Abstract
The MADS domain transcription factor AGAMOUS (AG) regulates floral meristem termination by preventing maintenance of the histone modification lysine 27 of histone H3 (H3K27me3) along the KNUCKLES (KNU) coding sequence. At 2 d after AG binding, cell division has diluted the repressive mark H3K27me3, allowing activation of KNU transcription prior to floral meristem termination. However, how many other downstream genes are temporally regulated by this intrinsic epigenetic timer and what their functions are remain unknown. Here, we identify direct AG targets regulated through cell cycle–coupled H3K27me3 dilution in Arabidopsis thaliana. Expression of the targets KNU, AT HOOK MOTIF NUCLEAR LOCALIZED PROTEIN18 (AHL18), and PLATZ10 occurred later in plants with longer H3K27me3-marked regions. We established a mathematical model to predict timing of gene expression and manipulated temporal gene expression using the H3K27me3-marked del region from the KNU coding sequence. Increasing the number of del copies delayed and reduced KNU expression in a polycomb repressive complex 2– and cell cycle–dependent manner. Furthermore, AHL18 was specifically expressed in stamens and caused developmental defects when misexpressed. Finally, AHL18 bound to genes important for stamen growth. Our results suggest that AG controls the timing of expression of various target genes via cell cycle–coupled dilution of H3K27me3 for proper floral meristem termination and stamen development.
Cell cycle–coupled H3K27me3 dilution at various target loci regulates floral development.
IN A NUTSHELL.
Background: For flowers to form, the floral meristem (floral stem cells) must irreversibly commit to becoming cells making up the various floral organs (sepals, petals, stamens, and carpels), a process known as floral meristem termination. Proper timing of floral meristem termination involves temporal activation of the transcription factor gene KNUCKLES (KNU) by its upstream regulator AGAMOUS (AG) via cell cycle–dependent dilution of the repressive histone modification at lysine 27 of histone H3 (H3K27me3) along the KNU coding sequence. This intrinsic “biotimer” will activate KNU at precisely the right time to ensure proper flower development.
Question: Are there other genes similarly regulated by AG during flower development making up a biotimer transcriptional regulatory network, and can we manipulate KNU gene activation timing based on the set of criteria that defines the biotimer mechanism?
Findings: Using the model plant Arabidopsis thaliana, we found that the genes AT HOOK MOTIF NUCLEAR LOCALIZED PROTEIN18 (AHL18) and PLATZ10 are direct biotimer-regulated AG targets among a set of 23 biotimer candidate genes (including KNU). AHL18 and PLATZ10 are likely involved during stamen development, specifically for proper stamen elongation and maturation, respectively. We also introduced a simple mathematical model correlating the length of H3K27me3-marked regions at biotimer genes with gene activation timing and correctly predicted the timing of activation for KNU, AHL18, and PZ10. We validated the model's predictions experimentally by modifying KNU gene length with tandem repeats of a H3K27me3-dense region in KNU's coding sequence named del resulting in delayed and reduced KNU expression in a PRC2- and cell cycle–dependent manner.
Next steps: Our current work provides insight into epigenetic approaches for tunable gene expression and provides a mechanistic framework to understand which aspects of transcriptional regulatory systems can be effectively manipulated for future work aimed at enhancing plant productivity and resilience.
Introduction
Plants continuously produce new organs throughout their life via groups of undifferentiated cells called meristems whose activity is dynamically regulated to balance cell proliferation and differentiation (Sablowski 2007). In Arabidopsis (Arabidopsis thaliana), floral meristems (FMs) form a fixed number of floral organs (Liu et al. 2011). At early stages, FMs proliferate to produce cells. At later stages of FM growth, the balance shifts toward cell differentiation to irreversibly terminate cell proliferation (Sun and Ito 2015; Shang et al. 2019; Xu et al. 2019). Proper timing of this developmental shift, designated FM termination, fixes the number of floral organs.
The MADS domain transcription factor AGAMOUS (AG) is the central regulator of FM termination (Yanofsky et al. 1990; Mayer et al. 1998; Lenhard et al. 2001; Lohmann et al. 2001; Pelayo et al. 2021). AG represses WUSCHEL (WUS), which maintains the stem cell population in FMs. AG (as a tetramer with SEPALLATA [SEP] proteins) can directly and indirectly repress WUS by recruiting polycomb repressive complex 2 (PRC2) to WUS (Liu et al. 2011) and indirectly via the 2 AG downstream targets CRABS CLAW (CRC, encoding a YABBY transcription factor) and KNUCKLES (KNU, encoding a C2H2-type zinc-finger protein). CRC regulates auxin homeostasis for proper FM termination and gynoecium formation through its targets TORNADO2 (TRN2) and YUCCA4 (YUC4), encoding a putative auxin transporter from the tetraspanin transmembrane protein family and an auxin biosynthetic enzyme, respectively (Yamaguchi et al. 2017, 2018).
AG also activates KNU, whose encoded protein transcriptionally represses WUS (Payne et al. 2004; Sun et al. 2009, 2014; Liu et al. 2011; Yamaguchi et al. 2017; Bollier et al. 2018; Sun et al. 2019; Huang et al. 2021; Kwaśniewska et al. 2021; Shang et al. 2021). The transcriptional activation of KNU shows a 2-d delay after AG recruitment to the KNU promoter (Sun et al. 2009). At stage 2 of flower development, KNU expression is initially repressed by PRC2, which establishes and maintains repressive trimethylation at lysine 27 of histone H3 (H3K27me3) along the KNU coding sequence (Sun et al. 2009, 2014; Ikeuchi et al. 2015). By floral stage 3, AG accumulates and displaces PRC2 from the KNU promoter, which decreases H3K27me3 deposition mediated by PRC2, thereby lowering H3K27me3 levels in a cell cycle–dependent manner. KNU transcription is activated after 2 d of cell divisions, just before FM termination (Sun et al. 2014). This intrinsic timing mechanism (referred to here as a “biotimer”) is essential for proper flower development, as altering temporal KNU expression results in either premature FM termination or indeterminacy (Sun et al. 2009; Yamaguchi 2022).
Although AG has about 2,000 known target genes, KNU is the only such target known to be regulated by the AG biotimer (Ó Maoiléidigh et al. 2013). Other AG targets associated with flower development and with regulatory roles include GIANT KILLER (GIK) and STYLISH1 (STY1) for proper carpel development by regulating auxin homeostasis as well as SPOROCYTLESS (SPL, also named NOZZLE [NZZ]) and DEFECTIVE ANTHER DEHISCIENCE1 (DAD1) for proper stamen development (Kuusk et al. 2002; Ito et al. 2004, 2007; Sohlberg et al. 2006; Ng et al. 2009; Ó Maoiléidigh et al. 2013). Many direct AG targets also remain uncharacterized. INDETERMINATE DOMAIN12 (IDD12) is associated with gibberellic acid (GA) biosynthesis and response. AT HOOK MOTIF NUCLEAR LOCALIZED PROTEIN (AHL18) is involved in regulating root system architecture (Širl et al. 2020). PLATZ10 encodes a member of a transcription factor class of plant-specific zinc-dependent DNA-binding proteins (Nagano et al. 2001; Sohlberg et al. 2006; Aoyanagi et al. 2020). How these genes are regulated by AG remains largely unknown.
PRC2 and other polycomb group proteins establish repressive H3K27me3 marks at target genes that result in transient and heritable silencing by chromatin compaction (Xiao et al. 2017; Yoon et al. 2018). In such cases, H3K27me3 is heritable across cell divisions and yet also responds dynamically to intracellular and extracellular cues (Eccleston et al. 2013). In plants, PRC2 regulates developmental transitions and cell type specification (Bemer and Grossniklaus 2012; Mozgova et al. 2015). Arabidopsis PRC2 forms specific protein complexes with different functions over a particular developmental phase (Bemer and Grossniklaus 2012; Kim and Sung 2014; Mozgova et al. 2015; Mozgova and Hennig 2015). For example, flower development is largely associated with the EMF2-PRC2 variant composed of CURLY LEAF (CLF, and its homolog SWINGER [SWN]), EMBRYONIC FLOWER2 (EMF2), FERTILIZATION-INDEPENDENT ENDOSPERM (FIE), and MULTICOPY SUPPRESSOR OF IRA1 (MSI1) (Bemer and Grossniklaus 2012).
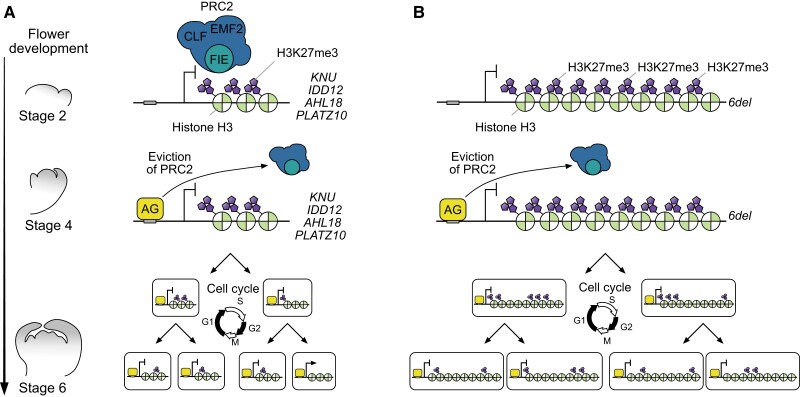
Although PRC2 function has been described in detail, questions remain regarding how it drives proper temporal transitions during development. Various models have been postulated for the stable propagation of H3K27me3 marks over generations and for their removal for activating gene expression (Alabert et al. 2015; Ramachandran and Henikoff 2015; Lai and Pugh 2017). Modes of chromatin reprogramming upon H3K27me3 removal can be controlled actively through histone demethylases, as well as passively when PRC2 is inactive at target loci (Bogliotti and Ross 2012). KNU transcriptional activation relies on cell division–dependent H3K27me3 removal, ensuring the proper timing of KNU expression and FM termination (Sun et al. 2014). We previously showed that del, a 231-bp region in the KNU coding sequence, also plays a role in proper KNU temporal activation. del is a H3K27me3-dense region bound directly by FIE, whose deletion causes early and ectopic KNU expression (Sun et al. 2009), suggesting that it is required for KNU repression through H3K27me3 deposition.
The transition from floral indeterminacy to determinacy signals the onset of cell differentiation and the cessation of cell proliferation, suggesting that FM cells become committed to a differentiation pathway (Zik and Irish 2003). Cell differentiation programs are tightly linked to cell cycle regulation to establish tissues and organs (Di Mambro et al. 2017). Cell cycle progression is driven mainly by the interaction of cyclins (CYCs) and cyclin-dependent kinases (CDKs), a class of Ser/Thr kinases (Harashima and Sekine 2020). CYCs and CDKs form complexes that phosphorylate various target proteins at specific phases of the cell cycle, namely, gap 1 (G1), synthesis (S), gap 2 (G2), and mitosis (M).
Among CDKs, B-type CDKs (CDKBs) are plant specific and are thought to be associated solely with cell cycle regulation (Harashima and Sekine 2020). CDKBs comprise 2 subgroups, CDKB1 and CDKB2, each represented by 2 members (CDKB1;1 and CDKB1;2, and CDKB2;1 and CDKB2;2). CDKB1 expression is associated with S, G2, and M phases, while CDKB2 expression is associated with G2 and M phases. CDKB activity is a marker for G2/M progression.
The action of CYC/CDK complexes can be regulated by CYC/CDK inhibitors (CKIs) (Kumar and Larkin 2017; Harashima and Sekine 2020), which may belong to 1 of 2 families: INTERACTOR/INHIBITOR OF CDK/KIP-RELATED PROTEINs (ICK/KRPs) and SIAMESE-RELATED PROTEINs (SMRs). ICK/KRPs are encoded by 7 genes in Arabidopsis, KRP1 (ICK1), KRP2 (ICK2), KRP3 (ICK6), KRP4 (ICK7), KRP5 (ICK3), KRP6 (ICK4), and KRP7 (ICK5), which are distantly related to the CKI p27Kip1, a member of the mammalian Kip/Cip CKI family (Veylder et al. 2001). KRPs function in a dose-dependent manner and inhibit CDKA1 during the G1/S phase (Kumar and Larkin 2017). The SMR family consists of 17 members in Arabidopsis; SIAMESE (SIM) was initially identified during a mutant screen for aberrant trichome formation in which trichomes underwent cell division instead of endoreduplication (Churchman et al. 2006; Kumar and Larkin 2017). KRPs inhibit entry into both M and S phases, while SMRs inhibit only M phase (Kumar and Larkin 2017). How cell cycle progression affects KNU transcriptional activation in the context of specific cell cycle regulators has yet to be described.
In this study, we identified biotimer genes during flower development and determined that the timing of their expression was correlated with the length of their H3K27me3-marked regions. We demonstrated that extension of the H3K27me3-marked region by adding copies of the region might be sufficient to repress gene expression in a cell cycle–dependent manner and increase the temporal lag to target activation. We also investigated the critical mechanistic determinants of the biotimer, particularly the interplay of PRC2 activity, chromatin environment, and the cell cycle, by manipulating the H3K27me3-marked region. Finally, we showed that 1 of the biotimer genes, AHL18, was expressed in stamens, and its precocious expression led to a short stamen filament phenotype, indicating that AHL18 controls key stamen regulators. Our results suggested that the AG-mediated biotimer controls proper FM determinacy and stamen development.
Results
Genome-wide identification of biotimer-regulated genes
Upon AG binding at floral stage 3, PRC2 eviction and H3K27me3 dilution start at biotimer-regulated genes (Fig. 1A). After sufficient H3K27me3 dilution through cell divisions, target genes can become expressed. To examine stage-specific protein binding at certain floral stages, we employed a previously described floral synchronization system, which allows the collection of numerous floral buds for biochemical experiments (Fig. 1A) (Wellmer et al. 2006; Ryan et al. 2015). The apetala1 cauliflower (ap1 cal) mutant has many inflorescence-like meristems containing stage 0 floral primordia. The ap1 cal double mutant used here carries a transgene encoding AP1 fused to the glucocorticoid receptor (GR). While the AP1-GR protein localizes to the cytosol in the absence of the steroid hormone dexamethasone (DEX), the exogenous application of DEX activates AP1 by inducing its relocation to the nucleus, where it can initiate the synchronous development of floral primordia. Furthermore, these induced primordia closely resemble those of wild-type (WT) floral buds. Thus, this floral synchronization system allows us to address biological events, such as stage-specific interactions between protein and DNA.
Figure 1.
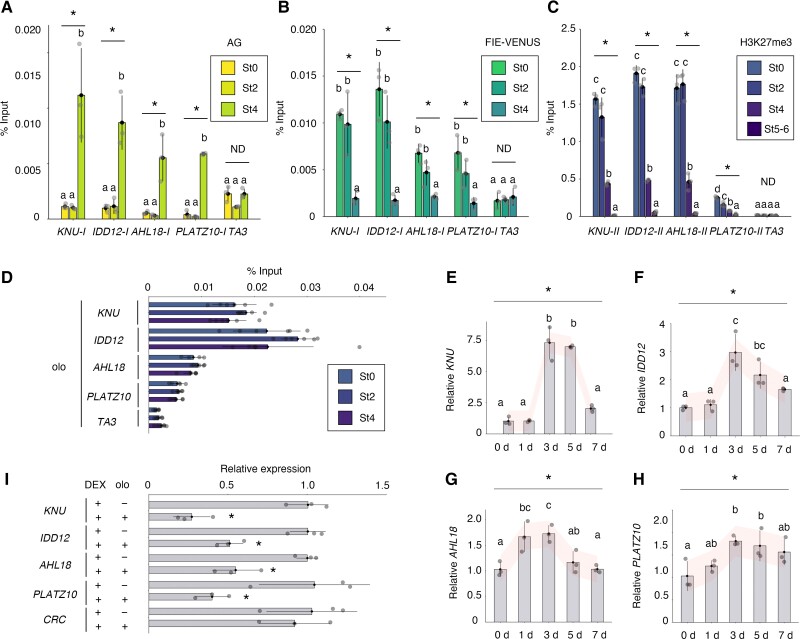
Genome-wide identification of biotimer-regulated genes. A) Schematic representation of the experimental design and selection criteria for the selection of biotimer-regulated genes of interest. Top: inflorescences of ap1 cal pro35S:AP1-GR plants before DEX treatment (0-d time point) and 1, 2, 3, and 4 d after DEX treatment. After DEX treatment, numerous synchronized floral buds at early developmental stages can be collected. Tissues were collected for AG binding, PRC2 removal, and H3K27me3 dilution by ChIP-seq and expression analysis by microarray using this floral synchronization system. B) Venn diagram showing the extent of overlap between AG-bound genes, FIE-evicted genes, EMF2-evicted genes, and H3K27me3-bound genes. The 242 genes indicated by asterisks were used for further analysis. C to E) FIE-VENUS C), EMF2-VENUS D), and H3K27me3 E) ChIP data in ap1 cal pro35S:AP1-GR without and with DEX treatment. Left: ap1 cal pro35S:AP1-GR without DEX treatment (St0, stage 0); middle: ap1 cal pro35S:AP1-GR tissues 1 d after DEX treatment (St2); right: ap1 cal pro35S:AP1-GR tissues 2 d after DEX treatment (St3). Upper panels show the average profile around the detected peak. Lower panels show read density heatmaps around each detected peak. F) Venn diagram showing the overlap between 242 genes bound by AG, PRC2, and H3K27me3, differentially expressed genes with and without the ag mutation at stages 3 to 4 and differentially expressed genes with and without the ag mutation at stages 6 to 7.
