Take Home Message
Preoperative prediction of the difficulty of partial nephrectomy is influenced by the surgical approach used. The RPN (Radius, Position of tumour, iNvasion of renal sinus) classification is a novel scoring system for specifically assessing the difficulty of robotic partial nephrectomy and was developed using input from high-volume robotic surgeons.
Keywords: Classification, Nephrometry scores, Partial nephrectomy, Preoperative difficulty assessment, Renal cell carcinoma, Robotic partial nephrectomy
Abstract
Background
The surgical difficulty of partial nephrectomy (PN) varies depending on the operative approach. Existing nephrometry classifications for assessment of surgical difficulty are not specific to the robotic approach.
Objective
To develop an international robotic-specific classification of renal masses for preoperative assessment of surgical difficulty of robotic PN.
Design, setting, and participants
The RPN classification (Radius, Position of tumour, iNvasion of renal sinus) considers three parameters: tumour size, tumour position, and invasion of the renal sinus. In an international survey, 45 experienced robotic surgeons independently reviewed de-identified computed tomography images of 144 patients with renal tumours to assess surgical difficulty of robot-assisted PN using a 10-point Likert scale. A separate data set of 248 patients was used for external validation.
Outcome measurements and statistical analysis
Multiple linear regression was conducted and a risk score was developed after rounding the regression coefficients. The RPN classification was correlated with the surgical difficulty score derived from the international survey. External validation was performed using a retrospective cohort of 248 patients. RPN classification was also compared with the RENAL (Radius; Exophytic/endophytic; Nearness; Anterior/posterior; Location), PADUA (Preoperative Aspects and Dimensions Used for Anatomic), and SPARE (Simplified PADUA REnal) scoring systems.
Results and limitation
The median tumour size was 38 mm (interquartile range 27–49). The majority (81%) of renal tumours were peripheral, followed by hilar (12%) and central (7.6%) locations. Noninvasive and semi-invasive tumours accounted for 37% each, and 26% of the tumours were invasive. The mean surgical difficulty score was 5.2 (standard deviation 1.9). Linear regression analysis indicated that the RPN classification correlated very well with the surgical difficulty score (R2 = 0.80). The R2 values for the other scoring systems were: 0.66 for RENAL, 0.75 for PADUA, and 0.70 for SPARE. In an external validation cohort, the performance of all four classification systems in predicting perioperative outcomes was similar, with low R2 values.
Conclusions
The proposed RPN classification is the first nephrometry system to assess the surgical difficulty of renal masses for which robot-assisted PN is planned, and is a useful tool to assist in surgical planning, training and data reporting.
Patient summary
We describe a simple classification system to help urologists in preoperative assessment of the difficulty of robotic surgery for partial kidney removal for kidney tumours.
1. Introduction
The American Urological Association (AUA) and European Association of Urology (EAU) recommend partial nephrectomy (PN) for clinical T1a and T1 renal masses when intervention is indicated [1], [2]. Historically, open PN was the only option available until laparoscopic PN was introduced and popularised at the turn of the 21st century [3]. With the increasing adoption of robotic technology and improvements in techniques to close parenchymal defects [4], robot-assisted PN (RAPN) is now widely performed [5], [6].
Several renal nephrometry systems have been described for assessing PN feasibility on the basis of a tumour complexity score and for reporting perioperative data. [7], [8], [9], [10], [11], [12], [13], [14]. Existing nephrometry systems seem to have a few major limitations. First, they are arguably too complex because of their multiple parameters and/or complex measurements, thereby limiting their use in routine clinical practice. Second, as urologists primarily decide the feasibility of PN from their perception of surgical difficulty after a review of imaging, it is therefore important that any tumour complexity score should correlate with surgeon-perceived surgical difficulty to validate their clinical use. Existing nephrometry systems lack this correlation. Third, current nephrometry systems are not approach-specific. The surgical difficulty of PN and associated complications for any given tumour may vary, depending on the surgical approach. With robotic platforms, more complex cases can be performed with greater ease and with lower complication rates in comparison to a laparoscopic approach [15]. Thus, renal scoring systems should be approach-specific.
As RAPN has now become the preferred approach, there is an unmet need to develop a robotic-specific nephrometry score that is simple and easy to use and that truly reflects the surgeon-perceived surgical difficulty of RAPN. Here we report a three-tiered classification, called RPN (Radius, Position of tumour, iNvasion of renal sinus), developed specifically for RAPN.
As a primary outcome, the aim of this study was to develop a simple, robotic-specific nephrometry classification to assess the preoperative surgical difficulty of RAPN to help in clinical decision-making and to provide consistency in reporting of data for RAPN series. We also aimed to predict the perioperative parameters of warm ischaemia time (WIT), operation time (OT), and estimated blood loss (EBL) for RAPN series as a secondary outcome. We compared the performance of RPN against the commonly used RENAL (Radius; Exophytic/endophytic; Nearness; Anterior/posterior; Location), PADUA (Preoperative Aspects and Dimensions Used for Anatomic), and SPARE (Simplified PADUA REnal) systems.
