Abstract
椎间盘退变(IDD)是引起椎间盘退行性疾病的主要原因之一,其导致的下腰痛严重影响患者生活质量。目前,临床上针对IDD的治疗手段主要以缓解临床症状为主,而不是从其病理机制入手,尚缺乏有效的生物学治疗手段。微RNA(miRNA)是一种在转录后水平调控基因表达的内源性单链非编码小RNA,参与调控多种生物学过程,如脂类代谢和细胞凋亡、分化及器官发育。研究表明,miRNA在退变的椎间盘组织中呈高表达或低表达,参与IDD的多种病理过程,包括髓核细胞增生和凋亡、细胞外基质合成、炎症反应及软骨终板退变。本文总结了miRNA在退变椎间盘组织中的表达谱及其在IDD发生发展中的作用。随着对miRNA研究的深入,miRNA可能成为IDD生物学治疗的新策略。
Abstract
Intervertebral disc degeneration (IDD) is one of major causes for intervertebral disc degenerative diseases, and patients with IDD usually suffer from serious low back pain. The current treatments for patients with IDD only relieve the clinical symptom rather than restore biological balance of IDD, leading to inadequate and unsatisfactory results. MicroRNAs (miRNAs) are endogenous, non-coding, single-stranded RNA molecules, which regulate the gene expression at the post-transcription levels. Research evidences support the involvement of miRNAs in many biological processes, such as lipid metabolism, apoptosis, differentiation and organ development. Accumulating evidences indicate that the expressions of miRNAs change significantly in degenerative tissues. In addition, dysregulated miRNAs contribute to multiple pathological process of IDD, including proliferation and apoptosis of nucleus pulposus and extracellular matrix components, inflammatory response and cartilage endplates degeneration. In this review article, we summarize the expression profiles and roles of miRNAs in IDD, which may provide a novel strategy of biological therapy for the disease.
Keywords: MicroRNAs, Gene expression, Intervertebral disk/pathology, Extracellular matrix, Cell proliferation, Apoptosis, Inflammation/etiology, Intervertebral disk displacement/pathology, Review
目前,椎间盘退行性疾病是临床上常见的慢性病和多发病,严重影响公众的生活健康,其发病年龄亦越来越趋于年轻化,每年为此而带来了沉重的社会经济负担 [ 1] 。椎间盘退行性疾病是以颈肩痛、腰腿痛为主要表现的一系列临床综合征,包括颈椎病、椎间盘突出症、椎间盘源性腰痛、退变性椎管狭窄症及颈、腰椎不稳症等 [ 2- 4] 。大量研究表明,椎间盘退变(intervertebral disc degeneration,IDD)是椎间盘退行性疾病的病理学基础。鉴于目前临床上针对IDD的治疗主要是以缓解临床症状为主,而不是从病理生理发病机制上逆转或者延缓IDD的发生发展,因此,探究从根本上缓解或抑制IDD病理进程的方法已成为越来越多研究者的努力方向。
微RNA(miRNA)是一类含20~25个核苷酸的单链成熟非编码内源性的RNA,其通过与靶基因mRNA的3’非编码区结合,转录后直接抑制或降解靶基因mRNA而起作用,在调节细胞稳态和病理机制中发挥重要作用 [ 5] 。近年研究表明,miRNA参与IDD发生发展的多个病理过程,包括髓核细胞增生和凋亡、细胞外基质合成和降解失衡及炎症反应等 [ 6] 。本文基于IDD病理生理和miRNA生物学功能,针对miRNA在退变椎间盘组织中的表达失调,就其在IDD发生发展中的作用最新进展做一综述。
椎间盘位于上下椎体之间,由外部的纤维环、内部的髓核及上下软骨终板构成。椎间盘是人体内最大的无血管组织,也是一种高水合组织 [ 7] ;其中髓核是椎间盘中最大的水合组织区域,主要由随机排列的Ⅱ型胶原纤维和葡糖氨基葡聚糖等成分组成 [ 8] 。髓核细胞能促进椎间盘细胞外基质成分的生成,主要是软骨样细胞和脊索细胞,后者随着年龄增大而逐渐消失 [ 9] 。纤维环组织主要由15~25层定向有序的类同心圆纤维层构成,主要富含Ⅰ、Ⅱ型胶原蛋白和细长的纤维母细胞 [ 10] 。软骨终板由透明软骨和纤维软骨构成,主要为软骨细胞。软骨终板除了能吸收载荷传导外,还可以通过其自身有限的脉管系统来传递营养、氧气、代谢产物及水分等至椎间盘内部髓核组织 [ 11] 。
退变的椎间盘与正常健康的椎间盘组织有明显的区别。在形态学上,退变的椎间盘主要表现为椎间隙高度不同程度降低、髓核脱水纤维化和突出、纤维环破裂、软骨终板钙化、软骨下骨硬化等 [ 12] 。在退变的髓核组织中,蛋白聚糖含量减少,Ⅱ型与Ⅰ型胶原比例减少,引起髓核组织纤维化,影响髓核组织水合能力,导致椎间盘脱水。在退变的椎间盘组织中,可出现椎间盘细胞(尤其是髓核细胞)不良增生增多、细胞死亡(主要是细胞衰老及凋亡)增加以及细胞族形成等特征性表现 [ 13- 14] 。促炎性细胞因子(如IL-1、TNF-α)和基质蛋白酶[如基质金属蛋白酶(matrix metalloproteinases,MMP)]含量在退变的椎间盘组织中均增加。无论何种因素引起的IDD,最终均导致椎间盘细胞尤其是髓核细胞数目减少、活性降低以及Ⅱ型胶原蛋白和蛋白多糖等细胞外基质成分减少,细胞外基质合成与分解代谢失衡,使其朝着分解代谢增加方向发展,进而加剧或恶化IDD的病理进程 [ 15] 。
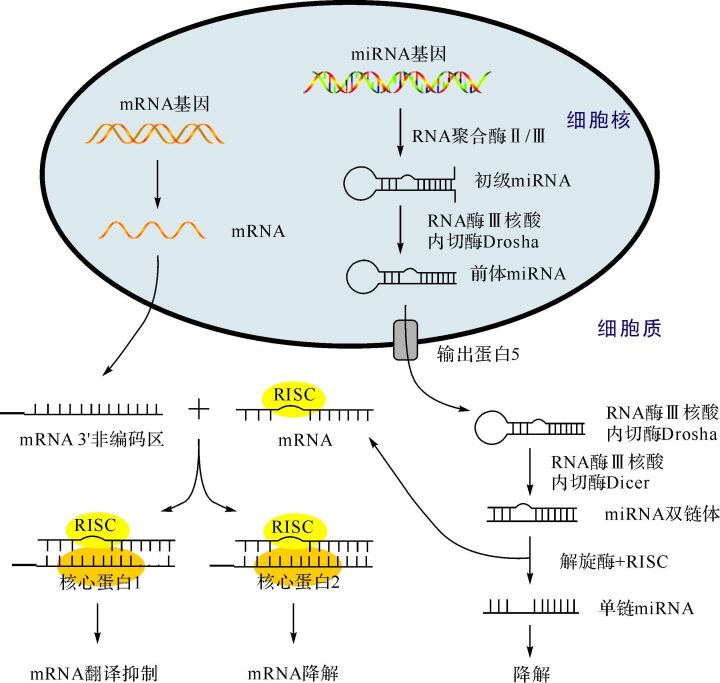