To characterize PRC2 eviction and H3K27me3 dilution upon AG binding from stage 3 onward, we conducted a time-course analysis of PRC2 and H3K27me3 deposition by chromatin immunoprecipitation (ChIP) followed by deep sequencing (ChIP-seq) using the floral synchronization system described above from stage 0 to stage 6. These time-course ChIP-seq experiments enabled us to look for genes with a lower enrichment of PRC2 and H3K27me3 only starting after stage 3 relative to stages 0 to 2, as seen at the KNU locus (Fig. 1, B to E). For PRC2 binding, we used the previously established transgenes proFIE:FIE-VENUS and proEMF2:EMF2-VENUS introduced in the ap1 cal pro35S:AP1-GR line (Sun et al. 2014, 2019). We observed a reduction of FIE and EMF2 enrichment as well as dilution of H3K27me3 from stage 3 across the genome (Fig. 1, C to E). We focused on genes that (i) are AG targets, (ii) showed a reduction of either FIE or EMF2 signal from stage 3, and (iii) are enriched in H3K27me3 (Zhang et al. 2007; Ó Maoiléidigh et al. 2013) (Supplemental Data Sets S1 and S2). This filtering yielded 242 genes that fulfilled our selection criteria (Fig. 1B).
We asked whether the expression of these 242 candidate genes may be regulated by AG with a time gap by comparing their expression in the ap1 cal pro35S:AP1-GR synchronization system with and without the ag mutation in stage 3 to 4 and stage 6 to 7 floral primordia (Ó Maoiléidigh et al. 2013) (Fig. 1F and Supplemental Data Sets S1 and S2). Among the 242 genes, we chose genes with no difference in their transcript levels at stages 3 to 4 with or without ag, but showing differential expression at stages 6 to 7 with and without ag, culminating in a list of 23 putative biotimer-regulated genes (Fig. 1F and Supplemental Table S1).
Characterization of 23 biotimer-regulated genes
To understand the possible role of these 23 biotimer-regulated genes, we conducted a Gene Ontology (GO) term enrichment analysis (Tian et al. 2017) (Supplemental Data Set 3). Most candidate genes encoded either transcription factors or proteins associated with DNA-binding transcription factor activity (Bosco et al. 2004; Ma et al. 2006; Hanada et al. 2011): the zinc-finger proteins KNU, IDD12, and STY1 (Riechmann et al. 2000; Payne et al. 2004; Sohlberg et al. 2006); the transcription factor PLATZ10; a homeodomain-like protein (encoded by At1g14600); and the AT hook domain-containing protein AHL18 (Riechmann et al. 2000; Gong et al. 2004; Liu et al. 2020; Širl et al. 2020). The functions of some of these genes or their homologs have previously been described during plant development (Nagano et al. 2001; Sohlberg et al. 2006; Aoyanagi et al. 2020; Širl et al. 2020). We also noticed that there are a few gibberellin-related genes such as ARABISOPSIS THALIANA GIBBERELLIN 20-OXIDASE 4 (ATGA2ox4) and ATGA3ox3 among our 23 biotimer-regulated genes (Frigerio et al. 2006). In addition, we found 4 KNOX genes that might be regulated by gibberellin (Jasinski et al. 2005).
The GO term enrichment analysis revealed that the GO terms with a lower P-value are mainly associated with either flower development or transcription (Fig. 2A). In good agreement with the function of AG during flower meristem termination and floral organ patterning, flower-related GO terms included “reproductive shoot system development,” “flower development,” “carpel development,” “gynoecium development,” “reproduction,” “floral whorl development,” “floral organ development,” and “meristem process” (Fig. 2A). We also noticed transcription-related GO terms such as “regulation of RNA metabolic process,” “regulation of RNA biosynthetic process,” “regulation of transcription, DNA-templated,” and “gene expression” (Fig. 2A). Consistent with the existence of hormone-related genes, other GO terms were also significantly enriched, including “signal transduction,” “signaling,” “response to abiotic stimulus,” and “cellular response to stimulus” (Fig. 2A). A further reduction of redundant GO terms by REVIGO categorized most of the GO terms above into “development” or “transcription” (Fig. 2B and Supplemental Data Set 2). The interactive graph view of 23 genes generated by REVIGO identified a cluster of GO terms that contains 6 different terms, including “plant organ development” and “meristem development” (Fig. 2C), suggesting that the 23 biotimer-regulated genes are involved in flower development via transcriptional control.
Figure 2.
Characterization of the 23 biotimer-regulated genes. A) Selected enriched GO terms for the identified biotimer-regulated genes generated using agriGO v2.0. B) Scatterplot showing representative clusters in a 2D space based on semantic similarities between GO terms. Color and size indicate P-value (P < 0.05) and the frequency of the GO term in the underlying GOA database, respectively. C) Graph-based visualization of refined GO terminology using REVIGO. Development-related data are shown. The node radius indicates the specificity, and the color shading corresponds to the P-values. Highly similar GO terms are linked by edges, with edge thickness indicating the degree of similarity. D) ChIP-seq signals for AG, the PRC2 components FIE and EMF2, and H3K27me3 at the KNU, AHL18, PLATZ10, IDD12, and SHP1 loci. The gene models are shown as black bars and lines at the bottom of each panel. Purple lines indicate PCR amplicons for Fig. 3. E) Heatmap representation of transcript levels for the 23 identified biotimer-regulated genes during early flower development. Expression levels of floral buds at stage 0 were set as 1.0, and fold change was calculated at stages 2, 4, and 8. Six clusters were identified by k-means clustering. F) Heatmap shows the log2 fold change of the 23 identified biotimer-regulated genes in a synchronization system with and without ag mutation. No change was observed in the synchronization system with and without ag mutation in stage 3 to 4 tissues. Significant transcriptional changes occurred for all genes at stages 6 to 7 (right) compared to stages 3 to 4 (left).
To understand the mechanistic determinants of the biotimer, we determined the enrichment for AG, PRC2, and H3K27me3 along the chromatin of selected candidate genes. At the KNU locus, as a positive control, the higher AG binding peak and moderate FIE and EMF2 peaks were located approximately 1 kb upstream of the transcription start site (TSS) (Fig. 2D). The KNU locus also exhibited similar FIE and EMF2 binding along the KNU gene body, as well as H3K27me3 deposition along the gene body, reflecting PRC2 enrichment at stages 0 and 2 of flower development (Sun et al. 2009, 2014) (Fig. 2D). At later stages, we detected reductions of FIE and EMF2 binding along the KNU promoter and gene body and the upstream polycomb response element at stage 3 (Fig. 2D). In addition, H3K27me3 signals were detected along the KNU gene body at stage 3 (Fig. 2D). Hence, each binding pattern at the KNU locus fulfilled our initial selection criteria.
Like KNU, other biotimer-regulated genes essentially showed similar patterns. For AHL18, PLATZ10, IDD12, and SHP1, we identified AG binding peaks upstream of or near the TSS (Fig. 2D). Additionally, moderate FIE and EMF2 binding peaks were also observed in the promoter region of AHL18 and PLATZ10, where AG bound (Fig. 2D). Regarding the IDD12 gene, we observed higher AG and moderate PRC2 binding in the promoter region (Fig. 2D). However, binding positions between AG and PRC2 were slightly different (Fig. 2D). At the SHP1 gene, cobinding of AG and PRC2 was not very clear (Fig. 2D). As with KNU, the other genes also displayed FIE and EMF2 binding peaks distributed along the gene body and corresponding H3K27me3 binding peaks in stage 0 to 2 flowers (Fig. 2D). In stage 3 flowers, we observed a decrease in FIE, EMF2, and H3K27me3 levels at all 4 loci.
To understand the role of the biotimer in temporal control of gene expression, we investigated published transcriptome data sets using the synchronized system during early flower development (Yan et al. 2019) (Fig. 2E). We set expression levels in stage 0 floral primordia to 1.0 and calculated fold change in expression across samples. We conducted a k-means clustering analysis of the 23 genes, defining 6 different clusters based on the direction of regulation (Fig. 2E). Genes in clusters 1 and 2 were transiently upregulated. Cluster 3 genes were downregulated from stage 0 to stage 8. The remaining clusters contained 15 genes, whose expression generally remained low until stage 4 before reaching their expression peak at stage 8. The positive control KNU, as well as other genes, such as PLATZ10 and SHP1, exhibited a delay in their expression after AG binding (Fig. 2E).
Furthermore, we examined whether expression of these 23 genes was regulated a few days after AG binding in an AG-dependent manner. To this end, we used the public transcriptome datasets using the synchronization system with and without ag (Ó Maoiléidigh et al. 2013). Their gene expression levels at stage 3 to 4 flowers were comparable regardless of the ag mutation (Fig. 2F). Using their fold change in expression with and without the ag mutation at stages 6 to 7, we sorted all 23 genes into 2 clusters as a function of the direction of regulation (Fig. 2F). As reported previously, KNU expression decreased from stage 6 to 7 floral primordia with the ag mutation. We observed a similar reduction only at the later stage for AHL18, IDD12, and PLATZ10 in the ag mutant background (Fig. 2F), suggesting that 3 genes are positively regulated by AG in a time-dependent manner.
Validation of biotimer regulation and its cell cycle dependence
To validate whether AHL18, IDD12, and PLATZ10 are controlled by the mechanism similar to KNU regulation, we tested their floral stage–specific AG binding, PRC2 eviction, and cell cycle–dependent H3K27me3 dilution at selected target sites using the synchronization system and ChIP followed by qPCR (ChIP–qPCR).
First, we tested and confirmed the timing of AG deposition with an anti-AG antibody during flower development. We detected no AG binding at KNU, IDD12, AHL18, or PLATZ10 promoters in floral primordia at stages 0 and 2 (Fig. 3A). However, we observed AG binding in floral primordia at stage 4 at all 4 loci, suggesting that AG binds to KNU, IDD12, AHL18, and PLATZ10 by stage 4.
Figure 3.
Cell cycle–dependent H3K27me3 dilution at biotimer-regulated genes. A to C) AG A), FIE-VENUS B), and H3K27me3 C) enrichment in ap1 cal pro35S:AP1-GR plants without and with DEX treatment. Data represent average of % input ± Se from 3 biological replicates (n = 3). One-way ANOVA test; *P < 0.05. ND, no difference. Different lowercase letters indicate significant differences based on a post hoc Tukey HSD test (P < 0.05). TA3 served as the negative control. PCR amplicons are shown in Fig. 2D. D) H3K27me3 enrichment in DEX-treated ap1 cal pro35S:AP1-GR without and with olo treatment. Data represent average of % input ± Se from 3 biological replicates (n = 3). TA3 served as the negative control. PCR amplicons are shown in Fig. 2D. E to H) Relative transcript levels for KNUE), IDD12F), AHL18G), and PLATZ10H) in ag-1 pro35S:AG-GR without (0 d) and with DEX treatment (1 to 7 d), as determined by RT-qPCR. Data represent average fold change ± Se of 3 biological replicates (n = 3). One-way ANOVA test; *P < 0.05. Different lowercase letters indicate significant differences based on a post hoc Tukey HSD test (P < 0.05). TUBULIN 2 (TUB2) served as the reference transcript. I) Relative expression levels for the biotimer-regulated candidates KNU, IDD12, AHL18, and PLATZ10 and the negative control CRC in DEX-treated ag-1 pro35S:AG-GR without and with olo treatment, as determined by RT-qPCR. Data represent average fold change ± Se of 3 biological replicates (n = 3). Significant differences were determined using a 2-tailed (*P < 0.05) or 1-tailed Student's t test (**P < 0.05). EIF4A1 served as the negative control.
Second, we examined the timing of FIE removal. FIE bound to the gene bodies of KNU, IDD12, AHL18, and PLATZ10 promoters in floral primordia at stages 0 and 2 (Fig. 3B). We observed no significant difference in FIE abundance at the KNU, IDD12, AHL18, and PLATZ10 loci in floral primordia at stages 0 and 2. At stage 4, FIE enrichment dropped at these loci. We conclude that AG binding and FIE removal cooccurred at all 4 loci tested.
Third, we tested the extent of cell cycle–dependent H3K27me3 dilution. H3K27me3 accumulated to high levels along the KNU, IDD12, and AHL18 gene bodies in floral primordia at stages 0 and 2, while we detected much lower H3K27me3 signals along the PLATZ10 gene body (Fig. 3C). Unlike FIE binding, H3K27me3 levels gradually decreased at all 4 genes during flower development. We observed a significant difference in H3K27me3 levels between stages 2 and 4 and between stages 4 and 6 at all 4 loci. We also asked whether H3K27me3 reduction required cell cycle progression by treating plants with the cell cycle inhibitor olomoucine (olo) prior to harvesting ChIP samples (Planchais et al. 2000; Sun et al. 2014). Although H3K27me3 levels gradually decreased at all 4 loci in the absence of olo treatment, olo treatment inhibited the drop in H3K27me3 levels (Fig. 3D), suggesting that these 4 genes are regulated by cell cycle–dependent H3K27me3 dilution.
To explore the effect of the biotimer on temporal control of gene expression, we conducted reverse transcription quantitative PCR (RT-qPCR) for all candidate genes over a time course. We used the transgenic line ag-1 pro35S:AG-GR for this purpose (Fig. 3, E to H). We collected samples over 7 d after exogenous DEX application and determined the relative transcript levels for KNU and the 3 other candidate genes. KNU expression showed an upregulation 3 d after DEX treatment, in agreement with the transcriptional delay characteristic of KNU activation (Fig. 3E). In contrast, we observed a rapid activation of SPOROCYTELESS (SPL) within 1 d after DEX treatment, as reported previously (Ito et al. 2004) (Supplemental Fig. S1), validating our experimental setup. We detected transcriptional delays for AHL18 and IDD12 at 3 d after treatment and for PLATZ10 at 3 to 7 d after treatment (Fig. 3, F to H). These results indicate that activation of IDD12, AHL18, and PLATZ10 transcription is similar to that of KNU, with a transcriptional delay upon AG induction.
To test whether the above delay in gene expression requires cell cycle progression, we exposed plants to olo prior to sample collection (Ross et al. 2008; Probst et al. 2009). After 3 d of DEX and olo treatment, we observed significant decrease in CDKB1;1 transcript levels, suggesting that cell cycle progression is inhibited by olo treatment (Supplemental Fig. S2). Under this condition, KNU, IDD12, AHL18, and PLATZ10 transcript levels rose in DEX-treated ag-1 pro35S:AG-GR floral primordia (Fig. 3I), but olo treatment significantly reduced the extent of this induction (P < 0.05, Fig. 3I). We also included the nonbiotimer AG target CRC as a control; this gene showed similar expression levels without and with olo treatment upon AG induction, suggesting that the observed reduction in expression levels in the selected genes are biotimer specific and related to their cell cycle regulation. Taken together, these findings suggest that AHL18, IDD12, and PLATZ10 are regulated by the AG-mediated epigenetic biotimer like KNU.
