Abstract
Acinetobacter baumannii is an opportunistic human pathogen that has become a global threat to healthcare institutions. This Gram-negative bacterium is one of the most successful human pathogens worldwide and responsible for hospital-acquired infections. This is due to its outstanding potential to adapt to very different environments, to persist in the human host and most important, its ability to develop multidrug resistance. Our combined approach of genomic and phenotypic analyses led to the identification of the envelope spanning Tol-Pal system in A. baumannii. We found that the deletion of the tolQ, tolR, tolA, tolB, and pal genes affects cell morphology and increases antibiotic sensitivity, such as the ∆tol-pal mutant exhibits a significantly increased gentamicin and bacitracin sensitivity. Furthermore, Galleria mellonella caterpillar killing assays revealed that the ∆tol-pal mutant exhibits a decreased killing phenotype. Taken together, our findings suggest that the Tol-Pal system is important for cell morphology, antibiotic resistance, and virulence of A. baumannii.
Keywords: Virulence, Tol-Pal system, Acinetobacter baumannii, Infection
Introduction
The Gram-negative opportunistic human pathogen Acinetobacter baumannii is one of the six most important multidrug-resistant nosocomial pathogens worldwide (Dijkshoorn et al. 2007; Antunes et al. 2014; World Health Organization 2017). One key to its success in hospital environments is its ability to persist and survive within hospital settings (Dijkshoorn et al. 2007). In particular, resistance against a broad range of antibiotics is responsible for the spread of A. baumannii in health care systems around the world (Dijkshoorn et al. 2007; Göttig et al. 2014). The increase in multidrug or even pan drug resistant A. baumannii strains is due to the acquisition of resistance mechanisms such as hydrolyzing enzymes (e.g., β-lactamases) and efflux pumps (Dijkshoorn et al. 2007; Kempf and Rolain 2012; Roca et al. 2012). In addition, there are intrinsic properties such as membrane permeability which affect antibiotic resistances of A. baumannii. For example, capsular polysaccharides and the OmpA porin decrease the membrane permeability of A. baumannii and thereby decrease susceptibility to antimicrobial agents (Roca et al. 2012; Lee et al. 2017). Analyses of colicin resistant Escherichia coli mutants led to the identification of Tol-Pal proteins important for outer membrane integrity and permeability (Kowata et al. 2016; Szczepaniak et al. 2020). Hetero-oligomeric Tol-Pal membrane protein complexes have been found in all clades of proteobacteria and play a role in charge transfer from the inner to the outer membrane and in cell division by remodeling septal peptidoglycan at division sites (Léonard et al. 2022; Webby et al. 2022). The Tol-Pal system is also important for pathogenesis and virulence such as it plays a role in type III secretion, bacterial motility, adhesion to epithelial cells, persister cell survival in the presence of antibiotics, and capsule formation of different pathogenic bacteria (Hirakawa et al. 2020). Information with respect to the Tol-Pal system in A. baumannii is very scarce. Here we report on the role of the Tol-Pal system in cell morphology, antibiotic resistance, and virulence of A. baumannii.
Materials and methods
Bacterial strains and culture conditions
E. coli DH5α was grown at 37 °C in LB medium (Bertani 1951). The A. baumannii wild type strain ATCC 19606T and the ∆tol-pal mutant were grown at 37 °C either in LB medium (Bertani 1951), in tryptic soy broth (TSB), or mineral medium with 20 mM succinate as sole carbon and energy source (Zeidler et al. 2017). For solid media, 1.8% agar was added. Growth experiments were started by inoculation of fresh medium with an overnight culture to an initial optical density (OD600nm) of 0.1. Growth was monitored photometrically (600 nm). Kanamycin (20 µg/ml) or tetracycline (30 µg/ml) was added from stock solutions when appropriate.
Generating the inserts for RecAB-mediated gene editing
Replacement of the tol-pal gene cluster by a kanamycin resistance cassette was performed by using the RecAB mediated recombineering system for A. baumannii (Tucker et al. 2014). Therefore, the recombinant plasmid pBIISK_∆tol-pal::kanR was generated. Approximately 300 bp upstream and downstream of the tol-pal gene cluster were amplified using the primer pairs uptolQ_fwd and uptolQ_rev or downpal_fwd and downpal_rev, respectively (primers are listed in Table 1). A kanamycin resistance cassette flanked by flippase recognition sites (FRT-sites) was amplified from the plasmid pKD4 using the primer pair kanR-FRT_fwd and kanR-FRT_rev. The plasmid pBIISK was amplified using the primers pBIISK_fw and pBIISK_rev. The resulting PCR products were assembled by Gibson assembly according to the instructions of the manufacturer (Gibson Assembly Master Mix, New England Biolabs, Ipswich, MA, USA). The resulting recombinant plasmid pBIISK_∆tol-pal::kanR was amplified using the primers lppΔtolQ-pal_fwd and lppΔtolQ-pal_rev. The PCR product was used for replacement of the tol-pal locus using a RecAB dependent recombineering system.
