Abstract
It has been extensively demonstrated that growth factors play a key role in the regulation of proliferation. Several lines of evidence support the hypothesis that for the induction of cell cycle progression in the absence of exogenous growth factors, oncogenes must either induce autocrine growth factor secretion or, alternatively, activate their receptors or their receptor substrates. Cells expressing polyomavirus large T antigen (PyLT) display reduced growth factor requirements, but the mechanisms underlying this phenomenon have yet to be explored. We conducted tests to see whether the reduction in growth factor requirements induced by PyLT was related to alterations of growth factor-dependent signals. To this end, we analyzed the phosphorylation status of a universal tyrosine kinase substrate, the transforming Shc adapter protein, in fibroblasts expressing the viral oncogene. We report that the level of Shc phosphorylation does not decrease in PyLT-expressing fibroblasts after growth factor withdrawal and that this PyLT-mediated effect does not require interaction with protein encoded by the retinoblastoma susceptibility gene. We also found that the chronic activation of the adapter protein is correlated with the binding of Shc to Grb-2 and with defects in the downregulation of mitogen-activated protein kinases. In fibroblasts expressing the nuclear oncoprotein, we also observed the formation of a PyLT-Shc complex that might be involved in constitutive phosphorylation of the adapter protein. Viewed comprehensively, these results suggest that the cell cycle progression induced by PyLT may depend not only on the direct inactivation of nuclear antioncogene products but also on the indirect induction, through the alteration of cytoplasmic pathways, of growth factor-dependent nuclear signals.
Polyomavirus large T antigen (PyLT) is a nuclear phosphoprotein with several distinct functions. In addition to its role in the regulation of viral replication and gene expression (84), PyLT can induce cellular DNA replication in the absence of other virus-transforming genes (24, 27). PyLT’s ability to affect cellular pathways of growth regulation is largely the result of its association with the retinoblastoma susceptibility gene product (Rb) (25, 36). PyLT can immortalize primary cells in culture in an Rb-dependent manner and cooperate with other oncogenes to trigger their transformation (33, 65). Our previous research showed that PyLT is the only early function of polyomavirus responsible for the inhibition of differentiation in polyomavirus-transformed C2 myoblast cells (42). Moreover, we showed that Rb inactivation plays a preponderant role in the inhibition of differentiation and cell cycle arrest, processes concomitantly blocked by PyLT in myoblasts (23, 43). Little evidence of Rb-independent functions has been reported for PyLT. Mutants of PyLT that fail to bind Rb still maintain some activities related to the stimulation of the cell cycle, such as the transactivation of the fos promoter (31) and the induction of high levels of cyclin D1 in myoblasts (our unpublished results).
Recently, another Rb-independent function, the interaction between the N-terminal J domain of PyLT and the cytoplasmic DnaK heat shock protein 70 (hsp70), has been reported (76). Moreover, it has been demonstrated that the interaction with the cytoplasmic chaperone is necessary for Rb inactivation. The binding to the chaperone was previously reported for simian virus 40 large T antigen (SV40LT), and for this viral oncoprotein the rearrangement activity of DnaK may also be involved in the modulation of the interaction with other cellular targets, such as p53 and p300 (39, 61, 80), suggesting a general role for hsp70 in the regulation of the interaction of viral oncoproteins with their cellular targets.
Some nuclear oncogenes have been shown to alter not only the function of nuclear antioncogenes but also the activity of cytoplasmic signalling pathways. The autocrine secretion of insulin-like growth factor 1 (IGF-1) by SV40LT-expressing fibroblasts has been reported (58, 59), as has the induction of an autocrine loop of hepatocyte growth factor in an epithelial cell line expressing the viral oncoprotein (45). SV40 alters the IGF-1 signalling pathway not only at the ligand level but also at downstream levels through the binding of insulin receptor substrate 1 (IRS-1). This interaction is essential for the SV40LT-dependent transformation of fibroblasts that do not express the IGF-1 receptor (22). In line with this observation, it has been recently demonstrated that cellular Ras is required for full neoplastic transformation by SV40LT (63). Other nuclear oncogenes, such as c-Myb, have been shown to alter the secretion of IGF-1 and the expression of its receptor (67). It is logical to hypothesize that in cells driven to proliferate by nuclear oncogenes the growth advantage is the consequence of the cooperation between oncogene-induced nuclear signals and the cytoplasmic signals controlled by growth factors. The growth properties of transformed cells under low-serum conditions are not so obvious. It has been proposed that, in principle, the nuclear functions of oncogenes are not sufficient to induce the cell cycle in the absence of growth factors and that some nuclear oncogenes have therefore evolved an ability to mimic the effects of growth factors (3).
Unlike its homologue SV40LT, PyLT does not induce transformation if it is not complemented by other oncogenes. This probably reflects a limited capacity of PyLT, compared to SV40LT, to alter the different independent cellular pathways that have been reported as being essential for full transformation. In fact, important nuclear targets of SV40LT, such as p53 and p300, have been shown not to interact with PyLT. With respect to nonnuclear alterations, in contrast to SV40LT, nothing has been reported with regard to PyLT. However, it has been demonstrated that PyLT increases the plating efficiency of rat fibroblasts under low-serum conditions and that this function depends on its N-terminal region (64). Fibroblasts immortalized by PyLT can grow to a low saturation density in the presence of 0.5% serum, much like those transformed by polyomavirus middle T antigen (PyMT). This demonstrates that in the absence of growth factors the transforming function of polyomavirus (i.e., PyMT) is not at an advantage with respect to its immortalizing function (i.e., PyLT) (64). Nothing is known as yet about the mechanism by which PyLT reduces the growth factor requirements, even though it is possible to infer that this reduction might well be the result of either the alteration in cytoplasmic signals under the control of an autocrine secretion of growth factors, a more efficient response to low levels of mitogens, or a combination of the two processes.
The purpose of this work was to determine whether PyLT induces alterations in signals related to receptor tyrosine kinases (TKs). To this end, we decided to analyze the activity of the Shc adapter proteins in cells expressing PyLT. These adapter proteins are involved in the transmission of activating signals to Ras (7) and in pathways related to all the TK receptors tested to date, including the epidermal growth factor (EGF) receptor (55), the platelet-derived growth factor receptor (88), the hepatocyte growth factor receptor (Met) (54), the erbB-2 receptor (68, 74), the insulin receptor (60, 78), the fibroblast growth factor receptor (86), and the nerve growth factor receptor (8, 81). Shc proteins are also involved in signalling from cytoplasmic TKs, since they are constitutively phosphorylated in cells that express activated Lck, Src, Fps, or Sea (2, 16, 19, 47, 56). These adapter proteins are also phosphorylated after ligand stimulation of surface receptors that lack intrinsic TK activity, and they are believed to signal by recruiting and activating cytoplasmic TKs (e.g., interleukin-2, erythropoietin, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, B- and T-cell receptors, CD4, or CD8) (2, 11, 17, 35, 46, 66). All of these data define Shc proteins as universal TK substrates. Upon phosphorylation by TKs, Shc proteins bind to Grb-2 (15, 41), an adapter protein engaged in a constitutive complex with Sos (10, 13, 21, 26, 38, 52, 69), a ubiquitously expressed Ras guanine nucleotide exchange factor for Ras (6, 9, 13, 77). This leads to the membrane relocalization of Sos, an event considered sufficient for Ras activation (1). p52Shc and p46Shc overexpression increase the proliferative response and mitogen-activated protein kinase (MAPK) activation by EGF and granulocyte-macrophage colony-stimulating factor (35, 48). Moreover, it has been demonstrated that Shc proteins are able to transform NIH 3T3 fibroblasts (55) and can be used as a tool for the identification of tumors with constitutive TK activity (56). On these bases, we hypothesized that if PyLT is responsible for any alteration in the secretion of growth factors and/or in the activity of their receptors, these changes could probably converge on the activation of Shc-dependent signals.
In this article, we show that Shc is phosphorylated in PyLT-expressing fibroblasts under low-serum conditions. Constitutive phosphorylation of Shc correlates with the chronic formation of the Shc–Grb-2 complex and with defects in the downregulation of MAPK activity after serum deprivation. This PyLT-mediated effect does not require interaction between PyLT and Rb. The same pattern of alterations was observed in fibroblasts expressing SV40LT but not in cytoplasmic mutants of either viral oncogene. Possible mechanisms for the activation of this pathway are discussed. We also report an indirect interaction between PyLT and Shc that might contribute to the induction of a growth factor-independent Shc activation or to the stabilization of the adapter molecule phosphorylation. These data, taken together, demonstrate that under conditions of growth factor withdrawal, PyLT alters cytoplasmic signalling that can contribute, along with the activation of nuclear signals, to the induction of cell cycle progression.
MATERIALS AND METHODS
Cell cultures.
