.
Eve Bernet, INRS-Centre Armand-Frappier Santé Biotechnologie, Bacterial Symbionts Evolution, Laval, QC H7V 1B7, QC, Canada.
Frederic Veyrier, INRS-Centre Armand-Frappier Santé Biotechnologie, Bacterial Symbionts Evolution, Laval, QC H7V 1B7, QC, Canada.
Arielle Elkrief, Laboratory of Immunotherapy and Onco-microbiome, University of Montreal Research Center (CRCHUM), Montreal, H2X 0A9, QC, Canada.
Louis-Marie Bobay, Department of Biology, University of North Carolina Greensboro, 321 Mclver Street, Greensboro, NC, United States.
Bertrand Routy, Laboratory of Immunotherapy and Onco-microbiome, University of Montreal Research Center (CRCHUM), Montreal, H2X 0A9, QC, Canada; Hematology-Oncology Service, Department of Medicine, University of Montreal Healthcare Centre (CHUM), Montreal, H2X 0A9, QC, Canada.
Khoudia Diop, Laboratory of Immunotherapy and Onco-microbiome, University of Montreal Research Center (CRCHUM), Montreal, H2X 0A9, QC, Canada.
Author contributions
Conceptualization: K.D. Methodology: B.R. and K.D. Validation: B.R. and K.D. Investigation: M.B., A.D., S.N., E.B., F.V., and K.D. Data Curation: A.D. and K.D. Formal Analysis: C.R., A.D., and K.D. Visualization: C.R., A.D., and K.D. Writing and original Draft Preparation: M.B., C.R., and K.D. Writing—Review & Editing: A.D., W.B., L.M.B., B.R., and K.D. Supervision: B.R. and K.D. Funding acquisition: K.D. and B.R. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
The study is funded by the Canadian Institutes of Health Research (CIHR) received by Bertrand Routy and the ‘Fonds de recherche du Québec–Santé’ received by Khoudia Diop, under agreements 420356 and 284894, respectively. Awa Diop and Louis M Bobay were supported by the National Science Foundation NSF grant DEB-1831730.
References
- Altschul SF, Madden TL, Schäffer AAet al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auch AF, von Jan M, Klenk H-Pet al. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci. 2010;2:117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A, Nurk S, Antipov Det al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Syst Biol. 2012;19:455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan Vet al. BLAST+: architecture and applications. BMC Bioinf. 2009;10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis R, Richards E, Rajan Jet al. BlobToolKit—interactive quality assessment of genome assemblies. G3 (Bethesda). 2020;10:1361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde Set al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- Dewhirst FE, Paster BJ, Tzellas Net al. Characterization of novel human oral isolates and cloned 16S rDNA sequences that fall in the family Coriobacteriaceae: description of Olsenella gen. nov., reclassification of Lactobacillus uli as Olsenella uli comb. nov. and description of Olsenella profusa sp. nov. Int J Syst Evol Microbiol. 2001;51:1797–804. [DOI] [PubMed] [Google Scholar]
- Galperin MY, Wolf YI, Makarova KSet al. COG database update: focus on microbial diversity, model organisms, and widespread pathogens. Nucleic Acids Res. 2021;49:D274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KI, Lee KC, Eom MK. et al. Olsenella faecalis sp. nov., an anaerobic actinobacterium isolated from human faeces. Int J Syst Evol Microbiol. 2019;69:2323–8. [DOI] [PubMed] [Google Scholar]
- Higgins DG, Thompson JD, Gibson TJ. Using CLUSTAL for multiple sequence alignments. Meth Enzymol. 1996;266:383–402. [DOI] [PubMed] [Google Scholar]
- Imhann F, Vich Vila A, Bonder MJet al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Oh HS, Park SCet al. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014;64:346–51. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li Met al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier JC, Armougom F, Million Met al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012;18:1185–93. [DOI] [PubMed] [Google Scholar]
- Lagier JC, Hugon P, Khelaifia Set al. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbio Rev. 2015;28:237–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier JC, Khelaifia S, Alou MTet al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee I, Ouk Kim Y, Park SCet al. OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 2016;66:1100–3. [DOI] [PubMed] [Google Scholar]
- Li W, O'Neill KR, Haft DHet al. RefSeq: expanding the prokaryotic genome annotation pipeline reach with protein family model curation. Nucleic Acids Res. 2021;49:D1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jensen RL, Højberg O. et al. Olsenella scatoligenes sp. nov., a 3-methylindole- (skatole) and 4-methylphenol- (p-cresol) producing bacterium isolated from pig faeces. Int J Syst Evol Microbiol. 2015;65:1227–33. [DOI] [PubMed] [Google Scholar]
- Meier-Kolthoff JP, Göker M, Spröer Cet al. When should a DDH experiment be mandatory in microbial taxonomy?. Arch Microbiol. 2013;195:413–8. [DOI] [PubMed] [Google Scholar]
- Milani C, Duranti S, Bottacini Fet al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81:e00036–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I, Johnson JL, Moore LVet al. Lactobacillus uli sp. nov. and Lactobacillus rimae sp. nov. from the human gingival crevice and emended descriptions of Lactobacillus minutus and Streptococcus parvulus. Int J Syst Bacteriol. 1991;41:261–6. [DOI] [PubMed] [Google Scholar]
- Pryszcz LP, Gabaldón T. Redundans: an assembly pipeline for highly heterozygous genomes. Nucleic Acids Res. 2016;44:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa Let al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- Routy B, Richard C, Benlaïfaoui Met al. Characterization of Alistipes montrealensis sp. nov., isolated from human feces of a patient with metastatic melanoma treated with immune checkpoint inhibitors. Microbiol Res. 2022;13:140–51. [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Iino T, Hamada M. et al. Parolsenella catena gen. nov., sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2018;68:1165–72. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Ikeyama N, Murakami Tet al. Comparative genomics of Parolsenella catena and Libanicoccus massiliensis: reclassification of Libanicoccus massiliensis as Parolsenella massiliensis comb. nov. Int J Syst Evol Microbiol. 2019;69:1123–9. [DOI] [PubMed] [Google Scholar]
- Schuijt TJ, Lankelma JM, Scicluna BPet al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng P, Abat C, Rolain JMet al. Identification of rare pathogenic bacteria in a clinical microbiology laboratory: impact of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2013;51:2182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng P, Drancourt M, Gouriet Fet al. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543–51. [DOI] [PubMed] [Google Scholar]
- Seppey M, Manni M, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness. Methods Mol Biol. 2019;1962:227–45. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today. 2006;33:152. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonneau M, Nolin-Lapalme A, Kazandjian S. et al. Helicobacter pylori serology is associated with worse overall survival in patients with melanoma treated with immune checkpoint inhibitors. Oncoimmunology. 2022;11:2096535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarza P, Yilmaz P, Pruesse Eet al. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol. 2014;12:635–45. [DOI] [PubMed] [Google Scholar]
- Zenner C, Hitch TCA, Riedel Tet al. Early-life immune system maturation in chickens using a synthetic community of cultured gut bacteria. Msystems. 2021;6:e01300–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgheib R, Anani H, Meng MMet al. New human-associated species of the family Atopobiaceae and proposal to reclassify members of the genus Olsenella. Int J Syst Evol Microbiol. 2021;71:4819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.