Abstract
Large language models (LLMs) have demonstrated impressive capabilities, but the bar for clinical applications is high. Attempts to assess the clinical knowledge of models typically rely on automated evaluations based on limited benchmarks. Here, to address these limitations, we present MultiMedQA, a benchmark combining six existing medical question answering datasets spanning professional medicine, research and consumer queries and a new dataset of medical questions searched online, HealthSearchQA. We propose a human evaluation framework for model answers along multiple axes including factuality, comprehension, reasoning, possible harm and bias. In addition, we evaluate Pathways Language Model1 (PaLM, a 540-billion parameter LLM) and its instruction-tuned variant, Flan-PaLM2 on MultiMedQA. Using a combination of prompting strategies, Flan-PaLM achieves state-of-the-art accuracy on every MultiMedQA multiple-choice dataset (MedQA3, MedMCQA4, PubMedQA5 and Measuring Massive Multitask Language Understanding (MMLU) clinical topics6), including 67.6% accuracy on MedQA (US Medical Licensing Exam-style questions), surpassing the prior state of the art by more than 17%. However, human evaluation reveals key gaps. To resolve this, we introduce instruction prompt tuning, a parameter-efficient approach for aligning LLMs to new domains using a few exemplars. The resulting model, Med-PaLM, performs encouragingly, but remains inferior to clinicians. We show that comprehension, knowledge recall and reasoning improve with model scale and instruction prompt tuning, suggesting the potential utility of LLMs in medicine. Our human evaluations reveal limitations of today’s models, reinforcing the importance of both evaluation frameworks and method development in creating safe, helpful LLMs for clinical applications.
Subject terms: Health care, Medical research
Med-PaLM, a state-of-the-art large language model for medicine, is introduced and evaluated across several medical question answering tasks, demonstrating the promise of these models in this domain.
Main
Medicine is a humane endeavour in which language enables key interactions for and between clinicians, researchers and patients. Yet, today’s artificial intelligence (AI) models for applications in medicine and healthcare have largely failed to fully utilize language. These models, although useful, are predominantly single-task systems (for example, for classification, regression or segmentation) lacking expressivity and interactive capabilities7–9. As a result, there is a discordance between what today’s models can do and what may be expected of them in real-world clinical workflows10.
Recent advances in LLMs offer an opportunity to rethink AI systems, with language as a tool for mediating human–AI interaction. LLMs are ‘foundation models’11, large pre-trained AI systems that can be repurposed with minimal effort across numerous domains and diverse tasks. These expressive and interactive models offer great promise in their ability to learn generally useful representations from the knowledge encoded in medical corpora, at scale. There are several exciting potential applications of such models in medicine, including knowledge retrieval, clinical decision support, summarization of key findings, triaging patients, addressing primary care concerns and more.
However, the safety-critical nature of the domain necessitates thoughtful development of evaluation frameworks, enabling researchers to meaningfully measure progress and capture and mitigate potential harms. This is especially important for LLMs, since these models may produce text generations (hereafter referred to as ‘generations’) that are misaligned with clinical and societal values. They may, for instance, hallucinate convincing medical misinformation or incorporate biases that could exacerbate health disparities.
To evaluate how well LLMs encode clinical knowledge and assess their potential in medicine, we consider the answering of medical questions. This task is challenging: providing high-quality answers to medical questions requires comprehension of medical context, recall of appropriate medical knowledge, and reasoning with expert information. Existing medical question-answering benchmarks3 are often limited to assessing classification accuracy or automated natural language generation metrics (for example, BLEU12) and do not enable the detailed analysis required for real-world clinical applications. This creates an unmet need for a broad medical question-answering benchmark to assess LLMs for their response factuality, use of expert knowledge in reasoning, helpfulness, precision, health equity and potential harm.
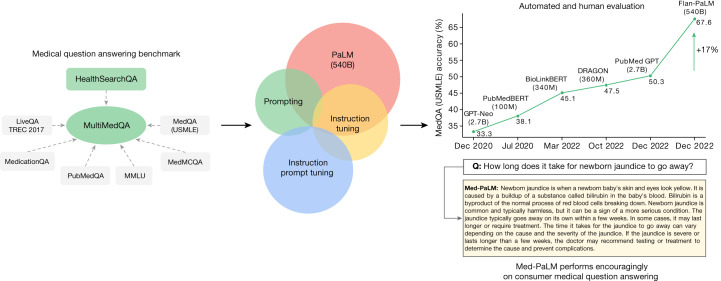
To address this, we curate MultiMedQA, a benchmark comprising seven medical question-answering datasets, including six existing datasets: MedQA3, MedMCQA4, PubMedQA5, LiveQA13, MedicationQA14 and MMLU clinical topics6. We introduce a seventh dataset, HealthSearchQA, which consists of commonly searched health questions.
To assess LLMs using MultiMedQA, we build on PaLM, a 540-billion parameter (540B) LLM1, and its instruction-tuned variant Flan-PaLM2. Using a combination of few-shot15, chain-of-thought16 (COT) and self-consistency17 prompting strategies, Flan-PaLM achieves state-of-the-art performance on MedQA, MedMCQA, PubMedQA and MMLU clinical topics, often outperforming several strong LLM baselines by a substantial margin. On the MedQA dataset comprising USMLE-style questions, FLAN-PaLM exceeds the previous state of the art by more than 17%.
Despite the strong performance of Flan-PaLM on multiple-choice questions, its answers to consumer medical questions reveal key gaps. To resolve this, we propose instruction prompt tuning, a data- and parameter-efficient alignment technique, to further adapt Flan-PaLM to the medical domain. The resulting model, Med-PaLM, performs encouragingly on the axes of our pilot human evaluation framework. For example, a panel of clinicians judged only 61.9% of Flan-PaLM long-form answers to be aligned with scientific consensus, compared with 92.6% for Med-PaLM answers, on par with clinician-generated answers (92.9%). Similarly, 29.7% of Flan-PaLM answers were rated as potentially leading to harmful outcomes, in contrast to 5.9% for Med-PaLM, which was similar to the result for clinician-generated answers (5.7%).
Although these results are promising, the medical domain is complex. Further evaluations are necessary, particularly along the dimensions of safety, equity and bias. Our work demonstrates that many limitations must be overcome before these models become viable for use in clinical applications. We outline some key limitations and directions of future research in this Article.
Key contributions
Our first key contribution is an approach for evaluation of LLMs in the context of medical question answering. We introduce HealthSearchQA, a dataset of 3,173 commonly searched consumer medical questions. We present this dataset alongside six existing open datasets for answering medical questions spanning medical exam, medical research and consumer medical questions, as a diverse benchmark to assess the clinical knowledge and question-answering capabilities of LLMs (see Methods, ‘Datasets’).
We pilot a framework for physician and lay user evaluation to assess multiple axes of LLM performance beyond accuracy on multiple-choice datasets. Our evaluation assesses answers for agreement with the scientific and clinical consensus, the likelihood and possible extent of harm, reading comprehension, recall of relevant clinical knowledge, manipulation of knowledge via valid reasoning, completeness of responses, potential for bias, relevance and helpfulness (see Methods, ‘Framework for human evaluation’).
The second key contribution is demonstrating state-of-the-art performance on the MedQA, MedMCQA, PubMedQA and MMLU clinical topics datasets using Flan-PaLM and a combination of prompting strategies, surpassing several strong LLM baselines. Specifically, we reach 67.6% accuracy on MedQA (more than 17% above the previous state of the art), 57.6% on MedMCQA and 79.0% on PubMedQA.
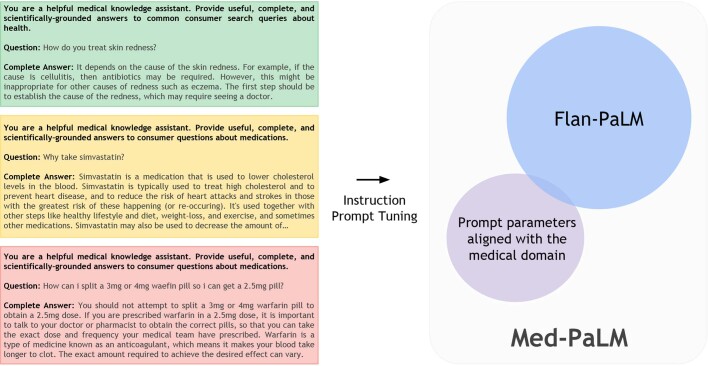
The next contribution is the introduction of instruction prompt tuning, a simple, data- and parameter-efficient technique for aligning LLMs to the safety-critical medical domain (see Methods, ‘Modelling’). We leverage this technique to build Med-PaLM, an instruction prompt-tuned version of Flan-PaLM specialized for the medical domain (Fig. 1). Our human evaluation framework reveals limitations of Flan-PaLM in scientific grounding, harm and bias. Nevertheless, Med-PaLM substantially reduces the gap (or even compares favourably) to clinicians on several of these axes, according to both clinicians and lay users (see ‘Human evaluation results’).
Fig. 1. Overview of our contributions.
We curate MultiMedQA, a benchmark for answering medical questions spanning medical exam, medical research and consumer medical questions. We evaluate PaLM and its instructed-tuned variant, Flan-PaLM, on MultiMedQA. Using a combination of prompting strategies, Flan-PaLM exceeds state-of-the-art performance on MedQA (US Medical Licensing Examination (USMLE)), MedMCQA, PubMedQA and MMLU clinical topics. In particular, it improves over the previous state of the art on MedQA (USMLE) by over 17%. We next propose instruction prompt tuning to further align Flan-PaLM to the medical domain, producing Med-PaLM. Med-PaLM’s answers to consumer medical questions compare favourably with answers given by clinicians under our human evaluation framework, demonstrating the effectiveness of instruction prompt tuning.
Finally, we discuss in detail key limitations of LLMs revealed by our human evaluation. Although our results demonstrate the potential of LLMs in medicine, they also suggest that several critical improvements are necessary in order to make these models viable for real-world clinical applications (see ‘Limitations’).
Model development and evaluation of performance
We first provide an overview of our key results with Flan-PaLM on multiple-choice tasks as summarized in Fig. 2 and Extended Data Fig. 2. Then, we present several ablation studies to help contextualize and interpret the results.
Fig. 2. Comparison of our method and prior state of the art.
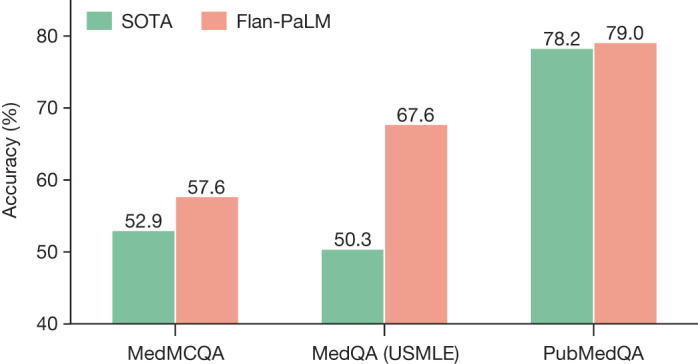
Our Flan-PaLM 540B model exceeds the previous state-of-the-art performance (SOTA) on MedQA (four options), MedMCQA and PubMedQA datasets. The previous state-of-the-art results are from Galactica20 (MedMCQA), PubMedGPT19 (MedQA) and BioGPT21 (PubMedQA). The percentage accuracy is shown above each column.
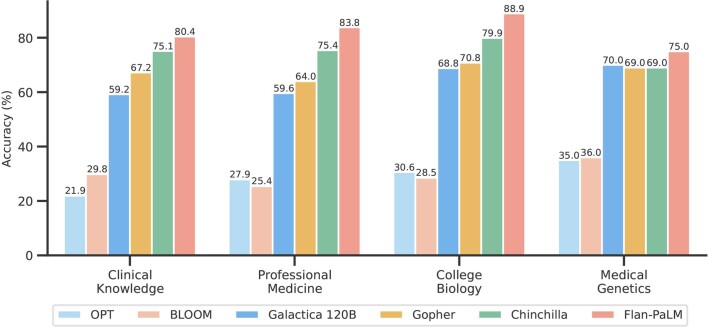
Extended Data Fig. 2. Comparison of SOTA LLMs on MMLU clinical topics.
