Abstract
Purpose
To evaluate the effectiveness of cemiplimab, a Programmed-cell-death-1(PD-1) protein inhibitor, for the treatment of cutaneous periocular-locally-advanced squamous-cell-carcinoma (POLA-SCC) with orbital-invasion.
Methods
Multicentre real-world retrospective study. Demographic and clinical data were collected and analysed for patients with biopsy-proven POLA-SCC(AJCC-T4) with orbital-invasion who were treated with cemiplimab at one of four tertiary medical centres in 2019–2022.
Results
The cohort included 13 patients, 8 males and 5 females, of median age 76 years (IQR65–86). The median duration of treatment was 5.0months (IQR3.5–10.5) and the median follow-up time, 15.0 months (IQR10.5–30). The overall response rate was 69.2%. Complete response was documented in seven patients (53.8%), partial response in two (15.4%), stable disease in one (7.7%), and progressive disease in two (15.4%); in one patient (7.7%), response was not evaluable. Six complete responders (46.1% of the cohort) received no further treatment and did not have a recurrence during an average follow-up of 6.14 (±6.9) months from treatment cessation. None of the patients underwent orbital-exenteration. The majority of adverse events were mild (grade-1), except for a moderate increase in creatinine level (grade-2), severe bullous dermatitis (grade-3), and myocarditis (grade-5) in one patient each. Four patients (30.7%) died during the follow-up period, all of whom had an Eastern-Cooperative-Oncology-Group score of 4 at presentation.
Conclusions
To our knowledge, this is the largest study to date on cemiplimab therapy for cutaneous POLA-SCC with orbital-invasion. Treatment was shown to be effective, with an overall response rate of 69.2%. Cemiplimab holds promise for the treatment of patients with tumours invading the orbit as it may alleviate the need for orbital exenteration.
Subject terms: Prognosis, Eye cancer
Introduction
Cutaneous periocular squamous cell carcinoma (SCC) is the second most common periocular tumour, accounting for 5%–10% of all eyelid cancers [1, 2]. The risk of developing SCC increases with age, fair skin, lifetime accumulation of ultraviolet radiation damage, and an immunosuppression state [2].
SCC can often be cured by local excision with margin control [3]. However, the infiltrating nature of the tumour, its high recurrence rate (6.8–37.9%) [4, 5], and its tendency for perineural invasion (up to 25%) [6, 7] limit the success of surgery as a single curative modality and may lead to a more locally advanced tumour stage that is often not amenable for surgery. Regional lymph node metastasis (1.3–24%) [4, 8–10] and distant metastasis (0.8%-6.2%) [10–12] may require adjuvant radiotherapy, concurrent chemotherapy, or immunotherapy [13–16].
The standard therapy for cutaneous periocular locally advanced SCC (POLA-SCC) is wide surgical excision, often resulting in local morbidity, loss of visual functions or the need for orbital exenteration (OE) [10, 17]. OE is a devastating consequence of treatment, which severely affects patients’ quality of life, functionality, and social interaction [18, 19]. Cutaneous POLA-SCC with orbital invasion is a leading indication for OE, despite its considerably lower (1:10) incidence compared to locally advanced basal cell carcinoma (BCC) [20–22].
Cemiplimab (Lybtayo, Regeneron) is a high-affinity human monoclonal antibody directed against the programmed death 1 (PD-1) protein. In recent years, cemiplimab has served as first-line treatment for metastatic or locally advanced SCC that is not amenable to surgery and/or radiation therapy [23]. It was approved for this indication by the US Federal Drug Administration in September 2018 [24] and soon thereafter by the Israel Ministry of Health [25]. An investigation of the effectiveness of cemiplimab for the treatment of locally advanced cutaneous SCC (all body sites) reported 13% complete response, 31% partial response, 36% stable disease, and 12% progression [26].
The potential to replace OE with cemiplimab immunotherapy would have a dramatic impact on the management of POLA-SCC. However, the evidence supporting this treatment is limited few case series and a single case report [6, 25, 27–29].
