Abstract
The vif gene of human immunodeficiency virus type 1 (HIV-1) is essential for the productive infection of primary blood-derived lymphocytes, macrophages, and certain human T-cell lines. It has been shown that Vif is associated with HIV-1 virions purified by sucrose density-equilibrium gradient analysis. However, the specificity of Vif incorporation into virions has not been determined. Moreover, recent studies have demonstrated that standard HIV-1 particle preparations created with sucrose density-equilibrium gradients are contaminated with cell-derived microvesicles. Here we demonstrate, as previously reported, that Vif cosediments with HIV-1 particles in sucrose density-equilibrium gradient analysis. However, we also found that, when Vif was expressed in the absence of all other HIV-1-encoded gene products and then isolated by sucrose density-equilibrium gradient centrifugation from extracellular supernatants, its sedimentation pattern was largely unaltered, suggesting that Vif can be secreted from cells. Using a newly developed OptiPrep velocity gradient method, we were able to physically separate most of the extracellular Vif from the HIV-1 virions without disrupting the infectivity of the virus. By titrating serial dilutions of purified Vif and Gag against the viral peak fraction in the OptiPrep gradient, we demonstrate that <1.0 Vif molecule per virion was present. This study shows that Vif is not significantly present in HIV-1 virions, a finding which is consistent with the idea that Vif functions predominantly in the virus-producing cells during virus assembly. The OptiPrep velocity gradient technique described here could be an easy and rapid way to purify HIV and other enveloped viruses from microvesicles and/or cell debris.
Retroviral Gag proteins, which constitute the primary structural components of the virus, are sufficient to drive viral particle assembly (30, 60). In addition to the Gag proteins, the surface virus glycoproteins, or envelope (Env) proteins, are embedded in the membrane of the viral particles and are necessary for fusion with and entry into new target cells (40). In the case of human immunodeficiency virus type 1 (HIV-1), Env is likely to be incorporated into virions by association of its cytoplasmic tail with the matrix protein (MA) of Gag (12, 14, 15, 20, 61, 62). Proteolytic processing of viral gene products, reverse transcription of the viral genome, and integration of proviral DNA into newly infected cellular chromosomes are achieved by the gene products encoded by the Pol open reading frame. Pol exists as a fusion protein with Gag prior to proteolytic cleavage and by virtue of the Gag-Gag association is included in virions (30, 60).
In addition to Gag, Pol, and Env, which are the fundamental proteins that constitute all retroviral particles, the virus-encoded molecules Vpr (11, 38, 45, 63, 65) and Nef (44, 59) have been shown to be present in HIV-1 virions. It has previously been reported that the p6 domain of Gag is critical for the incorporation of Vpr into particles (33, 37, 45). It has been suggested that Vpr acts during the early stage of the virus life cycle to enhance the nuclear import of the preintegration complex (46, 57) as well as serving as a global transcription transactivator (25). Although it has been suggested that the virus-encoded protease may account for the presence of a cleaved form of Nef in virions (44, 59), no molecular determinant has been identified for its incorporation into virions. It has also been proposed that Nef plays a role in modulating the infectivity of the virus upon entry (2).
Certain cellularly encoded molecules are also present in viral particles, including Cyclophilin A, HLA class I, HLA-DR, β2 microglobulin (β2m), and cytoskeletal proteins. Cyclophilin A was first shown to interact with HIV-1 Gag in a yeast two-hybrid screen (39). Additionally, Cyclophilin A could be found in significant quantities in purified HIV-1 virions (19, 42, 56); its binding site was mapped to the N terminus of CAp24 (22). Moreover, the use of the immunosuppressive drug cyclosporin, which binds Cyclophilin, could impair the replication of HIV-1 at a step prior to the initiation of reverse transcription (9). The biological significance of HLA class I, HLA-DR, and β2m on the surface of viral particles has been suggested by studies using antibodies directed against these molecules to suppress the replication of HIV-1 and simian immunodeficiency virus (3). Additionally, the cytoskeletal proteins actin, ezrin, moesin, and cofilin have been localized to the interior of HIV-1 virions (43), and actin has been found to bind Gag in vitro (48).
Lentiviruses encode several regulatory proteins that are involved in modulating virus replication, one of which is Vif (31, 34, 54). Studies using HIV-1 vif mutants revealed that Vif was essential for replication in nonpermissive cells such as primary blood-derived lymphocytes and CD4+ H9 cells (6, 13, 16, 17, 21, 50, 51, 53, 55, 58). Vif functions during the late stage of virus maturation. Studies have suggested several regulatory roles for Vif, including the modulation of proviral DNA synthesis (26, 55, 58), involvement in proper viral core structure in released virions (6, 29), and regulation of the efficiency of Env protein incorporation into released virions (6, 50). More recent studies have shown that vif mutant virions are blocked at a step prior to the initiation of reverse transcription, suggesting a role for Vif in the formation of infectious virions in virus-producing cells (6, 13). It has also been suggested that Vif plays a role in stabilizing the nucleoprotein complex upon entry into target cells (53). Additionally, some investigators have suggested that vif mutant virions display aberrant Gag cleavage (6, 51), while others have found no significant differences in the protein composition of the vif mutant compared to that of wild-type viruses (8, 18, 41, 58). Finally, several reports have indicated the presence of Vif in virions, although no molecular determinant for Vif incorporation has been found (6, 10, 18, 32, 36).
Two recent studies have shown that standard sucrose gradient-purified HIV-1 particles cosediment with cell-derived microvesicles (5, 24). Both cytosolic and membrane-embedded proteins, as well as cellular RNA, were present in extracellular vesicles. These reports raise questions as to what molecules make up true virion-containing components. In light of these data, we reexamined the question of Vif’s presence in virions. By generating highly purified virions, our results reveal a virtual absence of Vif in HIV-1 particles.
MATERIALS AND METHODS
DNA constructs.
