Abstract
Introduction
Metabolomics produces vast quantities of data but determining which metabolites are the most relevant to the disease or disorder of interest can be challenging.
Objectives
This study sought to demonstrate how behavioral models of psychiatric disorders can be combined with metabolomics research to overcome this limitation.
Methods
We designed a preclinical, untargeted metabolomics procedure, that focuses on the determination of central metabolites relevant to substance use disorders that are (a) associated with changes in behavior produced by acute drug exposure and (b) impacted by repeated drug exposure. Untargeted metabolomics analysis was carried out on liquid chromatography-mass spectrometry data obtained from 336 microdialysis samples. Samples were collected from the medial striatum of male Sprague-Dawley (N = 21) rats whilst behavioral data were simultaneously collected as part of a (±)-3,4-methylenedioxymethamphetamine (MDMA)-induced behavioral sensitization experiment. Analysis was conducted by orthogonal partial least squares, where the Y variable was the behavioral data, and the X variables were the relative concentrations of the 737 detected features.
Results
MDMA and its derivatives, serotonin, and several dopamine/norepinephrine metabolites were the greatest predictors of acute MDMA-produced behavior. Subsequent univariate analyses showed that repeated MDMA exposure produced significant changes in MDMA metabolism, which may contribute to the increased abuse liability of the drug as a function of repeated exposure.
Conclusion
These findings highlight how the inclusion of behavioral data can guide metabolomics data analysis and increase the relevance of the results to the phenotype of interest.
Keywords: Metabolomics, Behavior, Addiction, MDMA, Sensitization, LCMS
Introduction
There is an urgent need for new prevention and treatment strategies that can reduce the significant medical, financial, and emotional burden caused by substance use disorders (SUDs). It is now well accepted that SUDs are disorders of the brain, and some of the brain mechanisms that are altered by repeated exposure to drugs that are misused have been identified. Treatments that have targeted these mechanisms have not, however, been effective for many SUDs (Forray & Sofuoglu, 2014; Nestler, 2022; Volkow et al., 2016). Novel and more efficient means to identify relevant brain mechanisms that are altered by repeated drug exposure might lead to more effective treatment avenues.
Metabolomics is the large-scale analysis of small molecules (< 1500 Da) within cells, biofluids, tissues, or organisms (Fiehn, 2002; Mamas et al., 2011; Patti et al., 2012; Shulaev, 2006; Zhou et al., 2012). There are two main approaches within metabolomic research: targeted and untargeted. Targeted metabolomics quantitatively measures a limited number of known metabolites and is carried out using a list of target analytes and prepared external standards. Since the metabolites of interest must be known a priori, targeted metabolomics is partially hypothesis-driven, which prevents the discovery of novel or unexpected metabolites that might be relevant. Untargeted metabolomics, in contrast, aims to semi-quantitively measure all metabolites detectable within the sample. Thus, untargeted metabolomics is hypothesis-generating, and can be used as an efficient, high-throughput means for the potential discovery of novel diagnostic, prognostic, or therapeutic biomarkers of disease.
There have been significant advances in metabolomics research recently and both targeted and untargeted approaches have been used in a variety of applications including pharmacology, toxicology, food science and nutrition, and bioengineering (Giera et al., 2022). However, the application of metabolomics for the study of psychiatric disorders, particularly SUD, is still in its infancy. Several studies have been successful in identifying metabolomic changes associated with SUD in human blood/urine samples, or associated with drug exposure in laboratory animals, but the relevance of many of these metabolomic changes is limited (for reviews see Ghanbari and Sumner, 2018; Mussap et al., 2020; Sethi & Brietzke, 2016; Wang et al., 2016; Zaitsu et al., 2016). For example, in human studies, the analysis of peripheral metabolites from blood/urine samples may not provide the best insight into the underlying neurobiological mechanisms of psychiatric disorders such as SUDs. Additionally, studies in human subjects are limited because of the ethical constraints of repeatedly administering drugs with misuse potential and the inability to adequately control for critical environmental and genetic variables. These limitations are not relevant to preclinical studies. Nonetheless, only a small number of preclinical studies have examined the effect of acute versus repeated drug exposure on the metabolome, and little to no information about drug-produced changes in metabolite levels as a function of time have been obtained. Moreover, valuable behavioral data is rarely collected or utilized, which we suggest can be used to guide metabolomics data analysis in order to increase the relevance of the results to the disorder phenotype of interest and improve translation.
To this end, we have designed a preclinical, untargeted metabolomics procedure that focuses on the determination of central metabolites that are (a) associated with changes in behavior produced by acute drug exposure and (b) impacted by repeated drug exposure. A key component of this procedure is the use of microdialysis as the sampling method (Chefer et al., 2009; van Mever et al., 2021; Westerink & Cremers, 2007). Brain microdialysis involves the perfusion of artificial cerebrospinal fluid through a small probe equipped with a semi-permeable membrane that has been implanted into the brain region of interest. Neurotransmitters, metabolites, and other small molecules present in the extracellular space diffuse across this membrane and into the perfusate, which is subsequently analyzed for its constituents, typically by high performance liquid chromatography (HPLC) coupled with various detectors. As a sampling procedure for metabolomics, microdialysis offers several important advantages. First and foremost, microdialysis permits neurochemical samples to be collected from awake, freely moving subjects, which allows for the concurrent collection of valuable behavioral data. Second, microdialysis provides important temporal information since samples can be collected every ~ 5–30 min. Lastly, microdialysis only samples the extracellular fluid from a relatively discrete brain region, and therefore, offers results that are much more relevant to neurotransmission and behavior compared to whole tissue analysis.
As a proof of concept, we employed this procedure to study the effects of repeated exposure to the popular recreational drug of misuse, (±)-3,4-methylenedioxymethamphetamine (MDMA) on the neuro-metabolome of rats. Repeated intermittent exposure to MDMA increased MDMA-produced locomotor hyperactivity and facilitated the acquisition of MDMA self-administration, indicating that repeated exposure had sensitized these behavioral responses (van de Wetering & Schenk, 2017). This was not due to changes in MDMA-induced extracellular concentrations of serotonin (5-HT) or MDMA in the striatum, suggesting that other neurochemical mechanisms may be more important for the development of sensitization to the effects of MDMA (van de Wetering et al., 2022). In the current study, we use this untargeted, behavioral metabolomics procedure to identify other potential neurochemical correlates of sensitized MDMA-produced behavior following repeated exposure in rats.
Methods
In this study, untargeted metabolomics analysis was carried out on liquid chromatography–mass spectrometry (LC-MS) data obtained from 336 microdialysis samples collected during a previously conducted behavioral sensitization experiment in rats (van de Wetering et al., 2022). The animal treatment and sample collection/analysis methods are summarized below.
