Abstract
Background
Diabetes mellitus type 2 is a common disease that poses a challenge to the healthcare system. The disease is very often diagnosed late. A better understanding of the relationship between the gut microbiome and type 2 diabetes can support early detection and form an approach for therapies. Microbiome analysis offers a potential opportunity to find markers for this disease. Next-generation sequencing methods can be used to identify the bacteria present in the stool sample and to generate a microbiome profile through an analysis pipeline. Statistical analysis, e.g., using Student’s t-test, allows the identification of significant differences. The investigations are not only focused on single bacteria, but on the determination of a comprehensive profile. Also, the consideration of the functional microbiome is included in the analyses. The dataset is not from a clinical survey, but very extensive.
Results
By examining 946 microbiome profiles of diabetes mellitus type 2 sufferers (272) and healthy control persons (674), a large number of significant genera (25) are revealed. It is possible to identify a large profile for type 2 diabetes disease. Furthermore, it is shown that the diversity of bacteria per taxonomic level in the group of persons with diabetes mellitus type 2 is significantly reduced compared to a healthy control group. In addition, six pathways are determined to be significant for type 2 diabetes describing the fermentation to butyrate. These parameters tend to have high potential for disease detection.
Conclusions
With this investigation of the gut microbiome of persons with diabetes type 2 disease, we present significant bacteria and pathways characteristic of this disease.
Keywords: Type 2 diabetes, Gut, Butyrate, NGS, Statistical analysis
Background
Diabetes mellitus is one of the most prevalent diseases worldwide. In 2019, the World Health Organization (WHO) included diabetes mellitus in the top 10 leading causes of death (WHO 2020). Diabetes mellitus is a metabolic disease, with some hereditary predispositions. It is divided into two main variants, type 1 and type 2. Type 2 diabetes mellitus (T2D) is the most common form and accounts for over 90% of all cases. In this disease, the pancreas still provides sufficient insulin, but this can be increasingly poorly processed by the cells until a complete insulin resistance is developed (Zaccardi et al. 2016). Lack of exercise, diet, and obesity are regarded risk factors (Fletcher et al. 2002). In the past, this disease was considered to be a consequence of old age, but nowadays this disease is also increasingly occurring in children and youth. Diabetes mellitus type 2 disease can lead to the development of cardiovascular diseases, diabetic foot and damage to the kidneys and eyes, and can even lead to death (Cannon et al. 2018). Studies have shown that the disease can be pushed back or even defeated completely by changing the way of life (Lean et al. 2018).
The intestinal microbiota is a complex structure of bacteria, fungi and virus. Bacteria account for the largest share, with a total of about 100 trillion bacteria living in human intestines. In addition to digestion, the intestine is involved in many other processes and is also called the control center of the body. For example, it supports the immune system and controls inflammatory processes, among other things. The intestinal microbiome is not only influenced by nutrition, but also by many other factors, such as lifestyle, environment, age and gender. Thus, the composition of the intestinal microbiota varies depending on these factors. With a balanced diet, the diversity of bacteria in the intestine increases, and this leads to a wide formation of various metabolic products (Lozupone et al. 2012; Shreiner et al. 2015). In this context, the short-chain fatty acids (SCFA) are of particular importance (Valdes et al. 2018). An important representative is butyrate, which is produced by a variety of bacteria. Butyrate producers make up about 20% of the total bacterial community. Most frequently occurring representatives are members of the families Lachnospiraceae and Ruminococcaceae (Vital et al. 2017). Butyrate is formed during the fermentation of carbohydrates via various pathways (pyruvate, succinate, etc.) (Vital et al. 2014). In this process, it represents one of the most important sources of energy for intestinal cells. In addition, butyrate is considered to have a health-promoting effect. It not only ensures the functionality of intestinal cells, but also strengthens the intestinal barrier, regulates immune functions and controls metabolic processes. A reduction of butyrate-producing bacteria is associated with the development of diseases (Mallott and Amato 2022).
With the advent of research into the microbiome, links between gut bacteria and type 2 diabetes mellitus have also emerged. Although the question remains whether causality can be inferred between the correlations found. However, increased research in the field is leading to a better understanding. Existing correlations can be confirmed and deepened, and new ones can form the basis for further studies. Thus, consequences and causes of diseases and changes in the intestinal microbiome can be better understood and possible therapeutic approaches can be developed (Arora and Tremaroli 2021; Li et al. 2020; Sharma and Tripathi 2019; Gurung et al. 2020).
