Abstract
Optic neuritis (ON) is one of the most frequently seen neuro-ophthalmic causes of vision loss worldwide. Typical ON is often idiopathic or seen in patients with multiple sclerosis, which is well described in the landmark clinical trial, the Optic Neuritis Treatment Trial (ONTT). However, since the completion of the ONTT, there has been the discovery of aquaporin-4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) antibodies, which are biomarkers for neuromyelitis optica spectrum disorder (NMOSD) and MOG antibody-associated disease (MOGAD), respectively. These disorders are associated with atypical ON that was not well characterised in the ONTT. The severity, rate of recurrence and overall outcome differs in these two entities requiring prompt and accurate diagnosis and management. This review will summarise the characteristic neuro-ophthalmological signs in NMOSD and MOGAD, serological markers and radiographic findings, as well as acute and long-term therapies used for these disorders.
Subject terms: Visual system, Autoimmunity
Abstract
视神经炎(ON)是全球最易导致视力下降的神经眼科疾病。据标志性的视神经治疗试验(ONTT)描述, 典型的ON通常为特发性或见于多发性硬化症的患者。然而, 自ONTT完成以来, 已经发现了水通道蛋白-4(AQP4)和髓磷脂少突胶质细胞糖蛋白(MOG)抗体, 它们分别是视神经脊髓炎疾病(NMOSD)和MOG抗体相关疾病 (MOGAD) 的生物标志物。ONTT中并没有很好的描述这些与非典型ON相关的疾病。这两类疾病的严重程度、复发率和最终临床结局不同, 需要及时准确的诊断和治疗。这篇综述将总结NMOSD和MOGAD的特异性的神经眼科体征、血清学标志物以及放射影像学法相, 以及对于这些疾病的急性和长期治疗。
Introduction
Optic neuritis (ON) is one of the most common neuro-ophthalmological conditions worldwide leading to visual morbidity, especially in younger patients. Multiple sclerosis (MS) is the most recognised underlying aetiology of ON. Much of what we know about typical optic neuritis characteristics comes from the landmark Optic Neuritis Treatment Trial (ONTT) that was published in 1992 [1, 2]. The ONTT was designed to evaluate acute unilateral ON in patients with or without a history of MS and the influence of corticosteroid treatment on outcomes. Ultimately, it found that roughly 50% of ON is caused by MS and that high-dose intravenous methylprednisolone (IVMP) leads to faster recovery, but did not change the ultimate outcome [1, 3]. The ONTT found that typical ON usually presents as subacute monocular vision loss in a young adult, associated with eye pain worsened with eye movements, decreased contrast and colour vision, and evidence of relative afferent pupillary defect (RAPD) on examination. The degree of visual acuity loss and visual field defect can be variable. There is optic disc oedema on presentation in about a third of cases, which is typically mild because the lesions are often predominantly retrobulbar [1, 4].
Our understanding of other diseases associated with ON as part of their clinical presentation has tremendously grown in the past two decades with advances in serological antibody testing and imaging protocols. Since the completion of the ONTT, there has been the discovery of biomarkers of atypical ON, namely aquaporin-4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) antibodies, which are associated with neuromyelitis optica spectrum disorder (NMOSD) and MOG antibody-associated disease (MOGAD) respectively, that have different characteristics, prognoses, and treatment [5, 6]. A recent study found that among a third of blood samples remaining from the ONTT, none were positive for AQP4 antibodies and only 1.7% were found to have MOG antibodies [7]. These results indicate that the ONTT provides excellent details of typical ON, but not for the atypical ON seen in NMOSD or MOGAD. Our understanding of NMOSD and MOGAD has greatly expanded over the past decade, which extends our knowledge of ON beyond what was found in the ONTT.
In this review, we will summarise the neuro-ophthalmological findings, diagnostic measures, and acute and long-term therapeutics in NMOSD and MOGAD.
