Abstract
Mitral regurgitation (MR) is common in the critically unwell and encompasses a heterogenous group of conditions with diverging therapeutic strategies. MR may present acutely with haemodynamic instability or more insidiously with failure to wean from mechanical ventilation. Critical illness is associated with marked physiological stress and haemodynamic changes that dynamically influence the severity and implication of MR. The expanding role of critical care echocardiography uniquely positions the intensivist to apply advanced bedside valvular assessment to recognise haemodynanically significant MR, manipulate and optimise cardiopulmonary physiology and identify patients requiring urgent cardiology and surgical referral. This review will consider common clinical scenarios, therapeutic strategies and the pearls and pitfalls of echocardiographic assessment and quantification in the critically unwell.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-023-01163-4.
Pearls:
Detection of mitral regurgitation (MR) has significant treatment implications in the critically ill and is important not to miss.
A high level of suspicion and early echocardiography is key.
Acute, severe MR may be challenging to diagnose both with clinical examination and with echocardiography. A hyperdynamic left ventricle (LV) in the context of shock should prompt evaluation for life threatening acute severe MR.
In significant MR, a higher LV ejection fraction (EF) is expected. An EF of < 60% is abnormal and represents LV decompensation.
Explaining the mechanism is equally as important as grading severity.
Don’t be deceived by eccentric MR jets. A systematic approach is needed using all ultrasound modalities: 2D, colour, pulse wave Doppler (PWD) and continuous wave Doppler (CWD).
Introduction
Mitral regurgitation (MR) is commonly seen in the critically ill and recognition of haemodynamically significant MR using echocardiography (echo) is essential for tailoring management strategies. Issues arise as standard guidelines for the assessment and quantification of MR using echo are derived from the non-critical care population with stable and more predictable physiology [6–8]. The intensive care specialist is uniquely positioned to integrate advanced bedside valvular assessment to influence supportive therapy, prevent or recognise decompensation and guide early referral for definitive intervention [1]. Accordingly, comprehensive valvular assessment is an important facet of advanced critical care echocardiography.
MR may present acutely with cardiovascular instability and respiratory failure, or more insidiously as failure to wean from mechanical ventilation [2, 3]. Critical illness is associated with physiological stress and haemodynamic changes that dynamically influence the severity and implication of MR: adrenergic stimulation, catecholamines, positive pressure ventilation and heart–lung interactions all have the potential to affect MR severity [4–6]. Critical illness frequently tips the scales to favour mitral regurgitation into the left atrium over effective forward stroke volume (SV), leading to a declining spiral of worsening shock and left atrial/pulmonary venous hypertension.
We will discuss common clinical scenarios, the pearls and pitfalls of echo assessment and quantification of MR in critically ill patients.
Mitral valve anatomy
Any significant mitral regurgitation requires anatomical explanation. Disruption of any component of the valvular or subvalvular apparatus can lead to significant regurgitation. The saddle shaped mitral annulus forms part of the fibrous skeleton of the heart (Fig. 1). The mitral annulus provides an anchor for two asymmetrical valve leaflets—the anterior and posterior mitral valve leaflets (AMVL and PMVL, respectively). The AMVL inserts into the anterior one-third of the annulus but is longer than the PMVL and thus occupies two-thirds of the total mitral valve area. The PMVL is a ‘C’ shaped structure with indentations partitioning the valve into distinct scallops—P1, P2 and P3 [7]. Although the AMVL does not possess scallops, conventional nomenclature subdivides the valve into A1, A2 and A3 regions to correspond with its neighbouring PMVL [7]. A1 and P1 are the most anterolateral scallops located adjacent to the left atrial appendage. The valvular free edges meet at the coaptation zone where they overlap to provide a seal during ventricular systole. At the margins of the coaptation zone the AMVL and PMVL join to form the anterolateral and posteromedial commissures.
Fig. 1.
Mitral valve anatomy. Mitral valve anatomy and adjacent structures. Note that the anterior mitral valve leaflet does not possess scallops and its close proximity to the aortic valve. Each papillary muscle provides chordae tendineae to both valve leaflets. ALPM, anterolateral papillary muscle; PMPM, posteromedial papillary muscle; LAA, left atrial appendage; AV, aortic valve; AMVL, anterior mitral valve leaflet; PMVL, posterior mitral valve leaflet
Two papillary muscles, the anterolateral and posteromedial papillary muscles, provide chordae tendinae to both valve leaflets (Fig. 1). The single blood supply of the posteromedial papillary muscle by the posterior descending artery (typically a branch of the right coronary artery; less frequently the circumflex artery) renders it susceptible to ischaemia and rupture in the context of an inferior myocardial infarction. The anterolateral papillary muscle receives dual blood supply (left anterior descending and circumflex arteries) and is consequently less prone to ischaemia [8, 9].
Mechanism, mechanism, mechanism
Any significant mitral regurgitation requires mechanistic explanation (e.g. ruptured chordae, poor coaptation from annular dilation, etc.). MR may be categorised as acute versus chronic, primary versus secondary or using the Carpentier classification [10–12].
Acute versus chronic MR
Chronic MR should be considered in those with a history of mitral regurgitation or with risk factors such as hypertension, ischaemic heart disease, cardiomyopathy or renal failure [13]. MR leads to increased LA volume, increased LV preload and reduced forward stroke volume (SV) [14–16]. In response, the LV is remodelled through dilatation and eccentric hypertrophy with an aim to preserve forward SV and normalise afterload/wall stress [15, 16]. Importantly, despite progressive deterioration in LV contractile function, ejection fraction (EF) commonly remains within the normal range [17–19]. The adaptive mechanisms of the LV, which include serial increases in myocyte sarcomeres and myofibril slippage, are complex and beyond the scope of this article. The reader is directed to previous comprehensive and dedicated reviews of LV remodelling in MR [14, 16].
In the compensated phase of MR, LA compliance and LA SV increase in response to the increased volume (operating on the ‘ascending’ limb of the Frank–Starling curve) [20, 21]. This limits the rise in LA pressure (LAP) and enhances pulmonary venous drainage [22].
With progressive volume overload and wall stress, compensatory mechanisms are overwhelmed leading to progressive LV/LA dilation, impaired function and reduced forward SV [15, 16, 21]. LA volume overload results in maladaptive processes including myocyte growth, hypertrophy, necrosis and apoptosis alongside alterations in the extracellular matrix and interstitial fibrosis [18, 21]. The rise in LV end-diastolic volume (LVEDV) and LA volume are unable to increase cardiac output and may worsen MR due to annular dilatation (operating on the ‘descending’ limb of the Frank–Starling curve) [18, 20, 21, 23]. LAP increases and LA function/compliance diminishes, reducing the protective cushioning effect of the LA and adversely impacting pulmonary haemodynamics [18, 20, 22, 24]. Patients with chronic MR are therefore at significant risk of decompensation during the physiological stress and interventions associated with critical illness and are an important group to identify early [25].
Acute pathology, e.g. myocardial infarction, papillary muscle rupture or endocarditis, may result in acute MR. The rapidity of onset renders the heart incapable of compensating for the acute volume overload of the LV and LA leading to reduced forward SV, cardiogenic shock, raised LAP and acute pulmonary oedema [2, 26]. In addition to the structural abnormality causing acute MR, LV systolic function may appear normal or hyperdynamic with an absence of anatomical compensation such as LA/LV dilation or eccentric hypertrophy [27, 28]. This emphasises the need for intensivists to contextualise LV systolic function with cardiac output measurement. The absence of structural adaptation makes acute MR challenging to detect both clinically and with echocardiography [12, 29]. The MR jet may be underestimated despite significant regurgitant volume due to reduced MR driving pressure from a combination of hypotension and raised LAP [27]. Compensatory tachycardia also shortens the time for detection. Applying multiple imaging techniques, including Doppler to detect severity and upstream/downstream consequences, and transoesophageal echocardiography (TOE) may improve the detection of acute severe MR [30].
LV volume, LA volume and patient’s antecedent history may be used to differentiate chronic from acute MR. Indexing LA volume to body surface area improves the detection of LA enlargement independent of the patient’s body size and gender [31, 32]. The absence of LA dilation almost excludes the presence of chronic, severe MR [18, 28]. However, as LA dilation is present in many other conditions, including atrial fibrillation, hypertension and diastolic dysfunction, its presence does not necessarily indicate severe MR [27].