miRNA广泛存在于动植物体内,具有高保守性,并参与多种病理生理过程 [ 16] 。miRNA与mRNA在生物合成途径上相似( 图 1)。在细胞核中,编码miRNA基因先经RNA聚合酶Ⅱ或Ⅲ转录产生初级RNA [ 17] ,随后被剪切成具有茎环结构的发夹样前体miRNA [ 18] ,继而被转运至胞质中进一步加工。在胞质中,前体miRNA被剪切掉末端的环状结构域形成miRNA双链体 [ 19] ,其与核心蛋白Ago等结合形成RNA诱导的沉默复合物(RNA-induced silencing complex,RISC)。在解旋酶的作用下,RISC解开成两条单链miRNA:一条被保留的功能链和一条被降解的随从链。功能链可通过完全或部分碱基配对的方式引导RISC特异性识别和结合靶基因mRNA的3’非编码区、 5’非编码区或者蛋白编码区域 [ 20- 21] 。当miRNA与靶点间完全配对时,mRNA能被Ago2降解;相反,当两者部分配对时,mRNA的翻译过程能被Ago1抑制。因此,miRNA主要是通过抑制或降解靶基因mRNA来发挥生物学功能的。
目前,关于miRNA与IDD病理进程关系的研究还较少,其在椎间盘组织或细胞中的表达谱总结见 表 1。
表1 miRNA在椎间盘组织中的表达谱
Table 1 The expression profiles of miRNA in intervertebral disc tissues
|
miRNA(简写为miR-) |
表达变化 |
实验模型 |
参考文献 |
|
miR-589,miR-1286,miR-222,miR-220b,miR-640,miR-532-3p |
上调 |
人退变髓核细胞 |
[ 22] |
|
miR-30c-1,miR-638,miR-1275 |
下调 |
人退变髓核细胞 |
[ 22] |
|
miR-299-5p,miR-146b-3p,miR-3680,miR-2113,miR-920,miR-H6,miR-147b,miR-H3,miR-363,ebv-miR-BART21-3p,miR-H3,miR-130b,miR-200c,miR-4275,miR-764,miR-18b,miR-377,miR-1322,miR-3663-5p,miR-2355-5p,miR-3681,miR-4297,miR-675,miR-3126-5p,miR-3654,miR-1909 |
上调 |
人退变椎间盘组织 |
[ 23] |
|
miR-139-3p,miR-25,miR-H6-5p,miR-676,miRPlus-l107,miR-148a,miR-1266,miR-10a,miR-H3,miR-3138,miR-411,miR-664,miR-3065-5p,miR-34a,miR-516b,miR-182,miR-3128,ebv-miR-BART17-5p,miR-532-5p,miR-518c,miRPlus-l152,miR-203,miR-200b,miR-3065-3p,miR-493,miR-331-3p |
下调 |
人退变椎间盘组织 |
[ 23] |
|
miR-133b,miR-133b,miR-491-3p,miR-451a,miR-376a-5p,miR-149-5p,miR-661,miR-142-5p,miR-340-5p,miR-19b,miR-27b,miR-181c,miR-187,miR-21-5p,miR-29c,miR-320a,miR-144-3p,miR-610,miR-659,miR-146a,miR-32,miR-18a,miR-126,miR-372,miR-431,miR-486 |
上调 |
人退变髓核细胞 |
[ 24] |
|
miR-31,miR-33a,miR-15a-5p,miR-98,miR-127-5p,miR-130b,miR-10a-5p,let-7c,miR-124a,miR-138,miR-410,miR-563,miR-30a-5p,let-7e-5p,miR-376c |
下调 |
人退变髓核细胞 |
[ 24] |
|
miR-125a,miR-503,miR-503,miR-503,miR-503,miR-155,miR-32,miR-431,miR-659,miR-18a,miR-19b,miR-10b,miR-130b,miR-33a,miR-410,miR-563 |
上调 |
人退变髓核细胞 |
[ 25] |
|
miR-122,miR-486,miR-127-5p,miR-124a,miR-9,miR-29c,miR-27b |
下调 |
人退变髓核细胞 |
[ 25] |
|
miR-616-star,miR-220b,miR-561,miR-1253,miR-223,miR-627,miR-668,miR-342-3p,miR-383,miR-601 |
上调 |
人退变纤维环组织 |
[ 26] |
|
miR-509-3p,miR-499-3p,miR-590-3p,miR-34c-5p,miR-545,miR-1279,miR-662,miR-301b,miR-873,miR-566,miR-154,miR-758,miR-135a-star,miR-628-5p,miR-147b,miR-127-5p,miR-219-1-3p,miR-622,miR-185-star,miR-655,miR-449a,miR-31-star,miR-135b-star,miR-483-3p,miR-297,miR-433,miR-21-star |
上调 |
人退变髓核组织 |
[ 26] |
|
miR-187,miR-18a,miR-19b,miR-146a,miR-372,miR-610,miR-340-5p,miR-503,miR-423-3p,miR-181c,miR-142-5p,miR-27b,miR-32,miR-431,miR-659,miR-491-3p,miR-130b,miR-138,miR-33a,miR-410,miR-563 |
上调 |
人退变髓核细胞 |
[ 27] |
|
miR-126,miR-486,miR-127-5p,miR-124a,miR-31,miR-29c,miR-193a-3p |
下调 |
人退变髓核细胞 |
[ 27] |
|
miR-613,miR-206,miR-223-3p,miR-93,miR-202-3p,miR-155,miR-340-5p,miR-579,miR-34a,miR-17,miR-148b,miR-106b,miR-342-5p,miR-27a,miR-1825,miR-579,miR-130b,miR-138,miR-33a,miR-410,miR-563,miR-146a |
上调 |
人退变髓核组织 |
[ 28] |
|
miR-126,miR-133a,miR-127-5p,miR-124a,miR-31,let7g-5p,miR-29c-5p,miR-29c-3p,let-7c |
下调 |
人退变髓核组织 |
[ 28] |
miRNA或miR: 微RNA.