Table 1.
Primers used in this study
| Primer | Sequence 5′ → 3′ |
|---|---|
| pBIISK_fwd | atcgaattcctgcagccc |
| pBIISK_rev | atcaagcttatcgataccgtc |
| uptolQ_fwd | acggtatcgataagcttgatccattactggcatcaaaaag |
| uptolQ_rev | cacaatcgctcatagttacatatgccgg |
| kanR-FRT_fwd | tgtaactatgagcgattgtgtaggctgg |
| kanR−FRT_rev | ggatgttttaatatgaatatcctccttagttcctattc |
| downpal_fwd | atattcatattaaaacatcccaaaaaataaacg |
| downpal_rev | ccgggctgcaggaattcgattttacgtggtatgttgtttg |
| lppΔtolQ-pal_fwd | tgcttctggtgaggttgag |
| lppΔtolQ-pal_rev | gattatgaggcaaaacctg |
| ΔtolQ-pal_fwd | acagcagtcgcgattgaaag |
| ΔtolQ-pal_rev | caagtcgcagcaattgtgtc |
RecAB mediated gene replacement
The RecAB mediated gene replacement was performed according to Tucker et al. (2014). Therefore, A. baumannii ATCC 19606T was transformed with the plasmid pAT04 encoding the RecAB system using electroporation (2.5 kV, 200 Ω, and 25 μF). A. baumannii ATCC 19606T + pAT04 was selected on solid LB medium with tetracycline. The overnight culture was used to inoculate fresh LB medium followed by incubation at 37 °C for 1 h and addition of IPTG to a final concentration of 2 mM. After a further incubation at 37 °C for 3 h, cells were harvested and washed three times with ice-cold H2O containing 10% glycerol (50 ml) and finally resuspended in 200 µl H2O containing 10% glycerol. One hundred microliters of the resulting cell suspension was mixed with 5 µg of the PCR product and electroporated (2.5 kV, 200 Ω, and 25 μF). The electroporated cells were grown in LB medium with 2 mM IPTG for 4 h, followed by centrifugation and plating onto solid LB medium with kanamycin. Single colonies were obtained after overnight incubation at 37 °C. Replacement of the tol-pal genes by the FRT-flanked kanamycin resistance cassette led to a ∆tol-pal::kanR mutant. Replacement of the tol-pal genes was verified via PCR. The plasmid was cured by two transfers of the ∆tol-pal::kanR mutant in LB medium without tetracycline.
FLP mediated removal of the kanamycin resistance cassette
Generation of a markerless ∆tol-pal mutant was performed by flippase (FLP) mediated removal of the kanamycin cassette using the method of Tucker et al. (2014). Therefore, the ∆tol-pal::kanRmutant was transformed with pAT03_tet (Breisch et al. 2022) using electroporation (2.5 kV, 200 Ω, and 25 μF). Transformants were selected on solid LB medium in the presence of tetracycline. The resulting strain ATCC 19606T ∆tol-pal::kanR + pAT03_tet was grown overnight in LB medium with tetracycline and 0.1 mM IPTG. The overnight culture was plated onto solid LB medium with tetracycline and 0.1 mM IPTG. After overnight incubation at 37 °C, single colonies were obtained and analyzed with respect to kanamycin sensitivity. The ∆tol-pal mutant was verified via PCR.
Scanning electron microscopy
The A. baumannii wild type strain and the ∆tol-pal mutant were grown on glass coverslips in 12 well plates in TSB medium at 37 °C for 24 h. Samples were fixed (1.0% paraformaldehyde, 2.5% glutaraldehyde in 50 mM HEPES) for 24 h; dehydrated in 30, 50, 70, 90, 95, 100, and 100% ethanol; critical point dried; mounted on aluminum stubs; sputter coated with a 12 nm layer of gold–palladium; and finally examined in the SEM (ZEISS 1530 Gemini, Carl Zeiss Microscopy GmbH, Germany) operating at 3 kV using the in-lens electron detector.
Antibiotic and detergent resistance analyses
A. baumannii strains were grown overnight in LB medium. Cells were harvested and washed with sterile saline and adjusted to an OD600nm of 1. Serial dilutions were prepared using sterile saline and 4 µl of the cell suspensions was dropped onto solid LB medium containing SDS or different antibiotics. Cells were incubated overnight at 37 °C.