The aim of the present real-life study was to investigate the effectiveness, in terms of patient response and organ preservation, of primary treatment with cemiplimab in a relatively large cohort from four tertiary medical centres in Israel diagnosed with cutaneous POLA-SCC with orbital involvement (American Joint Committee on Cancer, AJCC, T4).
Methods
The study was approved by the local institutional review board. A multicentre retrospective design was used. The electronic databases of four tertiary medical centres in Israel (Rabin, Hadassah, Haemek, and Sheba Medical Centers) were searched for adult patients (age ≥18 years) diagnosed with biopsy-proven POLA-SCC with orbital involvement who were treated with cemiplimab between 2019 and 2022. Patients treated with other PD-1 inhibitors were excluded. Treatment was based on the standard protocol reported by Migden et al. [24]. In brief, cemiplimab was administered by a multidisciplinary tumour board that deals with lesions involving the orbit (stage T4, AJCC Cancer Staging Manual, eighth edition) or with local or distant metastasis for purposes of avoiding OE or as salvage in candidates for nonsurgical treatment (because of multiple comorbidities or local tumour extent or systemic spread). All patients received an intravenous injection of 350 mg every 3 weeks (Q3W) over 30 minutes. Reasons for discontinuation of cemiplimab were progressive disease, unacceptable toxicity, patient’s choice, and persistence of a complete clinical response over time, or physician discretion. Adverse events and toxicities were managed in clinical practice by the principal physician.
Data included patients’ demographics, prior treatments, tumour size, nodal involvement, and metastasis (TNM) staging (AJCC, eighth edition), orbital involvement, neural involvement, prior chemotherapy or radiation treatment, treatment duration, additional treatments, and duration of follow-up time. Response to treatment was assessed with the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. The RECIST criteria provides a simple and pragmatic methodology to evaluate the activity and efficacy of new cancer therapeutics in solid tumours, using validated and consistent criteria to assess changes in tumour burden. Adverse events were assessed with the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Level of functioning of each patient in terms of self-care ability, activity of daily living, and physical ability was evaluated with the Eastern Cooperative Oncology Group (ECOG) Performance Status scale. The ECOG performance status score is an attempt to quantify cancer patients’ general well-being and activities of daily life. It is an independent predictor of the response to treatment, overall survival and progression-free survival in oncology patients. An imaging specialist blinded to the clinical data reviewed the patients’ computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)-CT scans. The data were summarized as frequencies and percentages or medians and ranges, as appropriate. Statistical analyses were performed using R Studio (R Project for Statistical Computing), version 4.1.0.
Results
The cohort included 13 patients with orbital invasion of cutaneous POLA-SCC (AJCC T4), 8 males and 5 females, of median age 76 years (IQR 65-86). Their clinical data are summarized in Table 1. Five patients had lymph node involvement, four had metastatic spread, and three had perineural invasion. ECOG 0 or 1 was documented in six patients (46.1%), ECOG 2 in three patients (23%), and ECOG 4 in four patients (30.7%).
Table 1.
Characteristics of patients with locally advanced periocular biopsy-proven SCC with orbital invasion.