Materials and methods
Data
The available data sets consist of microbiome and individual lifestyle data. These are not from a clinical survey with medical supervision, but are extensive due to the low-threshold manner of the survey. More than 29,000 samples were available, each associated with individual lifestyle information. The data (only with indication of consent for scientific use) is provided by the company BIOMES NGS GmbH as a project partner within the scope of its business-like activity. BIOMES NGS GmbH offers a self-test for the analysis of the intestinal flora. Both the sampling and the answering of the questions about the individual lifestyle were performed by the customers themselves. It is a lifestyle product without any further verification of the customer’s information by a medical doctor.
The data on individual lifestyle includes information on age, center of life, height and body weight, as well as information on diet, diseases and medication intake. A total of 99 fields were covered.
The microbiome profile was composed of normalized counts per taxonomic level (kingdom, phylum, class, order, family, genus, species) from sequenced bacterial 16S ribosomal DNA (rDNA). Microbial DNA was analyzed using next-generation sequencing (NGS). This has advantages over classical gut analysis methods because the analysis is more accurate and thus the entirety of the intestinal microbiome can be determined. The 16S rDNA, a gene of the microorganisms, is analyzed.
One subset consisted of clients, who have a diabetes type 2 disease with an age between 18 and 80.
A total of 272 samples met the inclusion criteria, of which 143 were female and 127 were male, and two samples had no gender information.
The diabetes type 2 subgroup was compared with a healthy reference group. Samples for the healthy reference were selected based on the listed parameters.
Inclusion criteria (healthy):
Age 18-80
No diseases
No gastrointestinal complains
No allergies gluten intolerance
No medication
No antibiotic intake < 3 months
No probiotic intake < 3 months
BMI 18.5 to 27.5
Alcohol intake not daily
Good wellbeing (> = 5) & health score (>= 6 out of 10)
These criteria resulted in a group size of 674 healthy individuals. Of these, 340 were female, 318 were male, 1 are socially diverse, and 15 samples without gender information. Table 1 shows the distribution of the selected parameters age, gender, BMI, nutrition and sports for the two groups Healthy and T2D. There was no significant difference in nutrition between the two groups. The T2D group did slightly less sports than the Healthy group. This is consistent for the development of diabetes mellitus type 2 disease. For the investigated data set, further adjustment of the parameters in the two groups was not possible, as otherwise unacceptable group sizes would have resulted. The parameters age and BMI are known risk factors and crucial for the development of diabetes type 2. Thus, higher age and higher BMI are characteristic.
Table 1.
Distribution of the selected parameters age, gender, BMI, nutrition and sports for the two groups Healthy and T2D
| Parameter | Healthy group (n = 674) | T2D group (n = 272) |
|---|---|---|
| Age, years | 42,55 ± 12,12 | 59,71 ± 12,27 |
| Women/Men/Divers, n | 340/318/1 | 143/127/0 |
| BMI, kg/m2 | 23,13 ± 2,24 | 31.05 ± 6.38 |
| Nutrition | ||
| Omnivore | 517 | 245 |
| Vegan | 40 | 2 |
| Vegetarian | 74 | 10 |
| Pescetarian | 43 | 8 |
| Sports | ||
| Daily | 22 | 3 |
| 5–6 times per week | 61 | 5 |
| 3–4 times per week | 199 | 31 |
| 1–2 times per week | 172 | 28 |
| 1 time per week | 52 | 17 |
| 1 time per 2 weeks | 18 | 7 |
| Less 2 months | 2 | 5 |
| Never | 132 | 107 |
Methods
Sample preparation and sequencing
Sample storage and lysis
Collected stool samples are stored in 1000 μL DNA-stabilizing buffer at − 20 ∘C until use. For the lysis process, the samples are defrosted and will be centrifuged. Afterwards, warmed up PW buffer from the QIAamp 96 PowerFecal QIAcube HT Kit is added to each sample.
Extraction of stool samples
For the extraction we established the QIAamp 96 PowerFecal QIAcube HT Kit on our liquid handling systems (Hamilton StarLine & Tecan EVO) by using a vacuum chamber as well as a high-pressure chamber. The extracted gDNA is stored at − 20 ∘C until use.
Library preparation for sequencing with the Illumina MiSeq System
The library preparation follows the manual “16S Metagenomic Sequencing Library Preparation- Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System”. The mastermix reagents for the target and library amplification are from New England BioLabs, 16S V3V4 primer from Eurofins. For normalization of all samples, a fluorescent dye, and the Biotek Synergy HTX plate reader are used to measure DNA concentrations and to calculate the necessary dilution volume per sample. To ensure a high throughput, all the steps, from the first amplification to the library pooling, are nearly fully automated by using the liquid handling systems (Hamilton StarLine). Hence, we can process between 96 and 192 samples simultaneously and the normalization also works for up to 288 samples. The Library Denaturing and MiSeq Sample Loading is carried out manually.