Neuromyelitis optica spectrum disorder (NMOSD)
Historical overview and epidemiology
In 1894, Eugene Devic and his student Fernand Gault introduced the term ‘neuromyelitis optica acuta’ after describing a case of bilateral blindness and paraplegia with post-mortem pathology revealing demyelination of the optic nerves and demyelination and necrosis of a long portion of the spinal cord [8]. Over the years, the phenotypical differences, such as recurrence and severity of ON, as well as improvement in imaging techniques, led to many appreciating there was a difference between neuromyelitis optica (NMO) and MS. The first diagnostic criteria for NMO was devised in 1999 and required both ON and transverse myelitis for the diagnosis supported by imaging and CSF findings [9]. Despite phenotypic differences, it was initially unclear if NMO was a separate entity of MS or a more severe variant [10]. In 2004, AQP4-IgG was discovered [5], which is an antibody against a transmembrane water channel protein in the central nervous system (CNS) expressed on astrocytic end-feet that was found to be both a biomarker and pathologic cause of NMO. This discovery led to NMO becoming recognised as an independent clinical entity. Because of the availability of a specific biomarker of NMO, the spectrum of potential phenotypes has expanded. In 2015, the NMO diagnostic criteria was revised allowing for the molecular and clinical diagnosis of neuromyelitis optica spectrum disorder (NMOSD) [11–13], which is now known to represent a spectrum of clinical symptoms and MRI findings in addition to the seropositivity to AQP4-IgG. About 30% of NMOSD is seronegative for AQP4-IgG (Table 1).
Table 1.
Diagnostic criteria for neuromyelitis optica spectrum disorder (adapted from Wingerchuk et al. [13]).
| Core clinical characteristics | AQP4-IgG positive | AQP4-IgG negative or unknown AQP4-IgG status | Additional MRI requirements in AQP4-IgG negative or unknown AQP4-IgG status |
|---|---|---|---|
|
1. Optic neuritis 2. Acute myelitis 3. Area postrema syndrome: episode of otherwise unexplained hiccups or nausea and vomiting 4. Acute brainstem syndrome 5. Symptomatic narcolepsy or acute diencephalic clinical syndrome with NMOSD-typical diencephalic MRI lesions 6. Symptomatic cerebral syndrome with NMOSD-typical brain lesions |
1. At least 1 core clinical characteristic 2. Exclusion of alternative diagnoses |
1. At least 2 core clinical characteristics occurring as a result of 1 or more clinical attacks and meeting all the following requirements: a. At least 1 core clinical characteristic must be optic neuritis, acute myelitis with LETM, or area postrema syndrome b. Dissemination in space (2 or more different core clinical characteristics) c. Fulfilment of additional MRI requirements, as applicable 2. Negative tests for AQP4-IgG3. Exclusion of alternative diagnoses |
1. Acute optic neuritis: a brain MRI showing normal findings (or only non-specific white matter lesions), or an optic nerve MRI with a T2-hyperintense lesion or a T1-weighted gadolinium-enhanced lesion extending over more than one-half the optic nerve length or involving the optic chiasm 2. Acute myelitis: an associated intramedullary MRI lesion extending over either ≥3 contiguous segments (LETM) or ≥3 contiguous segments of focal spinal cord atrophy in patients with a history compatible with acute myelitis 3. Area postrema syndrome: associated dorsal medulla/area postrema lesions 4. Acute brainstem syndrome: associated periependymal brainstem lesions |
AQP4 aquaporin 4, IgG immunoglobulin G, LETM longitudinally extensive transverse myelitis, MRI magnetic resonance imaging, NMOSD neuromyelitis optica spectrum disorder.
AQP4-IgG positive NMOSD (AQP4-IgG + NMOSD) is a rare immune astrocytopathy with an incidence of 0.04–0.25 per 100,000 and prevalence of 0.70–1.91 per 100,000 in White populations and an incidence of 0.34–1.31 per 100,000 and prevalence of 0.86–4.25 per 100,000 in non-white populations [14]. Unlike MS, NMOSD is more prevalent in Asians and Blacks [15, 16]. The median age of onset is 39 years, about a decade later than MS, with a strong female preference (70–90%) [17]. It is generally a sporadic disorder, however, rare familial cases have been reported [18].