Temporal expression and generalization of biotimer-regulated genes
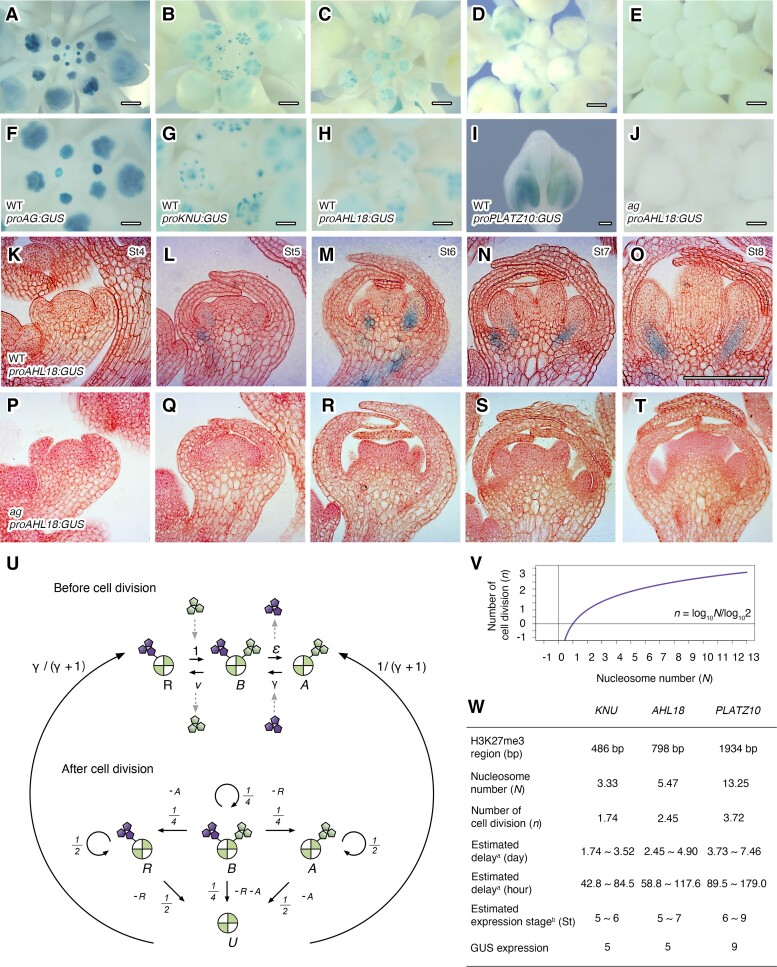
To understand spatiotemporal expression patterns of the 3 genes during flower development, we generated or obtained promoter reporter lines for biotimer genes using a GUS reporter and compared them to previously established AG and KNU reporter lines (Parcy et al. 1998; Sun et al. 2009; Širl et al. 2020) (Fig. 4, A to J). Consistent with previous findings, AG and KNU started to be expressed in FM from stage 3 and stage 6, respectively (Fig. 4, A, B, F, and G). Like KNU, we observed a delay in the activation of AHL18 and PLATZ10 transcription compared to that of AG (Fig. 4, A to J). In the WT background, AHL18 and PLATZ10 started to be expressed at stage 5 and stage 9, respectively (Fig. 4, C, H, D, and I). PLATZ10 was transiently expressed in anthers at stage 9 (Fig. 4I). Inspection of sections revealed no GUS staining from the proAHL18:GUS reporter in the WT until stage 4 (Fig. 4K). From stage 5 of flower development onward, we detected weak GUS staining for the proAHL18:GUS reporter construct at the base of stamen primordia (Fig. 4L). At stage 6, we detected a strong GUS signal in the presumptive stamen filaments along the vasculature (Fig. 4M). This expression remained at the same level after stage 6 (Fig. 4, N and O). In the ag mutant background, we failed to observe AHL18 expression in stamens from the proAHL18:GUS reporter (Fig. 4, E, J, and P to T). Based on RT-qPCR, AHL18, PLATZ10, and IDD12 expression levels were lower in the ag mutant compared to the WT (Supplemental Fig. S3). Taken together, these results suggest that AHL18 and PLATZ10 transcriptional activation is delayed after AG recruitment to their promoters and depends on AG function.
Figure 4.
Spatiotemporal gene expression for AG-mediated biotimer targets. A to E) GUS staining pattern using proAG:GUS in the WT A), proKNU:KNU-GUS in the WT B), proAHL18:GUS in the WT C), proPLATZ10:GUS in the WT D), and proAHL18:GUS in agE). Scale bars, 250 µm. F to J) Close-up views of A to E). For proPLATZ10:GUS in the WT I), a stage 9 flower is shown. A sepal was removed to show the inner structure. Scale bars, 100 µm. K to T) Representative longitudinal GUS sections from stage 4 to 8 floral buds from proAHL18:GUS in the WT and the ag mutant. K) P: stage 4. L) Q: stage 5. M) R: stage 6. N) S: stage 7. O) T: stage 8. Cell walls were stained with neutral red dye. Scale bar, 50 µm. U) Diagram of the mathematical model describing histone modification before and after cell division. There are 4 nucleosome states (R: repressive, B: bivalent, A: active, and U: unmodified) depending on the histone modification pattern. Purple and green symbols represent repressive and active marks, respectively. , the removal rate of repressive mark; γ, the addition rate of repressive mark; ν, the removal rate of repressive mark. V) Relationship between nucleosome number with H3K27me3 (N) and number of cell divisions (n) by mathematical modeling. W) Timing of KNU and AHL18 expression as predicted by the mathematical model. a, Duration of each floral stage is based on Smyth et al. (1990). b, Cell division frequency in FM is based on Reddy et al. (2004).
Passive dilution has been proposed as a possible mechanism for the removal of H3K27me3 via cell division in the absence of de novo H3K27me3 methylation activity. For example, in preimplantation embryos, this dilution mechanism is proposed to explain the global loss of H3K27me3, as the average nuclear staining decreased to ∼50% with each cell division (Ross et al. 2008). Since FMs consist of proliferating cells, constant division also provides a potential passive mechanism to dilute the H3K27me3 on target genes. To establish a mathematical model estimating the time lag for target activation, we made 2 assumptions: (i) AG evicts PRC2 and prevents further H3K27me3 deposition from target loci and (ii) H3K27me3 is diluted by half after each cell division. We considered N nucleosomes. Stochastic transitions occur between 4 nucleosome states (R, repressive; B, bivalent; A, active; and U, unmodified; Fig. 4U) in each N nucleosome. After cell division, histone modifications are transmitted to 2 daughter cells randomly, generating the unmodified nucleosome from R, B, and A states with rates depending on each state (Fig. 4U). When AG expression is low (i.e. ) under no cell division, the number of nucleosomes in each state at equilibrium under no cell division (0 cell division) is approximated as:
The above equation indicates that a repressive or bivalent state is dominant when AG expression is low prior to cell division (i.e. ). Cell division yields an unmodified nucleosome. The expected number of unmodified nucleosomes right after cell division is given as (Fig. 4U):
Because the unmodified nucleosome becomes active or takes on a repressive state with rate and , respectively, the expected number of repressive and bivalent nucleosomes after 1 cell division, , is formulated using the number of repressive or bivalent nucleosomes before cell division ( and ):
When AG is highly expressed and prevents further H3K27me3 deposition from target loci (i.e. ), R(1) is approximated as . Based on the formula, we have:
Because holds prior to the activation of AG, the expected number of nucleosomes in either state R or B after n cell divisions is given as:
The above equation shows that the number of repressive or bivalent nucleosomes is diluted by half after each round of cell division. H3K27me3 signals will not be detected and target genes will be activated when is below unity (i.e. ). This condition is calculated as n = LogN/Log2 (Fig. 4V).
To begin to validate the above mathematical model, we quantified H3K27me3 signals along the KNU gene body, as well as for that of the 3 other genes. We detected H3K27me3 signals throughout the 486-bp KNU gene body and the 798-bp AHL18 gene body (Fig. 4W). Because 1 nucleosome consists of ∼146 bp of DNA wrapped around the histone octamer, the KNU, AHL18, and PLATZ10 loci are expected to contain 3.3, 5.5, and 13.3 nucleosomes, respectively (Fig. 4W). When we applied the above equation to the regulation of KNU, AHL18, and PLATZ10, we determined that their transcriptional activation would require 1.7, 2.5, and 3.7 rounds of cell division, respectively. Since FM cells divide once every 1 to 2 d (Reddy et al. 2004), we calculated an estimated activation timing of 1.7 to 3.5 d (42.8 to 84.5 h) for KNU, 2.5 to 4.9 d (58.8 to 117.6 h) for AHL18, and 3.7 to 7.5 d (89.5 to 179.0 h) for PLATZ10 after AG binding. Based on floral developmental stages (Smyth et al. 1990), 42, 72, 96, 120, and 180 h are required for stage 3 flowers to reach stages 5, 6, 7, 8, and 9, respectively. Based on our calculations from the model, KNU and AHL18 induction is expected to start somewhere between stage 5 and stage 7. Indeed, KNU was reported to be activated from stage 5 (Kwaśniewska et al. 2021). Furthermore, our GUS reporter analysis showed that AHL18 and PLATZ10 were activated from stage 5 and stage 9, respectively (Fig. 4, I and L). The model highlighted the importance of a passive dilution mechanism.
Validation of the mathematical model by adding extra del copies
Based on our mathematical model, providing more copies of a region containing H3K27me3 would increase the level of repressive H3K27me3 marks and further delay timing of target expression during floral development. To explore this possibility, we first introduced 1 to 6 copies of the del region (1del to 6del) into the proKNU:KNU-GUS construct (Fig. 5A) and examined the effect of the del region on GUS signal in the generated primary transformants (T1) at floral stage 6 (Supplemental Fig. S4). We defined a signal as weak in the absence of GUS staining at stage 6, as intermediate with minimal signal detected at stage 6, and as strong with clear GUS signal at stages 6. We analyzed more than 50 independent primary transformants for each construct. We determined that proKNU:KNU-GUS exhibits the highest occurrence of strong GUS signals (61.8%), while the 6del construct showed the highest incidence of weak GUS signals (51.9%) (Fig. 5B). Intermediate GUS signals were most prevalent in primary transformants for the 4del construct (52.8%) followed closely by the 3del primary transformants (47.2%) (Fig. 5B). The lower GUS signal was not an indirect result of the longer coding region of the construct, as replacing 3del or 6del with DNA fragments of approximate equivalent lengths (1 or 2 copies of the cyan fluorescent protein [CFP] coding sequence) produced similar T1 GUS signal intensities as proKNU:KNU-GUS (Supplemental Fig. S5), suggesting that the change in GUS accumulation correlates well with the number of del regions.
Figure 5.
Extension of the KNU coding sequence prolongs the delay in KNU activation and reduces KNU expression. A) Schematic diagrams of the KNU-GUS series of constructs. Solid black line, KNU promoter region; white bars, 5′ untranslated region (5′ UTR); black bars, 255 bp of KNU coding sequence; gray, 231-bp del region (1 [1del] to 6 [6del] copies); blue bars, GUS gene. The line above the del and GUS gene junction is a PCR amplicon used for ChIP–qPCR. B) Variation in GUS activity levels in proKNU:KNU-GUS and del T1 plants. The dark blue, blue, and light blue bars represent strong, intermediate, and weak GUS signal intensity, respectively. A χ2 test was used to test for significant differences among the del lines (n > 52, *P < 0.05). C) Timing of 3del and 6del expression as predicted by the mathematical model. a, Duration of each floral stage is based on Smyth et al. (1990). b, Cell division frequency in FM is based on Reddy et al. (2004). D to R) Representative longitudinal GUS sections from stage 4 to 8 floral buds from proKNU:KNU-GUSD to H), 3delI to M), and 6delN to R). Cell walls were stained with neutral red dye. Scale bar, 50 µm. S) Relative GUS transcript levels in proKNU:KNU-GUS and 6del T1 plants, as determined by RT-qPCR. Data represent average fold change ± Se of 3 biological replicates (n = 3). Significant differences were determined using a 2-tailed Student's t test (*P < 0.05). TUB2 served as the reference transcript. T) GUS activity levels, as determined by MUG assay. Data represent average GUS activity (nmol/mg/h) ± Se from 9 biological replicates (n = 9). Significant differences were determined using a 2-tailed Student's t test (*P < 0.05). U) H3K27me3 enrichment in proKNU:KNU-GUS and 6del T1 plants. Data represent average % input ± Se from 4 biological replicates (n = 4). Significant differences were determined using a 2-tailed Student's t test (*P < 0.05). EIF4A1 served as the negative control. ND, no difference.
We also characterized the spatiotemporal expression pattern in representative proKNU:KNU-GUS, 3del, and 6del homozygous lines during flower development. Based on our mathematical model, expression from the 3del and 6del constructs should start somewhere between stages 5 to 6 and 9, and stages 6 and 9, respectively (Fig. 5C). We first selected representative lines among the proKNU:KNU-GUS, 3del, and 6del T1 plants based on their GUS signal from the strong, intermediate, and weak categories. Second, we confirmed that each T1 plant from the same category displayed a similar spatiotemporal GUS staining pattern (Supplemental Fig. S6). Finally, we made sure that these T2 plants harbor a single T-DNA insertion by checking the segregation ratio of the selection marker in their progeny. Only representative transgenic proKNU:KNU-GUS, 3del, and 6del lines exhibiting the expected Mendelian inheritance ratio for a single transgene were used for further study (Fig. 5, D to R).
The proKNU:KNU-GUS lines produced a moderate GUS signal starting from floral stage 5 (Fig. 5, D and E), which rose to a strong GUS signal at stage 6 (Fig. 5F) and later decreased to a moderate signal from stages 7 to 8 (Fig. 5, G and H). This GUS staining pattern followed the timing of KNU mRNA accumulation in WT plants (Payne et al. 2004). Both the timing of 3del/6del activation and their peak accumulation shifted. With the 3del construct, we detected only a very faint GUS signal at the center of the FM at stage 5 (Fig. 5, I and J), as predicted from the mathematical model. The GUS signal persisted into stage 6 before increasing to a moderate signal at stage 7 and returning to a weak signal at stage 8 (Fig. 5, K to M). Expression timing was further delayed in 6del. Unlike proKNU:KNU-GUS or 3del, 6del was not expressed by stage 5 (Fig. 5, N and O). Consistent with the prediction of the mathematical model, 6del exhibited a weak GUS signal initially at stage 6 until stage 7 that diminished by stage 8 (Fig. 5, P to R). Based on RT-qPCR analysis, GUS transcript levels were substantially lower in 6del lines compared to proKNU:KNU-GUS lines (Fig. 5S), while endogenous KNU mRNA levels were comparable (Supplemental Fig. S7). Furthermore, GUS activity was much lower in 6del lines relative to proKNU:KNU-GUS lines (Fig. 4T). These results demonstrate that adding more copies of an H3K27me3-rich region can extend the delay of gene expression, as predicted by mathematical modeling.
To investigate the trimethylation status of H3K27 in proKNU:KNU-GUS and 6del lines, we performed H3K27me3 ChIP–qPCR, which showed an enrichment for H3K27me3 levels in 6del lines compared to proKNU:KNU-GUS lines (Fig. 5U). Histone H3 levels were comparable between the lines, as evidenced by H3 ChIP to quantify H3 levels at the proKNU:KNU-GUS and 6del transgenes (Supplemental Fig. S8). Importantly, H3K27me3 ChIP signals were close to 3-fold higher in 6del lines relative to proKNU:KNU-GUS lines when normalized to the H3 ChIP signal (Supplemental Fig. S9). This finding indicated that the observed difference in H3K27me3 enrichment between proKNU:KNU-GUS and 6del lines is due to changes in H3K27me3 accumulation on the 6del regions rather than to changes in histone H3 deposition.