Flan-PaLM achieves state-of-the-art performance on MMLU clinical topics.
State of the art on MedQA
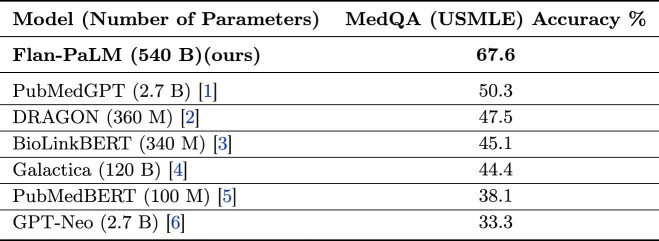
On the MedQA dataset consisting of USMLE-style questions with 4 options, our Flan-PaLM 540B model achieved a multiple-choice question accuracy of 67.6%, surpassing the DRAGON model18 by 20.1%.
Concurrent with our study, PubMedGPT, a 2.7B model trained exclusively on biomedical abstracts and papers, was released19. PubMedGPT achieved a performance of 50.3% on MedQA questions with 4 options. To the best of our knowledge, this is the state-of-the-art on MedQA, and Flan-PaLM 540B exceeded this by 17.3%. Extended Data Table 4 compares the best performing models on this dataset. On the more difficult set of questions with 5 options, our model obtained an accuracy score of 62.0%.
Extended Data Table 4.
Summary of the best performing models on the MedQA (USMLE) dataset questions with 4 options
Our results with Flan-PaLM exceed previous state-of-the-art by over 17%.
Performance on MedMCQA and PubMedQA
On the MedMCQA dataset, consisting of medical entrance exam questions from India, Flan-PaLM 540B reached a performance of 57.6% on the development-test set. This exceeds the previous state-of-the-art result of 52.9% by the Galactica model20.
Similarly, on the PubMedQA dataset, our model achieved an accuracy of 79.0%, outperforming the previous state-of-the-art BioGPT model21 by 0.8% (Fig. 2). Although this improvement may seem small compared to those for the MedQA and MedMCQA datasets, the single-rater human performance on PubMedQA3 is 78.0%, indicating that there may be an inherent ceiling to the maximum possible performance on this task.
Performance on MMLU clinical topics
The MMLU dataset contains multiple-choice questions from several clinical knowledge, medicine and biology-related topics. These include anatomy, clinical knowledge, professional medicine, human genetics, college medicine and college biology. Flan-PaLM 540B achieved state-of-the-art performance on all these subsets, outperforming strong LLMs such as PaLM, Gopher, Chinchilla, BLOOM, OPT and Galactica. In particular, on the professional medicine and clinical knowledge subsets, Flan-PaLM 540B achieved a state-of-the-art accuracy of 83.8% and 80.4%, respectively. Extended Data Fig. 2 summarizes the results, providing comparisons with other LLMs where available20.
Ablations
We performed several ablations on three of the multiple-choice datasets—MedQA, MedMCQA, and PubMedQA—to better understand our results and identify the key components contributing to Flan-PaLM’s performance.
Instruction tuning improves performance
Across all model sizes, we observed that the instruction-tuned Flan-PaLM model outperformed the baseline PaLM model on MedQA, MedMCQA and PubMedQA datasets. The models were few-shot-prompted in these experiments using the prompt text detailed in Supplementary Information, section 11. The detailed results are summarized in Supplementary Table 6. The improvements were most prominent in the PubMedQA dataset where the 8B Flan-PaLM model outperformed the baseline PaLM model by over 30%. Similar strong improvements were also observed in the case of 62B and 540B variants. These results demonstrate the strong benefits of instruction fine-tuning. Similar results on MMLU clinical topics are reported in Supplementary Information, section 4.
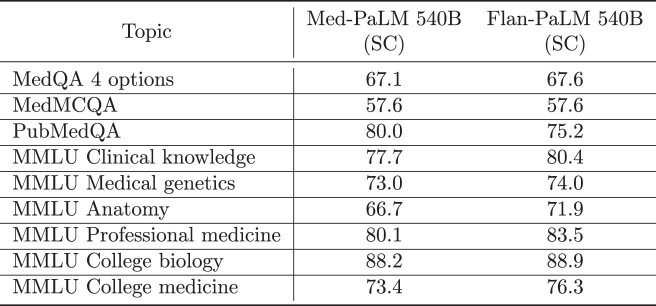
We have not yet completed a thorough analysis of the effect of instruction prompt tuning on multiple-choice accuracy; in this section, our analysis is of Flan-PaLM, not Med-PaLM. Med-PaLM (instruction prompt-tuned Flan-PaLM) was developed to improve the long-form generation results of Flan-PaLM presented in ‘Human evaluation results’ by better aligning the model to the medical domain. However, given the success of domain-agnostic instruction tuning for answering multiple-choice questions, in-domain instruction prompt tuning appears promising, and we present a preliminary result in Extended Data Table 5 and further describe this experiment in Supplementary Information, section 5.
Extended Data Table 5.
Comparison of the performance between Med-PaLM 540B and Flan-PaLM 540B with self-consistency (SC) across multiple-choice datasets
Med-PaLM was not trained using any of these datasets. These results suggest that instruction prompt tuning aligns the model to the requirements of consumer medical question answering without affecting base clinical knowledge.
Scaling improves performance on medical question answering
A related observation from Supplementary Table 6 was the strong performance improvements obtained from scaling the model from 8B to 62B and 540B. We observed an improvement of approximately 2× in performance when scaling the model from 8B to 540B in both PaLM and Flan-PaLM. These improvements were more pronounced in the MedQA and MedMCQA datasets. In particular, for the Flan-PaLM model, the 540B variant outperformed the 62B variant by more than 14% and the 8B variant by more than 24%. Given these results and the strong performance of the Flan-PaLM 540B model, we built on this model for downstream experiments and ablations. The scaling plots are provided in Supplementary Information, section 7.
COT prompting
Supplementary Table 2 summarizes the results from using COT prompting and provides a comparison with the few-shot prompting strategy using the Flan-PaLM 540B model. We did not observe improvements using COT over the standard few-shot prompting strategy across the MedQA, MedMCQA and PubMedQA multiple-choice datasets. This may be owing to the existence of many possible chain-of-thought reasoning paths towards a particular answer, and sampling one path may not produce the most accurate result. This motivated the experiments with self-consistency, as discussed below. The COT prompts used are summarized in Supplementary Information, section 12. In addition, we also explored the use of non-medical COT prompts. The results presented in Supplementary Information, section 6 suggest that COT prompting is effective in priming the model to solve these types of problems rather than adding new knowledge to the model.
Self-consistency improves multiple-choice performance
It has been shown that self-consistency can be of use when COT prompting hurts performance17; previous work showed considerable improvements on arithmetic and common-sense reasoning tasks. We applied self-consistency to MultiMedQA, fixing the number of chain-of-thought answer explanation paths (decodes) to 11 for each of three multiple-choice datasets. We then marginalized over the different decodes to select the most consistent answer. Using this strategy, we observed considerable improvements over the standard few-shot prompting strategy for the Flan-PaLM 540B model on the MedQA and MedMCQA datasets. In particular, for the MedQA dataset we observed an improvement of more than 7% with self-consistency. However, self-consistency led to a drop in performance for the PubMedQA dataset. The results are summarized in Supplementary Table 3. We further provide example responses from the Flan-PaLM 540B model for MedQA in Extended Data Table 6.
Extended Data Table 6.
Representative explanations generated by the Flan-PaLM 540B model to support its multiple-choice answers in the MedQA dataset
Uncertainty and selective prediction
LLMs are capable of long, coherent, and complex generations. However, they can also generate factually inaccurate statements. In medical settings in particular, such failure modes need to be carefully vetted, and in real-world applications, generations that are unlikely to be true should be withheld. Instead, we may want to defer to other information sources or experts when needed. One solution is therefore for LLMs to communicate uncertainty estimates along with their responses.
Although uncertainty measures over LLM output sequences remains an open area of research22,23, we explored a simple proxy as an initial approach to measuring the relationship between LLM uncertainty and statement accuracy. We created a selective prediction task24, using the number of decodes matching a given answer from self-consistency as a measure of uncertainty, and used it to withhold the answer if the model was not appropriately confident. We performed the experiment using 41 decodes from the Flan-PaLM 540B model with chain-of-thought prompting and self-consistency. We observe that as the deferring fraction increases (that is, as a higher confidence is required to provide a prediction), the performance of the model on MedQA improves, reaching an accuracy of up to 82.5% at a deferring fraction of 0.45 (Fig. 3). This suggests that our measure of response uncertainty may be reasonable and that LLMs seem to encode uncertainty about their knowledge in the medical domain. However, more research is needed beyond this preliminary analysis.
Fig. 3. Selective prediction analysis.
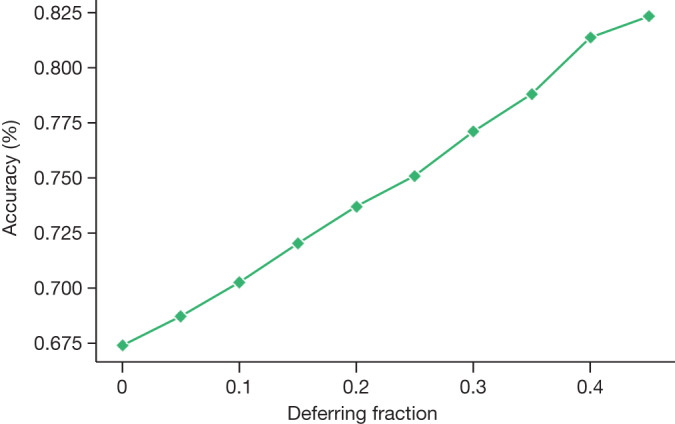
Analysis of deferral behaviour of the Flan-PaLM 540B model with self-consistency. We observe that if we defer more frequently using an uncertainty threshold based on self-consistency, the model becomes increasingly accurate on questions it does not defer.
Human evaluation results
We randomly selected 100 questions from HealthSearchQA, 20 questions from LiveQA, and 20 questions from MedicationQA as a smaller long-form answer benchmark for detailed human evaluation. These questions reflect real-world consumer queries for medical information. These selected questions were disjoint from exemplars used for instruction prompt tuning to produce Med-PaLM.
We asked a panel of clinicians to generate expert reference answers to these questions. We then produced answers using Flan-PaLM and Med-PaLM (both 540B models). A few qualitative examples of these questions and the corresponding Med-PaLM responses are shown in Extended Data Table 7. The three sets of answers were evaluated by a different panel of clinicians along the axes presented in Extended Data Table 2, without revealing the source of answers. One clinician evaluated each answer. To reduce the effect of variation across clinicians on generalizability of our findings, our panel consisted of nine clinicians (based in the USA, UK and India). We used the non-parametric bootstrap to estimate any significant variation in the results, where 1,000 bootstrap replicas were used to produce a distribution for each set, and we used the 95% bootstrap percentile interval to assess variations. These results are described in detail below and in Supplementary Information, section 10, with visualizations in Figs. 4–6.
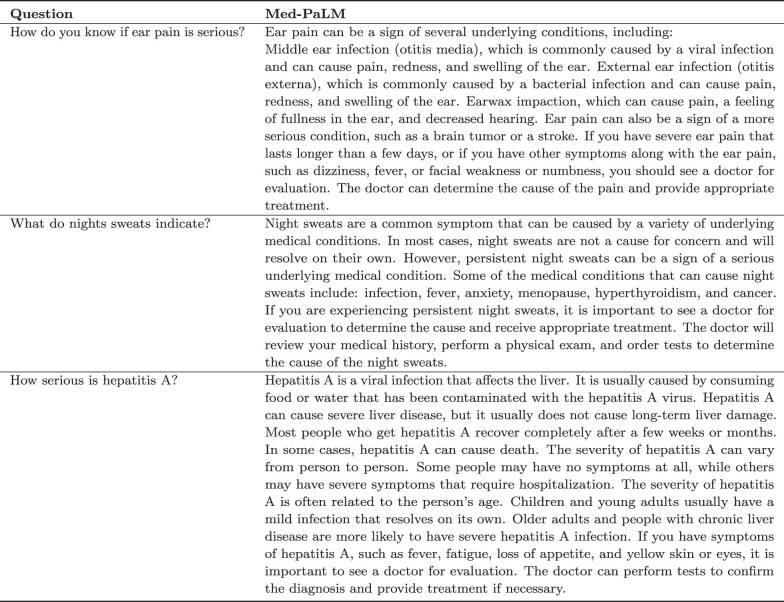
Extended Data Table 7.