Vectors expressing wild-type (wt) PyLT or a mutant PyLT that cannot bind Rb (PyLT Rb−) have been described elsewhere (42). PyLT-expressing cells lines, derived from NIH 3T3 and Rat-1 cells, were grown in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% fetal calf serum (FCS) (under constant selection with geneticin [400 μg/ml; Sigma Chemical Co., St. Louis, Mo.] for fibroblasts or with puromycin [5 μg/ml; Sigma] for Rat-1 derivatives) in a humidified 5% carbon dioxide atmosphere. Fibroblasts expressing the cytoplasmic mutant of PyLT were kindly provided by B. Schaffhausen (31), and the NIH 3T3 pools expressing SV40LT or its cytoplasmic mutant form were kindly provided by C. Vesco (82). The KG1 and SAA cell lines have been described elsewhere (18, 56). Cells were routinely passaged by standard trypsinization and seeded directly onto plastic tissue culture plates.
For 5-bromodeoxyuridine (BrdU) incorporation assays, 10 μM BrdU (Sigma) was added to cells kept in medium with 0.5% FCS 30 min or 1 h before fixation. BrdU-positive cells were detected by indirect immunofluorescence as described below.
Indirect immunofluorescence staining.
Cells grown on glass coverslips were fixed by immersion in methanol-acetone (3:7, vol/vol) for 15 min at −20°C and then air dried. Coverslips were incubated for 30 min in 1.5 N HCl at room temperature (RT). After three washes in phosphate-buffered saline (PBS), the coverslips were incubated for 1 h at room temperature or 30 min at 37°C with undiluted mouse monoclonal antibody BU-1 (Amersham, Arlington Heights, Ill.) in a humidified atmosphere. After three washes in PBS, they were then incubated for 1 h with secondary antibody (rhodamine-conjugated goat anti-mouse immunoglobulin G [IgG] fraction diluted 1:100 in PBS plus 3% bovine serum albumin [BSA]; Cappel Immunochemicals, Cochranville, Pa.). After repeated washes with PBS, a final staining of 10 min with a 1-μg/ml solution of the DNA-binding fluorochrome 4′,6-diamidino-2-phenylindole (DAPI; Boehringer Mannheim) was carried out to visualize total nuclei. After being washed with PBS, the coverslips were mounted with 70% glycerol in PBS. The samples were analyzed under a phase-contrast microscope with an appropriate fluorescent-light source.
Immunoprecipitations and Western blot procedures.
Cells were grown to confluence in 10% FCS and then shifted to a low-serum-containing medium (0.5% FCS). Cells were rinsed twice with PBS and lysed on ice in a solution containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EGTA (pH 8.0), 100 mM NaF (pH 8.0), 10% glycerol, 1 mM MgCl2, 1% (vol/vol) Triton X-100, and freshly added protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 μg of leupectin/ml, 5 μg of aproteinin/ml, and 1 mM sodium orthovanadate). Lysates were subjected to ultrasonic treatment for 15 s and then clarified by centrifugation at 4°C. Protein concentrations were determined with the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, Calif.).
To perform immunoprecipitations, the appropriate antibodies were adsorbed on protein A–Sepharose CL-4B (Pharmacia LKB, Uppsala, Sweden) and then incubated with precleaned cell lysates for 90 min at 4°C. Immunocomplexes were washed three times with cold NET-gel buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA [pH 8.0], 0.1% [vol/vol] Nonidet P-40, 0.25% gelatin, 1 mM sodium orthovanadate), eluted, and denaturated in reducing Laemmli buffer at room temperature or by heating for 5 min to avoid IgG comigration with the protein of interest. Proteins were resolved by sodium dodecyl sulfate (SDS)–12% polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose filters (Bio-Rad Laboratories). Blotting was stopped, and the blots were probed with specific antibodies after blockade of nonspecific reactivity. Primary antibodies were diluted in TBS-T (20 mM Tris-HCl [pH 7.8], 150 mM NaCl, 0.05% Tween 20) containing 0.2% gelatin. After being washed extensively, immunocomplexes were detected with horseradish peroxidase-conjugated species-specific secondary antiserum (Bio-Rad Laboratories) followed by enhanced chemiluminescence reaction (Amersham International plc, Little Chalfont, England).
For immunoprecipitations, the following antibodies were used: rabbit anti-Shc polyclonal antibodies (Transduction Laboratories, Lexington, Ky.), rabbit anti-Grb-2 (C-23) polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.), mouse anti-PyLT (LT1) monoclonal antibody (20), mouse anti-SV40LT (Pab 101; ATTC culture TIB-117) monoclonal antibody, and rabbit anti-Erk1 (C-16) and anti-Erk2 (C-14) polyclonal antibodies (both from Santa Cruz Biotechnology). For Western blotting, the following primary antibodies and dilutions were used: anti-Shc, a 1:1,000 dilution of the above-described rabbit polyclonal serum (Transduction Laboratories); antiphosphotyrosine, a 1:500 dilution of a mouse anti-phosphotyrosine-PY20 monoclonal antibody (Transduction Laboratories); anti-Grb-2, a 1:1,000 dilution of the above-described rabbit polyclonal serum (C-23); anti-T antigen (anti-PyLT plus anti-SV40LT), an undiluted mixture of the two monoclonal antibodies F4 (anti-PyLT; kindly provided by C. Prives) and Pab 101 (anti-SV40LT, made available by C. Vesco); anti-PyLT, a 1:1,000 dilution of a rabbit polyclonal serum (kindly provided by B. Schaffhausen); and anti-MAPK, a mixture of the two polyclonal antibodies anti-Erk1 (C-16) and anti-Erk2 (C-14) diluted 1:1,000.
In vitro binding assays.
The glutathione S-transferase (GST) fusion proteins used in these assays were described elsewhere (30, 35, 55, 70). Cultures of bacteria expressing either GST or GST fusion proteins were grown as previously described (35). Recombinant proteins were purified on glutathione-Sepharose 4B (Pharmacia LKB) and used for binding assays. For each reaction, about 25 μg of either GST or GST fusion protein bound to glutathione-Sepharose 4B was incubated for 90 min at 4°C with 1.5 to 3 mg of appropriate cell lysate. Protein complexes were washed five times in ice-cold lysis buffer (described above), eluted, denatured by heating at 95°C for 5 min in reducing Laemmli buffer, resolved by SDS-PAGE, and analyzed by immunoblotting.
For far-Western experiments with recombinant GST fusion proteins, blots were blocked in TBS-T containing 5% (wt/vol) BSA for at least 4 h at RT and then in TBS-T containing reduced glutathione (3 μM) and 5% BSA for 2 h at RT. The blots were then incubated with the appropriate fusion protein (10 nM) in TBS-T in the presence of reduced glutathione (3 μM) and BSA (5%) for 1 h at RT. After being extensively washed in TBS-T, fusion proteins on the blots were detected with the affinity-purified anti-GST antibody.
MAPK activation assays.
Starved and stimulated cell lines were washed twice in ice-cold PBS and lysed. After ultrasonic treatment, cell lysates were clarified and their protein concentrations were determined. MAPK activation was determined by immunocomplex kinase assays according to established procedures. Anti-Erk1 and -2 immunoprecipitates from lysates were tested for their capacity to phosphorylate myelin basic protein (MBP) as a substrate (0.25 mg/ml) in the presence of 50 μM ATP and 5 μCi of [γ-32P]ATP for 15 min at 30°C. The immunocomplex kinase reaction was stopped by addition of Laemmli buffer, and after 15 min of incubation at room temperature, samples were resolved by SDS–12% PAGE. The lower part of the gel was stained with Coomassie blue, dried, and subjected to autoradiography. The upper part was transferred to nitrocellulose and used for Western blot analysis with antibodies to MAPK (Erk-1 and Erk-2).
RESULTS
PyLT induces constitutive Shc phosphorylation in NIH 3T3 fibroblasts.
Our starting point was the test of the status of Shc phosphorylation in NIH 3T3 fibroblasts expressing PyLT. It has been demonstrated that while Shc phosphorylation is dependent on growth factors in normal tissues and in nontransformed cell lines, it is constitutive in some tumors and can be used as a marker for transformation events involving TK alterations (56).