Caution should be used to not dismiss acute on chronic MR which is commonly due to chordal rupture in a patient with moderate or severe MR [33, 34]. These cases present with acute features of pulmonary oedema and possibly haemodynamic instability with echocardiographic findings suggestive of chronic MR (e.g. LA/LV dilation) [35–37]. In such cases, reviewing previous imaging or performing TOE can help to identify new structural changes.
Primary versus secondary MR
MR is conventionally classified as primary, due to structural abnormalities of the valve or subvalvular apparatus, or secondary, due to left ventricular or atrial disease, causing tethering of the valve leaflets (also known as functional MR). It is possible for a mixed primary and secondary mechanism to be present, particularly in elderly patients with degenerative or calcific mitral valve disease, although a dominant mechanism can usually be identified that becomes the focus of therapeutic strategies [38].
Carpentier classification
Carpentier proposed a classification for MR in 1983 based upon leaflet motion to guide surgical intervention which has since been modified [10, 39]. Type I describes MR in the setting of normal leaflet motion that is typically centrally directed. In type II, the leaflet motion is increased with either mitral prolapse or flail through the coaptation line causing an eccentrically directed MR jet away from the affected leaflet. Type III is subdivided into type IIIa where leaflet motion is restricted during both systole and diastole (e.g. rheumatic mitral valve disease), whereas type IIIb the leaflet restriction is limited to systole causing eccentric MR towards the restricted leaflet. The MR jet in type III may be central or eccentric.
MR in critical illness
Certain clinical phenotypes should prompt the ICU clinician to consider the presence of haemodynamically significant MR. Whilst far from exhaustive, the following cases (Table 1) aim to emphasise the need to integrate clinical context (including loading conditions) with systematic echocardiography to evaluate the mechanism and severity of MR to direct management. The dynamic nature of MR necessitates repeat assessment after therapeutic interventions or altered loading conditions. Key principles of management are summarised in Fig. 2.
Table 1.
Cases demonstrating clinical phenotypes
| Case 1: A 62-year-old male presenting to ED with severe respiratory failure and shock | Case 2: A 67-year-old female with escalating noradrenaline and FiO2 requirements post-emergency laparotomy |
|---|---|
| 2-day history of fevers and feeling generally unwell | 12-h following laparotomy and bowel resection for small bowel obstruction |
| History of intravenous drug use (none for 3 months) | History of hypertension, type 2 diabetes mellitus and chronic kidney disease stage III |
| Rapid escalation in FiO2 requirement and shock in the Emergency Department requiring intubation | Progressive rise in vasopressor and FiO2 requirement with cool peripheries |
| Temperature 37.7°C, FiO2 0.6 (invasive mandatory ventilation), noradrenaline 0.2 mcg/kg/min, cool peripheries | Temperature 36.4°C, FiO2 0.5 at 50l flow via high flow nasal oxygenation, noradrenaline 0.4 mcg/kg/min |
| Mildly elevated troponin. Lactate 11 | Chest X-Ray demonstrated bilateral pulmonary infiltrates |
| Case 3: A 43-year-old with productive cough, chest discomfort and unilateral pulmonary infiltrates on chest X-Ray | Case 4: A 76-year-old female with non-retroviral pneumocystic jirovecii pneumonia with two failed spontaneous breathing trials |
|---|---|
| 24-h history of productive cough and fevers | 6 day admission to ICU requiring invasive ventilation for severe respiratory failure |
| Evolving breathlessness since symptom onset | History of hypertension, dyslipidaemia and smoking |
| History of antiphospholipid syndrome with multiple pulmonary emboli and an RCA myocardial infarction treated with drug eluting stents. Mildly elevated troponin, no new ECG changes | Previous TTE revealed mild mitral regurgitation |
| FiO2 0.5 on CPAP 10 cmH2O | Two spontaneous breathing trials resulted in tachycardia, hypertension, worsening hypoxaemia and bilateral pulmonary infiltrates |
| Right sided coarse crackles on examination |
Fig. 2.
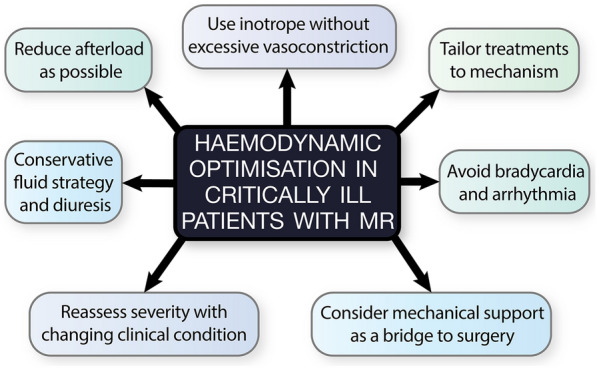
Management principles of MR in the critically ill
Clinical phenotype 1—shock with normal or hyperdynamic LV systolic function
Case 1 describes a case of native mitral valve endocarditis with severe/torrential MR in a patient with a history of intravenous drug use. This patient presented to the ED with scant clinical history and non-specific features of fevers, respiratory failure and shock. Initial management was guided by recommendations from the Surviving Sepsis Campaign including intravenous fluid boluses and early vasopressor therapy; both interventions that potentially exacerbate regurgitation fraction in MR [40–42]. The patient rapidly deteriorated requiring intubation, mechanical ventilation and escalating doses of noradrenaline. An urgent comprehensive TTE was performed and demonstrated mitral valve vegetations with severe MR (Fig. 3a)(Additional file 1: Figure 4 Video—Case 1—Infective Endocarditis). A TOE confirmed vegetations on P1/P2/P3 with a flail chord (Fig. 3b–e). In response to this new finding, noradrenaline was transitioned to adrenaline for enhanced inotropy and chronotropy, PEEP was increased, and an intra-aortic balloon pump (IABP) was sited to reduce afterload and promote forward SV. Adrenaline was selected at the preference of the treating intensivist and alternative strategies are available (e.g. adding dobutamine to noradrenaline for independent manipulation of inotropy and vasoconstriction). The patient was transferred to the regional cardiothoracic centre and underwent an urgent mitral valve replacement with a good post-operative recovery.
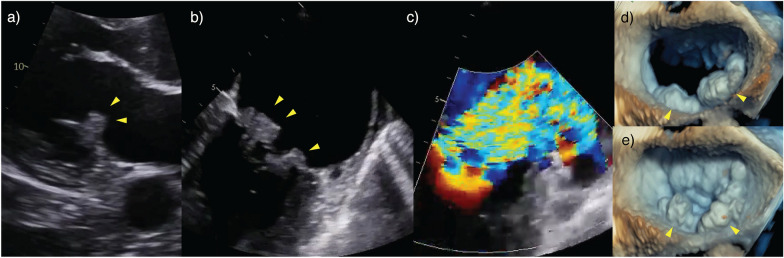
Fig. 3.
Case 1—Infective endocarditis. Vegetations highlighted with arrows on the a) parasternal long-axis (PLAX) TTE view and (b) mid-oesophageal commissural TOE view; c colour Doppler showing severe MR; d (diastole) and e (systole) demonstrates a 3D TOE image of the mitral valve vegetations (arrows)
Whilst this vignette showcases acute MR in infective endocarditis, similar principles may be applied for other causes of acute MR. The most notable example of this is acute ischaemic MR due to ruptured papillary muscle which commonly presents with severe shock in the context of recent myocardial infarction (Fig. 4) (Additional file 2: Figure 5 Video—Papillary Muscle Rupture). Echo will demonstrate a rapidly equalising, often eccentrically directed MR jet, with associated regional wall motion abnormalities. This requires management strategies aimed at augmenting contractility/heart rate (inotropic/chronotropic agents), reducing afterload (inodilators/IABP insertion) and urgent referral for surgical intervention [2, 26]. The IABP provides a rescue strategy for afterload reduction when haemodynamic instability limits the use of vasodilators [43]. VA ECMO and Impella devices are emerging alternatives to provide temporary mechanical support as a bridge to surgical intervention in acute MR [44, 45].
Fig. 4.
Posteromedial papillary muscle rupture. Mid-oesophageal TOE views demonstrating a ruptured posteromedial papillary muscle (arrows) in diastole (a) and systole (b) with flail leaflet and severe eccentric mitral regurgitation (c)
Clinical phenotype 2—rapid escalation in vasopressor requirements ± pulmonary oedema
Case 2 describes a progressive escalation in noradrenaline requirement and worsening oxygenation in the immediate post-operative period. The patient was managed with a conservative fluid strategy and early vasopressors due to the presumption of a mixed distributive (septic) and cardiogenic shock with early pulmonary infiltrates and hypoxaemia. A deteriorating trajectory prompted clinicians to consider second-line cardiovascular support agents with a clinical consensus favouring inotropy over further vasoconstriction due to pulmonary oedema and cool peripheries. Diuresis to achieve a negative fluid balance was also planned to limit worsening hypoxaemia and avoid re-intubation.