The parental virus used for this study was derived from the HXB2 clone (47). The EcoRI site in the cellular flanking region distal to the 3′ end of the viral genome was destroyed by digestion with XbaI and treatment with mung bean nuclease to create blunted ends, followed by ligation with T4 ligase. A ClaI site was created in the nef coding region (downstream from the env coding region) with a mutagenic oligonucleotide, 5′-TTT-TGC-TAT-AAC-ATC-GAT-GGC-AAG-TGG-TCA-3′ (restriction site underlined). Mutagenesis was performed according to the protocol of the manufacturer (Bio-Rad, Richmond, Calif.). The neomycin phosphotransferase gene (neoR) was amplified by PCR from pLXSN (23) with primers that incorporated ClaI and XhoI sites at the 5′ and 3′ ends, respectively, of neoR. PCR was performed according to conditions recommended by Perkin-Elmer with the primers 5′-ATGAGGATCGATCGATATGA TTGAACAAGA-3′ and 5′-AACCCCAGAGCTCGAGTCAGAAGAACTCGT-3′ (restriction sites underlined). PCR-amplified neoR was then digested with ClaI and XhoI and cloned into the modified HXB2 construct at the ClaI and XhoI sites of nef, generating HXB2NEO.
Cloning of the His-tagged Vif-encoding construct was performed by PCR amplification of Vif from the HXB2 clone. EcoRI sites were incorporated into the primers to flank Vif: 5′-AAGATCATTAGAATTCATGGAAAACAGATG-3′ and 5′-AGCTCCTCTAGAATTCCTAGTGTCCATTCA-3′ (restriction sites underlined). PCR-amplified Vif was then digested with EcoRI and cloned into pRSET B (Invitrogen, Carlsbad, Calif.) at the EcoRI site. The orientation of the PCR product in pRSET B was determined by DNA sequencing.
Cells, DNA transfection, and infection.
COS-7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum and antibiotics and passaged upon confluence. H9 cell lines were grown in RPMI 1640 with 10% fetal bovine serum, antibiotics, and G418 and maintained at a density of <1 × 106 cells per ml. Cell culture reagents were obtained from Life Technologies, Gaithersburg, Md.
COS-7 cells were transfected by the DEAE-dextran method. Briefly, COS-7 cells were trypsinized and seeded at 50% confluence 24 h prior to transfection. Cells (5 × 106) were then trypsinized, pelleted, and resuspended in 1 ml of TD buffer (25 mM Tris-HCl [pH 7.4], 140 mM NaCl, 5 mM KCl, 0.7 mM K2HPO4) containing 500 μg of DEAE-dextran and 5 μg of HXB2NEO or pNL4.3 DNA (1). Transfection was carried out at 37°C for 30 min; cells were washed in 5 ml of complete medium and reseeded in T-75 flasks.
Cell culture supernatants containing viral particles were harvested 3 days after transfection, precleared by centrifugation in a Sorvall RT 6000B centrifuge at 3,000 rpm for 30 min, filtered through a 0.2-μm-pore-size membrane, and used to infect H9 cell lines. At 24 h postinfection, cells were grown in the presence of 1.2 mg of G418 per ml for at least 2 weeks prior to analysis of cellular and viral protein profiles to ensure that all surviving cells contained viral genomes. Production of H9(LVifSN) cell lines has been described previously (58). H9 cell lines producing HIV-1 protease mutant (Pr−) virus (Pr−/H9) and HIV-1 pol deletion mutant virus (ΔPol/H9) have also been described previously (35). Pr55Gag was obtained from Gag particles produced by ΔPol/H9 cells, and the protein concentration was determined by Coomassie blue staining on sodium dodecyl sulfate (SDS) polyacrylamide gels against known standards.
RT assay and p24 ELISA.
Cell culture supernatants were cleared of cells and cellular debris by centrifugation in a Sorvall MC 12V centrifuge at 14,000 rpm for 2 min. For each sample, 250 μl of culture supernatant was mixed with 125 μl of 30% polyethylene glycol 8000–0.5 M NaCl at 4°C overnight. The samples were centrifuged at 2,500 rpm for 30 min (Sorvall RT 6000B centrifuge), viral pellets were dissolved in 25 μl of reverse transcriptase (RT) lysis buffer (1% Triton X-100, 20 mM Tris-HCl [pH 7.5], 60 mM KCl, 1 mM dithiothreitol, 30% glycerol), and 10 μl of each sample was used for the RT reaction. Viral lysates were combined with 90 μl of RT reaction cocktail {40 mM Tris-HCl [pH 7.8], 8 mM dithiothreitol, 10 mM MgCl2, 0.05 A260 unit of poly(rA)-(dT)15 [Boehringer Mannheim], 2.5 μCi of [3H]dTTP} at 37°C for 2 h. The reaction product was precipitated with 3 ml of chilled 10% (wt/vol) trichloroacetic acid, with tRNA as a carrier. Incorporation of [3H]dTTP was determined on the basis of material bound to GF/C glass microfiber filters following five washes with chilled 5% trichloroacetic acid and quantified with a Beckman LS 6500 scintillation counter. For gradient fractions, 50 μl of each sample was diluted with 200 μl of phosphate-buffered saline (PBS) and mixed with 125 μl of 30% polyethylene glycol 8000–0.5 M NaCl at 4°C overnight. Samples were then centrifuged and resuspended in RT lysis buffer, and reactions were conducted as described above. The p24 enzyme-linked immunosorbent assay (ELISA) was performed as described by the manufacturer (DuPont, Wilmington, Del.).
Immunoblotting.