2. Patients and methods
2.1. Study design
The study protocol received institutional review board approval (reference RMH-QA2021006). There were two steps involved in the development of the RPN model. In the first step, we identified three key parameters and their variables for the new classification following a review of various existing nephrometry systems. In the second step, all three variables for each parameter were assigned a score based on a regression coefficient from a multiple linear regression model using international survey data to help develop the three-tiered RPN classification system. The international survey involved 45 experienced robotic surgeons from 13 countries who reviewed 145 computed tomography (CT) images with renal tumours and marked their perceived surgical difficulty using a 10-point Likert scale.
To test the RPN model, we correlated the RPN tumour complexity score with the surgical difficulty scores from the international survey. For external validation, we used a separate retrospective cohort. All CT scans used in both cohorts of patients were scored independently by one senior urologist and a urology fellow, both experienced in robotic surgery, using the new RPN classification as well as the RENAL, PADUA, and SPARE systems. All of the steps involved in development and validation of the RPN system are described in detail in the following sections.
2.2. Development of the RPN model
We identified three key parameters and devised a new RPN classification system based on tumour radius (maximal diameter), position of the tumour, and invasion of the renal sinus. The individual scores were weighted on the basis of linear regression coefficients generated for each cutoff point, as discussed in the Results section. This classification requires review of contrast CT or magnetic resonance imaging (MRI) in both axial and coronal views according to a renal protocol.
2.2.1. Radius (maximal diameter) of the tumour
The RENAL, PADUA, and SPARE classifications use size categories of ≤4, >4–7, and ≥7 cm, in line with the TNM classification. However, TNM is an oncological rather than a clinical classification that does not necessarily correlate with the degree of surgical difficulty encountered by surgeons. We elected to use size categories of ≤2.5, >2.5–5, and >5 cm on the basis of the AUA and EAU guidelines and the tumour size distribution in large RAPN series. The AUA and EAU recommend PN for tumours of <4 cm and <7 cm, respectively [1], [2]. The mean or median tumour size was <4 cm in several large RAPN series [16], [17], [18], [19]. Thus, the three TNM categories appear to represent a wider spread, so we elected for lower cutoff values for our three categories.
2.2.2. Position of the tumour
The RPN classification categorises tumours as peripheral, hilar, or central. Hilar lesions are defined as renal tumours originating on the medial aspect of the kidney and abutting any of the hilar vessels and/or renal pelvis. Tumours that are not in direct contact with the hilar vessels and/or renal pelvis but arise from the parenchymal lips adjacent to the renal hilum are also considered hilar tumours. Central tumours are those defined as touching or crossing the central sinus point. The central sinus point is an imaginary point in the centre of the renal sinus: in the mid-coronal view it can be located as the centre of an imaginary circle in the renal sinus between two polar lines, and in the axial view as the centre of an imaginary circle in the renal sinus at the level of the renal axial midline (Fig. 1). The polar lines and the renal axial midline have been described previously [7]. The polar line is designated as the plane of the kidney above or below which the medial lip of parenchyma is interrupted by the renal sinus fat, vessels, or collecting system. The renal axial midline is defined as the axial cut on CT/MRI midway between the two polar lines. Tumours that are not hilar or central in location are classified as peripheral tumours.
Fig. 1.
RPN classification system. Table showing the details of the RPN scoring system. (A) Location of the renal hilum and renal sinus. (B) Central sinus point in mid-coronal view. (C) Central sinus point in axial view at the level of the renal axial midline. (D) Peripheral tumours (tumours that are not hilar or central). (E) Hilar tumours originating from the medial aspect of the kidney abutting hilar vessels/or the renal pelvis or arising from hilar lips. (F) Central tumours touching or crossing the central sinus point. (G) Noninvasive: exophytic peripheral tumours with a clear rim of parenchyma between the tumour and the renal sinus. (H) Semi-invasive: peripheral tumours touching the renal sinus or completely endophytic within the renal parenchyma or a hilar tumour not invading the renal sinus. (I) Invasive: tumours extending into the renal sinus. RPN = Radius, Position of tumour, iNvasion of renal sinus.
2.2.3. Invasion of the renal sinus
Tumours may be noninvasive, semi-invasive, or invasive. Noninvasive tumours are partly or predominantly exophytic peripheral tumours and have a clear rim of normal parenchyma between the tumour and the renal sinus. Semi-invasive tumours include three distinct types: (1) peripheral tumours touching the renal sinus; (2) completely endophytic tumours confined within the renal parenchyma; and (3) hilar tumours that are superficial and not invading the renal sinus. Invasive tumours are those that extend into the renal sinus. Thus, peripheral tumours can be noninvasive, semi-invasive, or invasive. Hilar tumours can be semi-invasive or invasive, and all central tumours are exclusively invasive (Fig. 1).