Processing sequence reads
The determined paired-end reads were filtered in the following. First, the forward/reverse reads were merged using PANDAseq (Masella et al. 2012). This was followed by an alignment using BLASTn (Altschul et al. 1990) against the SILVA rRNA database (version: 138.1) (Quast et al. 2013). Afterwards, filtering was performed. There must be at least 10,000 assigned reads for each sample, for further analysis. Different identity thresholds per taxonomic boundaries (phylum: 75.0%, class: 78.5%, order: 82.0%, family: 86.5%, genus: 94.5%, species: 97.0%) (Yarza et al. 2014) were used. The sequences were clustered according to their similarity (97%) using CD-HIT (Li and Godzik 2006; Fu et al. 2012). Biologically normalized abundances were calculated from the clustered reference sequences using the PICRUSt2 pipeline (Douglas et al. 2020). The output is a table of biologically normalized counts per taxonomic level. The PICRUSt2 pipeline was used to determine the available pathways (MetaCyc Caspi et al. 2016) for each sample applying a predictive model. The abundances of the identified pathways were calculated based on the gene families.
The alpha diversity measures Shannon entropy (Shannon 1948) and inverse Simpson correlation (Simpson 1949) were determined from the rarefied raw counts. The mathematically normalized values were calculated using QIIME2 (Bolyen et al. 2019). Shannon entropy is a measure of diversity and includes both the number of different species and the number of individuals per species. The Shannon index originated in information theory, as a measure of the information content of a message. The inverse Simpson index is also a measurement for describing diversity and, like the Shannon index, belongs to the alpha diversity measures. It is based on the Simpson index. This indicates the probability that two randomly selected individuals belong to different species. The inverse Simpson index is the reciprocal of the Simpson index.Further analysis steps were performed with custom Python scripts using the pandas (McKinney 2010; The pandas development team 2020), NumPy (Harris et al. 2020), scikit-learn (Pedregosa et al. 2011) and SciPy (Virtanen et al. 2020) libraries.
Statistical analysis
Various statistical tests were applied to detect significant differences between the two groups T2D and Healthy. To determine whether the numerical values of microbiome and lifestyle data follow a normal distribution, a test based on D’Agostino and Pearson (D’Agostino 1971; D’Agostino and Pearson 1973) was used. A Student’s t-test (Student 1908) was applied to normally distributed samples, and a Mann-Whitney U test (Wilcoxon 1945) was applied to non-normally distributed samples. To determine correlations of individual lifestyle categorical data, the Chi-Square Test of Independence was used. A p-value of > 0.05 was considered significant for all tests used.
Results
Diversity of the intestinal microbiome
To identify which bacteria/taxa are significant to each group, diversity was examined first. This was done by calculating the number of bacteria per taxonomic level that had at least one relative count. The quantity of different bacteria per subgroup for each taxonomic level is listed in Table 2. Occurrences over the entire group were also included. It can be seen that the values in the T2D group were about 10% lower than in the Healthy control group across all taxonomic levels.
Table 2.
Diversity: number of bacteria (at least one relative count) per taxonomic level for the groups Healthy and T2D and in the total of both groups
| Group | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|
| Total | 75 | 224 | 490 | 1045 | 2393 | 4326 |
| Healthy | 75 | 217 | 460 | 966 | 2140 | 3690 |
| T2D | 67 | 184 | 409 | 874 | 1911 | 3275 |
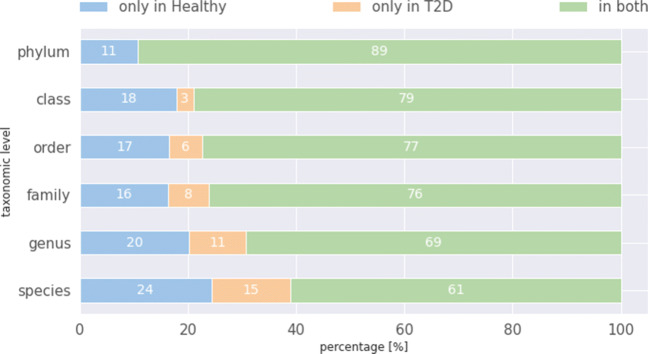
The uniqueness of bacteria in those groups was investigated afterwards. These percentage values are shown in Fig. 1. The majority of bacteria occur in both groups. In addition, more bacteria occur only in the group Healthy. To determine whether the unique bacteria are characteristic for diabetes, they were tested for significance. For this purpose, different hypothesis tests were applied, Student’s t-test for normally distributed samples and Mann-Whitney U test for non-normally distributed samples. A p-value of > 0.05 was assumed as significant. None of the unique bacteria were found to be characteristic of either group.
Fig. 1.