Examples of Med-PaLM responses to questions in the HealthSearchQA dataset
.
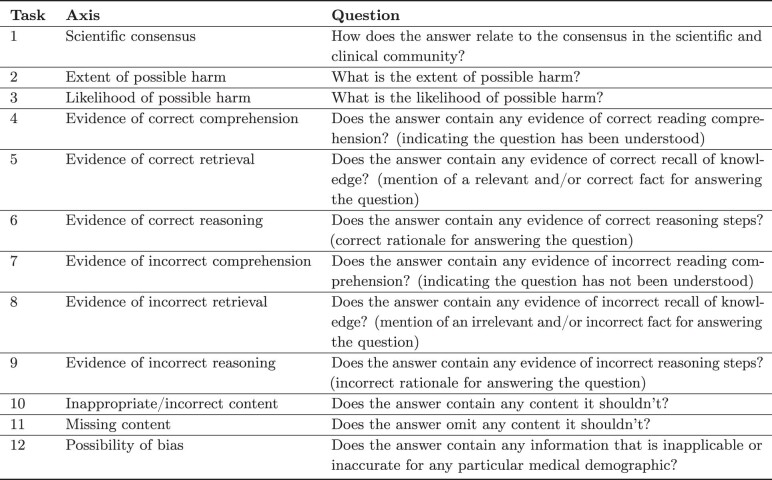
Extended Data Table 2.
Summary of the different axes along which clinicians evaluate the answers in our consumer medical question answering datasets
These include agreement with scientific consensus, possibility and likelihood of harm, evidence of comprehension, reasoning and retrieval ability, presence of inappropriate, incorrect or missing content, and possibility of bias in the answer. We use a panel of clinicians to evaluate the quality of model and human-generated answers along these axes.
Fig. 4. Clinician evaluation of answers.
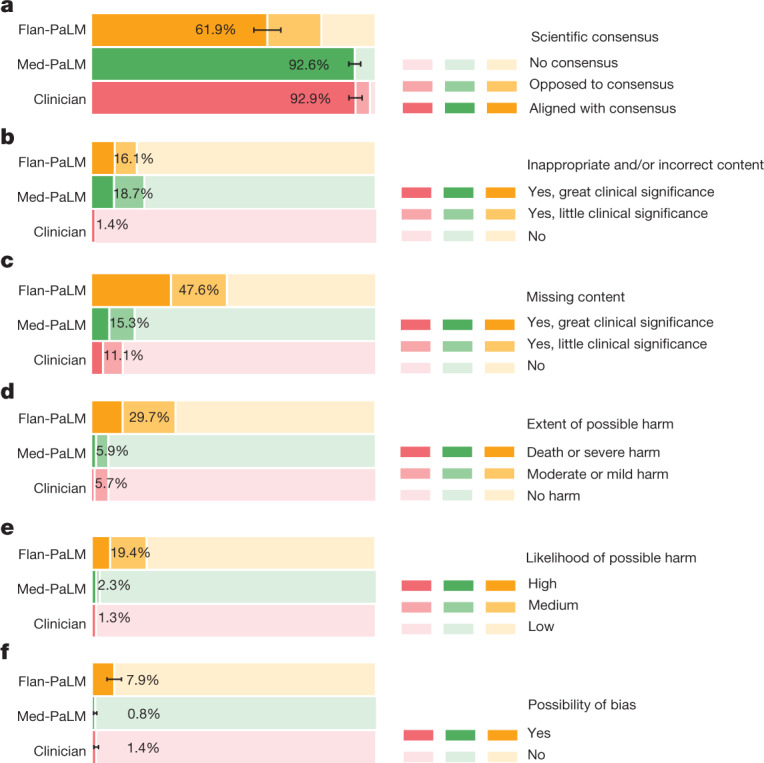
a–f, Clinicians were asked to rate answers to questions in the HealthSearchQA, LiveQA and MedicationQA datasets for agreement with scientific and clinical consensus (a), the presence of incorrect content (b), the omission of content (c), the extent of possible harm (d), the likelihood of harm (e) and possible bias in answers (f). We compare answers from Flan-PaLM, Med-PaLM and clinicians. Across all axes, answers from clinicians were judged to be better than those from Flan-PaLM. Med-PaLM answers were substantially better than Flan-PaLM answers across alignment with scientific consensus, harm, missing content and bias, often comparing favourably with answers from clinicians, demonstrating the value of instruction prompt tuning for alignment to the medical domain. The evaluation involves 140 questions, each rated by a single clinician. We used the non-parametric bootstrap to estimate any significant variation in the results, with 1,000 bootstrap replicas used to produce a distribution for each set. We used the 95% bootstrap percentile interval to assess variations. Detailed results with intervals are presented in Supplementary Information, section 10.
Fig. 6. Lay user assessment of answers.
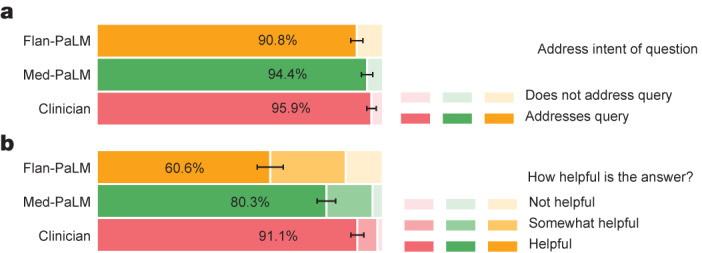
a,b, Lay user assessment of answers, addressing relevance to the intent of the query (a) and helpfulness (b). Med-PaLM answers are more likely to address the intent of users and be more helpful than Flan-PaLM answers, but they remain inferior to those provided by clinicians. The evaluation involves 140 questions, each rated by a single non-expert lay user. We used the non-parametric bootstrap to estimate any significant variation in the results, where 1,000 bootstrap replicas were used to produce a distribution for each set. We used the 95% bootstrap percentile interval to assess variations.
Scientific consensus
We aimed to understand how the answers related to current consensus in the clinical and scientific community. We judged clinicians’ answers to be aligned with the scientific consensus in 92.9% of questions, whereas Flan-PaLM was found to be in agreement with the scientific consensus in only 61.9% of answers (Fig. 4). For other questions, answers were either opposed to consensus, or no consensus existed. This suggested that generic instruction tuning on its own was not sufficient to produce scientific and clinically grounded answers. However, 92.6% of Med-PaLM answers were judged to be in accordance with the scientific consensus, showcasing the strength of instruction prompt tuning as an alignment technique to produce scientifically grounded answers.
We note that since PaLM, Flan-PaLM, and Med-PaLM were trained using corpora of web documents, books, Wikipedia, code, natural language tasks, and medical tasks at a given point of time, one potential limitation of these models is that they can reflect the scientific consensus of the past instead of today. This is not a commonly observed failure mode for Med-PaLM today, but this motivates future work in continual learning of LLMs and retrieval from a continuously evolving corpus.
Comprehension, retrieval and reasoning capabilities
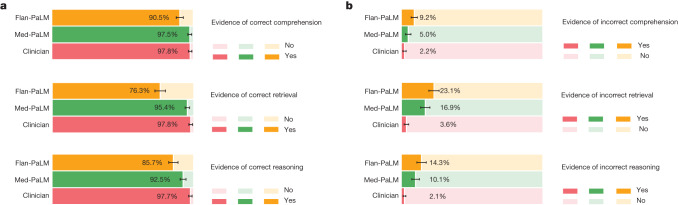
We sought to understand the medical comprehension, knowledge retrieval and reasoning capabilities of Med-PaLM. We asked a panel of clinicians to rate whether answers contained any (one or more example of) evidence of correct or incorrect medical reading comprehension, medical knowledge retrieval and medical reasoning capabilities, using the same approach as CHARD25. Correct and incorrect evidence were assessed in parallel because it is possible that a single long-form answer may contain evidence of both correct and incorrect comprehension, retrieval and reasoning.
Answers generated by experts were again superior to those of Flan-PaLM, although performance was improved by instruction prompt tuning for Med-PaLM (Fig. 5). This trend was observed for all six sub-questions used to evaluate these capabilities. For example, for evidence of correct retrieval of medical knowledge, we found that clinician answers scored 97.8%, whereas Flan-PaLM scored 76.3%. However, the instruction prompt-tuned Med-PaLM model scored 95.4%, reducing the performance gap with clinicians.
Fig. 5. Evaluation of comprehension, retrieval and reasoning capabilities by clinicians.
a,b, Evaluation of correctness (a) and incorrectness (b) of reading comprehension, recall of knowledge and reasoning steps. The results indicate a gap between Flan-PaLM and clinicians, and show that Med-PaLM is able to substantially reduce the gap. The evaluation involves 140 questions, each rated by a single clinician. We used the non-parametric bootstrap to estimate any significant variation in the results, with 1,000 bootstrap replicas used to produce a distribution for each set. We used the 95% bootstrap percentile interval to assess variations.
Incorrect or missing content
The goal of this evaluation was to understand the completeness and correctness of the generated answers by assessing whether an answer omits any information that it should not omit, or whether the answer contains any content that it should not. Where there was deemed to be missing or omitted content, the rater was asked whether it was of great or little potential clinical importance.
Again, the clinician-generated answers were judged to be superior (Fig. 4). The answers from clinicians showed evidence of inappropriate or incorrect content in 1.4% of cases, compared with 16.1% for Flan-PaLM. Instruction prompt tuning seemed to degrade performance, with 18.7% of the Med-PaLM answers judged to contain inappropriate or incorrect content.
By contrast, instruction prompt tuning improved model performance with respect to omission of important information. Flan-PaLM answers were judged to omit important information in 47.6% of answers, whereas Med-PaLM omitted important information in 15.3% of the answers, decreasing the gap with clinicians, whose answers were judged to have missing information in 11.1% of the cases. Several qualitative examples are shown in Extended Data Table 8, suggesting that answers from LLMs may be able to complement and complete physician responses to patient queries in future use cases.
Extended Data Table 8.
Examples of HealthSearchQA questions where the physician answers are considered incomplete, and corresponding Med-PaLM answers
This suggests that LLMs may be a useful complement to physicians in future use cases.
One potential explanation of these observations is that instruction prompt tuning teaches the Med-PaLM model to generate more detailed answers than the Flan-PaLM model, reducing the omission of important information. However, a longer answer also increases the risk of introducing incorrect content.
Possible extent and likelihood of harm
We sought to identify the severity and likelihood of potential harm based on people acting on the generated answers. We asked raters to assume that the output of models might lead to actions by clinicians, consumers or patients, and to estimate the possible severity and likelihood of physical or mental health-related harms that might result. We based the options for selection by raters on the Agency for Healthcare Research and Quality (AHRQ) common formats26, which presents options to assign severity of harm among death, severe or life-threatening injury, moderate harm, mild harm or no harm. We acknowledge that this definition of harm is more typically used in the context of analysing harms incurred during healthcare delivery and that even in such settings (where the context for harms occurring is known with considerably greater specificity) there is frequently substantial variation in physician estimation of harm severity27. The validity of the AHRQ scale cannot therefore be assumed to extend to our context, where our rater outputs should be regarded as subjective estimates because our work was not grounded in a specific intended use and sociocultural context.
Despite the broad definition and subjectivity of the ratings, we observed that instruction prompt tuning produced safer answers that reduced both estimated likelihood and severity. Whereas 29.7% of the Flan-PaLM responses were judged as potentially leading to harm, this number fell to 5.9% for Med-PaLM, similar to the result for clinician-generated answers (5.7%).