| Pt. no. | Age (y)/sex | Other tumours | Primary site of cutaneous SCC | Previous SCC treatment: Systemic/RT | PNI | T/N/M stage | Tx time (d) | Tx response | ECOG score | Other Tx | OE | Died | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65/M | Periocular | No/no | No | 4/1/0 | 422 | CR | 1 | No | No | |||
| 2 | 61/M | BCC | Periocular | Yes/yes | No | 4/0/1 | 460 | CR | 2 | No | No | ||
| 3 | 68M | BCC | Periocular | Yes/yes | Yes | 4/1/0 | 156 | CR | 2 | No | Yes | ||
| 4 | 83/F | BCC | Nose | No/yes | Yes | 4/1/0 | 21 | PR | 4 | No | Yes | Cardiac event | |
| 5 | 94/F | Periocular | No/yes | No | 4/0/0 | 21 | NE | 4 | No | No | Myocarditis | ||
| 6 | 82/M | Stomach adenocar-cinoma | Periocular | Yes/yes | No | 4/0/0 | 246 | SD | 2 | Chemo | No | No | |
| 7 | 64/F | Forearm melanoma | Periocular | Yes/yes | No | 4/1/1 | 105 | PD | 1 | No | No | ||
| 8 | 68/F | Forehead | Yes/yes | Yes | 4/0/0 | 531 | CR | 1 | No | No | |||
| 9 | 82/M | SCC other | Periocular | No/no | No | 4/0/0 | 252 | CR | 1 | Chemo | No | No | |
| 10 | 76/M | Periocular | No/no | No | 4/0/0 | 126 | CR | 1 | No | No | |||
| 11 | 90/M | Periocular | No/no | No | 4/0/1 | 146 | CR | 4 | No | Yes | Sepsis | ||
| 12 | 89/M | Periocular | No/no | No | 4/0/1 | 209 | PD | 4 | No | Yes | |||
| 13 | 65/F | Forehead | No/no | No | 4/1/0 | 135 | PR | 1 | No | No |
BCC basal cell carcinoma, SCC squamous cell carcinoma, PNI perineural invasion, Tx treatment, ECOG Eastern Cooperative Oncology Group, OE ocular exenteration, RT radiotherapy, Chemo chemotherapy.
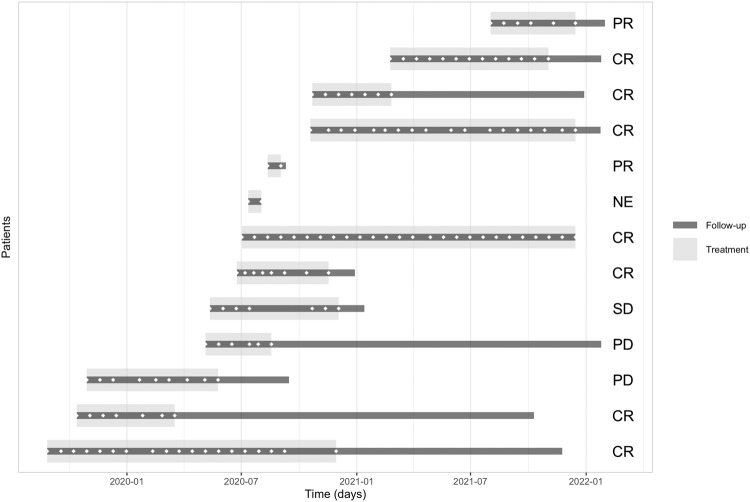
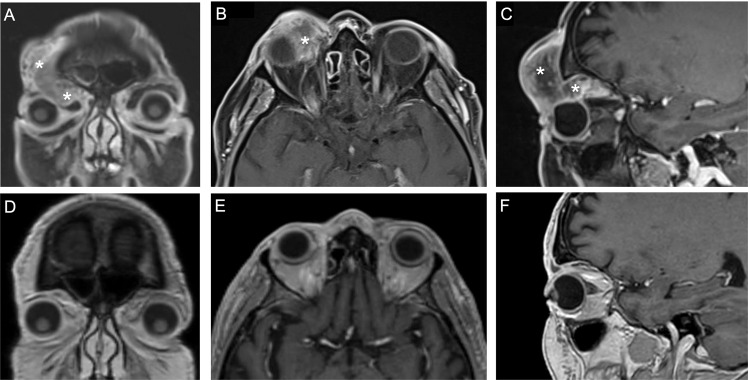
All patients were treated with cemiplimab for a median duration of 5.0 months (IQR 3.5–10.5) and followed for a median time of 15.0 months (IQR 10.5–30). The treatment timeline is presented in Fig. 1. Nine patients (69.2%) responded to treatment (Supplementary Table 1) of whom seven (53.8%) had a complete clinical response and two, a partial response. Six of the seven patients with a complete response (46.1% of the cohort) received no further treatment and did not have a recurrence during an average follow-up of 6.14 (±6.9) months from treatment cessation. Median treatment duration in the complete responders was 8 months (IQR 4–15). Two patients (15.4%) had progressive disease: one was switched from cemiplimab to chemotherapy with a subsequent complete response, and the other died of unknown cause. One patient (7.7%) with stable disease received chemotherapy as well. In one patient, response to treatment was not evaluable. None of the patients underwent OE. A representative photo of a lesion before and after treatment is shown in Supplementary Fig. 1, and the imaging scans of two patients before and after treatment are shown in Fig. 2 and Supplementary Fig. 2.