2.3. International surgical difficulty survey
In our survey, we included patients who presented to a tertiary centre with renal masses between 2010 and 2020. Patients with missing scans, poor-quality scans, and more than one tumour in the same kidney were excluded. Of 205 patients, 145 were considered eligible, and we collated their de-identified CT results for a renal protocol with axial and coronal views as video PowerPoint slides (Microsoft Corporation, Redmond, WA, USA).
The scans were then sent to 57 experienced urologists across Europe, North America, Australia, and Asia, who were able to independently scroll through each scan without a set time limit. They were asked to assess the degree of surgical difficulty for RAPN for their preferred approach on a scale from 1 to 10 (1 being easy and 10 being unsuitable for RAPN). All reviewers were provided detailed instructions on how to review the scans (Supplementary Video 1). The urologists were blinded to the scores calculated using the other scoring systems. All four scoring systems were compared with the surgeon’s perception of the surgical difficulty score. Data from 45 experienced surgeons (>50 RAPN procedures) were included for data analysis. Of the 145 CT scans, one was excluded from the analysis because of incorrect marking.
2.4. External validation for prediction of perioperative outcomes
The RPN classification was validated using a separate set of external, retrospectively collected data for 248 patients from the private practice of two experienced robotic surgeons. All patients underwent RAPN between November 2010 and June 2021. In addition to preoperative CT/MRI scans, perioperative and postoperative variables (WIT, OT, EBL, and complications) were collected. All complications were recorded using the Clavien-Dindo classification. Each scan was scored independently using the new RPN classification as well as the RENAL, PADUA and SPARE systems.
2.5. Statistical analysis
Inter-rater agreement for the surgical difficulty score for PN was assessed in terms of the intraclass correlation coefficient (ICC), which was estimated from a one-way analysis-of-variance model.
2.5.1. Model development
A linear regression model with degree of surgical difficulty as the outcome variable and each of the three potential predictors (tumour size, tumour position, and sinus invasion) in separate univariable models was initially used. Multiple linear regression was then conducted by including the three potential predictors. R2 values and the root mean square error (RMSE) were calculated and a risk score was developed by rounding the regression coefficients to the nearest integer. Separate regression models for variables included in the RENAL, PADUA, and SPARE scores were also fitted to the patient data and the performance of these models was compared with that of the RPN score.
2.5.2. Model testing
The RPN model was tested using bootstrapping (1000 replications) [20]. Optimism in performance of the model was estimated as the average of the differences in model performance between the bootstrap sample and the original data set. The optimism was then subtracted from the original performance measure (R2 and RMSE for the original model) to provide bias-corrected R2 and RMSE values for the final model.
2.5.3. External validation
The ability of the RPN classification to predict three surgical parameters (WIT, OT, and EBL) was assessed using linear regression models, and R2 and RMSE values were reported. The ability of the RPN classification to predict postoperative complications and an outcome trifecta was assessed using logistic regression analysis, and the areas under the receiver operator characteristic (ROC) curve were compared; p values for equality of the area under the curve were reported. In addition, the ability of risk scores from the RENAL, PADUA and SPARE systems to predict the above outcome parameters was also determined and compared with the performance of the RPN score.
All statistical analyses were performed using Stata v16 (StataCorp, College Station, TX, USA).
3. Results
3.1. International survey cohort
Scans were sent to 57 urologists, of whom 50 responded to the survey; data from 45 experienced surgeons were used in the analysis. Each scan was reviewed by an average of 43 surgeons (range 39–45). The ICC for the surgical difficulty score was 0.66 (95% confidence interval [CI] 0.60–0.71).
The preoperative characteristics of the renal masses in the 144 patients are shown in Table 1. The median tumour size was 38 mm (interquartile range [IQR] 27–49 mm). The mean surgical difficulty was 5.2 (standard deviation 1.9). The median nephrometry scores were 8.0 (IQR 7.0–9.0) for RENAL, 4.0 (IQR 2.0–6.0) for SPARE, and 9.0 (IQR 8.0–10.0) for PADUA. The estimated linear regression coefficients for tumour size, location, and invasiveness are shown in Supplementary Table 1. These cutoff points were then collapsed into a score of 0–2 (where 0 is the reference) based on the regression coefficient.
Table 1.