Virion-associated viral proteins were prepared from cell culture supernatants by removal of cellular debris by centrifugation at 3,000 rpm for 30 min in a Sorvall RT 6000B centrifuge and were then filtered through a 0.2-μm-pore-size membrane. Virus-particle-containing supernatants were concentrated by centrifugation through a 20% sucrose cushion at 100,000 × g for 2 h in a Sorvall Ultra80 centrifuge. Viral pellets were resuspended in PBS. Cell-associated viral proteins were analyzed from chronically infected H9 cell lines. Cells (105) were lysed in 1× loading dye (0.08 M Tris [pH 6.8], 2.0% SDS, 10% glycerol, 0.1 M dithiothreitol, 0.2% bromophenol blue). Samples were boiled for 10 min, and proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to two separate nitrocellulose membranes by passive diffusion for 48 h, producing identical mirror-image blots. Membranes were probed with either HIV-1-positive patient serum (1:200) or polyclonal serum against Vif (1:1,000), Nef (1:1,000), Cyclophilin A (1:1,000 [Affinity Bioreagents, Golden, Colo.]), or p6Gag (1:1,000). Generation of p6Gag antiserum has been described previously (64). Vif, Nef, and Cyclophilin A proteins were detected using by enhanced chemiluminescence (Amersham Life Science, Arlington Heights, Ill.), whereas other proteins were detected by an alkaline phosphatase reaction.
Sucrose and OptiPrep gradients.
Once viral supernatants were filtered through 20% sucrose and pelleted, they were resuspended in 200 μl of PBS and centrifuged in either a sucrose equilibrium gradient or an OptiPrep (60% [wt/vol] iodixanol; Life Technologies) velocity gradient. Sucrose gradients were prepared in PBS as 11 steps in 4% increments ranging from 20 to 60%. Virions were layered onto the top of the gradient and centrifuged for 14 h at 100,000 × g in an SW41 Ti rotor. Iodixanol gradients were prepared in PBS as 11 steps in 1.2% increments ranging from 6 to 18%. Virions were layered onto the top of the gradient and centrifuged for 1.5 h at 250,000 × g in an SW41 Ti rotor. Gradient fractions were collected from the top of the gradient and were either assayed for RT activity or analyzed for protein content. Densities of fractions were determined by weight of a 100-μl sample on an analytical balance. Gradient samples were precipitated with trichloroacetic acid, resuspended in 1× loading dye, and subjected to SDS-PAGE, followed by either Coomassie blue staining or immunoblot analysis.
Expression and purification of recombinant Vif.
His-tagged Vif was expressed and purified as described by the manufacturer (Invitrogen). Briefly, Vif-His was overexpressed in Escherichia coli BL21 and purified by Ni2+-affinity chromatography in the presence of 6 M guanidine hydrochloride. Column elutions were performed in 8 M urea by sequential reduction in pH. Concentrations of protein were determined by Coomassie blue staining on SDS-polyacrylamide gels against known standards.
RESULTS
Detection of extracellular Vif by sedimentation in a sucrose density-equilibrium gradient.
In order to examine whether the formation of viral particles is required for the presence of extracellular Vif, we utilized an H9 cell line which expresses Vif in the absence of all other HIV-1 gene products (58). We compared the gene expression pattern of H9 cells chronically infected with the HXB2-derived virus with that of H9 cells stably transfected with a Vif expression vector (Fig. 1). The HXB2-infected cells expressed Pr55Gag, Pr41Gag, and CAp24 (Fig. 1A), as well as Vif (Fig. 1B), whereas the H9(LVifSN) cell line expressed Vif (Fig. 1B) but not other viral proteins (Fig. 1A).
FIG. 1.
Protein expression pattern in HIV-1-infected and Vif-expressing cell lines. Cell lysates from mock-infected, HXB2NEO, and H9(LVifSN) cell lines were separated by SDS–12% polyacrylamide PAGE and stained with anti-HIV-1-positive serum (A) or anti-Vif serum (B).
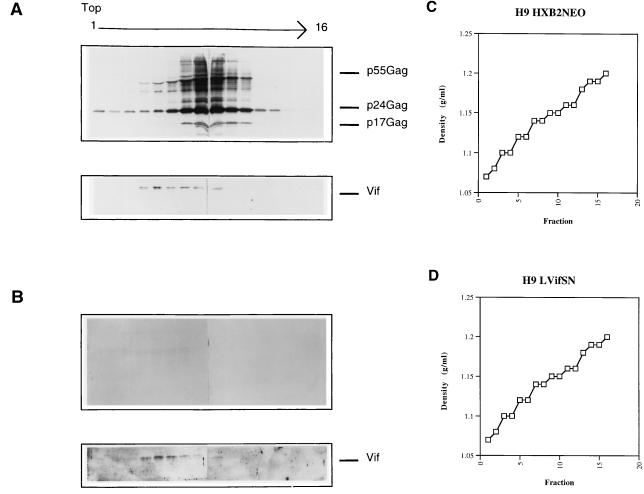
We next examined the extracellular sedimentation pattern of Vif relative to that of the viral Gag proteins in sucrose density-equilibrium gradients. Cell culture supernatants were harvested from H9 cells chronically infected with HXB2. Following centrifugation to remove large cellular debris, culture supernatants were passed through a 0.2-μm-pore-size filter and then pelleted through a 20% sucrose cushion. The pelleted material was then subjected to ultracentrifugation for 14 h through a 20 to 60% sucrose gradient. The sedimentation patterns from the HXB2-infected cells showed that Gag proteins peaked in fractions 8 and 9 (Fig. 2A), corresponding to densities of 1.14 to 1.16 g/ml (Fig. 2C). When the sedimentation pattern of Vif was examined from these culture supernatants, it was found in fractions 4 to 9 (Fig. 2A), corresponding to densities of 1.09 to 1.16 g/ml (Fig. 2C). Although Vif sedimentation overlaps with that of the virus particle, it also tends to be present in fractions which are less dense than those with mature virions.
FIG. 2.