Animals, treatments, and microdialysis
As previously reported (van de Wetering et al., 2022), adult, male Sprague-Dawley rats (n = 21) were stereotaxically implanted with intracerebral guide cannula (9.14. IC, Microbiotech, Sweden) in the medial striatum. One week later, rats began an 8-day MDMA sensitization experiment. On days 1–5, rats were placed into locomotor activity chambers (42 × 42 × 30 cm; Med Associates Inc., USA; model ENV-515) for 30 min prior to receiving an intraperitoneal (i.p.) injection of physiological saline (n = 11) or 10 mg/kg MDMA (n = 10) and remained in these chambers for an additional 60 min thereafter. This pre-treatment regimen was used as it has previously been shown to reliably induce behavioral sensitization as well as enhance the acquisition of MDMA self-administration (van de Wetering & Schenk, 2017; Wetering & Schenk, 2020). On the 8th day, microdialysis probes (MAB 9.14.3, Microbiotech, Sweden) were inserted into the guide cannula and rats were returned to the locomotor activity chambers. After a 3-hour stabilization period, microdialysis samples were collected at 30 min intervals for 8 h using a microinfusion pump (HD 2000 infusion, Harvard Apparatus) with a flow rate of 0.5 µL/min. During this time, all subjects received ascending doses of MDMA (0.0, 5.0, 10.0, mg/kg, i.p.) at 2-hour intervals. Locomotor activity was simultaneously recorded throughout and summed into 30 min intervals. These doses of MDMA produce moderate levels of locomotor activity following acute exposure and behavioral sensitization in response to these doses was apparent in MDMA pre-treated animals (van de Wetering & Schenk, 2017; Wetering & Schenk, 2020).
Sample preparation and LCMS analysis
As previously reported (van de Wetering et al., 2022), 2.5 µL of internal standard (100 nM D2-5-HT), 7.5 µL of borate buffer (sodium tetraborate, 100 nM), and 7.5 µL of BzCl (diluted to 5% in acetonitrile) was added to each 15 µL microdialysate sample, with mixing in between each addition, and then stored at -80 °C until analysis. Analysis was carried out using 30 µL of derivatized sample injected onto a Poroshell 120 SB-Aq 2.7 μm column (2.1 × 100 mm; Agilent Technologies) installed in an Agilent Technologies (Santa Clara, CA) 1260 HPLC connected to a 6530 quadrupole time-of-flight (Q-TOF) LC-MS equipped with a Jet-Stream electrospray ionization (ESI) source, using settings as previously described (van de Wetering et al., 2022).
Data processing
LC-MS metabolomics data were processed using the open-source software, MZmine (v2.51) (Li et al., 2018; Myers et al., 2017; Pluskal et al., 2010). Data processing parameters are shown in Table 1. Parameters were optimized to ensure accurate automated separation and integration of the internal standard (D2-5-HT) as well as some known analytes (5-HT, MDMA) that were quantified using targeted methods in our previous study (van de Wetering et al., 2022). Features that were not detected in at least 50% of samples were excluded from further analysis. The final feature list contained 737 features.
Table 1.
MZmine data processing parameters
| Mass detection (centroid): | |
| Noise level: 104 | |
| Chromatogram builder (ADAP): | |
| Minimum group size in # scans: 3 | |
| Group intensity threshold: 104 | |
| Minimum highest intensity: 104 | |
| m/z tolerance: 0.02 m/z | |
| Deconvolution (local minimum search): | |
| m/z center calculator: median | |
| Chromatographic threshold: 35% | |
| Search minimum in RT range: 0.01 min | |
| Minimum relative height: 5% | |
| Minimum absolute height: 104 | |
| Minimum ratio of peak top/edge: 2 | |
| Peak duration range: 0.02–0.5 min | |
| Isotope peak grouper: | |
| m/z tolerance: 0.005 m/z | |
| RT tolerance: 0.01 min absolute | |
| Monotonic shape: yes | |
| Maximum charge: 2 | |
| Representative isotope: lowest m/z | |
| Alignment (join aligner): | |
| m/z tolerance: 0.02 m/z | |
| Weight for m/z: 3 | |
| RT tolerance: 0.25 min absolute | |
| Weight for RT: 2 | |
| Gap-filling (same RT and m/z range): | |
| m/z tolerance: 0.01 m/z |
Statistical analysis
Principal components analysis (PCA) was used to provide an initial overview of the dataset. Because PCA is an unsupervised method that aims to explain the maximum variance in the data, differences between two treatments groups (e.g., drug-treated vs. drug naïve) will only be revealed if the between-group variance exceeds the within-group variance, which is often not the case with untargeted metabolomics data (Álvarez-Sánchez et al., 2010; Álvarez-Sánchez et al., 2010; Worley and Powers, 2013). Furthermore, our data set contains group × time-course metabolomics and behavioral data, which no single analysis is capable of fully describing.
Therefore, a two-step analytical procedure was carried out to determine which metabolites are of interest. First, in order to identify metabolites that were associated with changes in behavior produced by acute drug exposure, an orthogonal partial least squares (OPLS) model was fitted with the Y variable coded as the measured locomotor response and the relative concentrations of each detected feature/metabolite as X variables (Trygg & Wold, 2002; Wold et al., 1998). Variable importance on projection (VIP) scores were then used to determine which variables have the greatest impact on the model, with 1 being the threshold value (Galindo-Prieto et al., 2014, 2015). Second, in order to determine which metabolites were impacted by repeated drug exposure and differed as a function of MDMA pre-treatment group, 2 (pre-treatment group: saline, MDMA) × 16 (time: 0–480 min) analysis of variance (ANOVA), with time as a repeated measure, were carried out on select metabolites of interest (as determined by step one), followed by Šidák-corrected multiple comparisons.
All metabolomics data were normalized to the internal standard peak area. For multivariate analyses, data were log-transformed in order to achieve a normal distribution, and Pareto scaled so as to avoid highly abundant exogenous compounds such as MDMA from dominating the statistical models, as is recommended for MS metabolomics data (Livera et al., 2015; Sysi-Aho et al., 2007; van den Berg et al., 2006; Veselkov et al., 2011; Wheelock & Wheelock, 2013). Multivariate analyses were carried out using Soft Independent Modelling of Class Analogies (SIMCA; v17, Umetrics, Umeå Sweden). Both PCA and OPLS models were autofitted by SIMCA, and the default K-fold cross-validation procedure was performed. The overall fit of the model was determined by examining the cumulative R2X and R2Y, which represents the fraction of explained variation in X and Y, respectively. The predictive power of the model was assessed by examining Q2, which represents the fraction of variation explained by the cross-validated model. R2 and Q2 values that are relatively similar and above 0.5 represent a good model (Szymańska et al., 2012; Triba et al., 2015; Wheelock & Wheelock, 2013). Univariate testing was carried out using GraphPad Prism (v9.1.0, GraphPad Software, San Diego, California USA), with the level of significance set as p < .05.
Metabolite annotation and LC-MS/MS
Select metabolites of interest were identified or annotated using external standards or targeted LC-MS/MS, respectively. For LC-MS/MS, microdialysate samples were pooled and 10 µL was analyzed in duplicate using the same chromatography method as previously described (van de Wetering et al., 2022) and the following targeted Q-TOF ESI-MS/MS parameters: positive ion mode; gas temperature, 275 °C; gas flow, 8 L/m; nebulizer, 30 psi; capillary voltage, 2750 V; nozzle voltage, 0 V; fragmentor voltage, 130 V; acquisition rate, 2 spectra/s; mass range, m/z 50–500; collision-induced dissociation energies, 10, 20, and 40 eV. The resulting fragmentation patterns were compared to those recorded in the METLIN database (Smith et al., 2005) and with two previous studies that have characterized the MS/MS fragmentation of several BzCl-derivatized metabolites in microdialysis samples (Song et al., 2012; Wong et al., 2016).