A TTE was performed prior to initiating these measures which demonstrated a hyperdynamic, hypertrophied left ventricle with systolic anterior motion (SAM) of the mitral valve, dynamic LV outflow tract (LVOT) obstruction and posteriorly directed MR (Fig. 5) (Additional file 3: Figure 6 Video—Case 2—LVOT Obstruction). The planned interventions of diuresis and inotropy were likely to paradoxically worsen cardiogenic shock and acute pulmonary oedema in the setting of LVOT obstruction. Careful aliquots of fluid were administered to increase preload, LV and LVOT diameter, preventing SAM and led to improved haemodynamic and respiratory status.
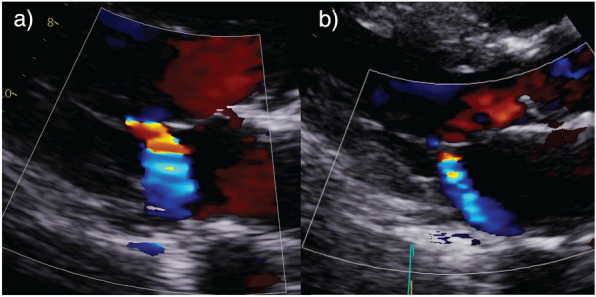
Fig. 5.
Case 2—LVOT obstruction. a Posteriorly directed MR jet (arrows) in the parasternal long-axis view; b M-mode demonstrating systolic anterior motion of the mitral valve (arrows); c spectral Doppler showing dynamic LVOT obstruction with a peak gradient of 71 mmHg
LVOT obstruction is common in the critically unwell [46]. SAM is caused by a combination of ‘drag’ phenomenon of the mitral valve apparatus and Venturi forces [47, 48]. Hypertrophic cardiomyopathy and hypertensive heart disease (particularly those with a ‘sigmoid’ basal septum) reduce the area of the LVOT and are predisposing risk factors for SAM. Mitral valve abnormalities, e.g. redundant anterior or posterior leaflet, papillary muscle displacement and anterior MV displacement, increase the forces acting to draw the valve towards the interventricular septum. Factors enhancing global or regional LV contractility such as distributive shock (particularly with volume depletion), Takotsubo cardiomyopathy or LAD infarction (both potentially leading to apical akinesis and hyperkinetic basal segments) may also cause LVOT obstruction [1, 46, 49–51]. Lastly, deviation of the interventricular septum towards the LV cavity, as is seen in acute cor pulmonale, predisposes individuals to SAM [52, 53].
This case emphasises that conventional management strategies applied to refractory hypoxaemia or shock (e.g. diuresis or inotropy) can worsen clinical parameters and should prompt consideration of an urgent comprehensive echo. Administration of fluids, pure vasoconstrictors (e.g. vasopressin/phenylephrine) and short-acting, titratable beta-blockade (e.g. esmolol), alongside discontinuation of inotropic agents and afterload reducing agents (including IABP), have all been demonstrated to reduce the incidence of LVOT obstruction [1, 46].
Clinical phenotype 3—unilateral pulmonary oedema
Unilateral pulmonary oedema (UPO) is a hallmark feature of severe eccentric MR. Nevertheless, UPO is frequently mistaken for pneumonia, particularly in cases with diagnostic uncertainty [54].
Case 3 describes a 43-year-old with a history of myocardial infarction due to RCA occlusion on a background of antiphospholipid syndrome. Previous imaging demonstrated inferior/inferolateral regional wall motion abnormalities with mild, posteriorly directed MR. He presented with a 2-day history of productive cough, fevers and shortness of breath that was treated as severe pneumonia. The absence of ECG changes did not suggest a primary cardiac aetiology for respiratory failure despite a mild rise in cardiac biomarkers.
Worsening oxygenation prompted referral to ICU. TTE demonstrated severe and posteriorly directed MR due to ischaemic tethering of the PMVL (Fig. 6) (Additional file 4: Figure 7 Video—Case 3—Acute on Chronic MR). His condition improved rapidly with diuresis and continuous positive airway pressure (CPAP) obviating the need for intubation.
Fig. 6.

Case 3—Acute on chronic MR. a Posteriorly directed MR jet on parasternal long-axis (PLAX) view; b MR jet on apical 4 chamber (A4C) view.
This case demonstrates how chronic MR can become physiologically significant during acute illness [55]. Conventional therapies, in this case fluid administration, can cause inadvertent worsening of physiological parameters. Whilst comprehensive echo does not eradicate diagnostic uncertainty, findings may prompt clinicians of potential alternative or contributory diagnoses to tailor and refine treatment strategies. [35]
Clinical phenotype 4—failure to wean from mechanical ventilation
Failure to wean from mechanical ventilation, defined as failure of a spontaneous breathing trial (SBT) or requirement for reintubation within 48 h of extubation, is common in the ICU [3, 56]. Case 4 describes a 76-year-old female with pneumocystis jirovecii pneumonia requiring invasive mechanical ventilation. Two SBTs resulted in tachycardia, hypertension and progressive tachypnoea with rising FiO2 requirements and bilateral pulmonary infiltrates. Repeat TTE demonstrated that the previously identified mild MR had worsened during the SBT and had become severe and posteriorly directed (Fig. 7). The patient was diuresed, commenced upon titratable afterload reducing medications and was successfully extubated directly to CPAP non-invasive ventilation.
Fig. 7.
Case 4—Failure to wean from mechanical ventilation. Reduction in MR severity between a (initial weaning study) and b following diuresis and afterload reduction
Cardiovascular disease (including LV systolic dysfunction, diastolic dysfunction and MR) is an important cause of failure to wean and is commonly under-recognised in critical illness [57–60]. In patients situated high on the Frank–Starling curve, positive pressure ventilation reduces preload and LV transmural pressure (LV afterload). The afterload reduction promotes LV ejection, cardiac output and reduces the severity of severe MR [61, 62].
A SBT increases preload and afterload due to a reduction in intrathoracic pressure. This is compounded by increased work of breathing, adrenergic tone and myocardial oxygen demand. SBTs have been demonstrated to increase the severity of MR particularly in the context of LV systolic dysfunction.[3, 55, 60]. An echo performed immediately prior to, and during the SBT can change management in this patient group. Lung ultrasound assessing presence and number of B-lines can also help elucidate severity of interstitial pulmonary fluid [63]. Worsening MR should prompt consideration of diuresis to reduce preload and vasodilators to reduce blood pressure and afterload [3]. These measures aim to decrease regurgitation fraction and promote forward flow. Careful monitoring of loading conditions with pre-emptive use of non-invasive ventilation after extubation serves to reduce the risk of weaning failure [64]. Rarely, percutaneous or surgical intervention for MR may be required to facilitate weaning from mechanical ventilation [65].
Assessment with echocardiography: tips and tricks
After considering the mechanism of the MR, two further questions are required to tailor management:
What is the severity of the MR?
What are the physiological consequences of the MR? (e.g. on LV/pulmonary haemodynamics and venous return)
Training level in critical care echocardiography
As critical care echocardiography (CCE) expands with more intensivists acquiring advanced echo qualifications, an understanding of the basic tenets behind comprehensive techniques will be of value to intensivists and trainees. Accordingly, CCE accreditation pathways have integrated such techniques into their curriculum [66, 67]. This is especially important as management decisions at the bedside are based upon MR severity and mechanism and require integration of echo findings with clinical history, examination and haemodynamics.
Whilst not all intensivists will need to conduct comprehensive mitral valve assessment, an understanding of the overarching principles of how severity is determined (including the dynamic nature of such measures and their potential pitfalls), the implication of the mechanism and exacerbating conditions will greatly assist in communication and patient management. For example, transfer of information such as ‘severe MR’ is less helpful to intensivists than acute severe MR due to mitral valve flail or acute on chronic severe MR due to LV dysfunction.