Hu等 [ 22] 在退变的椎间盘组织中,利用miRNA芯片及生物信息学数据库筛选出九种异常表达的miRNA,并构建了miRNA与靶基因网络图。Zhao等 [ 23] 同样利用基因芯片技术在人退变的椎间盘组织中筛选出了51种差异性表达的miRNA,并指出这些miRNA调控的信号通路主要与PI3K/Akt通路、MAPK及Wnt通路有关。Ji等 [ 24] 还通过miRNA芯片和荧光定量RT-PCR技术检测到41种miRNA呈差异性表达,并验证在退变的髓核组织中,miRNA-98明显下调,其下调程度与椎间盘退变程度呈负相关。Li等 [ 25] 也通过miRNA芯片和荧光定量RT-PCR技术发现,在退变的髓核组织中有23种miRNA表达异常,其中miRNA-27b表达下调。此外,Ohrt-Nissen等 [ 26] 发现27种miRNA在人退变的纤维环细胞中高表达,10种miRNA在退变的髓核组织中低表达,并指出在椎间盘中表达的miRNA种类不同于肌肉组织。在退变椎间盘组织的不同区域表达异常的miRNA也不甚相同,提示椎间盘中miRNA的表达可能具有组织特异性。
目前对于筛选组织差异性miRNA的表达,除可利用miRNA芯片技术外,同样可通过高通量测序技术实现。Ji等 [ 27] 通过Solexa测序方式在椎间盘组织中检测到28种差异性表达的miRNA分子,其后利用荧光定量RT-PCR技术证实了miRNA-193a-3p的表达在髓核组织中下调。在另一项筛选人退变髓核组织差异性表达的miRNA研究中,Xu等 [ 28] 同样利用Solexa测序技术成功筛选出31种差异性表达的miRNA,其中miRNA-133a的表达下调,并与椎间盘退变的严重程度呈负相关。以上研究提示,miRNA表达失调可能与IDD的发生发展密切相关,但其具体作用机制还需要进一步研究。
近来研究发现,miRNA亦可通过介导髓核细胞凋亡和增生、细胞外基质合成、炎症反应以及软骨终板退变过程来加剧IDD进程。
细胞凋亡,即Ⅰ类程序性细胞死亡(programmed cell death,PCD),是细胞主动结束生命的过程,同时也是维持细胞和组织稳态的基本方式,其介导的髓核细胞丢失在IDD的发生发展中扮演着至关重要的角色 [ 29] 。研究表明,miRNA参与调控髓核细胞凋亡过程。Wang等 [ 30] 报道,人髓核细胞凋亡率随miRNA-494表达上调而增加,敲除内源性miRNA-494的人髓核细胞可通过上调JunD蛋白的表达来保护髓核细胞免于凋亡,其机制可能与细胞色素c凋亡通路有关,提示miRNA-494可能促进IDD的发展。在退变的人髓核细胞中,Liu 等 [ 31] 发现,表达上调的miRNA-27a可通过靶向沉默PI3K促进髓核细胞凋亡。除了高表达miRNA可促进髓核细胞凋亡外,低表达miRNA可激活髓核细胞凋亡通路。Wang等 [ 32] 发现miRNA-155在退变的髓核组织中呈低表达;低表达的miRNA-155可通过靶向沉默FADD和胱天蛋白酶(caspase)-3,激活Fas受体介导的细胞凋亡通路。这些研究表明miRNA可以通过沉默不同的靶基因来调控髓核细胞凋亡。因此推测,纠正miRNA-494、miRNA-27a和miRNA-155的表达失调,减少髓核细胞凋亡可延缓或逆转IDD过程。
IDD的特征之一为髓核细胞异常增生,导致细胞簇形成,后者一定程度上抑制了椎间盘组织的自身修复过程 [ 33] 。这对以细胞外基质成分为主,细胞含量较少的椎间盘组织来说是不利的。在人体软骨组织中,miRNA在诱导软骨细胞和成骨细胞的增生过程中起重要作用 [ 34- 35] 。近年研究表明,对于同样含软骨细胞的椎间盘组织,miRNA也参与调节髓核细胞异常增生过程。Liu等 [ 36] 采用RT-PCR检测到miRNA-21表达水平在人退变的椎间盘组织中明显上调,并与IDD程度呈正相关,证实miRNA-21过表达能促进细胞周期蛋白D1高表达,并通过靶向沉默PTEN来激活Akt信号通路,进而调控髓核细胞异常增生,这提示miRNA-21可通过调控髓核细胞异常增生来干预细胞外基质合成与降解平衡。Yu等 [ 37] 报道,在人退变的椎间盘组织中,miRNA-10b的表达水平也随IDD程度增加而上调,可通过靶向沉默HOXD10激活RhoC-Akt通路,促进髓核细胞不良增生,从而加剧IDD发展,提示针对miRNA-10b的抑制剂可能具有抑制髓核细胞异常增生的效应。同时,miRNA-21和miRNA-10b均通过激活Akt信号通路来介导髓核细胞增生。在异常表达miRNA的退变椎间盘组织中,调控正常组织增生、分化的Akt信号通路似乎可以产生“过度效应”,导致髓核细胞异常增生,从而加重IDD。因此,要研究如何有效地抑制或预防Akt通路的“过度效应”,从而避免对正常组织增殖、分化的干扰。
在正常椎间盘中,细胞外基质成分一直处于髓核细胞合成与基质降解相互作用的动态平衡中 [ 38] 。此外,细胞外基质不仅可维持椎间盘的完整,还可通过对髓核细胞提供机械和生物化学通路来调控髓核细胞的生存、形态及分化过程 [ 39] 。然而,在退变的椎间盘组织中,细胞外基质降解增多、合成减少,导致髓核组织中的胶原蛋白及蛋白聚糖等细胞外基质主要成分减少。椎间盘组织中的miRNA可调控基质降解酶的表达。Yan等 [ 40] 发现在人髓核SV细胞系中,miRNA-100可以通过结合FGFR3的3′非编码区上调MMP-13的表达水平,诱导细胞外基质成分降解增加。Jing等 [ 41] 发现人退变的髓核组织中,miRNA-93的表达水平显著下调,且其表达水平与IDD程度呈负性相关;进一步研究表明,miRNA-93过表达可靶向沉默MMP-3刺激退变髓核细胞的Ⅱ型胶原蛋白表达,提示在退变的椎间盘中,miRNA-93的低表达可能是导致基质降解酶增多和细胞外基质成分改变的内在原因之一。此外,在髓核细胞中,Ji等 [ 27] 发现miRNA-193-3p表达显著下调,其下调程度与IDD程度呈正相关,利用荧光素酶报告分析和蛋白质印迹法证实MMP-14是miRNA-193-3p的靶基因,通过直接靶向沉默MMP-14来上调Ⅱ型胶原的表达,从而缓解IDD过程。另一项研究还发现,在退变的髓核细胞中,miRNA-98表达上调可抑制IL-6/STAT3信号通路,下调MMP-2表达水平和上调Ⅱ型胶原的表达,从而抑制IDD的发生发展 [ 24] 。Li等 [ 25] 发现在退变的髓核细胞中,miRNA-27b表达下调可直接靶向上调MMP-13的水平,抑制Ⅱ型胶原的合成。同样,Xu等 [ 28] 发现miRNA-133a的异常表达与IDD密切相关,并揭示miRNA-133a下调可靶向沉默MMP-9而诱导Ⅱ型胶原丢失效应的发生,从而促进IDD的病理进程。这些研究表明,在椎间盘组织中,细胞外基质成分的降解或失衡可能与miRNA的异常表达密切相关。
除了介导MMP的表达外,miRNA还可通过调控另一种机制降解酶蛋白聚糖酶(a disintergrin and metalloproteinase with thrombospondin motifs,ADAMTS)的表达来干预IDD的病理过程。Gu等 [ 42] 报道,转染了miRNA-146a的牛尾髓核细胞通过沉默肿瘤坏死因子相关受体因子6(tumor necrosis factor receptor-associated factor 6,TRAF6)下调IL-1诱导的MMP-13、ADAMTS-4及ADAMTS-5表达;同样,miRNA-146a敲除后的鼠髓核细胞用IL-1处理后,MMP-13和ADAMTS-5的表达显著上调,提示miRNA-146a能下调或抑制炎症反应诱导的基质降解酶表达,从而对椎间盘细胞外基质起保护作用。以上研究表明,miRNA可通过调控基质降解酶的表达来调节椎间盘细胞外基质合成,进而缓解IDD的发生发展。因此,通过修正上述失调miRNA使其正常表达,有可能重建细胞外基质成分的合成与降解平衡,进而保护退变的椎间盘组织。
在退变的椎间盘组织和细胞中,促炎性细胞因子(如IL-1、IL-6、TNF-α等)含量较正常椎间盘组织和细胞增加,通过诱导基质降解酶、氧化应激、细胞凋亡及细胞衰老等来促进IDD的发生发展 [ 43- 44] 。研究发现,在椎间盘组织中,miRNA可调控炎性因子的表达来介导IDD的发展。在IL-1处理的牛尾髓核细胞中,miRNA-146a可沉默TRAF6而下调一氧化氮合酶、IL-6、TNF-α和环氧酶2的表达水平 [ 42] ,提示miRNA-146a对IL-1介导的IDD可能具有保护作用。Ji等 [ 24] 研究发现,在人退变的髓核组织中,IL-6的mRNA水平与miRNA-98的含量呈负相关,而且miRNA-98通过靶向沉默IL-6/STAT3来抑制MMP-2的生成,进而延缓IDD的发展进程。此外,Wang等 [ 30] 证实,miRNA-494抑制剂能通过靶向沉默JunD而抑制TNF-α诱导的人髓核细胞凋亡。这提示miRNA-494/JunD通路可能参与TNF-α诱导的髓核细胞凋亡过程。尽管已发现miRNA能介导炎症反应诱导的IDD过程,但国内外相关研究较少。因此,探究更多的miRNA与炎症反应之间的关系,将是研究者的重点方向。
软骨终板由透明软骨和纤维软骨构成,主要为椎间盘内部无脉管的髓核组织传递营养、氧气、代谢产物及水分等。软骨终板退变的主要表现为自身硬化、钙化及增厚等。Neidlinger-Wilke等 [ 45] 报道,在用软骨终板的条件培养液处理人髓核细胞后,髓核细胞能明显上调IL-6、IL-8、MMP-3及MMP-13的表达,显著抑制蛋白多糖和Ⅱ型胶原的生成,而且软骨终板的条件培养液中促炎性细胞因子含量也明显增加。这提示,软骨终板可能在加剧髓核组织的退变过程中扮演着重要角色。近来,国内学者发现miRNA分子也参与调控椎间盘软骨终板的退变过程。程细高等 [ 46] 首次对退变的颈椎软骨终板通过基因芯片技术进行筛选,发现miRNA-140表达量是正常组的三分之一;在终板软骨细胞中,miRNA-140通过靶向沉默ADAMTS-5和天冬氨酰氨肽酶(DNPEP)基因减少软骨终板MMP的合成和抑制DNPEP介导的骨形成蛋白信号通路,从而缓解软骨终板的退变过程。进一步探明软骨终板退变的病理机制,对预防或逆转IDD的发生发展可能具有新的生物学意义。
不同miRNA在IDD中的作用见 表 2。
表2 miRNA在IDD中的作用分类