PRC2 deposits H3K27me3 on the extended del copies
We first detected GUS signal from the WT harboring the 6del transgene at stage 6 of floral development, later than for proKNU:KNU-GUS and constructs with fewer del copies (Fig. 5). We asked whether and to what extent the PRC2s FIE and CLF were responsible for H3K27me3 deposition on the extended KNU coding sequence by crossing our representative single-insertion homozygous 6del line with the FIE cosuppression line pro35S:GFP-FIE (hereafter referred to as fie) and the CLF T-DNA insertion mutant clf-28 (clf) (Fig. 6). We used the FIE cosuppression line, which retains some FIE transcripts, since loss-of-function fie mutants are embryonic lethal (Katz et al. 2004); the clf-28 mutant exhibits the typical phenotypes for loss of PRC2 function but with relatively stable seed production (Liu et al. 2016). We also crossed an already characterized 6del line rather than transforming the mutants with the transgene to avoid line selection and minimize position effects. In the WT, we failed to detect GUS signal from 6del at stages 4 and 5 (Fig. 6, A and B). GUS signal then appeared at stage 6 and remained weak until stage 7 (Fig. 6, C and D), with little to no GUS signal at stage 8 (Fig. 6E). Although GUS signal was absent from the fie and clf mutants at stage 4, both showed precocious expression at stage 5 (Fig. 6, G and L), with rising GUS signal intensity at stages 6 and 7 (Fig. 6, H, I, M, and N) in fie and clf. While the signal declined by stage 8 in clf (Fig. 6O), we detected GUS signal at stage 8 in the fie background (Fig. 6J). We hypothesize that the lower GUS signals observed in clf reflect the weaker phenotypes seen with CLF loss-of-function mutants (Doyle and Amasino 2009; Liu et al. 2016).
Figure 6.
PRC2 deposits H3K27me3 on the del region. A to O) Representative longitudinal GUS sections from stage 4 to 8 floral buds for WT A to E), fieF to J), and clfK to O) transformants harboring 6del. Cell walls were stained with neutral red dye. Scale bar, 50 µm. P) Relative GUS transcript levels derived from 6del in WT and fie transformants, as determined by RT-qPCR. Data represent average fold change ± Se of 3 biological replicates (n = 3). A 2-tailed Student’s t test was used to determine significant differences (*P < 0.05). TUB2 served as the reference transcript. Q) H3K27me3 enrichment over 6del in the WT compared to fie and clf. Data represent average % input ± Se from 3 independent experiments (n = 3). One-way ANOVA test; *P < 0.05. ND, no difference. Different lowercase letters indicate significant differences based on a post hoc Tukey HSD test (P < 0.05). EIF4A1 served as the negative control.
We confirmed that GUS signals are consistent with GUS transcript accumulation by RT-qPCR for 6del transgenic lines in the WT and fie backgrounds. Higher GUS signals correlated with the higher GUS transcript levels seen in fie compared to the 6del WT (Fig. 6P). Although AG expression was also upregulated in the fie background, GUS expression levels were close to 2-fold higher in the fie background relative to the WT when normalized to the AG results (Supplemental Fig. S10), suggesting that reduced GUS expression in 6del is at least in part due to reduced FIE activity.
We also investigated H3K27me3 levels in the fie background by ChIP–qPCR. We determined that H3K27me3 levels along the 6del transgene are substantially lower in fie and clf compared to the WT (Fig. 6Q). We conclude that the increase in GUS expression is due to a disruption in the PRC2 machinery, attenuating H3K27me3 deposition at del copies.
The cell cycle regulates H3K27me3 dilution on the extended del copies
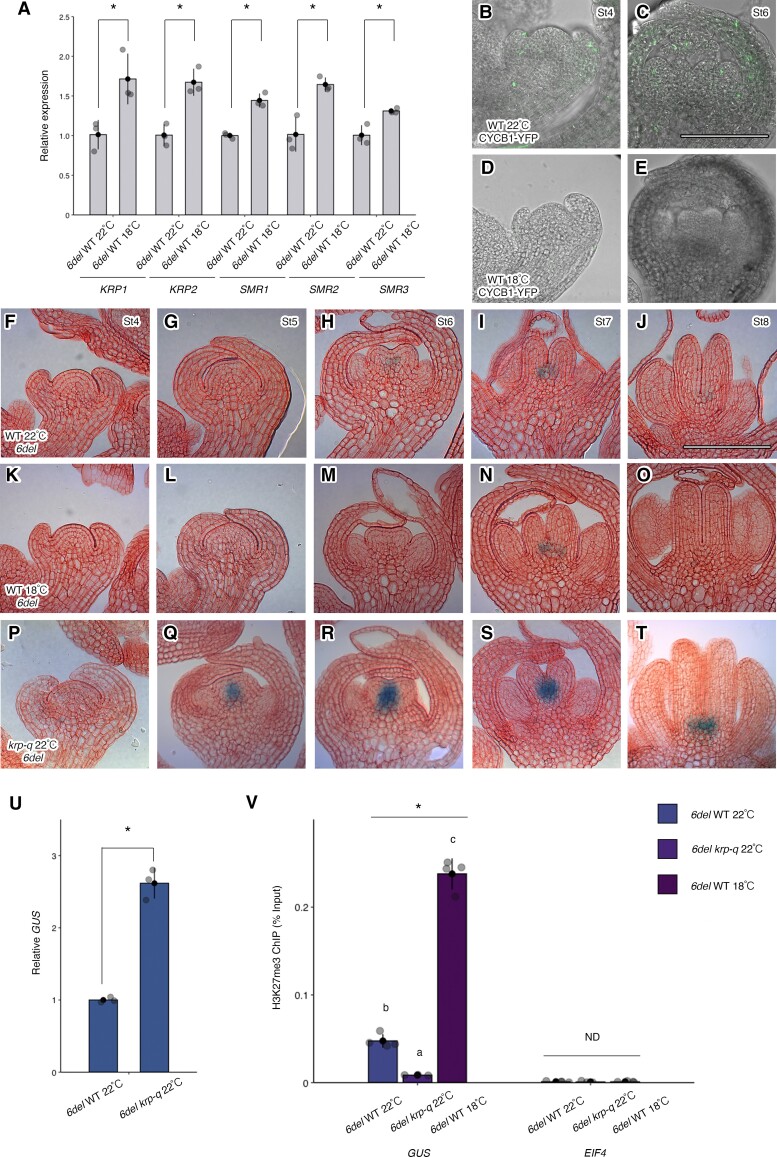
The cell cycle plays an important role in KNU biotimer regulation by diluting H3K27me3 along the KNU coding sequence. Arabidopsis grows optimally at 22 °C but can respond to changes in ambient temperature by adjusting growth rates, with lower temperatures slowing cellular processes including the cell cycle (Michael et al. 2008; Wigge 2013). We measured transcript levels for the selected CKI genes KRP1, KRP2, SMR1, SMR2, and SMR3 in 6del lines in the WT background grown at 22 °C or 18 °C. We determined that CKI expression is induced at 18 °C relative to 22 °C, suggesting that the cell cycle is inhibited (Fig. 7A). We also explored the stage-specific accumulation of the core cell cycle and G2/M transition regulator CYCB1 as a fusion protein to yellow fluorescent protein (YFP) at 22 °C and 18 °C (Fig. 7, B to E). We detected a prevalent CYCB1-YFP signal at stages 4 and 6 at 22 °C that is much weaker in plants grown at 18 °C, suggesting that CYC activity is blocked by lower temperature conditions, presumably due to higher CKI activity (Fig. 7, B to E).
Figure 7.
The dilution of H3K27me3 at del is cell cycle dependent. A) Relative transcript levels for cell cycle–related genes in WT lines harboring 6del, as determined by RT-qPCR. Data represent average fold change ± Se of 3 biological replicates (n = 3). A 2-tailed Student’s t test was used to determine significant differences (*P < 0.05). TUB2 served as the reference transcript. B to E) Representative longitudinal YFP sections from stage 4 B, D) and stage 6 C, E) floral buds from the WT harboring the CYCB1;1-YFP construct grown at 22 °C B, C) or at 18 °C D, E). F to T) Representative longitudinal GUS sections from stage 4 to 8 floral buds from the WT harboring the 6del construct F to J), 6del in the WT grown at 18 °C K to O), and 6del in the krp-q grown at 22 °C P to T). Cell walls were stained with neutral red dye. Scale bar, 50 µm. U) Relative GUS transcript levels in WT and krp-q transformants harboring the 6del transgene and grown at 22 °C, as determined by RT-qPCR using RNA isolated from floral bud clusters containing flowers until stage 12. Data represent average fold change ± Se of 3 biological replicates (n = 3). A 2-tailed Student's t test was used to determine significant differences (*P < 0.05). TUB2 served as the reference transcript. V) H3K27me3 enrichment at 6del in WT and krp-q transformants harboring 6del and grown at 22 °C or 18 °C. Samples used were floral bud clusters containing flowers until stage 12. Data represent average % input ± Se from 3 independent experiments (n = 3). One-way ANOVA test; *P < 0.05. ND, no difference. Different lowercase letters indicate significant differences based on a post hoc Tukey HSD test (P < 0.05). EIF4A1 served as the negative control.
We also characterized the consequences of a slower cell cycle on 6del activation by growing plants at 18 °C. We failed to detect GUS signal from 6del during stages 4 to 6 and only observed GUS signal at stage 7, which returned to below detection limits at stage 8 (Fig. 7, F to O). H3K27me3 levels showed an inverse correlation with GUS signal, with higher levels for 6del at 18 °C compared to 22 °C (Fig. 7V). These results suggest that lower temperatures further delay KNU activation in 6del, likely due to the slower dilution of H3K27me3 marks along the extended KNU region.
To investigate whether mutations in cell cycle genes affected KNU activation, we crossed 6del into the krp1 krp2 krp3 krp4 krp7 quintuple mutant (referred to as krp-q) (Cheng et al. 2013) (Fig. 7, P to T). The krp-q mutant exhibits increased cell proliferation, likely as a result of enhanced CDK activity leading to greater levels of phosphorylated RETINOBLASTOMA-RELATED1 (RBR1) and upregulation of the E2F pathway (Cheng et al. 2013). As with prc2 mutants, the krp-q mutant also showed precocious GUS signal from stage 5 floral buds (Fig. 7, P to T) that reached a peak at stages 6 and 7 (Fig. 7, R and S). Overall, GUS signal levels were higher in krp-q compared to that in the WT (Fig. 7, G to I and Q to S). Although 6del in the WT produced only a weak signal, krp-q mutants still possessed moderate GUS signal derived from 6del at stage 8 (Fig. 7, J and T). In addition, GUS transcripts accumulated to about 2.5-fold higher levels in krp-q relative to the WT (Fig. 7U), which correlated with lower H3K27me3 levels along the del sequence in krp-q compared to the WT (Fig. 7T). These results suggest that enhancing cell cycle activity by removing CDK inhibition results in accelerated H3K27me3 dilution along del.
To examine the effects of raising cell cycle activity on KNU and 6del activation, we used the pro35S:CYCD3;1 line (Supplemental Fig. S11), which has an extended G2 phase due to delayed activation of CYC B and G2/M gene expression (Zhou et al. 2003). We observed a delayed activation of KNU expression in pro35S:CYCD3;1, in both the proKNU:KNU-GUS and 6del backgrounds (Supplemental Fig. S11, A to T). Although 6del started to drive GUS expression at stage 6 of flower development in the WT, we detected no GUS signal and a faint signal in the pro35S:CYCD3;1 in 6del at stages 6 and 7, respectively (Supplemental Fig. S11, K to T). This finding further supports the notion that H3K27me3 dilution along the extended KNU coding sequence is cell cycle dependent.
Precocious AHL18 expression leads to reduced fertility due to shorter stamen filaments
To understand the biological relevance of temporal control via biotimer regulation during flower development, we used a previously established AHL18 overexpression line (pro35S:AHL18) and characterized its flower phenotype (Fig. 8) (Širl et al. 2020). We established that pro35S:AHL18 plants show reduced fertility compared to the WT (Fig. 8, A and B). Sepals and petals formed normally in both the WT and pro35S:AHL18 (Fig. 8, A and B). In the WT, long anthers extended above the stigma at stage 14 of flower development (Fig. 8, C, E, and G). However, pro35S:AHL18 stamens were too short to reach the stigma at that stage (Fig. 8, D, F, and H). We noticed the proper elongation of stigmatic papillae and many pollen grains attached to the stigma in the WT, but not in the pro35S:AHL18 background (Fig. 8, I to L). We also inspected stamens in the WT and pro35S:AHL18 at stage 14 (Fig. 8, M to R). By stage 14, we observed anther dehiscence and subsequent release of pollen grains in both backgrounds (Fig. 8, M and N). Notably, elongation of stamen filaments was different between the WT and pro35S:AHL18, with WT stamens at stage 14 having longer cells than anthers from pro35S:AHL18 (Fig. 8, O to R). Considering that AHL18 is expressed in stamen from stage 5, our results suggest that proper control of AHL18 expression is important for stamen development and subsequent fertilization. We did not observe differences between the WT and a loss-of-function ahl18 mutant during stamen elongation (Supplemental Fig. S12). Redundant genes other than AHL18 might thus compensate for proper stamen elongation.
Figure 8.
Precocious AHL18 expression leads to reduced fertility. A, B) Side views of primary inflorescences in WT A) and pro35S:AHL18B) plants. Asterisks indicate flowers failed to form seeds. Scale bar, 1 cm. C, D) Top views of stage 14 flowers in WT C) and pro35S:AHL18D) plants. Scale bar, 1 mm. E, F) Side views of stage 14 flowers in WT E) and pro35S:AHL18F) plants. Scale bar, 1 mm. G, H) Close-up views of stage 14 flowers in WT G) and pro35S:AHL18H) plants. Scale bar, 500 µm. I, J) Close-up views of stigma at stage 14 flowers in WT G) and pro35S:AHL18H) plants. Scale bar, 100 µm. K, L) Close-up views of stigma tip and pollens at stage 14 flowers in WT K) and pro35S:AHL18L) plants. Pollens are highlighted by pseudocolor (yellow). Images in K) and L) are magnified images of the boxed regions in I) and J), respectively. M, N) Close-up views of the adaxial surface of anthers at stage 14 flowers in WT G) and pro35S:AHL18H) plants. Scale bar, 200 µm. O, P) Close-up views of the adaxial surface of stamen filaments at stage 14 flowers in WT G) and pro35S:AHL18H) plants. Scale bar, 100 µm. Q, R) Adaxial surface of stamen filament cells at stage 14 flowers in WT G) and pro35S:AHL18H) plants. Several cells are outlined by black line. Images in O) and P) are magnified images of the boxed regions in Q) and R), respectively.