Human serum killing assay
Analysis of the sensitivity of A. baumannii and the Δtol-pal mutant to the human complement system was performed as described recently (Breisch et al. 2022). Therefore, overnight cultures of the A. baumannii wild type and the ∆tol-pal mutant were inoculated into fresh LB medium to an initial OD600nm of 0.1. Strains were grown at 37 °C to an OD600nm of 0.5–0.6, harvested, washed twice with sodium phosphate buffer (50 mM; pH 7) containing 0.9 mM CaCl2 and 0.5 mM MgCl2 (SPB+/+), and adjusted to an optical density of 0.4. Ten microliters of the cell suspension was added to 190 μl SPB+/+ with normal human serum concentrations in the range of 0–15%. The suspensions were incubated for 2 h at 37 °C. After incubation, 800 µl SPB+/+ was added to the samples, serial dilutions were prepared, and colony forming units were determined after growth on solid LB medium.
LL-37 killing assay
Resistance of A. baumannii and the Δtol-pal mutant to the human peptide LL-37 was analyzed as described by Lin et al. (2015). Overnight cultures of the A. baumannii wild type strain and the ∆tol-pal mutant were used to inoculate LB medium to an initial OD600nm of 0.1. Strains were grown at 37 °C to an OD600nm of 0.5–0.6, harvested, washed twice with sodium phosphate buffer (50 mM; pH 7), and adjusted to an optical density of 0.2. Ten microliters of the cell suspensions was added to sodium phosphate buffer containing different LL-37 concentrations. The cell suspensions were incubated at 37 °C for 3 h. After incubation, serial dilutions were prepared and the colony forming units were determined after growth on solid LB medium.
Galleria mellonella infection assay
G. mellonella infection assays were performed as described recently (Hubloher et al. 2021; König et al. 2021). The A. baumannii wild type strain and the ∆tol-pal mutant were grown in LB medium and harvested in the exponential growth phase (OD600nm = 0.5). Cells were washed and resuspended in sterile saline. For each infection assay, 16 caterpillars were injected with 10 µl cell suspensions (approximately 1*106 CFU) into the last proleg of preselected G. mellonella caterpillars (weight range between 0.35 and 0.45 g/larvae). A caterpillar control group was injected with 10 µl sterile saline and another control group was untreated. Caterpillars were incubated for 7 days in the dark at 37 °C. p values were determined by using an unpaired t-test (GraphPad Prism 6 Software) and p values of ≤ 0.05 were considered statistically significant.
Results
Tol-Pal homologs in A. baumannii ATCC 19606T
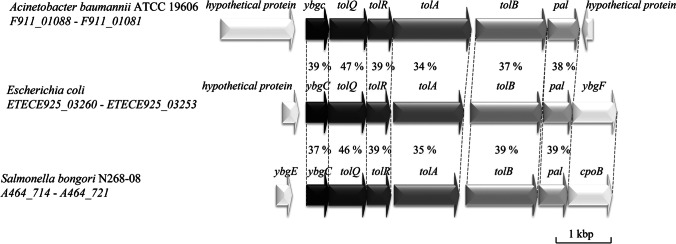
We searched the A. baumannii ATCC 19606T genome for potential genes of the Tol-Pal system and found genes of the Tol-Pal core system, annotated as tolQ, tolR, tolA, tolB, and pal. The presence of these core system subunits of the Tol-Pal system is conserved among proteobacteria (Szczepaniak et al. 2020). However, in addition to the conserved core genes some bacteria contain a ybgC and/ or a cpoB gene up- or downstream of the core gene cluster (Fig. 1). In A. baumannii, the tol-pal core genes are preceded a ybgC gene. A cpoB gene is not present in this gene cluster and was also not detected in any distant locus in the genome. Downstream of the tol-pal genes in opposite orientation a gene encoding a hypothetical protein which is not in any functional context with the Tol-Pal system was detected (Fig. 1). The tol-pal genes and the genetic organization of the tol-pal gene cluster is conserved in the Acinetobacter genus, e.g., in Acinetobacter baylyi and species of the A. baumannii-calcoaceticus complex (Acinetobacter calcoaceticus, A. baumannii, Acinetobacter nosocomialis, Acinetobacter pittii, and Acinetobacter seifertii). Sequence alignments of the deduced Tol-Pal proteins of A. baumannii revealed significant similarities to the Tol-Pal proteins of E. coli and Salmonella bongori, such as identities in the range of 47–34% were found (Fig. 1). These amino identities together with the conserved organization of the genes of the potential tol-pal gene cluster suggest that tolQ, tolR, tolA, tolB, and pal encode the core proteins of the Tol-Pal system in A. baumannii ATCC 19606T.
Fig. 1.