Results
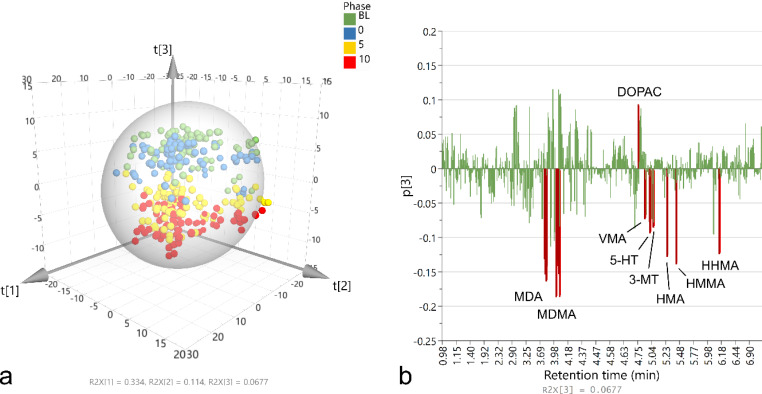
An overview of the full data set (737 features detected across 336 samples) was provided by PCA (R2X = 0.91, Q2 = 0.85). Figure 1 A shows the resulting 3D scores plot, which plots each sample’s score on the first 3 components (T[1–3]). Here, samples that cluster together represent those with a similar metabolomic profile. Some minor outliers were present, as determined by the ellipse/sphere representing the 95% confidence interval for Hotelling’s T2 test. No grouping was apparent on components 1 or 2 based on any experimental manipulation indicating that the primary variation in the dataset was uninduced. Clear grouping of the data along component 3 was observed based on acute MDMA dose, however. Figure 1B shows a histogram plotting the loading of each variable on component 3 (p[3]) as a function of LC-MS retention time. Several variables had a strong loading on component 3 and were driving the aforementioned grouping of samples based on acute MDMA dose. These included MDMA itself, 3,4-methylenedioxyamphetamine (MDA), 3,4-dihydroxymethamphetamine (HHMA), 4-hydroxy-3-methoxymethamphetamine (HMMA), 4-hydroxy-3-methoxyamphetamine (HMA), 5-HT, 3-methoxytyramine (3-MT), vanillylmandelic acid (VMA), and 3,4-dihydroxyphenylacetic acid (DOPAC). Note that the highly abundant exogenous compounds such as MDMA and MDA had multiple detected adducts and dimers (e.g., M + Na, 2M + H, 2M + Na etc.) as well as several fragments generated from in-source fragmentation (e.g., m/z 105 – benzoyl fragment).
Fig. 1.
Results of PCA on LC-MS metabolomics data collected from 336 microdialysis samples. a) 3D scores plot of the first three components with samples shaded according to acute MDMA dose (baseline [BL; green], 0.0 [blue], 5.0 [yellow], 10.0 [red] mg/kg). Ellipse/sphere represents Hotelling T2 (95%). b) Histogram showing variable loadings for component 3 as a function of retention time. R2X values of each component are shown at the bottom of each graph
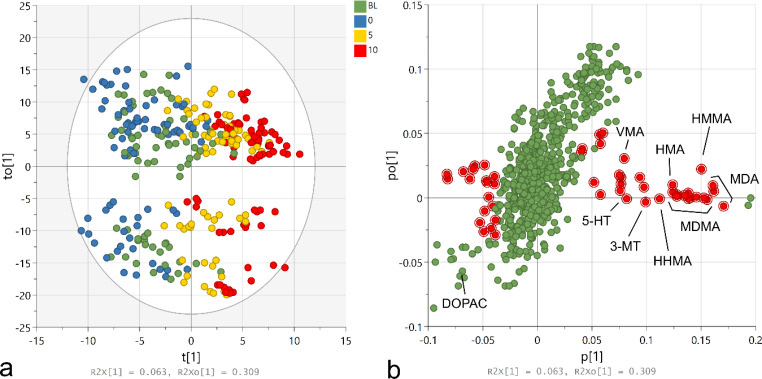
In order to determine which metabolites were dose-dependently associated with acute, MDMA-produced behavior over time, an OPLS model was fitted to the full data set with the Y variable coded as the locomotor activity (R2Y = 0.60, R2X = 0.57, Q2 = 0.46). Figure 2 A shows the resulting scores plot, where t[1] represents the predictive component and to[1] represents the first orthogonal component. Clear grouping of samples according to acute MDMA dose was observed along the predictive component as well as large orthogonal variation. Figure 2B shows the loadings plot, where each variable is plotted as a function of its predictive (p[1]) and orthogonal (po[1]) loadings. The variables that were the most strongly predictive of locomotor activity while also having little orthogonal variation, as determined by having predictive VIP scores above 1 and orthogonal VIP scores below 1, are highlighted in red. Figure 3 shows a summary of the metabolites of interest as identified by the OPLS model using these criteria, after filtering for non-unique metabolites (i.e., adducts, fragments) and a cluster of metabolites whose relative concentrations were impacted by ion suppression due to co-elution with the highly abundant MDMA. Of the metabolites/compounds that were detected in our samples, the strongest predictors of MDMA-induced locomotor activity were MDA, followed by MDMA, HMMA, 3-MT, 5-HT, and VMA. DOPAC was also a strong negative predictor of locomotor activity (VIP = 1.84) but had high orthogonal variation. Several unidentified/annotated metabolites were also predictive of locomotor activity.
Fig. 2.
Results of the OPLS model assessing variables predictive of locomotor activity. a) Scores plot showing samples shaded according to acute MDMA dose (baseline [BL; green], 0.0 [blue], 5.0 [yellow], 10.0 [red]). Ellipse represents Hotelling T2 (95%). b) Loadings plot with variables of interest highlighted in red as determined by predictive-VIP scores > 1 and orthogonal-VIP scores < 1. R2X values of each component are shown at the bottom of each graph
Fig. 3.
Mean (± standard error of the mean) locomotor activity and relative concentration of select metabolites of interest as a function of time and MDMA pre-treatment group. MDMA 0.0, 5.0, and 10.0 mg/kg i.p. was administered at 120, 240, and 360 min, respectively. aID based on external standards. bID based on MS/MS fragmentation data. cID based on mass and adduct pattern. *p < .05, **p < .01, ***p < .001, ****p < .0001 compared to control; two-way ANOVA followed by Šidák-corrected multiple comparisons
To determine which of these metabolites were impacted by repeated MDMA exposure, separate two-way ANOVAs (treatment × time) followed by Šidák-corrected multiple comparisons were used to assess changes in the relative concentration of these metabolites of interest as a function of MDMA pre-treatment group. Significant interaction effects were found for MDA, F(15, 285) = 1.87, p = .0256, and HMA, F(15, 285) = 4.08, p < .0001, and multiple comparisons indicated significantly higher MDMA-produced MDA and HMA concentrations were present in the MDMA pre-treated group at later time points (p < .05; Fig. 3).
Metabolite annotation
Ions detected at m/z 298.14, 385.15, and 394.12 were identified as the protonated molecular ions of benzoylated MDMA, 5-HT, and DOPAC + NH4, respectively, with the use of external standards. Targeted MS/MS was used to annotate other metabolites of interest in pooled microdialysate samples (Fig. 4). The ion at m/z 384.13 produced a fragmentation pattern similar to MDMA and was annotated as MDA, while m/z 404.19 was annotated as HMMA, having produced a unique fragment at m/z 269.12, which would be expected if it fragmented in the same location as both MDMA and MDA. The ion detected at m/z 376.15 was annotated as benzoylated 3-MT; the primary product was the benzoyl fragment (m/z 105) while the most abundant unique fragment was at m/z 151.07, which is consistent with previous reports (Wong et al., 2016) and the METLIN database. No conclusive MS/MS fragmentation data were obtained for other metabolites of interest, but m/z 494.19 and 390.17 were suspected to be benzoylated HHMA and HMA, respectively, based on their mass and adduct pattern. Lastly, m/z 320.09 was suspected to be benzoylated VMA + NH4 based on previous reports (Wong et al., 2016).