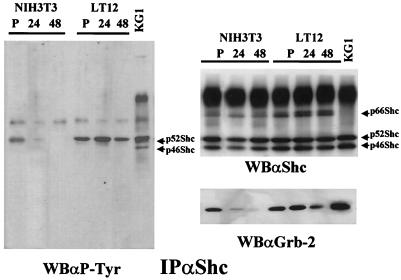
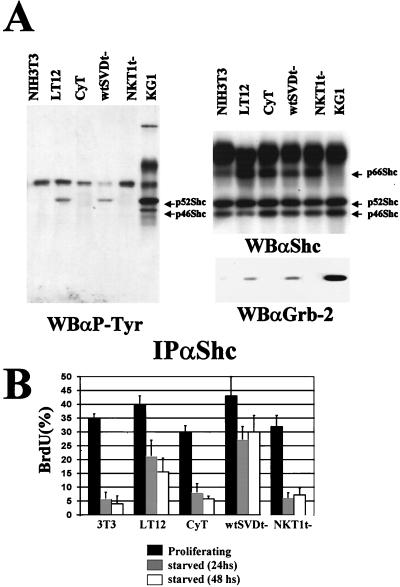
Proliferating and serum-starved cells were analyzed for phosphotyrosine-containing Shc proteins. To this end, a Western blot analysis performed with antibodies to phosphotyrosine was carried out after immunoprecipitation of Shc proteins from NIH 3T3 parental fibroblasts and a stable clone expressing PyLT (Fig. 1). Tyrosine-phosphorylated p52Shc was detected in proliferating NIH 3T3 cells, and in accordance with previous reports (56), no coimmunoprecipitating phosphotyrosine-containing proteins were detected. After growth factor removal, parental cells showed a decrease in Shc phosphorylation that became nondetectable after 24 h in low-serum medium. In the case of fibroblasts that stably express PyLT, we observed a constitutive phosphorylation of the p52Shc isoform that does not decrease within 48 h of growth factor withdrawal. As a positive control for Shc phosphorylation, we used the myeloblastic-leukemia-derived KG1 cell line, which shows a high-level constitutive phosphorylation of the p46 and the p52 isoforms of Shc. For this cell line, two phosphotyrosine-containing proteins, of 80 to 90 kDa and 140 kDa, are normally detected in Shc immunoprecipitates (56). As expected, the analysis of the same filter with antibodies to Shc showed no changes in Shc protein levels in PyLT fibroblasts (Fig. 1), demonstrating that the different levels of phosphotyrosine content in fact corresponded to different amounts of activated Shc proteins. We did not observe the coimmunoprecipitation of other tyrosine-phosphorylated proteins in Shc immunoprecipitates from PyLT-expressing fibroblasts. Shc proteins are phosphorylated on multiple sites (55, 70). To determine whether the constitutive phosphorylation of Shc observed in PyLT-expressing cells was induced in at least one tyrosine with a relevant biological function, we tested the association of Shc with Grb-2 proteins. For this purpose, we performed, on the same filter, Western blot analysis with antibodies to Grb-2. We observed a clear correlation between the maintenance of Shc activation and the formation of the Shc–Grb-2 complex (Fig. 1). The observed similarities between proliferating fibroblasts and starved PyLT derivatives in terms of Shc phosphorylation levels, the lack of phosphotyrosine-containing coimmunoprecipitating proteins, and Grb-2 coimmunoprecipitation suggest that these alterations might result from the maintenance of multiple activating pathways similar to those induced by serum rather than from the strong activation of a single deregulated signal. Since Grb-2 forms a very stable complex with Sos, our data suggest that the recruitment of Sos by Shc could take place in the absence of growth factors and that the Shc–Grb-2 complex detected in PyLT-expressing fibroblasts could be signalling to Ras.
FIG. 1.
Shc is constitutively phosphorylated in fibroblasts expressing PyLT. NIH 3T3 parental cells and a representative clone expressing PyLT (LT12) were grown to confluence in 10% FCS-containing medium and then shifted to a medium containing 0.5% FCS. Cells were lysed at different time points. Using anti-Shc serum (αShc), Shc proteins were immunoprecipitated (IP) from 5 mg of total cellular proteins, resolved by SDS–12% PAGE, transferred to nitrocellulose, and analyzed by Western blotting (WB) with antibodies to phosphotyrosine (αP-Tyr). The same filter was then analyzed with antibodies to Shc and Grb-2 proteins (αShc and αGrb-2, respectively). Shc isoforms are indicated by arrows. Lanes: P, subconfluent proliferating cultures; 24, cells starved for 24 h; 48, cells starved for 48 h.
Constitutive phosphorylation of Shc does not require interaction of PyLT with Rb.
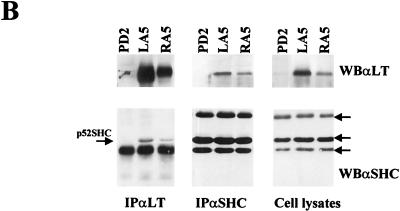
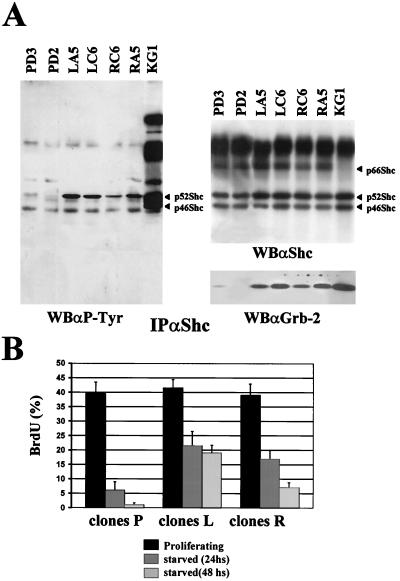
We were interested in determining the role of the PyLT-Rb interaction, the best-known biochemical activity of PyLT, in this novel function of the viral oncoprotein. Being unable to select an NIH 3T3 fibroblast expressing a PyLT mutant that fails to bind Rb (PyLT Rb−) at levels comparable to the wild type, we tested the status of Shc phosphorylation in Rat-1 fibroblasts, in which we were able to express the PyLT Rb− mutant at levels that were comparable to, albeit somewhat lower than, the wt level (see Fig. 5B). Serum-starved Rat-1 control subclones and clones expressing either wt or Rb−-mutant PyLT were lysed, and immunoprecipitations with antibodies to Shc were performed. Figure 2A shows that the level of Shc phosphorylation in Rat-1 control subclones was very low or not detectable after 48 h in medium containing 0.5% FCS. In Rat-1 subclones expressing not only wt but also mutant PyLT, on the other hand, a clearly detectable Shc phosphorylation that correlates with the formation of the Shc–Grb-2 complex was observed. Similar results were also observed in SAOS osteosarcoma cells, in which both wt and mutant PyLT induce constitutive Shc phosphorylation (data not shown). As expected, Rat-1 control parental cells were completely arrested in low-serum medium, while the stable expression of wt PyLT could clearly induce entry into S phase under these conditions (Fig. 2B). Interestingly enough, the Rat-1 subclones expressing the PyLT Rb− mutant showed a retardation in growth arrest with respect to control cells.
FIG. 5.
PyLT and SV40LT interact with Shc. (A) Parental NIH 3T3 and oncoprotein-derived cell lines (LT12, CyT, wtSVDt−, and NKT1t−) were grown to confluence, starved for 24 h, and then lysed. LTs (PyLT and SV40LT), Shc, and Grb-2 were immunoprecipitated (IP) from 10 mg of total cellular protein. After immunoprecipitation, each sample was divided into two identical fractions (one of which was heated and one which was not heated to avoid comigration of IgG with the protein of interest), resolved by SDS–12% PAGE, and analyzed by Western blotting (WB) with a mixture of monoclonal antibodies to PyLT and SV40 LT (αLT) or with polyclonal antibodies to Shc (αShc) or Grb-2 (αGrb-2). p52Shc and Grb-2 are indicated by arrows. (B) A representative control Rat-1 subclone (PD2) and PyLT wt (LA5)- and mutant PyLT (RA5)-derived cell lines were grown to confluence, starved for 48 h, and then lysed. PyLT and Shc were immunoprecipitated from 10 mg of total cellular proteins. After immunoprecipitation, each sample was divided into two identical fractions (one of which was heated and one which was not heated [to avoid comigration of IgG with the protein of interest]), resolved by SDS–12% PAGE, and analyzed by immunoblotting with antibodies to PyLT or Shc. Shc isoforms are indicated by arrows.
FIG. 2.
PyLT Rb− induces constitutive Shc activation. (A) Rat-1 control subclones (PD3 and PD2) and representative clones expressing either PyLT (LA5 and LC6) or PyLT Rb− (RA5 and RC6) were grown to confluence in 10% FCS-containing medium and then shifted to a medium containing 0.5% FCS. After 48 h, cells were lysed and Shc proteins were immunoprecipitated (IP) from 5 mg of total cellular protein with anti-Shc serum (αShc), resolved by SDS–12% PAGE, transferred to nitrocellulose, and analyzed by Western blotting (WB) with antibodies to phosphotyrosine (αP-Tyr), Shc (αShc), and Grb-2 (αGrb2). Shc isoforms are indicated by arrows. (B) PD2, PD3, LA5, LC6, RA5, and RC6 fibroblasts were treated as described above. Subconfluent proliferating cultures (P) and starved cultures (24 and 48 h) were analyzed for BrdU incorporation (added to the culture medium for the last hour). The percentages of BrdU-positive cells were calculated with respect to total nuclei, as visualized by DAPI staining. At least 400 nuclei were counted for each sample, and the results are the means ± standard deviations of data from three independent experiments. Since representative clones of each cell type show very similar growth properties, the data were represented as the averages of the data from clones expressing the same form of PyLT.
These data, seen as a whole, demonstrate not only that PyLT can also induce constitutive Shc phosphorylation in cellular systems other than NIH 3T3 fibroblasts but also that Shc activation is independent from Rb inactivation. From our data we can also infer that some biochemical functions cooperating with Rb inactivation are involved in the induction of entry into S phase. In this regard, however, we found that unlike in Rat-1 cells, PyLT Rb− does not induce changes in the kinetics of growth arrest in SAOS cells, thus suggesting that the contributions of different pathways to this potential cooperation depend on the cellular context in which the viral oncogene is expressed.
Constitutive phosphorylation of Shc is also induced by SV40LT but not by cytoplasmic LTs.