Sucrose density-equilibrium gradient analysis of extracellular supernatants from HXB2NEO and H9(LVifSN) cell lines. Extracellular supernatants were pelleted through 20% sucrose and then subjected to sucrose density-equilibrium gradient centrifugation in 20 to 60% sucrose. Fractions were collected, with fraction 1 referring to the top of the gradient, as indicated; the protein profiles were analyzed by Western blotting. Supernatants from HXB2NEO H9 cells (A) and H9(LVifSN) (B) were stained with anti-HIV-1-positive serum (top panels) or anti-Vif serum (bottom panels). Gradient fraction densities are given for HXB2NEO (C) and H9(LVifSN) (D) supernatants.
We then examined the sedimentation pattern of the cell culture supernatants from the cell line expressing Vif in the absence of other HIV-1 gene products. We observed that Vif sedimented in fractions 4 to 9 (Fig. 2B) in the 20 to 60% sucrose gradient with a density (1.09 to 1.16 g/ml [Fig. 2D]) similar to that of Vif from HIV-1-infected cells (Fig. 2C). These results indicate that Vif is present in extracellular supernatants irrespective of the presence of budding virions. Additionally, Vif is present in association with material sufficiently dense to be pelleted through 20% sucrose but has a broader yet overlapping density range with viral particles. These data suggest that some extracellular Vif may be contained in microvesicles, which have been previously described (5, 24).
Development of an OptiPrep velocity gradient technique to separate extracellular proteins from virions.
In order to determine whether Vif is a component of viral particles or contained in microvesicles, it was necessary to physically separate them. We then developed and evaluated a velocity-gradient-based method for this purpose. A velocity gradient was used for this study instead of a density gradient because previous observations suggested that microvesicles and HIV-1 virions have a similar density (5, 24). OptiPrep was selected for this purpose because it has been used to separate different intracellular membrane compartments such as Golgi, lysosomes, mitochondria, and peroxisomes (28). Unlike sucrose, OptiPrep can form iso-osmotic solutions at all densities and therefore can maintain the sizes of original membrane organelles, allowing better separation.
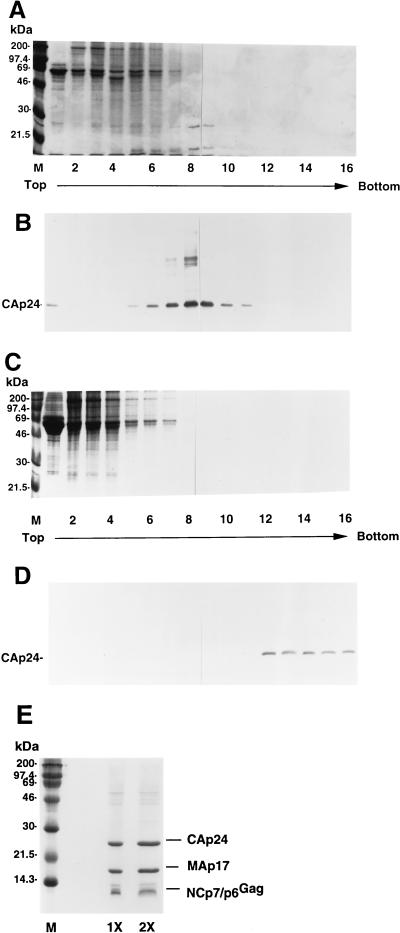
The distribution of extracellular proteins from culture supernatant in a typical 20 to 60% sucrose gradient was determined by staining proteins from each gradient fraction with Coomassie blue (Fig. 3A). The distribution of extracellular viral proteins in the same gradient was determined by immunoblotting with an HIV-1-positive human serum (Fig. 3B). Most of the extracellular non-viral proteins were found in fractions 1 to 7 (Fig. 3A). Viral Gag proteins peaked in fractions 7 through 9 (Fig. 3B).
FIG. 3.
Comparison of sedimentation patterns of extracellular nonviral and viral proteins by sucrose density-equilibrium gradient or OptiPrep gradient analysis. Culture supernatants from HXB2NEO H9 cells were first concentrated by being pelleted through a 20% sucrose cushion and then subjected to a 20 to 60% sucrose density-equilibrium gradient centrifugation (A and B) or a 6 to 18% iodixanol velocity gradient centrifugation (C and D). Fractions were collected, with fraction 1 referring to the top of the gradient, as indicated; the protein profiles were analyzed by Coomassie blue staining (A and C) or immunoblotting with an HIV-1-positive serum (B and D). (E) Proteins from fraction 13 of the OptiPrep velocity gradient were separated by SDS-PAGE and stained with Coomassie blue. Lanes 1X and 2X represent 2.5 μg and 5.0 μg of CAp24 viral equivalents, respectively.
When the similar starting materials used in the sucrose gradient were analyzed on an OptiPrep (6 to 18% iodixanol) velocity gradient, most of the extracellular proteins were present in fractions 1 to 7, as detected by Coomassie blue staining (Fig. 3C). On the other hand, most of the viral Gag proteins sedimented in fractions 12 to 16 (Fig. 3D), as detected by the HIV-1-positive human serum. Although some of the extracellular proteins (nonviral) overlap with viral proteins in the sucrose gradient, there is little overlap between the extracellular proteins (nonviral) and the viral proteins in the OptiPrep gradient. Analysis of virions obtained from the peak fractions of an OptiPrep velocity gradient indicated that viral Gag proteins CAp24, MAp17, and NCp7/p6Gag contributed more than 80% of the total protein content (Fig. 3E). This is in sharp contrast to virions purified by sucrose gradient (5) or gel filtration (49), in which viral products account for 40% or less of the total protein.
Analysis of extracellular Vif by the OptiPrep velocity gradient method.