Focused CCE (‘level 1’) has become increasingly established as a necessary skill for intensivists and is a mandatory component for ICU training in several countries [68, 69] Focused CCE has an important role in the bedside detection of significant 2D abnormalities and severe regurgitant colour jets and should prompt referral for urgent comprehensive valvular assessment [66, 68, 70]. Advanced CCE (‘level 2’) requires the clinician to be adequately trained in comprehensive measures of MR severity (using TTE ± TOE) including detailed 2D assessment, chamber size, colour jet analysis (including vena contracta, jet area and flow convergence) and spectral Doppler assessment (e.g. pulmonary vein flow, mitral inflow/regurgitant jet analysis and estimation of pulmonary pressure). Despite their inclusion in comprehensive CCE training, volumetric and semi-quantitative measures (e.g. proximal isovelocity surface area) are less applicable in the critically ill than in the outpatient setting. Expert CCE (‘level 3’) provides supervision and review of imaging performed by level 1 and 2 clinicians and apply specialised techniques including 3D assessment and procedural echo [66, 68].
Is the MR severe?
Determining the severity of MR using comprehensive CCE requires the use of multimodal echo techniques including 2D, colour Doppler, pulsed wave Doppler (PWD) and continuous wave Doppler (CWD). Errors with quantitative measures can occur in the critically ill, particularly given challenging imaging, however this should not preclude measurement [16]. A summary of the severity measurements for MR and pitfalls are listed in Table 2.
Table 2.
American Society of Echocardiography criteria for MR severity and potential pitfalls [27]
| MR severity | Pitfalls | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| MV morphology | None/mild leaflet abnormality | Moderate leaflet abnormality or moderate tenting | Severe valve lesions |
-Image quality often limited by mechanical ventilation and ability to optimise patient position -Requires low threshold for TOE if concerns for structural abnormalities |
|
LV size and function LA size |
Usually normal | Normal or mildly dilated |
Dilated An LV ejection fraction of 60% and LVESD of 40 mm is indicative of LV decompensation [71] |
- Poor endocardial definition in the critical care population - Limited access to contrast agents for more accurate measurement - May not be dilated in acute severe MR - The 60/40 rule (LVEF 60% and LVESD > 40 mm) is often used to guide surgical intervention in chronic severe MR |
| Colour jet area | Small, central, narrow, brief | Variable | Large central jet (> 50% of LA) or eccentric jet |
- Imprecise, particularly in eccentric, wall-hugging jets - Load dependent, jet size very dependent upon systolic BP - Overestimates when MR not holosystolic - Underestimated in acute severe MR due to low MR driving pressure (due to hypotension and very high LAP) |
| Flow convergence | Not visible, transient or small | Intermediate in size and duration | Large throughout systole |
- Problematic with multiple jets - Eccentric and constrained jets often underestimated - Non-hemispheric shape, particularly in secondary MR - Overestimates when MR not holosystolic |
| CWD jet | Faint, partial or parabolic | Dense but partial or parabolic | Holosystolic, dense or triangular |
- Qualitative - Angle dependent—central jets appear denser than eccentric jets of higher severity - Density is gain dependent |
| Vena contracta (cm) | < 0.3 | Intermediate | ≥ 0.7 |
- Problematic with multiple jets - Convergence zone needed for accurate measurement - Non-hemispheric shape, particularly in secondary MR |
| Pulmonary vein flow | Systolic dominance | Normal or systolic blunting | Systolic flow reversal or minimal systolic flow |
- MR may affect flow pattern in individual PVs - Challenging to image with TTE - Systolic blunting not specific for MR—also elevated LAP, AF - All pulmonary veins must be assessed when using TOE, particularly in the presence of eccentric MR jets |
| Mitral inflow | A-wave dominant | Variable | E-wave dominant (> 1.2 m/s) |
- Not specific to MR - Affected by severe LV dysfunction (low E wave even in severe MR) |
| Effective regurgitant orifice area (EROA), 2D proximal isovelocity surface area (PISA) (cm2) | < 0.20 | 0.2–0.39 | ≥ 0.40 |
- Less accurate with eccentric/multiple jets - Non-hemispheric shape, particularly in secondary MR. 3D PISA may improve accuracy but is seldom performed in the ICU - Significant amplification of small measurement errors through step-wise calculations |
| RVol (ml) | < 30 | 30–59 | ≥ 60 |
- Significant amplification of small measurement errors through step-wise calculations - Not valid if coexistent AR - Volumetric vs PWD methods may give different results |
| RF (%) | < 30 | 30–49 | ≥ 50 | |
MV, mitral valve; LV, left ventricle; LA, left atrium; CWD, continuous wave Doppler; TTE, transthoracic echocardiography; TOE, transoesophageal echocardiography; LVESD, LV end-systolic diameter; LAP, left atrial pressure; AF, atrial fibrillation; EROA, effective regurgitant orifice area; PISA, proximal isovelocity surface area; RVol, regurgitant volume; RF, regurgitant fraction; AR, aortic regurgitation; PWD, pulse wave Doppler
2D assessment
2D imaging (using conventional and zoomed views) is the key initial step to ‘fan’ through the valve and assess for clear structural abnormalities or coaptation defects (e.g. prolapse, flail, gap between leaflet tips) that would be suggestive of severe MR. LV and LA size also provide clues as to the acuity of regurgitation.
TOE provides superior resolution of the MV anatomy and clinicians should have a low threshold in cases of diagnostic or mechanistic uncertainty. This is particularly important with prosthetic mitral valves which are frequently challenging to image with TTE [72]. Assessment of prosthetic valves is beyond the scope of this article.
Newer 3D transducers facilitate multiplane reconstruction of the mitral valve which may be rotated to achieve a “surgical view” from the perspective of the left atrium. This allows simultaneous evaluation of the structure and function of the valve and subvalvular apparatus and has gained popularity in directing percutaneous and surgical intervention. 3D should be considered complementary to systematic 2D interrogation of the valve and relies upon good image resolution for image generation.
Colour Doppler
There are three components to a MR jet: the flow convergence proximal to the valve, the vena contracta (smallest jet diameter) and the jet area (Fig. 8). All three of these parameters can be measured to assess severity. In addition, colour Doppler helps to determine the optimal angle of interrogation for PWD and CWD (off-axis imaging may be required).
Fig. 8.
Jet area, vena contracta and PISA. a MR jet area measurement; b vena contracta measurement and c PISA measurement
MR jet area
The ‘colour flow jet area’ and ‘jet area: LA area ratio’ are standard parameters to determine MR severity, with >10 cm2 and >50%, respectively, considered to be severe. Particularly in the critically ill, there is an unpredictable relationship between jet area and severity and the American Society of Echocardiography (ASE) recommend against its use for severity assessment [27, 73]. Increased driving pressure across the valve (e.g. raised LVEDP, fluid overload) will increase the jet area for a given regurgitant orifice area [38]. Conversely, systemic hypotension will reduce the driving pressure across the valve and thus underestimate MR severity. Eccentric, wall-hugging jets will appear considerably smaller than non-constrained central jets due to the Coanda effect. The presence of an abnormal, horizontal ‘splay’ signal of colour Doppler along the atrial surface of the valve may be the only feature of severe MR in an otherwise benign or equivocal looking jet [74].
Vena contracta
The vena contracta (VC) is the narrowest portion of the jet with the highest velocity—located immediately downstream of the regurgitant orifice area. VC is less load dependent that other measures and therefore may be particularly useful in critical care. VC diameter should be measured using a zoomed view (often the parasternal long-axis view), a narrow colour box (to optimise temporal resolution) and with jet direction perpendicular to the insonation beam (applying axial measurement accuracy) [12]. 2D measurement of VC diameter relies upon the concept that the regurgitant orifice area is circular. Whilst this is mostly the case in primary MR, the regurgitant orifice area is more elongated along the coaptation line in secondary MR, meaning VC diameter may both overestimate or underestimate MR severity in the same patient depending upon the imaging plane [75, 76]. The flow convergence zone of the regurgitant jet should be visualised for accurate measurement of the VC. It is not recommended to add the VC of multiple jets together to determine a total VC if multiple orifices exist [27, 38].
Flow convergence (proximal isovelocity surface area; PISA) and EROA
As blood flow converges towards a regurgitant orifice, it forms concentric ‘shells’ of increasing velocity and decreasing surface area. The presence of a flow convergence zone is likely to indicate moderate or severe MR even in the absence of a significant colour jet when using the recommended Nyquist limit range (50–70 cm/s). Manipulation of the colour scale baseline enables visualisation of an aliasing threshold and measurement of the proximal isovelocity surface area (PISA): from VC to point of colour aliasing. Numerous assumptions (e.g. PISA assumed to be hemispherical), measurement error and amplification of errors through step-wise calculation lessens the accuracy of PISA and therefore is used less frequently in the critically ill [38]. Accuracy may be improved by applying only in central MR jets and using TOE. If performed, the effective regurgitant orifice area (EROA) can be calculated and considered severe if >0.4 cm2. The EROA is subsequently multiplied by the MR velocity time integral (VTI) to calculate the regurgitant volume (RVol) [12, 28].