Genetic organization of tol-pal gene clusters. The amino acid identities between the Tol-Pal (in percent) of A. baumannii ATCC 19606T and other Tol-Pal proteins are stated between the distinct gene clusters
The Tol-Pal system is important for cell morphology
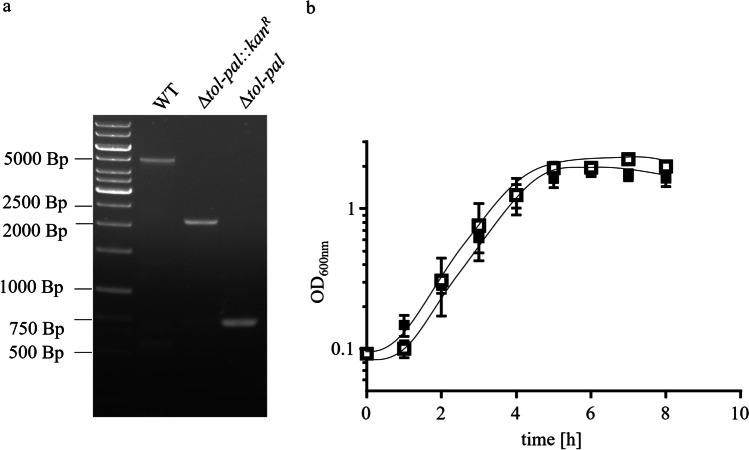
The functionality of the Tol-Pal system of A. baumannii in cell morphology was addressed by deleting the genes of the Tol-Pal core system (tolQ, tolR, tolA, tolB, and pal). RecAB-mediated replacement of tol-pal locus by a kanamycin resistance cassette led to a ∆tol-pal::kanR mutant (Tucker et al. 2014). Excision of the kanamycin resistant cassette was mediated by the FLP and resulted in a markerless A. baumannii ATCC 19606T ∆tol-pal mutant. The generated mutants ∆tol-pal::kanRand ∆tol-pal were verified by PCR using the primers ΔtolQ-pal_fwd and ΔtolQ-pal_rev. Amplification of the tol-pal locus in the wild type resulted in a PCR product with the size of 5172 bp, whereas amplification of the tol-pal locus from the genome of the ∆tol-pal::kanR mutant led to a PCR product of 2123 bp and the PCR product generated with genomic DNA of the ∆tol-pal mutant comprises of 619 bp (Fig. 2a). These PCR fragments correspond to the expected DNA fragments and confirm that the mutants are correct. A polar effect of the markerless deletion of the tol-pal genes can be excluded since the gene downstream of the tol-pal genes is divergently transcribed and the hypothetical protein product is not in any functional context with the Tol-Pal system.
Fig. 2.
PCR verification of the tol-pal mutants (a) and growth of the wild type and the ∆tol-pal mutant in mineral medium with succinate (b). Precultures of the A. baumannii wild type strain (□) and the ∆tol-pal mutant (■) were grown overnight in mineral medium with succinate and were used to inoculate prewarmed fresh mineral medium to an initial OD600nm of 0.1. Each value is the mean ± SEM of at least three independent experiments
Analysis of the growth phenotype of the ∆tol-pal mutant revealed that growth in mineral medium was unaffected (Fig. 2b). A comparable growth was also found in LB medium (data not shown).
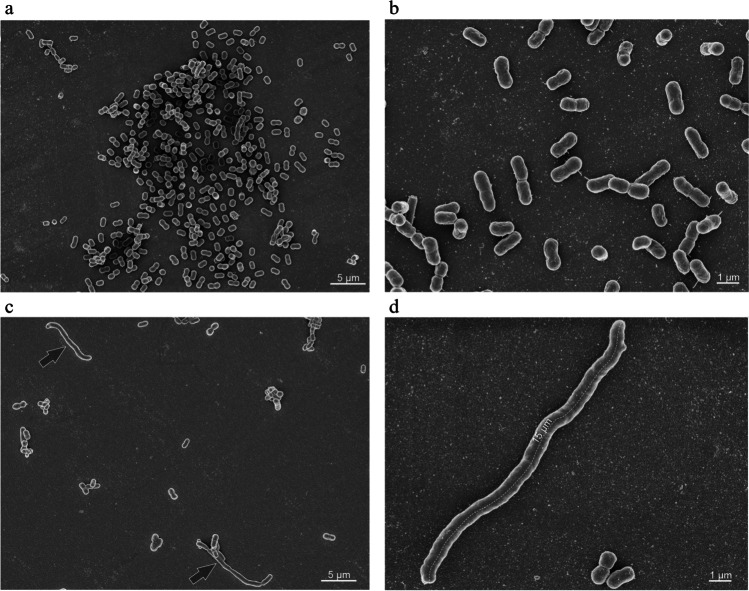
To analyze the role of the Tol-Pal system in cell morphology of the A. baumannii wild type strain and ∆tol-pal mutant was analyzed by scanning electron microscopy. These studies revealed that many cells of the ∆tol-pal mutant exhibited an extraordinary length up to twenty-five times of their normal length (Fig. 3c and d). This elongated phenotype was never observed with the wild type strain (Fig. 3a and b). These findings suggest that the Tol-Pal system of A. baumannii plays a role in cell morphology.