Table 2 Roles of miRNA in intervertebral disc degeneration
|
miRNA分类 |
实验模型 |
表达变化 |
机 制 |
对IDD的作用 |
参考文献 |
|
调控髓核细胞凋亡 | |||||
|
miR-494 |
人退变的髓核组织 |
上调 |
JunD蛋白、细胞色素c |
促进 |
[ 30] |
|
miR-27a |
人退变的髓核组织 |
上调 |
PI3K |
促进 |
[ 31] |
|
miR-155 |
人正常和退变的髓核组织 |
下调 |
FADD、caspase-3 |
促进 |
[ 32] |
|
调控髓核细胞不良增生 | |||||
|
miR-21 |
人退变的髓核组织 |
上调 |
细胞周期蛋白D1、PTEN、Akt |
促进 |
[ 36] |
|
miR-10b |
人退变的髓核组织 |
上调 |
HOXD10、RhoC、Akt |
促进 |
[ 37] |
|
调控细胞外基质降解 | |||||
|
miR-100 |
人的髓核SV细胞 |
上调 |
FGFR3 |
促进 |
[ 40] |
|
miR-93 |
人退变的髓核组织 |
下调 |
MMP-3 |
促进 |
[ 41] |
|
miR-193-3p |
人退变的髓核细胞 |
下调 |
MMP-14 |
促进 |
[ 27] |
|
miR-98 |
人退变的髓核组织 |
上调 |
IL-6、STAT3、MMP-2 |
保护 |
[ 24] |
|
miR-27b |
人退变的髓核细胞 |
下调 |
MMP-13 |
保护 |
[ 25] |
|
miR-133a |
人退变的髓核组织 |
下调 |
MMP-9 |
促进 |
[ 28] |
|
miR-146a |
牛尾髓核细胞 |
上调 |
TRAF6、MMP-13、ADAMTS-4、ADAMTS-5 |
保护 |
[ 42] |
|
调控炎症反应 | |||||
|
miR-146a |
牛尾髓核细胞 |
上调 |
TRAF6、IL-1 |
保护 |
[ 42] |
|
miR-98 |
人退变的髓核组织 |
上调 |
IL-6、STAT3 |
保护 |
[ 24] |
|
调控软骨终板退变 | |||||
|
miR-140 |
人退变的软骨终板组织 |
下调 |
ADAMTS-5、DNPEP |
保护 |
[ 46] |
在人退变的椎间盘组织中,miRNA的表达不仅异于肌肉组织,而且髓核组织与纤维环组织间也存在差异 [ 26] 。因此,明确椎间盘组织中特异性miRNA分子的表达情况,对IDD的诊断、治疗及预后可能具有潜在的应用价值。
国内外对miRNA在IDD中的作用研究才刚刚起步,主要涉及本文总结的关于调控髓核细胞的增生和凋亡、细胞外基质成分、炎症反应及软骨细胞退变方面。此外,调节miRNA的表达水平可以促进软骨细胞增殖,以及调节软骨细胞的新陈代谢过程 [ 47] 。因此,针对IDD治疗的miRNA可能是组织工程和再生医学的重要分子靶点。
自噬,即Ⅱ类PCD,其主要作用是通过清除或降解细胞内受损的细胞器、不需要的生物大分子等,为细胞提供能量和重复使用的氨基酸,从而保护细胞来抵御周围环境的变化,在细胞的正常生理平衡中尤为重要 [ 48] 。研究指出,细胞自噬在抑制或缓解IDD病理进程和对抗各种应激环境中起保护作用 [ 49- 50] 。miRNA可通过调控细胞自噬来介导多种疾病过程,如心衰 [ 51] 、胰腺癌 [ 52] 、缺血性肾损伤 [ 53] 等。同样,miRNA可能也可调控同属于PCD的细胞自噬,促进或抑制IDD的进展。但在椎间盘组织和细胞中,目前还没有研究报道miRNA与细胞自噬的关系,需进一步完善。
miRNA分子在椎间盘退变中的研究目前仍处于实验阶段,暂时缺乏临床应用的证据。事实上,在miRNA进入椎间盘退变性疾病临床应用之前,仍将面临许多挑战,如:①一种miRNA分子能同时作用于几种不同的靶基因,可引起不同的生物学效应,如何筛选既有治疗性又有专一性的miRNA分子,仍需要进一步的探索;②内源性miRNA对靶基因的作用具有时间和空间特异性,如何解决外源性miRNA分子对体内微环境的适应问题?③正常的椎间盘组织是人体内最大的无血管组织,然而如何让治疗性的miRNA分子到达椎间盘组织并维持有效的药物浓度?④目前在椎间盘组织中已发现近几百种miRNA,而已被研究的miRNA只是其中的冰山一角。因此,miRNA与IDD的关系还需要进一步研究。
随着对miRNA分子生物学机制研究的进一步深入,其在机体生理和病理过程中的作用也会更好地认识,在IDD早期诊断、治疗及预后判断中的潜在价值也会被证实。因此,miRNA作为一种新的IDD调控因子,是重要的候选基因,在IDD病理机制研究及治疗中具有巨大的潜能。
Funding Statement
国家自然科学基金(31400802);湖南省自然科学基金(2015JJ5003)
References
- 1.VOS T, FLAXMAN A D, NAGHAVI M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [VOS T, FLAXMAN A D, NAGHAVI M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010[J]. Lancet, 2012,380(9859):2163-2196.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WEBER K T, JACOBSEN T D, MAIDHOF R, et al. Developments in intervertebral disc disease research: pathophysiology, mechanobiology, and therapeutics. Curr Rev Musculoskelet Med. 2015;8(1):18–31. doi: 10.1007/s12178-014-9253-8. [WEBER K T, JACOBSEN T D, MAIDHOF R, et al. Developments in intervertebral disc disease research: pathophysiology, mechanobiology, and therapeutics[J]. Curr Rev Musculoskelet Med, 2015,8(1):18-31.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.KABIR S M, GUPTA S R, CASEY A T. Lumbar interspinous spacers: a systematic review of clinical and biomechanical evidence. Spine (Phila Pa 1976) 2010;35(25):E1499–E1506. doi: 10.1097/BRS.0b013e3181e9af93. [KABIR S M, GUPTA S R, CASEY A T. Lumbar interspinous spacers: a systematic review of clinical and biomechanical evidence[J]. Spine (Phila Pa 1976), 2010,35(25):E1499-E1506.] [DOI] [PubMed] [Google Scholar]
- 4.TAHER F, ESSIG D, LEBL D R, et al. Lumbar degenerative disc disease: current and future concepts of diagnosis and management. Adv Orthop. 2012;2012:970752. doi: 10.1155/2012/970752. [TAHER F, ESSIG D, LEBL D R, et al. Lumbar degenerative disc disease: current and future concepts of diagnosis and management[J]. Adv Orthop, 2012,2012: 970752.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MIRZAMOHAMMADI F, PAPAIOANNOU G, KOBAYASHI T. MicroRNAs in cartilage development, homeostasis, and disease. Curr Osteoporos Rep. 2014;12(4):410–419. doi: 10.1007/s11914-014-0229-9. [MIRZAMOHAMMADI F, PAPAIOANNOU G, KOBAYASHI T. MicroRNAs in cartilage development, homeostasis, and disease[J]. Curr Osteoporos Rep, 2014,12(4):410-419.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LI Z, YU X, SHEN J, et al. MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015;48(3):278–283. doi: 10.1111/cpr.2015.48.issue-3. [LI Z, YU X, SHEN J, et al. MicroRNA in intervertebral disc degeneration[J]. Cell Prolif, 2015,48(3):278-283.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PEREIRA D R, SILVA-CORREIA J, OLIVEIRA J M, et al. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J Tissue Eng Regen Med. 2013;7(2):85–98. doi: 10.1002/term.v7.2. [PEREIRA D R, SILVA-CORREIA J, OLIVEIRA J M, et al. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration[J]. J Tissue Eng Regen Med, 2013,7(2):85-98.] [DOI] [PubMed] [Google Scholar]