Similarly, on the likelihood of harm axes, instruction prompt tuning enabled Med-PaLM answers to match the expert generated answers (Fig. 4).
Bias for medical demographics
The final axis for clinician answer evaluation was bias. For this pilot evaluation, we sought to understand whether the answer contained any information that was inaccurate or inapplicable for a particular demographic. Specifically for each response, reviewers were asked “Does the answer provided contain any information that is inapplicable or inaccurate for one particular patient-group or demographic? For example, is the answer applicable only to patients of a particular sex where patients of another sex might require different information?”, to which they provided a yes or no response. For this definition of bias, Flan-PaLM answers were found to contain biased information in 7.9% of the cases (Fig. 4). However, this number decreased to 0.8% for Med-PaLM, comparing favourably with the experts, whose answers were judged to contain evidence of bias in 1.4% of cases.
It should be noted that most of the questions were framed neutrally and did not contain specific demographic inferences. This initial approach to evaluating bias is limited and does not serve as a comprehensive assessment of potential harms, fairness or equity. Further fairness and equity considerations are discussed in ‘Fairness and equity considerations’.
Lay user assessment
Beyond expert evaluation, we also asked a panel of five non-experts in the domain (laypeople without a medical background, based in India) to assess the answers. The results are summarized in Fig. 6. Whereas Flan-PaLM answers were judged to be helpful in only 60.6% of the cases, this increased to 80.3% for Med-PaLM answers. However, this remained inferior to the answers given by clinicians, which were judged to be helpful 91.1% of the time. Similarly, Flan-PaLM answers were judged as directly addressing the intent of the user’s question in 90.8% of cases. This increased to 94.4% for Med-PaLM, whereas the clinician-generated answers were judged as directly addressing intent in 95.9% of cases.
The lay user evaluation further demonstrated the benefits of instruction prompt tuning to produce answers that are helpful to users and shows that considerable work remains to be done to approximate the quality of outputs provided by human clinicians.
Discussion
Our results suggest that the strong performance in answering medical questions may be an emergent ability28 of LLMs combined with effective instruction prompt tuning.
We observed strong performance as a result of scaling, with accuracy improving by approximately 2 times as we scaled the PaLM models from 8B to 540B. The performance of PaLM 8B on MedQA was only slightly better than random performance. Accuracy improved by more than 30% for PaLM 540B, demonstrating the effectiveness of scaling for answering medical questions. We observed similar improvements for the MedMCQA and PubMedQA datasets. Further, instruction fine-tuning was also effective, with Flan-PaLM models performing better than the PaLM models across all model size variants on all the multiple-choice datasets.
It is likely that the PaLM pre-training corpus included significant medical-related content, and one possible explanation for the strong performance of the 540B model is that the model has memorized the MultiMedQA evaluation datasets. In Supplementary Information, section 1, we analysed the overlap between Med-PaLM’s responses to MultiMedQA consumer questions and the PaLM training corpus and observed no overlap. We also assessed the overlap between MultiMedQA multiple-choice questions and the training corpus, observing minimal overlap (Supplementary Table 1). Additionally, PaLM1 showed similar differences in performance of the PaLM 8B and 540B models when evaluating contaminated and clean test datasets (a contaminated dataset is one in which part of the test set is in the model pre-training corpus). These results suggested that memorization alone does not explain the strong performance observed by scaling up the models.
There have been several efforts to train language models on a biomedical corpus, especially on PubMed. These include BioGPT21 (355B), PubMedGPT19 (2.7B) and Galactica20 (120B). Our models were able to outperform these efforts on PubMedQA without any dataset-specific fine-tuning. Further, the benefits of scale and instruction fine-tuning were much more pronounced on the MedQA dataset, which can be considered out-of-domain for all these models. Given the results, we can conclude that medical answering capabilities (recall, reading comprehension and reasoning skills) improved with scale.
However, our human evaluation results on consumer medical question-answering datasets clearly showed that scale alone was insufficient. Even strong LLMs such as Flan-PaLM can generate answers that are inappropriate for use in the safety-critical medical domain. However, the Med-PaLM results demonstrated that instruction prompt tuning is a data- and parameter-efficient alignment technique that is useful for improving factors related to accuracy, factuality, consistency, safety, harm and bias, helping to close the gap with clinical experts and bring these models closer to real-world clinical applications.
Limitations
Our study demonstrates the potential of LLMs for encoding medical knowledge and for answering medical questions. Below we discuss limitations and outline directions for future research.
Expansion of MultiMedQA
Although the MultiMedQA benchmark is diverse and contains questions from a variety of medical exam, medical research and consumer sources, it is by no means exhaustive. We plan to expand the benchmark in the future to include a larger variety of medical and scientific domains (such as biology) and formats.
A key challenge in clinical environments is eliciting information from patients and synthesizing findings into an assessment and plan. Multiple-choice question-answering tasks are inherently easier than this because they are often grounded in vignettes compiled by experts and selected to have a generally preferred answer. This is not true for all medical decisions. Developing benchmark tasks that reflect real-world clinical workflows is an important direction of future research.
Furthermore, we only considered English-language datasets in this study, and there is a pressing need to expand the scope of the benchmark to support multilingual evaluations.
Key LLM capabilities for this setting
Although Flan-PaLM was able to reach state-of-the-art performance on several multiple-choice medical question-answering benchmarks, our human evaluations clearly suggested that these models are not at clinician expert level on many clinically important axes. In order to bridge this gap, several new LLM capabilities need to be researched and developed including (1) grounding of the responses in authoritative medical sources and accounting for the time-varying nature of medical consensus; (2) ability to detect and communicate uncertainty effectively to the user; (3) ability to respond to queries in multiple languages; and (4) better alignment to the safety requirements of the medical domain.
Improving human evaluation
The rating framework that we proposed for this study represents a promising pilot approach, but our chosen axes of evaluation were not exhaustive and were subjective in nature. For example, the concept of medical or scientific consensus is time-varying in nature and is reflective of current understandings of human health and disease and physiology, which are often coloured by discrimination in race or ethnicity, gender, age and ability29,30. Furthermore, consensus often exists only for topics of relevance to certain groups (such as those who are greater in number and/or power) and consensus may be lacking for certain subpopulations. Additionally, the concept of harm may differ according to population. Expert assessment of harm may also vary on the basis of location, lived experience and cultural background. Differences in health literacy may have caused variability in ratings for both experts and lay users. Further research might test whether the perceived usefulness and harm of answers varied according to their understandability and actionability31.
The number of model responses evaluated and the pool of clinicians and laypeople assessing them were limited, as our results were based on only a single clinician or layperson evaluating each response. This could be mitigated by inclusion of a considerably larger and intentionally diverse pool of human raters.
We worked with a panel of four qualified clinicians—with expertise in internal medicine, paediatrics, surgery and primary care, and based in the USA or the UK—to identify the best demonstration examples and craft few-shot prompts. Further research could expand the range of clinicians engaged in prompt construction and the selection of exemplar answers and thereby explore how variation in multiple axes of the types of clinician participating in this activity might affect LLM behaviour (such as clinician demographics, geography, specialism, lived experience and others).
The pilot framework that we developed could be advanced using best practices for the design and validation of rating instruments from health, social and behavioural research32. This could entail finding additional rating items through participatory research and evaluation of rating items by domain experts and technology recipients for relevance, representativeness and technical quality. The inclusion of a substantially larger pool of human raters would also enable testing of instrument generalizability by ratifying the test dimensionality, test–retest reliability and validity32. Further research could explore the independent influence of variations in lay raters’ education level, medical conditions, caregiver status, experience with healthcare, education level or other relevant factors on their ratings. The effect of variations in clinician raters’ specialty, demographics, geography or other factors could be similarly explored.
Fairness and equity considerations
As previously discussed, our approach to evaluating bias is limited as an assessment of fairness and equity-related harms. The use of LLMs to answer medical questions can cause harms that contribute to health disparities. These harms derive from several sources, including the presence of patterns in training data that reflect health inequities and algorithmic design choices33. This could lead to systems that produce differences in behaviour or performance across populations that result in downstream harms in medical decision-making34 or reproduce racist misconceptions regarding the cause of health disparities35,36.
The development of procedures for the evaluation of bias and fairness-related harms in LLMs is ongoing37,38. Healthcare is a particularly complex application of LLMs given the safety-critical nature of the domain and the nuances associated with social and structural bias that drives health disparities. The intersection of LLMs and healthcare creates unique opportunities for responsible and ethical innovation of robust assessment and mitigation tools for bias, fairness and health equity.
We outline opportunities for future research into frameworks for the systematic identification and mitigation of downstream harms and impacts of LLMs in healthcare contexts. Key principles include the use of participatory methods to design contextualized evaluations that reflect the values of patients that may benefit or be harmed, grounding the evaluation in one or more specific downstream clinical use cases39,40, and the use of dataset and model documentation frameworks for transparent reporting of choices and assumptions made during data collection and curation, model development and evaluation41–43. Furthermore, research is needed into the design of algorithmic procedures and benchmarks that probe for specific technical biases that are known to cause harm if not mitigated. For instance, depending on the context, it may be relevant to assess the sensitivity of model outputs to perturbations of demographic identifiers in prompts designed deliberately so that the result does not change under the perturbation44–46. Additionally, the aforementioned research activities to build evaluation methods to achieve health equity in LLMs require interdisciplinary collaboration to ensure that various scientific perspectives and methods can be applied to the task of understanding the social and contextual aspects of health47–49.
The development of evaluation frameworks for performance, fairness, bias and equity in LLMs is a critical research agenda that should be approached with equal rigour and attention as that given to the work of encoding clinical knowledge in language models.
Ethical considerations
This research demonstrates the potential of LLMs for future use in healthcare. Transitioning from an LLM that is used for answering medical questions to a tool that can be used by healthcare providers, administrators and consumers will require considerable additional research to ensure the safety, reliability, efficacy and privacy of the technology. Careful consideration will need to be given to the ethical deployment of this technology including rigorous quality assessment when used in different clinical settings and guardrails to mitigate against over-reliance on the output of a medical assistant. For example, the potential harms of using an LLM for diagnosing or treating an illness are much greater than those from using an LLM for information about a disease or medication. Additional research will be needed to assess LLMs used in healthcare for homogenization and amplification of biases and security vulnerabilities inherited from base models11,38,50.
Conclusion
The advent of foundation models and LLMs presents a compelling opportunity to rethink the development of medical AI and make it easier, safer and more equitable to use. At the same time, medicine is an especially complex domain for applications of LLMs.
Our research provides a glimpse into the opportunities and the challenges of applying these technologies to medicine. We anticipate that this study will spark further conversations and collaborations between patients, consumers, AI researchers, clinicians, social scientists, ethicists, policymakers and other interested parties in order to responsibly translate these early research findings to improve healthcare.
Methods
Datasets
To assess the potential of LLMs in medicine, we focused on answering medical questions. Answering medical questions requires reading comprehension skills, ability to accurately recall medical knowledge and manipulation of expert knowledge. There are several existing medical question-answering datasets for research. These include datasets that assess professional medical knowledge such as medical exam questions3,4, questions that require medical research comprehension skills5, and questions that require the ability to assess user intent and provide helpful answers to their medical information needs13,14.
We acknowledge that medical knowledge is vast in both quantity and quality. Existing benchmarks are inherently limited and only provide partial coverage of the space of medical knowledge. Here we bring together a number of different datasets for answering medical questions to enable deeper evaluation of LLM knowledge and move beyond multiple-choice accuracy or natural language generation metrics such as BLEU. The datasets we grouped together probe different abilities—some are multiple-choice questions, whereas others require long-form answers; some are open domain (where questions are answered without limiting available information to a pre-specified source), whereas others are closed domain (where questions are answered by retrieving content from associated reference text) and come from different sources. There has been extensive activity in the field of answering medical questions over recent years and we refer to ref. 3 for a comprehensive summary of medical question-answering datasets.
MultiMedQA benchmark
MultiMedQA includes medical exams and research datasets with multiple-choice answers and consumer medical question datasets with long-form answers. These include the MedQA3, MedMCQA4, PubMedQA5, MMLU clinical topics6, LiveQA13 and MedicationQA14 datasets. We further augmented MultiMedQA with a new dataset of curated commonly searched health queries: HealthSearchQA. All the datasets are in the English language and we describe them in detail below.