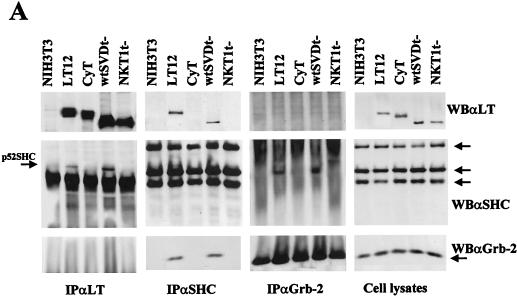
SV40LT and PyLT exhibit some common activities. It had been demonstrated that these viral proteins can alter some cytoplasmic signalling pathways (22, 45, 58, 59, 63), and so we decided to determine whether SV40LT could also induce constitutive Shc activation. We determined the status of Shc phosphorylation in serum-starved NIH 3T3 fibroblasts expressing SV40LT. As expected, the constitutive phosphorylation of Shc previously observed in PyLT-expressing fibroblasts was also detected in these cells, and this activation correlated with Shc binding to Grb-2 (Fig. 3A).
FIG. 3.
Constitutive Shc phosphorylation and increased DNA synthesis are induced by both SV40LT and PyLT but not by cytoplasmic mutants of the two viral oncogenes. (A) NIH 3T3 parental cells, a clone expressing wt PyLT (LT12), a representative clone expressing a cytoplasmic PyLT mutant (CyT), a pool expressing wt SV40LT (wtSVDt−), and a pool expressing a cytoplasmic SV40LT mutant (NKT1t−) were grown to confluence and shifted to low-serum medium (0.5% FCS). After 24 h, cells were lysed and immunoprecipitations (IP) with anti-Shc antibodies (αShc) were performed on 5 mg of total cell lysate proteins, resolved by SDS–12% PAGE, transferred to nitrocellulose, and analyzed by Western blotting (WB) with antibodies to phosphotyrosine (αP-Tyr), Shc (αShc), and Grb-2 (αGrb-2). Shc isoforms are indicated by arrows. (B) NIH 3T3, LT12, CyT, wtSVDt−, and NKT1t− fibroblasts were grown as described above. Subconfluent proliferating cells (P) and starved cultures (24 and 48 h) were analyzed for incorporation of BrdU (added to the culture medium for the last 30 min). The percentages of BrdU-positive cells were calculated with respect to total nuclei, as visualized by DAPI staining. At least 400 nuclei were counted for each sample, and the results are the means ± standard deviations of data from three independent experiments.
On the basis of the different intracellular localizations of Shc and LT(s), as well the fact that the cytoplasmic mutant of SV40LT might maintain the ability to induce some cytoplasmic signals previously described for the wt form (22), we tested the effect of cytoplasmic mutants of the viral oncogenes on Shc activation. Cytoplasmic forms of PyLT and SV40LT with mutations in the nuclear localization signals were used for this purpose. It has been reported that such mutations are responsible for defects in the phosphorylation of the viral oncoproteins that presumably require nuclear localization to be completed (14, 31, 37, 87). The cytoplasmic mutant of PyLT used in these experiments (CyT) failed to immortalize primary rat embryo fibroblasts, was defective in both pRb-dependent and -independent transactivation activities, and failed to relocalize Rb in the cytoplasm (31). The cytoplasmic mutant of SV40LT (NKT1t−), mutated in the unique nuclear localization signal, could still transform NIH 3T3 cells in culture (34, 82). This property, however, was not correlated with the level of induction of entry into S phase in low-serum medium, which was clearly reduced with respect to the that of the wt form (Fig. 3B).
Serum-starved NIH 3T3 fibroblasts expressing the cytoplasmic mutants mentioned above were analyzed for the phosphorylation status of Shc proteins. As shown in Fig. 3A, in contrast to cells expressing the wt forms of the viral oncogenes, no tyrosine-phosphorylated Shc proteins were detected in cell lines expressing high levels of either of the cytoplasmic mutants. It has been suggested that transport-defective mutants of SV40LT could probably bind IRS-1 to a cytoplasmic target of the wt viral oncogene (22). In this way, the cytoplasmic mutants of SV40LT could maintain the ability to alter at least one nonnuclear signalling pathway. In accordance with this, we observed that NKT1t− fibroblasts showed the expected SV40LT–IRS-1 complex formation (data not shown). The results obtained with the SV40LT cytoplasmic mutant and the observation that PyLT does not bind IRS-1 (our unpublished data) led to the hypothesis that IRS-1 binding and the induction of Shc phosphorylation are independent functions and that PyLT can alter only part of the cytoplasmic pathways affected by SV40LT. We hypothesize that the Shc activation mechanism requires completed posttranslational modifications of PyLT and SV40LT that cannot be achieved by the cytoplasmic forms of these viral proteins.
Constitutive Shc phosphorylation induced by PyLT and SV40LT correlates with alterations in MAPK activity.
The Ras-MAPKs are a converging point of growth factor-dependent signals in the cytoplasm and the nucleus, as is Shc in the membrane. These kinases become phosphorylated in response to Ras-dependent and -independent signals, and it has been demonstrated that the selective activation of Shc by TK receptors is sufficient to activate them (4, 7, 50, 53). Moreover, the overexpression of the p46 and p52 isoforms of Shc increases the MAPK activity in response to growth factor stimulation (48).
It has been repeatedly proposed that the conditions necessary for Shc to activate the Ras-MAPK pathway are the formation of the Grb-2–Shc complex and the relocalization of this complex to the plasma membrane. The general rule is that Shc phosphorylation is coupled to its localization, as in the case of receptor TKs. In other cases, however, such as the already-described phosphorylation of Shc by activated cytoplasmic TKs (2, 16, 47, 56) or the proposed Shc activities in other cellular compartments (40, 83), this coupling is not so clear.
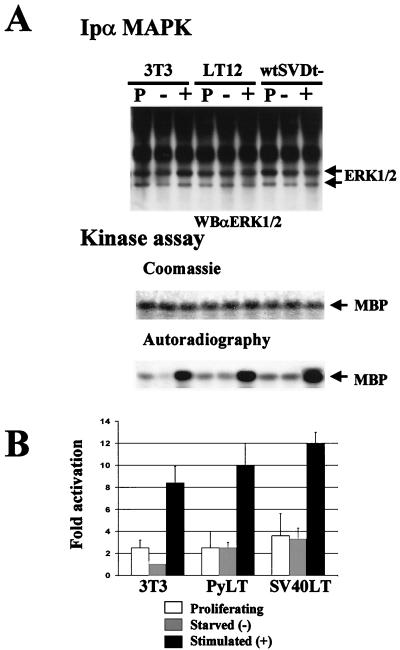
Regardless of Shc localization, and since we had observed the constitutive formation of an Shc–Grb-2 complex in fibroblasts expressing PyLT and SV40LT oncogenes, we decided to test the biological functionality of this complex through the analysis of a possible connection with the Ras-MAPK pathway in PyLT-expressing fibroblasts. The effects of PyLT and SV40LT expression on endogenous MAPK activation were tested by immunoprecipitation of MAPKs and determination of their phosphorylating activities on MBP (Fig. 4A). Cells were grown to confluence, serum starved for 24 h, and then either treated or not treated with serum for 5 min. In accordance with the literature (49), Fig. 4A shows that the MAPK activity of starved NIH 3T3 cells is reduced to half that of proliferating cells. In the case of PyLT- and SV40LT-expressing cells, the levels of MAPK activity do not change upon transfer from growth to starvation conditions, recapitulating the chronic serum exposure of the NIH 3T3 cells. These alterations in MAPK downregulation somehow do not affect the sensitivity of starved cells to serum stimulation. Our data on Shc and MAPK activation, taken together, show that regardless of where the constitutive interaction of Shc and Grb-2, induced by LT, takes place, this interaction is sufficient for the alteration of MAPK activity and, what is more, lead to the interesting hypothesis that PyLT and SV40LT affect multiple cytoplasmic signals during starvation by interfering with the downregulation of multiple activating pathways that are supposed to be active during long periods of serum exposure.
FIG. 4.
Effects of PyLT and SV40LT expression on MAPK activity. NIH 3T3, LT12, and wtSVDt− cells were grown to confluence and then shifted to low-serum medium (0.5%). After 24 h, cells were treated or not treated with 10% FCS-containing medium. Cells were then lysed, and MAPKs were immunoprecipitated from 500 mg of proliferating (P), starved (−), or stimulated (+) cells. (A) Anti-MAPK immunoprecipitates (IP) were evaluated for their ability to phosphorylate the MBP substrate. The kinase assay was stopped after 15 min by the addition of Laemmli buffer, and samples were resolved by SDS-PAGE. Coomassie staining revealed total MBP levels, and autoradiography showed the 32P incorporation in each sample. The amounts of MAPK in the immunoprecipitates were revealed by Western blotting (WB) with antibodies to Erk-1 and Erk-2. p44 and p42 MAPKs are indicated by arrows. (B) Representation of the means ± standard deviations of data from three independent experiments performed in triplicate. Data are expressed as fold increases in MAPK activation with respect to that of starved NIH 3T3 cells.