Fig. 4.
a) Q-TOF-ESI MS/MS spectra of benzoylated MDMA, MDA, HMMA, and 3-MT at 10, 20, and 40 eV collision energies. b) Proposed fragmentation of MDMA, MDA, HMMA, and 3-MT
Discussion
In the current study, we designed a new method to improve the translatability of preclinical metabolomics research and help filter through potentially overwhelming and often noisy datasets. Using a combination of microdialysis and behavioral measures, we show a small number of behaviorally relevant changes in metabolites that are impacted by MDMA exposure.
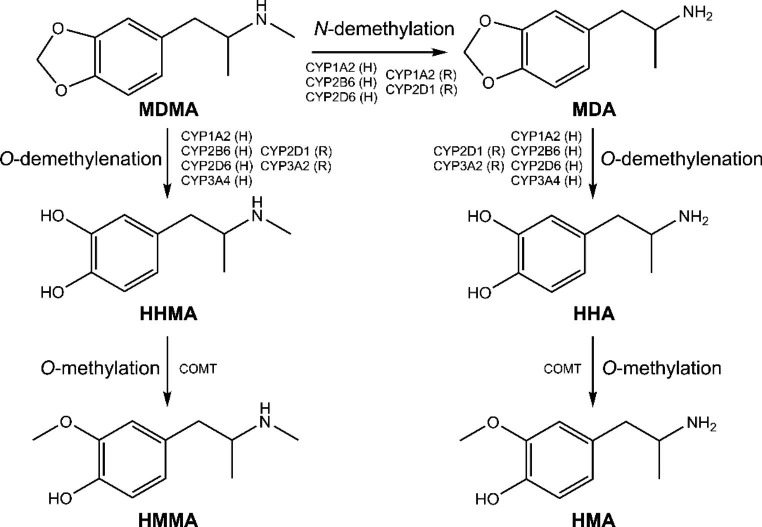
In the first step of our analysis, we fitted an OPLS model with locomotor activity as the Y variable and the metabolomics data as X variables. By doing so, we were effectively able to determine which metabolites were most associated with dose-dependent increases in MDMA-produced locomotor activity over time. MDMA and its derivatives, MDA, HMA, HHMA, and HMMA (Fig. 5), were among the highest predictors of MDMA-induced locomotor activity, as was expected. MDMA is a potent psychostimulant (Cole & Sumnall, 2003). It induces the release and prevents the reuptake of the monoamine neurotransmitters, 5-HT, dopamine (DA), and norepinephrine (NE) (Green et al., 2003), all of which have well-established locomotor-activating effects (Ball et al., 2003; Baumann et al., 2008b; Berger et al., 1992; Bubar et al., 2004; Callaway et al., 1990, 1992; Daniela et al., 2004; Fantegrossi et al., 2004; Gold et al., 1989; Hekmatpanah & Peroutka, 1990; Kehne et al., 1996; Selken & Nichols, 2007). In line with this, our results indicated that 5-HT and several DA/NE-derived metabolites including 3-MT, VMA, and DOPAC, were also important predictors of MDMA-induced locomotor activity, though we were not able to directly detect DA or NE in our samples due to methodological parameters. The relative concentrations of these metabolites were all associated with dose-dependent increases in MDMA-induced locomotor activity, either positively or negatively, and changed as a function of acute MDMA administration in a manner consistent with previous quantitative studies (Baumann, Clark, & Rothman, 2008; Baumann, Clark, Franken, Baumann et al., 2008a, b; Bradbury et al., 2013; Colussi-Mas et al., 2010; Fernández-Galaz et al., 1993; Gough et al., 2006; Kankaanpää et al., 1998; Kurling et al., 2008; Morales-Villagrán et al., 1999; Nash, 1990; O’Shea et al., 2005).
Fig. 5.
Primary metabolic pathways of MDMA and the CYP enzymes involved in both rats (R) and humans (H) (de la Torre & Farré, 2004)
Of the 700 + features detected in our samples, approximately 50 were associated with MDMA-produced behavior, and fewer than 20 of these were unique metabolites/compounds. Given that our data set was already reduced to a manageable size, we proceeded to use univariate statistics in our second analytical step, which aimed to evaluate these ~ 20 behaviorally relevant metabolites for differences as a function of MDMA pre-treatment group and identify those that were also impacted by repeated MDMA exposure1. Our results suggest potential changes in MDMA metabolism. Although the concentration of MDMA itself did not change as a function of repeated MDMA exposure, the concentration of both MDA (a primary MDMA metabolite) and HMA (a metabolite of MDA) were significantly higher in MDMA pre-treated rats compared to drug naïve controls. These results were mirrored, albeit non-significantly, by decreases in HHMA (a primary MDMA metabolite) and HMMA (a metabolite of HHMA). Together, these results might suggest an increase in MDMA N-demethylation relative to other metabolism pathways as a function of repeated MDMA exposure (Fig. 5) and could be due to changes in cytochrome P450 (CYP) enzymatic activity associated with each metabolic pathway (de la Torre et al., 2004; de la Torre & Farré, 2004).
While most metabolites of MDMA (including HMA) have been shown to have little direct effect on locomotor activity (Schindler et al., 2014; Yeh & Hsu, 1991), MDA is a particularly potent psychostimulant and has been shown to be even more effective at stimulating locomotor activity than equivalent doses of MDMA (20 mg/kg i.p.; Bexis and Docherty, 2006). MDA also has greater potency to increase synaptic overflow of DA compared to MDMA (Johnson et al., 1986; Nash & Nichols, 1991). DA is an important driver of locomotor activity, and it is well-established that the development of sensitized behavioral responses to psychostimulants, including MDMA, are driven by sensitized DAergic mechanisms (Ball et al., 2006, 2009; Bradbury et al., 2012; Kalivas et al., 1998; Morgan et al., 1997; van de Wetering & Schenk, 2017; Vanderschuren & Kalivas, 2000; Vezina, 2007). Although we were not able to detect DA itself in our samples, we did see trending effects of repeated MDMA exposure on both 3-MT and DOPAC (both primary DA metabolites), as well as VMA (an end-stage catecholamine metabolite) that would be consistent with a sensitized DAergic response. Our findings, therefore, suggest that behavioral sensitization to MDMA may, in part, be driven by increased MDA concentrations as a function of repeated MDMA exposure and the more potent effect of MDA on DAergic mechanisms associated with psychostimulant sensitization.
We have previously shown that rats that develop sensitization to the locomotor activating effects of MDMA, also develop sensitization to the rewarding effects of MDMA, as indicated by significant increases in both the rate and proportion of rats that acquire MDMA self-administration (van de Wetering & Schenk, 2017). Sensitization to the effects of psychostimulants drugs has long been suggested as an important factor underlying SUDs (Robinson & Berridge, 1993; Vanderschuren & Pierce, 2010; Vezina, 2007). Thus, the current findings provide valuable insight on the mechanisms underlying the development of sensitization to the rewarding effects of MDMA and suggest that increased turnover of MDMA into MDA as a function of repeated exposure might increase the abuse liability of the drug. These findings contribute to the growing area of research that focusses on the development of pharmacokinetics-based treatments for SUDs (Gorelick, 2012; Lin et al., 2021; Zheng et al., 2022).