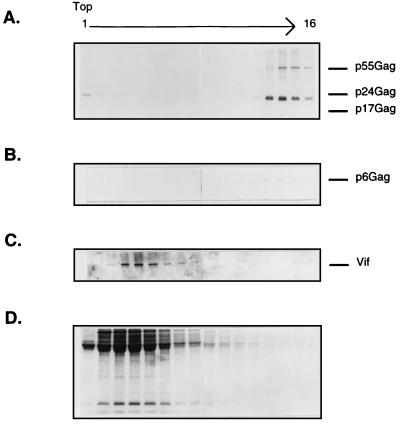
By the OptiPrep velocity gradient method, the sedimentation pattern of extracellular Vif in relationship to that of other viral proteins was examined. Analysis of HXB2-derived virions in 6 to 18% iodixanol gradients revealed a protein sedimentation pattern in which the majority of Vif was physically separated from the HIV-1 Gag proteins (Fig. 4A to C). The viral Gag proteins sedimented in fractions 12 to 16, whereas Vif was present in fractions 2 to 7. Additionally, the RT activity peaked in fraction 13, which overlaps with the peak of CAp24, while no RT activity was detected in the fractions containing Vif (Fig. 4E). The sedimentation profiles of the extracellular proteins in this OptiPrep gradient were studied by SDS-PAGE and stained with Coomassie blue (Fig. 4D). The majority of these proteins were nonviral and were present in fractions 2 to 7, which overlap with fractions where Vif was found.
FIG. 4.
OptiPrep gradient analysis of extracellular Vif from HXB2NEO H9 cells. Extracellular supernatants were pelleted through a 20% sucrose cushion and then subjected to velocity gradient centrifugation in 6 to 18% iodixanol. Fractions were collected, with fraction 1 referring to the top of the gradient, as indicated; the protein profiles were analyzed by immunoblotting with an HIV-1-positive serum (A), anti-p6Gag serum (B), and anti-Vif serum (C). Aliquots of OptiPrep gradient fractions were stained with Coomassie blue (D) and assayed for RT activity (E) and infectivity on SupT1 cells by syncytium formation (F).
We then assessed whether the gradient conditions disrupted viral infectivity. Aliquots from each gradient fraction were used to initiate a syncytium formation assay on SupT1 cell lines (Fig. 4F). Fractions 12 to 16 yielded syncytia within 3 to 5 days, which demonstrates the functional integrity of these viral particles. In contrast, peak Vif-containing fractions failed to produce syncytia. These data show that the majority of extracellular Vif fails to associate with infectious virions.
Analysis of virion-associated proteins.
Since the majority of the extracellular Vif appeared to be excluded from virions, we next examined the presence of other known virion-associated proteins. OptiPrep-gradient-purified virions derived from H9 cells acutely infected with the NL4.3 strain of HIV-1 were analyzed for incorporation of Nef and Cyclophilin A. Peak gradient fractions were collected and analyzed by immunoblotting (Fig. 5). Both Nef and Cyclophilin A had sedimentation patterns that overlapped with those of the virion Gag proteins, whereas Vif was not detected (Fig. 5D).
FIG. 5.
Virion-associated protein composition from the OptiPrep gradient. Peak fractions were separated by SDS-PAGE and immunoblotted with an HIV-1-positive serum (A), anti-Nef serum (B), anti-Cyclophilin A serum (C), and anti-Vif serum (D).
Quantitation of Vif in purified virions.
Previous reports have shown the number of Vif molecules that are present in virions to range from 7 to 100 per particle (10, 18, 36). By separating infectious virions from the majority of the extracellular Vif, we were able to determine the number of Vif molecules present in highly purified virions. Viral particles from fraction 13 of an OptiPrep velocity gradient were quantitated by p24 ELISA, proteins were separated by SDS-PAGE, and their concentrations were compared with known concentrations of CAp24 and recombinant Vif. After electrophoresis, proteins were allowed to passively diffuse onto two separate membranes, generating identical mirror-image blots from the same gel, which were then probed with either anti-HIV-1 or anti-Vif antiserum (Fig. 6A). Purified virus consisting of 3,000 ng of CAp24 yielded <0.63 ng of Vif. After the weights of Gag and Vif were converted to numbers of molecules, and assuming an average of 2,750 CA molecules per virion, the number of Vif molecules per virion was determined to be 0.70, or one Vif molecule per 4,000 Gag molecules. These purified virions were subsequently shown to be infectious in H9 cell lines (data not shown).
FIG. 6.
Quantitation of Vif in OptiPrep-gradient-purified virions. (A) H9-derived HXB2NEO virions from fraction 13 of the OptiPrep velocity gradient were separated by SDS-PAGE and analyzed by immunoblotting. p24Gag and Vif-His proteins, shown in nanogram quantities, were run side by side with OptiPrep-velocity-gradient-purified HIV-1 (3,000 ng of p24Gag) on SDS–12% polyacrylamide PAGE. (B) H9-derived protease mutant (Pr−) virions from fraction 13 of the OptiPrep velocity gradient were separated by SDS-PAGE and analyzed by immunoblotting. P55Gag and Vif-His proteins, shown in nanogram quantities, were run side by side with OptiPrep-velocity-gradient-purified HIV-1 (3,000 ng of p24Gag) on SDS–12% polyacrylamide PAGE. Protein profiles were analyzed by immunoblotting with anti-HIV-1-positive serum (top panels) and anti-Vif serum (bottom panels).
We further examined the quantities of Vif in immature virions by use of a viral protease mutant. Virions from Pr−/H9 cell culture supernatants were purified by the OptiPrep gradient method. Viral proteins were separated by SDS-PAGE, and the concentrations were compared with known concentrations of p55Gag and recombinant Vif as standards (Fig. 6B). Purified Pr− virus, consisting of 3000 ng of p55Gag, revealed <0.31 ng of Vif. Using an estimation similar to that for the wild-type virus, we found there to be 0.74 Vif molecule per virion, or one Vif molecule per 3,700 Gag molecules.