Fig. 1. Treatment timeline and follow-up of each of the 13 patients with locally advanced squamous cell carcinoma treated with cemiplimab.
Patient’s response to treatment is stated on the right (CR complete response, PR partial response, SD stable disease, PD progressive disease, NE not evaluable). ◇ represents cemiplimab treatments given during follow-up.
Fig. 2. MRI scans of an 82-year-old man with squamous cell carcinoma with orbital involvement.
A Before treatment; post-contrast coronal T1 fat-saturated image showing an ill-defined low enhancing mass penetrating the right orbit with globe compression. B Before treatment; post-contrast axial T1 fat-saturated image showing an ill-defined low enhancing mass penetrating the right orbit. C Before treatment; post-contrast sagittal T1 fat-saturated image showing an ill-defined low enhancing mass extending from the right forehead to the left orbit, advancing along the orbital and penetrating the right orbit. D After treatment; post-contrast coronal T1 image showing a complete response. E After treatment; post-contrast axial T1 image showing a complete response. F After treatment; post-contrast sagittal T1 image, showing a complete response. *indicates the tumor before treatment.
In one patient with a very large tumour who had a complete response to cemiplimab, despite orbital preservation, regression of the tumour left the globes without eyelid protection which resulted in corneal melting due to exposure.
Eight patients (61.5%) had a total of 20 adverse events. Most were mild, with the exception, in one patient each, of a moderate increase in creatinine level (grade 2), severe bullous dermatitis (grade 3), and myocarditis (grade 5).
During the follow-up period, four patients (30.7%) died. One was the patient with myocarditis which was considered a treatment-related death, and another patient died of sepsis unrelated to the cemiplimab treatment. Two patients died at home, and the cause of death was unknown because the families refused autopsy. The ECOG score of all the deceased patients was 4.
Discussion
This multicentre retrospective real-life study is the largest to date of primary cemiplimab treatment for cutaneous POLA-SCC with orbital involvement. The results show that cemiplimab seems to be effective, and all our patients were spared orbital exenteration.
The overall response rate in our cohort was 69.2%, and the complete response rate was 53.8%. These results are better than in earlier controlled trials of cemiplimab treatment in all body sites, wherein the overall and complete response rates were 44% and 13%, respectively [26], and similar to some initial real-world series that reported an overall response rate of 77% [30]. Our good results might be explained by the better response to immuno-checkpoint inhibitors of tumours arising in the head and neck area relative to other body sites [6, 28, 31–34], possibly attributable to their sun-exposure-induced high mutational burden [32, 33].
OE along with adjuvant radiotherapy is considered the standard treatment for POLA-SCC with orbital involvement. SCC along with BCC are leading reasons for oncologic OE [20]. For the last decade Locally Advanced BCC can be successfully treated with Hedgehog pathway inhibitors, yet a prevalence decline in OE has today been demonstrated in only one study [20, 35, 36]. Multiple studies have shown that patients experience a significant reduction in quality of life following exenteration [18, 19], and an improved one if treated medically [37]. Hence, an alternative treatment that preserves the orbit should be the ultimate goal in any patient with orbital involvement. All of our patients were spared OE with cemplimab treatment, either as a monotherapy (11 patients) or in conjunction with chemotherapy (2 patients). Of our two patients with progressive disease, one died early in the course of treatment and the other was switched to chemotherapy which proved successful.
Because of the retrospective design of the study, we were unable to extract sufficient ophthalmic data to establish a correlation of orbital preservation with visual function preservation. Nevertheless, in the VISORB study in which patients with locally advanced BCC, including 56% with orbital involvement, were treated with vismodegib as an alternative to OE [38], all subjects maintained visual functions. Further prospective studies are needed to determine if similar findings may be achieved with the more aggressive SCC.