Fig. 3.
Scanning electron micrographs of the A. baumannii wild type strain and the ∆tol-pal mutant. A. baumannii wild type cells and the ∆tol-pal mutant were grown on glass coverslips in 12 well plates in TSB medium at 37 °C for 24 h. The wild type cells formed typical short rods (a, b), whereas many mutant cells were found to form cells with extraordinary length (arrows) (c, d). One out of 50 ∆tol-pal cells showed an dramatic increase in cell length. The analysis has been done by quantifying 7 randomly picked images containing 50–100 cells. Images a and c were taken at a magnification of 5,500 fold, and b and d at 16,000 fold
The ∆tol-pal mutant is impaired in antibiotic and SDS resistance
The role of the Tol-Pal system in membrane integrity prompted us to analyze the resistance of the ∆tol-pal mutant to antibiotics and detergents via drop dilution assays (Fig. 4). Both strains grew comparably up to the highest dilution (10−7) on LB agar. Addition of SDS or antibiotics reduced the viability of the cells in a strain-dependent manner. In the presence of 1% SDS, the viability of wild type cells was reduced such as growth was only observed up to a dilution of 10−2 whereas the ∆tol-pal mutant did not grow at all. A dramatic decrease in viability of the ∆tol-pal mutant was also observed in the presence of antibiotics such as 20 µg/ml gentamicin or 100 µg/ml bacitracin. The wild type strain still grew up to a dilution of 10−7. In contrast, there were only a few colonies of the ∆tol-pal mutant at a dilution of 10−3 in presence of these antibiotics and even less at higher dilutions. Furthermore, the viability of the ∆tol-pal mutant was slightly decreased in the presence of colistin in comparison to the wild type. In contrast, there was no difference in viability of the wild type and the ∆tol-pal mutant in presence of polymyxin B, ampicillin, or streptomycin.
Fig. 4.
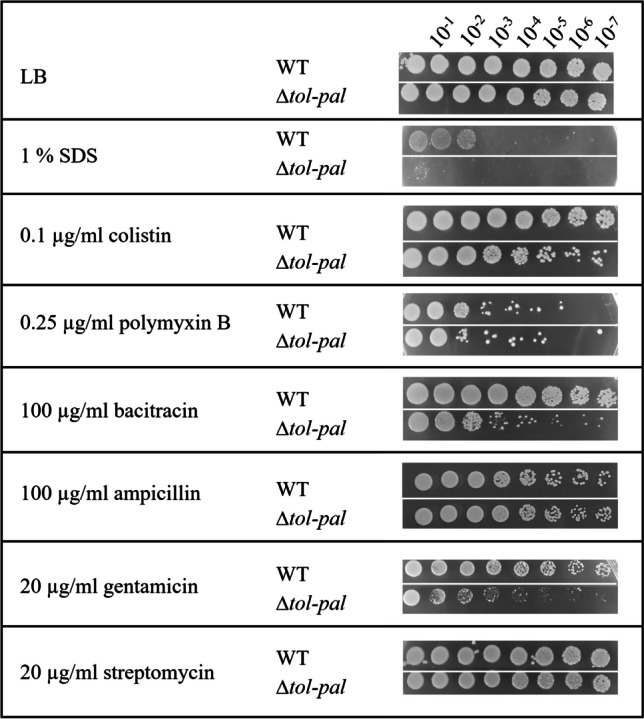
Effect of the tol-pal deletion on the viability of A. baumannii in presence of SDS or antibiotics. Serial dilutions of A. baumannii and the ∆tol-pal mutant were prepared and 4 µl of the cell suspensions was dropped onto solid LB medium containing either SDS or different antibiotics followed by overnight incubation at 37 °C. One representative experiment of at least three independent replicates is shown
A. baumannii ATCC 19606T ∆tol-pal mutant is not impaired in complement resistance
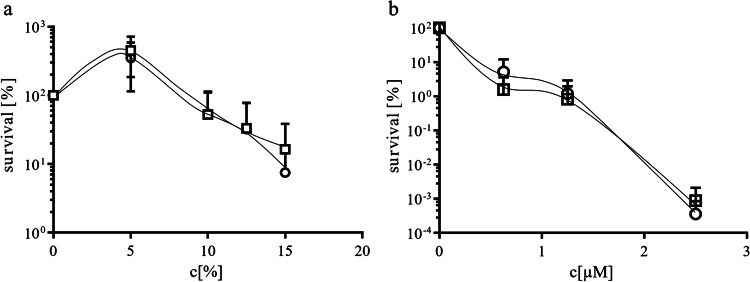
Next, we analyzed whether the ∆tol-pal mutant has also a decreased resistance to human antimicrobial compounds. Therefore, we compared the sensitivity of the A. baumannii wild type and the ∆tol-pal mutant to the human complement system (Fig. 5a) and to the antimicrobial human peptide LL-37 (Fig. 5b) which is part of the complement system. Incubation of A. baumannii either with human serum or with LL-37 decreased the viability in a concentration-dependent manner. Deletion of the tol-pal system did not further increase the susceptibility to human serum or LL-37. This suggests that the Tol-Pal core system does not play a role in complement resistance. This is consistent with the results obtained with the pal mutant of E. coli which also did not show an increased sensitivity to human serum (Diao et al. 2017).