Preoperative characteristics of 144 renal masses in the data set for the international survey on surgical difficulty as assessed preoperatively by experienced surgeons
| Variable | Result a |
|---|---|
| Radius (maximal diameter, mm) | 38.2 (27.5–48.8) |
| Radius (maximal diameter), n (%) | |
| ≤2.5 cm | 30 (21) |
| >2.5–5.0 cm | 82 (57) |
| >5.0 cm | 32 (22) |
| Position of tumour, n (%) | |
| Peripheral | 116 (81) |
| Hilar | 17 (12) |
| Central | 11 (7.6) |
| Invasion of renal sinus, n (%) | |
| Noninvasive | 53 (37) |
| Semi-invasive | 53 (37) |
| Invasive | 38 (26) |
| Surgical difficulty score | 5.2 ± 1.9 |
| RENAL score | 8.0 (7.0–9.0) |
| SPARE score | 4.0 (2.0–6.0) |
| PADUA score | 9.0 (8.0–10.0) |
Data are presented as the mean ± standard deviation or median (interquartile range) for continuous variables.
3.2. New RPN classification
The new three-tier RPN classification system is shown in Figure 1 and in Supplementary Video 2. Based on the regression coefficient, each variable was assigned a score of 0–2. The scores range from zero to six, with zero representing the lowest and six the highest degree of complexity for RAPN. A renal mass with size ≤2.5 cm was scored 0 points, >2.5–5 cm, 1 point, and >5 cm, 2 points. Peripheral tumours were scored 0 points, hilar tumours, 1 point, and central tumours, 2 points. Noninvasive tumours were scored 0 points, semi-invasive tumours, 1 point, and invasive tumours, 2 points. Examples of RPN classification are shown in Figure 2. Although treating tumour size as a continuous variable is preferable, we opted for categorisation in line with established scores for simplicity, practicality, and memorability, as supported by our observation that clear categories are more readily used in clinical practice than complex calculations.
Fig. 2.
Examples of RPN classification with computed tomography scans in axial and coronal views. RPN = Radius, Position of tumour, iNvasion of renal sinus.
3.3. Analysis of RPN model testing
Analysis of the multiple linear regression model of perioperative renal mass characteristics in predicting mean surgical difficulty scores showed values of R2 = 0.80 and RMSE = 0.87 for the RPN model. Bootstrapping analysis with 1000 replications showed bias-corrected R2 and RMSE values of 0.80 (95% CI 0.72–0.85) and 0.90 (95% CI 0.80–0.99), respectively, for the RPN model. R2 and RMSE values were 0.66 and 1.2 for RENAL, 0.75 and 0.99 for PADUA, and 0.70 and 1.1 for SPARE, respectively (Supplementary Table 2). These data indicate that the RPN model correlated very well with preoperative prediction of the surgical difficulty of renal masses for which RAPN was planned, despite having only three parameters. Violin plots for correlation of the surgical difficulty score with the RPN and other three classifications are shown in Figure 3.
Fig. 3.
Violin plots comparing the mean surgical difficulty score (determined from patient scans and averaged across scores from multiple surgeon assessors) for the newly developed RPN score and RENAL, PADUA, and SPARE scores. RPN = Radius, Position of tumour, iNvasion of renal sinus; RENAL = Radius, Exophytic/endophytic, Nearness, Anterior/posterior, Location; PADUA = Preoperative Aspects and Dimensions Used for Anatomic; SPARE = Simplified PADUA REnal.
3.4. Analysis of external validation for the RPN model
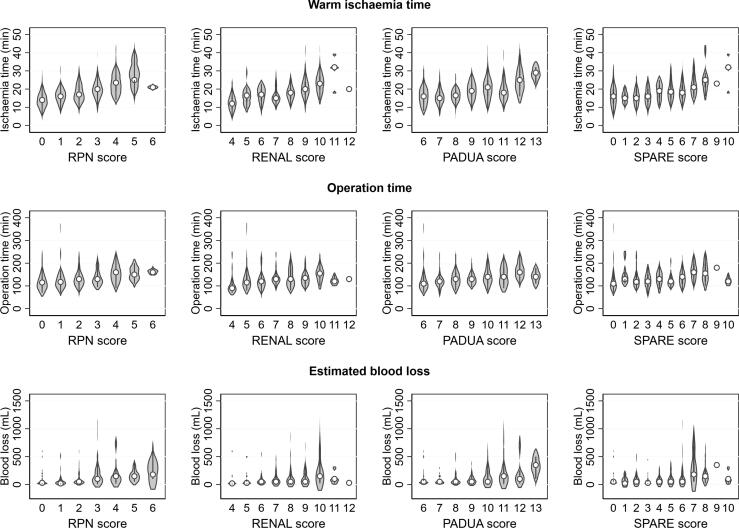
The association of the RPN model with perioperative measures of surgical difficulty was evaluated in an independent cohort of 248 patients. The preoperative characteristics of these patients are shown in Table 2. Comparison of WIT, OT, and EBL between the RPN, RENAL, PADUA and SPARE scoring systems is shown in Figure 4. Linear regression model summaries for the RPN, RENAL, PADUA and SPARE systems in predicting WIT, OT, and EBL are shown in Supplementary Table 3. All four classifications had similar performance in predicting perioperative outcomes, with low R2 values and high RMSE values and no significant correlation.