DISCUSSION
Previous reports have demonstrated that microvesicles are major contaminants in preparations of HIV-1 particles (5, 24). Cell-derived RNA as well as protein cosediments with HIV-1 particles in conventional sucrose density-equilibrium gradients. In this report, we have developed a velocity-gradient-based method for purification of HIV-1 virions and compared it to the routine sucrose density-equilibrium gradient method. Since microvesicles have a density similar to that of HIV-1 virions (5, 24), a velocity gradient is more likely to separate microvesicles from virions. OptiPrep (60% iodixanol in water) was used instead of sucrose because it offers several advantages. OptiPrep can form iso-osmotic solutions at all densities (up to 1.32 g/ml), whereas the osmolality increases as the density of sucrose increases. Hyperosmotic conditions may lead to the loss of water from organelles, vesicles, and virions and therefore reduce the power of separation. OptiPrep is less viscous than sucrose and requires less sedimentation time for separation. Iodixanol was developed as an X-ray contrast medium for injection into humans, and the lack of toxicity to a variety of biological materials has been confirmed after rigorous testing (28).
By comparing the sucrose density-equilibrium gradient and the OptiPrep velocity gradient, we found that the OptiPrep velocity gradient tends to separate HIV-1 virions away from most of the extracellular nonviral proteins more effectively than the routine sucrose density-equilibrium gradient (Fig. 3). Therefore, virions obtained by OptiPrep velocity gradient fractionation are less likely to be contaminated with nonviral extracellular proteins than the routine sucrose density-equilibrium gradient method. We have also found that extracellular Vif sediments in fractions in the sucrose density-equilibrium gradient which overlap with those of viral particles irrespective of the expression of all other HIV-1 gene products (Fig. 2). Utilization of the OptiPrep velocity gradient instead of the conventional sucrose density-equilibrium gradient permitted the separation of most extracellular Vif from viral particles. This new technique for purification of HIV-1 may represent a rapid and easy method to separate the majority of the nonviral extracellular molecules from infectious virions.
Previous studies have claimed that the HIV-1-encoded molecule, Vif, copurifies with viral particles (6, 10, 18, 32, 36). However, to date no structural domain within the viral genome confers specific incorporation. In fact HIV-1 Vif has been shown to be incorporated in murine leukemia virus, which lacks an open reading frame for Vif (10). Results presented here indicated that the vast majority of Vif cosedimented with nonviral extracellular proteins. Quantitatively, it was determined that there is <1 Vif molecule per virion, which raises the question of its functional significance in particles. On the other hand, significant amounts of Cyclophilin A and Nef cosedimented with infectious virions, suggesting that these molecules are components of HIV-1 particles (Fig. 5). In light of these data, it would be difficult to envision Vif functioning directly within viral particles or during the early stages in newly infected target cells. It remains more plausible that Vif influences the infectivity of virions from its effects in the virus-producing cells.
The reported discrepancies in the amount of Vif cosedimenting with virions, 7 to 100 molecules of Vif per virion (10, 18, 36), may be accounted for in part by the number of CA molecules each investigator assigns to a given viral particle, 1,500 to 2,750. In this report we assumed 2,750 CA per virion to calculate the level of Vif virion incorporation. An additional factor may be the varying numbers and sizes of microvesicles released from the cell surface at any given time. More numerous and larger budding vesicles may produce more Vif in the extracellular supernatants. It should be considered that if there were a viral determinant for incorporation of Vif into virions, the amount of Vif in particles should be constant. Since trace amounts of Vif are still present in highly purified particles (Fig. 6A), it remains to be determined whether Vif is actively excluded from viral particles or is only randomly incorporated into virions at low concentrations.
It has been suggested that Vif both colocalizes with (52) and binds directly to (7) Pr55Gag within infected cells. This notion seems intriguing since Vif appears to regulate steps in reverse transcription (6, 13, 26, 55, 58), which can be influenced by NCp7 (4). Also, if Vif were regulating early steps in the penetration of the core into newly infected cells (53), it could be doing so by modulating the Gag molecules. However, since Vif does not appear to be present within virions in significant amounts, its association with Gag suggests a number of possibilities. Vif may associate with Pr55Gag in the cell and fail to bind the fully cleaved form of Gag. However, this possibility is less likely, since no significant enhancement of virion incorporation of Vif was observed in the case of a protease mutant virus (Fig. 6B). Alternatively, the association of Vif with Gag may be transient in the cell and may involve additional molecules, which function as a molecular bridge. This latter notion might be considered since the coimmunoprecipitation of Vif with an anti-NCp7 antibody was performed in the presence of only 0.2% Triton (7).
This study indicates that Vif does not constitute a significant component of mature or immature HIV-1 virions. In light of these data, Vif may be interacting with cellular factors during viral particle formation in virus-producing cells. It has been suggested that Vif colocalizes with vimentin in transfected HeLa cells (32), although these data were not confirmed with HIV-1-infected H9 cells (52). In addition, the C terminus of Vif appears to be critical for its association with canine pancreatic microsomes, suggesting that Vif binds membranes directly (27). These data are consistent with the cosedimentation of Vif with membranes from HIV-1-infected cells (27), even when expressed in the absence of other viral proteins, including Gag (52). In contrast, it appears as though the same C-terminal Vif mutants which disrupt membrane binding also disrupted the ability of Vif to associate with Gag (7). By considering these data, it remains to be determined whether the C terminus of Vif is responsible for its association with Gag, membranes, or other, yet to be determined, factors. Identification of cellular components which interact with Vif will be useful to elucidate its function.
ACKNOWLEDGMENTS
We are grateful to Zene Matsuda, Tun-Hou Lee, and Max Essex for supplying us with the modified HXB2 DNA constructs; Didier Trono for providing the H9(LVifSN) cells; and Richard Markham for critical review of the manuscript.
The following reagents were obtained through the AIDS Research Reference and Reagents Program, Division of AIDS, NIAID, NIH: antiserum to HIV-1 Vif (catalog number 2745), antiserum to HIV-1 Nef (catalog number 2121), and purified p24Gag (catalog number 382). M.D. was supported in part by a training grant from the NIEHS (ES07141).