Ratio of bacteria present only in the Healthy group (blue), those present only in the T2D group (orange), and in both groups (green), in percent for each taxonomic level
A hypothesis test was performed for bacteria present in both groups to check for significance. For normally distributed samples, a Student’s t-test was applied, and Mann-Whitney U test was used for samples not following a Gaussian distribution. A p-value of 0.05 was assumed as significance threshold. Of 2393 different genera, 25 were identified as significant. Of these, the count values for 4 genera (Bacteroides, Blautia, Lachnoclostridium, and Prevotella) were increased in the T2D group in comparison to the Healthy group. The other 19 genera (Faecalibacterium, Lachnospira, Roseburia, Ruminococcus, etc.) showed decreased count values in T2D compared to Healthy. Bacteria that could not be precisely assigned (non-specific, etc.) were not included. Table 3 lists significant bacteria (genus level) with respecting p-value and relative abundance in the T2D group.
Table 3.
Bacteria at genus level determined to be significant, with p-value and inclusion of the T2D group relative to the Healthy group (⇑ — increases; ⇓ — decreases)
| Genus | Occurrence in diabetes group | p-value |
|---|---|---|
| Lachnoclostridium | ⇑ | 7.751e-22 |
| Bacteroides | ⇑ | 1.308e-05 |
| Blautia | ⇑ | 2.169e-03 |
| Prevotella | ⇑ | 1.644e-03* |
| Lachnospiraceae FCS020 group | ⇓ | 2.460e-22 |
| Lachnospiraceae ND3007 group | ⇓ | 6.416e-14 |
| Faecalibacterium | ⇓ | 8.775e-12 |
| Ruminococcus | ⇓ | 7.398e-11 |
| Clostridium sensu stricto 1 | ⇓ | 3.000e-10* |
| Lachnospiraceae UCG-001 | ⇓ | 2.885e-08 |
| Coprococcus | ⇓ | 5.091e-07 |
| Subdoligranulum | ⇓ | 4.141e-06 |
| Lachnospira | ⇓ | 3.639e-06 |
| Lachnospiraceae NC2004 group | ⇓ | 2.348e-06 |
| Lachnospiraceae NK4A136 group | ⇓ | 2.284e-06 |
| Fusicatenibacter | ⇓ | 4.077e-05 |
| Lachnospiraceae UCG-006 | ⇓ | 2.310e-05 |
| UCG-002 | ⇓ | 7.378e-03 |
| Roseburia | ⇓ | 5.050e-03 |
| Marvinbryantia | ⇓ | 3.677e-03 |
| Lachnospiraceae UCG-008 | ⇓ | 2.881e-03 |
| Agathobacter | ⇓ | 2.438e-03* |
| Alistipes | ⇓ | 2.560e-02* |
| Butyricicoccus | ⇓ | 1.747e-02* |
| Anaerostipes | ⇓ | 1.288e-02 |
*Mann-Whitney U test
The significant genera belong to 9 different families (out of 1045 different families). Thereby, the family of Lachnospiracaeae was the most represented one (16 genera out of 25), followed by Ruminococcacaeae (3 out of 25). For the remaining 6 genera, each belongs to different families.
Quantification by alpha diversity
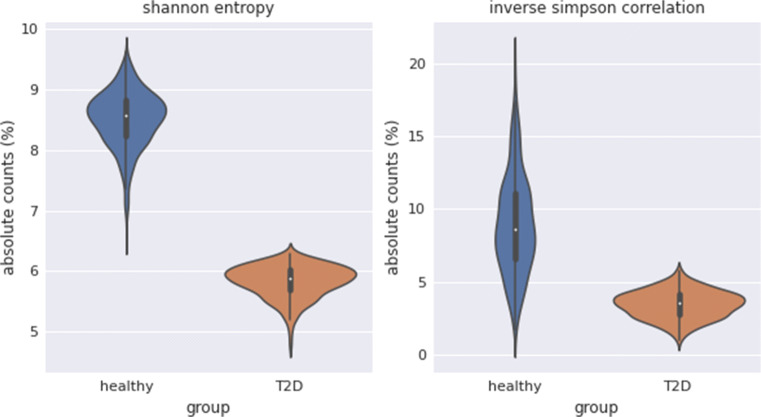
To describe the diversity of the groups, the Shannon entropy and the inverse Simpson correlation were calculated. Figure 2 shows the results as a violin plot for both groups. Both parameters for alpha diversity are significantly increased in the Healthy group compared to the T2D group. This is particularly visible for the Shannon entropy.
Fig. 2.
Alpha diversity: violin plot for the group Healthy (blue) and the group T2D (orange) of absolute counts (%) for Shannon entropy (left) and inverse Simpson correlation (right)
Functional microbiome: consideration of the butyrate production pathways
Furthermore, the identified pathways were analyzed for their significance for type 2 diabetes mellitus. Special attention was paid to pathways in which butyrates are produced. The significant pathways are listed in Table 4. These are six pathways in which fermentation to butanoate occurs. High significance was found for three of six pathways (PWY-5677, P163-PWY, CENTFERM-PWY; cf. Table 4). The three other pathways (P162-PWY, PWY-5676, PWY-5022) showed weak significance.