Data on cemiplimab treatment for POLA-SCC with or without orbital invasion is limited to small series [6, 27, 28] and one case report [25]. The first case series included four patients, three of whom responded to treatment with cemiplimab [27]. The second series included six patients with POLA-SCC without orbital invasion (AJCC T2-3) of whom five received cemiplimab as neoadjuvant treatment followed by surgery and only one, as primary treatment [6]. In all six cases, tumour control was achieved. The third series presented seven patients with POLA-SCC with orbital invasion (AJCC T4) who received immunotherapy after declining OE [28]. All six treated with cemiplimab responded, and five of them avoided OE. The patient who ultimately underwent OE showed rapid clinical progression, although a pseudo-progression (mainly an inflammatory reaction) was subsequently suggested histologically. A recent work exhibited a good tumour control in most patients, adding to the importance of cemiplimab treatment in POLA-SCC patients [29]. Thus, the data from our study strongly confirm observations from previous smaller retrospective case series. We believe that the information from our study supported by previous case series emphasizes the important role of cemiplimab in preserving the orbit and may lead to a major paradigm shift in clinical practice for patients with cutaneous POLA-SCC invading the orbit.
Immune checkpoint inhibitors are generally considered safe, with low incidence of fatal events [39]. In the present study, four patients (30.7%) died during the follow-up period, as shown in Supplementary Fig. 3. One death (7.7%) was due to an adverse event related to treatment, which is comparable to the rates of 1.3–5.6% reported in other studies [24, 40, 41]. The high total death rate in our study, although troubling, is similar to the 32.0–43.3% found in other real-life studies with a long follow-up period [30, 40, 41] It might be at least partly explained by the very advanced disease of our patients to begin with, as cemiplimab treatment is authorized for use by the FDA and the Israel Ministry of Health only for stage T4 POLA-SCC [25]. However, the main reason for the high mortality rate is most probably related to the nature of real-life patients with SCC who are often older and frail, with a poor performance status, multiple comorbidities, and iatrogenic immunosuppressive conditions. Indeed, every patient in our study who died had an ECOG score of 4. Although alternatives to cemiplimab, such as platinum-based chemotherapies, are heavily toxic themselves, given the fragile nature of the population in need, caution is warranted and careful patient and tumour selection is required in every case.
Our study was limited mainly by the retrospective design and small number of patients. In addition, our study database does not include full ocular examinations and the primary follow-up staff did not include an ophthalmologist, nor did the follow-up focus on preservation of vision. Furthermore, efficacy was based on RECIST classification and not on post-treatment biopsies. Moreover, although not short compared to other studies, our median time of follow-up was limited, and as a result the response rates might have been different if it was longer. However, we presume that the results would not alter much as complete responses usually persist over time when using immunotherapies [42]. Finally, the patients for this study were attending four different medical centres. Despite efforts to adhere to a uniform standard protocol [24], variations in treatment and follow-up between different teams may have occurred. This, however, might also be considered a strength of the study, as it better reflects a real-life setting wherein patient demographics are less homogeneous, which may make the results more relevant to different populations.
There is no consensus to date regarding the best course of action to control the disease and preserve vision in these complicated cases. Several biological treatment have also been reported (other PD-1 inhibitors and anti-EGFR). The role of cemiplimab and its integration with other available treatments such as radiation or other biological drugs has yet to be determined. Both may have a synergistic or an abscopal effect. It is possible that the ongoing prospective multicentre trials examining different treatment protocols and approaches for utilizing cemiplimab for advanced SCC will answer some of these questions. In the meanwhile, we strongly recommend first attempting cemiplimab treatment in patients with lesions that could lead to OE, as the response is usually rapid and not much time would be wasted were OE or chemotherapy eventually needed. In addition, cemiplimab can be of great benefit in patients who are unable to withstand surgical intervention.
In conclusion, in this real-world multicentre setting study, treatment with cemiplimab for cutaneous POLA-SCC seems to be effective and holds great hope for patients with tumours invading the orbit, as it may alleviate the need for orbital exenteration.