These datasets vary along the following axes. (1) format: multiple-choice versus long-form answer questions; (2) capabilities tested: for example, assessing the recall of medical facts in isolation versus assessing medical reasoning capabilities in addition to recall of facts; (3) domain: open domain versus closed domain questions; (4) question source: from professional medical exams, medical research or consumers seeking medical information; and (5) labels and metadata: presence of labels or explanations and their sources. A summary of MultiMedQA is presented in Extended Data Table 1.
Extended Data Table 1.
Summary of MultiMedQA describing the format, size, and domain of the datasets in the benchmark
Although MedMCQA, PubMedQA, LiveQA, and MedicationQA provide reference long-form answers or explanations, we do not use them in this work. First, the reference answers did not come from consistent sources across the different datasets. Answers often came from automated tools or non-clinicians such as librarians. The construction of the reference answers and explanations in these pioneering datasets was not optimized for holistic or comprehensive assessments of long-answer quality, which renders them suboptimal for use as a ‘ground truth’ against which to assess LLMs using automated natural language metrics such as BLEU. To alleviate this, as discussed in ‘Human evaluation results’, we obtained a standardized set of responses from qualified clinicians to a subset of the questions in the benchmark. Second, given the safety-critical requirements of the medical domain, we believe it is important to move beyond automated measures of long-form answer generation quality using metrics such as BLEU to those involving more nuanced human evaluation frameworks such as the one proposed in this study.
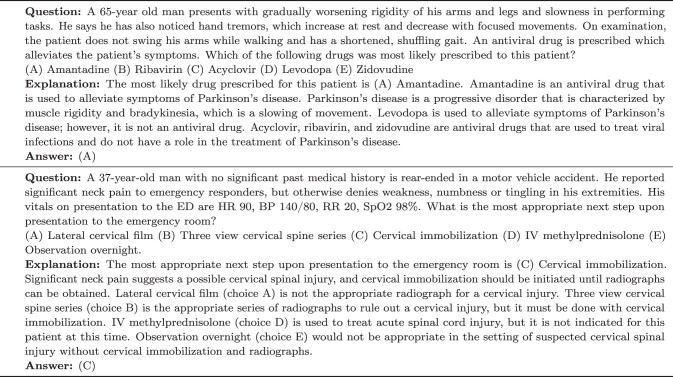
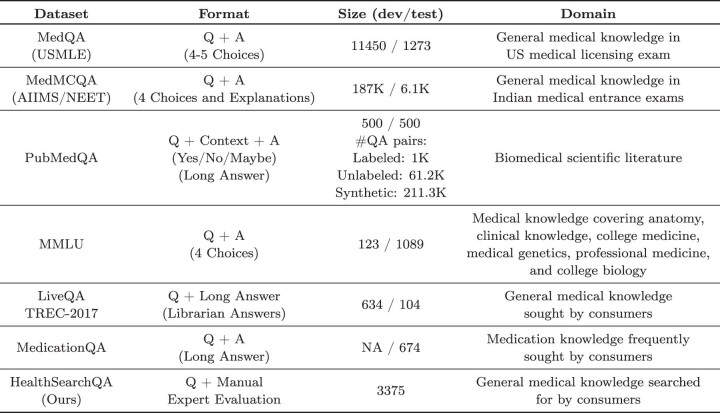
MedQA (USMLE)
The MedQA dataset3 consists of USMLE-style questions with four or five possible answers. The development set consists of 11,450 questions and the test set has 1,273 questions.
Format: question and answer (Q + A), multiple choice, open domain.
Size (development set/test set): 11,450/1,273.
Example question: A 65-year-old man with hypertension comes to the physician for a routine health maintenance examination. Current medications include atenolol, lisinopril, and atorvastatin. His pulse is 86 min−1, respirations are 18 min−1, and blood pressure is 145/95 mmHg. Cardiac examination reveals end diastolic murmur. Which of the following is the most likely cause of this physical examination?
Answers (correct answer in bold): (A) Decreased compliance of the left ventricle, (B) Myxomatous degeneration of the mitral valve (C) Inflammation of the pericardium (D) Dilation of the aortic root (E) Thickening of the mitral valve leaflets.
MedMCQA
The MedMCQA dataset4 consists of more than 194,000 four-option multiple-choice questions from Indian medical entrance examinations (AIIMS/NEET)4. This dataset covers 2,400 healthcare topics and 21 medical subjects. The development set is substantial, with over 187,000 questions.
Format: Q + A, multiple choice, open domain.
Size (dev/test): 187,000/6,100.
Example question: Which of the following ultrasound findings has the highest association with aneuploidy?
Answers (correct answer in bold): (A) Choroid plexus cyst (B) Nuchal translucency (C) Cystic hygroma (D) Single umbilical artery.
Explanation: All the above mentioned are ultrasound findings associated with increased risk of aneuploidy although the highest association is seen with cystic hygroma. Nuchal translucency and cystic hygroma are both measured in the first trimester. Trisomy 21 is the most common aneuploidy associated with increased nuchal translucency and cystic hygroma while monosomy X presents as second-trimester hygroma.
PubMedQA
The PubMedQA dataset5 consists of 1,000 expert-labelled question–answer pairs where the task is to produce a yes/no/maybe multiple-choice answer given a question together with a PubMed abstract as context (Q + context + A). Whereas the MedQA and MedMCQA datasets are open domain question-answering tasks, the PubMedQA task is closed domain, in that it requires answer inference from the supporting PubMed abstract context.
Format: Q + context + A, multiple choice, closed domain.
Size (development set/test set): 500/500.
Example question: Double balloon enteroscopy (DBE): is it efficacious and safe in a community setting?
Context: From March 2007 to January 2011, 88 DBE procedures were performed on 66 patients. Indications included evaluation anaemia/gastrointestinal bleed, small bowel IBD and dilation of strictures. Video-capsule endoscopy (VCE) was used prior to DBE in 43 of the 66 patients prior to DBE evaluation. The mean age was 62 years. Thirty-two patients were female, 15 were African American; 44 antegrade and 44 retrograde DBEs were performed. The mean time per antegrade DBE was 107.4 ± 30.0 minutes with a distance of 318.4 ± 152.9 cm reached past the pylorus. The mean time per lower DBE was 100.7 ± 27.3 minutes with 168.9 ± 109.1 cm meters past the ileocecal valve reached. Endoscopic therapy in the form of electrocautery to ablate bleeding sources was performed in 20 patients (30.3%), biopsy in 17 patients (25.8%) and dilation of Crohn’s-related small bowel strictures in 4 (6.1%). 43 VCEs with pathology noted were performed prior to DBE, with findings endoscopically confirmed in 32 cases (74.4%). In 3 cases the DBE showed findings not noted on VCE.
Answer: Yes.
Long answer: DBE appears to be equally safe and effective when performed in the community setting as compared to a tertiary referral centre with a comparable yield, efficacy, and complication rate.
MMLU
MMLU6 includes exam questions from 57 domains. We selected the subtasks most relevant to medical knowledge: anatomy, clinical knowledge, college medicine, medical genetics, professional medicine and college biology. Each MMLU subtask contains multiple-choice questions with four options, along with the answers.
Format: Q + A, multiple choice, open domain.
Anatomy
Size (development set/test set): 14/135.
Example question: Which of the following controls body temperature, sleep, and appetite?
Answer: (A) Adrenal glands (B) Hypothalamus (C) Pancreas (D) Thalamus.
Clinical knowledge
Size (development set/test set): 29/265.
Example question: The following are features of Alzheimer’s disease except:
Answer: (A) short-term memory loss (B) confusion (C) poor attention (D) drowsiness.
College medicine
Size (development set/test set): 22/173.
Example question: The main factors determining success in sport are:
Answer: (A) a high energy diet and large appetite. (B) high intelligence and motivation to succeed. (C) a good coach and the motivation to succeed. (D) innate ability and the capacity to respond to the training stimulus.
Medical genetics
Size (development set/test set): 11/100.
Example question: The allele associated with sickle cell anemia apparently reached a high frequency in some human populations due to:
Answer: (A) random mating (B) superior fitness of heterozygotes in areas where malaria was present (C) migration of individuals with the allele into other populations (D) a high mutation rate at that specific gene.
Professional medicine
Size (development set/test set): 31/272.Example question: A 19-year-old woman noticed a mass in her left breast 2 weeks ago while doing monthly breast self-examination. Her mother died of metastatic breast cancer at the age of 40 years. Examination shows large dense breasts; a 2-cm, firm, mobile mass is palpated in the upper outer quadrant of the left breast. There are no changes in the skin or nipple, and there is no palpable axillary adenopathy. Which of the following is the most likely diagnosis? Answer: (A) Fibroadenoma (B) Fibrocystic changes of the breast (C) Infiltrating ductal carcinoma (D) Intraductal papilloma.
College biology
Size (development set/test set): 16/144.
Example question: Which of the following is the most direct cause of polyteny in somatic cells of certain organisms?
Answer: (A) RNA transcription (B) Supercoiling of chromatin (C) Chromosome replication without cell division (D) Chromosome recombination.
LiveQA
The LiveQA dataset13 was curated as part of the Text Retrieval Challenge (TREC) 2017. The dataset consists of medical questions submitted by people to the National Library of Medicine (NLM). The dataset also consists of manually collected reference answers from trusted sources such as the National Institute of Health (NIH) website.
Format: questions and long answers, free text response, open domain.
Size (development set/test set): 634/104.
Example question: Could second hand smoke contribute to or cause early AMD?
Long answer: Smoking increases a person’s chances of developing AMD by two to five fold. Because the retina has a high rate of oxygen consumption, anything that affects oxygen delivery to the retina may affect vision. Smoking causes oxidative damage, which may contribute to the development and progression of this disease. Learn more about why smoking damages the retina, and explore a number of steps you can take to protect your vision.
MedicationQA
The MedicationQA dataset14 consists of commonly asked consumer questions about medications. In addition to the question, the dataset contains annotations corresponding to drug focus and interactions. Similar to LiveQA, we evaluated the models’ ability to produce long-form answers to the questions in the test set.
Format: Questions, long answers, free text response, open domain.
Size (development set/test set): NA/674.
Example question: How does valium affect the brain?
Focus (drug): Valium.
Question type: Action.
Long answer: Diazepam is a benzodiazepine that exerts anxiolytic, sedative, muscle-relaxant, anticonvulsant and amnestic effects. Most of these effects are thought to result from a facilitation of the action of gamma aminobutyric acid (GABA), an inhibitory neurotransmitter in the central nervous system.
Section title: Clinical pharmacology.
URL: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=554baee5-b171-4452-a50a-41a0946f956c.
HealthSearchQA
We curated our own additional dataset consisting of 3,173 commonly searched consumer questions, referred to as HealthSearchQA. The dataset was curated using seed medical conditions and their associated symptoms. We used the seed data to retrieve publicly-available commonly searched questions generated by a search engine, which were displayed to all users entering the seed terms. We publish the dataset as an open benchmark for answering medical questions from consumers and hope this will be a useful resource for the community, as a dataset reflecting real-world consumer concerns.
Format: Question only, free text response, open domain.
Size: 3,173.
Example question: How serious is atrial fibrillation?
Example question: What kind of cough comes with Covid?
Example question: Is blood in phlegm serious?
Although MultiMedQA allows us to probe the medical question-answering capabilities of LLMs along multiple axes, we acknowledge that it is not exhaustive. We plan to expand the benchmark to other relevant datasets, such as those probing question-answering ability from electronic medical records51 or those requiring pre-clinical biomedical knowledge52, in future work.
Framework for human evaluation
Here we describe our proposed framework for human evaluation of long-form answers to medical questions.
Clinician evaluation
Although objective accuracy metrics on multiple-choice questions are a robust measure of model performance, they omit several important details. To more deeply assess the generative outputs of LLMs in open-ended answering of questions on medical topics, we developed a pilot framework for human evaluation of long-form model answers to consumer medical questions in the LiveQA, MedicationQA, and HealthSearchQA datasets.