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur L O, Bess J W, Jr, Sowder R C D, Benveniste R E, Mann D L, Chermann J C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 4.Berthoux L, Pechoux C, Ottmann M, Morel G, Darlix J L. Mutations in the N-terminal domain of human immunodeficiency virus type 1 nucleocapsid protein affect virion core structure and proviral DNA synthesis. J Virol. 1997;71:6973–6981. doi: 10.1128/jvi.71.9.6973-6981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bess J W, Jr, Gorelick R J, Bosche W J, Henderson L E, Arthur L O. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- 6.Borman A M, Quillent C, Charneau P, Dauguet C, Clavel F. Human immunodeficiency virus type 1 Vif− mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J Virol. 1995;69:2058–2067. doi: 10.1128/jvi.69.4.2058-2067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouyac M, Courcoul M, Bertoia G, Baudat Y, Gabuzda D, Blanc D, Chazal N, Boulanger P, Sire J, Vigne R, Spire B. Human immunodeficiency virus type 1 Vif protein binds to the Pr55Gag precursor. J Virol. 1997;71:9358–9365. doi: 10.1128/jvi.71.12.9358-9365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouyac M, Rey F, Nascimbeni M, Courcoul M, Sire J, Blanc D, Clavel F, Vigne R, Spire B. Phenotypically Vif− human immunodeficiency virus type 1 is produced by chronically infected restrictive cells. J Virol. 1997;71:2473–2477. doi: 10.1128/jvi.71.3.2473-2477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bratten D, Franke E K, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camaur D, Trono D. Characterization of human immunodeficiency virus type 1 Vif particle incorporation. J Virol. 1996;70:6106–6111. doi: 10.1128/jvi.70.9.6106-6111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen E A, Dehni G, Sodroski J G, Haseltine W A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosson P. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 1996;15:5783–5788. [PMC free article] [PubMed] [Google Scholar]
- 13.Courcoul M, Patience C, Rey F, Blanc D, Harmache A, Sire J, Vigne R, Spire B. Peripheral blood mononuclear cells produce normal amounts of defective Vif− human immunodeficiency virus type 1 particles which are restricted for the preretrotranscription steps. J Virol. 1995;69:2068–2074. doi: 10.1128/jvi.69.4.2068-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorfman T, Mammano F, Haseltine W A, Gottlinger H G. Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1994;68:1689–1696. doi: 10.1128/jvi.68.3.1689-1696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubay J W, Roberts S J, Hahn B H, Hunter E. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J Virol. 1992;66:6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan L, Peden K. Cell-free transmission of Vif mutants of HIV-1. Virology. 1992;190:19–29. doi: 10.1016/0042-6822(92)91188-z. [DOI] [PubMed] [Google Scholar]
- 17.Fisher A G, Ensoli B, Ivanoff L, Chamberlain M, Petteway S, Ratner L, Gallo R C, Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987;237:888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- 18.Fouchier R A, Simon J H, Jaffe A B, Malim M H. Human immunodeficiency virus type 1 Vif does not influence expression or virion incorporation of gag-, pol-, and env-encoded proteins. J Virol. 1996;70:8263–8269. doi: 10.1128/jvi.70.12.8263-8269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franke E K, Yuan H E, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 20.Freed E O, Martin M A. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J Virol. 1996;70:341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabuzda D H, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine W A, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamble T R, Vajdos F F, Yoo S, Worthylake D K, Houseweart M, Sundquist W I, Hill C P. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- 23.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 24.Gluschankof P, Mondor I, Gelderblom H R, Sattentau Q J. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virology. 1997;230:125–133. doi: 10.1006/viro.1997.8453. [DOI] [PubMed] [Google Scholar]
- 25.Goh W C, Rogel M E, Kinsey C M, Michael S F, Fultz P N, Nowak M A, Hahn B H, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 26.Goncalves J, Korin Y, Zack J, Gabuzda D. Role of Vif in human immunodeficiency virus type 1 reverse transcription. J Virol. 1996;70:8701–8709. doi: 10.1128/jvi.70.12.8701-8709.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goncalves J, Shi B, Yang X, Gabuzda D. Biological activity of human immunodeficiency virus type 1 Vif requires membrane targeting by C-terminal basic domains. J Virol. 1995;69:7196–7204. doi: 10.1128/jvi.69.11.7196-7204.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham J, Ford T, Rickwood D. The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol. Anal Biochem. 1994;220:367–373. doi: 10.1006/abio.1994.1351. [DOI] [PubMed] [Google Scholar]
- 29.Hoglund S, Ohagen A, Lawrence K, Gabuzda D. Role of vif during packing of the core of HIV-1. Virology. 1994;201:349–355. doi: 10.1006/viro.1994.1300. [DOI] [PubMed] [Google Scholar]
- 30.Hunter E. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin Virol. 1994;5:71–83. [Google Scholar]
- 31.Kan N C, Franchini G, Wong-Staal F, DuBois G C, Robey W G, Lautenberger J A, Papas T S. Identification of HTLV-III/LAV sor gene product and detection of antibodies in human sera. Science. 1986;231:1553–1555. doi: 10.1126/science.3006245. [DOI] [PubMed] [Google Scholar]