The MAPK cascade seems to be one of the main ways in which growth factors (and many other signalling pathways) induce cell proliferation, and it appears to be activated by most, if not all, nonnuclear oncogenes (75), including the polyomavirus early function PyMT (19, 85). Furthermore, it has been determined that not only the induction but also the duration of MAPK activation influences the cellular response (28, 29, 44). It has been reported that defects in the downregulation of MAPK activity after the shift to low-serum conditions represent a means by which some mutated receptors can transduce their oncogenic signals (49) and that weak increases in their activity can play a role in important processes such as muscle differentiation (5). Also in the case of PyLT and SV40LT, we observed defects in the regulation of MAPK activity that could probably play a role in the production of constitutive nuclear signals that might cooperate with the nuclear functions of the viral oncogenes in cell cycle progression.
PyLT and SV40LT interact with Shc adapter proteins.
Some constitutive phosphorylations induced by viral oncoproteins turn out to be related to the association of the viral oncoprotein itself with the altered target molecule, as in the case of the chronic phosphorylation induced in the platelet-derived growth factor receptor by its interaction with the bovine papillomavirus E5 transforming protein (3, 51, 57) and the constitutive Shc phosphorylation induced by its binding to PyMT (12, 19). In other cases, such as in the association between IRS-1 and SV40LT, the interaction and the constitutive phosphorylation of the docking protein have been attributed to different, independent mechanisms (22). We decided to investigate the possibility of an association between Shc and LT in NIH 3T3 fibroblasts. The results of this analysis are reported in Fig. 5. Immunoprecipitations of starved cell cultures with either LT- or Shc-specific antibodies were performed. Lysates from each cell line were immunoprecipitated with a mixture of PyLT and SV40LT monoclonal antibodies and blotted with Shc antibodies. A band that comigrates with p52Shc was visible only in the samples obtained from cells expressing the wt forms of the two viral proteins and not in cells expressing the cytoplasmic mutants. The coimmunoprecipitation of Shc observed in SV40LT immunoprecipitates did not agree with previously reported negative results for this interaction (22), probably due to the different technical approaches with regard to, for example, the cellular system, lysis protocol, and amount of immunoprecipitated protein. In any case, wt LT proteins (PyLT and SV40LT) were clearly detected after the immunoprecipitation with antibodies to Shc, thus confirming our finding. Similar results were obtained under cell proliferation conditions and after 48 h in low-serum medium (data not shown), suggesting that the binding is constitutive.
Although we clearly detected an interaction between Shc and Grb-2 in the same cells in which an LT-Shc complex is revealable (by both Shc and Grb-2 immunoprecipitation), we did not detect the formation of a complex between LT and Grb-2. This may be explained either by a low stoichiometry or stability of the complex formed between these three molecules (LT–Shc–Grb-2) or, alternatively, by the occurrence of interactions that do not necessarily require the simultaneous binding of Shc to its partners.
We also confirmed this interaction in Rat-1 fibroblasts for both wt PyLT and its Rb− mutant (Fig. 5B). These data demonstrate that neither Shc phosphorylation nor Shc binding is dependent on interaction of PyLT with Rb.
Phosphotyrosine domains of Shc are involved in the interaction with LT.
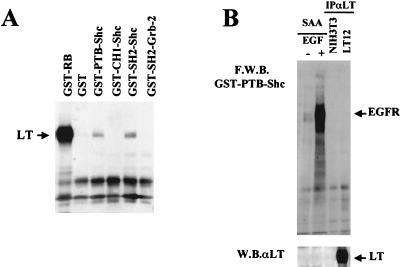
Since PyLT is a nuclear protein and Shc adapters are cytosolic proteins, we were interested in determining which domain(s) of the adapters are required for the interaction. We took advantage of the GST fusion protein system, with which it is possible to produce and purify the three domains of p52Shc: the phosphotyrosine binding domain (PTB), the collagen homology 1 domain (CH1), and the Src homology 2 domain (SH2). In these experiments, we tested the interaction of the three domains of p52Shc with the PyLT molecules present in cell lysates from starved PyLT-expressing fibroblasts (Fig. 5A). As a positive control, we chose an Rb domain that contains the LT binding site. We observed (Fig. 6A) that LT interacts only with the two phosphotyrosine binding domains of Shc, PTB and SH2, while it fails to bind the CH1 domain of the adapter molecule. However, in agreement with the coimmunoprecipitation data shown in Fig. 5, another phosphotyrosine-binding domain, the SH2 domain of Grb-2, did not interact with LT in these assays, supporting the specificity of the binding. Similar results were obtained with cell lysates obtained from proliferating PyLT-expressing cells (data not shown).
FIG. 6.
In vitro binding of Shc and PyLT. (A) The SH2 and PTB domains of SHC bind LT in vitro. LT12 fibroblasts were grown to confluence, starved for 24 h, and then lysed. Total cell lysates were incubated with equal amounts of bacterium-expressed fusion proteins corresponding to the protein domain Rb(379–928), Shc-SH2, Shc-CH1, Shc-PTB, and Grb-2–SH2 and GST alone as a control. All were then resolved by SDS–10% PAGE and analyzed by immunoblotting with antibodies to LT. LT proteins are indicated by arrows. (B) Anti-PyLT (αLT) immunoprecipitates (IP) from NIH 3T3 or LT12 fibroblasts were resolved by SDS–9% PAGE. The blot was then probed with the GST-PTB-Shc fusion protein and resolved by far-Western blotting (F.W.B.) with anti-GST antibodies. As controls, cell lysates from EGF-starved (−) and EGF-stimulated (+) SAA cells were used. The same filter was then analyzed by Western blotting (W.B.) with polyclonal antibodies to PyLT.
To further characterize this interaction, far-Western assays were performed with the purified recombinant fusion proteins Shc-PTB and Shc-SH2. The results obtained for Shc-PTB are represented in Fig. 6B, and similar results were obtained for Shc-SH2 (data not shown). NIH 3T3 cells overexpressing the EGF receptor (18) were used as a control, and as expected, a specific interaction with the phosphorylated receptor was observed. The discrepancy between these two in vitro approaches could be the consequence of nonclassical interactions for the PTB and SH2 domains of Shc that cannot be revealed by far-Western blotting techniques. Alternatively, the interaction between the two phosphotyrosine binding domains of Shc and LT might be indirect. In this regard, however, no interaction with other proteins potentially present in the PyLT immunoprecipitates was detected. More work will be required to obtain information regarding any other protein(s) that might be involved in the PyLT-Shc interaction.
DISCUSSION
In the last few years it has become clear that environmental signals regulate in vivo and in vitro cell growth and that some transforming oncogenes have evolved strategies aimed at the alteration of growth factor-dependent signals. It has been reported, in fact, that several cell-derived oncogenes are homologous to growth factors, growth factor receptors, or their substrates. Some evidence, though still fragmentary, indicates an indirect but nevertheless effective strategy involving viral and nonviral nuclear oncoproteins, such as SV40LT and c-myb, in the alteration of growth factor-related signals (3, 22, 58, 59, 67). Other viral proteins, such as PyMT and SV40LT, bind to receptor substrates and alter their activity (12, 19). It has also been reported that with regard to many other oncogenes, such as PyLT (64), reduced requirements for growth factors might be only apparent and, in reality, simply the consequence of oncogene-dependent alterations of growth factor-related signals. Our starting hypothesis was that the Shc adapter proteins might reflect the presence of any of these alteration(s). In this work, we have reported the constitutive phosphorylation of p52Shc in fibroblasts expressing PyLT. Our data suggest that constitutive Shc phosphorylation in these cells is followed by the activation of downstream pathways and the generation of nuclear signals (MAPK) that are capable of cooperating with the nuclear functions of the oncogenic viral protein in the induction of entry into S phase. We have shown that the constitutive Shc activation is a PyLT effect independent of the binding to Rb. This suggests the possibility of a cooperation between different PyLT functions in the induction of cell cycle progression. Of course, there is no question about the preponderant role of Rb inactivation, but the participation of cytoplasmic alterations in successful cell cycle progression in the absence of mitogens constitutes an extremely interesting possibility that deserves further investigation.