Table 2.
Preoperative characteristics of the patients and renal masses and perioperative outcomes in the external validation data set (n = 248)
| Variable | Result a |
|---|---|
| Age (yr) | 59.1 ± 13 |
| Body mass index (kg/m2) | 28.8 ± 5.8 |
| Radius (maximal diameter, mm) | 35.9 ± 15 |
| Radius (maximal diameter), n (%) | |
| ≤2.5 cm | 65 (26) |
| >2.5–5.0 cm | 151 (61) |
| >5.0 cm | 32 (13) |
| Position of tumour, n (%) | |
| Peripheral | 204 (82) |
| Hilar | 38 (15) |
| Central | 6 (2.4) |
| Invasion of renal sinus, n (%) | |
| Noninvasive | 75 (30) |
| Semi-invasive | 83 (34) |
| Invasive | 90 (36) |
| RENAL score | 8.0 (6.0–9.0) |
| PADUA score | 9.0 (7.0–10.0) |
| SPARE score | 5.0 (2.0–6.0) |
| Operation time (min) | 130 (105–155) |
| Estimated blood loss (ml) | 50 (20–150) |
| Warm ischaemia time (min) | 19 ± 6.3 |
| Warm ischaemia time <25 min | |
| No | 40 (16) |
| Yes | 206 (83) |
| Data missing | 2 (0.8) |
| Complications, n (%) | |
| No | 229 (92) |
| Yes | 19 (7.7) |
| Positive surgical margin, n (%) | |
| No | 235 (95) |
| Yes | 9 (3.6) |
| Data missing | 4 (1.6) |
| Trifecta outcome, n (%) | |
| No | 60 (24) |
| Yes | 182 (73) |
| Data missing | 6 (2) |
| Surgical approach, n (%) | |
| Transperitoneal | 178 (72) |
| Retroperitoneal | 70 (28) |
| RPN score, n (%) | |
| 0 | 39 (16) |
| 1 | 46 (19) |
| 2 | 63 (25) |
| 3 | 59 (24) |
| 4 | 29 (12) |
| 5 | 9 (4) |
| 6 | 3 (1) |
Data are presented as the mean ± standard deviation or median (interquartile range) for continuous variables.
Fig. 4.
Violin plots comparing warm ischaemia time, operating time, and estimated blood loss for RPN, RENAL, PADUA, and SPARE scores in the external validation data set. RPN = Radius, Position of tumour, iNvasion of renal sinus; RENAL = Radius, Exophytic/endophytic, Nearness, Anterior/posterior, Location; PADUA = Preoperative Aspects and Dimensions Used for Anatomic; SPARE = Simplified PADUA REnal.
Complications occurred in 19 patients. Of these, 12 patients had significant (Clavien-Dindo grade 3–4) complications. All classifications performed similarly and did not show any significant difference (p = 0.47). The area under the ROC curve for significant complications was 0.65 for RPN, 0.61 for RENAL, 0.68 for PADUA, and 0.63 for SPARE (Supplementary Table 4).
4. Discussion
With only three parameters, the new RPN classification is the one of the simplest nephrometry systems to use. In comparison, other systems may have up to six parameters with several complex measurements, which may limit their routine use because of time constraints [8], [9], [12]. The RPN score ranges from 0 to 6. Tumours with a score of 0–1 may be considered as having low complexity, a score of 2–4 may be considered as moderate complexity, and a score of 5–6 may be considered as high complexity for an experienced surgeon.
With advances in robotic technology, PN for hilar and central tumours is becoming feasible [21], [22], [23]. The existing classifications do not recognise central/hilar tumours as a separate entity. By contrast, the RPN system allows distinct scoring for hilar and central tumours. Our definition of hilar tumours is an amended one from earlier definitions [21], [22]. Significant inconsistencies in the definition of central renal tumours exist in the literature [23]. Our definition of central tumours, as described in Section 2.2.2, is simple and objective and can provide consistency for future reference.
Owing to its unique simplicity and robotic-specific validation, the RPN score may have several clinical applications. Our surgical difficulty score correlates well according to highly experienced surgeons, indicating that the system can be used by less experienced surgeons for case selection while ascending the learning curve. This should help in monitoring progress and standardising the learning curve for RAPN. In addition, the RPN system may provide an objective tool for accrediting surgeons for the RAPN program. The RPN score can be routinely used in multidisciplinary meetings to discuss the treatment plan for patients with renal masses and assess their suitability for RAPN. The RPN system should also help in reporting and comparing perioperative data from RAPN series.