Clinical presentation
Based on International Panel for NMO Diagnosis criteria, one core clinical characteristic of NMOSD with positive serum AQP4-IgG is sufficient to make a diagnosis of AQP4-IgG + NMOSD. Core clinical characteristics include: (1) Acute ON; (2) Acute transverse myelitis; (3) Area postrema syndrome presenting with intractable nausea, vomiting, and/or hiccups; (4) Acute brainstem syndrome; (5) Acute diencephalic clinical syndrome or symptomatic narcolepsy; (6) Symptomatic cerebral syndrome [13]. A diagnosis of AQP4-IgG seronegative NMOSD can be made with the presence of 2 core clinical characteristics with at least one of them being ON, transverse myelitis, or area postrema syndrome (Table 1). It was initially thought that NMOSD was a monophasic disorder, though it is now recognised that NMOSD usually has a relapsing course, which is seen in up to 90% of patients. Relapses more commonly occur in the first year after initial attack, however, remote relapses beyond 10 years have also been reported [19].
Prior studies have reported ON as the initial presentation in 42% of NMOSD patients. It has also been found that close to two-third of patients with NMOSD will eventually develop ON during the disease course [20]. Distinguishing NMOSD ON (NMOSD-ON) from other forms of ON can be challenging at the time of initial presentation, but there are some clinical clues that should raise the suspicion of NMOSD. NMOSD-ON often causes severe vision loss with over 75% having a visual acuity of 20/200 or worse at nadir. Severe visual acuity loss of 20/200 or worse at nadir was also reported in 35.9% of ONTT patients and therefore severe vision loss alone at presentation does not perfectly stratify NMOSD-ON from other forms of ON [21–25]. However, NMOSD-ON outcomes tend to be worse with less recovery. Over one-third of patients with NMOSD-ON have a final visual outcome of 20/200 or worse, which is a significant morbidity compared to improvement to 20/40 or better in over 90% of patients in the ONTT [1, 26] (Table 2).
Table 2.
Comparison of clinical and paraclinical findings in NMOSD vs. MOGAD.
| NMOSD | MOGAD | |
|---|---|---|
| Characteristics | ||
| Median age (years) | 30–40s | 30s (adults) and children (<18) |
| Gender (F:M) | 9:1 | 1:1 |
| Ethnicity preference | Asian, African American | No clear predilection |
| Clinical and imaging features | ||
| Optic neuritis: | ||
| • Optic disc oedema | + | +++ |
| • Bilateral optic nerve involvement | ++ | ++ |
| • Pain | ++ | +++ |
| • Severe vision loss at nadir | +++ | +++ |
| • Recurrent visual loss | +++ | +++ |
| • Steroid dependence | + | ++ |
| • Visual recovery | Poor | Favourable |
| • Optic chiasm involvement | +++ | + |
| • MRI enhancement location | Posterior optic nerve | Anterior optic nerve |
| • MRI perineural enhancement | Rare | ++ |
| Myelitis: | ||
| • LETM | +++ | ++ |
| • Conus medullaris involvement | + | +++ |
| • MRI gadolinium enhancement | ++ | + |
| • H sign | + | ++ |
| Area postrema syndrome | ++ | Rare |
| Seizure | Rare | + |
| Encephalopathy | Rare | ++ |
| Diencephalic symptoms | ++ | Rare |
| Brainstem syndromes | + | ++ |
| ADEM | Rare | ++ |
| CSF | ||
| White blood cell count (cells/µl) | Normal to mild elevation | Normal to mild elevation |
| • >50 cells/µl | 35% | 13–35% |
| Protein mg/dl | Normal to mild/moderate elevation | Normal to mild elevation |
| Oligoclonal bands | <20% | <20% |
Rare or less than 5%, + infrequent, ++ frequent, +++ very frequent.