Fig. 5.
Resistance of the A. baumannii wild type strain and the Δtol-pal mutant to the human complement system (a) and the antimicrobial peptide LL-37 (b). The A. baumannii wild type strain (□) and the ∆tol-pal mutant (○) were incubated either with human serum or with the antimicrobial peptide LL-37 at 37 °C, followed by plating onto LB agar to determine the colony forming units. Each value is the mean ± SEM of at least three independent experiments
Tol-Pal system plays an important role in infection of G. mellonella larvae
Next, we addressed the role of the Tol-Pal system in virulence of A. baumannii by performing G. mellonella caterpillar infection studies with the wild type strain and the ∆tol-pal mutant (Fig. 6). The ∆tol-pal mutant was reduced in virulence, such as 57% and 45% of the caterpillars infected with the ∆tol-pal mutant survived after 2 and 3 days, respectively, whereas only 34% and 21% of the larvae survived after 2 and 3 days, respectively, after infection with wild type cells (Fig. 6). This strain dependent difference has been diminished after a prolonged incubation of 5–7 days.
Fig. 6.
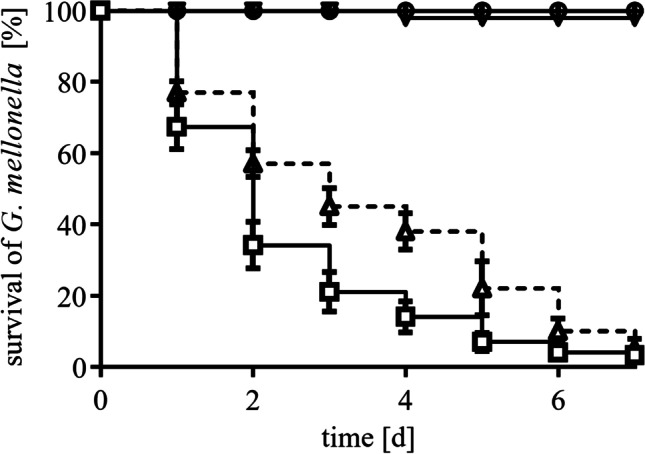
The ∆tol-pal mutant displays a reduced virulence towards G. mellonella larvae. For infection assays, 10 µl of either the A. baumannii wild type strain (□) or the Δtol-pal (△) mutant (approximately 1*106 CFU) was injected into preselected G. mellonella caterpillars followed by incubation at 37 °C for 7 days. A control group was injected with 10 µl sterile saline (▽) and another control group was untreated (○). Each value is the mean ± SEM of at least three independent measurements. The p values for the G. mellonella survival rates at days 3 and 4 are 0.0138 and 0.0072, respectively
Discussion
The Tol-Pal system plays an important role in cell division by remodeling septal peptidoglycan at division sites but is also involved in pathogenesis and virulence of pathogenic bacteria (Hirakawa et al. 2020; Webby et al. 2022). Moreover, deletions of Tol-Pal systems induce pleiotropic effects such as release of periplasmic proteins into the extracellular medium and hypersensitivities to detergents and antibiotics (Lazzaroni et al. 1989; Masilamani et al. 2018). The hypersensitivities of the tol-pal mutants led to the conclusion that deletion of the Tol-Pal system leads to a disturbed outer membrane cell barrier. This is also consistent with the finding that tol-pal deficient bacteria often have an increased sensitivity to surface active compounds, such as SDS or bile salts. In the present study, we identified the Tol-Pal system in A. baumannii and report that a ∆tol-pal mutant exhibits a decreased SDS and antibiotic resistance. This suggests that the Tol-Pal system in A. baumannii is also important for membrane integrity and permeability.