PISA has become the preferred technique for quantification of EROA and RVol in the outpatient setting but is limited by numerous assumptions and prone to significant error. In the critically ill, where specific volumes are dynamic and conditions suboptimal, PISA is arguably less applicable and should be applied with caution and only in combination with other parameters.
Pulse wave Doppler
PWD assessment of flow through the mitral valve provides supporting evidence of MR severity. This is represented by an elevated E wave velocity (≥1.5 m/s). These are non-specific findings and may be seen in many conditions including mitral stenosis, diastolic dysfunction/restrictive heart disease, hyperdynamic circulation or tachycardia [77]. Similarly, E wave velocity is often low (<1 m/s) in the setting of severe LV dysfunction even if significant MR is present. The rise in MV inflow velocity in MR occurs independently of the diastolic function. Severe MR therefore negates the use of MV inflow velocity and E/e’ as a marker of LVEDP and diastolic dysfunction [32, 77].
Flow characteristics within the pulmonary veins is the most important PWD parameter to determine MR severity. This is discussed in more detail in the section on physiological consequences of MR.
Continuous wave Doppler
The density of CWD trace provides qualitative evidence of MR severity. As severity increases, a greater quantity of blood flows through the regurgitant orifice—represented by an increasingly dense CWD signal. The angle dependency of CWD is a potential pitfall to this and central jets may appear denser than more severe eccentric jets due to malalignment. Similarly, density is gain dependent, meaning users should take care to avoid excessive gain when optimising the spectral Doppler trace.
The shape of the MR jet is also important. A dense, holosystolic, triangular shaped CWD trace is indicative of raised LAP or significant regurgitant flow (Fig. 9) and is analogous to a large V wave on a pressure waveform. Many of the aforementioned techniques will either over- or under-estimate MR severity if MR is not holosystolic, e.g. in mitral valve prolapse (late systolic), severe LV dysfunction (biphasic) or diastolic MR [27]. Diastolic MR is likely to be under-recognised in the critically ill particularly given the frequency of atrioventricular conduction abnormalities [27, 78, 79].
Fig. 9.

Continuous wave Doppler. Incomplete, parabolic Doppler signal in mild MR (a) versus dense, triangular shaped Doppler signal in severe MR (b)
CWD analysis of the TR jet is useful for the identification of raised pulmonary artery pressure as a consequence of MR.
Additional volumetric measures of MR
An alternative method for calculating regurgitant volume is by subtracting the LV SV (calculated using LVOT area and PWD LVOT VTI) from the Simpson’s biplane-derived stroke volume or using PWD technique at the mitral annulus. Simpson’s biplane is known to underestimate LV volumes compared with contrast echo, 3D echo and cardiac MRI and may lead to errors that are compounded in subsequent calculations. Volumetric calculations are challenging and less applicable to the critical care population.
Physiological consequences of MR
The intensivist should consider the upstream and downstream consequences of MR to be of equal importance to the regurgitation severity. Chronic MR leads to dilation and volume overload of the LA and LV, causing elevated mean LAP that is transmitted to the pulmonary venous circulation. With time, LA hypertension leads to remodelling, fibrosis and reduced compliance and is an important cause of post-capillary pulmonary hypertension (PHT). Ultimately this may lead to right ventricular remodelling and failure. Assessment of PHT and the RV is beyond the scope of this paper and the reader is directed elsewhere [80, 81]. In acute MR, the LA is unable to acutely dilate to accommodate an increased volume precipitating a rapid elevation in LAP and pulmonary pressures.
PWD of the pulmonary venous flow provides a useful adjunctive measure of MR severity. This is performed by placing PWD within one of the right pulmonary veins in the far field of the A4C view on TTE [12, 82]. It is possible to assess all pulmonary veins when using TOE; an omniplane angle of 110° can locate left-sided veins and 60–80° used for right sided veins. A minimum TOE dataset should always include assessment of all pulmonary veins where possible. Increasing MR severity reduces the systolic component of pulmonary venous flow and eventually leads to systolic flow reversal (Fig. 10). Technical difficulty and limited image quality unfortunately renders this assessment challenging using TTE within the ICU [83]. Furthermore, recommendations suggest more than one pulmonary vein should be assessed in MR due to the possibility of the MR jet selectively entering a single vein [12, 28]. This is usually only achievable with TOE.
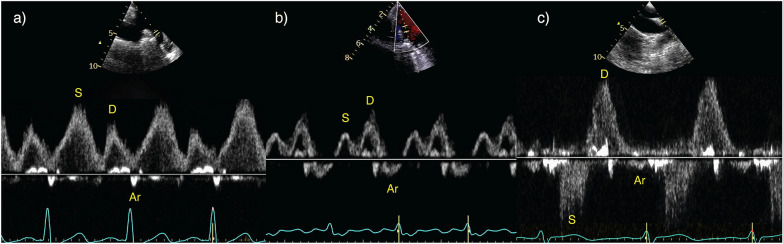
Fig. 10.
Pulmonary venous flow patterns. a Normal systolic dominant flow pattern with a low velocity and short duration of flow reversal during atrial systole (Ar). b Blunting of systolic flow (S) with dominant diastolic flow (D) and a higher velocity and increased duration of Ar, indicative of raised left atrial pressure. c Systolic flow reversal (S) suggestive of severe MR or severely elevated left atrial pressure
Pulmonary venous flow alterations are not specific for mitral valve disease and are seen in other causes of raised LAP. In addition to the conventional chronic causes of raised LAP (e.g. diastolic dysfunction, mitral stenosis), considerations in the critically unwell include any cause of volume overload or acute systolic/diastolic dysfunction (e.g. septic cardiomyopathy) [83, 84]. A reduced IVRT of <60 ms and mitral ‘L’ wave of >20 cm/s are supporting features of raised LAP but are beyond the scope of this article [32]. The reader is directed elsewhere for excellent review articles of the echo assessment of LAP [32, 83, 84].
Despite its lack of sensitivity for MR, the presence of blunted systolic pulmonary venous flow provides the clinician with a window into the patient’s cardiopulmonary physiology. The ageing critical care population with an increased incidence of degenerative mitral valve disease and functional MR is leading to the frequent coexistence of multiple causes of raised LAP (e.g. MV calcification with functional MR, alongside LV hypertrophy and diastolic dysfunction). These complementary factors may lower the threshold for decompensation during acute illness. Fortunately, many management principles in these conditions overlap (e.g. diuresis) allowing clinicians to use the upstream and downstream consequences to guide ongoing management.
As previously mentioned, acute MR may lead to the preservation of EF even in the setting of profound shock. Doppler-derived assessment of LV stroke volume is a simple technique that should be within the armamentarium of all critical care sonographers and integrated into conventional assessments of LV function. Peak velocity of the MR CWD signal also provides indirect evidence of haemodynamic compromise. Maximum peak MR velocity is usually between 4–6 m/s due to the high systolic gradient between LV and LA. Low peak MR velocity (less than 4 m/s) is suggestive of a reduced gradient due to hypotension and raised LAP [27].
Take home points for MR in critical care
Serial assessment is crucial and allows recognition of physiologically significant MR and the development of tailored management plans (e.g. diuresis, avoiding bradycardia and hypertension, extubation directly to CPAP).
Identifying the mechanism of MR is of equal importance as the severity. Severe structural abnormalities should prompt referral for definitive intervention (e.g. MV replacement/repair). Early implementation of mechanical haemodynamic interventions (IABP, Impella) to stabilise physiology as a bridge to intervention should be considered.
Secondary MR is likely to be more common in the ICU. Critical illness will lower the threshold for decompensation and early imaging can recognise and limit iatrogenic worsening of MR.
Quantitative parameters of MR severity are limited by issues with precision, reproducibility and amplification of small measurement errors. Provided the pitfalls of these measures are considered, quantification of MR is useful for serial assessment and provides a common language during specialist referral.
The presence of a flow convergence zone at the recommended Nyquist limit range of 50–70 cm/s is likely to indicate moderate or severe MR even in the absence of a significant colour jet.
The upstream and downstream consequences of MR, including reduced cardiac output, pulmonary venous hypertension and RV dysfunction, are important to recognise and quantify in the critically ill patient.