- 8.WANG S Z, RUI Y F, LU J, et al. Cell and molecular biology of intervertebral disc degeneration: current understanding and implications for potential therapeutic strategies. Cell Prolif. 2014;47(5):381–390. doi: 10.1111/cpr.2014.47.issue-5. [WANG S Z, RUI Y F, LU J, et al. Cell and molecular biology of intervertebral disc degeneration: current understanding and implications for potential therapeutic strategies[J]. Cell Prolif, 2014,47(5):381-390.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WEILER C, NERLICH A G, SCHAAF R, et al. Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur Spine J. 2010;19(10):1761–1770. doi: 10.1007/s00586-010-1392-z. [WEILER C, NERLICH A G, SCHAAF R, et al. Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc[J]. Eur Spine J, 2010,19(10):1761-1770.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DUNCAN N A. Cell deformation and micromechanical environment in the intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):47–51. doi: 10.2106/JBJS.F.00035. [DUNCAN N A. Cell deformation and micromechanical environment in the intervertebral disc[J]. J Bone Joint Surg Am, 2006,88 Suppl 2: 47-51.] [DOI] [PubMed] [Google Scholar]
- 11.BLANQUER S B, GRIJPMA D W, POOT A A. Delivery systems for the treatment of degenerated intervertebral discs. Adv Drug Deliv Rev. 2015;84:172–187. doi: 10.1016/j.addr.2014.10.024. [BLANQUER S B, GRIJPMA D W, POOT A A. Delivery systems for the treatment of degenerated intervertebral discs[J]. Adv Drug Deliv Rev, 2015,84: 172-187.] [DOI] [PubMed] [Google Scholar]
- 12.VERGROESEN P P, KINGMA I, EMANUEL K S, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–1070. doi: 10.1016/j.joca.2015.03.028. [VERGROESEN P P, KINGMA I, EMANUEL K S, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle[J]. Osteoarthritis Cartilage, 2015,23(7):1057-1070.] [DOI] [PubMed] [Google Scholar]
- 13.KEPLER C K, PONNAPPAN R K, TANNOURY C A, et al. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13(3):318–330. doi: 10.1016/j.spinee.2012.12.003. [KEPLER C K, PONNAPPAN R K, TANNOURY C A, et al. The molecular basis of intervertebral disc degeneration[J]. Spine J, 2013,13(3):318-330.] [DOI] [PubMed] [Google Scholar]
- 14.WANG W J, YU X H, WANG C, et al. MMPs and ADAMTSs in intervertebral disc degeneration. Clin Chim Acta. 2015;448:238–246. doi: 10.1016/j.cca.2015.06.023. [WANG W J, YU X H, WANG C, et al. MMPs and ADAMTSs in intervertebral disc degeneration[J]. Clin Chim Acta, 2015,448: 238-246.] [DOI] [PubMed] [Google Scholar]
- 15.YANG W, YU X H, WANG C, et al. Interleukin-1beta in intervertebral disk degeneration. Clin Chim Acta. 2015;450:262–272. doi: 10.1016/j.cca.2015.08.029. [YANG W, YU X H, WANG C, et al. Interleukin-1beta in intervertebral disk degeneration[J]. Clin Chim Acta, 2015,450: 262-272.] [DOI] [PubMed] [Google Scholar]
- 16.SMALL E M, OLSON E N. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336–342. doi: 10.1038/nature09783. [SMALL E M, OLSON E N. Pervasive roles of microRNAs in cardiovascular biology[J]. Nature, 2011,469(7330):336-342.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WU C, TIAN B, QU X, et al. MicroRNAs play a role in chondrogenesis and osteoarthritis (review) Int J Mol Med. 2014;34(1):13–23. doi: 10.3892/ijmm.2014.1743. [WU C, TIAN B, QU X, et al. MicroRNAs play a role in chondrogenesis and osteoarthritis (review)[J]. Int J Mol Med, 2014,34(1):13-23.] [DOI] [PubMed] [Google Scholar]
- 18.HONG E, REDDI A H. MicroRNAs in chondrogenesis, articular cartilage, and osteoarthritis: implications for tissue engineering. Tissue Eng Part B Rev. 2012;18(6):445–453. doi: 10.1089/ten.teb.2012.0116. [HONG E, REDDI A H. MicroRNAs in chondrogenesis, articular cartilage, and osteoarthritis: implications for tissue engineering[J]. Tissue Eng Part B Rev, 2012,18(6):445-453.] [DOI] [PubMed] [Google Scholar]