It should be noted that the metabolism of MDMA is complex, however. Although MDMA metabolism is qualitatively similar across different animal species, and involves the same primary metabolic pathways and homologous CYP isoenzymes (Fig. 5), these enzymes are not functionally identical, and can have different Vmax values resulting in different metabolic pathways being prevalent at different doses in different species (Bogaards et al., 2008; de la Torre & Farré, 2004). MDMA can also inhibit its own metabolism, through the formation of complexes with CYP enzymes (de la Torre et al., 2000; Delaforge et al., 1999). This may further interact with genetic polymorphisms in CYP enzymes, which have been shown to impact the metabolism of MDMA into MDA in humans (de la Torre et al., 2012; Vizeli et al., 2017). Finally, to add to this complexity, our findings suggest that the metabolism of MDMA can change as a function of repeated exposure. Therefore, future studies using targeted, hypothesis-driven approaches are needed to clarify the role of MDMA metabolism, MDA, and DA in the development of sensitization to the effects of MDMA and the impact of this on MDMA use disorders in humans.
Limitations
There are some limitations to the current study that should be discussed. Firstly, the derivatization and LC-MS parameters were optimized for the targeted quantification of 5-HT and MDMA for a previous, hypothesis-driven study (van de Wetering et al., 2022), and not for untargeted metabolomics. Metabolomics analysis of the samples was retrospective and as a result, the dataset was noisy and limited; several relevant metabolites (e.g., DA) that should be detectable in BzCl derivatized microdialysate samples were not detected (Song et al., 2012; Wong et al., 2016), and there was a large amount of uninduced variation. Nevertheless, the study served as a proof of concept. The use of supervised OPLS helped to identify behaviorally relevant results amongst significant noise. With some optimization of the derivatization and LC-MS procedures for untargeted metabolomics, detection of a wider range of metabolites could be achieved in order to cast a larger ‘metabolomic net’ and generate a larger dataset for analysis. Secondly, while microdialysis offers several advantages as a neurochemical sampling procedure, it is technically challenging due to the low sample volumes obtained and low concentrations of metabolites (for reviews see van Mever et al., 2021; Zestos & Kennedy, 2017). To overcome this, we adapted BzCl derivatization procedures from a previous targeted study in order to increase metabolite stability and improve detection limits (Song et al., 2012; Wong et al., 2016). Of course, the issue with derivatization and untargeted metabolomics is metabolite identification. BzCl reacts with both primary and secondary amines, phenols, and ribose-hydroxyl groups (Song et al., 2012; Wong et al., 2016). Because BzCl derivatization adds a varying number of benzoyl groups to metabolites, determination of the original mass becomes challenging, and the results cannot be simply compared to large databases. A potential means to address this issue is to use elementally labelled BzCl (i.e., bromo- or chloro-benzoyl chloride), which could isotopically elucidate the number of BzCl adducts of each metabolite in order to more readily calculate the original mass for comparison against metabolomics databases (Castro-Falcón et al., 2016; Schäfer et al., 2023).
Conclusions
There is an increasing interest in monitoring dynamic changes in the metabolome over time (Rusilowicz et al., 2018; Smilde et al., 2010). Microdialysis provides the means to achieve this in vivo by sampling central metabolites with relatively high temporal resolution when compared to other metabolomics sampling techniques. While a handful of studies have developed targeted metabolomics procedures using microdialysis (Bongaerts et al., 2018; Song et al., 2012; Wong et al., 2016), the current study is the first untargeted metabolomics study. This is also the first metabolomics study to collect and analyze behavioral data alongside metabolomics data. A commonly reported limitation of -omics research, including metabolomics, is the poor translation of results to the disease or disorder phenotype (Humer et al., 2020; Sethi & Brietzke, 2016; Wang et al., 2016). By utilizing behavioral models of psychiatric disorders, and incorporating the behavioral data into the metabolomics analyses, we show that it is possible to determine which metabolites are most associated with the behavior of interest. This novel approach will be applicable to the study of drug effects and other research areas in all subjects regardless of species, age, or sex, and can improve the relevance and translatability metabolomics research.
Statements and declarations.
Acknowledgements
The authors gratefully acknowledge the technical contributions of Joyce Colussi-Mas, Michael Roberts, and Aimee Culverhouse.
Abbreviations
- 3-MT
3-methoxytyramine
- 5-HT
Serotonin
- ANOVA
Analysis of variance
- CYP
Cytochrome P450
- DA
Dopamine and
- DOPAC
3,4-dihydroxyphenylacetic acid
- ESI
Electrospray ionization
- HHMA
3,4-dihydroxymethamphetamine
- HMA
4-hydroxy-3-methoxyamphetamine
- HMMA
4-hydroxy-3-methoxymethamphetamine
- HPLC
High performance liquid chromatography
- LC-MS
Liquid chromatography–mass spectrometry
- MDA
3,4-methylenedioxyamphetamine
- MDMA
(±)-3,4-methylenedioxymethamphetamine
- NE
Norepinephrine
- OPLS
Orthogonal partial least squares
- PCA
Principal components analysis
- Q-TOF
Quadrupole time-of-flight
- SUD
Substance use disorder
- VIP
Variable importance on projection
- VMA
Vanillylmandelic acid
Author contributions
RVDW and SS conceived and designed research. RVDW conducted experiments, analyzed data, and wrote the manuscript. JEH, RAK, & SS provided supervision. All authors provided resources, reagents, or analytical tools and contributed to experiment methodology and validation. All authors reviewed and approved the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was funded in part by University Research Fund grants from Victoria University of Wellington.
Data availability
The availability of all data presented in this study is from authors upon request.