Using a large international survey, we objectified the subjective surgeon-perceived surgical difficulty as the RPN score. On external validation, the performance of all four classifications was similar in predicting perioperative outcomes, with low R2 values and high RMSE values with no significant correlation. Patients in this cohort underwent RAPN performed by two experienced surgeons. Major complications occurred in only 4.8%, the mean WIT was only 18.8 min, and the median EBL and OT were 50 ml and 130 min, respectively. With such favourable perioperative outcomes and low complication rates, it is highly unlikely that any classification would provide a meaningful clinical correlation with perioperative outcomes.
There is significant inconsistency in the literature on the role of nephrometry systems in predicting perioperative outcomes. While several studies found that nephrometry systems were helpful in predicting perioperative outcomes [17], [24], [25], [26], [27], others found no such correlation. In a large multicentre study of more than 500 RAPN cases, Ubrig et al [16] found that the PADUA score was unhelpful in predicting perioperative outcomes. Similarly, Mufarrij et al [18] reported that RENAL scores did not correlate with perioperative outcomes in an RAPN series of 92 patients. Yeon et al [19] also found that the RENAL and PADUA nephrometry scoring systems did not predict perioperative outcomes in a series of 113 patients who underwent RAPN. In a collaborative review involving 29 series, RENAL, PADUA and C-index systems were inconsistent in predicting perioperative outcomes [28].
With cutting-edge robotic technology, it appears that experienced robotic surgeons are able to perform PN in high-complexity cases with favourable outcomes. Buffi et al [29] reported RAPN outcomes for complex tumours (PADUA score ≥10) in a multicentre study of 255 patients. The mean OT was 165 min, mean WIT was 19 min, and Clavien-Dindo grade >2 complications were observed only in 5.1% of patients [29]. Ge et al [30] reported clinical data for 22 patients with a renal hilar tumour who underwent RAPN. The mean WIT and OT were 18 ± 4.0 min and 134 ± 44 min, respectively. The mean EBL was 136 ± 131 ml and no patient needed an intraoperative blood transfusion. There was no conversion to open surgery [30]. Data from these RAPN series, consistent with our findings, suggest that tumour complexity may not necessarily correlate with perioperative outcomes, especially for experienced surgeons. However, we hope that future studies will demonstrate correlation of the RPN score with perioperative outcomes in heterogeneous series involving surgeons of varying levels of experience.
Given their inconsistent predictive ability, the sole use of nephrometry systems should not be for prediction of perioperative outcomes. Rather, they should be considered a clinical tool to assign a tumour complexity score for the various clinical applications discussed earlier in this section. Owing to its simple application because it comprises just three parameters, we suggest that RPN is the nephrometry system most suitable and practical for use by urologists.
The findings from our study should be interpreted within the context of its limitations. First, we focused only on tumour factors. Additional factors such as BMI, previous abdominal surgery, perinephric fat thickness, and surgical approach, among many others, may also influence the difficulty of treating an individual patient. However, inclusion of all the factors that influence difficulty would be impossible and would produce a scoring system that is difficult to use with limited applications. Second, there was moderate agreement in the international survey between surgeons (ICC 0.66); however, this represents real-world data, as perfect agreement on surgical difficulty evaluation among surgeons is almost impossible. Third, we used a 10-point Likert scale in our international survey because of its simplicity, but this is not a validated tool. Fourth, we rounded the regression coefficients for simplicity, which we acknowledge may result in less precise prediction of surgical difficulty. However, our main intention with this system is to create a practical tool that can be easily implemented in a busy clinical setting, and scores that require an interactive calculator or have discontinuous numerical scores may not be intuitive for users. Finally, we used retrospective data in the external validation, and further prospective external validation may be required to confirm our findings and to assess the reproducibility and interobserver reliability of the RPN score in the future.
5. Conclusions
The RPN classification is the first nephrometry system developed to reflect the surgical difficulty of renal masses for which RAPN is planned. With three parameters, this new RPN system is intuitive, simple to implement, and applicable to RAPN.
Author contributions: Dinesh K. Agarwal had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: D.K. Agarwal, Corcoran.
Acquisition of data: Mulholland, Dundee, Moon, Giudice, Wang, Furrer, Pan.
Analysis and interpretation of data: D.K. Agarwal, Koye, Corcoran, Yao, Sathianathen, Mulholland, Simpson.
Drafting of the manuscript: D.K. Agarwal, Mulholland, Koye, Yao, Corcoran.
Critical revision of the manuscript for important intellectual content: Corcoran, Koye, Yao, Dundee, Sathianathen, Simpson, Wagner, Harke, A. Agarwal.
Statistical analysis: Koye, Simpson.
Obtaining funding: D.K. Agarwal.
Administrative, technical, or material support: Mulholland.
Supervision: D.K. Agarwal.