Table 4.
Pathways with butyrate as end product
| BioCyc ID | Pathways | p-value |
|---|---|---|
| PWY-5677 | Succinate fermentation to butanoate | 1.570e-12 |
| P163-PWY | l-Lysine fermentation to acetate and butanoate | 4.804e-10 |
| CENTFERM-PWY | Pyruvate fermentation to butanoate | 2.312e-05 |
| P162-PWY | l-Glutamate degradation V (via hydroxyglutarate) | 6.015e-02 |
| PWY-5676 | Acetyl-CoA fermentation to butanoate | 8.599e-02 |
| PWY-5022 | 4-Aminobutanoate degradation V | 2.278e-01 |
Discussion
This work analyzed the intestinal microbiome of 272 individuals with diabetes mellitus type 2 disease and 674 healthy control subjects. Significant differences in the alpha diversity measures, Shannon entropy and inverse Simpson correlation, were found between the two groups. It became clear that the diversity in the diabetes mellitus type 2 group is lower than in the Healthy control group. This was also confirmed by other studies, which show that the microbiome diversity of persons suffering a disease is lower compared to healthy persons (Zhang et al. 2019; Larsen et al. 2010).
Furthermore, the analyses demonstrated that there are bacteria that are only present in one of the two comparison groups. However, the occurrences of these bacteria were not significant for diabetes mellitus type 2 disease. Characteristic bacteria for distinguishing the diabetes microbiome from healthy microbiome were present in both groups. The only significant difference between the genera was in the amount of their occurrence. Four genera (Bacteroides, Blautia, Lachnoclostridium and Prevotella) showed an increased occurrence in the diabetes mellitus type 2 group than in the Healthy control group. The other significant genera (Anaerostipes, Coprococcus, Fusicatenibacter, Lachnospira, Marvinbryantia, Roseburia, Faecalibacterium, Ruminococcus, Subdoligranulum, UCG-002, Agathobacter, Butyricicoccus, Alistipes, Clostridium sensu stricto 1 and all Lachnospiraceae) showed the opposite.
The genus Blautia was one of the most represented genera in both groups enriched in group T2D. Previous studies confirm this behavior (Egshatyan et al. 2015; Zhang et al. 2013).
Lachnoclostridium were more abundant in the microbiome profiles of diabetic patients. This association has not yet been verified in other studies. However, there are studies in relation to other diseases (Kang et al. 2021). Further research is necessary to identify Lachnoclostridium as a marker for type 2 diabetes mellitus. There are anomalies in the genera Bacteroides and Prevotella, both of which are increased in the T2D group, compared to the healthy group. Although similar behavior was detected in other studies, both genera are propionate producers. Propionate, like butyrate, belongs to the short-chain fatty acids. These are associated with a healthy status of the microbiome (Wu et al. 2010; Candela et al. 2016).
A significant reduction of genera Alistipes, Anaerostipes, Ruminococcus was detected in the T2D group. This decrease was associated with diabetes mellitus type 2 and was supported by previous studies. Ruminococcus and Anaerostipes were associated with a healthy state of the microbiome and showed increased values in the Healthy control group (Gao et al. 2018; Doumatey et al. 2020). Furthermore, various studies also indicated a decrease in genera in the group T2D (Zhang et al. 2019; Gaike et al. 2020; Liu et al. 2020; Salamon et al. 2018; Das et al. 2021).
A decrease of Alistipes counts was also identified in the data of group T2D (Thingholm et al. 2019).
Additionally, the analysis pointed out a reduction in the Lachnospira, Roseburia, Faecalibacterium and Coprococcus genera in the T2D group compared to the healthy group. These genera were butyrate producers and were associated with healthy gut flora. The decrease of these genera favored obesity and the development of diseases. This fact and other studies supported the assumption that these were genera associated with diabetes mellitus type 2 disease. In these studies, a reduction was also detected in the T2D group (Zhang et al. 2019; Larsen et al. 2010; Kang et al. 2021; Candela et al. 2016; Gao et al. 2018; Liu et al. 2020; Salamon et al. 2018; Das et al. 2021; Anand et al. 2016).
The genus Subdoligranulum is closely related to the genera Faecalibacterium, both from the family Ruminococcaceae. Like Faecalibacterium, Subdoligranulum is also a butyrate producer. Furthermore, this genus was associated with a healthy metabolic status, but the exact physiological role is yet unknown (Van Hul et al. 2020). The results (cf. Table 3) show that the occurrence of Subdoligranulum is reduced in the group with type 2 diabetes mellitus. The reduction of Subdoligranulum can also be assumed to have a negative impact and is thus characteristic of type 2 diabetes mellitus disease.
In four other genera (Fusicatenibacter, Agathobacter, Butyricicoccus, Marvinbryantia), a decrease in the T2D group could also be detected. Those are currently not associated with type 2 diabetes mellitus. All genera produce short-chain fatty acids, e.g., butyrates, and are associated with a healthy intestinal flora (Kang et al. 2021; Lu et al. 2019; Ma et al. 2020; Chen et al. 2021). The reduction of genera leads to a lower proportion of short-chain fatty acids in the organism. This favors obesity and the development of diseases. Thus, these genera may be elements of a profile for the detection of type 2 diabetes mellitus. The exact relationship between the genera Fusicatenibacter, Agathobacter, Butyricicoccus and Marvinbryantia and type 2 diabetes mellitus disease also needs to be investigated in further trials.
The consideration of the functional microbiome has received greater attention in recent years. It is assumed that not only individual bacteria/taxa, but also their interactions are decisive. So characteristic profiles, e.g., diseases and lifestyle, can be found. Thus, pathway analyses are becoming more and more important. In this work, pathways were investigated in which fermentation to butanoate takes place (Qin et al. 2012; Reichardt et al. 2014). Six different pathways (PWY-5677, P163-PWY, CENTFERM-PWY, P162-PWY, PWY-5676, PWY-5022) could be determined. Through significance analysis, these were examined with respect to their relevance for the group T2D. Furthermore, significant, determined taxa (e.g., Lachnospira, Roseburia, Faecalibacterium, and Coprococcus) are also identified as butyrate producers. These facts support the assumption that the functional microbiome has a high importance for the analysis of the microbiome. Further research must verify the potential found for associations with diabetes mellitus type 2 disease or even other diseases or lifestyles. For this purpose, additional pathways need to be examined. These may also be approaches for early detection and therapies (Arora and Tremaroli 2021).
Acknowledgements
Thanks to the laboratory team from BIOMES NGS GmbH, especially Philipp Franke. Further thanks go to Christian Rockmann and Prof. Dr. Marcus Frohme for the project acquisition.
Abbreviations
- BMI
body mass index
- NGS
next-generation sequencing
- rDNA
ribosomal deoxyribonucleic acid
- SCFA
short-chain fatty acids
- T2D
type 2 diabetes
- WHO
World Health Organization
Author contribution
J.S. and H.P.: conceptualization. J.S.: statistical analysis, writing — original draft. O.M. and C.K.: providing the data (sample preparation and sequencing; processing sequence reads). I.K. and J.A.: writing — review and editing
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Federal Ministry of Education and Research of Germany under grant number 13FH209PX8.
Availability of data and materials
The data used are not publicly available because the research project was carried out in collaboration with the company BIOMES NGS GmbH. They can be requested with a reasoned request to the corresponding author.
Declarations
Ethics approval and consent to participate
The data are provided by the company BIOMES NGS GmbH. Customers pay for the microbiome test and give their approval for further scientific use in the purchase process. Only data with granted consent was used.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Julienne Siptroth, Email: siptroth@th-wildau.de.
Olga Moskalenko, Email: olga.moskalenko@biomes.world.
Carsten Krumbiegel, Email: carsten.krumbiegel@biomes.world.
Jörg Ackermann, Email: j.ackermann@bioinformatik.uni-frankfurt.de.
Ina Koch, Email: ina.koch@bioinformatik.uni-frankfurt.de.