The hetero-oligomeric Tol-Pal system of Gram-negative bacteria spans the inner and outer membrane. TolQ, TolR, and TolA are located in the inner membrane and TolB is located in the outer membrane. The lipoprotein Pal is associated with the peptidoglycan and is attached to the outer membrane on the periplasmic side. All Tol-Pal proteins are thought to be involved in energy transduction from the inner to the outer membrane via a cycle of Tol-Pal complex formation and dissociation (Yakhnina and Bernhardt 2020). Thereby the proton motive force at the inner membrane is used. The Tol-Pal dependent energy transduction from the inner to the outer membrane is important for energy driven reactions such as transport through the outer membrane and coordination of peptidoglycan restructuring and septum formation at the division site (Yakhnina and Bernhardt 2020). Our finding that an A. baumannii ∆tol-pal mutant formed cells with extraordinary length suggests that this mutant is defect in cell division. This is consistent with the findings in E. coli where the Tol-Pal system is crucial for efficient cell division, such as deletion of tol-pal genes resulted in cells with unequal and extended lengths. The same holds true for Vibrio cholerae where deletion of tol genes led to mutants which formed long filaments with unequal length (Meury and Devilliers 1999; Heilpern and Waldor 2000; Llamas et al. 2000; Tan and Chng 2021).
Our results show that deletion of the Tol-Pal system in A. baumannii leads to a decreased killing of G. mellonella. This could be due to a decreased secretion of virulence factors or a decreased adherence to host cells. A role of the Tol-Pal system in secretion of virulence factors has already been reported, such as Hirakawa reported that the deletion of tolB in enterohemorrhagic E. coli (EHEC) decreased the type III secretion system mediated secretion of virulence factors thereby lowering virulence. Moreover, flagella-dependent motility in broth and adhesion to epithelial cells was also impaired (Hirakawa et al. 2020). A role of the Tol-Pal system in virulence has also been reported for Citrobacter rodentium which causes lethality in mice, such as a tolB mutation of C. rodentium abolished lethality in mice (Hirakawa et al. 2020). The authors proposed that the decreased virulence is caused by the dysregulation of the type III secretion system and flagellar activity. The role of the tol-pal system in virulence of A. baumannii has not been elucidated so far. However, our finding that a ΔtolQ-pal mutant is impaired in G. mellonella killing suggests that the Tol-Pal system is also implicated in virulence of A. baumannii. Whether the decreased virulence of the ΔtolQ-pal mutant is due to an altered membrane permeability, decreased adhesion to host cells, or even an impaired ability to evade the host defense system will be subject of future studies.
Conclusions
We have shown here that the Tol-Pal system of A. baumannii is required for resistance to antibiotics as well as important for cell morphology and virulence in G. mellonella caterpillars. We suggest that the deletion of the Tol-Pal system reduces intrinsic resistance to antibiotics by increasing the membrane permeability, thereby causing an increased influx of antibiotics. Our finding that the ∆tol-pal mutant was impaired in killing of G. mellonella larvae suggests that the Tol-Pal system is also important for virulence of A. baumannii and it is tempting to speculate that it plays a role in host cell adhesion and/or evasion of host defense mechanisms.
Acknowledgements
The authors are indebted to the Deutsche Forschungsgemeinschaft for financial support via DFG Research Unit FOR 2251.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Lisa van der Sande, Josephine Hubloher, and Christoph Schaudinn. The first draft of the manuscript was written by Josephine Joy Hubloher, Volker Müller, and Beate Averhoff. All authors commented on previous versions of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Financial support was received from the Deutsche Forschungsgemeinschaft (project FOR 2251, grant number 258351992).
Availability of data and material
Data are available from the authors.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Josephine Joy Hubloher and Lisa van der Sande contributed equally to the study.