AHL18 binds to open chromatin and controls key regulators for stamen development
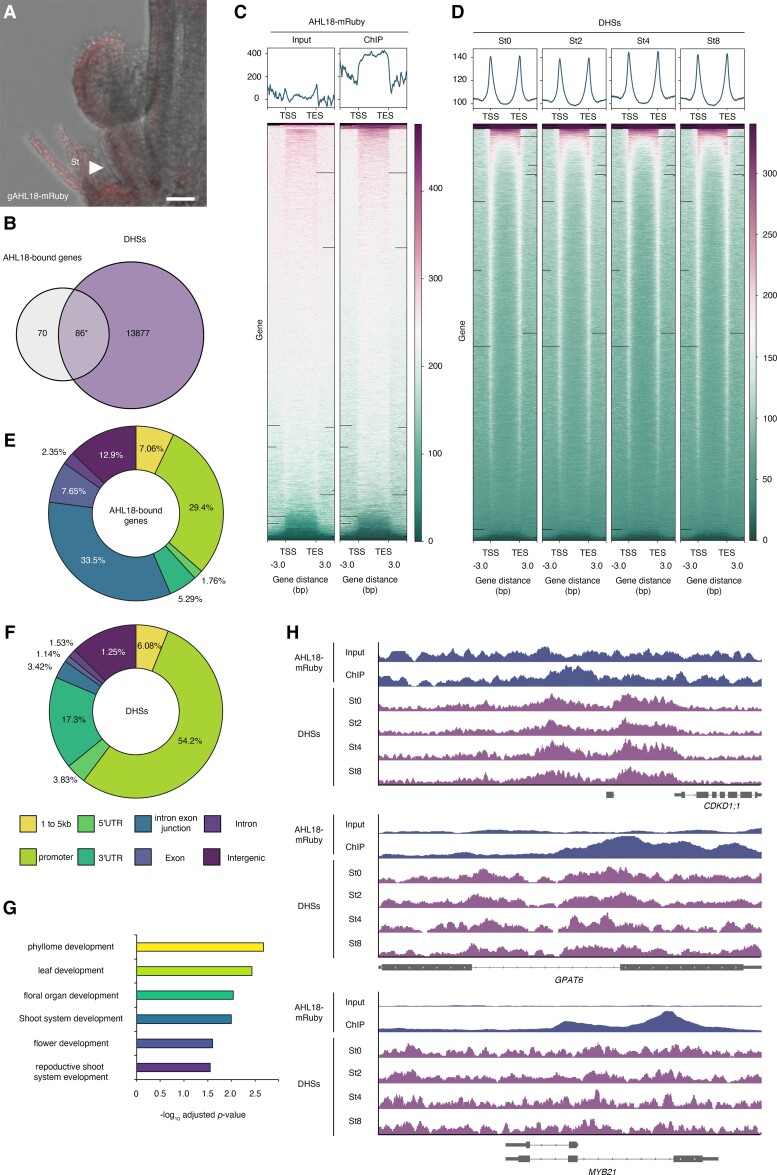
To identify direct AHL18 targets, we conducted ChIP-seq using the previously established line gAHL18-mRuby (Fig. 9) (Širl et al. 2020). We detected the accumulation of AHL18-mRuby in stamens (Fig. 9A). AHL18-mRuby ChIP identified 156 direct targets (Fig. 9B and Supplemental Data Set 4). A previous study revealed that AHL family proteins interact with transcription factors to control gene expression (Zhao et al. 2013). Because transcription factor binding sites are often correlated with DNase I hypersensitive sites (DHSs), we compared AHL18-bound sites and DHSs. Accordingly, we used the previously published floral stage–specific DHS data (Pajoro et al. 2014; Yan et al. 2019) and assessed changes in accessibility levels at AHL18-bound regions. Of 156 AHL18-bound genes, 86 also harbored DHSs (Fig. 9B). We detected AHL18 binding along the gene body (Fig. 9, C and E), at intron–exon junctions (Fig. 9E). In contrast, more than half of DHSs during flower development mapped to the promoter, as well as in intergenic regions and untranslated regions (UTRs) (Fig. 9, D and F). Based on GO term enrichment analysis, the 86 direct AHL18 targets with DHSs were involved in developmental decisions, including floral organ development and flower development (Fig. 9G). Among key targets, MYB21 and GLYCEROL-3-PHOSPHATE SN-2-ACYLTRANSFERASE6 (GPAT6) are both required for stamen development (Li et al. 2012; Zhang et al. 2021b). For GPAT6 and MYB21 genes, we observed AHL18 binding peaks and DHSs at intron–exon junctions (Fig. 9H). We observed AHL18 binding sites and DHSs at the 5′ intergenic region of the CDKD1;1 locus (Fig. 9H). Importantly, the chromatin of all loci harboring AHL18 binding sites stayed open across all 4 time points prior to AHL18 expression (Fig. 4). These results suggest that AHL18 accesses opened chromatin regions for subsequent control of target expression.
Figure 9.
AHL18-bound genes and chromatin accessibility. A) Side view of WT flowers harboring the gAHL18-mRuby construct. B) Venn diagram showing the extent of overlap between AHL18-bound genes and DHSs. The 86 genes indicated by asterisks were used for further analysis. C) AHL18-mRuby ChIP data in the WT background. Left, input sample; right, ChIP sample. Upper panels show the average profile around the detected peak. Lower panels show read density heatmaps around each detected peak. D) DHSs in ap1 cal pro35S:AP1-GR without and with DEX treatment. St0, St2, St4, and St8 were examined previously. Upper panels show the average profile around the detected peak. Lower panels show read density heatmaps around each detected peak. E, F) Pie chart showing the percentage of AHL18-bound peaks E) and DHSs F) according to their functional genomic distribution. AnnotatePeak was used for assignment. G) Selected GO terms for the identified biotimer-regulated genes generated using agriGO v2.0. H) AHL18 ChIP-seq and DHS signals at the CDKD1;1, GPAT6, and MYB21 loci. The gene models are shown as black bars and lines at the bottom of each panel.
Discussion
In this study, we identified 23 high-confidence biotimer genes that are direct AG targets. Our time-course and genome-wide AG, PRC2, and H3K27me3 binding analysis along the chromatin of 23 biotimer-regulated genes revealed similar characteristics in the chromatin environment between these targets. In most cases, we detected AG binding peaks in the 5′ intergenic regions or near the TSS. The distribution of PRC2 components was localized in both the promoter and gene body, while H3K27me3 were often found over the gene body. We further confirmed the cell cycle–dependent dilution of H3K27me3 at the biotimer loci AHL18, PLATZ10, and IDD12 after AG binding and delayed transcriptional activation of these 3 genes, as observed with KNU (Figs. 3 and 4). These results suggested that AHL18, PLATZ10, and IDD12 could be regulated by an AG-mediated biotimer like KNU. Previously, it was shown that KNU is regulated by competitive binding between AG and PRC2 at its promoter (Sun et al. 2014). Consistent with this result, our ChIP-seq experiment showed higher AG binding as well as moderate FIE and EMF2 binding was observed at the KNU promoter. Additionally, we observed higher AG and moderate PRC2 binding at the AHL18, PLATZ10, and IDD12 promoters, suggesting that expression of those targets could be regulated by competitive binding between AG and PRC2. However, the binding positions between AG and PRC2 at the IDD12 promoter were slightly different. Although AG bound to the promoter of SHP1, only a faint signal of FIE and EMF2 binding was observed. Thus, a direct competition between AG and PRC2 may not be the only mechanism for biotimer regulation. Previously, it was shown that AG can access its target DNA sequence inside a nucleosome by opening up the chromatin and indirectly allow other factors to bind (Yamaguchi et al. 2018). Such secondary factors may also contribute to the regulation of biotimer.
AHL family members have been implicated in the initiation of leaf senescence, hypocotyl elongation, phytohormone biosynthesis, and flowering time regulation (Lim et al. 2007; Matsushita et al. 2007; Street et al. 2008; Ng et al. 2009; Yadeta et al. 2011; Zhou et al. 2013; Lee and Seo 2017; Wong et al. 2019). AHL18 was previously shown to be a regulator of lateral root development (Širl et al. 2020). In this study, we revealed that timing of AHL18 activation was important for stamen filament elongation and subsequent fertilization. At stage 3, AG bound to the AHL18 locus and PRC2 was evicted (Figs. 2 and 3). Once H3K27me3 at the AHL18 locus was diluted through cell division (Figs. 2 and 3), AHL18 expression was activated in the presumptive stamen filaments at stage 6 (Fig. 4). Misexpression of AHL18 from a constitutive promoter prevented proper stamen filament elongation (Fig. 8), suggesting that the timing of AHL18 expression controlled by the biotimer is biologically relevant to stamen development and fertilization. As is often the case with multigene families, we did not observe a difference between the WT and the ahl18 loss-of-function mutant during flower development (Supplemental Fig. S12), likely due to functional redundancy between family members. The closest homolog to AHL18 is AHL22 (Zhang et al. 2021a). The overexpression of AHL22 revealed that AHL22 controls timing of FLOWERING LOCUS T expression via chromatin remodeling (Yun et al. 2012). Although loss-of-function ahl22 mutants show no clear flowering phenotype, silencing of 4 AHL genes (AHL22, AHL18, AHL27, and AHL29) results in early flowering (Xiao et al. 2009). It would be interesting to examine whether these 4 genes are also redundantly involved in stamen filament elongation.
Because AHLs interact with transcription factors and histone variants and control the activation and repression of genes (Ng et al. 2009; Zhou et al. 2013), an analysis of the dynamics of chromatin accessibility and AHL18 binding sites in flower development indicated that AHL18 bound to open chromatin regions for subsequent action, rather than opening chromatin by itself. Several key AHL18 targets may have roles in stamen development. For example, MYB21, MYB24, and MYB57 redundantly control elongation of stamen filaments (Cheng et al. 2009). The myb21 myb24 myb57 triple mutant displays a short stamen phenotype leading to male sterility. Overexpression of MYB21 also results in retarded stamen development (Song et al. 2011). GPAT6 also plays multiple roles in stamen development and fertility. gpat6 exhibits male-sterile and short filament phenotypes (Song et al. 2011). MYB21 and GPAT6 are both expressed in stamen filaments. The overlapping expression pattern between AHL18, MYB21, and GPAT6 also supports the possibility of MYB21 and GPAT6 regulation by AHL18. Other targets might have important roles during stamen development. Further functional characterization is needed for the rest of the targets.
PLATZ10 is a transcription factor belonging to a class of plant-specific zinc-dependent DNA-binding proteins originally identified in pea (Pisum sativum) (Nagano et al. 2001; Liu et al. 2020) that selectively bind A/T-rich DNA sequences resulting in transcriptional repression (Nagano et al. 2001). Various PLATZ proteins have since been characterized in both model and agriculturally important plant species such as Arabidopsis, cotton (Gossypium hirsutum), maize (Zea mays), and rice (Oryza sativa), where they are associated mainly with abiotic stress responses, namely, seed desiccation tolerance, salt stress response, and endosperm development (Liu et al. 2020; Zhang et al. 2018; Wang et al. 2018, 2019, 6; Li et al. 2017; González-Morales et al. 2016). In Arabidopsis, PLATZ1 and PLATZ2 play important roles in seed desiccation tolerance as major nodes of the underlying transcriptional networks (González-Morales et al. 2016), while PLATZ2 also negatively affects the salt stress response by directly repressing genes from the salt overly sensitive pathway (Liu et al. 2020).
Like KNU and AHL18, PLATZ10 expression was controlled by the AG-mediated biotimer. We detected AG binding and PRC2 eviction at KNU, AHL18, and PLATZ10 loci after stage 3 (Figs. 2 and 3). A major difference between KNU, AHL18, and PLATZ10 loci was the length of their H3K27me3 region. Approximately 2 kb of PLATZ10 gene body was entirely covered with H3K27me3 (Figs. 2 and 3). Hence, more cell division should be needed to passively dilute H3K27me3 before PLATZ10 activation based on our mathematical model (Fig. 4). Indeed, PLATZ10 was expressed in anthers from stage 9 of flower development, with a significantly later timing than KNU and AHL18. Although many AG targets have been identified and characterized (Pelayo et al. 2021), knowledge about their roles during floral organ development is still limited. Thus, functional analysis of PLATZ10 will be critical to dissect its precise roles during flower development.
IDD12 is a member of the IDD family of transcription factors, a conserved subfamily of the C2H2 family in plants (Kumar et al. 2019). IDDs are distinguished by their 4 zinc-finger motifs that are important for DNA and RNA binding (Kumar et al. 2019). Recent studies have elucidated their roles in various aspects of plant growth and development, namely, sugar signaling modulation in the photoperiodic transition to flowering, regulation of starch metabolism in response to cold stress, seed development, plant architecture, phytohormone signaling, and ammonium metabolism (Kumar et al. 2019). Like KNU and AHL18, IDD12 was regulated by direct AG binding, PRC2 removal, and cell cycle–dependent H3K27me3 dilution (Figs. 2 and 3). IDD12 was induced 2 d after AG induction, suggesting that IDD12 was activated at stages 5 to 6 (Fig. 4). IDD12 was recently identified as an upstream regulator of CRC (Gross and Becker 2021). Consistent with our expression analysis and mathematical modeling, IDD12 expression is highest at stage 5 based on laser microdissection followed by RNA-seq (Gross and Becker 2021). Yeast 1-hybrid analysis revealed that IDD12 binds to the proximal region of the CRC promoter (Gross and Becker 2021). In addition, CRC expression was reduced in idd12 mutants (Gross and Becker 2021). Although it is not clear what the role of IDD12 is during flower development, AG may promote CRC expression via direct IDD12 activation for FM determinacy. In this regard, the crc mutant is a sensitized genetic tool to understand biological function, as it often strongly enhances mutant phenotypes (Prunet et al. 2008; Yamaguchi et al. 2017). The crc idd12 double mutant would therefore be an interesting material to generate for molecular genetic analysis.
In this study, we developed a mathematical model to predict stochastic dynamics of repressive histone modifications by the AG-mediated epigenetic biotimer in a cell cycle–dependent manner (Fig. 4). The model predicted not only timing of KNU expression, but also that of AHL18 and PLATZ10 (Fig. 4). In addition, our findings are supported by manipulating the number of H3K27me3 domains within the KNU locus driving the expression of a GUS reporter. More copies of del resulted in a progressive delay of KNU temporal activation (Fig. 5). Using the proKNU:KNU-GUS reporter, we confirmed that the GUS staining pattern paralleled that of endogenous KNU expression, with staining during stages 5 to 8 (Fig. 5, E to H), in agreement with the typical time delay observed for KNU expression after AG activation beginning at floral stage 3 (Sun et al. 2014). The KNU coding sequence is 451 bp in length, equivalent to ∼3 nucleosomes of ∼150 to 200 bp of DNA each wrapped around the histone octamer and each marked with H3K27me3. The del region from the second half of the KNU coding sequence consists of 231 bp, roughly corresponding to 1 nucleosome, is bound by FIE, and is decorated by H3K27me3 marks. Each cell division cycle from floral stage 3 onward may result in the removal of H3K27me3 on a marked nucleosome at KNU, suggesting that stage 4 proliferating cells may be a mixture of cells with 1 nucleosome without H3K27me3 (initial cells) or with 2 unmarked nucleosomes (cell progeny cells) (Fig. 10A). We also hypothesize that the H3K27me3 marks diluted during cell division reside along the del region due to the demonstrated role of this region in regulating KNU temporal activation. By stage 5, H3K27me3 marks may thus be completely removed in some cells, which start to exhibit KNU expression (Fig. 10A). By stage 6, KNU expression reaches its peaks as most proliferating cells are now unmarked by H3K27me3 (Fig. 10A). This time frame corresponds to the proper timing of FM termination to ensure the development of proper numbers of floral organs. By stage 7 and until stage 8, KNU expression decreases (Fig. 5, G and H). Whether KNU repression is also modulated by the balance between AG and PRC2 after stage 8 remains to be demonstrated.
Figure 10.
Proposed model for biotimer-regulated genes and their manipulation. A) Biotimer regulation for KNU, IDD12, AHL18, and PLATZ10 loci. Prior to AG accumulation, PRC2 deposits H3K27me3 along the gene bodies of KNU, IDD12, AHL18, and PLATZ10 loci for transcriptional silencing. At stage 3 of flower development, AG accumulation leads to PRC2 displacement at the KNU, IDD12, AHL18, and PLATZ10 loci. After sufficient cell proliferation, cells free from H3K27me3 marks at the KNU, IDD12, AHL18, and PLATZ10 loci are produced and biotimer-regulated genes are transcribed. B) Biotimer manipulation by iterative addition of H3K27me3-enriched regions. The first 2 steps are identical to the endogenous regulation of KNU, IDD12, AHL18, and PLATZ10 expression. Even at stage 6, H3K27me3 dilution in most cells remains incomplete; hence, 6del expression is delayed.
When using 6del, we observed a prolonged delay in KNU activation (Fig. 5, N to R), with the first signs of transcriptional activation at stage 6 instead of stage 5 for proKNU:KNU-GUS. We propose that this prolonged delay is due to a greater number of nucleosomes marked with H3K27me3 occupying the KNU locus, leading to a partial dilution of H3K27me3 marks by stage 5 (Fig. 10B). Based on this model, H3K27me3 removal would still be incomplete by stage 6 in most cells, with the minimal GUS signal observed at this stage likely originating from a small pool of dividing cells in which H3K27me3 and FIE have been removed from the del copies (Fig. 10B). At stage 7, H3K27me3 removal may still be incomplete in dividing cells, explaining the reduced GUS signal detected in 6del peaks compared to the peak KNU expression in the WT at stage 6. KNU repression by stage 7 may also contribute to this reduction. By stage 8, KNU expression is undetectable in 6del, suggesting that the additional del regions augment KNU repression via H3K27me3 deposition. We have not examined whether GUS regions in the proKNU:KNU-GUS and 6del transgenic lines are enriched with H3K27me3. Because of our prediction by mathematical modeling, we are currently thinking that these 2 transgenic lines do not contain H3K27me3 in the GUS regions. However, our proAHL18:GUS or proPLATZ10:GUS constructs, which do not include coding regions, showed proper timing of gene activation as predicted by mathematical modeling. Thus, H3K27me3 could be introduced into the GUS regions at least in these 2 constructs. Therefore, each regulatory component may function differently when we use them for epigenetic manipulation of gene expression. Further analysis is required for a precise understanding of regulatory components. H3K27me3 deposition on the extended KNU region appears to be modulated by the PRC2 complex, based on the earlier and stronger KNU induction of 6del in the fie and clf backgrounds. An intact PRC2 machinery is thus necessary for proper KNU temporal activation.