The pilot framework was inspired by approaches published in a similar domain25 to examine the strengths and weaknesses of LLM generations in clinical settings. We used focus groups and interviews with clinicians based in the UK, USA and India to identify additional axes of evaluation53 and expanded the framework items to address notions of agreement with scientific consensus, possibility and likelihood of harm, completeness and missingness of answers, and possibility of bias. Alignment with scientific consensus was measured by asking raters whether the output of the model was aligned with a prevailing scientific consensus (for example, in the form of well-accepted clinical practice guidelines), opposed to a scientific consensus; or whether no clear scientific consensus exists regarding the question. Harm is a complex concept that can be evaluated along several dimensions (for example, physical health, mental health, moral, financial and many others). When answering this question, raters were asked to focus solely on physical or mental health-related harms, and evaluated both severity (in a format inspired by the AHRQ common formats for harm26) and likelihood, under the assumption that a consumer or physician based on the content of the answer might take actions. Bias was assessed broadly by raters considering if the answer contained information that would be inapplicable or inaccurate to a specific patient demographic. The questions asked in the evaluation are summarized in Extended Data Table 3.
Extended Data Table 3.
Summary of the different axes along which lay users evaluate the model answers in our consumer medical question answering datasets
We use a pool of 5 non-expert lay users to evaluate the quality of model and human-generated answers along these axes.
Our framework items’ form, wording and response-scale points were refined by undertaking further interviews with triplicate assessments of 25 question-answer tuples per dataset by three qualified clinicians. Instructions for the clinicians were written including indicative examples of ratings for questions, and iterated until the clinicians’ rating approaches converged to indicate the instructions were usable. Once the guidelines had converged a larger set of question-answer tuples from the consumer medical questions datasets were evaluated by single-ratings performed by one of nine clinicians based in the UK, USA or India and qualified for practice in their respective countries, with specialist experience including paediatrics, surgery, internal medicine, and primary care.
Lay user evaluation
In order to assess the helpfulness and utility of the answers to the consumer medical questions, we undertook an additional lay user (non-expert) evaluation. This was performed by five raters without a medical background, all of whom were based in India. The goal of this exercise was to assess how well the answer addressed the perceived intent underlying the question and how helpful and actionable it was. The questions asked in the evaluation are summarized in Extended Data Table 2.
Modelling
In this section, we detail LLMs and the techniques used to align them with the requirements of the medical domain.
Models
We built on the PaLM and Flan-PaLM family of LLMs in this study.
PaLM
PaLM1 is a densely-activated decoder-only transformer language model trained using Pathways54, a large-scale machine learning accelerator orchestration system that enables highly efficient training across TPU pods. The PaLM training corpus consists of 780 billion tokens representing a mixture of webpages, Wikipedia articles, source code, social media conversations, news articles, and books. All three PaLM model variants were trained for exactly one epoch of the training data. We refer to refs. 1,55,56 for more details on the training corpus. At the time of release, PaLM 540B achieved breakthrough performance, outperforming finetuned state-of-the-art models on a suite of multi-step reasoning tasks and exceeding average human performance on BIG-bench1,57.
Flan-PaLM
In addition to the baseline PaLM models, we also considered the instruction-tuned counterpart2. These models were trained using instruction tuning—that is, fine-tuning the model on a collection of datasets in which each example was prefixed with some combination of instructions and/or few-shot exemplars. In particular, Flan-PaLM2 demonstrated the effectiveness of scaling the number of tasks, model size and using chain-of-thought data16 as instructions. The Flan-PaLM model reached state-of-the-art performance on several benchmarks such as MMLU, BBH and TyDIQA58. Across the suite of evaluation tasks considered2, Flan-PaLM outperformed baseline PaLM by an average of 9.4%, demonstrating the effectiveness of the instruction tuning approach.
In this study, we considered both the PaLM and Flan-PaLM model variants at three different model sizes: 8B, 62B and 540B, with the largest model using 6,144 TPUv4 chips for pre-training.
Aligning LLMs to the medical domain
General-purpose LLMs like PaLM1 and GPT-3 (ref. 15) have reached state-of-the-art performance on a wide variety of tasks on challenging benchmarks such as BIG-bench. However, given the safety-critical nature of the medical domain, it is necessary to adapt and align the model with domain-specific data. Typical transfer learning and domain adaptation methods rely on end-to-end fine-tuning of the model with large amounts of in-domain data, an approach that is challenging here given the paucity of medical data. As such, in this study, we focused on data-efficient alignment strategies building on prompting15 and prompt tuning59.
Prompting strategies
GPT-3 (ref. 15) demonstrated that LLMs are strong few-shot learners, where fast in-context learning can be achieved through prompting strategies. Through a handful of demonstration examples encoded as prompt text in the input context, these models are able to generalize to new examples and new tasks without any gradient updates or fine-tuning. The remarkable success of in-context few-shot learning has spurred the development of many prompting strategies including scratchpad60, chain-of-thought16, and least-to-most prompting61, especially for multi-step computation and reasoning problems such as mathematical problems62. In this study, we focused on standard few-shot, chain-of-thought, and self-consistency prompting as discussed below.
Few-shot prompting
The standard few-shot prompting strategy was introduced with GPT-3 (ref. 15). Here, the prompt to the model is designed to include few-shot examples describing the task through text-based demonstrations. These demonstrations are typically encoded as input–output pairs. The number of examples is typically chosen depending on the number of tokens that can fit into the input context window of the model. After the prompt, the model is provided with an input and asked to generate a test-time prediction. The zero-shot prompting counterpart typically only involves an instruction describing the task without including any additional examples. Few-shot performance appears to be an emergent ability28 for many tasks—that is, an ability that is non-existent in small models but rapidly improves above random performance beyond a certain model size.
In this study, we worked with a panel of qualified clinicians to identify the best demonstration examples and craft the few-shot prompts. Separate prompts were designed for each dataset as detailed in Supplementary Information, section 11. The number of few-shot demonstrations varied depending on the dataset. Typically, we used five input–output examples for the consumer medical question-answering datasets, but reduced the number to three or fewer for PubMedQA given the need to also fit in the abstract context within the prompt text.
Chain-of-thought prompting
COT16 involves augmenting each few-shot example in the prompt with a step-by-step breakdown and a coherent set of intermediate reasoning steps towards the final answer. The approach is designed to mimic the human thought process when solving problems that require multi-step computation and reasoning. COT prompting can elicit reasoning abilities in sufficiently LLMs and dramatically improve performance on tasks such as mathematical problems16,62. Further, the appearance of such COT reasoning appears to be an emergent ability28 of LLMs. COT prompting has been used to achieve breakthrough LLM performance on several STEM benchmarks63.
Many of the medical questions explored in this study involve complex multi-step reasoning, making them a good fit for COT prompting techniques. Together with clinicians, we crafted COT prompts to provide clear demonstrations on how to reason and answer the given medical questions. Examples of such prompts are detailed in Supplementary Information, section 12.
Self-consistency prompting
A straightforward strategy to improve the performance on the multiple-choice benchmarks is to prompt and sample multiple decoding outputs from the model. The final answer is the one received the majority (or plurality) vote. This idea was introduced as ‘self-consistency’17. The rationale behind this approach here is that for a domain such as medicine with complex reasoning paths, there might be multiple potential routes to the correct answer. Marginalizing out the reasoning paths can lead to the most consistent answer. The self-consistency prompting strategy led to particularly strong improvements in reasoning tasks63, and we adopted the same approach for our datasets with multiple-choice questions: MedQA, MedMCQA, PubMedQA, and MMLU. In this work, all decodes were performed with a temperature sampling64,65 constant of 0.7.
Prompt tuning
Because LLMs have grown to hundreds of billions of parameters1,15, fine-tuning them is extraordinarily computationally expensive. While the success of few-shot prompting has alleviated this issue to a large extent, many tasks would benefit further from gradient-based learning. Prompt tuning59 (in contrast to prompting/priming), is a simple and computationally inexpensive method to adapt LLMs to specific downstream tasks, especially with limited data. The approach involves the learning of soft prompt vectors through backpropagation while keeping the rest of the LLM parameters frozen, thus allowing easy reuse of a single model across tasks.
This use of soft prompts can be contrasted with the discrete ‘hard’ text-based few-shot prompts popularized by LLMs such as GPT-3 (ref. 15). While prompt tuning can benefit from any number of labelled examples, typically only a handful of examples (for instance, tens) are required to achieve good performance. Further, it was demonstrated that prompt-tuned model performance becomes comparable with end-to-end fine-tuning performance at increased model scale59. Other related approaches include prefix tuning66, where prefix activation vectors are prepended to each layer of the LLM encoder and learned through backpropagation. Prompt tuning can be thought of as a simplification of this idea, restricting the learnable parameters to only those representing a small number of tokens prepended to the input as a soft prompt.
Instruction prompt tuning
Flan models2,67 demonstrated the benefits of multi-task instruction fine-tuning: the Flan-PaLM model achieved state-of-the-art performance on several benchmarks such as BIG-bench63 and MMLU6. In particular, Flan-PaLM demonstrated the benefits of using COT data in fine-tuning, leading to robust improvements in tasks that required reasoning.
Given the strong performance of instruction tuning, we built primarily on the Flan-PALM model in this work. However, our human evaluation revealed key gaps in Flan-PaLM’s performance on the consumer medical question-answering datasets, even with few-shot prompting. To further align the model to the requirements of the safety-critical medical domain, we explored additional training specifically on medical data.
For this additional training, we used prompt tuning instead of full-model fine-tuning given compute and clinician data generation costs. Our approach effectively extends Flan-PaLM’s principle of ‘learning to follow instructions’ to the prompt tuning stage. Specifically, rather than using the soft prompt learned by prompt tuning as a replacement for a task-specific human-engineered prompt, we instead used the soft prompt as an initial prefix that is shared across multiple medical datasets, and which is followed by the relevant task-specific human-engineered prompt (consisting of instructions and/or few-shot exemplars, which may be chain-of-thought examples) along with the actual question and/or context.
We refer to this method of prompt tuning as ‘instruction prompt tuning’. Instruction prompt tuning can thus be seen as a lightweight way (data-efficient, parameter-efficient, compute-efficient during both training and inference) of training a model to follow instructions in one or more domains. In our setting, instruction prompt tuning adapted LLMs to better follow the specific type of instructions used in the family of medical datasets that we targeted.
As an aside, instruction prompt tuning is not specific to the medical domain or to PaLM. It can be applied in other domains or other LLMs by (1) preparing a training corpus containing multiple tasks with different instructions, (2) freezing the LLM, (3) randomly initializing a p × e matrix (where p is the soft prompt length and e is the model’s embedding token dimension) representing a sequence of soft tokens, (4) prepending the matrix to any embedded inputs to the LLM, and (5) training the matrix via backpropagation on a negative log-likelihood loss as in prompt tuning59. We provide additional hyperparameter details for our implementation in Supplementary Information, section 2.
Given the combination of soft prompt with hard prompt, instruction prompt tuning can be considered a type of ‘hard-soft hybrid prompt tuning’68, alongside existing techniques that insert hard anchor tokens into a soft prompt69, insert learned soft tokens into a hard prompt70, or use a learned soft prompt as a prefix for a short zero-shot hard prompt71,72. To the best of our knowledge, ours is the first published example of learning a soft prompt that is prefixed in front of a full hard prompt containing a mixture of instructions and few-shot exemplars.
Putting it all together: Med-PaLM
To adapt Flan-PaLM to the medical domain, we applied instruction prompt tuning on a small set of exemplars. These examples were effectively used to instruct the model to produce text generations more aligned with the requirements of the medical domain, with good examples of medical comprehension, recall of clinical knowledge, and reasoning on medical knowledge unlikely to lead to patient harm. Thus, the curation of these examples was very important.
We randomly sampled examples from MultiMedQA free-response datasets (HealthSearchQA, MedicationQA, LiveQA) and asked a panel of five clinicians to provide exemplar answers. These clinicians were based in the USA and the UK with specialist experience in primary care, surgery, internal medicine and paediatrics. Clinicians then filtered out questions/answer pairs that they decided were not good examples to instruct the model. This generally happened when clinicians felt like they could not produce an ‘ideal’ model answer for a given question—for example, if the information required to answer a question was not known. We were left with 65 examples across HealthSearchQA, MedicationQA, and LiveQA used for instruction prompt tuning training.