Supplementary information is available at nature.com/eye
Summary
What was known before
The standard therapy for periocular locally advanced SCC (POLA-SCC) is wide surgical excision, often resulting in local morbidity, loss of visual functions or the need for orbital exenteration. In recent years, cemiplimab has served as first-line treatment for metastatic or locally advanced SCC that is not amenable to surgery and/or radiation therapy. However, the evidence supporting this treatment for periocular locally advanced SCC is limited.
What this study adds
Cemiplimab treatment was shown to be effective, with an overall response rate of 69.2%. Cemiplimab holds promise for the treatment of patients with tumours invading the orbit as it may alleviate the need for orbital exenteration.
Supplementary information
Author contributions
AG and AT acquired the data and drafted the manuscript. AT and MB-I analysed the data and aided in interpreting the results. YC, GM, NK, AP and GBS aided in data acquisition. GBS and IY designed the current study and revised the manuscript. All authors read and approved the final manuscript.
Data availability
The data that support the findings of this study are not openly available due to the hospital’s patient privacy policy. The patient shown in Supplementary Fig. 1 has provided written consent for their image to be used in published media.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Assaf Gershoni, Iftach Yassur.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-02358-y.
References
- 1.Reifler DM, Hornblass A. Squamous cell carcinoma of the eyelid. Surv Ophthalmol. 1986;30:349–65. doi: 10.1016/0039-6257(86)90089-5. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson MJ, Sullivan TJ, Whitehead KJ, Williamson RM. Squamous cell carcinoma of the eyelids. Br J Ophthalmol. 2002;86:1161–5. doi: 10.1136/bjo.86.10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemet AY, Deckel Y, Martin PA, Kourt G, Chilov M, Sharma V, et al. Management of periocular basal and squamous cell carcinoma: a series of 485 cases. Am J Ophthalmol. 2006;142:293–7. doi: 10.1016/j.ajo.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Nasser QJ, Roth KG, Warneke CL, Yin VT, El Sawy T, Esmaeli B. Impact of AJCC ‘T’ designation on risk of regional lymph node metastasis in patients with squamous carcinoma of the eyelid. Br J Ophthalmol. 2014;98:498–501. doi: 10.1136/bjophthalmol-2013-304434. [DOI] [PubMed] [Google Scholar]
- 5.Sun MT, Andrew NH, O’Donnell B, McNab A, Huilgol SC, Selva D. Periocular squamous cell carcinoma: TNM staging and recurrence. Ophthalmology. 2015;122:1512–6. doi: 10.1016/j.ophtha.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb JA, Ferrarotto R, Gross N, Goepfert R, Debnam JM, Gunn B, et al. Immune checkpoint inhibitors for treatment of periorbital squamous cell carcinoma. Br J Ophthalmol. 2021; 10.1136/bjophthalmol-2021-319417. [DOI] [PubMed]
- 7.Malhotra R, Huilgol SC, Huynh NT, Selva D. The Australian Mohs database: periocular squamous cell carcinoma. Ophthalmology. 2004;111:617–23. doi: 10.1016/j.ophtha.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Faustina M, Diba R, Ahmadi MA, Esmaeli B. Patterns of regional and distant metastasis in patients with eyelid and periocular squamous cell carcinoma. Ophthalmology. 2004;111:1930–2. doi: 10.1016/j.ophtha.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Ridenhour CE, Spratt JS., Jr Epidermoid carcinoma of the skin involving the parotid gland. Am J Surg. 1966;112:504–7. doi: 10.1016/0002-9610(66)90312-6. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan TJ. Squamous cell carcinoma of eyelid, periocular, and periorbital skin. Int Ophthalmol Clin. 2009;49:17–24. doi: 10.1097/IIO.0b013e3181b7ecd1. [DOI] [PubMed] [Google Scholar]