As H3K27me3 removal along KNU is cell division dependent, more cell cycles are needed to completely dilute H3K27me3 marks along the extended region in 6del, contributing to the observed delay and reduction in KNU expression. In agreement with this notion, we observed an earlier and stronger KNU induction from the 6del construct in the krp-q mutant background, indicating that proper cell cycle progression is necessary for KNU temporal activation by facilitating H3K27me3 removal. We also observed a delay in KNU activation by growing plants at 18 °C, likely due to slower growth kinetics. This observation emphasizes the dynamic regulation of H3K27me3 in response to extracellular and intracellular cues and suggests a role for the cell cycle–dependent biotimer in coordinating the balance between cell proliferation and differentiation. Additional experiments will be necessary to clarify the effect of lower temperature conditions on the cell cycle and on biotimer regulation.
Overall, our findings highlight the complex and dynamic nature of H3K27me3 regulation for transcriptional activation in the context of FM termination. We identified candidate biotimer genes and proposed a mechanistic perspective of the biotimer regulation modulated by PRC2 activity, chromatin environment, and the cell cycle. Future work will focus on modulating biotimer regulation based on targets other than KNU by investigating the minimal sequences marked with H3K27me3.
Materials and methods
Plant material and growth conditions
The Arabidopsis (A. thaliana) accessions Ler and Col-0 were used in this study. The ap1 cal pro35S:AP1-GR, ap1 cal FIE-VENUS pro35S:AP1-GR, ap1 cal EMF2-VENUS pro35S:AP1-GR, ag-1 pro35S:AG-GR, ag (SALK_14999), proCYCB1;2:CYCB1;2-YFP pro35S:CYCD3, ahl18 (SAIL_346_C06), proAHL18:GUS, gAHL18-mRuby, and pro35S:AHL18 lines were described previously (Zhou et al. 2003; Ito et al. 2004; Wellmer et al. 2006; Iwata et al. 2011; Sun et al. 2014; Širl et al. 2020). proKNU:KNU-GUS (Sun et al. 2009), proPLATZ10:GUS, and the del series, as well as the CFP and 2×CFP controls, were in the Ler background. proAHL18:GUS, gAHL18-mRuby, and pro35S:AHL18-GFP were in the Col-0 background. The PRC2 and cell cycle mutants used for crossing with proKNU:KNU-GUS and 6del were in Col-0. The pro35S:FIE-GFP line was described previously (Katz et al. 2004). clf-28 is a SALK T-DNA insertion line (SALK_139371) (Doyle and Amasino 2009), while krp1 krp2 krp3 krp4 krp7 was previously generated by crossing single SALK and GABI-kat lines (SALK_026391, SALK_130744, GABI-kat 185C07, SALK_102417, and GABI-kat 841D12, respectively) (Cheng et al. 2013). 6del plants were typically used as mother plants in the crosses to generate the fie and krp-q lines. For fie, lines of interest were selected by Basta treatment (from F1s) and then selected by both Basta treatment and phenotype from F2 generations onward. For krp-q, lines of interest were selected by Basta treatment and genotyping from F1s onward. fie lines used in the study were from the F4 to F5 generation, while krp-q lines were from the F5 to F6 generation. Seeds were sown in pots containing a mixture of soil and vermiculite (1:2, w/w) supplemented with Hyponex (1/1,000) upon germination. Sown seeds were stratified at 4 °C in the dark for 3 to 4 d before being transferred to long-day (16-h light/8-h dark) conditions at 22 °C or kept at 18 °C in constant light. For further plant growth, plant growth chambers with Plant Flec LED lamp (15,000 lx; Nippon Medial & Chemical instruments) were used. Primers used for genotyping are listed in Supplemental Table S2.
Chemical treatments
For ap1 cal pro35S:AP1-GR, ap1 cal FIE-VENUS pro35S:AP1-GR, ap1 cal EMF2-VENUS pro35S:AP1-GR, and ag-1 pro35S:AG-GR chemical treatments, a working concentration of 1 µm DEX aqueous solution was used with 0.015% (v/v) Silwet L-77. Plants used as negative controls were not treated.
For olo treatment, ap1 cal pro35S:AP1-GR and ag-1 pro35S:AG-GR plants (no more than 8 cm in height) were cut at the base of the stem upon bolting and placed in half-strength liquid Murashige and Skoog (MS) solution in 50-mL Falcon tubes for 3 d under long-day conditions at 22 °C. A solution consisting of half-strength liquid MS medium with 10 µm DEX was used for the control treatment, while the olo treatment contained 10 µm DEX and 750 µm olo. Inflorescences were collected on the third day and were trimmed to contain floral buds up to stage 12 before flash-freezing in liquid nitrogen.
ChIP-seq
ChIP-seq was conducted using 2 biological replicates, as described previously (Yamaguchi et al. 2021). For immunoprecipitation, anti-GFP (SAB4701015), anti-H3K27me3 (MABI0323), and anti-RFP (for mRuby; M208-3) antibodies were used. Quality check and trimming of the raw sequencing reads were conducted by FastQC (version 0.11.7) and Trimmomatic (version 0.38), respectively. Using the resulting fastq files, mapping was performed by Bowtie2 (version 2.3.4.2). Then, broad peaks were called by SICER (version 1.1). Reads were counted using featureCounts (version 1.6.3). Heatmaps were generated by deeptools (version 3.2.1). The ChIP-seq data have been deposited in the DDBJ database (DRA015419 and DRA15595).
Data analysis
For the ag-1 microarray experiments, we obtained data using ag-1 ap1-1 cal-1 pro35S:AP1-GR from Ó Maoiléidigh et al. (2013) (GSE45939). According to their publication, 4 biological replicates were generated. The data were filtered by setting the P-value cutoff to 0.05 and selecting the resulting genes from stages 3 to 4 and stages 6 to 7. The AG ChIP-seq data were also obtained from Ó Maoiléidigh et al. (2013) through NCBI (GSE45939). According to their publication, 2 biological replicates were prepared. The corresponding AG binding data were combined and sorted by setting the q-value cutoff to 1.0 × 10−4. Each filtered data set was then uploaded to a web-based custom Venn diagram drawing tool (http://bioinformatics.psb.ugent.be/webtools/Venn/). The heatmap was generated using WebMeV (http://mev.tm4.org). The browser views of ChIP sequencing signals for AG and PRC2 binding sites and H3K27me3 were generated using Integrative Genomics Viewer (IGV) (Thorvaldsdóttir et al. 2013). GO term analysis and visualization were performed using agriGO v2.0 (http://systemsbiology.cau.edu.cn/agriGOv2/) followed by GO term refinement using REVIGO (http://revigo.irb.hr/).
Plasmid construction
To generate the KNU reporter with tandem repeats, site-directed mutagenesis was performed to insert BglII and BamHI restriction sites at positions +594 and +1 (with the translation start site counting as +1) before the GUS reporter gene to create proKNU:KNU-GUS (BglII/BamHI). The KNU tandem repeat includes a partial KNU coding sequence from nucleotides +367 to +594 (1del); the 2× and 3× repeats were synthesized by GenScript Services (www.genscript.com). The synthesized 2× and 3× repeats were digested with BglII and BamHI. The proKNU:KNU-GUS (BglII/BamHI) construct was digested in a similar fashion. Subsequently, ligation was performed to create constructs carrying different numbers of tandem repeats, 2del to 6del. These constructs were recombined into Gateway vectors (Thermo Fisher Scientific) by LR recombination according to the manufacturer's instructions (ATMI). Primers used for cloning are listed in Supplemental Table S2.
To generate the PLATZ10 promoter construct, the 5′ intergenic region of the PLATZ10 promoter was amplified by PCR using PrimeSTAR GXL polymerase (Takara). The resulting PCR product was gel-purified and TOPO-cloned into the pENTR/dTOPO vector. After sequencing, the DNA fragment was recombined into the destination vector pGBWSF7.0 by LR clonase (Thermo Fisher Scientific).
To create gKNU-CFP-GUS and gKNU-2xCFP-GUS constructs, 1xECFP and 2xECFP were amplified from pMD20-2XECFP. The resulting PCR fragments were then inserted into pCR8-proKNU:KNU-GUS (Sun et al. 2009) via a 2-step omega-PCR strategy as described (Chen et al. 2013) to create pCR8-proKNU:KNU-1XECFP-GUS and pCR8-proKNU:KNU-2XECFP-GUS. Next, the vectors were introduced into the CD3-694 destination vector (or pEarleyGate303) via LR reaction (Thermo Fisher Scientific).
Plant transformation and selection
All resulting constructs were transformed into the Ler background via the floral dip method using Agrobacterium (Agrobacterium tumefaciens) strain GV3101 as described previously (Weigel and Glazebrook 2002). Basta resistance and GUS staining were used to select transgenic T1 transformants. Single-copy transgenic lines were selected by checking the segregation ratio for Basta resistance among the T2 progeny produced by self-pollination consistent with the expected Mendelian inheritance ratio for 1 transgene (3:1) (Lebedev 2022). After identifying single-insertion T2 lines, they were propagated to the T3 generation.
GUS staining
The primary floral bud cluster from the main inflorescence shoot of plants ∼3 to 10 cm in height was collected and fixed in ice-cold 90% (v/v) acetone for 20 min. The samples were then rinsed 2 to 3 times with Milli-Q H2O and then washed 2 to 3 times with GUS staining buffer (without X-Gluc). The tissues were then infiltrated with GUS staining solution (with X-Gluc) under vacuum for 30 to 60 s and incubated at 37 °C for 16 h. After incubation, the staining solution was replaced with 70% (v/v) ethanol 2 to 3 times until the tissues cleared (∼1 wk at room temperature).
4-Methylumbelliferyl β-d-glucuronide assay
To quantitatively measure GUS activity, fluorescence measurements were taken for the conversion of 4-methylumbelliferyl β- d-glucuronide (4-MUG) to 4-methyl umbelliferone (4-MU) in the presence of GUS. Up to 10 primary inflorescences containing floral buds until stage 12 were collected per biological replicate and flash-frozen in liquid nitrogen. The procedure described by Weigel and Glazebrook (2002) was followed with minor modifications. For protein extraction, the tissues were crushed in GUS extraction buffer on ice. After centrifugation, the supernatants were stored at −80 °C until use. An aliquot was also set aside to measure protein concentration with Qubit 4 (Thermo Fisher Scientific).
For the MUG assay, the extract was added to extraction buffer mixed with 1 mm 4-MUG, hereafter referred to as the reaction mix. Half of the reaction mix was taken out immediately and stopped by the addition of 0.2 m Na2CO3 and designated as the 0-h reaction. The remaining reaction mix was incubated at 37 °C for 1 h. After incubation, the reaction mix was added to the stop buffer and designated as the 1-h reaction. The 0- and 1-h reaction mixes were dispensed in triplicate in 96-well plates, and fluorescence measurements were taken with a TriStar2 LB942 microplate reader (Berthold Technologies). Excitation and emission were at 355 and 460 nm, respectively. A 4-MU dilution series was prepared to create a 4-MU fluorescence standard curve to determine GUS activity from all samples as the amount of substrate converted to 4-MU relative to the starting protein concentration, reaction volume, and reaction duration.
Sectioning
Following GUS staining, plant tissues were dehydrated in a graded ethanol series (80% [v/v], 90%, 95%, and 100%) and embedded in Technovit 7100 resin (Heraeus). Tissue sections (10 µm) were produced using a RM2255 microtome (Leica). The sections were dried on a 42 °C hot plate (Sakura Finetek), stained with 0.05% (w/v) neutral red, and mounted on a microscope slide with 500 µL MountQuick (Daido Sangyo). An Axio Scope A1 microscope (ZEISS) was used to observe the sections of 3 or more floral buds per flower developmental stage.
RT-qPCR
Total RNA was extracted from primary floral bud clusters of the main inflorescence shoot (up to 10 per biological replicate), collected, and ground in liquid nitrogen. RNA extraction and clean-up was performed using an RNeasy kit (Qiagen) with an in-column DNase I digest. PrimeScript RT Master Mix system (Takara) was used for first-strand cDNA synthesis. The subsequent qPCR reaction mix was prepared using a FastStart Essential DNA Green Master (Roche). qPCR was performed with a Light Cycler 480 (Roche) instrument using the Light Cycler 480 release 1.5.1.62 SP software (Roche). Relative transcript abundance across 3 biological replicates was calculated using the comparative CT method; statistical analyses were performed using a 2-tailed Student’s t test. The sequences of primer pairs used for genes of interest and the reference gene TUBULIN2 are listed in Supplemental Table S2.
ChIP
To quantify H3K27me3 and H3 enrichment, the ChIP protocol described by Yamaguchi et al. (2014) was followed with minor modifications. Primary inflorescences (300 to 600 mg) were fixed in 1% (w/v) formaldehyde for 15 min, flash-frozen in liquid nitrogen, and stored at −80 °C until use. Chromatin was extracted by nuclei isolation and sonicated to yield 500-bp fragments. Protein A beads (Thermo Fisher Scientific) were used for preclearing the chromatin by incubation for 1 h at 4 °C on a rotating device. The beads were separated from the solution using a magnetic stand, and an aliquot (2%) was taken out from the cleared solution as 2% input sample. For immunoprecipitation, anti-GFP (SAB4701015), anti-H3K27me3 (ab6002), anti-H3 (ab1791), and anti-AG (Ito et al. 2004) antibodies were used. The antibody of interest was added to the remaining cleared solution and incubated overnight at 4 °C. For immunoprecipitation, beads were added to the samples and incubated at 4 °C for 6 h, followed by washing 2 times each with low-salt buffer, high-salt buffer, LiCl buffer, and Tris-HCl EDTA buffer. Samples were incubated at 65 °C for 1 h to elute the DNA. Both the ChIP and input samples were incubated overnight at 65 °C for reverse crosslinking. DNA was purified with a QIAquick PCR purification kit (Qiagen). For qPCR, primers spanning the del–GUS junction yielding a 500-bp amplicon were used. EIF4 and TA3 served as negative controls. The percent input method was used to normalize the resulting qPCR data. Primers used for qPCR are listed in Supplemental Table S2.
Confocal microscopy
To observe GFP signals in FMs, vibratome sections were produced using the primary floral bud clusters of the main inflorescence shoot. After removal of young floral buds up to stage 8 with forceps, tissues were embedded in 5% (w/v) agar. The resulting agar blocks were sliced with a DTK-1000N vibratome (Dosaka). Sections were placed on glass slides (Matsunami), mounted with a drop of water, covered by a cover glass (Matsunami), and observed with a TCS SP8 confocal microscope (Leica). Image acquisition parameters for GFP were as follows: 488 white laser 6%, HyD 500 to 590 nm, and detector gain 100. At least 6 FMs from different floral developmental stages and temperature conditions were observed. Representative images are shown in the figures.
Scanning electron microscopy
Scanning electron microscopy (SEM) was conducted as described previously (Yamaguchi et al. 2017). Briefly, flowers at stage 14 were harvested from 5- to 10-cm-long inflorescences and fixed in FAA overnight. After dehydration using a graded ethanol series, tissues were incubated in acetone. Critical drying and spattering were conducted with an HCP-2 critical point dryer (Hitachi) and gold-coated with E-1010 (Hitachi). The resulting tissues were observed with a S-4700 microscope (Hitachi).