From what we have learned about other nonviral oncogenes (3), we hypothesize that the mechanism by which LT induces constitutive Shc phosphorylation involves alterations in (i) the secretion of growth factors, (ii) the expression of receptors, and (iii) the activities of kinases or phosphatases involved in the control of Shc phosphorylation, as well as, of course, a simultaneous alteration of the above-mentioned mechanisms. It has been reported that PyLT does not induce the secretion of transforming growth factors (32), and our own observations demonstrate that the conditioned medium from starved PyLT-expressing cells does not contain the minimum concentration or the right combination of growth factors required for the induction of NIH 3T3 cell proliferation. On the other hand, we observed that the same conditioned medium contains a scattering activity that has been detectable only in the case of extremely sensitive scatter assays performed on BN14 epithelial cells (data not shown). Furthermore, this scattering activity is not observed in the conditioned medium from NIH 3T3 cells expressing the cytoplasmic mutant of PyLT, which also fails to induce constitutive Shc phosphorylation and DNA synthesis in low-serum medium. A possible explanation for this observation is that PyLT induces a minimal secretion of one or more growth factors that offer advantages only to those cells that can use them efficiently, either because of high local concentrations of the secreted growth factors or because of cooperation with nuclear alterations. A second possible explanation for chronic Shc activation is an increase in the level of some TK receptors that can induce a higher sensitivity of PyLT-expressing cells to low levels of growth factors. A third possible explanation for constitutive Shc phosphorylation is that the interaction of LT with Shc is responsible for a deregulated kinase activity on the adapter molecule or, alternatively, for interference with the Shc dephosphorylation events. Even if the interaction between Shc and LT is indirect and involves the phosphotyrosine-interacting domains of Shc and probably also the complete maturation of the viral oncoprotein, we still do not know precisely which domain of LT participates in the interaction, and, more importantly, we still have no information about other molecules that can be recruited into the complex. We did not detect any phosphotyrosine-containing protein in Shc and LT immunoprecipitates (data not shown), and unfortunately, far-Western blot analyses performed with the PTB and SH2 domains of Shc did not reveal any interaction between these Shc domains and any protein contained in PyLT immunoprecipitates (data not shown). Phosphotyrosine-independent interactions have been reported for SH2 domains, and in particular, a non-phosphotyrosine-dependent interaction has been reported for the amino-terminal region of Shc (63). Moreover, the PTB of Shc exhibits structural homology to PH domains, suggesting the possibility of other interactions for Shc-PTB that do not directly involve the phosphotyrosine-binding site (89). On this basis, it can be hypothesized that these domains engage in other, as-yet-uncharacterized types of interactions that might involve the folded structure of the target protein and/or the posttranslational regulation of the domains themselves, revealing a limit to the analyses that can be carried out by far-Western techniques. Any information about any other molecule(s) that participates in the complex will help to determine the role of the interaction in the constitutive activation of the adapter molecule. This will also help to selectively block the PyLT-induced Shc phosphorylation so that the contribution of this new function to the maintenance of the cycling phenotype can be evaluated.
Although we cannot propose a direct link between Shc activation and an interaction with the viral oncoproteins, some evidence suggests that both events take place in the cytoplasm. (i) Even though SV40LT is a nuclear oncogene, a fraction of the total protein is found in the cytoplasm (71, 72), and cell fractionation experiments have revealed that PyLT is not completely localized in the nucleus (reference 31 and our unpublished results). (ii) Nuclear Shc localization has not been reported. (iii) Cytoplasmic proteins other than Shc have been reported to interact with SV40LT (i.e., IRS-1 and hsp70 [22, 80]) and PyLT (i.e., hsp70 [76]). (iv) The interaction of SV40LT with IRS-1, a clearly cytosolic function, suggests that the cytoplasmic fraction of the oncoprotein participates actively and is required for cell transformation. The apparent contradiction between the proposed cytoplasmic interaction and the experimental data obtained with cytoplasmic mutants of both oncoproteins may be the consequence of differences between the cytoplasmic fraction of the wt protein and the cytoplasmic mutant. For example, it has been demonstrated that PyLT and SV40LT transport-defective mutants show an incomplete phosphorylation pattern (31, 73). Furthermore, it has been reported that some LT functions are regulated by phosphorylations (31, 37) and that some phosphorylations require nuclear localization (14, 31, 87). A second possibility is that the nuclear fraction of LT is indirectly involved in transcriptional modifications affecting either other factors that participate in the complex or Shc itself.
We have already discussed (see above) the different strategies generated by SV40LT to specifically alter the IGF-1-dependent signal. In particular, the IGF-1 receptor forms a complex with SV40LT and the docking protein, IRS-1. It has been proposed that the binding of SV40LT to IRS-1 does not directly induce its phosphorylation but rather amplifies its dependent signal. Our results show that PyLT does not interact with IRS-1 in experiments in which SV40LT and its cytoplasmic mutant do interact (data not shown). Intriguingly, the cytoplasmic mutant of SV40LT partially retains tumorigenic potential in the presence of growth factors (82) and forms a complex with IRS-1 but fails to induce either constitutive Shc phosphorylation after growth factor removal or DNA synthesis under low-serum conditions. PyLT is not tumorigenic and does not bind IRS-1 but induces both cell cycle progression after growth factor removal and constitutive Shc activation. These observations support the hypothesis that the alteration of Shc phosphorylation is a function of SV40LT that is independent of the signal generated by IRS-1 binding (even if they are probably convergent) and that PyLT has evolved the ability to induce only one of these two possible cytoplasmic signal alterations. This, once again, reveals a limit in the capacity of PyLT to activate all of the independent pathways known to be essential for SV40LT in cell transformation.
Our data demonstrate that regardless of the mechanism that induces Shc phosphorylation in PyLT-expressing cells, the phosphorylation of the adapter molecule is sufficient to ensure the formation of the Shc–Grb-2 complex which presumably recruits also Sos. The enhanced MAPK activity observed in LT-expressing cells after growth factor removal may be the consequence of Ras activation. Of course, it is impossible to exclude the possibility that there are other, still-unknown cytoplasmic signals induced by PyLT which converge on MAPK activity. The further increase in MAPK activity observed in SV40LT-expressing cells may be the result of a more significant Ras activation induced by a cooperative Shc phosphorylation- and IRS-1-dependent signalling.
The constitutive MAPK activation, though weak, must not be overlooked (see “Constitutive Shc phosphorylation induced by PyLT and SV40LT correlates with alterations in MAPK activity” in Results). We cannot exclude the possibility that the modest alterations in the kinetics of MAPK activity represent one means by which the constitutive activated isoform of Shc transduces its signal. In this regard, it has been reported that PyLT can activate the fos promoter (31), one of the best-characterized target genes of MAPKs, once these enzymes translocate to the nucleus, suggesting that a MAPK-dependent activation of early genes could easily cooperate with the nuclear functions of PyLT and SV40LT in the maintenance of actively cycling cells. The observations that both the transactivation of the fos promoter and the induction of Shc phosphorylation by PyLT are independent of Rb binding and that the cytoplasmic mutant of PyLT fails in both these functions suggest that the constitutive Shc phosphorylation induced by PyLT is effectively transducing signals that are altering the Ras-MAPK pathway. Finally, we must not forget that activated Shc may also be affecting Ras-independent pathways (7) that can contribute to alterations in growth control.
An interesting consequence of our findings is that they add SV40LT and PyLT to the list of oncogenes that are “interested” in altering MAPK activities. We must not forget that PyMT is a potent activator of this pathway (85) and that it has been demonstrated that SV40 small t antigen indirectly activates MAPK because of the inactivation of PP2A (79). Since polyomavirus small T antigen also binds PP2A, the third polyomavirus early function also probably alters MAPK activity. It will be interesting to find out whether this is a consequence of an incomplete evolution in the division of early protein function or, even more interesting, a combination of different kinetics of activation of MAPKs is required for viral infections, thus suggesting yet another phenomenon that could be added to the long list of processes in which these kinases play a preponderant role.
ACKNOWLEDGMENTS
We acknowledge A. E. Salcini and E. Migliaccio for helpful discussions and B. Schaffhausen, C. Vesco, and C. Prives for generous gifts of plasmids, antibodies, and cell lines. We also thank N. Falcone for technical assistance.
This work was supported by grants from the Associazione Italiana Ricerca sul Cancro (AIRC) and MURST, Rome, Italy (to P.A.). V.G. was the recipient of a fellowship from UNIDO-ICGEB, Trieste, Italy.