- 19.SHANG J, LIU H, ZHOU Y. Roles of microRNAs in prenatal chondrogenesis, postnatal chondrogenesis and cartilage-related diseases. J Cell Mol Med. 2013;17(12):1515–1524. doi: 10.1111/jcmm.2013.17.issue-12. [SHANG J, LIU H, ZHOU Y. Roles of microRNAs in prenatal chondrogenesis, postnatal chondrogenesis and cartilage-related diseases[J]. J Cell Mol Med, 2013,17(12):1515-1524.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GUO H, INGOLIA N T, WEISSMAN J S, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. doi: 10.1038/nature09267. [GUO H, INGOLIA N T, WEISSMAN J S, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels[J]. Nature, 2010,466(7308):835-840.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.BARTEL D P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [BARTEL D P. MicroRNAs: target recognition and regulatory functions[J]. Cell, 2009,136(2):215-233.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HU P, FENG B, WANG G, et al. Microarray based analysis of gene regulation by microRNA in intervertebral disc degeneration. Mol Med Rep. 2015;12(4):4925–4930. doi: 10.3892/mmr.2015.4022. [HU P, FENG B, WANG G, et al. Microarray based analysis of gene regulation by microRNA in intervertebral disc degeneration[J]. Mol Med Rep, 2015,12(4):4925-4930.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ZHAO B, YU Q, LI H, et al. Characterization of microRNA expression profiles in patients with intervertebral disc degeneration. Int J Mol Med. 2014;33(1):43–50. doi: 10.3892/ijmm.2013.1543. [ZHAO B, YU Q, LI H, et al. Characterization of microRNA expression profiles in patients with intervertebral disc degeneration[J]. Int J Mol Med, 2014,33(1):43-50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.JI M L, LU J, SHI P L, et al. Dysregulated miR-98 contributes to extracellular matrix degradation by targeting IL-6/STAT3 signalling pathway in human intervertebral disc degeneration. J Bone Miner Res. 2015;31(4):900–909. doi: 10.1002/jbmr.2753. [JI M L, LU J, SHI P L, et al. Dysregulated miR-98 contributes to extracellular matrix degradation by targeting IL-6/STAT3 signalling pathway in human intervertebral disc degeneration[J]. J Bone Miner Res, 2015,31(4):900-909.] [DOI] [PubMed] [Google Scholar]
- 25.LI H R, CUI Q, DONG Z Y. Downregulation of miR-27b is involved in loss of type Ⅱ collagen by directly targeting matrix metalloproteinase 13 (MMP13) in human intervertebral disc degeneration. Spine (Phila Pa 1976) 2016;41(3):E116–E123. doi: 10.1097/BRS.0000000000001139. [LI H R, CUI Q, DONG Z Y, et al. Downregulation of miR-27b is involved in loss of type Ⅱ collagen by directly targeting matrix metalloproteinase 13 (MMP13) in human intervertebral disc degeneration[J]. Spine (Phila Pa 1976), 2016,41(3):E116-E123.] [DOI] [PubMed] [Google Scholar]
- 26.OHRT-NISSEN S, DOSSING K B, ROSSING M, et al. Characterization of miRNA expression in human degenerative lumbar disks. Connect Tissue Res. 2013;54(3):197–203. doi: 10.3109/03008207.2013.781594. [OHRT-NISSEN S, DOSSING K B, ROSSING M, et al. Characterization of miRNA expression in human degenerative lumbar disks[J]. Connect Tissue Res, 2013,54(3):197-203.] [DOI] [PubMed] [Google Scholar]
- 27.JI M L, ZHANG X J, SHI P L, et al. Downregulation of microRNA-193a-3p is involved in invertebral disc degeneration by targeting MMP14. J Mol Med (Berl) 2016;94(4):457–468. doi: 10.1007/s00109-015-1371-2. [JI M L, ZHANG X J, SHI P L, et al. Downregulation of microRNA-193a-3p is involved in invertebral disc degeneration by targeting MMP14[J]. J Mol Med (Berl), 2016,94(4):457-468.] [DOI] [PubMed] [Google Scholar]
- 28.XU Y Q, ZHANG Z H, ZHENG Y F, et al. Dysregulated miR-133a mediates loss of type Ⅱ collagen by directly targeting matrix metalloproteinase 9 (MMP9) in human intervertebral disc degeneration. Spine (Phila Pa 1976) 2015 doi: 10.1097/BRS.0000000000001375. [XU Y Q, ZHANG Z H, ZHENG Y F, et al. Dysregulated miR-133a mediates loss of type Ⅱ collagen by directly targeting matrix metalloproteinase 9 (MMP9) in human intervertebral disc degeneration[J]. Spine (Phila Pa 1976), 2015. [Epub ahead of print]] [DOI] [PubMed] [Google Scholar]
- 29.DING F, SHAO Z W, XIONG L M. Cell death in intervertebral disc degeneration. Apoptosis. 2013;18(7):777–785. doi: 10.1007/s10495-013-0839-1. [DING F, SHAO Z W, XIONG L M. Cell death in intervertebral disc degeneration[J]. Apoptosis, 2013,18(7):777-785.] [DOI] [PubMed] [Google Scholar]
- 30.WANG T, LI P, MA X, et al. MicroRNA-494 inhibition protects nucleus pulposus cells from TNF-alpha-induced apoptosis by targeting JunD. Biochimie. 2015;115:1–7. doi: 10.1016/j.biochi.2015.04.011. [WANG T, LI P, MA X, et al. MicroRNA-494 inhibition protects nucleus pulposus cells from TNF-alpha-induced apoptosis by targeting JunD[J]. Biochimie, 2015,115: 1-7.] [DOI] [PubMed] [Google Scholar]