ADEM acute disseminated encephalomyelitis, CSF cerebrospinal fluid, LETM longitudinally extensive transverse myelitis, MOGAD myelin oligodendrocyte glycoprotein antibody-associated disease, MRI magnetic resonance imaging, NMOSD neuromyelitis optica spectrum disorder.
Other helpful distinguishing factors is simultaneous or rapidly sequential bilateral ON that are seen more commonly associated with NMOSD-ON and MOGAD optic neuritis (MOGAD-ON), while rare in MS and typical ON [27–30]. Bilateral simultaneous ON in NMOSD is 20% in NMOSD compared to almost 50% in MOGAD. However, both are far higher than MS where it is likely 1% at most (Table 2). NMOSD-ON has higher optic chiasm involvement ranging from 20–64%, which is rare in MS (Fig. 1) [31]. Optic disc oedema in only noted in 5–33% of NMOSD-ON cases given its propensity to involve the more posterior aspects of the optic nerve [32].
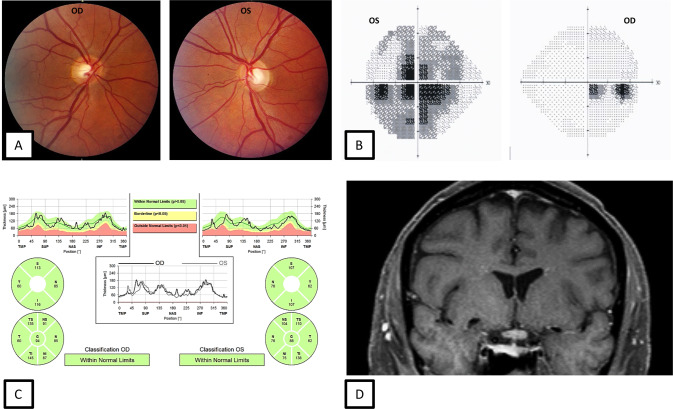
Fig. 1. 57-year-old male with AQP4-IgG positive neuromyelitis optica spectrum disorder (NMOSD) optic neuritis.
During the acute optic neuritis attack, fundus photographs demonstrate no optic disc oedema (A). Automated visual field testing shows a junctional scotoma (B). OCT shows a normal pRNFL thickness (C). Coronal, fat saturated T1-weighted post contrast MRI of the orbits shows enhancement of the optic chiasm and left optic nerve at the junction of the chiasm correlating with the visual field defects (D).
Diagnosis and testing
Visual fields and OCT
NMOSD-ON can lead to any pattern of visual field loss on automated perimetry, with central scotoma being the most common pattern followed by altitudinal hemianopia and less commonly bitemporal hemianopsia from chiasmal involvement [33, 34]. However, often the vision is so severe at nadir that automated visual fields cannot be performed.
Neuroaxonal damage is often significant in NMOSD-ON, which can be evaluated by optical coherence tomography (OCT) after ON. This damage leads to an average peripapillary retinal nerve fibre layer (pRNFL) loss that is almost two-fold higher than seen in MS-ON (38.4 µm thinning in NMOSD-ON compared to 20 µm thinning in MS-ON). Macular ganglion cell and inner plexiform layer (GCIPL) thinning in NMOSD-ON has been reported as 1.5-fold higher than MS-ON [35, 36]. Severe pRNFL and GCIPL loss correlates with the worse visual outcomes in NMOSD-ON compared to MS-ON or MOGAD-ON [37].
Neuroimaging
Simultaneous bilateral optic nerve enhancement, longitudinally extensive enhancement (≥50% of the optic nerve) and chiasmal involvement [38], should raise the suspicion for atypical ON associated with NMOSD (Fig. 1). The most commonly described intracranial brain lesions have non-specific characteristics presenting as small hyperintensities on T2-weighted or FLAIR sequences in the deep white matter; however, certain localisation of lesions make them more unique for NMOSD [39]. NMOSD brain lesions have increased preference to the areas with high AQP4 expression such as diencephalon surrounding the third ventricle and cerebral aqueduct, posterior brainstem adjacent to the fourth ventricle, and periependymal adjacent to lateral ventricles [40, 41]. Involvement of the area postrema is frequent in NMOSD, which is the third most common presentation of NMOSD behind ON and transverse myelitis. Periventricular white matter lesions that are typically seen in MS are rare in NMOSD.