REFERENCES
- 1.Aronheim A, Engelberg D, Li N, al Alawi N, Schlessinger J, Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994;78:949–961. doi: 10.1016/0092-8674(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 2.Baldari C T, Pelicci G, Di Somma M M, Milia E, Giuli S, Pelicci P G, Telford J L. Inhibition of CD4/p56lck signaling by a dominant negative mutant of the Shc adaptor protein. Oncogene. 1995;10:1141–1147. [PubMed] [Google Scholar]
- 3.Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994;79:927–930. doi: 10.1016/0092-8674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 4.Basu T, Warne P H, Downward J. Role of Shc in the activation of Ras in response to epidermal growth factor and nerve growth factor. Oncogene. 1994;9:3483–3491. [PubMed] [Google Scholar]
- 5.Benett A M, Tonks N K. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1998;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- 6.Bonfini L, Karlovich C A, Dasgupta C, Banerjee U. The son of sevenless gene product: a putative activator of Ras. Science. 1992;255:603–606. doi: 10.1126/science.1736363. [DOI] [PubMed] [Google Scholar]
- 7.Bonfini L, Migliaccio E, Pelicci G, Lanfrancone L, Pelicci P G. Not all of Shc’s roads lead to Ras. Trends Biochem Sci. 1996;21:257–261. [PubMed] [Google Scholar]
- 8.Borrello M G, Pelicci G, Arighi E, De Filippis L, Greco A, Bongarzone I, Rizzetti M, Pelicci P G, Pierotti M A. The oncogenic versions of the Ret and Trk tyrosine kinases bind Shc and Grb2 adaptor proteins. Oncogene. 1994;9:1661–1668. [PubMed] [Google Scholar]
- 9.Bowtell D, Fu P, Simon M, Senior P. Identification of murine homologues of the Drosophila son of sevenless gene: potential activators of ras. Proc Natl Acad Sci USA. 1992;89:6511–6515. doi: 10.1073/pnas.89.14.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 11.Burns L A, Karnitz L M, Sutor S L, Abraham R T. Interleukin-2-induced tyrosine phosphorylation of p52shc in T lymphocytes. J Biol Chem. 1993;268:17659–17661. [PubMed] [Google Scholar]
- 12.Campbell K S, Ogris E, Burke B, Su W, Auger K R, Druker B J, Schaffhausen B S, Roberts T M, Pallas D C. Polyoma middle tumor antigen interacts with SHC protein via the NPTY (Asn-Pro-Thr-Tyr) motif in middle tumor antigen. Proc Natl Acad Sci USA. 1994;91:6344–6348. doi: 10.1073/pnas.91.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chardin P, Camonis J H, Gale N W, van Aelst L, Schlessinger J, Wigler M H, Bar Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee A, Bockus B J, Gjørup O V, Schaffhausen B S. Phosphorylation sites in polyomavirus large T antigen that regulate its function in viral, but not cellular, DNA synthesis. J Virol. 1997;71:6472–6478. doi: 10.1128/jvi.71.9.6472-6478.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark S G, Stern M J, Horvitz H R. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- 16.Crowe A J, McGlade J, Pawson T, Hayman M J. Phosphorylation of the SHC proteins on tyrosine correlates with the transformation of fibroblasts and erythroblasts by the v-sea tyrosine kinase. Oncogene. 1994;9:537–544. [PubMed] [Google Scholar]
- 17.Damen J E, Liu L, Cutler R L, Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993;82:2296–2303. [PubMed] [Google Scholar]
- 18.Di Fiore P P, Pierce J H, Fleming T P, Hazan R, Ullrich A, King C R, Schlessinger J, Aaronson S A. Overexpression of the human EGF receptor confers an EGF-dependent transformation phenotype to NIH3T3. Cell. 1987;51:1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- 19.Dilworth S M, Brewster C E, Jones M D, Lanfrancone L, Pelicci G, Pelicci P G. Transformation by polyoma virus middle T-antigen involves the binding and tyrosine phosphorylation of Shc. Nature. 1994;367:87–90. doi: 10.1038/367087a0. [DOI] [PubMed] [Google Scholar]
- 20.Dilworth S M, Griffin B E. Monoclonal antibodies against polyomavirus tumor antigens. Proc Natl Acad Sci USA. 1982;79:1059–1063. doi: 10.1073/pnas.79.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egan S E, Giddings B W, Brooks M W, Buday L, Sizeland A M, Weinberg R A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 22.Fei Z-L, D’Ambrosio C, Li S, Surmacz E, Baserga R. Association of insulin receptor substrate 1 with simian virus 40 large T antigen. Mol Cell Biol. 1995;15:4232–4239. doi: 10.1128/mcb.15.8.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fimia G M, Gottifredi V, Bellei B, Ricciardi M R, Tafuri A, Amati P, Maione R. The activity of differentiation factors induces apoptosis in polyomavirus large T-expressing myoblasts. Mol Biol Cell. 1998;9:1449–1463. doi: 10.1091/mbc.9.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francke B, Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973;55:127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- 25.Freund R, Bronson R T, Benjamin T L. Separation of immortalization from tumor induction with polyoma large T mutants that fail to bind the retinoblastoma gene product. Oncogene. 1992;7:1979–1987. [PubMed] [Google Scholar]
- 26.Gale N W, Kaplan S, Lowenstein E J, Schlessinger J, Bar Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- 27.Gjørup O V, Rose P E, Holman P S, Bockus B J, Schaffhausen B S. Protein domains connect cell cycle stimulation directly to initiation of DNA replication. Proc Natl Acad Sci USA. 1994;91:12125–12129. doi: 10.1073/pnas.91.25.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grammer T C, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–1642. doi: 10.1038/sj.onc.1201000. [DOI] [PubMed] [Google Scholar]
- 29.Greulich H, Reichman C, Hanafusa H. Delay in serum stimulation of Erk activity caused by oncogenic transformation. Oncogene. 1996;12:1689–1695. [PubMed] [Google Scholar]
- 30.Holman P S, Gjoerup O V, Davin T, Schaffhausen B S. Characterization of an immortalizing N-terminal domain of polyomavirus large T antigen. J Virol. 1994;68:668–673. doi: 10.1128/jvi.68.2.668-673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howes S H, Bockus B J, Schaffhausen B S. Genetic analysis of polyomavirus large T nuclear localization: nuclear localization is required for productive association with pRb family members. J Virol. 1996;70:3581–3588. doi: 10.1128/jvi.70.6.3581-3588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan P L, Ozanne B. Polyoma virus-transformed cells produce transforming growth factor(s) and grow in serum-free medium. Virology. 1982;123:372–380. doi: 10.1016/0042-6822(82)90270-7. [DOI] [PubMed] [Google Scholar]
- 33.Land H, Parada L F, Weinberg R A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 34.Lanford R E, Wong C, Butel J S. Differential ability of a T-antigen transport-defective mutant of simian virus 40 to transform primary and established rodent cells. Mol Cell Biol. 1985;5:1043–1050. doi: 10.1128/mcb.5.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanfrancone L, Pelicci G, Brizzi M F, Aronica M G, Casciari C, Giuli S, Pegoraro L, Pawson T, Pelicci P G, Arouica M G A. Overexpression of Shc proteins potentiates the proliferative response to the granulocyte-macrophage colony-stimulating factor and recruitment of Grb2/SoS and Grb2/p140 complexes to the beta receptor subunit. Oncogene. 1995;10:907–917. [PubMed] [Google Scholar]
- 36.Larose A, Dyson N, Sullivan M, Harlow E, Bastin M. Polyomavirus large T mutants affected in retinoblastoma protein binding are defective in immortalization. J Virol. 1991;65:2308–2313. doi: 10.1128/jvi.65.5.2308-2313.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Bhattacharyya S, Prives C. Cyclin-dependent kinase regulation of the replication functions of polyomavirus large T antigen. J Virol. 1997;71:6479–6485. doi: 10.1128/jvi.71.9.6479-6485.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar Sagi D, Margolis B, Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- 39.Lill N L, Tevethia M J, Eckner R, Livingston D M, Modjtahedi N. p300 family members associate with the carboxyl terminus of simian virus 40 large tumor antigen. J Virol. 1997;71:129–137. doi: 10.1128/jvi.71.1.129-137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lotti L V, Lanfrancone L, Migliaccio E, Zompetta C, Pelicci G, Salcini A E, Falini B, Pelicci P G, Torrisi M R. Shc proteins are localized on endoplasmic reticulum membranes and are redistributed after tyrosine kinase receptor activation. Mol Cell Biol. 1996;16:1946–1954. doi: 10.1128/mcb.16.5.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowenstein E J, Daly R J, Batzer A G, Li W, Margolis B, Lammers R, Ullrich A, Skolnik E Y, Bar Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 42.Maione R, Fimia G M, Amati P. Inhibition of in vitro myogenic differentiation by a polyomavirus early function. Oncogene. 1992;7:85–93. [PubMed] [Google Scholar]
- 43.Maione R, Fimia G M, Holman P, Schaffhausen B, Amati P. Retinoblastoma antioncogene is involved in the inhibition of myogenesis by polyomavirus large T antigen. Cell Growth Differ. 1994;5:231–237. [PubMed] [Google Scholar]
- 44.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 45.Martel C, Harper F, Cereghini S, Noe V, Mareel M, Cremisi C. Inactivation of retinoblastoma family proteins by SV40 T antigen results in creation of a hepatocyte growth factor/scatter factor autocrine loop associated with an epithelial-fibroblastoid conversion and invasiveness. Cell Growth Differ. 1997;8:165–178. [PubMed] [Google Scholar]
- 46.Matsuguchi T, Salgia R, Hallek M, Eder M, Druker B, Ernst T J, Griffin J D. Shc phosphorylation in myeloid cells is regulated by granulocyte macrophage colony-stimulating factor, interleukin-3, and steel factor and is constitutively increased by p210BCR/ABL. J Biol Chem. 1994;269:5016–5021. [PubMed] [Google Scholar]