- 32.Karczewski M K, Strebel K. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein. J Virol. 1996;70:494–507. doi: 10.1128/jvi.70.1.494-507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo E, Mammano F, Cohen E A, Gottlinger H G. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J Virol. 1995;69:2759–2764. doi: 10.1128/jvi.69.5.2759-2764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee T H, Coligan J E, Allan J S, McLane M F, Groopman J E, Essex M. A new HTLV-III/LAV protein encoded by a gene found in cytopathic retroviruses. Science. 1986;231:1546–1549. doi: 10.1126/science.3006243. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y M, Yu X F. Identification and characterization of virus assembly intermediate complexes in HIV-1 infected CD4+ T cells. Virology. 1998;243:78–93. doi: 10.1006/viro.1998.9064. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Wu X, Newman M, Shaw G M, Hahn B H, Kappes J C. The Vif protein of human and simian immunodeficiency viruses is packaged into virions and associates with viral core structures. J Virol. 1995;69:7630–7638. doi: 10.1128/jvi.69.12.7630-7638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y L, Bennett R P, Wills J W, Gorelick R, Ratner L. A leucine triplet repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J Virol. 1995;69:6873–6879. doi: 10.1128/jvi.69.11.6873-6879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y L, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luban J, Bossolt K L, Franke E K, Kalpana G V, Goff S P. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 40.Luciw P A. Human immunodeficiency viruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1881–1952. [Google Scholar]
- 41.Ochsenbauer C, Wilk T, Bosch V. Analysis of vif-defective human immunodeficiency virus type 1 (HIV-1) virions synthesized in ’non-permissive’ T lymphoid cells stably infected with selectable HIV-1. J Gen Virol. 1997;78:627–635. doi: 10.1099/0022-1317-78-3-627. [DOI] [PubMed] [Google Scholar]
- 42.Ott D E, Coren L V, Johnson D G, Sowder R C N, Arthur L O, Henderson L E. Analysis and localization of cyclophilin A found in the virions of human immunodeficiency virus type 1 MN strain. AIDS Res Hum Retroviruses. 1995;11:1003–1006. doi: 10.1089/aid.1995.11.1003. [DOI] [PubMed] [Google Scholar]
- 43.Ott D E, Coren L V, Kane B P, Busch L K, Johnson D G, Sowder R C N, Chertova E N, Arthur L O, Henderson L E. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J Virol. 1996;70:7734–7743. doi: 10.1128/jvi.70.11.7734-7743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandori M W, Fitch N J, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paxton W, Connor R I, Landau N R. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gag and mutational analysis. J Virol. 1993;67:7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popov S, Rexach M, Zybarth G, Reiling N, Lee M A, Ratner L, Lane C M, Moore M S, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratner L, Haseltine W, Patarca R, Livak K J, Starcich B, Josephs S F, Doran E R, Rafalski J A, Whitehorn E A, Baumeister K, et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 48.Rey O, Canon J, Krogstad P. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology. 1996;220:530–534. doi: 10.1006/viro.1996.0343. [DOI] [PubMed] [Google Scholar]
- 49.Richieri S P, Bartholomew R, Aloia R C, Savary J, Gore R, Holt J, Ferre F, Musil R, Tian H R, Trauger R, Lowry P, Jensen F, Carlo D J, Maigetter R Z, Prior C P. Characterization of highly purified, inactivated HIV-1 particles isolated by anion exchange chromatography. Vaccine. 1998;16:119–129. doi: 10.1016/s0264-410x(97)00196-5. [DOI] [PubMed] [Google Scholar]
- 50.Sakai H, Shibata R, Sakuragi J, Sakuragi S, Kawamura M, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1993;67:1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simm M, Shahabuddin M, Chao W, Allan J S, Volsky D J. Aberrant Gag protein composition of a human immunodeficiency virus type 1 vif mutant produced in primary lymphocytes. J Virol. 1995;69:4582–4586. doi: 10.1128/jvi.69.7.4582-4586.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon J H, Fouchier R A, Southerling T E, Guerra C B, Grant C K, Malim M H. The Vif and Gag proteins of human immunodeficiency virus type 1 colocalize in infected human T cells. J Virol. 1997;71:5259–5267. doi: 10.1128/jvi.71.7.5259-5267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon J H, Malim M H. The human immunodeficiency virus type 1 Vif protein modulates the postpenetration stability of viral nucleoprotein complexes. J Virol. 1996;70:5297–5305. doi: 10.1128/jvi.70.8.5297-5305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sodroski J, Goh W C, Rosen C, Tartar A, Portetelle D, Burny A, Haseltine W. Replicative and cytopathic potential of HTLV-III/LAV with sor gene deletions. Science. 1986;231:1549–1553. doi: 10.1126/science.3006244. [DOI] [PubMed] [Google Scholar]
- 55.Sova P, Volsky D J. Efficiency of viral DNA synthesis during infection of permissive and nonpermissive cells with vif-negative human immunodeficiency virus type 1. J Virol. 1993;67:6322–6326. doi: 10.1128/jvi.67.10.6322-6326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh C T, Sodroski J, Gottlinger H G. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 57.Vodicka M A, Koepp D M, Silver P A, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Schwedler U, Song J, Aiken C, Trono D. Vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welker R, Kottler H, Kalbitzer H R, Krausslich H G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral protease. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- 60.Wills J W, Craven R C. Form, function, and use of retroviral gag proteins (editorial) AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Yu X, Yuan X, Matsuda Z, Lee T H, Essex M. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol. 1992;66:4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu X, Yuan X, McLane M F, Lee T H, Essex M. Mutations in the cytoplasmic domain of human immunodeficiency virus type 1 transmembrane protein impair the incorporation of Env proteins into mature virions. J Virol. 1993;67:213–221. doi: 10.1128/jvi.67.1.213-221.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu X F, Matsuda M, Essex M, Lee T H. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J Virol. 1990;64:5688–5693. doi: 10.1128/jvi.64.11.5688-5693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu X F, Matsuda Z, Yu Q C, Lee T H, Essex M. Role of the C terminus Gag protein in human immunodeficiency virus type 1 virion assembly and maturation. J Gen Virol. 1995;76:3171–3179. doi: 10.1099/0022-1317-76-12-3171. [DOI] [PubMed] [Google Scholar]
- 65.Yuan X, Matsuda Z, Matsuda M, Essex M, Lee T H. Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res Hum Retroviruses. 1990;6:1265–1271. doi: 10.1089/aid.1990.6.1265. [DOI] [PubMed] [Google Scholar]