Other (survey completion): Dundee, Moon, Kearsley, Norris, Zargar, Mottrie, Fuller, Mottaran, Challacombe, Kua, Metcalfe, Wagner, Dubey, Gomez Sancha, Bruyère, Gautam, Pooleri, Bozzini, Lau, Thyer, Teoh, Vass, Vivian, McDermott, Winter, Ragavan, Campbell, Harke, Richard, Teloken, Dekuyper, Sutherland, Ahlawat, Nair, Pemberton, Catterwell, Oomen, Weston, Moritz, Krishnappa, Leslie, Van Appledorn, Yuvaraja, Meert, Dujardin, Gross, Walton, Huang, Caumartin, Lawrentschuk.
Financial disclosures: Dinesh K. Agarwal certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was supported by Device Technologies (Australian distributor for da Vinci robotic systems), who provided financial support for the costs related to artwork and statistical analysis. The company played no direct role in the study.
Acknowledgments
We would like to acknowledge MISCH (Methods and Implementation Support for Clinical and Health Research Hub), Faculty of Medicine, Dentistry and Health Sciences at University of Melbourne for its support while conducting this research. We would also like to extend special thanks to Dr. Levent Efe for the creation of all illustrations for this article.
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2023.05.007.
Contributor Information
Dinesh K. Agarwal, Email: dineshagarwal@bigpond.com.
International Collaborative Group of Urologists:
Alex Mottrie, Andrew Fuller, Angelo Mottaran, Ben Challacombe, Boon Kua, Charles Metcalfe, Christian Wagner, Deepak Dubey, Fernando Gomez Sancha, Franck Bruyère, Gagan Gautam, Ginil K. Pooleri, Giorgio Bozzini, Howard Lau, Isaac Thyer, Jeremy Teoh, Justin Vass, Justin Vivian, Kara McDermott, Mathew Winter, Narasimhan Ragavan, Nicholas Campbell, Nina N. Harke, Patrick O. Richard, Patrick Teloken, Peter Dekuyper, Peter Sutherland, Rajesh Ahlawat, Rajesh Nair, Richard Pemberton, Rick Catterwell, Robert J.A. Oomen, Robin Weston, Rudolf Moritz, Raghunath S. Krishnappa, Scott Leslie, Scott Van Appledorn, T.B. Yuvaraja, Thibault Meert, Thierry Dujardin, Tobias Gross, Tom Walton, William C. Huang, and Yves Caumartin
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Campbell S.C., Clark P.E., Chang S.S., Karam J.A., Souter L., Uzzo R.G. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA guideline: part I. J Urol. 2021;206:199–208. doi: 10.1097/JU.0000000000001911. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B., Albiges L., Abu-Ghanem Y., et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82:399–410. doi: 10.1016/j.eururo.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Gill I.S., Kavoussi L.R., Lane B.R., et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Silagy A.W., Young R., Kelly B.D., et al. Surgical innovation revisited: a historical narrative of the minimally invasive “Agarwal sliding-clip renorrhaphy” technique for partial nephrectomy and its application to an Australian cohort. BJUI Compass. 2021;2:211–218. doi: 10.1002/bco2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukavina L., Mishra K., Calaway A., Ponsky L. Robotic partial nephrectomy: update on techniques. Urol Clin North Am. 2021;48:81–90. doi: 10.1016/j.ucl.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Choi J.E., You J.H., Kim D.K., Rha K.H., Lee S.H. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol. 2015;67:891–901. doi: 10.1016/j.eururo.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Kutikov A., Uzzo R.G. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Ficarra V., Novara G., Secco S., et al. Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56:786–793. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Simmons M.N., Ching C.B., Samplaski M.K., Park C.H., Gill I.S. Kidney tumor location measurement using the C index method. J Urol. 2010;183:1708–1713. doi: 10.1016/j.juro.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Simmons M.N., Hillyer S.P., Lee B.H., Fergany A.F., Kaouk J., Campbell S.C. Diameter-axial-polar nephrometry: integration and optimization of R.E.N.A.L. and centrality index scoring systems. J Urol. 2012;188:384–390. doi: 10.1016/j.juro.2012.03.123. [DOI] [PubMed] [Google Scholar]
- 11.Hakky T.S., Baumgarten A.S., Allen B., et al. Zonal NePhRO scoring system: a superior renal tumor complexity classification model. Clin Genitourin Cancer. 2014;12:e13–e18. doi: 10.1016/j.clgc.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Leslie S., Gill I.S., de Castro Abreu A.L., et al. Renal tumor contact surface area: a novel parameter for predicting complexity and outcomes of partial nephrectomy. Eur Urol. 2014;66:884–893. doi: 10.1016/j.eururo.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Spaliviero M., Poon B.Y., Karlo C.A., et al. An arterial based complexity (ABC) scoring system to assess the morbidity profile of partial nephrectomy. Eur Urol. 2016;69:72–79. doi: 10.1016/j.eururo.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ficarra V., Porpiglia F., Crestani A., et al. The Simplified PADUA Renal (SPARE) nephrometry system: a novel classification of parenchymal renal tumours suitable for partial nephrectomy. BJU Int. 2019;124:621–628. doi: 10.1111/bju.14772. [DOI] [PubMed] [Google Scholar]