- 47.McGlade J, Cheng A, Pelicci G, Pelicci P G, Pawson T. Shc proteins are phosphorylated and regulated by the v-Src and v-Fps protein-tyrosine kinases. Proc Natl Acad Sci USA. 1992;89:8869–8873. doi: 10.1073/pnas.89.19.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Migliaccio E, Mele S, Salcini A E, Pelicci G, Lai K M, Superti Furga G, Pawson T, Di Fiore P P, Lanfrancone L, Pelicci P G. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montgomery R B, Moscatello D K, Wong A J, Cooper J A, Stahl W L. Differential modulation of mitogen-activated protein (MAP) kinase/extracellular signal-related kinase and MAP kinase activities by a mutant epidermal growth factor receptor. J Biol Chem. 1995;270:30562–30566. doi: 10.1074/jbc.270.51.30562. [DOI] [PubMed] [Google Scholar]
- 50.Myers M G, Jr, Wang L-M, Sun X J, Zhang Y, Yenush L, Schlessinger J, Pierce J H, White M F. Role of IRS-1–GRB-2 complexes in insulin signaling. Mol Cell Biol. 1994;14:3577–3587. doi: 10.1128/mcb.14.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nilson L A, DiMaio D. Platelet-derived growth factor receptor can mediate tumorigenic transformation by the bovine papillomavirus E5 protein. Mol Cell Biol. 1993;13:4137–4145. doi: 10.1128/mcb.13.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olivier J P, Raabe T, Henkemeyer M, Dickson B, Mbamalu G, Margolis B, Schlessinger J, Hafen E, Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993;73:179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- 53.Ouwens D M, van der Zon G C, Pronk G J, Bos J L, Moller W, Cheatham B, Kahn C R, Maassen J A. A mutant insulin receptor induces formation of an Shc-growth factor receptor bound protein 2 (Grb2) complex and p21ras-GTP without detectable interaction of insulin receptor substrate 1 (IRS1) with Grb2. Evidence for IRS1-independent p21ras-GTP formation. J Biol Chem. 1994;269:33116–33122. [PubMed] [Google Scholar]
- 54.Pelicci G, Giordano S, Zhen Z, Salcini A E, Lanfrancone L, Bardelli A, Panayotou G, Waterfield M D, Ponzetto C, Pelicci P G, et al. The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene. 1995;10:1631–1638. [PubMed] [Google Scholar]
- 55.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Pawson T, Pelicci P G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 56.Pelicci G, Lanfrancone L, Salcini A E, Romano A, Mele S, Grazia Borrello M, Segatto O, Di Fiore P P, Pelicci P G. Constitutive phosphorylation of Shc proteins in human tumors. Oncogene. 1995;11:899–907. [PubMed] [Google Scholar]
- 57.Petti L, DiMaio D. Specific interaction between the bovine papillomavirus E5 transforming protein and the β receptor for platelet-derived growth factor in stably transformed and acutely transfected cells. J Virol. 1994;68:3582–3592. doi: 10.1128/jvi.68.6.3582-3592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porcu P, Ferber A, Pietrzkowski Z, Roberts C T, Adamo M, LeRoith D, Baserga R. The growth-stimulatory effect of simian virus 40 T antigen requires the interaction of insulinlike growth factor 1 with its receptor. Mol Cell Biol. 1992;12:5069–5077. doi: 10.1128/mcb.12.11.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porcu P, Grana X, Li S, Swantek J, De Luca A, Giordano A, Baserga R. An E2F binding sequence negatively regulates the response of the insulin-like growth factor 1 (IGF-I) promoter to simian virus 40 T antigen and to serum. Oncogene. 1994;9:2125–2134. [PubMed] [Google Scholar]
- 60.Pronk G J, McGlade J, Pelicci G, Pawson T, Bos J L. Insulin-induced phosphorylation of the 46- and 52-kDa Shc proteins. J Biol Chem. 1993;268:5748–5753. [PubMed] [Google Scholar]
- 61.Quartin R S, Cole C N, Pipas J M, Levine A J. The amino-terminal functions of the simian virus 40 large T antigen are required to overcome wild-type p53-mediated growth arrest of cells. J Virol. 1994;68:1334–1341. doi: 10.1128/jvi.68.3.1334-1341.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raffel G D, Parmar K, Rosenberg N. In vivo association of v-Abl with Shc mediated by a non-phosphotyrosine-dependent SH2 interaction. J Biol Chem. 1996;271:4640–4645. doi: 10.1074/jbc.271.9.4640. [DOI] [PubMed] [Google Scholar]
- 63.Raptis L, Brownell H L, Corbley M J, Wood K W, Wang D, Haliotis T. Cellular ras gene activity is required for full neoplatic transformation by the large tumor antigen of SV40. Cell Growth Differ. 1997;8:891–901. [PubMed] [Google Scholar]
- 64.Rassoulzadegan M, Cowie A, Carr A, Glaichenhaus N, Kamen R, Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982;300:713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- 65.Rassoulzadegan M, Naghashfar Z, Cowie A, Carr A, Grisoni M, Kamen R, Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of “normal” rodent fibroblast cell lines. Proc Natl Acad Sci USA. 1983;80:4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ravichandran K S, Lee K K, Songyang Z, Cantley L C, Burn P, Burakoff S J. Interaction of Shc with the zeta chain of the T cell receptor upon T cell activation. Science. 1993;262:902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- 67.Reiss K, Porcu P, Sell C, Pietrzkowski Z, Baserga R. The insulin-like growth factor 1 receptor is required for the proliferation of hemopoietic cells. Oncogene. 1992;7:2243–2248. [PubMed] [Google Scholar]
- 68.Ricci A, Lanfrancone L, Chiari R, Belardo G, Pertica C, Natali P G, Pelicci P G, Segatto O. Analysis of protein-protein interactions involved in the activation of the Shc/Grb-2 pathway by the ErbB-2 kinase. Oncogene. 1995;11:1519–1529. [PubMed] [Google Scholar]
- 69.Rozakis Adcock M, van der Geer P, Mbamalu G, Pawson T. MAP kinase phosphorylation of mSos1 promotes dissociation of mSos1-Shc and mSos1-EGF receptor complexes. Oncogene. 1995;11:1417–1426. [PubMed] [Google Scholar]
- 70.Salcini A E, McGlade J, Pelicci G, Nicoletti I, Pawson T, Pelicci P G. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene. 1994;9:2827–2836. [PubMed] [Google Scholar]
- 71.Santos M, Butel J S. Detection of a complex of SV40 large tumor antigen and 53K cellular protein on the surface of SV40-transformed mouse cells. J Cell Biochem. 1982;19:127–144. doi: 10.1002/jcb.240190204. [DOI] [PubMed] [Google Scholar]
- 72.Santos M, Butel J S. Association of SV40 large tumor antigen and cellular proteins on the surface of SV40-transformed mouse cells. Virology. 1982;120:1–17. doi: 10.1016/0042-6822(82)90002-2. [DOI] [PubMed] [Google Scholar]
- 73.Scheidtmann K H, Schickedanz J, Walter G, Lanford R E, Butel J S. Differential phosphorylation of cytoplasmic and nuclear variants of simian virus 40 large T antigen encoded by simian virus 40-adenovirus 7 hybrid viruses. J Virol. 1984;50:636–640. doi: 10.1128/jvi.50.2.636-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segatto O, Pelicci G, Giuli S, Digiesi G, Di Fiore P P, McGlade J, Pawson T, Pelicci P G. Shc products are substrates of erbB-2 kinase. Oncogene. 1993;8:2105–2112. [PubMed] [Google Scholar]
- 75.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 76.Sheng Q, Denis D, Ratnofsky M, Roberts T M, DeCaprio J A, Schaffhausen B. The DnaJ domain of polyomavirus large T antigen is required to regulate Rb family tumor suppressor function. J Virol. 1997;71:9410–9416. doi: 10.1128/jvi.71.12.9410-9416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simon M A, Bowtell D D, Dodson G S, Laverty T R, Rubin G M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991;67:701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 78.Skolnik E Y, Lee C H, Batzer A, Vicentini L M, Zhou M, Daly R, Myers M J J, Backer J M, Ullrich A, White M F, et al. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 80.Srinivasan A, McClellan A J, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky J L, Pipas J M. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stephens R M, Loeb D M, Copeland T D, Pawson T, Greene L A, Kaplan D R. Trk receptors use redundant signal transduction pathways involving SHC and PLC-γ-1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 82.Tedesco D, Fischer Fantuzzi L, Vesco C. Limits of transforming competence of SV40 nuclear and cytoplasmic large T mutants with altered Rb binding sequences. Oncogene. 1993;8:549–557. [PubMed] [Google Scholar]
- 83.Thomas D, Patterson S D, Bradshaw R A. Src homologous and collagen (Shc) protein binds to F-actin and translocates to the cytoskeleton upon nerve growth factor stimulation in PC12 cells. J Biol Chem. 1995;270:28924–28931. doi: 10.1074/jbc.270.48.28924. [DOI] [PubMed] [Google Scholar]
- 84.Tooze J. Molecular biology of tumor viruses: DNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1981. [Google Scholar]
- 85.Urich M, el Shemerly M Y, Besser D, Nagamine Y, Ballmer Hofer K. Activation and nuclear translocation of mitogen-activated protein kinases by polyomavirus middle-T or serum depend on phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:29286–29292. doi: 10.1074/jbc.270.49.29286. [DOI] [PubMed] [Google Scholar]
- 86.Vainikka S, Joukov V, Wennstrom S, Bergman M, Pelicci P G, Alitalo K. Signal transduction by fibroblast growth factor receptor-4 (FGFR-4). Comparison with FGFR-1. J Biol Chem. 1994;269:18320–18326. [PubMed] [Google Scholar]
- 87.Wang E H, Bhattacharyya S, Prives C. The replication functions of polyomavirus large tumor antigen are regulated by phosphorylation. J Virol. 1993;67:6788–6796. doi: 10.1128/jvi.67.11.6788-6796.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yokote K, Mori S, Hansen K, McGlade J, Pawson T, Heldin C H, Claesson Welsh L. Direct interaction between Shc and the platelet-derived growth factor beta-receptor. J Biol Chem. 1994;269:15337–15343. [PubMed] [Google Scholar]
- 89.Zhou M M, Ravichandran K S, Olejniczak E F, Petros A M, Meadows R P, Sattler M, Harlan J E, Wade W S, Burakoff S J, Fesik S W. Structure and ligand recognition of the phosphotyrosine binding domain of Shc. Nature. 1995;378:584–592. doi: 10.1038/378584a0. [DOI] [PubMed] [Google Scholar]