Heike Pospisil, Email: heike.pospisil@th-wildau.de.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anand S, Kaur H, Mande SS. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front Microbiol. 2016;7:1945. doi: 10.3389/fmicb.2016.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora T, Tremaroli V. Therapeutic potential of butyrate for treatment of type 2 diabetes. Front Endocrinol. 2021;12:761834. doi: 10.3389/fendo.2021.761834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu Y-X, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela M, Biagi E, Soverini M, Consolandi C, Quercia S, Severgnini M, Peano C, Turroni S, Rampelli S, Pozzilli P, Pianesi M, Fallucca F, Brigidi P. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80–93. doi: 10.1017/S0007114516001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon A, Handelsman Y, Heile M, Shannon M. Burden of illness in type 2 diabetes Mellitus. J Manage Care Specialty Pharm. 2018;24(9-a Suppl):5–13. doi: 10.18553/jmcp.2018.24.9-a.s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R, Billington R, Ferrer L, Foerster H, Fulcher CA, Keseler IM, Kothari A, Krummenacker M, Latendresse M, Mueller LA, Ong Q, Paley S, Subhraveti P, Weaver DS, Karp PD. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016;44(Database issue):471–480. doi: 10.1093/nar/gkv1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Radjabzadeh D, Chen L, Kurilshikov A, Kavousi M, Ahmadizar F, Ikram MA, Uitterlinden AG, Zhernakova A, Fu J, Kraaij R, Voortman T. Association of insulin resistance and type 2 diabetes with gut microbial diversity. JAMA Netw Open. 2021;4(7):2118811. doi: 10.1001/jamanetworkopen.2021.18811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino RB. An omnibus test of normality for moderate and large size samples. Biometrika. 1971;58(2):341–348. doi: 10.1093/biomet/58.2.341. [DOI] [Google Scholar]
- D’Agostino R, Pearson ES. Tests for departure from normality. Empirical results for the distributions of b2 and $\sqrt {b1}$b1. Biometrika. 1973;60(3):613–622. [Google Scholar]
- Das T, Jayasudha R, Chakravarthy S, Prashanthi GS, Bhargava A, Tyagi M, Rani PK, Pappuru RR, Sharma S, Shivaji S. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci Rep. 2021;11(1):2738. doi: 10.1038/s41598-021-82538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MGI. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38(6):685–688. doi: 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumatey AP, Adeyemo A, Zhou J, Lei L, Adebamowo SN, Adebamowo C, Rotimi CN (2020) Gut microbiome profiles are associated with type 2 diabetes in urban Africans. Front Cell Infect Microbiol 10 [DOI] [PMC free article] [PubMed]
- Egshatyan L, Kashtanova D, Popenko A, Tkacheva O, Tyakht A, Alexeev D, Karamnova N, Kostryukova E, Babenko V, Vakhitova M, Boytsov S. Gut microbiota and diet in patients with different glucose tolerance. Endocr Connect. 2015;5(1):1–9. doi: 10.1530/EC-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs. 2002;16(2):17–23. doi: 10.1097/00005082-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28(23):3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaike AH, Paul D, Bhute S, Dhotre DP, Pande P, Upadhyaya S, Reddy Y, Sampath R, Ghosh D, Chandraprabha D, Acharya J, Banerjee G, Sinkar VP, Ghaskadbi SS, Shouche YS. The gut microbial diversity of newly diagnosed diabetics but not of prediabetics is significantly different from that of healthy nondiabetics. mSystems. 2020;5(2):00578–19. doi: 10.1128/mSystems.00578-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Zhu C, Li H, Yin M, Pan C, Huang L, Kong C, Wang X, Zhang Y, Qu S, Qin H. Dysbiosis signatures of gut microbiota along the sequence from healthy, young patients to those with overweight and obesity. Obesity. 2018;26(2):351–361. doi: 10.1002/oby.22088. [DOI] [PubMed] [Google Scholar]
- Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, Wieser E, Taylor J, Berg S, Smith NJ, Kern R, Picus M, Hoyer S, van Kerkwijk MH, Brett M, Haldane A, del Río JF, Wiebe M, Peterson P, Gérard-Marchant P, Sheppard K, Reddy T, Weckesser W, Abbasi H, Gohlke C, Oliphant TE. Array programming with NumPy. Nature. 2020;585(7825):357–362. doi: 10.1038/s41586-020-2649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Li P, Wang D, Wang T, Hao D, Qu X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci Rep. 2021;11:4628. doi: 10.1038/s41598-021-84031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N, Vogensen FK, van den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5(2):9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Stefanetti R, Trenell M, Welsh P, Kean S, Ford I, McConnachie A, Sattar N, Taylor R. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- Li W, Godzik A. CD-HIT: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics (Oxford, England) 2006;22(13):1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Li W-Z, Stirling K, Yang J-J, Zhang L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J Diabetes. 2020;11(7):293–308. doi: 10.4239/wjd.v11.i7.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Shao W, Gao M, Liu J, Guo Q, Jin J, Meng F. Changes in intestinal flora in patients with type 2 diabetes on a low-fat diet during 6 months of follow-up. Exp Ther Med. 2020;20(5):40. doi: 10.3892/etm.2020.9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H-F, Ren Z-G, Li A, Zhang H, Xu S-Y, Jiang J-W, Zhou L, Ling Q, Wang B-H, Cui G-Y, Chen X-H, Zheng S-S, Li L-J. Fecal microbiome data distinguish liver recipients with normal and abnormal liver function from healthy controls. Front Microbiol. 2019;10:1518. doi: 10.3389/fmicb.2019.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Li Y, Wang J, Li P, Duan Y, Dai H, An Y, Cheng L, Wang T, Wang C, Wang T, Zhao B. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomed Pharmacother. 2020;124:109873. doi: 10.1016/j.biopha.2020.109873. [DOI] [PubMed] [Google Scholar]