TOE provides superior resolution of mitral valve anatomy and clinicians should have a low threshold in cases of diagnostic or mechanistic uncertainty. This is particularly important with prosthetic mitral valves [72].
Conclusions
Critical care echo is an expanding subspecialty that allows recognition and management of MR during acute illness. The intensivist is uniquely positioned to apply advanced valvular assessment at the bedside to optimise cardiorespiratory physiology and identify patients requiring percutaneous or surgical intervention. MR is dynamic and repeated assessment is key. Comprehensive assessment in the ICU should integrate loading factors and clearly describe the mechanism, severity and the upstream/downstream complications.
Supplementary Information
Additional file 1. Videos to accompany images for Case 1—Infective Endocarditis with severe mitral regurgitation.
Additional file 2. Videos to accompany images for Figure 5—Papillary Muscle Rupture.
Additional file 3. Videos to accompany images for Figure 6—LVOT Obstruction.
Additional file 4. Videos to accompany images for Figure 7—Acute on Chronic MR.
Acknowledgements
Nil.
Abbreviations
- A4C
Apical four chamber view
- ACEI
ACE inhibitors
- AF
Atrial fibrillation
- ALPM
Anterolateral papillary muscle
- AMVL
Anterior mitral valve leaflet
- AR
Aortic regurgitation
- ARBs
Angiotensin receptor blockers
- ARDS
Acute respiratory distress syndrome
- ASE
American Society of Echocardiography
- AV
Aortic valve
- CCBs
Calcium channel blockers
- CM
Cardiomyopathy
- CWD
Continuous wave Doppler
- ECG
Electrocardiogram
- Echo
Echocardiography
- ED
Emergency department
- EROA
Effective regurgitant orifice area
- FiO2
Fraction of inspired oxygen
- HCM
Hypertrophic cardiomyopathy
- IABP
Intra-aortic balloon pump
- IPPV
Invasive positive pressure ventilation
- IVRT
Isovolumic relaxation time
- LA
Left atrium
- LAA
Left atrial appendage
- LAD
Left anterior descending coronary artery
- LAP
Left atrial pressure
- LV
Left ventricle
- LVEDP
LV end-diastolic pressure
- LVH
Left ventricle hypertrophy
- MR
Mitral regurgitation
- MS
Mitral stenosis
- PE
Pulmonary embolism
- PEEP
Positive end expiratory pressure
- PHT
Pulmonary hypertension
- PISA
Proximal isovelocity surface area
- PLAX
Parasternal long-axis view
- PMPM
Posteromedial papillary muscle
- PMVL
Posterior mitral valve leaflet
- PWD
Pulse wave Doppler
- RCA
Right coronary artery
- RF
Regurgitant fraction
- RVol
Regurgitant volume
- SAM
Systolic anterior motion of the mitral valve
- SBT
Spontaneous breathing trial
- SV
Stroke volume
- SVR
Systemic vascular resistance
- TOE
Transoesophageal echocardiogram
- TEE
Transesophageal echocardiogram
- TR
Tricuspid regurgitation
- TTE
Transthoracic echocardiogram
- UPO
Unilateral pulmonary oedema
- VC
Vena contracta
- VTI
Velocity time integral
Author contributions
CD, EB and SO conceived the article. CD prepared the main manuscript. All authors contributed and edited the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Competing interests
No competing interests of declarations for all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Orde S, Slama M, Hilton A, Yastrebov K, McLean A. Pearls and pitfalls in comprehensive critical care echocardiography. Crit Care. 2017;21:279. doi: 10.1186/s13054-017-1866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe N. Acute mitral regurgitation. Heart. 2019;105:671. doi: 10.1136/heartjnl-2018-313373. [DOI] [PubMed] [Google Scholar]
- 3.Vignon P. Cardiovascular failure and weaning. Ann Transl Medicine. 2018;6:354–354. doi: 10.21037/atm.2018.05.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanfilippo F, Johnson C, Bellavia D, Morsolini M, Romano G, Santonocito C, et al. Mitral regurgitation grading in the operating room: a systematic review and meta-analysis comparing preoperative and intraoperative assessments during cardiac surgery. J Cardiothor Vasc An. 2017;31:1681–1691. doi: 10.1053/j.jvca.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 5.Gisbert A, Soulière V, Denault AY, Bouchard D, Couture P, Pellerin M, et al. Dynamic quantitative echocardiographic evaluation of mitral regurgitation in the operating department. J Am Soc Echocardiog. 2006;19:140–146. doi: 10.1016/j.echo.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Grewal KS, Malkowski MJ, Piracha AR, Astbury JC, Kramer CM, Dianzumba S, et al. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am J Cardiol. 2000;85:199–203. doi: 10.1016/S0002-9149(99)00644-X. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy KP, Ring L, Rana BS. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr. 2010;11:i3–9. doi: 10.1093/ejechocard/jeq153. [DOI] [PubMed] [Google Scholar]
- 8.Chiechi MA, Lees WM, Thompson R. Functional anatomy of the normal mitral valve. J Thorac Surg. 1956;32:378–398. doi: 10.1016/S0096-5588(20)30404-9. [DOI] [PubMed] [Google Scholar]
- 9.Harari R, Bansal P, Yatskar L, Rubinstein D, Silbiger JJ. Papillary muscle rupture following acute myocardial infarction: Anatomic, echocardiographic, and surgical insights. Echocardiogr. 2017;34:1702–1707. doi: 10.1111/echo.13739. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier A. Cardiac valve surgery—the “French correction”. J Thorac Cardiovasc Surg. 1983;86:323–337. doi: 10.1016/S0022-5223(19)39144-5. [DOI] [PubMed] [Google Scholar]
- 11.Agricola E, Oppizzi M, Pisani M, Meris A, Maisano F, Margonato A. Ischemic mitral regurgitation: mechanisms and echocardiographic classification. Eur J Echocardiogr. 2008;9:207–221. doi: 10.1016/j.euje.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Zoghbi WA, Asch FM, Bruce C, Gillam LD, Grayburn PA, Hahn RT, et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement a report from the american society of echocardiography developed in collaboration with the society for cardiovascular angiography and interventions, japanese society of echocardiography, and society for cardiovascular magnetic resonance. J Am Soc Echocardiog. 2019;32:431–475. doi: 10.1016/j.echo.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Bartko PE, Pavo N, Pérez-Serradilla A, Arfsten H, Neuhold S, Wurm R, et al. Evolution of secondary mitral regurgitation. European Hear J - Cardiovasc Imaging. 2018;19:622–629. doi: 10.1093/ehjci/jey023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carabello BA, Crawford FA. Valvular Heart Disease. New Engl J Medicine. 1997;337:32–41. doi: 10.1056/NEJM199707033370107. [DOI] [PubMed] [Google Scholar]
- 15.Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–1394. doi: 10.1016/S0140-6736(09)60692-9. [DOI] [PubMed] [Google Scholar]
- 16.Keir M, Pravin M. Left ventricular remodelling in chronic primary mitral regurgitation: implications for medical therapy. Cardiovasc J Afr. 2018;29:51–64. doi: 10.5830/CVJA-2017-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starling MR, Kirsh MM, Montgomery DG, Gross MD. Impaired left ventricular contractile function in patients with long-term mitral regurgitation and normal ejection fraction. J Am Coll Cardiol. 1993;22:239–250. doi: 10.1016/0735-1097(93)90840-W. [DOI] [PubMed] [Google Scholar]
- 18.Cameli M, Incampo E, Mondillo S. Left atrial deformation: useful index for early detection of cardiac damage in chronic mitral regurgitation. Ijc Hear Vasc. 2017;17:17–22. doi: 10.1016/j.ijcha.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaasch WH, Meyer TE. Left ventricular response to mitral regurgitation. Circulation. 2008;118:2298–2303. doi: 10.1161/CIRCULATIONAHA.107.755942. [DOI] [PubMed] [Google Scholar]
- 20.Kihara Y, Sasayama S, Miyazaki S, Onodera T, Susawa T, Nakamura Y, et al. Role of the left atrium in adaptation of the heart to chronic mitral regurgitation in conscious dogs. Circ Res. 2018;62:543–553. doi: 10.1161/01.RES.62.3.543. [DOI] [PubMed] [Google Scholar]
- 21.Ren B, Laat LE de G, Geleijnse ML. Left atrial function in patients with mitral valve regurgitation. Am J Physiol-heart C. 2014;307:H1430–7. [DOI] [PubMed]