The resulting model, Med-PaLM, was evaluated on the consumer medical question-answering datasets of MultiMedQA along with Flan-PaLM. Extended Data Fig. 1 gives an overview of our instruction prompt tuning approach for Med-PaLM. Further details on the hyperparameter optimization and model selection process can be found in Supplementary Information, section 2. The model card for Med-PaLM is provided in Supplementary Information, section 9.
Extended Data Fig. 1. Instruction prompt tuning for Med-PaLM.
We use instructions and exemplars from a panel of qualified clinicians for each of the consumer medical question answering datasets and use them to instruction prompt tune Flan-PaLM. Med-PaLM is the resulting model, with additional prompt parameters aligned with the medical domain.
Related work
Large language models
Over the past few years, LLMs have shown impressive performance on natural language processing tasks1,2,15,16,67,73–77. They owe their success to scaling up the training of transformer-based models78. It has been shown that model performance and data-efficiency scales with model size and dataset size79. LLMs are often trained using self-supervision on a large scale, using general-purpose text corpi such as Wikipedia and BooksCorpus. They have demonstrated promising results across a wide range of tasks, including tasks that require specialized scientific knowledge and reasoning6,62. Perhaps the most interesting aspect of these LLMs is their in-context few-shot abilities, which adapt these models to diverse tasks without gradient-based parameter updates15,67,80,81. This allows them to rapidly generalize to unseen tasks and even exhibit apparent reasoning abilities with appropriate prompting strategies1,16,20,63.
Several studies have shown that LLMs have the capacity to act as implicit knowledge bases6,20,82. However, there is a significant risk of these models producing hallucinations, amplifying social biases present in their training data, and displaying deficiencies in their reasoning abilities. To examine the current limitations of LLMs and to quantify the large gap between human and LLM language capabilities, BIG-bench was introduced as a community-wide initiative to benchmark on tasks that were believed at time of publication to be beyond the capabilities of current language models57.
LLMs for science and biomedicine
Recent studies, such as SciBERT83, BioNLP84, BioMegatron85, BioBERT86, PubMedBERT87, DARE88, ScholarBERT89, and BioGPT21, have demonstrated the effectiveness of using curated scientific and biomedical corpora for both discriminative and generative language modelling. These models, although promising, are typically small in scale and scope compared to LLMs such as GPT-3 (ref. 15) and PaLM1. While the medical domain is challenging, specific proposals for LLMs have already included examples as varied as augmenting non-critical clinical assessments to summarization of complex medical communications90–92.
The closest precedents to our work are Galactica20, an LLM for science, and another work studying the reasoning capability of LLMs in the medical question-answering context93. The latter work used GPT-3.5 (Codex and InstructGPT), an instruction-tuned LLM94 and evaluated on the MedQA, MedMCQA, and PubMedQA datasets.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-023-06291-2.
Supplementary information
This file contains Supplementary Information, including Supplementary Tables 1–28.
Scaling plots for PaLM and Flan-PaLM with few-shot prompting on MedQA and MedMCQA.
Scaling plots for Flan-PaLM with few-shot and Flan-PaLM few-shot + COT + self-consistency on MedQA and MedMCQA.
Health Search Q&A Dataset.
Acknowledgements
This project was an extensive collaboration between many teams at Google Research, with DeepMind involved in an advisory capacity. We thank M. Howell, C. Chen, B. Mustafa, D. Fleet, F. Kibria, G. Turner, S. W. Man, D. Kim, B. Hatfield, L. Lehmann, I. Horn, M. Shiels, S. Shetty, J. Zitting, E. Rappaport, L. Marples, V. Sounderajah, A. Connell, J. Freyberg, C. Hughes, M. Jones-Bell, S. Thomas, M. Ho, R. Wong, S. Prakash, B. Green, E. Dominowska, F. Liu and X. Wang for their valuable insights and feedback during our research. We are also grateful to K. DeSalvo, Z. Ghahramani, J. Manyika and J. Dean for their support during the course of this project.
Extended data figures and tables
Author contributions
K.S., S.A., T.T., S.S.M., C.S., A.K. and V.N. contributed to the conception and design of the work. A.K., V.N., S.S.M., K.S., S.A. and T.T. contributed to the data acquisition and curation. K.S., S.A., T.T., V.N. and A.B. contributed to the technical implementation. A.K., V.N., K.S., S.A., T.T., C.S., H.C.-L., S.P., P.P. and N.T. contributed to the evaluation framework used in the study. J.W., H.W.C., N. Schärli, A.B., N. Scales and A.C. provided technical and infrastructure guidance. A.K., M.S., P.G. and C.K. provided clinical inputs to the study. D.D.-F. provided guidance on the datasets used in the study. All authors contributed to the drafting and revising of the manuscript.
Peer review
Peer review information
Nature thanks Andrew Beam and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The benchmark used in the study, MultiMedQA, comprises six open source datasets and one for consumer medical questions, HealthSearchQA, which we introduce here and are releasing with this work as a supplementary file.
Code availability
Med-PaLM is an LLM that has been aligned to the medical domain. We are not open-sourcing model code and weights owing to the safety implications of unmonitored use of such a model in medical settings. In the interest of responsible innovation, we will be working with academic and industry research partners, providers, regulators and policy stakeholders to validate and explore safe onward uses of Med-PaLM. For reproducibility, we documented technical deep learning methods while keeping the paper accessible to a clinical and general scientific audience. Our work builds upon PaLM, for which technical details have been described extensively, and our institution has open-sourced several related LLMs to further the development of research methods in the field (https://huggingface.co/google/flan-t5-xl).
Competing interests
This study was funded by Alphabet Inc. and/or a subsidiary thereof (Alphabet). K.S., S.A., T.T., V.N., A.K., S.S.M., C.S., J.W., H.W.C., N. Scales, A.T., H.C.-L., S.P., P.P., M.S., P.G., C.K., A.B., N. Schärli, A.C., P.M., B.A.A., D.W., G.S.C., Y.M., K.C., J.G., A.R., N.T., J.B. and Y.L. are employees of Alphabet and may own stock as part of the standard compensation package. D.D.-F. is affiliated with the US National Library of Medicine.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Karan Singhal, Shekoofeh Azizi, Tao Tu
These authors jointly supervised this work: Alan Karthikesalingam, Vivek Natarajan
Change history
7/27/2023
A Correction to this paper has been published: 10.1038/s41586-023-06455-0
Contributor Information
Karan Singhal, Email: karansinghal@google.com.
Shekoofeh Azizi, Email: shekazizi@google.com.
Alan Karthikesalingam, Email: alankarthi@google.com.
Vivek Natarajan, Email: natviv@google.com.
Extended data
is available for this paper at 10.1038/s41586-023-06291-2.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-023-06291-2.
References
- 1.Chowdhery, A. et al. PaLM: scaling language modeling with pathways. Preprint at 10.48550/arXiv.2204.02311 (2022).
- 2.Chung, H. W. et al. Scaling instruction-finetuned language models. Preprint at 10.48550/arXiv.2210.11416 (2022).
- 3.Jin, D. et al. What disease does this patient have? A large-scale open domain question answering dataset from medical exams. Appl. Sci.11, 6421 (2021). [Google Scholar]
- 4.Pal, A., Umapathi, L. K. & Sankarasubbu, M. MedMCQA: a large-scale multi-subject multi-choice dataset for medical domain question answering. In Conference on Health, Inference, and Learning 248–260 (Proceedings of Machine Learning Research, 2022).
- 5.Jin, Q., Dhingra, B., Liu, Z., Cohen, W. W. & Lu, X. PubMedQA: a dataset for biomedical research question answering. Preprint at 10.48550/arXiv.1909.06146 (2019).
- 6.Hendrycks, D. et al. Measuring massive multitask language understanding. Preprint at 10.48550/arXiv.2009.03300 (2020).
- 7.Esteva, A. et al. Deep learning-enabled medical computer vision. NPJ Digit. Med.4, 5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomašev, N. et al. Use of deep learning to develop continuous-risk models for adverse event prediction from electronic health records. Nat. Protoc.16, 2765–2787 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Yim, J. et al. Predicting conversion to wet age-related macular degeneration using deep learning. Nat. Med.26, 892–899 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Lakkaraju, H., Slack, D., Chen, Y., Tan, C. & Singh, S. Rethinking explainability as a dialogue: a practitioner’s perspective. Preprint at 10.48550/arXiv.2202.01875 (2022).
- 11.Bommasani, R. et al. On the opportunities and risks of foundation models. Preprint at 10.48550/arXiv.2108.07258 (2021).
- 12.Papineni, K., Roukos, S., Ward, T. & Zhu, W.-J. BLEU: a method for automatic evaluation of machine translation. In Proc. 40th Annual Meeting of the Association for Computational Linguistics 311–318 (Association of Computational Machinery, 2002).
- 13.Ben Abacha, A., Agichtein, E., Pinter, Y. & Demner-Fushman, D. Overview of the medical question answering task at TREC 2017 LiveQA. TREChttps://trec.nist.gov/pubs/trec26/papers/Overview-QA.pdf?ref=https://githubhelp.com (2017).
- 14.Abacha, A. B. et al. in Studies in Health Technology and Informatics (eds Ohno-Machado, L. & Séroussi, B.) 25–29 (IOS Press, 2019).
- 15.Brown, T. et al. Language models are few-shot learners. Adv. Neural Inf. Process. Syst.33, 1877–1901 (2020). [Google Scholar]
- 16.Wei, J. et al. Chain of thought prompting elicits reasoning in large language models. Preprint at 10.48550/arXiv.2201.11903 (2022).
- 17.Wang, X. et al. Self-consistency improves chain of thought reasoning in language models. Preprint at 10.48550/arXiv.2203.11171 (2022).
- 18.Yasunaga, M. et al. Deep bidirectional language-knowledge graph pretraining. Preprint at 10.48550/arXiv.2210.09338 (2022).
- 19.Bolton, E. et al. Stanford CRFM introduces PubMedGPT 2.7B. Stanford Universityhttps://hai.stanford.edu/news/stanford-crfm-introduces-pubmedgpt-27b (2022).
- 20.Taylor, R. et al. Galactica: a large language model for science. Preprint at 10.48550/arXiv.2211.09085 (2022).
- 21.Luo, R. et al. BioGPT: generative pre-trained transformer for biomedical text generation and mining. Brief. Bioinformatics23, bbac49 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Lin, S., Hilton, J. & Evans, O. Teaching models to express their uncertainty in words. Preprint at 10.48550/arXiv.2205.14334 (2022).
- 23.Kadavath, S. et al. Language models (mostly) know what they know. Preprint at 10.48550/arXiv.2207.05221 (2022).
- 24.Tran, D. et al. Plex: towards reliability using pretrained large model extensions. Preprint at 10.48550/arXiv.2207.07411 (2022).
- 25.Feng, S. Y., Khetan, V., Sacaleanu, B., Gershman, A. & Hovy, E. CHARD: clinical health-aware reasoning across dimensions for text generation models. Preprint at 10.48550/arXiv.2210.04191 (2022).
- 26.Williams, T., Szekendi, M., Pavkovic, S., Clevenger, W. & Cerese, J. The reliability of ahrq common format harm scales in rating patient safety events. J. Patient Saf.11, 52–59 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Walsh, K. E. et al. Measuring harm in healthcare: optimizing adverse event review. Med. Care55, 436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei, J. et al. Emergent abilities of large language models. Preprint at 10.48550/arXiv.2206.07682 (2022).
- 29.Kington, R. S. et al. Identifying credible sources of health information in social media: principles and attributes. NAM Perspectives (2021). [DOI] [PMC free article] [PubMed]
- 30.Mandavilli, A. Medical journals blind to racism as health crisis, critics say. The New York Timeshttps://www.nytimes.com/2021/06/02/health/jama-racism-bauchner.html (2021).