- Mallott EK, Amato KR. Butyrate production pathway abundances are similar in human and nonhuman primate gut microbiomes. Mol Biol Evol. 2022;39(1):279. doi: 10.1093/molbev/msab279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinforma. 2012;13(1):31. doi: 10.1186/1471-2105-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney W (2010) Data structures for statistical computing in Python, Austin, Texas, pp 56–61. 10.25080/Majora-92bf1922-00a, https://conference.scipy.org/proceedings/scipy2010/mckinney.html
- Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J, Passos A, Cournapeau D, Brucher M, Perrot M, Duchesnay É. Scikit-learn: Machine Learning in Python. J Mach Learn Res. 2011;12(85):2825–2830. [Google Scholar]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto J-M, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(D1):590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, Flint HJ, Louis P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8(6):1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon D, Sroka-Oleksiak A, Kapusta P, Szopa M, Mrozińska S, Ludwig-Słomczyńska AH, Wołkow PP, Bulanda M, Klupa T, Małecki MT, Gosiewski T. Characteristics of gut microbiota in adult patients with type 1 and type 2 diabetes based on next-generation sequencing of the 16S rRNA gene fragment. Pol Arch Intern Med. 2018;128(6):336–343. doi: 10.20452/pamw.4246. [DOI] [PubMed] [Google Scholar]
- Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27(3):379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem. 2019;63:101–108. doi: 10.1016/j.jnutbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31(1):69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH. Measurement of diversity. Nature. 1949;163(4148):688–688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- Student The probable error of a mean. Biometrika. 1908;6(1):1–25. doi: 10.2307/2331554. [DOI] [Google Scholar]
- The pandas development team (2020) pandas-dev/pandas: Pandas 1.0.3. Zenodo. 10.5281/zenodo.3715232
- Thingholm LB, Rühlemann MC, Koch M, Fuqua B, Laucke G, Boehm R, Bang C, Franzosa EA, Hübenthal M, Rahnavard A, Frost F, Lloyd-Price J, Schirmer M, Lusis AJ, Vulpe CD, Lerch MM, Homuth G, Kacprowski T, Schmidt CO, Nöthlings U, Karlsen TH, Lieb W, Laudes M, Franke A, Huttenhower C. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe. 2019;26(2):252–26410. doi: 10.1016/j.chom.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hul M, Le Roy T, Prifti E, Dao MC, Paquot A, Zucker J-D, Delzenne NM, Muccioli G, Clément K, Cani PD. From correlation to causality: the case of Subdoligranulum. Gut Microbes. 2020;12(1):1–13. doi: 10.1080/19490976.2020.1849998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J, van der Walt SJ, Brett M, Wilson J, Millman KJ, Mayorov N, Nelson ARJ, Jones E, Kern R, Larson E, Carey CJ, Polat İ, Feng Y, Moore EW, VanderPlas J, Laxalde D, Perktold J, Cimrman R, Henriksen I, Quintero EA, Harris CR, Archibald AM, Ribeiro AH, Pedregosa F, van Mulbregt P. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020;17(3):261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (Meta)genomic Data. mBio. 2014;5(2):00889–14. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital M, Karch A, Pieper DH. Colonic butyrate-producing communities in humans: an overview using omics data. mSystems. 2017;2(6):00130–17. doi: 10.1128/mSystems.00130-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon F. Individual comparisons by ranking methods. Biom Bull. 1945;1(6):80–83. doi: 10.2307/3001968. [DOI] [Google Scholar]
- Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, Yu P, Zhao C, Li L, Zhou A, Wang J, Moore JE, Cherie Millar B, Xu J. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010;61(1):69–78. doi: 10.1007/s00284-010-9582-9. [DOI] [PubMed] [Google Scholar]
- WHO, WHO (2020) WHO reveals leading causes of death and disability worldwide: 2000-2019. fact sheet. https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019
- Yarza P, Yilmaz P, Pruesse E, Glöckner FO, Ludwig W, Schleifer K-H, Whitman WB, Euzéby J, Amann R, Rosselló-Móra R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol. 2014;12(9):635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2016;92(1084):63–69. doi: 10.1136/postgradmedj-2015-133281. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. 2013;8(8):71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang M, Yang J, Xu Q, Liang C, Chen B, Zhang J, Yang Y, Wang H, Shang Y, Wang Y, Mu X, Zhu D, Zhang C, Yao M, Zhang L. Response of gut microbiota in type 2 diabetes to hypoglycemic agents. Endocrine. 2019;66(3):485–493. doi: 10.1007/s12020-019-02041-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used are not publicly available because the research project was carried out in collaboration with the company BIOMES NGS GmbH. They can be requested with a reasoned request to the corresponding author.