Serum and cerebrospinal fluid
Detection of serum AQP4-IgG by live cell-based assays has been shown to have a sensitivity of ~75% and specificity of >99%, which is superior to other assays such as ELISA, and therefore are the gold standard for the detection of AQP4-IgG [42]. Serological testing for AQP4-IgG is crucial for treatment as well as prognostication. Prior studies have shown a statistically significant correlation between AQP4 seropositivity and higher risk of ON and myelitis recurrence, thus leading to poorer visual outcome and overall disability [43]. Cerebrospinal fluid (CSF) evaluation for AQP4-IgG was determined to be less sensitive and not cost effective, and is therefore not recommended in the setting of ON or suspected NMOSD [44].
There are no unique CSF characteristics in NMOSD, however, up to 35% of AQP4-IgG + NMOSD cases have shown pleocytosis with >50 cells/µl of either neutrophilic or monocular predominance [45]. Protein elevations can be seen in up to 44% of cases. Oligoclonal bands is only seen in 15–30% of cases, compared to 90% in MS. High levels of glial fibrillary acidic protein (GFAP) in the CSF of NMOSD patients during acute attack further suggest astrocytic damage [46]. Interestingly, studies have also shown that higher serum GFAP level is associated with 3-fold increased risk of relapses [47, 48].
Treatment
While the ONTT showed that steroids do not alter the visual outcome of typical ON, NMOSD-ON has a worse prognosis and therefore treatment with both IVMP and plasma exchange are recommended. Early treatment of NMOSD-ON is likely important in reducing the risk of visual morbidity as prior retrospective studies have shown better preservation of vision and pRNFL in NMOSD-ON when treated with early IVMP [49–51]. IVMP is usually used at 1000 mg/day for 3 to 5 consecutive days with or without an oral prednisone taper. High-dose oral corticosteroids (1250 mg prednisone, which is the bioequivalent of 1000 mg IVMP) has been shown to be equivalent to high-dose IVMP for the treatment of ON [52], and is therefore also an acute treatment option for NMOSD-ON. In addition, multiple case series and retrospective studies have demonstrated benefits of using plasma exchange as first line or in corticosteroid-refractory NMOSD-ON [53]. Plasma exchange is typically administered every other day for a total of 5 to 7 sessions with most benefits in visual outcomes when initiated within 7 days from symptom onset [54]. Retrospective studies have shown that the use of IVMP and plasma exchange in combination leads to a better final visual acuity than IVMP alone [55]. Therefore, most experts recommend both high-dose corticosteroids and plasma exchange as the first line treatment for acute NMOSD-ON.
All NMOSD patients require long-term immunosuppressive therapy (IST) given the high rate of relapses (67–90%) and high risk of morbidity and even potential for mortality [38]. Traditional off label ISTs, such as mycophenolate mofetil and azathioprine, have been shown to be associated with a reduction in relapses based on multiple retrospective studies [56]. Rituximab, a chimeric monoclonal antibody targeting CD20, has been shown to very effective in relapse prevention based on retrospective and randomised clinical trials [57, 58], and therefore has traditionally been the most commonly employed treatment for NMOSD. More recently, results of four randomised clinical trials have led to Food and Drug Administration (FDA) approval of three ISTs demonstrating lower relapse rates in NMOSD patients compared to placebo. These three ISTs include eculizumab—an anti-C5 complement inhibitor [59], inebilizumab—a CD19 monoclonal antibody [60], and satralizumab—a interleukin 6 receptor (IL-6) monoclonal antibody [61], which all greatly improve our treatment armamentarium for NMOSD.