- 15.Leow J.J., Heah N.H., Chang S.L., Chong Y.L., Png K.S. Outcomes of robotic versus laparoscopic partial nephrectomy: an updated meta-analysis of 4,919 patients. J Urol. 2016;196:1371–1377. doi: 10.1016/j.juro.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Ubrig B., Roosen A., Wagner C., et al. Tumor complexity and the impact on MIC and trifecta in robot-assisted partial nephrectomy: a multi-center study of over 500 cases. World J Urol. 2018;36:783–788. doi: 10.1007/s00345-018-2191-0. [DOI] [PubMed] [Google Scholar]
- 17.Diana P., Lughezzani G., Uleri A., et al. Multi-institutional retrospective validation and comparison of the Simplified PADUA Renal nephrometry system for the prediction of surgical success of robot-assisted partial nephrectomy. Eur Urol Focus. 2021;7:1100–1106. doi: 10.1016/j.euf.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Mufarrij P.W., Krane L.S., Rajamahanty S., Hemal A.K. Does nephrometry scoring of renal tumors predict outcomes in patients selected for robot-assisted partial nephrectomy? J Endourol. 2011;25:1649–1653. doi: 10.1089/end.2011.0003. [DOI] [PubMed] [Google Scholar]
- 19.Yeon J.S., Son S.J., Lee Y.J., et al. The nephrometry score: is it effective for predicting perioperative outcome during robot-assisted partial nephrectomy? Korean J Urol. 2014;55:254–259. doi: 10.4111/kju.2014.55.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steyerberg E.W., Harrell F.E., Jr, Borsboom G.J., Eijkemans M.J., Vergouwe Y., Habbema J.D. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 21.Gill I.S., Colombo J.R., Jr, Frank I., Moinzadeh A., Kaouk J., Desai M. Laparoscopic partial nephrectomy for hilar tumors. J Urol. 2005;174:850–854. doi: 10.1097/01.ju.0000169493.05498.c3. [DOI] [PubMed] [Google Scholar]
- 22.Dulabon L.M., Kaouk J.H., Haber G.P., et al. Multi-institutional analysis of robotic partial nephrectomy for hilar versus nonhilar lesions in 446 consecutive cases. Eur Urol. 2011;59:325–330. doi: 10.1016/j.eururo.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Frank I., Colombo J.R., Jr, Rubinstein M., Desai M., Kaouk J., Gill I.S. Laparoscopic partial nephrectomy for centrally located renal tumors. J Urol. 2006;175:849–852. doi: 10.1016/S0022-5347(05)00346-0. [DOI] [PubMed] [Google Scholar]
- 24.Long J.A., Arnoux V., Fiard G., et al. External validation of the RENAL nephrometry score in renal tumours treated by partial nephrectomy. BJU Int. 2013;111:233–239. doi: 10.1111/j.1464-410X.2012.11339.x. [DOI] [PubMed] [Google Scholar]
- 25.Ficarra V., Bhayani S., Porter J., et al. Predictors of warm ischemia time and perioperative complications in a multicenter, international series of robot-assisted partial nephrectomy. Eur Urol. 2012;61:395–402. doi: 10.1016/j.eururo.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Lista G., Buffi N.M., Lughezzani G., et al. Margin, ischemia, and complications system to report perioperative outcomes of robotic partial nephrectomy: a European Multicenter Observational Study (EMOS project) Urology. 2015;85:589–595. doi: 10.1016/j.urology.2014.09.068. [DOI] [PubMed] [Google Scholar]
- 27.Casale P., Lughezzani G., Buffi N., et al. Evolution of robot-assisted partial nephrectomy: techniques and outcomes from the Transatlantic Robotic Nephron-sparing Surgery Study Group. Eur Urol. 2019;76:222–227. doi: 10.1016/j.eururo.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 28.Klatte T., Ficarra V., Gratzke C., et al. A literature review of renal surgical anatomy and surgical strategies for partial nephrectomy. Eur Urol. 2015;68:980–992. doi: 10.1016/j.eururo.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buffi N.M., Saita A., Lughezzani G., et al. Robot-assisted partial nephrectomy for complex (PADUA score ≥10) tumors: techniques and results from a multicenter experience at four high-volume centers. Eur Urol. 2020;77:95–100. doi: 10.1016/j.eururo.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Ge G.J., Ding G.Q., Zhao W.P., et al. Robot-assisted partial nephrectomy for treating renal hilar tumors: a clinical study of 22 cases. Zhonghua Yi Xue Za Zhi. 2018;98:2438–2440. doi: 10.3760/cma.j.issn.0376-2491.2018.30.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.