Declarations
Ethical statement
All experimental procedures involving animals were approved by the Victoria University of Wellington Animal Ethics Committee (Approval ID: 23853).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
With larger data sets, it would be recommended to first conduct supervised, class-based multivariate analyses (i.e., PLS-DA/OPLS-DA) and filter the results for behaviourally relevant metabolites before proceeding to any univariate analyses.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Álvarez-Sánchez B, Priego-Capote F, de Castro MDL. Metabolomics analysis II. Preparation of biological samples prior to detection. TrAC Trends in Analytical Chemistry. 2010;29(2):120–127. doi: 10.1016/J.TRAC.2009.12.004. [DOI] [Google Scholar]
- Álvarez-Sánchez B, Priego-Capote F, de Luque MD. Metabolomics analysis I. Selection of biological samples and practical aspects preceding sample preparation. TrAC Trends in Analytical Chemistry. 2010;29(2):111–119. doi: 10.1016/J.TRAC.2009.12.003. [DOI] [Google Scholar]
- Ball KT, Budreau D, Rebec G. Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: Differential involvement of dopamine D1 and D2 receptors. Brain Research. 2003;994(2):203–215. doi: 10.1016/j.brainres.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Ball KT, Budreau D, Rebec G. Context-dependent behavioural and neuronal sensitization in striatum to MDMA (ecstasy) administration in rats. The European Journal of Neuroscience. 2006;24(1):217–228. doi: 10.1111/j.1460-9568.2006.04885.x. [DOI] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Fortenberry E, Rebec G. Sensitizing regimens of (±)3, 4-methylenedioxymethamphetamine (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neuroscience. 2009;160(2):264–274. doi: 10.1016/J.NEUROSCIENCE.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience. 2008;152(3):773–784. doi: 10.1016/j.neuroscience.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacology Biochemistry and Behavior. 2008;90(2):208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger UV, Gu XF, Azmitia EC. The substituted amphetamines 3,4-methylenedioxymethamphetamine, methamphetamine, p-chloroamphetamine and fenfluramine induce 5-hydroxytryptamine release via a common mechanism blocked by fluoxetine and cocaine. European Journal of Pharmacology. 1992;215(2–3):153–160. doi: 10.1016/0014-2999(92)90023-W. [DOI] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Effects of MDMA, MDA and MDEA on blood pressure, heart rate, locomotor activity and body temperature in the rat involve α -adrenoceptors. British Journal of Pharmacology. 2006;147(8):926–934. doi: 10.1038/sj.bjp.0706688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaards JJP, Bertrand M, Jackson P, Oudshoorn MJ, Weaver RJ, Van Bladeren PJ, Walther B. Determining the best animal model for human cytochrome P450 activities: A comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Http://Dx Doi Org/10 1080/00498250010021684. 2008;30(12):1131–1152. doi: 10.1080/00498250010021684. [DOI] [PubMed] [Google Scholar]
- Bongaerts J, de Bundel D, Mangelings D, Smolders I, Heyden, van Eeckhaut A. Sensitive targeted methods for brain metabolomic studies in microdialysis samples. Journal of Pharmaceutical and Biomedical Analysis. 2018;161:192–205. doi: 10.1016/J.JPBA.2018.08.043. [DOI] [PubMed] [Google Scholar]
- Bradbury S, Gittings D, Schenk S. Repeated exposure to MDMA and amphetamine: sensitization, cross-sensitization, and response to dopamine D1- and D2-like agonists. Psychopharmacology (Berl) 2012;223(4):389–399. doi: 10.1007/s00213-012-2726-9. [DOI] [PubMed] [Google Scholar]
- Bradbury S, Bird J, Colussi-Mas J, Mueller M, Ricaurte GA, Schenk S. Acquisition of MDMA self-administration: Pharmacokinetic factors and MDMA-induced serotonin release. Addiction Biology. 2013;19(5):874–884. doi: 10.1111/adb.12069. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Pack KM, Frankel PS, Cunningham KA. Effects of dopamine D1- or D2-like receptor antagonists on the hypermotive and discriminative stimulus effects of (+)-MDMA. Psychopharmacology (Berl) 2004;173(3–4):326–336. doi: 10.1007/s00213-004-1790-1. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4- methylenedioxymethamphetamine in rats. Journal Of Pharmacology And Experimental Therapeutics. 1990;254(2):456–464. [PubMed] [Google Scholar]
- Callaway CW, Rempel NL, Peng RY, Geyer MA. Serotonin 5-HT1-like receptors mediate hyperactivity in rats induced by 3,4-methylenedioxymethamphetamine. Neuropsychopharmacology : Official Publication Of The American College Of Neuropsychopharmacology. 1992;7(2):113–127. [PubMed] [Google Scholar]
- Castro-Falcón G, Hahn D, Reimer D, Hughes CC. Thiol Probes to Detect Electrophilic Natural Products based on their mechanism of action. ACS Chemical Biology. 2016;11(8):2328–2336. doi: 10.1021/acschembio.5b00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of Brain Microdialysis. Current Protocols in Neuroscience. 2009;47(1):711–7128. doi: 10.1002/0471142301.ns0701s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA) Neuroscience & Biobehavioral Reviews. 2003;27(3):199–217. doi: 10.1016/S0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Colussi-Mas J, Wise RJ, Howard A, Schenk S. Drug seeking in response to a priming injection of MDMA in rats: Relationship to initial sensitivity to self-administered MDMA and dorsal striatal dopamine. International Journal Of Neuropsychopharmacology. 2010;13(10):1315–1327. doi: 10.1017/S1461145710000283. [DOI] [PubMed] [Google Scholar]
- Daniela E, Brennan KA, Gittings D, Hely L, Schenk S. Effect of SCH 23390 on (+/-)-3,4-methylenedioxymethamphetamine hyperactivity and self-administration in rats. Pharmacology Biochemistry and Behavior. 2004;77(4):745–750. doi: 10.1016/j.pbb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M. Neurotoxicity of MDMA (ecstasy): The limitations of scaling from animals to humans. Trends in Pharmacological Sciences. 2004;25(10):505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Ortuño J, Mas M, Brenneisen R, Roset PN, Segura J, Camí J. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. British Journal of Clinical Pharmacology. 2000;49(2):104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Camí J. Human pharmacology of MDMA: Pharmacokinetics, metabolism, and disposition. Therapeutic Drug Monitoring. 2004;26(2):137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- de la Torre, R., Yubero-Lahoz, S., Pardo-Lozano, R., & Farré, M. (2012). MDMA, methamphetamine, and CYP2D6 pharmacogenetics: What is clinically relevant? Frontiers in Genetics, 3(NOV), 10.3389/FGENE.2012.00235. [DOI] [PMC free article] [PubMed]
- Delaforge M, Jaouen M, Bouille G. Inhibitory metabolite complex formation of methylenedioxymethamphetamine with rat and human cytochrome P450. Particular involvement of CYP 2D. Environmental Toxicology and Pharmacology. 1999;7(3):153–158. doi: 10.1016/S1382-6689(99)00007-1. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, Leach PT, van Martin C, Karabenick RL, Chen X, Ohizumi Y, Ullrich T, Rice KC, Woods JH. Nantenine: An antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology (Berl) 2004;173(3–4):270–277. doi: 10.1007/S00213-003-1741-2/FIGURES/6. [DOI] [PubMed] [Google Scholar]
- Fernández-Galaz C, Herbison AE, Dyer RG. Characterization of Tritiated Noradrenaline Release from the rat preoptic area with Microdialysis in vivo. Journal of Neurochemistry. 1993;60(5):1806–1815. doi: 10.1111/J.1471-4159.1993.TB13407.X. [DOI] [PubMed] [Google Scholar]
- Fiehn, O. (2002). Metabolomics — the link between genotypes and phenotypes. Functional Genomics (pp. 155–171). Springer Netherlands. http://link.springer.com/10.1007/978-94-010-0448-0_11. [PubMed]
- Forray A, Sofuoglu M. Future pharmacological treatments for substance use disorders. British Journal of Clinical Pharmacology. 2014;77(2):382–400. doi: 10.1111/j.1365-2125.2012.04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Prieto B, Eriksson L, Trygg J. Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS) Journal of Chemometrics. 2014;28(8):623–632. doi: 10.1002/cem.2627. [DOI] [Google Scholar]
- Galindo-Prieto B, Eriksson L, Trygg J. Variable influence on projection (VIP) for OPLS models and its applicability in multivariate time series analysis. Chemometrics and Intelligent Laboratory Systems. 2015;146:297–304. doi: 10.1016/J.CHEMOLAB.2015.05.001. [DOI] [Google Scholar]
- Ghanbari R, Sumner SJ. Using metabolomics to investigate biomarkers of drug addiction. Trends in Molecular Medicine. 2018;24(2):197–205. doi: 10.1016/J.MOLMED.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Giera M, Yanes O, Siuzdak G. Metabolite discovery: Biochemistry’s scientific driver. Cell Metabolism. 2022;34(1):21–34. doi: 10.1016/J.CMET.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Hubner CB, Koob GF. A role for the mesolimbic dopamine system in the psychostimulant actions of MDMA. Psychopharmacology (Berl) 1989;99(1):40–47. doi: 10.1007/BF00634450. [DOI] [PubMed] [Google Scholar]
- Gorelick, D. A. (2012). Pharmacokinetic strategies for treatment of drug overdose and addiction. Http://Dx.Doi.Org/10.4155/Fmc.11.190, 4(2), 227–243. 10.4155/FMC.11.190. [DOI] [PMC free article] [PubMed]
- Gough B, Imam SZ, Blough BE, Slikker W, Ali SF. Comparative Effects of substituted amphetamines (PMA, MDMA, and METH) on Monoamines in Rat Caudate. Annals of the New York Academy of Sciences. 2006;965(1):410–420. doi: 10.1111/j.1749-6632.2002.tb04182.x. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacological Reviews. 2003;55(3):463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Hekmatpanah CR, Peroutka SJ. 5-Hydroxytryptamine uptake blockers attenuate the 5-hydroxytryptamine-releasing effect of 3,4-methylenedioxymethamphetamine and related agents. European Journal of Pharmacology. 1990;177(1–2):95–98. doi: 10.1016/0014-2999(90)90555-K. [DOI] [PubMed] [Google Scholar]
- Humer E, Probst T, Pieh C. Metabolomics in Psychiatric Disorders: What we learn from animal models. Metabolites 2020. 2020;10(2):72. doi: 10.3390/METABO10020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Hoffman AJ, Nichols DE. Effects of enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices. European Journal of Pharmacology. 1986;132(2–3):269–276. doi: 10.1016/0014-2999(86)90615-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, White SR. MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 1998;18(6):469–479. doi: 10.1016/S0893-133X(97)00195-4. [DOI] [PubMed] [Google Scholar]
- Kankaanpää A, Meririnne E, Lillsunde P, Seppälä T. The Acute Effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus Accumbens. Pharmacology Biochemistry and Behavior. 1998;59(4):1003–1009. doi: 10.1016/S0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Ketteler HJ, McCloskey TC, Sullivan CK, Dudley MW, Schmidt CJ. Effects of the selective 5-HT2A receptor antagonist MDL 100,907 on MDMA-induced locomotor stimulation in rats. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 1996;15(2):116–124. doi: 10.1016/0893-133X(95)00160-F. [DOI] [PubMed] [Google Scholar]
- Kurling S, Kankaanpää A, Seppälä T. Sub-chronic nandrolone treatment modifies neurochemical and behavioral effects of amphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in rats. Behavioural Brain Research. 2008;189(1):191–201. doi: 10.1016/j.bbr.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Li Z, Lu Y, Guo Y, Cao H, Wang Q, Shui W. Comprehensive evaluation of untargeted metabolomics data processing software in feature detection, quantification and discriminating marker selection. Analytica Chimica Acta. 2018;1029:50–57. doi: 10.1016/J.ACA.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Lin M, Ellis B, Eubanks LM, Janda KD. Pharmacokinetic Approach to Combat the Synthetic Cannabinoid PB-22. ACS Chemical Neuroscience. 2021;12(14):2573–2579. doi: 10.1021/acschemneuro.1c00360. [DOI] [PubMed] [Google Scholar]
- Livera AM, De, Sysi-Aho M, Jacob L, Gagnon-Bartsch JA, Castillo S, Simpson JA, Speed TP. Statistical methods for handling unwanted variation in Metabolomics Data. Analytical Chemistry. 2015;87(7):3606–3615. doi: 10.1021/ac502439y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamas M, Dunn WB, Neyses L, Goodacre R. The role of metabolites and metabolomics in clinically applicable biomarkers of disease. Archives of Toxicology. 2011;85(1):5–17. doi: 10.1007/s00204-010-0609-6. [DOI] [PubMed] [Google Scholar]
- Morales-Villagrán A, López-Pérez S, Medina-Ceja L, Tapia R. Cortical catecholamine changes and seizures induced by 4-aminopyridine in awake rats, studied with a dual microdialysis-electrical recording technique. Neuroscience Letters. 1999;275(2):133–136. doi: 10.1016/S0304-3940(99)00759-4. [DOI] [PubMed] [Google Scholar]
- Morgan AE, Horan B, Dewey SL, Ashby CR. Repeated administration of 3,4-methylenedioxymethamphetamine augments cocaine’s action on dopamine in the nucleus accumbens: A microdialysis study. European Journal Of Pharmacology. 1997;331(1):R1–3. doi: 10.1016/S0014-2999(97)01035-2. [DOI] [PubMed] [Google Scholar]
- Mussap, M., Loddo, C., Fanni, C., & Fanos, V. (2020). Metabolomics in pharmacology - a delve into the novel field of pharmacometabolomics. Expert Review of Clinical Pharmacology, 1–20. 10.1080/17512433.2020.1713750. [DOI] [PubMed]
- Myers OD, Sumner SJ, Li S, Barnes S, Du X. Detailed investigation and comparison of the XCMS and MZmine 2 Chromatogram Construction and Chromatographic Peak Detection Methods for Preprocessing Mass Spectrometry Metabolomics Data. Analytical Chemistry. 2017;89(17):8689–8695. doi: 10.1021/acs.analchem.7b01069. [DOI] [PubMed] [Google Scholar]
- Nash JF. Ketanserin pretreatment attenuates MDMA-induced dopamine release in the striatum as measured by microdialysis. Life Sciences. 1990;47(26):2401–2408. doi: 10.1016/0024-3205(90)90484-9. [DOI] [PubMed] [Google Scholar]
- Nash JF, Nichols DE. Microdialysis studies on 3,4-methylenedioxyamphetamine and structurally related analogues. European Journal of Pharmacology. 1991;200(1):53–58. doi: 10.1016/0014-2999(91)90664-C. [DOI] [PubMed] [Google Scholar]
- Nestler, E. J. (2022). Cellular basis of memory for addiction. Dialogues in Clinical Neuroscience, 15(4), 431–443. 10.31887/DCNS.2013.15.4/ENESTLER [DOI] [PMC free article] [PubMed]
- O’Shea E, Escobedo I, Orio L, Sanchez V, Navarro M, Green AR, Colado MI. Elevation of ambient room temperature has Differential Effects on MDMA-Induced 5-HT and dopamine release in striatum and nucleus accumbens of rats. Neuropsychopharmacology : Official Publication Of The American College Of Neuropsychopharmacology. 2005;30(7):1312–1323. doi: 10.1038/sj.npp.1300673. [DOI] [PubMed] [Google Scholar]
- Patti GJ, Yanes O, Siuzdak G. Metabolomics: The apogee of the omics trilogy. Nature Reviews Molecular Cell Biology. 2012;13(4):263–269. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskal T, Castillo S, Villar-Briones A, Orešič M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. Bmc Bioinformatics. 2010;11(1):395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Rusilowicz MJ, Dickinson M, Charlton AJ, O’Keefe S, Wilson J. MetaboClust: Using interactive time-series cluster analysis to relate metabolomic data with perturbed pathways. PLOS ONE. 2018;13(10):e0205968. doi: 10.1371/journal.pone.0205968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer RJB, Wilson K, Biedermann M, Moore BS, Sieber S, Wennemers H. Identification of Isonitrile-Containing Natural Products in Complex Biological Matrices through Ligation with Chlorooximes. Chemistry – A European Journal. 2023;29(6):e202203277. doi: 10.1002/CHEM.202203277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Blough BE, Tella SR, Goldberg SR, Baumann MH. Effects of 3,4-methylenedioxymethamphetamine (MDMA) and its main metabolites on cardiovascular function in conscious rats. British Journal of Pharmacology. 2014;171(1):83–91. doi: 10.1111/BPH.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selken J, Nichols DE. α1-Adrenergic receptors mediate the locomotor response to systemic administration of (±)-3,4-methylenedioxymethamphetamine (MDMA) in rats. Pharmacology Biochemistry and Behavior. 2007;86(4):622–630. doi: 10.1016/J.PBB.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Brietzke E. Omics-Based biomarkers: Application of Metabolomics in Neuropsychiatric Disorders. International Journal of Neuropsychopharmacology. 2016;19(3):1–13. doi: 10.1093/IJNP/PYV096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev V. Metabolomics technology and bioinformatics. Briefings in Bioinformatics. 2006;7(2):128–139. doi: 10.1093/bib/bbl012. [DOI] [PubMed] [Google Scholar]