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD)
Historical overview and epidemiology
MOGAD is a recently described inflammatory demyelinating condition, which can present with ON and other CNS demyelinating phenotypes, including transverse myelitis, acute disseminated encephalomyelitis (ADEM), brainstem syndromes, seizure and cerebral cortical encephalitis [62, 63]. While MOG-IgG was previously erroneously thought to be a marker of MS based on non-specific older generation assays using MOG in its denatured form [64], the use of transfected cell-based assays with MOG in its native conformation has led to MOG-IgG becoming a reliable biomarker for a new entity, MOGAD, which is distinct from both MS and NMOSD [62, 63, 65, 66].
The median age of onset for MOGAD is mid-30s, but can affect any age, including a predilection for children [62, 67]. Unlike MS and NMOSD, there is no clear gender or racial predilection [62, 68]. However, studies in predominantly White populations suggest that it is likely 2 to 3-fold more common than AQP4-IgG + NMOSD [69, 70].
Clinical presentation
Optic neuritis is the most common phenotype of MOGAD in adults, while ADEM is more frequent in the paediatric population [62]. In adults, MOGAD accounts for ~5% of ON [68, 70, 71] while in children, MOGAD accounts for a much higher percentage of ON, ranging from 20–50% [68, 72–74]. While there are overlapping features of ON with other demyelinating conditions, there are some salient clinical characteristics that should alert the clinician to the possibility of MOGAD (Table 2). Unlike other forms of ON where visible optic disc oedema is present in only a third of cases, optic disc oedema is present in ~80% of MOGAD-ON, which can sometimes be severe and associated with peripapillary haemorrhages [66, 67, 75–77] (Fig. 2). Up to 50% can be bilateral at presentation [66, 67, 76, 78]. Pain is a prominent feature of most MOGAD-ON attacks, and can be severe enough to manifest as headache [79]. MOGAD-ON typically has severe vision loss at nadir (similar to AQP4-IgG + NMOSD ON), but often has significant recovery leading to better visual outcomes, with only 5–14% of patients with a final visual acuity of 20/200 or worse [29, 62, 67, 76, 80].
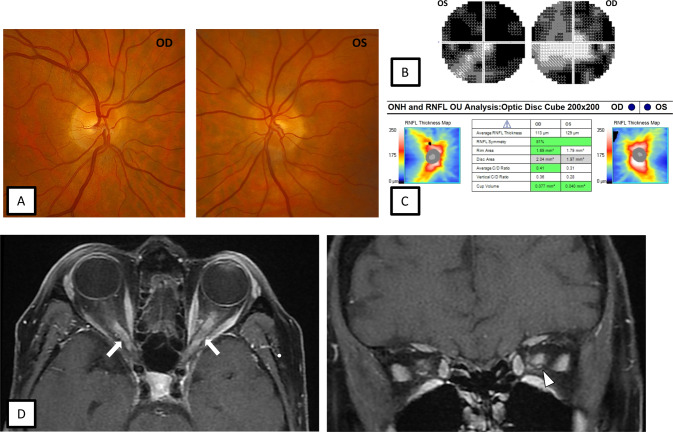
Fig. 2. 67-year-old female with myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) optic neuritis.
During the acute optic neuritis attack, fundus photographs show trace bilateral optic disc oedema (A). Automated visual field testing demonstrates bilateral generalised constriction (B). OCT shows mild bilateral pRNFL thickening (C). Axial and coronal, fat saturated T1-weighted post contrast MRI of the orbits shows bilateral longitudinally optic nerve enhancement (white arrows) with mild perineural enhancement on the left (white arrowhead) (D).