References
- Antunes LCS, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71:292–301. doi: 10.1111/2049-632x.12125. [DOI] [PubMed] [Google Scholar]
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/JB.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breisch J, Huber LS, Kraiczy P, Hubloher J, Averhoff B. The ß-ketoadipate pathway of Acinetobacter baumannii is involved in complement resistance and affects resistance against aromatic antibiotics. Environ Microbiol Rep. 2022;14:170–178. doi: 10.1111/1758-2229.13042. [DOI] [PubMed] [Google Scholar]
- Diao J, Bouwman C, Yan D, Kang J, Katakam AK, Liu P, et al. Peptidoglycan association of murein lipoprotein is required for kpsd-dependent group 2 capsular polysaccharide expression and serum resistance in a uropathogenic Escherichia coli isolate. mBio. 2017;8:e00603–00617. doi: 10.1128/mBio.00603-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- Göttig S, Gruber TM, Higgins PG, Wachsmuth M, Seifert H, Kempf VAJ. Detection of pan drug-resistant Acinetobacter baumannii in Germany. J Antimicrob Chemother. 2014;69:2578–2579. doi: 10.1093/jac/dku170. [DOI] [PubMed] [Google Scholar]
- Heilpern AJ, Waldor MK. Infection of Vibrio cholerae requires the tolQRA gene products. J Bacteriol. 2000;182:1739–1747. doi: 10.1128/JB.182.6.1739-1747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa H, Suzue K, Takita A, Awazu C, Kurushima J, Tomita H. Roles of the Tol-Pal system in the type III secretion system and flagella-mediated virulence in enterohemorrhagic Escherichia coli. Sci Rep. 2020;10:15173. doi: 10.1038/s41598-020-72412-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubloher JJ, Schabacker K, Müller V, Averhoff B. CsrA coordinates compatible solute synthesis in Acinetobacter baumannii and facilitates growth in human urine. Microbiol Spectr. 2021;9:e0129621 . doi: 10.1128/Spectrum.01296-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf M, Rolain J-M. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents. 2012;39:105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- König P, Averhoff B, Müller V. K+ and its role in virulence of Acinetobacter baumannii. Int J Med Microbiol. 2021;311:151516. doi: 10.1016/j.ijmm.2021.151516. [DOI] [PubMed] [Google Scholar]
- Kowata H, Tochigi S, Kusano T, Kojima S. Quantitative measurement of the outer membrane permeability in Escherichia coli lpp and tol–pal mutants defines the significance of Tol-Pal function for maintaining drug resistance. J Antibiot. 2016;69:863–870. doi: 10.1038/ja.2016.50. [DOI] [PubMed] [Google Scholar]
- Lazzaroni JC, Fognini-Lefebvre N, Portalier R. Cloning of the excC and excD genes involved in the release of periplasmic proteins by Escherichia coli K12. Mol Gen Genet. 1989;218:460–464. doi: 10.1007/bf00332410. [DOI] [PubMed] [Google Scholar]
- Lee C-R, Lee JH, Park M, Park KS, Bae IK, Kim YB, et al. Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front Cell Infect Microbiol. 2017;7:55. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard RR, Sauvage E, Lupo V, Perrin A, Sirjacobs D, Charlier P, et al. Was the last bacterial common ancestor a monoderm after all? Genes. 2022;13:376. doi: 10.3390/genes13020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M-F, Tsai P-W, Chen J-Y, Lin Y-Y, Lan C-Y. OmpA binding mediates the effect of antimicrobial peptide LL-37 on Acinetobacter baumannii. PLoS ONE. 2015;10:e0141107 . doi: 10.1371/journal.pone.0141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas MA, Ramos JL, Rodríguez-Herva JJ. Mutations in each of the tol genes of Pseudomonas putida reveal that they are critical for maintenance of outer membrane stability. J Bacteriol. 2000;182:4764–4772. doi: 10.1128/JB.182.17.4764-4772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masilamani R, Cian MB, Dalebroux ZD. Salmonella Tol-Pal reduces outer membrane glycerophospholipid levels for envelope homeostasis and survival during bacteremia. Infect Immun. 2018;86:e00173–e118. doi: 10.1128/IAI.00173-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meury J, Devilliers G. Impairment of cell division in tolA mutants of Escherichia coli at low and high medium osmolarities. Biol Cell. 1999;91:67–75. doi: 10.1111/j.1768-322X.1999.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Roca I, Espinal P, Vila-Farrés X, Vila J. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front Microbiol. 2012;3:148–148. doi: 10.3389/fmicb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepaniak J, Press C, Kleanthous C. The multifarious roles of Tol-Pal in Gram-negative bacteria. FEMS Microbiol Rev. 2020;44:490–506. doi: 10.1093/femsre/fuaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WB, Chng S-S. Genetic interaction mapping highlights key roles of the Tol-Pal complex. Mol Microbiol. 2021;117(4):921–936. doi: 10.1101/2021.09.13.460050. [DOI] [PubMed] [Google Scholar]
- Tucker AT, Nowicki EM, Boll JM, Knauf GA, Burdis NC, Trent MS, Davies BW. Defining gene-phenotype relationships in Acinetobacter baumannii through one-step chromosomal gene inactivation. mBio. 2014;5:e01313–01314. doi: 10.1128/mBio.01313-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby MN, Williams-Jones DP, Press C, Kleanthous C. Force-generation by the trans-envelope Tol-Pal system. Front Microbiol. 2022;13:852176 . doi: 10.3389/fmicb.2022.852176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2017) WHO priority pathogens list for R&D of new antibiotics https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed [october 2022].
- Yakhnina AA, Bernhardt TG. The Tol-Pal system is required for peptidoglycan-cleaving enzymes to complete bacterial cell division. Proc Natl Acad Sci USA. 2020;117:6777–6783. doi: 10.1073/pnas.1919267117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler S, Hubloher J, Schabacker K, Lamosa P, Santos H, Müller V. Trehalose, a temperature- and salt-induced solute with implications in pathobiology of Acinetobacter baumannii. Environ Microbiol. 2017;19:5088–5099. doi: 10.1111/1462-2920.13987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors.