Abstract
In this study, the active components of the plant were carefully extracted and identified using three solvent systems. After extraction, we used solvent systems to further purify the main flavonoid chemical constituent. As a result of our analytical strategy, which included HPLC analysis, MS/MS spectroscopic analysis, and NMR data-based constructions, quercetin was determined to be the main chemical constituent. Our study suggests the potential therapeutic advantages of quercetin, a compound found in the leaves of Acalypha indica, for treating breast cancer cell lines MCF-7 and MDA-MB-231. Our comparison of Quercetin to the regularly prescribed medicine Doxorubicin shows that it has the capacity to inhibit MCF-7 and MDA-MB-231 cells. Measurements of apoptosis and cell cycle phase showed this to be the case. Furthermore, a ladder that formed as a result of cellular damage brought on by ROS provided further proof of the drug's impact on DNA integrity. Notably, pro-apoptotic proteins displayed increased apoptosis activity in cells treated with quercetin. Given that it is extracted from plants and has less adverse effects than other compounds, quercetin is a viable option for further clinical study. The objective is to fight breast cancer, one of the most prevalent diseases in the world and a main cause of death for women. Thus, our research makes a significant addition to the ongoing search for potent, plant-based breast cancer treatments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03705-w.
Keywords: Acalypha indicia, Anti-cancer compound, Apoptosis, MCF-7, MDA-MB-231, Plant extract, Quercetin
Introduction
World cancer incidence and mortality estimate that 14.1 million people will be diagnosed with cancer in the upcoming years. 8.2 million people were estimated to die of cancer, while 32.6 million will live with cancer as a chronic related issue (Bertucci et al. 2019; Waks and Winer 2019). According to estimates, there will be 26 million new cancer diagnoses and 17 million cancer-related deaths by 2030 (Hassanpour and Dehghani 2017). Despite significant efforts, cancer remains the leading cause of death worldwide (Mortezaee et al. 2019). Additionally, novel synthetic chemotherapeutic medicines in clinical trials have not met expectations regardless of their actual development costs (Chandrasekar et al. 2018). The increasing number of cancer cases has resulted in the constant need for practical and cost-effective anti-cancer drugs (Hassanpour and Dehghani 2017). Compounds derived from plants have been used to cure human diseases since the dawn of medicine (Bryda and Stadnytska 2021). Due to their potential as cancer prevention and treatment agents, natural products have received much attention in the last 30 years (Gangola et al. 2017; Chavda et al. 2021). Further evidence shows that plant-derived compounds such as polyphenols (Ameer et al. 2017), flavonoids (Niranjan et al. 2011), lignin (Formica and Regelson 1995), alkaloids (Yun et al. 2021), terpenoids (Kamran et al. 2022), carotenoids (Ávila-Román et al. 2021), and other bioactive compounds with importance in cancer prevention and treatment (Roy et al. 2017; Azmir et al. 2013).
Around 60% of modern cancer treatments are natural products, with plants being the most significant source (Shoemaker et al. 2005). Medicinal plants are used in many countries worldwide to treat cancer (Huang et al. 2021). Currently, over 3000 plants have been identified as having anti-cancer properties globally (Yun et al. 2021). Plant-derived products are used for treatment by 10–40% of cancer patients worldwide, with a 50% rate among Asian patients (Kainsa et al. 2012). Next-generation plant-based bioactive compounds must have fewer adverse effects, are less expensive, and are more effective in the present situation. There has been much interest in biologically potential extracts and their therapeutic chemicals obtained from various medicinal plants in recent years (Kainsa et al. 2012; Mickymaray et al. 2016).
Several types of cancer cells were strongly inhibited by Quercetin, including those from the breast, lungs, stomach, ovarian, colorectal, and liver (Manach et al. 1995; Boots et al. 2007; Molani Gol and Kheirouri 2022). The anti-cancer effects of Quercetin ascribe to several mechanisms, including cell death, the blockage of angiogenesis, the blocking of P-gp channels, the reduction of oncogene expression, and the regulation of signal pathways (Li et al. 2016). Rheumatoid arthritis, ulcers, and other diseases are worsened by inflammation because it produces inflammatory mediators (Rauf et al. 2018). Following research, Quercetin exerts an anti-inflammatory effect by suppressing proinflammatory cytokines, ATP, and nuclear factor kappa B (IkB) binding sites (Kashyap et al. 2022; Becer and Vatansever 2022).
The plant Acalypha indica has medicinal properties that can benefit human health. Many cultures in Asia and Africa use A. indica as traditional medicine (Solomon et al. 2005; Govindarajan et al. 2008). There are several places in western and southern Africa where it can be found, including Ethiopia, Somalia, and other countries (Dineshkumar et al. 2010; Takle et al. 2011). There are medicinal plants in many countries in Asia, Europe, and South and North America (Teklani and Perera 2016). This plant's leaves, roots, and stems treat eye infections, respiratory issues, rheumatism, skin issues and lower blood sugar levels (Venkatachalam et al. 2017). Various methods are accessible to extract active components from A. indica. It is generally true that Soxhlet extraction has high efficiency and accuracy, but thermal stress may lead to the degradation of target photochemical components (Zahidin et al. 2017; Ninave and Patil 2022; De Castro and Priego-Capote 2010).
Novel therapies are needed to minimize the numerous complications associated with existing therapeutic drugs in treating breast cancer. Our hypothesis posits that the isolation of bioactive components from A. indica has the potential to serve as a viable therapeutic option for the treatment of breast cancer. Examining potential bioactive compounds, such as Quercetin, through systematic extraction methods, established cytotoxicity, and anti-tumour treatment methods for breast cancer cell lines include MDA-MB-231 and MCF-7.
Materials and methods
Chemicals
In our study, we purchased analytical grade solvents, including Methanol (CH3OH), Chloroform (CHCl3), Ethyl acetate (C4H8O2), n-Butanol (C4H10O), and Dichloromethane (CH2Cl2), were purchased from Loba Chemie Pvt. Ltd. Chemicals for phytochemical studies which include, 1, 1-diphenyl-2- picrylhydrazyl (DPPH), Vanillin (C8H8O3), Aluminium trichloride (AlCl3), Naphthyl ethylene diamine dichloride (C12H16Cl2N2), and tannic acid from Himedia. Hydrogen peroxide (H2O2) and Lead acetate (Pb(C2H3O2)2) were purchased from Finar Chemicals. For cell culture studies and immunomodulatory assay Dulbecco’s modified Eagle’s medium (DMEM), Roswell Park Memorial Institute Medium (RPMI- 1640), Penicillin (Pen), Streptomycin (Sm), Dimethyl sulfoxide (DMSO), and (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) (MTT) were purchased from Himedia. Fetal bovine serum (FBS) from Gibco. The chemical-based kits were purchased from Invitrogen.
Preparation of plant extract
The A. indica plant material was collected from the Central Institute of Medicinal and Aromatic Plants (CIMAP) in Hyderabad, India (BSI/DRC/2021–2022/Tech/Identification/287). Methanol, Ethyl acetate, and chloroform are used to extract 500 g of powdered leaf material in 1000 mL of organic solvent using Soxhlet extraction for 8 h (The polarity index for Methanol, Ethyl acetate and chloroform are 5.1, 4.5, and 4.0 respectively). Powdered leaves were dissolved in an organic solvent at 50 °C continuously stirring. Three extracts were filtered using 5 M Whatman paper, and all three extracts were evaporated at 40 °C using a vacuum rotary evaporator (Heidolph, Germany). After evaporation, the crude extract was kept in a desiccator. After extraction with three solvents, the residual residue was removed, and each dried crude extract was weighed. Physical properties and percentage yields of various extracts are documented.
Isolation and characterization of quercetin
The crude methanolic extract was considered a potential source of Quercetin due to its relative cytotoxicity. A crude methanolic leaf extract of A. indica was weighed and fractionated. Methanol fractions were collected, and aqueous methanol phases were evaporated under reduced pressure to form semisolids. In 200 mL of distilled water, the semisolid portion was dissolved and fractionated with aqueous methanol (MeOH), dichloromethane (DCM) and n-butanol (BuOH). For further analysis, the dried fractions were dissolved in methanol and stored in amber-coloured vials after fractionation. The concentration of extracts used in all experiments was 10 mg/mL.
The relative cytotoxicity of flavonoids, total phenols, triterpenoids, terpenes, and saponins from methanolic extracts of A. indica leaves was assessed. In order to quantify biomolecules, a defatted sample is essential. The extraction procedure (explained in Supplementary Information) was followed to prepare a defatted sample. Using a Soxhlation approach, 300 mL diethyl ether was dissolved in powdered material for 2 h.
GC–MS spectral studies
GC–MS analysis was performed later to examine the bio-molecular distribution of methanolic leaf extracts of A. indica. Perkin-Elmer (Auto System XL) Clarus 500 Gas Chromatograph equipped with a Turbomas 5.2 spectrometer and an Elite-1 (100% Dimethyl Polysiloxane) capillary column were used for GC–MS analysis. By utilizing the National Institute of Standards and Technology Mass Spectral information base (NIST-MS), discontinuity examples of mass spectra were contrasted and stored in the spectrometer information base. The percentage of each component was calculated based on the relative peak areas of each component in the chromatogram. The NIST library’s known and unknown components were compared (Mohan et al. 2012; Chekuri et al. 2016).
HPLC spectral studies
Characterization of methanolic aq was performed using HPLC. To validate the identity of individual compounds and verify the purity of fractions obtained after successive column purification of A. indica leaf extract. The HPLC system consists of two LC pumps (Perkin-Elmer Series 200 and a high-pressure mixer), an autosampler (Norwalk, CT) with a 10 L sample loop and Chromolith® Performance RP-18, and a Phenomenex column (100 × 4.6 mm, 3 mm particles). Thermo Scientific Chromeleon software edition 6.80 was used for data processing and analysis (Qin et al. 2021; Jackson Seukep et al. 2020).
HR-MS/MS spectrum
The high-resolution mass spectrometer was operated in positive ion mode and had the following requirements: capillary voltage, 2200 V; source temperature, 100 °C; desolation temperature, 350 °C; cone voltage, 30 V. Over a mass spectrum between 300 and 750 m/z, the spectra are scanned at 2.0 s per cycle. NIST's molecular profile database detects individual substances under common chromatographic conditions for standardized compounds. Results were interpreted and processed using Chromeleon Client version 6.80 (Thermo Scientific) (Ballesteros-Vivas et al. 2019).
Proton NMR
Proton NMR spectra of Quercetin were taken on a Bruker MSL 500 NMR spectrometer. Data were processed in MestReNova-11.0.3, and 1H-NMR spectra were compared with available research literature data (Martini et al. 2008).
Cell culture
Three tumour cell lines were selected to screen A. indica leaf extracts in MeOH, Ethyl acetate (EAc) and chloroform (Chl). Subcultures of the cell lines include A549 (lung adenocarcinoma), MCF-7 (breast adenocarcinoma), and A-375 (human melanoma cells) cell lines opted from the PGP Life Sciences, Hyderabad, TS. The cell line's initial source is the ATCC (American Type Culture Collection). A humidified atmosphere of 5% CO2 was maintained at 37 °C in 75 cm2 bottle-necked vented flasks (Corning) with DMEM. The cell lines were grown in DMEM with 10% FBS, 1% non-essential amino acids (NAA), 1% Pen (1000 U/mL), 1% Sm (1000 g/mL), and 1% amphotericin (Am) (250 U/mL). On 75 cm2 plastic flasks, cells were sub-cultivated at a density of 2.2 × 104 cells/cm2 using 0.25% trypsin-1 mM EDTA enzymatically. We have changed the medium every two days. Microscopic observation confirms cell confluence (80%).
To estimate the cytotoxicity with the MeOH fractions, 96-well tissue culture plates with clear bottoms, we plated and cultured MCF-7, MDA-MB-453, and MDA-MB-231 human breast adenocarcinoma cells. The number of cells was 7 × 103 per well. Various concentrations of aqueous (Aq.), dichloromethane (DCM), and n-butanol (n-BuOH) fractions of A. indica leave extracts were applied to the cells after 24 h of seeding, and the cells were incubated for 24 h at 37 °C, 5% CO2. The incubation medium was removed from all wells of a plate and washed three times with PBS. MTT reagent was made up in PBS medium to a final concentration of 0.5 mg/mL, then added to each well. Reagent volume should be adjusted based on cell culture volume. Optical density (OD) was measured for each well on an absorbance plate reader at 570 nm for 3 h at 37 °C until intracellular purple formazan crystals were visible (Azizi et al. 2017; Vyshnava et al. 2020a, 2022).
Cell cytotoxicity assay
The MTT Cell Viability (A549, MCF-7 and A-375 cell lines) assay provides a convenient, sensitive, quantitative, and dependable way to measure how many viable cells are in a culture. MTT assay measures cell viability, proliferation, and cytotoxicity by measuring metabolic activity. A homogeneous colorimetric assay transfers the tetrazolium salt MTT, a pale-yellow substance, to formazan, a purple compound. NADH/NADPH cofactors are present during cellular reduction, and only living cells can catalyze the reaction. The formazan substance is not soluble in water and is purple. Dissolving the resulting formazan in a solubilization buffer allows convenient quantification of substance formulation (Burton 2005). A substance's colour strength is directly proportional to the number of cells living in the culture at 550–620 nm. The increasing proliferation of cells corresponds to an increase in the signal, whereas decreasing proliferation of cells can indicate toxic compounds or suboptimal conditions. Under a microscope, intracellular purple formazan crystals were visible after 3 h at 37 °C. Each well was measured on an absorbance plate reader at 570 nm optical density (OD) (Mbaveng et al. 2018). In all experimentations of the cytotoxicity studies, we used 1% DMSO as the vehicle control.
Anti-cancer activity
Human breast cancer cells, including MCF-7, MDA-MB-453, and MDA-MB-231, were plated and cultured in DMEM of growth medium per well of a transparent flat bottom 96-well tissue culture plate (There were 7 × 103 cells per well). Following 24 h of seeding, the cells were exposed to incremental 0.1 to 50 µg/mL concentrations of Quercetin isolated from A. indica leaves and incubated at 37 °C with 5% CO2. After incubation, we removed the growth medium from all wells and washed them three times with 1 × PBS. Each well was treated with 15 µL of MTT reagent in PBS medium at a final concentration of 0.5 mg/mL. Reagent volume should be adjusted based on cell culture volume. After 3 h at 37 °C, intracellular purple formazan crystals could be seen under a microscope. At 570 nm optical density, each well was evaluated using an absorbance plate reader (OD).
MTT assay was used to determine Quercetin’s time-dependent cytotoxicity after incubation for 24, 48, and 72 h. In 96-well tissue culture plates with a flat bottom, we cultured human breast cancer cells MCF-7, MDA-MB-453, and MDA-MB-231. (Each well had 7 × 103 cells). After 24 h of seeding, cells were exposed to different concentrations of Quercetin made from Acalypha indica leaves, ranging from 0.1 to 50 mM solutions. They were incubated for 24, 48, and 72 h at 37 °C and 5% CO2. Incubated wells were washed with 1xPBS three times after removing the growth medium. In each well, 15 µL of MTT reagent was added in 1xPBS medium at a 0.5 mg/mL concentration. Depending on the cell culture volume, adjust the magnitude of the reagent. A solution of 10% acidified Sodium dodecyl sulphate (SDS-HCl) was added to the cells after 3 h at 37 °C to dissolve the purple formazan crystals formed within the cells (Kumar et al. 2018). The absorbance was measured at 570 nm for each well using an absorbance plate reader (Ghagane et al. 2017).
Apoptosis induction
The initial apoptotic effects were assessed using an Annexin V-FITC and PI kit (BD Biosciences, San Jose, CA, USA) as directed by the manufacturer. A six-well plate containing approximately 3 × 105 cells was plated the night before and given the night to attach. Cells were treated for 48 h with Quercitin at doses equal to the IC50, then trypsinized and resuspended in 1xPBS at a cell density of 1 × 106 per ml. After mixing the suspension with binding buffer, Annexin V-FITC, and propidium iodide (PI), it was incubated at room temperature for 20 more minutes in the dark. The binding buffer, a volume of 500 µL of sample volume, was treated. A BD FACS Calibur flow cytometer (BD BioSciences, USA) was used to analyze samples after vortexing (Abd Razak et al. 2019).
Apoptosis by DNA laddering assay
Apoptosis is characterized by irreversible DNA fragmentation, which happens before changes in plasma membrane permeability (prelytic DNA fragmentation). An endonuclease cleavage product of apoptosis can be visualized using DNA laddering. DNA is extracted from a lysed cell homogenate in this assay and then electrophoresed on an agarose gel (Alkahtani et al. 2022).
Analysis of cell cycle arrest
MCF-7 and MDA-MB-231 were stained with PI (Propidium iodide). Later, flow cytometry was used to assess the effect of Quercetin. We plated 5.3 × 105 cell lines and let them attach overnight. The medium was replaced with a fresh medium containing the desired amount of Quercetin. Incubated at 37 °C for 48 h, floating and adherent cells were collected, washed twice with cold 1xPBS, and fixed overnight with ice-cold 70% EtOH. The cells were then incubated for 30 min and treated with 80 mg/L RNase A and 50 mg/L PI. BD FACS Calibur flow cytometer was used to analyze stained cells. We reanalyzed the data using BD MultiSET (Prasad et al. 2020).
Western blot analysis
To further verify the apoptosis process in Quercetin treated cells through SDS-PAGE followed by western blotting to investigate the protein expression involved in apoptosis. SDS-PAGE was done under reducing conditions using polyacrylamide slab gels at 12.5%. A semidry electroblotting tank was used to transfer proteins from the gel to the PVDF membrane. After washing three times (each time 5 min) with wash buffer (0.05% Tween 20 in PBS), the membrane was soaked in the primary antibody (Abcam, USA) with a dilution of 1/1000 on the shaker for two hours, followed by three more washes with wash buffer. After 2 h of shaking, the membrane was incubated for 0.03% HRP conjugated secondary antibody (Abcam, USA, 3 mL in 10 mL wash buffer, at 1/5000 dilution). With wash buffer, the membrane was washed three times after discarding the secondary antibody. A radiography film was developed with an ECL substrate to establish the protein bands (Leng et al. 2018; Hargraves et al. 2016).
VEGFR-1 and Aurora kinase A Genes Expression
RT-PCR was performed to confirm Western blotting results of VEGFR-1 and Aurora kinase A expression. Therefore, using mRNA from MCF-7 and MDA-MB-231 cells, PCR was carried out on synthesized cDNAs. Electrophoresis through a 2% agarose gel followed by ethidium bromide (EtBr) staining visualized all RT-PCR products (Mohajeri et al. 2020).
Molecular docking and analysis
The structures of the compounds were sketched in ChemDraw and used as ligands. In Open Babel, nine SD (.sdf) files were converted to their corresponding three-dimensional (.pdb/.mol2) structures. The Gasteiger charges were added, and non-polar hydrogens were merged with AutoDock 4.2. Using the Research Collaboratory for Structural Bioinformatics (RCSB) and protein data bank (PDB), the 3D crystal structures of human Cyclin B (2JGZ), Cyclin D (2W96), Cyclin E (1W98), VEGFR (2VWE), IKKB (3BRV), GSK3 (4NM0), PI3 (7POP), BCL-2 (4C5D), and BCL-XL (7LH7) were retrieved, without complexed ligands. Hydrogens were removed from protein structures using AutoDock4.2, Gasteiger charges were calculated, polar hydrogens were added, and non-polar hydrogens merged using AutoDock4.2 (Rizvi et al. 2013). Both receptor and ligand structures were converted to PDBQT files using AutoDock4.2, which contain atomic charges, atom types, and topological information (rotatable bonds) for ligands. In addition to ensuring coverage of the binding site of the structure, a grid was used to ensure that the binding site was covered. AutoDock4.2 generated nine conformations of the ligand in complex with the receptor to perform docking simulations, which were then ranked (Vyshnava et al. 2020b).
Results and discussion
Isolation of crude extracts from Acalypha indica dried leaves
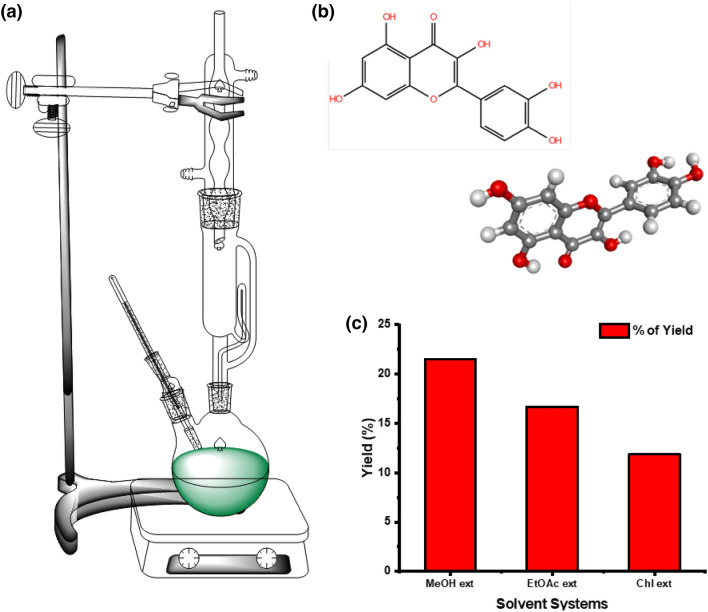
Using the phytochemical extraction from the A. indica leaves extract, we systematically observed Quercetin's effect on breast cancer cell lines. Standard procedures were followed to extract phytochemicals using various organic solvents. A indica leaves were allowed to settle for 24 to 72 h at a temperature closest to their near boiling points of organic solvents, as shown in Fig. 1a. After observing the dissociation of the plant compounds into the solvents Fig. 1b, we allowed the solvents to filter through a 45 μm filter and evaporate in a vacuum rotavapor. As shown in Table 1, the percentage yields of the organic solvents are shown, with MeOH showing the highest yield at around 21.51%, followed by semi-polar organic solvent, such as EAc, at 16.69%, and chlor, a non-polar organic solvent, with an estimated yield of 11.84%. (Fig. 1c).
Fig. 1.
a Soxhlet apparatus used for the plant leaf extraction for Quercetin b Structure of the Quercetin in 2d and 3d perspectives c Three solvent systems showed the percentage of yield for plant leaf extraction, where methanolic extraction shows the promising percentage of bioactive phytochemical compounds
Table 1.
Percentage of Yield in various organic solvent systems of Acalypha indica. L leaves
| Solvent | Wt. of dry leaf powder in (g) | Wt. of Yield (g) |
Yield (%) |
|---|---|---|---|
| Methanol | 500 | 107.59 | 21.518 |
| Ethyl acetate | 500 | 83.46 | 16.692 |
| Chloroform | 500 | 59.22 | 11.844 |
Phytochemical analysis of the crude extracts
Based on the previous research reports, a qualitative analysis was carried out using standard chemical methods (see Supplementary Information methodology) to assess the nature of phytochemical compounds or secondary metabolites found in the crude extracts (Azmir et al. 2013; Popov et al. 2017; Bryda and Stadnytska 2021). MeOH crude extract of A. indica contains several preliminary phytochemicals, including alkaloids, saponins, terpenoids, flavonoids, tannins, steroids, glycosides, and phenols. However, a few chemical constituents have mild concentrations in the EAc and Chl extracts, as listed in Supplementary Information Table S1.
Cytotoxicity of crude extracts
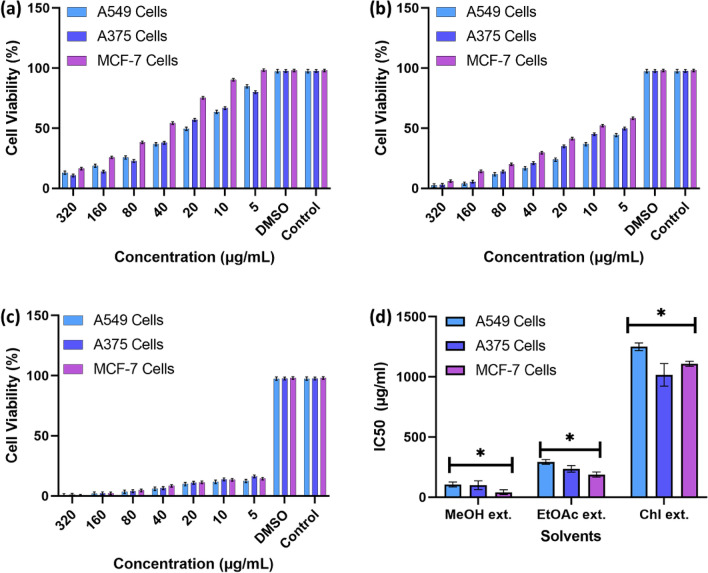
Further doses of the organic crude extracts from 5 to 320 µg/mL were added to the adenocarcinoma cell lines to observe their relative composition and cytotoxic efficacy. We observed that methanolic crude extract shows the potential cytotoxicity at a comparable concentration of 160 µg/mL in MCF-7 cell lines, followed by A549 and A375 cell lines, as shown in Fig. 2a. In the case of the EAc extract, the data revealed a moderate amount of cytotoxicity compared with 40% less inhibition than the MeOH extract, as mentioned in Fig. 2b. The Chl extracts show relatively negligible cytotoxicity compared with the other two solvent systems, which is 80% lower, as shown in Fig. 2c. The relative IC50 values of the three solvent extracts indicated a lower dosage of methanolic extract when treated with the experimental cell lines of about 200 µg/mL. In contrast, IC50 values reported for ethyl acetate extract were mild to moderate about 400 µg/mL and the chloroform extracts showed higher IC50 values depicted in Fig. 2d.
Fig. 2.
Bar chart representation for cytotoxicity of Acalypha indica leaves organic solvent include the (a) Methanolic extracts (b) Ethyl acetate extracts (c) Chloroform extracts (d) respective IC50 values on adenocarcinoma cell lines including A549, MCF-7, and A375. (With a non-significant value of P > 0.05 (observed P value of 0.9863))
The first step in determining the crude extract activity of a plant is to identify its polar chemical constituents, which inhibit MCF-7 and two other cell lines when compared to ethyl acetate and chloroform extracts, which contain fewer polar chemical constituents with a non-significant value of P > 0.05 (observed P value of 0.9863). These results agree with previous research observations regarding the phytochemical composition of methanolic and ethyl acetate extracts of A. indica, which suppress cancer cells (Zahidin et al. 2017; Suresh et al. 2021; Sophia et al. 2022). By analysing the phytochemical components of the crude methanolic extract, we isolated Quercetin as the molecule of interest. We further quantified crude methanol extract based on gravimetric measurements reported earlier (Raaman 2006; Kokate 2001).
GC–MS analysis of the methanolic extract
Based on the gravimetric data, the methanolic extraction yields abundant in flavonoids, phenolics, triterpenoids, tannins, and saponins are show in Fig. 3a. Figure 3b shows the relative spectral peak area in the GC–MS chromatogram of methanolic extract. Table 2 lists isolated phytochemical compounds corresponding to the NIST-MS library and the compounds previously reported with known functions mentioned in the Ruslan group, which were identified as antibacterial, anti-cancer, and anti-inflammatory compounds (Ruslan 2015). In contrast, Quercetin was observed in the spectrum at 32.19 min retention time, our compound of interest obtained with fewer functional applications. Previous research reports suggest that the anti-cancer properties of Quercetin compound are relatively for fewer cancers (Murakami et al. 2008; Hashemzaei et al. 2017; Rauf et al. 2018; Erdoğan et al. 2022; Becer and Vatansever 2022). Based on this experimental observation, we further proceeded to isolate the Quercetin in its pure form based on fractionation with organic solvents based on the polarity.
Fig. 3.
a Gravimetric content for bioactive compounds from the methanolic extraction, where flavonoids are dominant (b) GC–MS spectrum of methanolic leaf bioactive phytochemical content in flavonoids, where the observed presence of the Quercetin at 32.19 Retention time (Rt) of the spectrum
Table 2.
GC–MS spectrum of methanolic leaf extract of Acalypha indica, concerning various organic contents based on the spectral peaks
| S. No | *Rt | Name of the compound | Mol. Formula | Mol.wt (g/Mol) |
Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 9.76 | 4-C-methyl-myo-inositol | C7H14O6 | 194.22 | 15.85 |
| 2 | 11.92 | Proline,3,4-didehydro | C5H7NO2 | 113.67 | 100.00 |
| 3 | 15.06 | 3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran4-one | C6H8O4 | 144.09 | 18.78 |
| 4 | 16.34 | Cysteine | C3H7NO2S | 121.16 | 17.49 |
| 5 | 17.97 | Ellagic acid | C14H6O8 | 302.19 | 56.92 |
| 6 | 18.18 | Diethyl phthalate | C12H14O4 | 222.23 | 14.02 |
| 7 | 19.16 | 1H-Pyrrole-2,5-dione,1- ethenyl | C6H7NO2 | 123.42 | 35.71 |
| 8 | 19.95 | Chebulic acid | C14H12O11 | 356.23 | 48.81 |
| 9 | 21.44 | Gallic acid | C7H6O5 | 170.12 | 39.24 |
| 10 | 22.84 | 4-Amino-3- methoxypyrazolo[3,4- d]pyrimidine, | C6H7N5O | 165.29 | 35.28 |
| 11 | 23.03 | Propanenitrile,3-(5- diethylamino-1- methoxy3-pentynyloxy) | C13H22N2O | 223.16 | 13.91 |
| 12 | 24.89 | Didodecyl phthalate | C32H54O4 | 502.14 | 47.65 |
| 13 | 26.04 | Trifluoromethyl t-butyl disulfide | C5H9S2F3 | 190.21 | 33.67 |
| 14 | 27.18 | Ethyl pentanate | C10H20O2 | 172.48 | 46.12 |
| 15 | 28.15 | Ethyl decanate | C12H24O2 | 200.31 | 55.37 |
| 16 | 28.42 | Rutin | C27H30O16 | 610.51 | 63.63 |
| 17 | 31.02 | Kaempfeorl | C15H10O6 | 286.23 | 89.07 |
| 18 | 31.14 | Phytol | C20H40O | 296.18 | 89.34 |
| 19 | 32.19 | Quercetin | C15H10O7 | 302.23 | 91.06 |
| 20 | 33.27 | Squalene | C30H5O | 410.57 | 78.17 |
| 21 | 35.06 | 3,8-Nanodiene-2-one | C9H14O | 138.24 | 76.20 |
*Rt retention time
Methanolic fraction extraction
Approximately equal quantities of crude methanolic extract were weighed and fractioned into three portions, dissolved in methanol and evaporated under reduced pressure to form a semisolid. The induvial fraction was made into methanol, dichloromethane (DCM) and n-butanol fractions. The dried fractions are collected and stored at ambient temperatures.
Cytotoxicity of the methanolic fraction
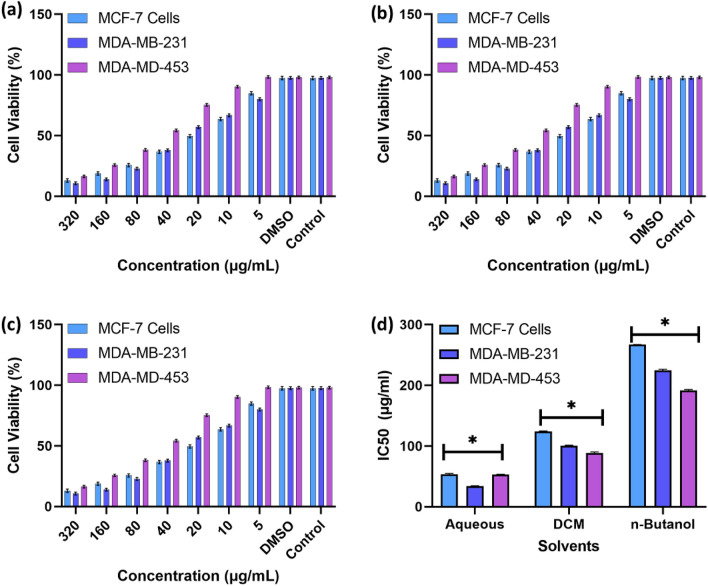
Further cytotoxicity was evaluated by comparing the methanolic crude extract fractionation to the individual cancerous cells originating from breast cancers. With a steady state increase in the concentration of the solvent fractions, the aqueous fraction of plant extract showed maximum toxicity towards MDA-MB-231 cells with 99% inhibition, as shown in Fig. 4a. A similar trend regarding the doxorubicin treatment for MCF-7 and MDA-MB-453 cells as shown in Fig. 4b, 50% of the relative toxicity was observed in the DCM fraction, and similar trends were observed across all cell lines. However, the observations in the n-butanol fractions show no significant cytotoxicity, which may be negligible, with similar trends in all the treated cell lines making up less than 20%, as shown in Fig. 4c. Regarding the DCM, the IC50 of the aqueous fraction is approximately 200 µg/mL, whereas the IC50 of the n-butanol is > 200 µg/mL with a non-significant value of P > 0.05 (observed P value of 0.8821). Based on the observation, we confirmed that the aqueous methanolic fraction contained most of the phytochemical to be isolated, which was further refined and chemically analyzed.
Fig. 4.
Bar chart representation for cytotoxicity of Acalypha indica leaves in a solvent extract with (a) Aqueous fraction (b) Di-chloromethane fraction (c) n-Butanol fraction (d) respective to IC50 values MCF-7, MDA-MB-453, and MDA-MB-231 cells. (With a non-significant value of P > 0.05 (observed P value of 0.8821))
Colum chromatography and HPLC for the methanolic extract
Aqueous methanolic extracts were purified in the first instance using activated silica gel in conjunction with eluting solvents and various solvent systems, as shown in the Supplementary information in Table S2. As a result of starting with the corresponding fractions with the solvent systems, it is possible to estimate the gravimetric percentage of yield. Methanol is the most promising eluent for further experimentation, with a maximum harvest of 27.39% at 17.69 mg mass weight, compared to 50 mg mass weight in the initial aqueous methanolic fraction in Fig. 5.
Fig. 5.
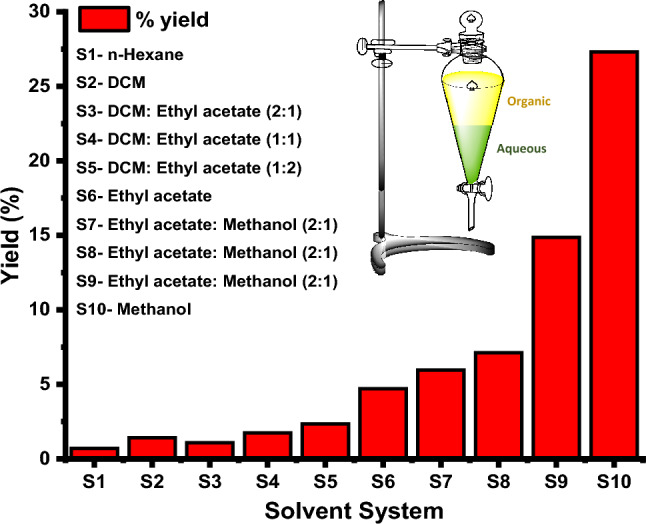
Percentage of yield for the various organic solvent systems to be executed for the aqueous methanolic fractions for the column chromatography, where methanol shows better for the extraction fractions
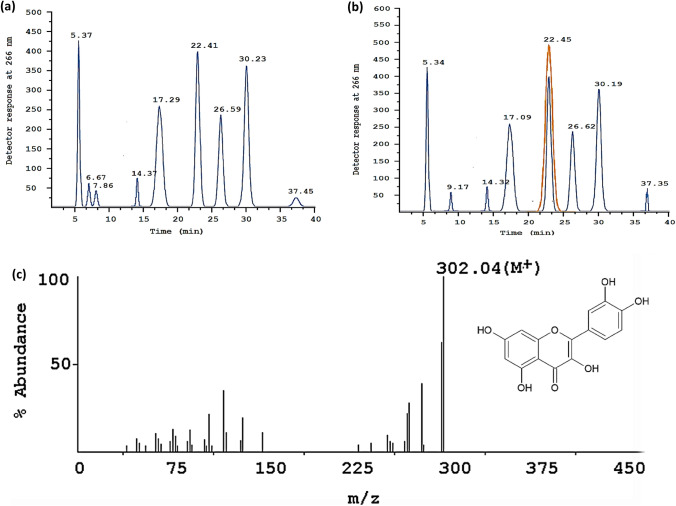
Following the Column mentioned above chromatography purification, the methanolic aqueous fraction was subjected to HPLC for further purification. As part of the analysis, standards are purchased for Quercetin for HPLC runs. As soon as the peaks were obtained, Quercetin was identified at 22.41 min retention time (Rt), all of which were analyzed through Fig. 6a. Based on the peak Rt, which includes the peak height and the peak breadth, Quercetin was identified for purity and quality, as represented in Fig. 6b, including peak 22.45 min applicable to Quercetin. Figure 6c shows that the MS/MS spectral data are used to analyze the HPLC purified fractions and determine their structural characteristics. MS/MS spectrophotometers run samples through to elute the data based on the NIST database. Figure 6c depicts the peaks obtained from the 302.04 m/z peaks for Quercetin, consistent with the previous research reports (Pinho et al. 2014; Jirge et al. 2014; Niranjan et al. 2011; Manach et al. 1995).
Fig. 6.
Isolation and identification of the compound of interest through HPLC runs for the Column purified aqueous methanolic fraction of Acalypha indica leaves extract, where with a structural correlation of (a) Commercially available compounds (b) Quercetin (c) MS/MS spectral analysis of the Quercetin from the HPLC based isolates, where the MS/MS peak shows the (ESI, M +): 302.04; found 302.04 m/z
NMR for the methanolic extract
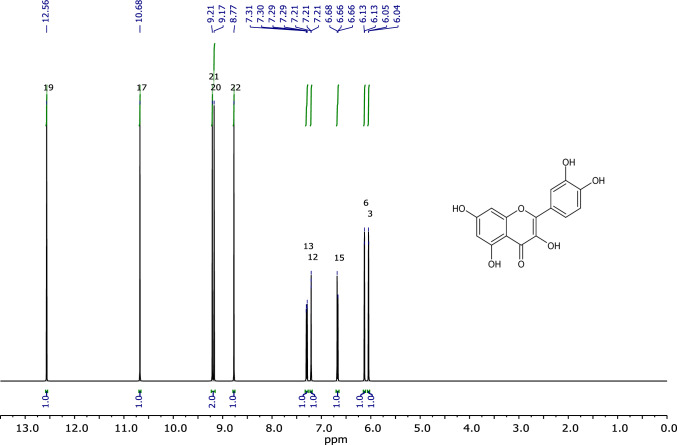
We correlated the structural data sets from HPLC fractions with the NMR data sets, as shown in Fig. 7, to generate the respective constructs for Quercetin. The 1H protonated spectra of Quercetin exhibit chemical shifts of 1H NMR (400 MHz, CDCl3): δ 12.6 (s, 1H), 10.7 (s, 1H), 9.3 (m, 2H), 8.6 (s, 1H), 7.3 (m, 1H), 7.1 (s, 1H), 6.6 (m, 1H), 6.2 (m, 1H), 6.1 (m, 1H). Based on these data sets, the NMR analysis for plant-based extracts is consistent with earlier research (Wiese et al. 2013; Chandradevan et al. 2020).
Fig. 7.
1H NMR spectral data of Quercetin (400 MHz, CDCl3) [.1H NMR (400 MHz, CDCl3): δ 12.6 (s, 1H), 10.7 (s, 1H), 9.3 (m, 2H), 8.6 (s, 1H), 7.3 (m, 1H), 7.1 (s, 1H), 6.6 (m, 1H), 6.2 (m, 1H), 6.1 (m, 1H)]
Cytotoxicity of the quercetin
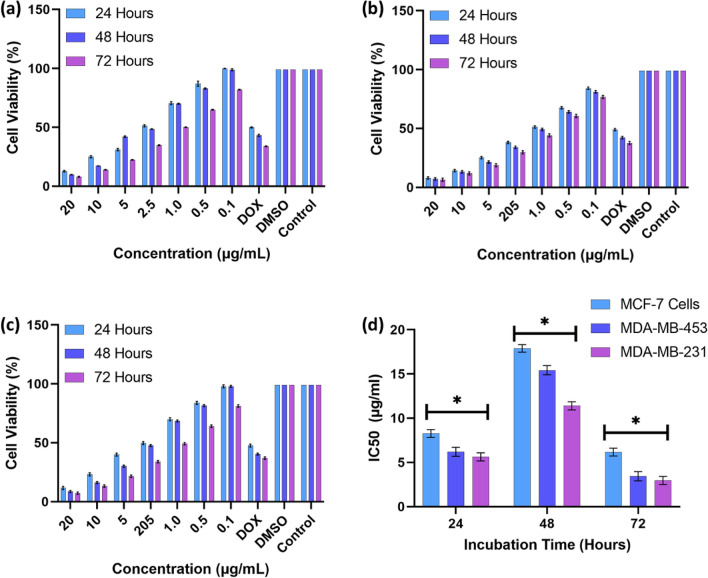
Quercetin isolated from sequential extraction has potent anti-cancer activity, and the aqueous methanolic fraction, compared to the other molecules identified in this study, had a lower IC50 value in inhibiting MCF-7, MDA-MB-453 and MDA-MB-231. According to Fig. 8a, MCF-7 cells treated with Quercetin for 24 h, 48 h and 72 h demonstrated a gradual increase in cytotoxicity with the different concentrations of Quercetin, ranging from 0.1 to 50 µg/mL. The bar chart Fig. 8a representations show similar trends, with no significant change in cytotoxicity in MCF-7 cell lines, which is 99% of cellular inhibition based on exposure time. MDA-MD-453 and MDA-MD-231 show similar trends as shown in Fig. 8(b, c), respectively, which indicate that inhibition at higher concentrations remains over 90% based on their time and dosage of Quercetin. As shown in Fig. 8d, the Quercetin's IC50 values against MCF-7, MDA-MB-453, and MDA-MB-231 cells lines are relative, where the Quercetin treated with MDA-MB-231 for the 72 h showed a lower IC50 value at 5 µg/mL compared with the 24 h and 48 h periods of exposure, while the MCF-7 requires 10 µg/mL and the MDA-MB-453 requires > 20 µg/mL.
Fig. 8.
Bar graph representation of IC50 values of Quercetin against (a) MCF-7 (b) MDA-MB-453 and (c) MDA-MB231 cell lines treated for a period of 24, 48 and 72 h of treatment, (d) IC50 values. (With a non-significant value of P > 0.05 (observed P value of 0.7136))
Quercetin appeared to be a potential inhibitor for the MDA-MB-231 cancer cell lines with lower IC50 values for different time slots and acting as a gradual inhibitor or death for the cancer cells as the time increased with a non-significant value of P > 0.05 (observed P value of 0.7136). The first study looked at the MDA-MB-231 cell line, which was not discussed in previous studies.
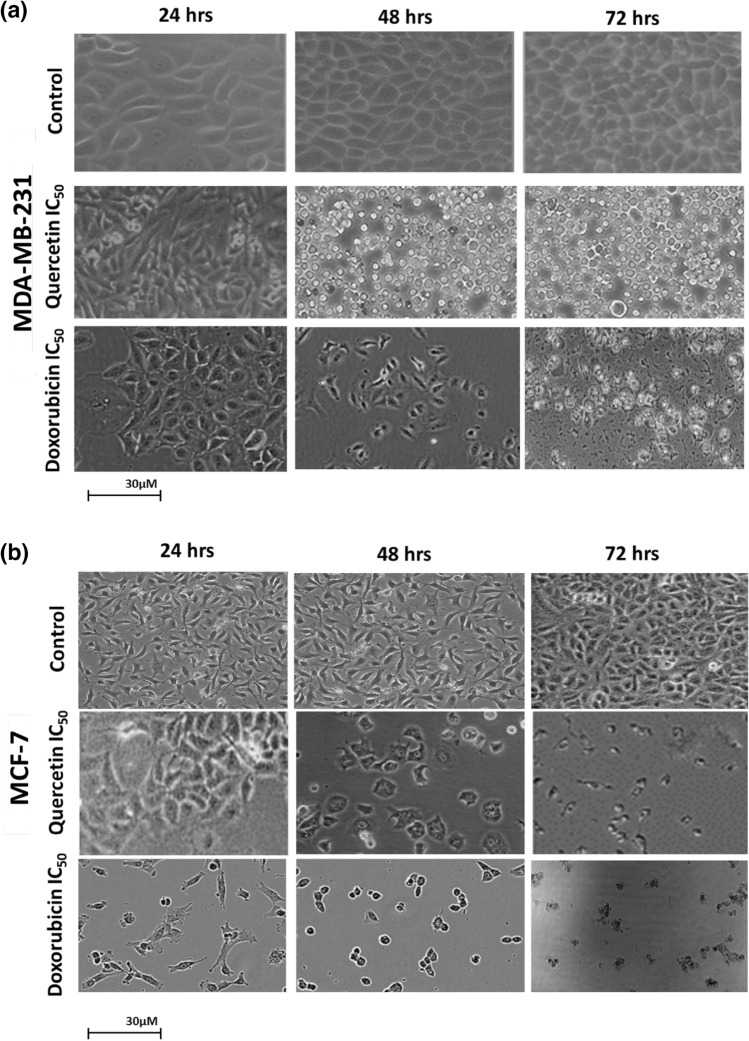
Cell morphology studies with quercetin compounds
The molecular analysis and biocompatible applications of the isolated fractions from Acalypha indicia leaves, the compound of interest, Quercetin, was further examined for cellular morphological studies. The cytotoxicity and morphological characteristics of MCF-7 and MDA-MB-231 cells were studied by exposing these cell lines to Quercetin for 24 h, 48 h, and 72 h as Doxorubicin at their IC50 value concentrations. Quercetin displays significant inhibitions with damaged cell borders and osteolytic appearance. Based on the observations in Fig. 9a, the MCF-7 and Fig. 9b, the MDA-MB-231 cell lines showed confluency of 20–40% and damage to cell borders when exposed to Quercetin and Doxorubicin for 24 h to 72 h. As we confirmed the change in the cellular morphology, i.e., damaging the cellular integral structure after treatment with Quercetin for the respective cell lines, we further assessed the stages of cellular death, also known as apoptosis; this activity was studied with the flow cytometric technique.
Fig. 9.
The morphological characteristics of the (a) MCF-7, (b) MDA-MB-231 when treated with the Quercetin at a concentration corresponding to their IC50 dosages and the changes are observed for a period of 24, 48, and 72 h of treatment
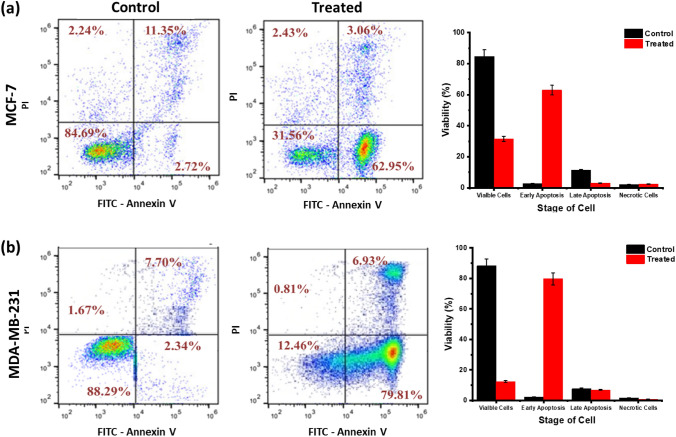
Apoptosis studies with quercetin
A concentration of Quercetin at the IC50 value was applied for 48 h to MCF-7 and MDA-MB-231 cells in a six-well microtiter plate at a density of about 3 × 105 cells per well. In both treated and untreated cell lines, cells are harvested and incubated with Annexin V-FITC and PI, followed by flow cytometry analysis to determine whether they have displayed apoptotic activity. As shown in Fig. 10a, MCF-7 showed that 62.95% of cells were in the early stages of apoptosis, followed by 31.56% intact cells and 2.43% necrotic cells. Observing Fig. 10b, we watched the 12.46% unchanged cell profile in a culture of MDA-MB-231 that has not been treated, while in the treated part of the culture, 79.81% of the cells are at the early apoptotic stage, as indicated by the frequency of the cells distributed with the FITC-Annexin V uptake. The most effective suppressed cell line for Quercetin treatment is MDA-MB-231. Based on these results, we further investigated the genomic DNA constituents in cells treated with Quercetin was based on these results.
Fig. 10.
Apoptosis study of the cell lines showing the control and treated with Quercetin (a) MCF-7, (b) MDA-MB-231 cell lines
DNA ladder studies with Quercetin
MCF-7 and MDA-MB-231 cellular DNA electrophoretic gel images depict the DNA ladder formation as shown in Fig. 11a, b. The comparison of gels showed that DNA ladders formed in the gels of MDA-MB-231 rather than MCF-7 as the concentration of Doxorubicin increased the number of DNA fragments. Quercetin, thus, may be involved in DNA damage, directly engaging cellular infrastructures and stimulating ROS-mediated cell membrane damage cascades to cause apoptosis.
Fig. 11.
DNA ladder formation when cells, including MDA-MB-231 and MCF-7 cells, are treated with Quercetin with various concentrations relative to its IC50 dosage
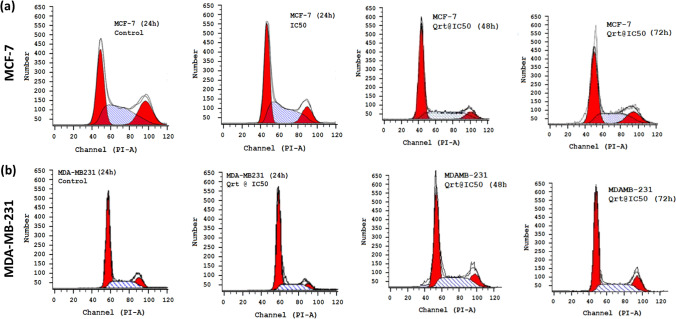
Cell cycle phases studies with Quercetin
A cell cycle study was conducted with the MCF-7 and MDA-MB-231 cell lines treated with Quercetin. MCF-7 and MDA-MB-231 graphical peaks show time-dependent percentages of cell cycle generations in Fig. 12a, b. In the MCF-7 and MDA-MB-231 cell lines, most cells are in the G1 phase, which is evident even though the time extends from 24 to 72 h. The Quercetin concentration increased from the IC50 to double the concentration of the IC50 values. When comparing the detailed analyses of the cell cycle phases for the MCF-7 cells for a while of 24 h to 72 h, in these cells, we found an increase in the percentage of cell distribution in the G1 phase when compared to control to treated samples. The same trend was observed in 42 h and 72 h for the G1-Phase, while the G2/M, S, and sub-G1 phases were consistent in the control sample rather than the treated sample, suggesting that the MCF-7.
Fig. 12.
Cell cycle phases of (a) MCF-7 and (b) MDA-MB-231 breast cancer cells treated with purified Quercetin compound for 24 h Cell cycle phases of MCF-7 and MDA-MB-231 breast cancer cells treated with purified Quercetin compound for 48 h
In contrast to MCF-7 cells, the MDA-MB-231 cell lines showed similar distributions when treated and untreated. Inhibition rates increased from 0.5 to 2 times with an increase in the IC50 dose, indicating that Quercetin inhibited cells at the G1 phase more efficiently than the MCF-7 cells, which showed a slightly slower inhibition rate. In contrast, MDA-MB-231 cell lines show dramatic reductions in Sub G1, S, and G2/M phased compared to untreated controls. The MDA-MB-231 showed a faster growth inhibition when treated with Quercetin than the MCF-7. Our observation concerning the time and concertation dependent of Quercetin was distinct to represent a not published data sets. Our study would be novel in approaching the plant-based extracts that will be leading sources for the treatment of cancer and related disorders.
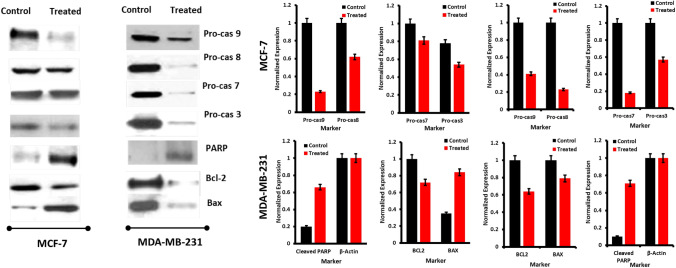
Western blotting studies with Quercetin
Our approach was on the right track in understanding the cellular events for the treatment and application of Quercetin; now we approach the molecular level, including confirming the apoptosis with a base cellular expression of the proteins for apoptosis which will be caspase systems viz., procaspase-9, -8, -7, -3 which are best studied in the previous reports (Movsesyan et al. 2002; Fearnhead et al. 1998; Cui et al. 2007), Cleaved PARP (Bressenot et al. 2009; Casao et al. 2015), Bcl-2 (Dai et al. 2016; Vogler 2012), Bax (PORĘBSKA et al. 2006), VEGFR1 (Zhang et al. 2010), Aurora Kinase-A (Katayama et al. 2012) and standard cell protein β-actin (Lei et al. 2017; Khan et al. 2017). Figure 13 shows the western blot data for expressed proteins when treated with the Quercetin IC50 value concentration; all the pro-caspases, including pro-caspase 9,8,7 and 3, are expressed in both MCF-7, and MDA-MB-231 cell lines, which represents the activation of the apoptosis activity, along with the caspases, Bcl-2, cleaved PARP, Bax, VEGFR-1 and Aurora Kinase A was expressed relatively depicting the activity of the apoptosis activation, the cell viability was shown with the standard expression of the β-actin protein in the respective cell lines.
Fig. 13.
Western blot data sets of the pro-apoptotic protein’s expression in MCF-7 and MDA-MB-231 cell lines when treated with the Quercetin
Aurora Kinase A and VEGFR-1 studies with Quercetin
Similarly, the relative intensities of the expressed protein and controls for MCF-7 and MDA-MB-231 the normalized intensities are shown in Fig. 14. Comparing MCF-7 with MDA-MB-231, PARP, Bax, Bcl-2, and VEGFR-1 are expressed at a significant level. As a further step, protein expression was analyzed on the MCF-7 and MDA-MB-231 cell lines with and without Quercetin and control treatment, according to IC50 values. These results are compared with the viability of the cell lines with high expression of the β-actin protein. For the control, untreated cells, and treated cells, our analysis shows the augmented incremental expression of VEGFR-1 and Aurora Kinase A is higher in the MDA-MB-231 cells in comparison-to-comparison MCF-7 cells.
Fig. 14.
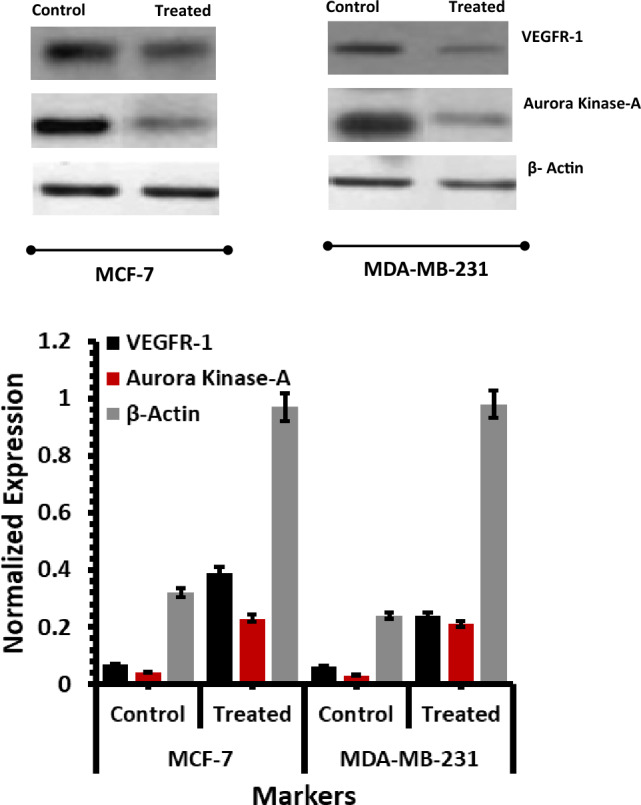
VEGFR-1, Aurora Kinase-A, and β-Actin protein expression analysis of the MCF-7 and MDA-MB-231 treated with the Quercetin in IC50 dosage
RT PCR studies with quercetin
We performed RT-PCR with the appropriate VEGFR-1 and Aurora Kinase -A genes primers to understand their expression in cells treated with the IC50 Quercetin concentration. In addition, after cell harvesting for the mRNA from the individual cell lines, concerning the treated and untreated Quercetin, RT-PCR was undergone to know the expression of the VEGFR-1 and Aurora kinase respective to the standard GAPDH as shown in the figure for the DNA gel electrophoretic gel Fig. 15, the appearance of the gels with 234 bp and 196 bp based for the respective cell lines include MCF-7 and MDA-MB-231 which are consistent with the previous results (Manikandan et al. 2010; Bakhashab et al. 2018), which refers the active formation of the apoptotic gene express for the Quercetin treated samples. The relative expressions of the VEGFR-1 and Aurora kinase A with the standard of the GADPH gene levels reveal the apoptotic genes' active formation.
Fig. 15.
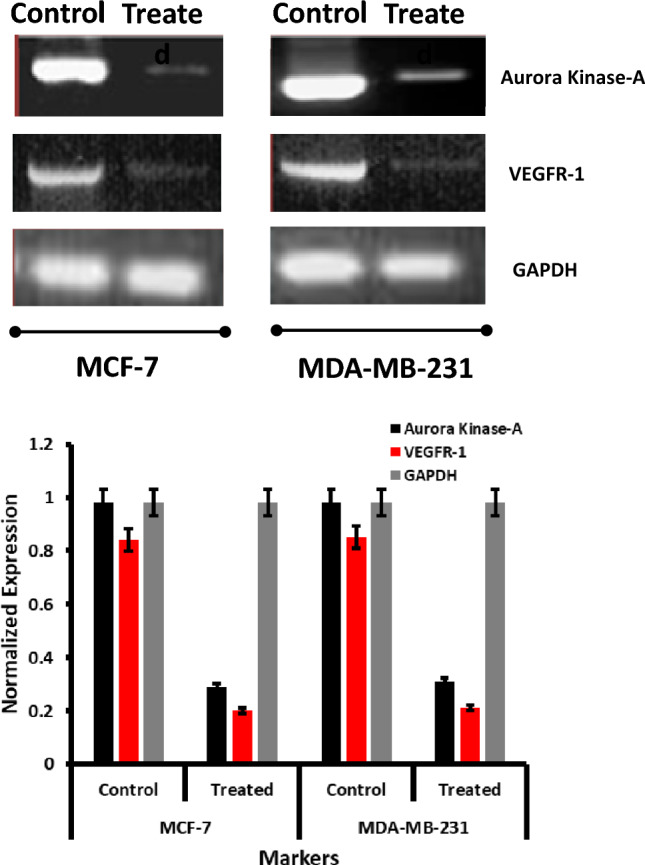
RT-PCR-based VEGFR-1 and Aurora kinase-A genes expression studies when Quercetin treated on MCF-7 and MDA-MB-231
Molecular docking analysis
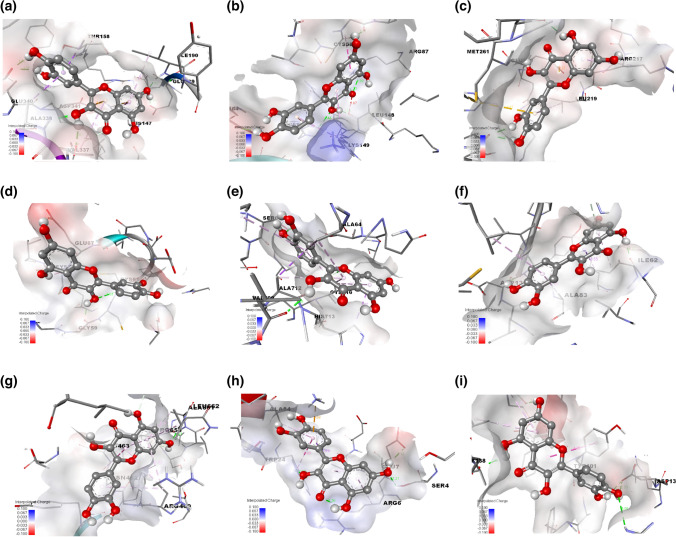
Among all Quercetin and apoptosis–anti–apoptosis complexes, the minimum binding energy was observed with BCL-XL complex with Binding affinity (ΔG) maxima of − 8.24 kcal/Mol with inhibition constant (Ki) of 916.33 nM followed by PI3 protein about ΔG − 7.97 kcal/Mol with Ki of 1.45 µM. Approximately nine amino acid residues interacted with the ligand molecule, including THR158, HIS147, GLU189, ILE190, GLY336, VAL337, ALA338, GLU340, and ASP341 from the receptor of interest with the Quercetin which is shown in Table 3. THR, HIS, GLU, ILE, GLY, VAL, ALA, GLU, ASP, and TYR interacted with strong hydrogen bonds with Quercetin. Alkyl and van der Waals interaction was another interaction observed with CYS, LEU, ALA, LEU, and LYS bonded with Quercetin via an alkyl group, as shown in Fig. 16a,i. Along with the weak interactions, there were four distinct types of bond interaction: THR and LEU exhibited pi-sigma interaction with the Quercetin. A typical carbon-hydrogen bond was created by ASP, LYS, VAL, LEU, LEU, LEU, and MET and reacted with the ligand by interacting with alkyl bonds. The van der Waal forces of the remaining residues reinforced these interactions.
Table 3.
Molecular docking of Quercetin with the function apoptosis and anti-apoptosis proteins
| Receptor | Ligand | Binding Affinity (Kcal/Mol) |
Inhibition Constant (Ki) |
Cluster RMSD | Number H-bonds |
Active amino acids |
|---|---|---|---|---|---|---|
| Cyclin B | Quercetin | − 6.86 | 9.34 µM | 1.22 | 6 | THR158; HIS147; GLU189; ILE190; GLY336; VAL337; ALA338; GLU340; ASP341 |
| Cyclin D | Quercetin | − 6.89 | 8.86 µM | 2.10 | 7 | CYS38; ALA39; ARG87; LEU148;LYS149; ARG61; GLU64 |
| Cyclin E | Quercetin | − 6.30 | 23.91 µM | 1.57 | 3 | THR158; ARG217; LEU219; GLU260; MET261; TYR262 |
| VEGFR | Quercetin | − 6.20 | 28.61 µM | 1.89 | 5 | GLY59; CYS61; GLU67; CYS68 |
| IKKB | Quercetin | − 6.80 | 10.30 µM | 2.93 | 3 | ILE65; SER68; VAL709; ALA712; HIS713; CYS716; ALA64 |
| GSK3 | Quercetin | − 7.32 | 4.31 µM | 3.10 | 2 | ILE62; ALA83; ASP133; ARG141; LEU188 |
| PI3 | Quercetin | − 7.97 | 1.45 µM | 0.63 | 2 | LYS123; ARG460; ASN462; PRO463; PRO658; ALA661; LEU662 |
| BCL-2 | Quercetin | − 5.86 | 50.32 µM | 3.18 | 4 | SER4; ARG6; GLU7; VAL10; TRP24; ALA84; LYS87 |
| BCL-XL | Quercetin | − 8.24 | 916.33 nM | 0.37 | 5 | ALA93; TYR101; GLY138; VAL141; ASP133; ARG139 |
Fig. 16.
3D representation of molecular docking of the Quercetin with the function apoptosis and anti-apoptosis proteins include (a) Cyclin B (b) Cyclin D (c) Cyclin E (d) VEGFR (e) IKKB (f) GSK3 (g) PI3 (h) BCL-2 and (i) BCL-XL
Conclusions
Our research showed a therapeutic drug made from the leaves of the Acalypha indicia plant that may treat breast cancer cell lines, including MCF-7 and MDA-MB-231. We systematically separated and identified the component of interest using the three solvent systems for crude extracts. The plant extracts were then processed using solvent systems for flavonoid chemical component purification. The identifying chemical of interest was Quercetin, deduced using HPLC analysis, HR-MS/MS spectroscopic analysis, and NMR data-based constructs to infer purity and structural correlations. Compared to the standard drug Doxorubicin, Quercetin showed potential inhibitions in MCF-7 and MDA-MB-231 cells, as evaluated by apoptosis and cell cycle phase measurements. The DNA integrity was demonstrated by forming a ladder because of ROS-induced cell damage. Cells treated with Quercetin, pro-apoptotic proteins, revealed enhanced apoptosis activity. Overall research, we concluded that Quercetin would be many drugs of interest in treating breast cancer cell linage.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank for the support from the Department of Genetics, Osmania University, for providing the resources and moral support to complete this research.
Author contributions
SC, and SSV equally contributed in this research and involved in Conceptualization, Methodology, Investigation, Formal analysis, Writing—Original Draft, SSV managed to curate the software applications, Validation, Formal analysis, Investigation, Resources was provided by Ms. SLS, and Prof. RRA, Data Curation was done by Ms. SBKC, and Mr. CG and finally Supervision, Project administration, Writing—Review and Editing of this research was supported by Prof. RRA.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The complete data is provided in supporting information.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Sudhakar Chekuri and Satyanarayana Swamy Vyshnava have contributed equally to this work.
References
- Abd Razak N, Abu N, Ho WY, Zamberi NR, Tan SW, Alitheen NB, Long K, Yeap SK. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-018-37796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkahtani SA, Alshabi AM, Shaikh IA, Orabi MA, Abdel-Wahab BA, Walbi IA, Habeeb MS, Khateeb MM, Shettar AK, Hoskeri JH. In Vitro cytotoxicity and spectral analysis-based phytochemical profiling of methanol extract of barleria hochstetteri, and molecular mechanisms underlying its apoptosis-inducing effect on breast and lung cancer cell lines. Separations. 2022;9(10):298. doi: 10.3390/separations9100298. [DOI] [Google Scholar]
- Ameer K, Shahbaz HM, Kwon JH. Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Comprehensive Rev Food Sci Food Safety. 2017;16(2):295–315. doi: 10.1111/1541-4337.12253. [DOI] [PubMed] [Google Scholar]
- Ávila-Román J, García-Gil S, Rodríguez-Luna A, Motilva V, Talero E. Anti-inflammatory and anticancer effects of microalgal carotenoids. Mar Drugs. 2021;19(10):531. doi: 10.3390/md19100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi M, Ghourchian H, Yazdian F, Bagherifam S, Bekhradnia S, Nyström B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci Rep. 2017;7(1):5178. doi: 10.1038/s41598-017-05461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmir J, Zaidul ISM, Rahman MM, Sharif K, Mohamed A, Sahena F, Jahurul M, Ghafoor K, Norulaini N, Omar A. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117(4):426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Bakhashab S, Ahmed F, Schulten H-J, Ahmed FW, Glanville M, Al-Qahtani MH, Weaver JU. Proangiogenic effect of metformin in endothelial cells is via upregulation of VEGFR1/2 and their signaling under hyperglycemia-hypoxia. Int J Mol Sci. 2018;19(1):293. doi: 10.3390/ijms19010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros-Vivas D, Álvarez-Rivera G, Ibáñez E, Parada-Alfonso F, Cifuentes A. A multi-analytical platform based on pressurized-liquid extraction, in vitro assays and liquid chromatography/gas chromatography coupled to high resolution mass spectrometry for food by-products valorisation. Part 2: Characterization of bioactive compounds from goldenberry (Physalis peruviana L.) calyx extracts using hyphenated techniques. J Chromatogr A. 2019;1584:144–154. doi: 10.1016/j.chroma.2018.11.054. [DOI] [PubMed] [Google Scholar]
- Becer E, Vatansever H. Senescence-mediated anticancer effects of quercetin. Nutrition Research (new York, NY) 2022;104:82–90. doi: 10.1016/j.nutres.2022.04.007. [DOI] [PubMed] [Google Scholar]
- Bertucci F, Ng CK, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, Soria JC, Dien AT, Adnani Y, Kamal M. Genomic characterization of metastatic breast cancers. Nature. 2019;569(7757):560–564. doi: 10.1038/s41586-019-1056-z. [DOI] [PubMed] [Google Scholar]
- Boots AW, Li H, Schins RP, Duffin R, Heemskerk JW, Bast A, Haenen GR. The quercetin paradox. Toxicol Appl Pharmacol. 2007;222(1):89–96. doi: 10.1016/j.taap.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F, Plénat F. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem. 2009;57(4):289–300. doi: 10.1369/jhc.2008.952044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryda O, Stadnytska N (2021) Extraction Methods of Extractive Substances from Medicinal Plant Raw Materials: Advantages and Limitations. Annals of the Romanian Society for Cell Biology:1737–1751
- Burton JD (2005) The MTT assay to evaluate chemosensitivity. Chemosensitivity: Volume 1 In Vitro Assays:69–78 [DOI] [PubMed]
- Casao A, Mata-Campuzano M, Ordas L, Cebrián-Pérez J, Muiño-Blanco T, Martínez-Pastor F. Cleaved PARP-1, an apoptotic marker, can be detected in ram spermatozoa. Reprod Domest Anim. 2015;50(4):688–691. doi: 10.1111/rda.12549. [DOI] [PubMed] [Google Scholar]
- Chandradevan M, Simoh S, Mediani A, Ismail IS, Abas F. 1 H NMR-based metabolomics approach in investigating the chemical profile, antioxidant and anti-inflammatory activities of Gynura procumbens and Cleome gynandra. Plant Foods Hum Nutr. 2020;75(2):243–251. doi: 10.1007/s11130-020-00805-3. [DOI] [PubMed] [Google Scholar]
- Chandrasekar R, Sivagami B, Babu MN. Pharmacoeconomic focus on medicinal plants with anticancer activity. Res J Pharma Phytochemistry. 2018;10(1):91–100. [Google Scholar]
- Chavda VP, Ertas YN, Walhekar V, Modh D, Doshi A, Shah N, Anand K, Chhabria M Advanced computational methodologies used in the discovery of new natural anticancer compounds. Frontiers in Pharmacology (2021) [DOI] [PMC free article] [PubMed]
- Chekuri S, Vankudothu N, Panjala S, Babu Rao N, Anupali R. Phytochemical analysis, anti-oxidant and anti-microbial activity of “Acalypha indica” leaf extracts in different organic solvents. Int J Phytomedicine. 2016;8(3):444–452. doi: 10.5138/09750185.1882. [DOI] [Google Scholar]
- Cui Q, Yu J-h, Wu J-n, Tashiro S-i, Onodera S, Minami M, Ikejima T. P53-mediated cell cycle arrest and apoptosis through a caspase-3-independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharmacol Sin. 2007;28(7):1057–1066. doi: 10.1111/j.1745-7254.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Dai H, Meng W, Kaufmann S. BCL2 family, mitochondrial apoptosis, and beyond. Cancer Trans Med. 2016;2(1):1. doi: 10.4103/2395-3977.177558. [DOI] [Google Scholar]
- De Castro ML, Priego-Capote F. Soxhlet extraction: past and present panacea. J Chromatogr A. 2010;1217(16):2383–2389. doi: 10.1016/j.chroma.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Dineshkumar B, Vigneshkumar P, Bhuvaneshwaran S, Mitra A. Phyto-pharmacology of Acalypha indica: a review. Inter J Biosci, Alternative Holistic Med. 2010;1(2):27. [Google Scholar]
- Erdoğan MK, Ağca CA, Aşkın H. Quercetin and luteolin improve the anticancer effects of 5-fluorouracil in human colorectal adenocarcinoma in vitro model: a mechanistic insight. Nutr Cancer. 2022;74(2):660–676. doi: 10.1080/01635581.2021.1900301. [DOI] [PubMed] [Google Scholar]
- Fearnhead HO, Rodriguez J, Govek E-E, Guo W, Kobayashi R, Hannon G, Lazebnik YA. Oncogene-dependent apoptosis is mediated by caspase-9. Proc Natl Acad Sci. 1998;95(23):13664–13669. doi: 10.1073/pnas.95.23.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica J, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33(12):1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Gangola S, Khati P, Bhatt P, Sharma A. India as the heritage of medicinal plant and their use. Current Trends Biomed Eng Biosci. 2017;4(4):50–51. [Google Scholar]
- Ghagane SC, Puranik SI, Kumbar VM, Nerli RB, Jalalpure SS, Hiremath MB, Neelagund S, Aladakatti R. In vitro antioxidant and anticancer activity of Leea indica leaf extracts on human prostate cancer cell lines. Integrative Med Res. 2017;6(1):79–87. doi: 10.1016/j.imr.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan M, Jebanesan A, Reetha D, Amsath R, Pushpanathan T, Samidurai K. Antibacterial activity of Acalypha indica L. Eur Rev Med Pharmacol Sci. 2008;12(5):299–302. [PubMed] [Google Scholar]
- Hargraves KG, He L, Firestone GL. Phytochemical regulation of the tumor suppressive microRNA, miR-34a, by p53-dependent and independent responses in human breast cancer cells. Mol Carcinog. 2016;55(5):486–498. doi: 10.1002/mc.22296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, Sadegh SE, Tsarouhas K, Kouretas D, Tzanakakis G. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol Rep. 2017;38(2):819–828. doi: 10.3892/or.2017.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour SH, Dehghani M. Review of cancer from perspective of molecular. J Cancer Res Practice. 2017;4(4):127–129. doi: 10.1016/j.jcrpr.2017.07.001. [DOI] [Google Scholar]
- Huang M, Lu J-J, Ding J. Natural products in cancer therapy: past, present and future. Natural Products Bioprospecting. 2021;11(1):5–13. doi: 10.1007/s13659-020-00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Seukep A, Zhang Y-L, Xu Y-B, Guo M-Q. In vitro antibacterial and antiproliferative potential of Echinops lanceolatus Mattf (Asteraceae) and identification of potential bioactive compounds. Pharmaceuticals. 2020;13(4):59. doi: 10.3390/ph13040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirge S, Tatke P, Gabhe S. Simultaneous estimation of kaempferol, rutin, and quercetin in various plant products and different dosage forms of Bhuiamla and Amla. JPC-J Planar Chromatography-Modern TLC. 2014;27(4):267–273. doi: 10.1556/JPC.27.2014.4.6. [DOI] [Google Scholar]
- Kainsa S, Kumar P, Rani P. Medicinal plants of Asian origin having anticancer potential: short review. Asian J Biomed Pharm Sci. 2012;2(10):1–11. [Google Scholar]
- Kamran S, Sinniah A, Abdulghani MA, Alshawsh MA. Therapeutic potential of certain terpenoids as anticancer agents: a scoping review. Cancers. 2022;14(5):1100. doi: 10.3390/cancers14051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap A, Umar SM, Mendiratta M, Prasad CP. In vitro anticancer efficacy of a polyphenolic combination of Quercetin, Curcumin, and Berberine in triple negative breast cancer (TNBC) cells. Phytomedicine plus. 2022;2(2):100265. doi: 10.1016/j.phyplu.2022.100265. [DOI] [Google Scholar]
- Katayama H, Wang J, Treekitkarnmongkol W, Kawai H, Sasai K, Zhang H, Wang H, Adams HP, Jiang S, Chakraborty SN. Aurora kinase-A inactivates DNA damage-induced apoptosis and spindle assembly checkpoint response functions of p73. Cancer Cell. 2012;21(2):196–211. doi: 10.1016/j.ccr.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Ansari AA, Rolfo C, Coelho A, Abdulla M, Al-Khayal K, Ahmad R. Evaluation of in vitro cytotoxicity, biocompatibility, and changes in the expression of apoptosis regulatory proteins induced by cerium oxide nanocrystals. Sci Technol Adv Mater. 2017;18(1):364–373. doi: 10.1080/14686996.2017.1319731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate C. Practical pharmacognosy, 2001. Vallabh Prakashan, Delhi. 2001;107(108–111):123. [Google Scholar]
- Kumar P, Nagarajan A, Uchil PD (2018) Analysis of cell viability by the MTT assay. Cold spring harbor protocols 2018 (6):pdb. prot095505 [DOI] [PubMed]
- Lei Y, Wang S, Ren B, Wang J, Chen J, Lu J, Zhan S, Fu Y, Huang L, Tan J. CHOP favors endoplasmic reticulum stress-induced apoptosis in hepatocellular carcinoma cells via inhibition of autophagy. PLoS ONE. 2017;12(8):e0183680. doi: 10.1371/journal.pone.0183680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng E, Xiao Y, Mo Z, Li Y, Zhang Y, Deng X, Zhou M, Zhou C, He Z, He J. Synergistic effect of phytochemicals on cholesterol metabolism and lipid accumulation in HepG2 cells. BMC Complement Altern Med. 2018;18:1–10. doi: 10.1186/s12906-018-2189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8(3):167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manach C, Morand C, Texier O, Favier M-L, Agullo G, Demigné C, Régérat F, Rémésy C. Quercetin metabolites in plasma of rats fed diets containing rutin or quercetin. J Nutr. 1995;125(7):1911–1922. doi: 10.1093/jn/125.7.1911. [DOI] [PubMed] [Google Scholar]
- Manikandan P, Murugan RS, Priyadarsini RV, Vinothini G, Nagini S. Eugenol induces apoptosis and inhibits invasion and angiogenesis in a rat model of gastric carcinogenesis induced by MNNG. Life Sci. 2010;86(25–26):936–941. doi: 10.1016/j.lfs.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Martini S, Bonechi C, Rossi C. Interaction of quercetin and its conjugate quercetin 3-o-β-D-glucopyranoside with albumin as determined by NMR relaxation data. J Nat Prod. 2008;71(2):175–178. doi: 10.1021/np070285u. [DOI] [PubMed] [Google Scholar]
- Mbaveng AT, Fotso GW, Ngnintedo D, Kuete V, Ngadjui BT, Keumedjio F, Andrae-Marobela K, Efferth T. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants Garcinia epunctata and Ptycholobium contortum towards multi-factorial drug resistant cancer cells. Phytomedicine. 2018;48:112–119. doi: 10.1016/j.phymed.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Mickymaray S, Al Aboody MS, Rath PK, Annamalai P, Nooruddin T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac J Trop Biomed. 2016;6(3):185–191. doi: 10.1016/j.apjtb.2015.12.005. [DOI] [Google Scholar]
- Mohajeri M, Bianconi V, Ávila-Rodriguez MF, Barreto GE, Jamialahmadi T, Pirro M, Sahebkar A. Curcumin: a phytochemical modulator of estrogens and androgens in tumors of the reproductive system. Pharmacol Res. 2020;156:104765. doi: 10.1016/j.phrs.2020.104765. [DOI] [PubMed] [Google Scholar]
- Mohan C, Dinakar S, Anand T, Elayaraja R, SathiyaPriya B. Phytochemical, GC-MS analysis and Antibacterial activity of a medicinal plant Acalypha indica. Int J Pharm Tech Res. 2012;4(3):1050–1054. [Google Scholar]
- Molani Gol R, Kheirouri S. The effects of quercetin on the apoptosis of human breast cancer Cell Lines MCF-7 and MDA-MB-231: A Systematic Review. Nutr Cancer. 2022;74(2):405–422. doi: 10.1080/01635581.2021.1897631. [DOI] [PubMed] [Google Scholar]
- Mortezaee K, Najafi M, Farhood B, Ahmadi A, Potes Y, Shabeeb D, Musa AE. Modulation of apoptosis by melatonin for improving cancer treatment efficiency: An updated review. Life Sci. 2019;228:228–241. doi: 10.1016/j.lfs.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Movsesyan VA, Yakovlev AG, Dabaghyan EA, Stoica BA, Faden AI. Ceramide induces neuronal apoptosis through the caspase-9/caspase-3 pathway. Biochem Biophys Res Commun. 2002;299(2):201–207. doi: 10.1016/S0006-291X(02)02593-7. [DOI] [PubMed] [Google Scholar]
- Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269(2):315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Ninave PB, Patil SD (2022) Pharmacological screening of Acalypha indica L.: Possible role in the treatment of asthma. Journal of Ethnopharmacology 290:115093 [DOI] [PubMed]
- Niranjan A, Pandey A, Misra P, Trivedi PK, Lehri A, Amla D. Development and optimization of HPLC-PDA-MS-MS method for simultaneous quantification of three classes of flavonoids in legume seeds, vegetables, fruits, and medicinal plants. J Liq Chromatogr Relat Technol. 2011;34(16):1729–1742. doi: 10.1080/10826076.2011.578324. [DOI] [Google Scholar]
- Pinho E, Ferreira IC, Barros L, Carvalho AM, Soares G, Henriques M (2014) Antibacterial potential of northeastern Portugal wild plant extracts and respective phenolic compounds. BioMed research international 2014 [DOI] [PMC free article] [PubMed]
- Popov V, Khabarov S, Kadochnikova G, Poznyakovsky V. Improvement of the methods of extraction of plant raw materials. Int J Appl Eng Res. 2017;12(15):5421–5429. [Google Scholar]
- Porębska I, Wyrodek E, Kosacka M, Adamiak J, Jankowska R, Harłozińska-Szmyrka A. Apoptotic markers p53, Bcl-2 and Bax in primary lung cancer. In Vivo. 2006;20(5):599–604. [PubMed] [Google Scholar]
- Prasad SK, Veeresh PM, Ramesh PS, Natraj SM, Madhunapantula SV, Devegowda D. Phytochemical fractions from Annona muricata seeds and fruit pulp inhibited the growth of breast cancer cells through cell cycle arrest at G0/G1 phase. J Cancer Res Ther. 2020;16(6):1235–1249. doi: 10.4103/jcrt.JCRT_494_19. [DOI] [PubMed] [Google Scholar]
- Qin Z, Liu H-M, Ma Y-X, Wang X-D. Developments in extraction, purification, and structural elucidation of proanthocyanidins (2000–2019) Stud Nat Prod Chem. 2021;68:347–391. doi: 10.1016/B978-0-12-819485-0.00008-6. [DOI] [Google Scholar]
- Raaman N (2006) Phytochemical techniques. New India Publishing,
- Rauf A, Imran M, Khan IA, ur‐Rehman M, Gilani SA, Mehmood Z, Mubarak MS, (2018) Anticancer potential of quercetin: A comprehensive review. Phytotherapy Research 32 (11): 2109-2130 [DOI] [PubMed]
- Rizvi SMD, Shakil S, Haneef M (2013) A simple click by click protocol to perform docking: AutoDock 4.2 made easy for non-bioinformaticians. EXCLI journal 12:831 [PMC free article] [PubMed]
- Roy A, Ahuja S, Bharadvaja N. A review on medicinal plants against cancer. J Plant Sci Agricultural Res. 2017;2(1):008. [Google Scholar]
- Ruslan NF (2015) Evaluation of Acalypha indica extracts for antioxidant and antibacterial activities. Universiti Teknologi Malaysia,
- Shoemaker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytotherapy Res Inter J Devoted Pharmacological Toxicological Evaluation Natural Product Derivatives. 2005;19(7):649–651. doi: 10.1002/ptr.1702. [DOI] [PubMed] [Google Scholar]
- Solomon RJ, Kallidass S, Vimalan J. Isolation, identification and study of antimicrobial property of a bioactive compound in an Indian medicinal plant Acalypha indica (Indian-nettle) World J Microbiol Biotechnol. 2005;21(6):1231–1236. doi: 10.1007/s11274-005-1479-6. [DOI] [Google Scholar]
- Sophia A, Faiyazuddin M, Alam P, Hussain MT, Shakeel F. GC–MS characterization and evaluation of antimicrobial, anticancer and wound healing efficiency of combined ethanolic extract of Tridax procumbens and Acalypha indica. J Mol Struct. 2022;1250:131678. doi: 10.1016/j.molstruc.2021.131678. [DOI] [Google Scholar]
- Suresh M, Alfonisan M, Alturaiki W, Al Aboody MS, Alfaiz FA, Premanathan M, Vijayakumar R, Umamagheswari K, Al Ghamdi S, Alsagaby SA (2021) Investigations of bioactivity of Acalypha indica (L.), Centella asiatica (L.) and croton bonplandianus (Baill) against multidrug resistant bacteria and cancer cells. Journal of Herbal Medicine 28:100359
- Takle V, Savad R, Kandalkar A, Akarte A, Patel A. Pharmacognostic and Phytochemical investigations of aerial parts of Acalypha indica Linn. Pharmacognosy Journal. 2011;3(21):33–35. doi: 10.5530/pj.2011.21.6. [DOI] [Google Scholar]
- Teklani P, Perera B (2016) The important biological activities and phytochemistry of Acalypha indica. International Journal of Research in Pharmacy and Science (1)
- Venkatachalam P, Jayalakshmi N, Geetha N, Sahi SV, Sharma NC, Rene ER, Sarkar SK, Favas PJ. Accumulation efficiency, genotoxicity and antioxidant defense mechanisms in medicinal plant Acalypha indica L. under lead stress. Chemosphere. 2017;171:544–553. doi: 10.1016/j.chemosphere.2016.12.092. [DOI] [PubMed] [Google Scholar]
- Vogler M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 2012;19(1):67–74. doi: 10.1038/cdd.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyshnava SS, Pandluru G, Kanderi DK, Panjala SP, Banapuram S, Paramasivam K, Anupalli RR, Bontha RR, Dowlatabad MR. Gram scale synthesis of QD 450 core–shell quantum dots for cellular imaging and sorting. Appl Nanosci. 2020;10:1257–1268. doi: 10.1007/s13204-020-01261-w. [DOI] [Google Scholar]
- Vyshnava SS, Pandluru G, Kumar KD, Panjala S, Paramasivam K, Banapuram S. A Computational approach for protein-protein interactions of bacterial surface layer Protein with human Erb3 and αIIB-β3 receptors. Biointerface Res Appl Chem. 2020;12(1):420–430. [Google Scholar]
- Vyshnava SS, Pandluru G, Kumar KD, Panjala SP, Banapuram S, Paramasivam K, Devi KV, Anupalli RR, Dowlatabad MR. Quantum dots based in-vitro co-culture cancer model for identification of rare cancer cell heterogeneity. Sci Rep. 2022;12(1):5868. doi: 10.1038/s41598-022-09702-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waks AG, Winer EP. Breast cancer treatment: a review. Jama. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- Wiese S, Wubshet SG, Nielsen J, Staerk D. Coupling HPLC-SPE-NMR with a microplate-based high-resolution antioxidant assay for efficient analysis of antioxidants in food–Validation and proof-of-concept study with caper buds. Food Chem. 2013;141(4):4010–4018. doi: 10.1016/j.foodchem.2013.06.115. [DOI] [PubMed] [Google Scholar]
- Yun D, Yoon SY, Park SJ, Park YJ. The anticancer effect of natural plant alkaloid isoquinolines. Int J Mol Sci. 2021;22(4):1653. doi: 10.3390/ijms22041653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahidin NS, Saidin S, Zulkifli RM, Muhamad II, Ya'akob H, Nur H. A review of Acalypha indica L. (Euphorbiaceae) as traditional medicinal plant and its therapeutic potential. J Ethnopharmacol. 2017;207:146–173. doi: 10.1016/j.jep.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nör JE. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ. 2010;17(3):499–512. doi: 10.1038/cdd.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete data is provided in supporting information.