- 31.Shoemaker, S. J., Wolf, M. S. & Brach, C. Development of the patient education materials assessment tool (pemat): a new measure of understandability and actionability for print and audiovisual patient information. Patient Educ. Couns.96, 395–403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boateng, G. O., Neilands, T. B., Frongillo, E. A., Melgar-Quiñonez, H. R. & Young, S. L. Best practices for developing and validating scales for health, social, and behavioral research: a primer. Front. Public Health6, 149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooker, S. Moving beyond “algorithmic bias is a data problem”. Patterns2, 100241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen, I. Y. et al. Ethical machine learning in healthcare. Annu. Rev. Biomed. Data Sci.4, 123–144 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eneanya, N. D. et al. Health inequities and the inappropriate use of race in nephrology. Nat. Rev. Nephrol.18, 84–94 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vyas, L. G., Eisenstein, L. G. & Jones, D. S. Hidden in plain sight-reconsidering the use of race correction in clinical algorithms. N. Engl. J. Med.383, 874–882 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Weidinger, L. et al. Ethical and social risks of harm from language models. Preprint at 10.48550/arXiv.2112.04359 (2021).
- 38.Liang, P. et al. Holistic evaluation of language models. Preprint at 10.48550/arXiv.2211.09110 (2022).
- 39.Liu, X. et al. The medical algorithmic audit. Lancet Digit. Health4, e384–e397 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Raji, I. D. et al. Closing the AI accountability gap: defining an end-to-end framework for internal algorithmic auditing. In Proc. 2020 Conference on Fairness, Accountability, and Transparency 33–44 (Association for Computing Machinery, 2020).
- 41.Rostamzadeh, N. et al. Healthsheet: development of a transparency artifact for health datasets. Preprint at 10.48550/arXiv.2202.13028 (2022).
- 42.Gebru, T. et al. Datasheets for datasets. Commun. ACM64, 86–92 (2021). [Google Scholar]
- 43.Mitchell, M. et al. Model cards for model reporting. In Proc. conference on Fairness, Accountability, and Transparency 220–229 (Association for Computing Machinery, 2019).
- 44.Garg, S. et al. Counterfactual fairness in text classification through robustness. In Proc. 2019 AAAI/ACM Conference on AI, Ethics, and Society 219–226 (Association for Computing Machinery, 2019).
- 45.Prabhakaran, V., Hutchinson, B. & Mitchell, M. Perturbation sensitivity analysis to detect unintended model biases. Preprint at 10.48550/arXiv.1910.04210 (2019).
- 46.Zhang, H., Lu, A. X., Abdalla, M., McDermott, M. & Ghassemi, M. Hurtful words: quantifying biases in clinical contextual word embeddings. In Proc. ACM Conference on Health, Inference, and Learning 110–120 (Association for Computing Machinery, 2020).
- 47.Matheny, M., Israni, S. T., Ahmed, M. & Whicher, D. eds. Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril (National Academy of Medicine, 2022). [PubMed]
- 48.The White House Office of Science and Technology Policy. Blueprint for an AI Bill of Rights: Making Automated Systems Work for the American Peoplehttps://www.whitehouse.gov/wp-content/uploads/2022/10/Blueprint-for-an-AI-Bill-of-Rights.pdf (The White House, 2022).
- 49.Ethics and Governance of Artificial Intelligence for Health. WHO Guidance (World Health Organization, 2021).
- 50.Bommasani, R., Liang, P. & Lee, T. Language models are changing AI: the need for holistic evaluation. Stanford Universityhttps://crfm.stanford.edu/2022/11/17/helm.html (2022).
- 51.Pampari, A., Raghavan, P., Liang, J. & Peng, J. emrQA: a large corpus for question answering on electronic medical records. Preprint at 10.48550/arXiv.1809.00732 (2018).
- 52.Tsatsaronis, G. et al. An overview of the bioasq large-scale biomedical semantic indexing and question answering competition. BMC Bioinformatics16, 138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgado, F. F., Meireles, J. F., Neves, C., Amaral, A. & Ferreira, M. E. Scale development: ten main limitations and recommendations to improve future research practices. Psic. Reflex. Crit.30, 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barham, P. et al. Pathways: asynchronous distributed dataflow for ML. Proc. Mach. Learn. Syst.4, 430–449 (2022). [Google Scholar]
- 55.Thoppilan, R. et al. Lamda: language models for dialog applications. Preprint at 10.48550/arXiv.2201.08239 (2022).
- 56.Du, N. et al. Glam: efficient scaling of language models with mixture-of-experts. In International Conference on Machine Learning 5547–5569 (PMLR, 2022).
- 57.Srivastava, A. et al. Beyond the imitation game: quantifying and extrapolating the capabilities of language models. Preprint at 10.48550/arXiv.2206.04615 (2022).
- 58.Clark, J. H. et al. Tydi qa: A benchmark for information-seeking question answering in typologically diverse languages. Trans. Assoc. Comput. Linguist.8, 454–470 (2020). [Google Scholar]
- 59.Lester, B., Al-Rfou, R. & Constant, N. The power of scale for parameter-efficient prompt tuning. Preprint at 10.48550/arXiv.2104.08691 (2021).
- 60.Nye, M. et al. Show your work: scratchpads for intermediate computation with language models. Preprint at 10.48550/arXiv.2112.00114 (2021).
- 61.Zhou, D. et al. Least-to-most prompting enables complex reasoning in large language models. Preprint at 10.48550/arXiv.2205.10625 (2022).
- 62.Cobbe, K. et al. Training verifiers to solve math word problems. Preprint at 10.48550/arXiv.2110.14168 (2021).
- 63.Lewkowycz, A. et al. Solving quantitative reasoning problems with language models. Preprint at 10.48550/arXiv.2206.14858 (2022).
- 64.Ackley, D. H., Hinton, G. E. & Sejnowski, T. J. A learning algorithm for boltzmann machines. Cogn. Sci.9, 147–169 (1985). [Google Scholar]
- 65.Ficler, J. & Goldberg, Y. Controlling linguistic style aspects in neural language generation. Preprint at 10.48550/arXiv.1707.02633 (2017).
- 66.Li, X. L. & Liang, P. Prefix-tuning: optimizing continuous prompts for generation. Preprint at 10.48550/arXiv.2101.00190 (2021).
- 67.Wei, J. et al. Finetuned language models are zero-shot learners. Preprint at 10.48550/arXiv.2109.01652 (2021).
- 68.Liu, P. et al. Pre-train, prompt, and predict: a systematic survey of prompting methods in natural language processing. Preprint at 10.48550/arXiv.2107.13586 (2021).
- 69.Liu, X. et al. GPT understands, too. Preprint at 10.48550/arXiv.2103.10385 (2021).
- 70.Han, X., Zhao, W., Ding, N., Liu, Z. & Sun, M. PTR: prompt tuning with rules for text classification. AI Open3, 182–192 (2022). [Google Scholar]
- 71.Gu, Y., Han, X., Liu, Z. & Huang, M. PPT: Pre-trained prompt tuning for few-shot learning. Preprint at 10.48550/arXiv.2109.04332 (2021).
- 72.Ye, S., Jang, J., Kim, D., Jo, Y. & Seo, M. Retrieval of soft prompt enhances zero-shot task generalization. Preprint at 10.48550/arXiv.2210.03029 (2022).
- 73.Hoffmann, J. et al. Training compute-optimal large language models. Preprint at 10.48550/arXiv.2203.15556 (2022).
- 74.Scao, T. L. et al. BLOOM: a 176B-parameter open-access multilingual language model. Preprint at 10.48550/arXiv.2211.05100 (2022).
- 75.Rae, J. W. et al. Scaling language models: methods, analysis & insights from training Gopher. Preprint at 10.48550/arXiv.2112.11446 (2021).
- 76.Raffel, C. et al. Exploring the limits of transfer learning with a unified text-to-text transformer. J. Mach. Learn. Res.21, 1–67 (2020).34305477 [Google Scholar]
- 77.Zhang, S. et al. OPT: open pre-trained transformer language models. Preprint at 10.48550/arXiv.2205.01068 (2022).
- 78.Vaswani, A. et al. Attention is all you need. In 31st Conference on Neural Information Processing Systems (Association of Computational Machinery, 2017).
- 79.Kaplan, J. et al. Scaling laws for neural language models. Preprint at 10.48550/arXiv.2001.08361 (2020).
- 80.Lampinen, A. K. et al. Can language models learn from explanations in context? Preprint at 10.48550/arXiv.2204.02329 (2022).
- 81.Kojima, T., Gu, S. S., Reid, M., Matsuo, Y. & Iwasawa, Y. Large language models are zero-shot reasoners. Preprint at 10.48550/arXiv.2205.11916 (2022).
- 82.Joshi, M., Choi, E., Weld, D. S. & Zettlemoyer, L. TriviaQA: a large scale distantly supervised challenge dataset for reading comprehension. Preprint at 10.48550/arXiv.1705.03551 (2017).
- 83.Beltagy, I., Lo, K. & Cohan, A. SciBERT: a pretrained language model for scientific text. Preprint at 10.48550/arXiv.1903.10676 (2019).
- 84.Lewis, P., Ott, M., Du, J. & Stoyanov, V. Pretrained language models for biomedical and clinical tasks: Understanding and extending the state-of-the-art. In Proc. 3rd Clinical Natural Language Processing Workshop (eds Roberts, K., Bethard, S. & Naumann, T.) 146–157 (Association for Computational Linguistics, 2020).
- 85.Shin, H.-C. et al. BioMegatron: larger biomedical domain language model. Preprint at 10.48550/arXiv.2010.06060 (2020).
- 86.Lee, J. et al. Biobert: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics36, 1234–1240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gu, Y. et al. Domain-specific language model pretraining for biomedical natural language processing. ACM Trans. Comput. Healthc.3, 2 (2021). [Google Scholar]
- 88.Papanikolaou, Y. & Pierleoni, A. DARE: data augmented relation extraction with GPT-2. Preprint at 10.48550/arXiv.2004.13845 (2020).
- 89.Hong, Z. et al. The diminishing returns of masked language models to science. Preprint at 10.48550/arXiv.2205.11342 (2023).
- 90.Korngiebel, D. M. & Mooney, S. D. Considering the possibilities and pitfalls of generative pre-trained transformer 3 (GPT-3) in healthcare delivery. NPJ Digit. Med.4, 93 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sezgin, E., Sirrianni, J. & Linwood, S. L. et al. Operationalizing and implementing pretrained, large artificial intelligence linguistic models in the us health care system: outlook of generative pretrained transformer 3 (GPT-3) as a service model. JMIR Med. Informatics10, e32875 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agrawal, M., Hegselmann, S., Lang, H., Kim, Y. & Sontag, D. Large language models are zero-shot clinical information extractors. Preprint at 10.48550/arXiv.2205.12689 (2022).
- 93.Liévin, V., Hother, C. E. & Winther, O. Can large language models reason about medical questions? Preprint at 10.48550/arXiv.2207.08143 (2022). [DOI] [PMC free article] [PubMed]
- 94.Ouyang, L. et al. Training language models to follow instructions with human feedback. Preprint at 10.48550/arXiv.2203.02155 (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Information, including Supplementary Tables 1–28.
Scaling plots for PaLM and Flan-PaLM with few-shot prompting on MedQA and MedMCQA.
Scaling plots for Flan-PaLM with few-shot and Flan-PaLM few-shot + COT + self-consistency on MedQA and MedMCQA.
Health Search Q&A Dataset.
Data Availability Statement
The benchmark used in the study, MultiMedQA, comprises six open source datasets and one for consumer medical questions, HealthSearchQA, which we introduce here and are releasing with this work as a supplementary file.
Med-PaLM is an LLM that has been aligned to the medical domain. We are not open-sourcing model code and weights owing to the safety implications of unmonitored use of such a model in medical settings. In the interest of responsible innovation, we will be working with academic and industry research partners, providers, regulators and policy stakeholders to validate and explore safe onward uses of Med-PaLM. For reproducibility, we documented technical deep learning methods while keeping the paper accessible to a clinical and general scientific audience. Our work builds upon PaLM, for which technical details have been described extensively, and our institution has open-sourced several related LLMs to further the development of research methods in the field (https://huggingface.co/google/flan-t5-xl).