- 22.Inciardi RM, Rossi A, Bergamini C, Benfari G, Maffeis C, Greco C, et al. Mitral regurgitation, left atrial structural and functional remodelling and the effect on pulmonary haemodynamics. Eur J Heart Fail. 2020;22:499–506. doi: 10.1002/ejhf.1677. [DOI] [PubMed] [Google Scholar]
- 23.Bergstra A, Simsek C, van den Heuvel AFM. Mitral regurgitation: when to intervene? Neth Heart J. 2020;28:266–271. doi: 10.1007/s12471-020-01417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratanasit N, Karaketklang K, Krittayaphong R. Left atrial volume index as an independent determinant of pulmonary hypertension in patients with chronic organic mitral regurgitation. Bmc Cardiovasc Disor. 2016;16:141. doi: 10.1186/s12872-016-0306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe Y, Akamatsu K, Furukawa A, Ito K, Matsumura Y, Haze K, et al. Pre-load–induced changes in forward lv stroke and functional mitral regurgitation echocardiographic detection of the descending limb of starling’s curve. Jacc Cardiovasc Imaging. 2017;10:611–618. doi: 10.1016/j.jcmg.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Güvenç RÇ, Güvenç TS. Clinical presentation, diagnosis and management of acute mitral regurgitation following acute myocardial infarction. J Acute Dis. 2016;5:96–101. doi: 10.1016/j.joad.2015.11.001. [DOI] [Google Scholar]
- 27.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation a report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiog. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Robinson S, Ring L, Augustine DX, Rekhraj S, Oxborough D, Harkness A, et al. The assessment of mitral valve disease: a guideline from the british society of echocardiography. Echo Res Pract. 2021;8:G87–136. doi: 10.1530/ERP-20-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stout KK, Verrier ED. Acute valvular regurgitation. Circulation. 2009;119:3232–3241. doi: 10.1161/CIRCULATIONAHA.108.782292. [DOI] [PubMed] [Google Scholar]
- 30.Hagendorff A, Knebel F, Helfen A, Stöbe S, Haghi D, Ruf T, et al. Echocardiographic assessment of mitral regurgitation: discussion of practical and methodologic aspects of severity quantification to improve diagnostic conclusiveness. Clin Res Cardiol. 2021;110:1704–1733. doi: 10.1007/s00392-021-01841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the european association of cardiovascular imaging. European Hear J - Cardiovasc Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 32.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiog. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Orszulak TA, Schaff HV, Danielson GK, Piehler JM, Pluth JR, Frye RL, et al. Mitral regurgitation due to ruptured chordae tendineae Early and late results of valve repair. J Thorac Cardiovasc Surg. 1985;89:491–498. doi: 10.1016/S0022-5223(19)38752-5. [DOI] [PubMed] [Google Scholar]
- 34.Gabbay U, Yosefy C. The underlying causes of chordae tendinae rupture: a systematic review. Int J Cardiol. 2010;143:113–118. doi: 10.1016/j.ijcard.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Yang M-J, Kang S-J, Yoon M-H, Hwang Y-H, Lim H-S, Choi B-J, et al. Acute mitral regurgitation due to spontaneous chordal rupture in a patient with obstructive hypertrophic cardiomyopathy. Korean Circ J. 2009;39:292–294. doi: 10.4070/kcj.2009.39.7.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampert J, Halista M, Pujadas E, Alexander S, Bier B, Hadley M, et al. Cardiogenic shock and mitral valve chord rupture a rare presentation of libman-sacks endocarditis. Jacc Case Reports. 2020;2:1988–1991. doi: 10.1016/j.jaccas.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura Y, Komatsu J, Sugane H, Hosoda H, Imai R, Nakaoka Y, et al. Unilateral pulmonary edema in patients with acute mitral regurgitation caused by chordal rupture. Circulation Reports. 2022;4:571–578. doi: 10.1253/circrep.CR-22-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grayburn PA, Thomas JD. Basic principles of the echocardiographic evaluation of mitral regurgitation. Jacc Cardiovasc Imaging. 2021;14:843–853. doi: 10.1016/j.jcmg.2020.06.049. [DOI] [PubMed] [Google Scholar]
- 39.Shah PM, Raney AA. New echocardiography-based classification of mitral valve pathology: relevance to surgical valve repair. J Hear valve Dis. 2012;21:37–40. [PubMed] [Google Scholar]
- 40.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes K, Gibbison B, Vohra HA. Mitral valve and mitral valve disease. Bja Educ. 2017;17:1–9. doi: 10.1093/bjaed/mkw032. [DOI] [Google Scholar]
- 42.Richter EW, Shehata IM, Elsayed-Awad HM, Klopman MA, Bhandary SP. Mitral regurgitation in patients undergoing noncardiac surgery. Seminars Cardiothorac Vasc Anesthesia. 2022;26:54–67. doi: 10.1177/10892532211042827. [DOI] [PubMed] [Google Scholar]
- 43.Kettner J, Sramko M, Holek M, Pirk J, Kautzner J. Utility of intra-aortic balloon pump support for ventricular septal rupture and acute mitral regurgitation complicating acute myocardial infarction. Am J Cardiol. 2013;112:1709–1713. doi: 10.1016/j.amjcard.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 44.Vandenbriele C, Balthazar T, Wilson J, Adriaenssens T, Davies S, Droogne W, et al. Left Impella®-device as bridge from cardiogenic shock with acute, severe mitral regurgitation to MitraClip®-procedure: a new option for critically ill patients. European Hear J Acute Cardiovasc Care. 2020;10:zuaa031. [DOI] [PubMed]
- 45.Imaoka S, Kainuma S, Toda K, Miyagawa S, Yoshioka D, Kawamura T, et al. Impella Support as a Bridge to Surgery for Severe Mitral Regurgitation With Cardiogenic Shock. Circulation Reports. 2021;3:178–181. doi: 10.1253/circrep.CR-21-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slama M, Tribouilloy C, Maizel J. Left ventricular outflow tract obstruction in ICU patients. Curr Opin Crit Care. 2016;22:260–266. doi: 10.1097/MCC.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 47.Ibrahim M, Rao C, Ashrafian H, Chaudhry U, Darzi A, Athanasiou T. Modern management of systolic anterior motion of the mitral valve. Eur J Cardio-thorac. 2012;41:1260–1270. doi: 10.1093/ejcts/ezr232. [DOI] [PubMed] [Google Scholar]
- 48.Raut M, Maheshwari A, Swain B. Awareness of ‘systolic anterior motion’ in different conditions. Clin Medicine Insights Cardiol. 2018;12:1179546817751921. doi: 10.1177/1179546817751921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chauvet J-L, El-Dash S, Delastre O, Bouffandeau B, Jusserand D, Michot J-B, et al. Early dynamic left intraventricular obstruction is associated with hypovolemia and high mortality in septic shock patients. Crit Care. 2015;19:262. doi: 10.1186/s13054-015-0980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozaki K, Okubo T, Hagiya K, Kubota N, Tsuchida K, Takahashi K, et al. Unstable angina complicated with dynamic left ventricular outflow tract obstruction. J Cardiol Cases. 2021;23:181–188. doi: 10.1016/j.jccase.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balik M, Novotny A, Suk D, Matousek V, Maly M, Brozek T, et al. vasopressin in patients with septic shock and dynamic left ventricular outflow tract obstruction. Cardiovasc Drug Ther. 2020;34:685–688. doi: 10.1007/s10557-020-06998-8. [DOI] [PubMed] [Google Scholar]
- 52.Evans JS, Huang SJ, McLean AS, Nalos M. Left ventricular outflow tract obstruction—be prepared! Anaesth Intens Care. 2016;45:12–20. doi: 10.1177/0310057X1704500103. [DOI] [PubMed] [Google Scholar]
- 53.Kim S, Kim SJ, Kim J, Yoon P, Park J, Moon J. Dynamic obstruction induced by systolic anterior motion of the mitral valve in a volume-depleted left ventricle: an unexpected cause of acute heart failure in a patient with chronic obstructive pulmonary disease. J Thorac Dis. 2015;7:E365–E369. doi: 10.3978/j.issn.2072-1439.2015.09.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin JH, Kim SH, Park J, Lim Y-H, Park H-C, Choi SI, et al. Unilateral pulmonary edema: a rare initial presentation of cardiogenic shock due to acute myocardial infarction. J Korean Med Sci. 2011;27:211–214. doi: 10.3346/jkms.2012.27.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piérard LA, Lancellotti P. The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. New Engl J Medicine. 2004;351:1627–1634. doi: 10.1056/NEJMoa040532. [DOI] [PubMed] [Google Scholar]