Statistical analysis
Statistical tests were performed as described in the figure legends and text. Statistical data are provided in Supplemental Data Set 5.
Accession numbers
Sequencing data were deposited at the DDBJ under accession number DRA015419 (FIE, EMF2, and H3K27me3 ChIP-seq) and DRA015595 (AHL18 ChIP-seq). The ag microarray and AG ChIP-seq data were obtained from Ó Maoiléidigh et al. (2013) through NCBI (GSE45939). Sequence data of the genes used in this study can be found on TAIR website under the following accession numbers: AG (At4g18960), KNU (At5g14010), FIE (At3g20740), EMF2 (At5g51230), AHL18 (At3g60870), PLATZ10 (At3g60670), IDD12 (At4g02670), MYB21 (At3g27810), GPAT6 (At2g38110), and CDKD1;1 (At1g73690).
Supplementary Material
Acknowledgments
We thank Akie Takahashi, Hiroko Egashira, Rie Shimano, and Mizuki Shinoda for technical assistance, Sachi Ando for checking the draft of this manuscript, Yukiko Nakayama for revising the model figures, and Dr. Sun Bo for sharing pro35S:FIE-GFP. Computations were partially performed on the National Institute of Genetics (NIG) supercomputer at the Research Organization of Information and Systems, NIG.
Contributor Information
Margaret Anne Pelayo, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan.
Fumi Morishita, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan.
Haruka Sawada, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan.
Kasumi Matsushita, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan.
Hideaki Iimura, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan.
Zemiao He, Temasek Life Sciences Laboratory, National University of Singapore, Singapore 117604, Singapore.
Liang Sheng Looi, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan; Temasek Life Sciences Laboratory, National University of Singapore, Singapore 117604, Singapore.
Naoya Katagiri, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan.
Asumi Nagamori, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan.
Takamasa Suzuki, Department of Biological Chemistry, College of Bioscience and Biotechnology, Chubu University, Kasugai 487-8501, Japan.
Marek Širl, Department of Experimental Plant Biology, Faculty of Science, Charles University, Prague 12844, Czech Republic.
Aleš Soukup, Department of Experimental Plant Biology, Faculty of Science, Charles University, Prague 12844, Czech Republic.
Akiko Satake, Department of Biology, Faculty of Science, Kyushu University, Nishi-ku 819-0395, Japan.
Toshiro Ito, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan; Temasek Life Sciences Laboratory, National University of Singapore, Singapore 117604, Singapore.
Nobutoshi Yamaguchi, Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, Ikoma 630-0192, Japan.
Author contributions
The contributions in detail are as follows: M.A.P., A.S., T.I., and N.Y. designed the research; M.A.P., F.M., H.S., K.M., H.I., Z.H., L.S.L., N.K., A.N., T.S., M.Š., Al.S., Ak.S., and N.Y. performed the research and analyzed the data; M.A.P. and N.Y. wrote the manuscript with the help from F.M., H.S., K.M., H.I., Z.H., L.S.L., N.K., A.N., T.S., M.Š., Al.S., Ak.S., and T.I.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. SPL/NZZ is immediately induced in DEX-treated ag-1 pro35S:AG-GR plants.
Supplemental Figure S2. CDKB1;1 is repressed by olo in DEX-treated ag-1 pro35S:AG-GR plants.
Supplemental Figure S3. Expression of biotimer-regulated genes in the ag-1 mutant.
Supplemental Figure S4. Classification of the GUS staining pattern in T1 plants into 3 categories.
Supplemental Figure S5. KNU fused to CFP and 2XCFP results in similar T1 GUS signal strength as proKNU:KNU-GUS.
Supplemental Figure S6. The spatiotemporal GUS staining pattern is similar for proKNU:KNU-GUS, 3del, and 6del T1 plants in the same signal category.
Supplemental Figure S7. Endogenous KNU mRNA levels are similar in proKNU:KNU-GUS and 6del transgenic plants.
Supplemental Figure S8. Occurrence of the histone variant H3 is comparable in proKNU:KNU-GUS and 6del transgenic plants.
Supplemental Figure S9. 6del has a significantly higher H3K27me3 enrichment than proKNU:KNU-GUS when H3K27me3 ChIP results are normalized to H3 ChIP results.
Supplemental Figure S10. AG expression in fie background.
Supplemental Figure S11. proKNU:KNU-GUS and 6del expression pattern in the WT and pro35S:CYCD3;1.
Supplemental Figure S12. Flower phenotypes in the WT and ahl18.
Supplemental Table S1. Candidates of biotimer-regulated genes.
Supplemental Table S2. Primers used in this study.
Supplemental Data Set 1. Results of time-course ChIP experiments.
Supplemental Data Set 2. Genome-wide annotation of biotimer-regulated genes.
Supplemental Data Set 3. GO term analysis of 242 biotimer candidate genes.
Supplemental Data Set 4. Results of AHL18 ChIP experiments.
Supplemental Data Set 5. Statistical data for this study.
Funding
This work was supported by a grant from a JSPS KAKENHI Grant-in-Aid for Scientific Research B (nos. 18H02465 and 23H02503), Grant-in-Aid for Transformative Research Areas (A) (nos. 21H05663 and 23H04968), a grant from the SECOM Science and Technology Foundation to N.Y., a grant from the Ohsumi Frontier Science Foundation to N.Y., a JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas (20H04888), a JSPS KAKENHI Grant-in-Aid for Scientific Research A (20H00470), a Grant-in-Aid for challenging Exploratory Research (no. 21K19266), and a Grant-in-Aid for Transformative Research Area (A) (no. 22H05176) to T.I.
References
- Alabert C, Barth TK, Reverón-Gómez N, Sidoli S, Schmidt A, Jensen ON, Imhof A, Groth A. Two distinct modes for propagation of histone PTMs across the cell cycle. Genes Dev. 2015:29(6):585–590. 10.1101/gad.256354.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyanagi T, Ikeya S, Kobayashi A, Kozaki A. Gene regulation via the combination of transcription factors in the INDETERMINATE DOMAIN and GRAS families. Genes (Basel) 2020:11(6):613. 10.3390/genes11060613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemer M, Grossniklaus U. Dynamic regulation of polycomb group activity during plant development. Curr Opin Plant Biol. 2012:15(5):523–529. 10.1016/j.pbi.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Bogliotti YS, Ross PJ. Mechanisms of histone H3 lysine 27 trimethylation remodeling during early mammalian development. Epigenetics 2012:7(9):976–981. 10.4161/epi.21615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollier N, Sicard A, Leblond J, Latrasse D, Gonzalez N, Gévaudant F, Benhamed M, Raynaud C, Lenhard M, Chevalier C, et al. At-MINI ZINC FINGER2 and SI-INHIBITOR OF MERISTEM ACTIVITY, a conserved missing link in the regulation of floral meristem termination in Arabidopsis and tomato. Plant Cell 2018:30(1):83–100. 10.1105/tpc.17.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco CD, Lezhneva L, Biehl A, Leister D, Strotmann H, Wanner G, Meurer J. Inactivation of the chloroplast ATP synthase γ subunit results in high non-photochemical fluorescence quenching and altered nuclear gene expression in Arabidopsis thaliana. J Biol Chem. 2004:279(2):1060–1069. 10.1074/jbc.M308435200 [DOI] [PubMed] [Google Scholar]
- Chen L, Wang F, Wang X, Liu Y-G. Robust one-tube Ω-PCR strategy accelerates precise sequence modification of plasmids for functional genomics. Plant Cell Physiol. 2013:54(4):634–642. 10.1093/pcp/pct009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Cao L, Wang S, Li Y, Shi X, Liu H, Li L, Zhang Z, Fowke LC, Wang H, et al. Downregulation of multiple CDK inhibitor ICK/KRP genes upregulates the E2F pathway and increases cell proliferation, and organ and seed sizes in Arabidopsis. Plant J. 2013:75(4):642–655. 10.1111/tpj.12228 [DOI] [PubMed] [Google Scholar]
- Cheng H, Song S, Xiao L, Soo HM, Cheng Z, Xie D, Peng J. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 2009:5(3):e1000440. 10.1371/journal.pgen.1000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman ML, Brown ML, Kato N, Kirik V, Hülskamp M, Inzé D, De Veylder L, Walker JD, Zheng Z, Oppenheimer DG, et al. SIAMESE, a plant-specific cell cycle regulator, controls endoreplication onset in Arabidopsis thaliana. Plant Cell 2006:18(11):3145–3157. 10.1105/tpc.106.044834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mambro R, De Ruvo M, Pacifici E, Salvi E, Sozzani R, Benfey PN, Busch W, Novak O, Ljung K, Di Paola L, et al. Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc Natl Acad Sci USA. 2017:114(36):E7641–E7649. 10.1073/pnas.1705833114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MR, Amasino RM. A single amino acid change in the enhancer of zeste ortholog CURLY LEAF results in vernalization-independent, rapid flowering in Arabidopsis. Plant Physiol. 2009:151(3):1688–1697. 10.1104/pp.109.145581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston A, Cesari F, Skipper M. Transcription and epigenetics. Nature 2013:502(7472):461. 10.1038/502461a [DOI] [PubMed] [Google Scholar]
- Frigerio M, Alabadí D, Pérez-Gómez J, García-Cárcel L, Phillips AL, Hedden P, Blázquez MA. Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol. 2006:142(2):553–563. 10.1104/pp.106.084871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Shen YP, Ma LG, Pan Y, Du YL, Wang DH, Yang JY, Hu LD, Liu XF, Dong CX, et al. Genome-Wide ORFeome cloning and analysis of Arabidopsis transcription factor genes. Plant Physiol. 2004:135(2):773–782. 10.1104/pp.104.042176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Morales SI, Chávez-Montes RA, Hayano-Kanashiro C, Alejo-Jacuinde G, Rico-Cambron TY, de Folter S, Herrera-Estrella L. Regulatory network analysis reveals novel regulators of seed desiccation tolerance in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2016:113(35):E5232–E5241. 10.1073/pnas.1610985113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross T, Becker A. Transcription factor action orchestrates the complex expression pattern of CRABS CLAW in Arabidopsis. Genes (Basel) 2021:12(11):1663. 10.3390/genes12111663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Sawada Y, Kuromori T, Klausnitzer R, Saito K, Toyoda T, Shinozaki K, Li W-H, Hirai MY. Functional compensation of primary and secondary metabolites by duplicate genes in Arabidopsis thaliana. Mol Biol Evol. 2011:28(1):377–382. 10.1093/molbev/msq204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima H, Sekine M. Cyclin-dependent protein kinases in the control of cell cycle in plants. In: Protein kinases and stress signaling in plants. New York: John Wiley & Sons, Ltd; 2020. p. 347–368. [Google Scholar]
- Huang R, Huang T, Irish VF. Do epigenetic timers control petal development? Front Plant Sci. 2021:12:709360. 10.3389/fpls.2021.709360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Iwase A, Sugimoto K. Control of plant cell differentiation by histone modification and DNA methylation. Curr Opin Plant Biol. 2015:28:60–67. 10.1016/j.pbi.2015.09.004 [DOI] [PubMed] [Google Scholar]
- Ito T, Ng K-H, Lim T-S, Yu H, Meyerowitz EM. The homeotic protein AGAMOUS controls late stamen development by regulating a jasmonate biosynthetic gene in Arabidopsis. Plant Cell 2007:19(11):3516–3529. 10.1105/tpc.107.055467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Wellmer F, Yu H, Das P, Ito N, Alves-Ferreira M, Riechmann JL, Meyerowitz EM. The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 2004:430(6997):356–360. 10.1038/nature02733 [DOI] [PubMed] [Google Scholar]
- Iwata E, Ikeda S, Matsunaga S, Kurata M, Yoshioka Y, Criqui M-C, Genschik P, Ito M. GIGAS CELL1, a novel negative regulator of the anaphase-promoting complex/cyclosome, is required for proper mitotic progression and cell fate determination in Arabidopsis. Plant Cell 2011:23(12):4382–4393. 10.1105/tpc.111.092049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol. 2005:15(17):1560–1565. 10.1016/j.cub.2005.07.023 [DOI] [PubMed] [Google Scholar]
- Katz A, Oliva M, Mosquna A, Hakim O, Ohad N. FIE And CURLY LEAF polycomb proteins interact in the regulation of homeobox gene expression during sporophyte development. Plant J. 2004:37(5):707–719. 10.1111/j.1365-313X.2003.01996.x [DOI] [PubMed] [Google Scholar]
- Kim D-H, Sung S. Polycomb-mediated gene silencing in Arabidopsis thaliana. Mol Cells. 2014:37(12):841–850. 10.14348/molcells.2014.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Larkin JC. Why do plants need so many cyclin-dependent kinase inhibitors? Plant Signal Behav. 2017:12(2):e1282021. 10.1080/15592324.2017.1282021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Le DT, Hwang S, Seo PJ, Kim HU. Role of the INDETERMINATE DOMAIN genes in plants. Int J Mol Sci. 2019:20(9):2286. 10.3390/ijms20092286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusk S, Sohlberg JJ, Long JA, Fridborg I, Sundberg E. STY1 and STY2 promote the formation of apical tissues during Arabidopsis gynoecium development. Development 2002:129(20):4707–4717. 10.1242/dev.129.20.4707 [DOI] [PubMed] [Google Scholar]
- Kwaśniewska K, Breathnach C, Fitzsimons C, Goslin K, Thomson B, Beegan J, Finocchio A, Prunet N, Ó’Maoiléidigh DS, Wellmer F. Expression of KNUCKLES in the stem cell domain is required for its function in the control of floral meristem activity in Arabidopsis. Front Plant Sci. 2021:12:704351. 10.3389/fpls.2021.704351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WKM, Pugh BF. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat Rev Mol Cell Biol. 2017:18(9):548–562. 10.1038/nrm.2017.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev V. Stability of transgene inheritance in progeny of field-grown pear trees over a 7-year period. Plants 2022:11(2):151. 10.3390/plants11020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Seo PJ. Coordination of matrix attachment and ATP-dependent chromatin remodeling regulate auxin biosynthesis and Arabidopsis hypocotyl elongation. PLoS One 2017:12(7):e0181804. 10.1371/journal.pone.0181804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard M, Bohnert A, Jürgens G, Laux T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 2001:105(6):805–814. 10.1016/S0092-8674(01)00390-7 [DOI] [PubMed] [Google Scholar]
- Li Q, Wang J, Ye J, Zheng X, Xiang X, Li C, Fu M, Wang Q, Zhang Z, Wu Y. The maize imprinted gene Floury3 encodes a PLATZ protein required for tRNA and 5S rRNA transcription through interaction with RNA polymerase III. Plant Cell 2017:29(10):2661–2675. 10.1105/tpc.17.00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-C, Zhu J, Yang J, Zhang G-R, Xing W-F, Zhang S, Yang Z-N. Glycerol-3-phosphate acyltransferase 6 (GPAT6) is important for tapetum development in Arabidopsis and plays multiple roles in plant fertility. Mol Plant. 2012:5(1):131–142. 10.1093/mp/ssr057 [DOI] [PubMed] [Google Scholar]
- Lim PO, Kim Y, Breeze E, Koo JC, Woo HR, Ryu JS, Park DH, Beynon J, Tabrett A, Buchanan-Wollaston V, et al. Overexpression of a chromatin architecture-controlling AT-hook protein extends leaf longevity and increases the post-harvest storage life of plants. Plant J. 2007:52(6):1140–1153. 10.1111/j.1365-313X.2007.03317.x [DOI] [PubMed] [Google Scholar]