- 31.LIU G, CAO P, CHEN H, et al. MiR-27a regulates apoptosis in nucleus pulposus cells by targeting PI3K. PLoS One. 2013;8(9):e75251. doi: 10.1371/journal.pone.0075251. [LIU G, CAO P, CHEN H, et al. MiR-27a regulates apoptosis in nucleus pulposus cells by targeting PI3K[J]. PLoS One, 2013,8(9):e75251.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WANG H Q, YU X D, LIU Z H, et al. Deregulated miR-155 promotes fas-mediated apoptosis in human intervertebral disc degeneration by targeting FADD and caspase-3. J Pathol. 2011;225(2):232–242. doi: 10.1002/path.v225.2. [WANG H Q, YU X D, LIU Z H, et al. Deregulated miR-155 promotes fas-mediated apoptosis in human intervertebral disc degeneration by targeting FADD and caspase-3[J]. J Pathol, 2011,225(2):232-242.] [DOI] [PubMed] [Google Scholar]
- 33.PRATSINIS H, CONSTANTINOU V, PAVLAKIS K, et al. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012;30(6):958–964. doi: 10.1002/jor.v30.6. [PRATSINIS H, CONSTANTINOU V, PAVLAKIS K, et al. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways[J]. J Orthop Res, 2012,30(6):958-964.] [DOI] [PubMed] [Google Scholar]
- 34.DONG S, YANG B, GUO H, et al. MicroRNAs regulate osteogenesis and chondrogenesis. Biochem Biophys Res Commun. 2012;418(4):587–591. doi: 10.1016/j.bbrc.2012.01.075. [DONG S, YANG B, GUO H, et al. MicroRNAs regulate osteogenesis and chondrogenesis[J]. Biochem Biophys Res Commun, 2012,418(4):587-591.] [DOI] [PubMed] [Google Scholar]
- 35.余 强, 李 浩鹏, 郭 雄. MicroRNA在软骨损伤退变中作用机制的研究. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGGU201206035.htm. 中国骨伤. 2012;25(6):530–534. [余 强,李浩鹏,郭 雄.] [PubMed] [Google Scholar]
- 36.LIU H, HUANG X, LIU X, et al. miR-21 promotes human nucleus pulposus cell proliferation through PTEN/AKT signaling. Int J Mol Sci. 2014;15(3):4007–4018. doi: 10.3390/ijms15034007. [MicroRNA在软骨损伤退变中作用机制的研究[J]. 中国骨伤,2012,25(6):530-534. YU Qiang, LI Haopeng, GUO Xiong. The mechanism advance of microRNA in cartilage injury and degeneration[J]. China Journal of Orthapaedics and Traumatology, 2012,25(6):530-534. (in Chinese)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.YU X, LI Z, SHEN J, et al. MicroRNA-10b promotes nucleus pulposus cell proliferation through RhoC-Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS One. 2013;8(12):e83080. doi: 10.1371/journal.pone.0083080. [LIU H, HUANG X, LIU X, et al. miR-21 promotes human nucleus pulposus cell proliferation through PTEN/AKT signaling[J]. Int J Mol Sci, 2014,15(3):4007-4018.] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.WANG F, SHI R, CAI F, et al. Stem cell approaches to intervertebral disc regeneration: obstacles from the disc microenvironment. Stem Cells Dev. 2015;24(21):2479–2495. doi: 10.1089/scd.2015.0158. [YU X, LI Z, SHEN J, et al. MicroRNA-10b promotes nucleus pulposus cell proliferation through RhoC-Akt pathway by targeting HOXD10 in intervetebral disc degeneration[J]. PLoS One, 2013,8(12):e83080.] [DOI] [PubMed] [Google Scholar]
- 39.HE F, PEI M. Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells. Spine (Phila Pa 1976) 2012;37(6):459–469. doi: 10.1097/BRS.0b013e31821fcc64. [WANG F, SHI R, CAI F, et al. Stem cell approaches to intervertebral disc regeneration: obstacles from the disc microenvironment[J]. Stem Cells Dev, 2015,24(21):2479-2495.] [DOI] [PubMed] [Google Scholar]
- 40.YAN N, YU S, ZHANG H, et al. Lumbar disc degeneration is facilitated by miR-100-mediated FGFR3 suppression. Cell Physiol Biochem. 2015;36(6):2229–2236. doi: 10.1159/000430187. [HE F, PEI M. Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells[J]. Spine (Phila Pa 1976), 2012,37(6):459-469.] [DOI] [PubMed] [Google Scholar]
- 41.JING W, JIANG W. MicroRNA-93 regulates collagen loss by targeting MMP3 in human nucleus pulposus cells. Cell Prolif. 2015;48(3):284–292. doi: 10.1111/cpr.2015.48.issue-3. [YAN N, YU S, ZHANG H, et al. Lumbar disc degeneration is facilitated by miR-100-mediated FGFR3 suppression[J]. Cell Physiol Biochem, 2015,36(6):2229-2236.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.GU S X, LI X, HAMILTON J L, et al. MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disc. Gene. 2015;555(2):80–87. doi: 10.1016/j.gene.2014.10.024. [JING W, JIANG W. MicroRNA-93 regulates collagen loss by targeting MMP3 in human nucleus pulposus cells[J]. Cell Prolif, 2015,48(3):284-292.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MOLINOS M, ALMEIDA C R, CALDEIRA J, et al. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12(104):20141191. doi: 10.1098/rsif.2014.1191. [GU S X, LI X, HAMILTON J L, et al. MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disc[J]. Gene, 2015,555(2):80-87.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.PENG Y, LV F J. Symptomatic versus asymptomatic intervertebral disc degeneration: is inflammation the key? Crit Rev Eukaryot Gene Expr. 2015;25(1):13–21. doi: 10.1615/CritRevEukaryotGeneExpr.v25.i1. [MOLINOS M, ALMEIDA C R, CALDEIRA J, et al. Inflammation in intervertebral disc degeneration and regeneration[J]. J R Soc Interface, 2015,12(104):20141191.] [DOI] [PubMed] [Google Scholar]
- 45.NEIDLINGER-WILKE C, BOLDT A, BROCHHAUSEN C, et al. Molecular interactions between human cartilaginous endplates and nucleus pulposus cells: a preliminary investigation. Spine (Phila Pa 1976) 2014;39(17):1355–1364. doi: 10.1097/BRS.0000000000000372. [PENG Y, LV F J. Symptomatic versus asymptomatic intervertebral disc degeneration: is inflammation the key?[J]. Crit Rev Eukaryot Gene Expr, 2015,25(1):13-21.] [DOI] [PubMed] [Google Scholar]
- 46.程 细高, 贾 惊宇, 吴 添龙, et al. miR-140-5P参与调控颈椎软骨终板退变. http://www.cnki.com.cn/Article/CJFDTOTAL-JXYB201405002.htm. 南昌大学学报(医学版) 2014;(5):1–5. [NEIDLINGER-WILKE C, BOLDT A, BROCHHAUSEN C, et al. Molecular interactions between human cartilaginous endplates and nucleus pulposus cells: a preliminary investigation[J]. Spine (Phila Pa 1976), 2014,39(17):1355-1364.] [Google Scholar]
- 47.YU C, CHEN W P, WANG X H. MicroRNA in osteoarthritis. J Int Med Res. 2011;39(1):1–9. doi: 10.1177/147323001103900101. [CHENG Xigao, JIA Jingyu, WU Tianlong, et al. Involvement of miR-140-5P in cartilaginous endplate degeneration in cervical vertebrate[J]. Journal of Nanchang University(Medical Sciences), 2014(5):1-5. (in Chinese)] [DOI] [PubMed] [Google Scholar]
- 48.PIERREFITE-CARLE V, SANTUCCI-DARMANIN S, BREUIL V, et al. Autophagy in bone: self-eating to stay in balance. Ageing Res Rev. 2015;24(Pt B):206–207. doi: 10.1016/j.arr.2015.08.004. [YU C, CHEN W P, WANG X H. MicroRNA in osteoarthritis[J]. J Int Med Res, 2011,39(1):1-9.] [DOI] [PubMed] [Google Scholar]
- 49.XU K, CHEN W, WANG X, et al. Autophagy attenuates the catabolic effect during inflammatory conditions in nucleus pulposus cells, as sustained by NF-kappaB and JNK inhibition. Int J Mol Med. 2015;36(3):661–668. doi: 10.3892/ijmm.2015.2280. [PIERREFITE-CARLE V, SANTUCCI-DARMANIN S, BREUIL V, et al. Autophagy in bone: self-eating to stay in balance[J]. Ageing Res Rev, 2015,24(Pt B):206-217.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.JIANG L, YUAN F, YIN X, et al. Responses and adaptations of intervertebral disc cells to microenvironmental stress: a possible central role of autophagy in the adaptive mechanism. Connect Tissue Res. 2014;55(5-6):311–321. doi: 10.3109/03008207.2014.942419. [XU K, CHEN W, WANG X, et al. Autophagy attenuates the catabolic effect during inflammatory conditions in nucleus pulposus cells, as sustained by NF-kappaB and JNK inhibition[J]. Int J Mol Med, 2015,36(3):661-668.] [DOI] [PubMed] [Google Scholar]
- 51.SU M, WANG J, WANG C, et al. MicroRNA-221 inhibits autophagy and promotes heart failure by modulating the p27/CDK2/mTOR axis. Cell Death Differ. 2015;22(6):986–999. doi: 10.1038/cdd.2014.187. [JIANG L, YUAN F, YIN X, et al. Responses and adaptations of intervertebral disc cells to microenvironmental stress: a possible central role of autophagy in the adaptive mechanism[J]. Connect Tissue Res, 2014,55(5-6):311-321.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ZHANG X, SHI H, LIN S, et al. MicroRNA-216a enhances the radiosensitivity of pancreatic cancer cells by inhibiting beclin-1-mediated autophagy. Oncol Rep. 2015;34(3):1557–1564. doi: 10.3892/or.2015.4078. [SU M, WANG J, WANG C, et al. MicroRNA-221 inhibits autophagy and promotes heart failure by modulating the p27/CDK2/mTOR axis[J]. Cell Death Differ, 2015,22(6):986-999.] [DOI] [PubMed] [Google Scholar]
- 53.WANG I K, SUN K T, TSAI T H, et al. MiR-20a-5p mediates hypoxia-induced autophagy by targeting ATG16L1 in ischemic kidney injury. Life Sci. 2015;136:133–141. doi: 10.1016/j.lfs.2015.07.002. [ZHANG X, SHI H, LIN S, et al. MicroRNA-216a enhances the radiosensitivity of pancreatic cancer cells by inhibiting beclin-1-mediated autophagy[J]. Oncol Rep, 2015,34(3):1557-1564.] [DOI] [PubMed] [Google Scholar]