- Smilde AK, Westerhuis JA, Hoefsloot HCJ, Bijlsma S, Rubingh CM, Vis DJ, Jellema RH, Pijl H, Roelfsema F, van der Greef J. Dynamic metabolomic data analysis: A tutorial review. Metabolomics. 2010;6(1):3–17. doi: 10.1007/s11306-009-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Maille GO, Want EJ, Qin C, Trauger SA, Brandon TR, Custodio DE, Abagyan R, Siuzdak G. METLIN Therapeutic Drug Monitoring. 2005;27(6):747–751. doi: 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]
- Song P, Mabrouk OS, Hershey ND, Kennedy RT. In vivo neurochemical monitoring using Benzoyl Chloride Derivatization and Liquid Chromatography–Mass Spectrometry. Analytical Chemistry. 2012;84(1):412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysi-Aho M, Katajamaa M, Yetukuri L, Orešič M. Normalization method for metabolomics data using optimal selection of multiple internal standards. Bmc Bioinformatics. 2007;8(1):93. doi: 10.1186/1471-2105-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymańska E, Saccenti E, Smilde AK, Westerhuis JA. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics. 2012;8(S1):3–16. doi: 10.1007/s11306-011-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triba MN, Le Moyec L, Amathieu R, Goossens C, Bouchemal N, Nahon P, Rutledge DN, Savarin P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Molecular BioSystems. 2015;11(1):13–19. doi: 10.1039/C4MB00414K. [DOI] [PubMed] [Google Scholar]
- Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS) Journal of Chemometrics. 2002;16(3):119–128. doi: 10.1002/cem.695. [DOI] [Google Scholar]
- van de Wetering R, Schenk S. Repeated MDMA administration increases MDMA-produced locomotor activity and facilitates the acquisition of MDMA self-administration: Role of dopamine D2 receptor mechanisms. Psychopharmacology (Berl) 2017;234(7):1155–1164. doi: 10.1007/s00213-017-4554-4. [DOI] [PubMed] [Google Scholar]
- van de Wetering R, Schenk S. Regional changes in ∆FosB expression in rat brain following MDMA self-administration predict increased sensitivity to effects of locally infused MDMA. Addiction Biology. 2020;25(5):e12814. doi: 10.1111/ADB.12814. [DOI] [PubMed] [Google Scholar]
- van de Wetering R, Vorster JA, Geyrhofer S, Harvey JE, Keyzers RA, Schenk S. The role of extracellular serotonin and MDMA in the sensitizing effects of MDMA. Behavioural Brain Research. 2022;430:113936. doi: 10.1016/J.BBR.2022.113936. [DOI] [PubMed] [Google Scholar]
- van den Berg RA, Hoefsloot HCJ, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. Bmc Genomics. 2006;7(1):142. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mever M, Segers K, Mangelings D, Hankemeier T, vander Heyden Y, van Eeckhaut A, Ramautar R. Mass spectrometry based metabolomics of volume-restricted in-vivo brain samples: Actual status and the way forward. TrAC Trends in Analytical Chemistry. 2021;143:116365. doi: 10.1016/J.TRAC.2021.116365. [DOI] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology (Berl) 2000;151(2–3):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Pierce RC. Sensitization processes in drug addiction. In: Self David JK, Gottschalk S, editors. Behavioral neuroscience of drug addiction. Berlin Heidelberg: Springer; 2010. pp. 179–195. [DOI] [PubMed] [Google Scholar]
- Veselkov KA, Vingara LK, Masson P, Robinette SL, Want EJ, Li JV, Barton RH, Boursier-Neyret C, Walther B, Ebbels TM, Pelczer I, Holmes E, Lindon JC, Nicholson JK. Optimized preprocessing of Ultra-Performance Liquid Chromatography/Mass Spectrometry urinary metabolic profiles for Improved Information Recovery. Analytical Chemistry. 2011;83(15):5864–5872. doi: 10.1021/ac201065j. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization, drug addiction and psychopathology in animals and humans. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31(8):1553–1555. doi: 10.1016/J.PNPBP.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizeli P, Schmid Y, Prestin K, Meyer zu Schwabedissen HE, Liechti ME. Pharmacogenetics of ecstasy: CYP1A2, CYP2C19, and CYP2B6 polymorphisms moderate pharmacokinetics of MDMA in healthy subjects. European Neuropsychopharmacology. 2017;27(3):232–238. doi: 10.1016/J.EURONEURO.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the Brain Disease Model of Addiction. New England Journal of Medicine. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wu N, Zhao TY, Li J. The potential biomarkers of drug addiction: Proteomic and metabolomics challenges. Biomarkers. 2016;21(8):678–685. doi: 10.1080/1354750X.2016.1201530. [DOI] [PubMed] [Google Scholar]
- Westerink, B. H. C., & Cremers, T. I. F. H. (2007). Handbook of microdialysis: Methods, applications and perspectives (16 vol.). Academic Press.
- Wheelock ÅM, Wheelock CE. Trials and tribulations of ‘omics data analysis: Assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Molecular BioSystems. 2013;9(11):2589. doi: 10.1039/c3mb70194h. [DOI] [PubMed] [Google Scholar]
- Wold S, Antti H, Lindgren F, Öhman J. Orthogonal signal correction of near-infrared spectra. Chemometrics and Intelligent Laboratory Systems. 1998;44(1–2):175–185. doi: 10.1016/S0169-7439(98)00109-9. [DOI] [Google Scholar]
- Wong JMT, Malec PA, Mabrouk OS, Ro J, Dus M, Kennedy RT. Benzoyl chloride derivatization with liquid chromatography–mass spectrometry for targeted metabolomics of neurochemicals in biological samples. Journal of Chromatography A. 2016;1446:78–90. doi: 10.1016/j.chroma.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley B, Powers R. Multivariate analysis in Metabolomics. Current Metabolomics. 2013;1(1):92–107. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SY, Hsu FL. The neurochemical and stimulatory effects of putative metabolites of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine in rats. Pharmacology Biochemistry and Behavior. 1991;39(3):787–790. doi: 10.1016/0091-3057(91)90165-X. [DOI] [PubMed] [Google Scholar]
- Zaitsu K, Hayashi Y, Kusano M, Tsuchihashi H, Ishii A. Application of metabolomics to toxicology of drugs of abuse: A mini review of metabolomics approach to acute and chronic toxicity studies. Drug Metabolism and Pharmacokinetics. 2016;31(1):21–26. doi: 10.1016/J.DMPK.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Zestos AG, Kennedy RT. Microdialysis coupled with LC-MS/MS for in vivo neurochemical monitoring. AAPS Journal. 2017;19(5):1284–1293. doi: 10.1208/S12248-017-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Jin Z, Deng J, Chen X, Zheng X, Wang G, Kim K, Shang L, Zhou Z, Zhan CG. Development of a highly efficient long-acting Cocaine Hydrolase Entity to accelerate Cocaine Metabolism. Bioconjugate Chemistry. 2022;33(7):1340–1349. doi: 10.1021/acs.bioconjchem.2c00210. [DOI] [PubMed] [Google Scholar]
- Zhou B, Xiao JF, Tuli L, Ressom HW. LC-MS-based metabolomics. Mol BioSyst. 2012;8(2):470–481. doi: 10.1039/C1MB05350G. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The availability of all data presented in this study is from authors upon request.