- 11.McCord CD, Jr, Cavanagh HD. Microscopic features and biologic behavior of eyelid tumors. Ophthalmic Surg. 1980;11:671–81. [PubMed] [Google Scholar]
- 12.Rowe DE, Carroll RJ, Day CL., Jr Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976–90. doi: 10.1016/0190-9622(92)70144-5. [DOI] [PubMed] [Google Scholar]
- 13.Yin VT, Pfeiffer ML, Esmaeli B. Targeted therapy for orbital and periocular basal cell carcinoma and squamous cell carcinoma. Ophthalmic Plast Reconstr Surg. 2013;29:87–92. doi: 10.1097/IOP.0b013e3182831bf3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu S, Sagiv O, Rubin ML, Sa HS, Tetzlaff MT, Nagarajan P, et al. Validation study of the AJCC cancer staging manual, eighth edition, staging system for eyelid and periocular squamous cell carcinoma. JAMA Ophthalmol. 2019;137:537–42. doi: 10.1001/jamaophthalmol.2019.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazarevic D, Ramelyte E, Dummer R, Imhof L. Radiotherapy in periocular cutaneous malignancies: a retrospective study. Dermatology. 2019;235:234–9. doi: 10.1159/000496539. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick PJ, Thompson GA, Easterbrook WM, Gallie BL, Payne DG. Basal and squamous cell carcinoma of the eyelids and their treatment by radiotherapy. Int J Radiat Oncol Biol Phys. 1984;10:449–54. doi: 10.1016/0360-3016(84)90023-3. [DOI] [PubMed] [Google Scholar]
- 17.Gerring RC, Ott CT, Curry JM, Sargi ZB, Wester ST. Orbital exenteration for advanced periorbital non-melanoma skin cancer: prognostic factors and survival. Eye. 2017;31:379–88. doi: 10.1038/eye.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen ML, Ekholm O, Prause JU, Toft PB. Quality of life of eye amputated patients. Acta Ophthalmol. 2012;90:435–40. doi: 10.1111/j.1755-3768.2010.02092.x. [DOI] [PubMed] [Google Scholar]
- 19.Ye J, Lou L, Jin K, Xu Y, Ye X, Moss T, et al. Vision-related quality of life and appearance concerns are associated with anxiety and depression after eye enucleation: a cross-sectional study. PLoS One. 2015;10:e0136460. doi: 10.1371/journal.pone.0136460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baum SH, Pfortner R, Manthey A, Bechrakis NE, Mohr C. Periorbital, conjunctival and primary intraorbital carcinomas: Survival and risk factors after orbital exenteration. Eye. 2021;35:1365–76. doi: 10.1038/s41433-020-1055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martel A, Baillif S, Nahon-Esteve S, Gastaud L, Bertolotto C, Lassalle S, et al. Orbital exenteration: an updated review with perspectives. Surv Ophthalmol. 2021;66:856–76. doi: 10.1016/j.survophthal.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Kiratli H, Koc I. Orbital exenteration: Institutional review of evolving trends in indications and rehabilitation techniques. Orbit. 2018;37:179–86. doi: 10.1080/01676830.2017.1383466. [DOI] [PubMed] [Google Scholar]
- 23.Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841–6. doi: 10.1007/s40265-018-1012-5. [DOI] [PubMed] [Google Scholar]
- 24.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl J Med. 2018;379:341–51. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 25.Schaffer M, Simonovich A, I O, Batash R, Asna N. Use of cemiplimab in locally advanced cutaneous squamous cell carcinoma. J Clin Case Rep. 2020;10:1320.