- Liu J, Deng S, Wang H, Ye J, Wu H-W, Sun H-X, Chua N-H. CURLY LEAF regulates gene sets coordinating seed size and lipid biosynthesis. Plant Physiol. 2016:171(1):424–436. 10.1104/pp.15.01335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim YJ, Müller R, Yumul RE, Liu C, Pan Y, Cao X, Goodrich J, Chen X. AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of polycomb group proteins. Plant Cell 2011:23(10):3654–3670. 10.1105/tpc.111.091538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Yang R, Liu M, Zhang S, Yan K, Yang G, Huang J, Zheng C, Wu C. PLATZ2 Negatively regulates salt tolerance in Arabidopsis seedlings by directly suppressing the expression of the CBL4/SOS3 and CBL10/SCaBP8 genes. J Exp Bot. 2020:71(18):5589–5602. 10.1093/jxb/eraa259 [DOI] [PubMed] [Google Scholar]
- Lohmann JU, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 2001:105(6):793–803. 10.1016/S0092-8674(01)00384-1 [DOI] [PubMed] [Google Scholar]
- Ma C, Haslbeck M, Babujee L, Jahn O, Reumann S. Identification and characterization of a stress-inducible and a constitutive small heat-shock protein targeted to the matrix of plant peroxisomes. Plant Physiol. 2006:141(1):47–60. 10.1104/pp.105.073841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita A, Furumoto T, Ishida S, Takahashi Y. AGF1, an AT-Hook protein, is necessary for the negative feedback of AtGA3ox1 encoding GA 3-oxidase. Plant Physiol. 2007:143(3):1152–1162. 10.1104/pp.106.093542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998:95(6):805–815. 10.1016/S0092-8674(00)81703-1 [DOI] [PubMed] [Google Scholar]
- Michael TP, Mockler TC, Breton G, McEntee C, Byer A, Trout JD, Hazen SP, Shen R, Priest HD, Sullivan CM, et al. Network discovery pipeline elucidates conserved time-of-day–specific cis-regulatory modules. PLoS Genet. 2008:4(2):e14. 10.1371/journal.pgen.0040014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozgova I, Hennig L. The polycomb group protein regulatory network. Annu Rev Plant Biol. 2015:66(1):269–296. 10.1146/annurev-arplant-043014-115627 [DOI] [PubMed] [Google Scholar]
- Mozgova I, Köhler C, Hennig L. Keeping the gate closed: functions of the polycomb repressive complex PRC2 in development. Plant J. 2015:83(1):121–132. 10.1111/tpj.12828 [DOI] [PubMed] [Google Scholar]
- Nagano Y, Furuhashi H, Inaba T, Sasaki Y. A novel class of plant-specific zinc-dependent DNA-binding protein that binds to A/T-rich DNA sequences. Nucleic Acids Res. 2001:29(20):4097–4105. 10.1093/nar/29.20.4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K-H, Yu H, Ito T. AGAMOUS controls GIANT KILLER, a multifunctional chromatin modifier in reproductive organ patterning and differentiation. PLoS Biol. 2009:7(11):e1000251. 10.1371/journal.pbio.1000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó’Maoiléidigh DS, Wuest SE, Rae L, Raganelli A, Ryan PT, Kwaśniewska K, Das P, Lohan AJ, Loftus B, Graciet E, et al. Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS. Plant Cell 2013:25(7):2482–2503. 10.1105/tpc.113.113209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoro A, Madrigal P, Muiño JM, Matus J, Jin J, Mecchia MA, Debernardi JM, Palatnik JF, Balazadeh S, Arif M, et al. Dynamics of chromatin accessibility and gene regulation by MADS-domain transcription factors in flower development. Genome Biol. 2014:15(3):R41. 10.1186/gb-2014-15-3-r41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F, Nilsson O, Busch MA, Lee I, Weigel D. A genetic framework for floral patterning. Nature 1998:395(6702):561–566. 10.1038/26903 [DOI] [PubMed] [Google Scholar]
- Payne T, Johnson SD, Koltunow AM. KNUCKLES (KNU) encodes a C2H2 zinc-finger protein that regulates development of basal pattern elements of the Arabidopsis gynoecium. Development 2004:131(15):3737–3749. 10.1242/dev.01216 [DOI] [PubMed] [Google Scholar]
- Pelayo MA, Yamaguchi N, Ito T. One factor, many systems: the floral homeotic protein AGAMOUS and its epigenetic regulatory mechanisms. Curr Opin Plant Biol. 2021:61:102009. 10.1016/j.pbi.2021.102009 [DOI] [PubMed] [Google Scholar]
- Planchais S, Glab N, Inzé D, Bergounioux C. Chemical inhibitors: a tool for plant cell cycle studies. FEBS Lett. 2000:476(1–2):78–83. 10.1016/S0014-5793(00)01675-6 [DOI] [PubMed] [Google Scholar]
- Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009:10(3):192–206. 10.1038/nrm2640 [DOI] [PubMed] [Google Scholar]
- Prunet N, Morel P, Thierry AM, Eshed Y, Bowman JL, Negrutiu I, Trehin C. REBELOTE, SQUINT, and ULTRAPETALA1 function redundantly in the temporal regulation of floral meristem termination in Arabidopsis thaliana. Plant Cell. 2008:20(4):901–919. 10.1105/tpc.107.053306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Henikoff S. Replicating nucleosomes. Sci Adv. 2015:1(7):e1500587. 10.1126/sciadv.1500587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GV, Heisler MG, Ehrhardt DW, Meyerowitz EM. Real-time lineage analysis reveals oriented cell divisions associated with morphogenesis at the shoot apex of Arabidopsis thaliana. Development 2004:131(17):4225–4237. 10.1242/dev.01261 [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Heard J, Martin G, Reuber L, Jiang C-Z, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 2000:290(5499):2105–2110. 10.1126/science.290.5499.2105 [DOI] [PubMed] [Google Scholar]
- Ross PJ, Ragina NP, Rodriguez RM, Iager AE, Siripattarapravat K, Lopez-Corrales N, Cibelli JB. Polycomb gene expression and histone H3 lysine 27 trimethylation changes during bovine preimplantation development. Reproduction 2008:136(6):777–785. 10.1530/REP-08-0045 [DOI] [PubMed] [Google Scholar]
- Ryan PT, Ó’Maoiléidigh DS, Drost HG, Kwaśniewska K, Gabel A, Grosse I, Graciet E, Quint M, Wellmer F. Patterns of gene expression during Arabidopsis flower development from the time of initiation to maturation. BMC Genom. 2015:16:488. 10.1186/s12864-015-1699-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R. Flowering and determinacy in Arabidopsis. J Exp Bot. 2007:58(5):899–907. 10.1093/jxb/erm002 [DOI] [PubMed] [Google Scholar]
- Shang E, Ito T, Sun B. Control of floral stem cell activity in Arabidopsis. Plant Signal Behav. 2019:14(11):1659706. 10.1080/15592324.2019.1659706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang E, Wang X, Li T, Guo F, Ito T, Sun B. Robust control of floral meristem determinacy by position-specific multifunctions of KNUCKLES. Proc Natl Acad Sci USA. 2021:118(36):e2102826118. 10.1073/pnas.2102826118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Širl M, Šnajdrová T, Gutiérrez-Alanís D, Dubrovsky JG, Vielle-Calzada JP, Kulich I, Soukup A. At-Hook motif nuclear localised protein 18 as a novel modulator of root system architecture. Int J Mol Sci. 2020:21(5):1886. 10.3390/ijms21051886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. Plant Cell. 1990:2(8):755–767. 10.1105/tpc.2.8.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg JJ, Myrenås M, Kuusk S, Lagercrantz U, Kowalczyk M, Sandberg G, Sundberg E. STY1 regulates auxin homeostasis and affects apical–basal patterning of the Arabidopsis gynoecium. Plant J. 2006:47(1):112–123. 10.1111/j.1365-313X.2006.02775.x [DOI] [PubMed] [Google Scholar]
- Song S, Qi T, Huang H, Ren Q, Wu D, Chang C, Peng W, Liu Y, Peng J, Xie D. The jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis. Plant Cell 2011:23(3):1000–1013. 10.1105/tpc.111.083089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street IH, Shah PK, Smith AM, Avery N, Neff MM. The AT-hook-containing proteins SOB3/AHL29 and ESC/AHL27 are negative modulators of hypocotyl growth in Arabidopsis. Plant J. 2008:54(1):1–14. 10.1111/j.1365-313X.2007.03393.x [DOI] [PubMed] [Google Scholar]
- Sun B, Ito T. Regulation of floral stem cell termination in Arabidopsis. Front Plant Sci. 2015:6:17. 10.3389/fpls.2015.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Looi L-S, Guo S, He Z, Gan E-S, Huang J, Xu Y, Wee W-Y, Ito T. Timing mechanism dependent on cell division is invoked by polycomb eviction in plant stem cells. Science 2014:343(6170):1248559. 10.1126/science.1248559 [DOI] [PubMed] [Google Scholar]
- Sun B, Xu Y, Ng K-H, Ito T. A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev. 2009:23(15):1791–1804. 10.1101/gad.1800409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Zhou Y, Cai J, Shang E, Yamaguchi N, Xiao J, Looi L-S, Wee W-Y, Gao X, Wagner D, et al. Integration of transcriptional repression and polycomb-mediated silencing of WUSCHEL in floral meristems. Plant Cell 2019:31(7):1488–1505. 10.1105/tpc.18.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013:14(2):178–192. 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, Xu W, Su Z. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017:45(W1):W122–W129. 10.1093/nar/gkx382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veylder LD, Beeckman T, Beemster GTS, Krols L, Terras F, Landrieu I, Schueren EVD, Maes S, Naudts M, Inzé D. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 2001:13(7):1653–1668. 10.1105/TPC.010087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Hou Q, Si L, Huang X, Luo J, Lu D, Zhu J, Shangguan Y, Miao J, Xie Y, et al. The PLATZ transcription factor GL6 affects grain length and number in rice1[OPEN]. Plant Physiol. 2019:180(4):2077–2090. 10.1104/pp.18.01574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ji C, Li Q, Zhou Y, Wu Y. Genome-wide analysis of the plant-specific PLATZ proteins in maize and identification of their general role in interaction with RNA polymerase III complex. BMC Plant Biol. 2018:18(1):221. 10.1186/s12870-018-1443-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. Arabidopsis: a laboratory manual. CSHL Press; 2002. [Google Scholar]
- Wellmer F, Alves-Ferreira M, Dubois A, Riechmann JL, Meyerowitz EM. Genome-wide analysis of gene expression during early Arabidopsis flower development. PLoS Genet. 2006:2(7):e117. 10.1371/journal.pgen.0020117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge PA. Ambient temperature signalling in plants. Curr Opin Plant Biol. 2013:16(5):661–666. 10.1016/j.pbi.2013.08.004 [DOI] [PubMed] [Google Scholar]
- Wong MM, Bhaskara GB, Wen T-N, Lin W-D, Nguyen TT, Chong GL, Verslues PE. Phosphoproteomics of Arabidopsis highly ABA-induced1 identifies AT-Hook–Like10 phosphorylation required for stress growth regulation. Proc Natl Acad Sci USA. 2019:116(6):2354–2363. 10.1073/pnas.1819971116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Chen F, Yu X, Lin C, Fu Y-F. Over-expression of an AT-hook gene, AHL22, delays flowering and inhibits the elongation of the hypocotyl in Arabidopsis thaliana. Plant Mol Biol. 2009:71(1–2):39–50. 10.1007/s11103-009-9507-9 [DOI] [PubMed] [Google Scholar]
- Xiao J, Jin R, Yu X, Shen M, Wagner JD, Pai A, Song C, Zhuang M, Klasfeld S, He C, et al. Cis and trans determinants of epigenetic silencing by polycomb repressive complex 2 in Arabidopsis. Nat Genet. 2017:49(10):1546–1552. 10.1038/ng.3937 [DOI] [PubMed] [Google Scholar]
- Xu Y, Yamaguchi N, Gan E-S, Ito T. When to stop: an update on molecular mechanisms of floral meristem termination. J Exp Bot. 2019:70(6):1711–1718. 10.1093/jxb/erz048 [DOI] [PubMed] [Google Scholar]
- Yadeta KA, Hanemian M, Smit P, Hiemstra JA, Pereira A, Marco Y, Thomma BPHJ. The Arabidopsis thaliana DNA-binding protein AHL19 mediates Verticillium wilt resistance. MPMI. 2011:24(12):1582–1591. 10.1094/MPMI-04-11-0090 [DOI] [PubMed] [Google Scholar]
- Yamaguchi N. The epigenetic mechanisms regulating floral hub genes and their potential for manipulation. J Exp Bot. 2022:73(5):1277–1287. 10.1093/jxb/erab490 [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Huang J, Tatsumi Y, Abe M, Sugano SS, Kojima M, Takebayashi Y, Kiba T, Yokoyama R, Nishitani K, et al. Chromatin-mediated feed-forward auxin biosynthesis in floral meristem determinacy. Nat Commun. 2018:9(1):5290. 10.1038/s41467-018-07763-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Huang J, Xu Y, Tanoi K, Ito T. Fine-tuning of auxin homeostasis governs the transition from floral stem cell maintenance to gynoecium formation. Nat Commun. 2017:8(1):1125. 10.1038/s41467-017-01252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Matsubara S, Yoshimizu K, Seki M, Hamada K, Kamitani M, Kurita Y, Nomura Y, Nagashima K, Inagaki S, et al. H3K27me3 demethylases alter HSP22 and HSP17.6C expression in response to recurring heat in Arabidopsis. Nat Commun. 2021:12(1):616. 10.1038/s41467-021-23766-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Winter CM, Wu M-F, Kwon CS, William DA, Wagner D. PROTOCOLS: chromatin immunoprecipitation from Arabidopsis tissues. Arabidopsis Book 2014:12:e0170. 10.1199/tab.0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Chen D, Schumacher J, Durantini D, Engelhorn J, Chen M, Carles CC, Kaufmann K. Dynamic control of enhancer activity drives stage-specific gene expression during flower morphogenesis. Nat Commun. 2019:10(1):1705. 10.1038/s41467-019-09513-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 1990:346(6279):35–39. 10.1038/346035a0 [DOI] [PubMed] [Google Scholar]
- Yoon K-J, Vissers C, Ming G, Song H. Epigenetics and epitranscriptomics in temporal patterning of cortical neural progenitor competence. J Cell Biol. 2018:217(6):1901–1914. 10.1083/jcb.201802117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Kim Y-S, Jung J-H, Seo PJ, Park C-M. The AT-hook motif-containing protein AHL22 regulates flowering initiation by modifying FLOWERING LOCUS T chromatin in Arabidopsis. J Biol Chem. 2012:287(19):15307–15316. 10.1074/jbc.M111.318477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Clarenz O, Cokus S, Bernatavichute YV, Pellegrini M, Goodrich J, Jacobsen SE. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 2007:5(5):e129. 10.1371/journal.pbio.0050129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W-M, Fang D, Cheng X-Z, Cao J, Tan X-L. Insights into the molecular evolution of AT-hook motif nuclear localization genes in Brassica napus. Front Plant Sci. 2021a:12:714305. 10.3389/fpls.2021.714305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, He Y, Li L, Liu H, Hong G. Involvement of the R2R3-MYB transcription factor MYB21 and its homologs in regulating flavonol accumulation in Arabidopsis stamen. J Exp Bot. 2021b:72(12):4319–4332. 10.1093/jxb/erab156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Yang R, Huo Y, Liu S, Yang G, Huang J, Zheng C, Wu C. Expression of cotton PLATZ1 in transgenic Arabidopsis reduces sensitivity to osmotic and salt stress for germination and seedling establishment associated with modification of the abscisic acid, gibberellin, and ethylene signalling pathways. BMC Plant Biol. 2018:18(1):218. 10.1186/s12870-018-1416-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Favero DS, Peng H, Neff MM. Arabidopsis thaliana AHL family modulates hypocotyl growth redundantly by interacting with each other via the PPC/DUF296 domain. Proc Natl Acad Sci USA. 2013:110(48):E4688–E4697. 10.1073/pnas.1219277110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang H, Gilmer S, Whitwill S, Fowke LC. Effects of co-expressing the plant CDK inhibitor ICK1 and D-type cyclin genes on plant growth, cell size and ploidy in Arabidopsis thaliana. Planta 2003:216(4):604–613. 10.1007/s00425-002-0935-x [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang X, Lee J-Y, Lee J-Y. Cell-to-cell movement of two interacting AT-hook factors in Arabidopsis root vascular tissue patterning. Plant Cell 2013:25(1):187–201. 10.1105/tpc.112.102210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zik M, Irish VF. Flower development: initiation, differentiation, and diversification. Annu Rev Cell Dev Biol. 2003:19(1):119–140. 10.1146/annurev.cellbio.19.111301.134635 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.