- 56.Boles J-M, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 57.Cabello B, Thille AW, Roche-Campo F, Brochard L, Gómez FJ, Mancebo J. Physiological comparison of three spontaneous breathing trials in difficult-to-wean patients. Intensive Care Med. 2010;36:1171–1179. doi: 10.1007/s00134-010-1870-0. [DOI] [PubMed] [Google Scholar]
- 58.Perren A, Brochard L. Managing the apparent and hidden difficulties of weaning from mechanical ventilation. Intensive Care Med. 2013;39:1885–1895. doi: 10.1007/s00134-013-3014-9. [DOI] [PubMed] [Google Scholar]
- 59.Sanfilippo F, Scolletta S, Morelli A, Vieillard-Baron A. Practical approach to diastolic dysfunction in light of the new guidelines and clinical applications in the operating room and in the intensive care. Ann Intensive Care. 2018;8:100. doi: 10.1186/s13613-018-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruiz-Bailén M, Cobo-Molinos J, Castillo-Rivera A, Pola-Gallego-de-Guzmán MD, Cárdenas-Cruz A, Martínez-Amat A, et al. Stress echocardiography in patients who experienced mechanical ventilation weaning failure. J Crit Care. 2017;39:66–71. doi: 10.1016/j.jcrc.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Corp A, Thomas C, Adlam M. The cardiovascular effects of positive pressure ventilation. Bja Educ. 2021;21:202–209. doi: 10.1016/j.bjae.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vieillard-Baron A, Matthay M, Teboul JL, Bein T, Schultz M, Magder S, et al. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med. 2016;42:739–749. doi: 10.1007/s00134-016-4326-3. [DOI] [PubMed] [Google Scholar]
- 63.Ferré A, Guillot M, Lichtenstein D, Mezière G, Richard C, Teboul J-L, et al. Lung ultrasound allows the diagnosis of weaning-induced pulmonary oedema. Intensive Care Med. 2019;45:601–608. doi: 10.1007/s00134-019-05573-6. [DOI] [PubMed] [Google Scholar]
- 64.Kuhn BT, Bradley LA, Dempsey TM, Puro AC, Adams JY. Management of mechanical ventilation in decompensated heart failure. J Cardiovasc Dev Dis. 2016;3:33. doi: 10.3390/jcdd3040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis C. Percutaneous mitral valve repair in a ventilator-dependant patient. Anaesthesia. 2012;67:420–423. doi: 10.1111/j.1365-2044.2011.06982.x. [DOI] [PubMed] [Google Scholar]
- 66.Nanjayya VB, Orde S, Hilton A, Yang Y, Costello C, Evans J, et al. Levels of training in critical care echocardiography in adults. Recommendations from the College of Intensive Care Medicine Ultrasound Special Interest Group. Australas J Ultrasound Medicine. 2019;22:73–9. [DOI] [PMC free article] [PubMed]
- 67.Flower L, Dempsey M, White A, Sanfilippo F, Olusanya O, Madhivathanan PR. Training and accreditation pathways in critical care and perioperative echocardiography. J Cardiothor Vasc An. 2021;35:235–247. doi: 10.1053/j.jvca.2020.07.046. [DOI] [PubMed] [Google Scholar]
- 68.Vieillard-Baron A, Millington SJ, Sanfilippo F, Chew M, Diaz-Gomez J, McLean A, et al. A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med. 2019;45:770–788. doi: 10.1007/s00134-019-05604-2. [DOI] [PubMed] [Google Scholar]
- 69.Rajamani A, Knudsen S, Huynh KNBH, Huang S, Wong W-T, Ting I, et al. Basic echocardiography competence program in intensive care units: A multinational survey of intensive care units accredited by the College of Intensive Care Medicine. Anaesth Intens Care. 2020;48:150–154. doi: 10.1177/0310057X20911663. [DOI] [PubMed] [Google Scholar]
- 70.Hindocha R, Garry D, Short N, Ingram TE, Steeds RP, Colebourn CL, et al. A minimum dataset for a Level 1 echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2020;7:G51–G58. doi: 10.1530/ERP-19-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Otto CM, Nishimura RA, Bonow RO, Carabello BA, III, JPE, Gentile F, , et al. ACC/AHA guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2020;2021(143):e72–227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 72.Blauwet LA, Miller FA. Echocardiographic assessment of prosthetic heart valves. Prog Cardiovasc Dis. 2014;57:100–110. doi: 10.1016/j.pcad.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 73.Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of cardiovascular imaging. European Hear J - Cardiovasc Imaging. 2013;14:611–644. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 74.Wiener PC, Friend EJ, Bhargav R, Radhakrishnan K, Kadem L, Pressman GS. Color doppler splay: a clue to the presence of significant mitral regurgitation. J Am Soc Echocardiog. 2020;33:1212–1219.e1. doi: 10.1016/j.echo.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Song J-M, Kim M-J, Kim Y-J, Kang S-H, Kim J-J, Kang D-H, et al. Three-dimensional characteristics of functional mitral regurgitation in patients with severe left ventricular dysfunction: a real-time three-dimensional colour Doppler echocardiography study. Heart. 2008;94:590. doi: 10.1136/hrt.2007.119123. [DOI] [PubMed] [Google Scholar]
- 76.Matsumura Y, Fukuda S, Tran H, Greenberg NL, Agler DA, Wada N, et al. Geometry of the proximal isovelocity surface area in mitral regurgitation by 3-dimensional color doppler echocardiography: difference between functional mitral regurgitation and prolapse regurgitation. Am Heart J. 2008;155:231–238. doi: 10.1016/j.ahj.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Sunderji I, Singh V, Fraser AG. When does the E/e’ index not work? The pitfalls of oversimplifying diastolic function. Echocardiogr. 2020;37:1897–1907. doi: 10.1111/echo.14697. [DOI] [PubMed] [Google Scholar]
- 78.Aksu U, Topcu S, Gulcu O, Kalkan K, Tanboga IH. Diastolic mitral and tricuspid regurgitation in a patient with 2:1 AV block. Int J Cardiol. 2015;195:111–112. doi: 10.1016/j.ijcard.2015.05.091. [DOI] [PubMed] [Google Scholar]
- 79.Boriani G, Fauchier L, Aguinaga L, Beattie JM, Lundqvist CB, Cohen A, et al. European heart rhythm association (EHRA) consensus document on management of arrhythmias and cardiac electronic devices in the critically ill and post-surgery patient, endorsed by heart rhythm society (HRS), Asia pacific heart rhythm society (APHRS), cardiac arrhythmia society of Southern Africa (CASSA), and Latin American heart rhythm society (LAHRS) Ep Europace. 2018;21:7–8. doi: 10.1093/europace/euy110. [DOI] [PubMed] [Google Scholar]
- 80.Orde S, Slama M, Yastrebov K, Mclean A, Huang S, Nyikovics A, et al. Subjective right ventricle assessment by echo qualified intensive care specialists: assessing agreement with objective measures. Crit Care. 2019;23:70. doi: 10.1186/s13054-019-2375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hockstein MA, Haycock K, Wiepking M, Lentz S, Dugar S, Siuba M. transthoracic right heart echocardiography for the intensivist. J Intensive Care Med. 2021;36:1098–1109. doi: 10.1177/08850666211003475. [DOI] [PubMed] [Google Scholar]
- 82.Baba Y, Ochi Y, Hirota T, Arima N, Sugiura K, Kawaguchi J, et al. What we believed to be a right upper pulmonary vein by transthoracic echocardiography is actually a right lower pulmonary vein. Echocardiogr. 2021;38:427–434. doi: 10.1111/echo.14986. [DOI] [PubMed] [Google Scholar]
- 83.Nagueh SF. Non-invasive assessment of left ventricular filling pressure. Eur J Heart Fail. 2018;20:38–48. doi: 10.1002/ejhf.971. [DOI] [PubMed] [Google Scholar]
- 84.Bowcock EM, Mclean A. Bedside assessment of left atrial pressure in critical care: a multifaceted gem. Crit Care. 2022;26:247. doi: 10.1186/s13054-022-04115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Videos to accompany images for Case 1—Infective Endocarditis with severe mitral regurgitation.
Additional file 2. Videos to accompany images for Figure 5—Papillary Muscle Rupture.
Additional file 3. Videos to accompany images for Figure 6—LVOT Obstruction.
Additional file 4. Videos to accompany images for Figure 7—Acute on Chronic MR.
Data Availability Statement
Not applicable.