- 26.Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21:294–305. doi: 10.1016/S1470-2045(19)30728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nageli M, Mangana J, Chaloupka K, Dummer R. Cutaneous SCC with orbital invasion: case series. J Eur Acad Dermatol Venereol. 2022;36:59–62. doi: 10.1111/jdv.17529. [DOI] [PubMed] [Google Scholar]
- 28.McLean LS, Lim AM, Webb A, Cavanagh K, Thai A, Magarey M, et al. Immunotherapy to avoid orbital exenteration in patients with cutaneous squamous cell carcinoma. Front Oncol. 2021;11:796197. doi: 10.3389/fonc.2021.796197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steren B, Burtness B, Bhatia A, Demirci H, Shinder R, Yoo D, et al. Cemiplimab for orbital squamous cell carcinoma in 11 cases. Ophthalmic Plast Reconstr Surg. 2022;38:496–502. [DOI] [PubMed]
- 30.Strippoli S, Fanizzi A, Quaresmini D, Nardone A, Armenio A, Figliuolo F, et al. Cemiplimab in an elderly frail population of patients with locally advanced or metastatic cutaneous squamous cell carcinoma: a single-center real-life experience from Italy. Front Oncol. 2021;11:686308. doi: 10.3389/fonc.2021.686308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salzmann M, Leiter U, Loquai C, Zimmer L, Ugurel S, Gutzmer R, et al. Programmed cell death protein 1 inhibitors in advanced cutaneous squamous cell carcinoma: real-world data of a retrospective, multicenter study. Eur J Cancer. 2020;138:125–32. doi: 10.1016/j.ejca.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Hanna GJ, Ruiz ES, LeBoeuf NR, Thakuria M, Schmults CD, Decaprio JA, et al. Real-world outcomes treating patients with advanced cutaneous squamous cell carcinoma with immune checkpoint inhibitors (CPI) Br J Cancer. 2020;123:1535–42. doi: 10.1038/s41416-020-01044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.In GK, Vaidya P, Filkins A, Hermel DJ, King KG, Ragab O, et al. PD-1 inhibition therapy for advanced cutaneous squamous cell carcinoma: a retrospective analysis from the University of Southern California. J Cancer Res Clin Oncol. 2021;147:1803–11. doi: 10.1007/s00432-020-03458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hober C, Fredeau L, Pham-Ledard A, Boubaya M, Herms F, Celerier P, et al. Cemiplimab for locally advanced and metastatic cutaneous squamous-cell carcinomas: real-life experience from the French CAREPI Study Group. Cancers 2021;13:3547. [DOI] [PMC free article] [PubMed]
- 35.Ben Ishai M, Tiosano A, Fenig E, Ben Simon G, Yassur I. Outcomes of vismodegib for periocular locally advanced basal cell carcinoma from an open-label trial. JAMA Ophthalmol. 2020;138:749–55. doi: 10.1001/jamaophthalmol.2020.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eiger-Moscovich M, Reich E, Tauber G, Berliner O, Priel A, Ben Simon G, et al. Efficacy of vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol. 2019;207:62–70. doi: 10.1016/j.ajo.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Gershoni A, Tiosano A, Ben Ishai M, Barayev E, G JBS, Yassur I. Vismodegib improves quality of life in patients with periocular locally advanced basal cell carcinoma: subgroup analysis, STEVIE trial. Eye. 2022;36:407–13. doi: 10.1038/s41433-021-01493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahana A, Unsworth SP, Andrews CA, Chan MP, Bresler SC, Bichakjian CK, et al. Vismodegib for preservation of visual function in patients with advanced periocular basal cell carcinoma: The VISORB Trial. Oncologist. 2021;26:e1240–e1249. doi: 10.1002/onco.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guillaume T, Puzenat E, Popescu D, Aubin F, Nardin C. Cemiplimab-rwlc in advanced cutaneous squamous cell carcinoma: real-world experience in a French dermatology department. Br J Dermatol. 2021;185:1056–8. doi: 10.1111/bjd.20569. [DOI] [PubMed] [Google Scholar]
- 41.Valentin J, Gerard E, Ferte T, Prey S, Dousset L, Dutriaux C, et al. Real-world safety outcomes using cemiplimab for cutaneous squamous cell carcinoma. J Geriatr Oncol. 2021;12:1110–3. doi: 10.1016/j.jgo.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 42.Rischin D, Khushalani NI, Schmults CD, Guminski A, Chang ALS, Lewis KD, et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow-up of outcomes and quality of life analysis. J Immunother Cancer. 2021;9:e002757. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to the hospital’s patient privacy policy. The patient shown in Supplementary Fig. 1 has provided written consent for their image to be used in published media.