Approximately 50% of patients with MOGAD will have relapsing disease, which is most commonly in the form of ON [62, 81]. The ON attacks are often steroid responsive, but some are steroid dependent and can follow a chronic relapsing inflammatory optic neuropathy (CRION)-like phenotype. Recent studies have shown that MOGAD accounts for many cases of CRION that were previously thought to be idiopathic [82–84]. Less common ophthalmic manifestations reported in MOGAD include uveitis, macular neuro-retinopathy, neuroretinitis, retinopathy secondary to venous stasis and increased intracranial pressure, orbital inflammatory syndrome, and cranial neuropathies [85].
Diagnosis and testing
Visual fields and OCT
Automated perimetry during an acute MOGAD-ON attack can reveal any pattern of field loss, including central scotoma or severe generalised depression, which can affect one or both eyes.
OCT during the acute phase of ON typically shows pRNFL thickening consistent with the optic disc oedema [86, 87]. A study comparing MOGAD-ON to MS-ON found that a pRNFL thickness of ≥175 µm had a 96% specificity of distinguishing MOGAD-ON from MS-ON [88]. Progressive optic atrophy with pRNFL and GCIPL thinning is seen in the chronic phase despite usually having significant recovery of vision [37]. Although the visual acuity loss at nadir is often as severe as NMOSD-ON, recurrent attacks are often required in MOGAD-ON to reach similar severe levels of pRNFL and GCIPL thinning seen in NMOSD [80, 89].
Neuroimaging
Optic nerve gadolinium enhancement is typically longitudinally extensive (≥50% of the optic nerve) (90% of cases) with evidence of perineural enhancement involving the optic nerve sheath and peribulbar fat (50% of cases) [67, 77] (Fig. 2). Chiasmal involvement occurs in ~15% of cases [76], which is often from a longitudinally extensive lesion involving the entire optic nerve, unlike NMOSD, which more commonly causes isolated chiasmal involvement [31]. Intracranial lesions can be present in both white and deep grey matter and are typically characterised as large T2-weighted hyperintensities with indistinct margins [90].
Serum and cerebrospinal fluid
The detection of MOG-IgG should be done in the serum and tested with a cell-based assay, which provides a specificity of about 98%. The sensitivity and specificity of cell-based assays in identifying MOG-IgG are significantly higher than other assays, such as ELISA [91, 92]. Despite an excellent specificity of 98%, a study looking at MOG-IgG testing over 2 years at a tertiary centre found that the positive predictive value was only 72%, which was even poorer for lower titres of 1:20 and 1:40. Higher titres of ≥1:100 had higher a positive predictive value [93]. Therefore, patients with a low MOG-IgG titre should be reviewed in detail to make sure the disease is compatible with a MOGAD phenotype.
While CSF testing for AQP4-IgG has been shown to be not clinically useful [44], the utility of MOG-IgG testing is still being evaluated. There may be some cases of MOGAD that are negative in the serum, but positive in the CSF [48, 94]. However, the specificity of CSF MOG-IgG testing is still being elucidated. Overall, testing the serum for MOG-IgG remains the gold standard, but CSF MOG-IgG testing can be considered in patients with a MOGAD phenotype who are seronegative for MOG-IgG.
During an acute attack, CSF can show mild pleocytosis (>5 cells/µl) in more than 50% of patients (less commonly with isolated ON clinical phenotype when compared to myelitis or multifocal CNS involvement) [95]. Marked pleocytosis of >50 cells/µl has been reported in about 30% of patients during acute attacks, which is an uncommon finding in comparison to MS [96]. Oligoclonal bands are seen in <20%, which can help differentiate MOGAD from MS.
Treatment
Acute MOGAD-ON is often very responsive to high-dose corticosteroids. In addition, retrospective studies have suggested that early treatment with corticosteroids may lead to better outcomes [51, 97]. Because some patients are both steroid responsive and steroid dependent, an oral prednisone taper over 1–2 months is often recommended [38]. However, it is important to note that spontaneous improvement without steroid treatment can occur as well [7, 97, 98]. In a small percentage of the patient with significant visual loss and no improvement after the high-dose corticosteroid administration, additional treatment with plasma exchange should be considered [97].
Because only 50% of MOGAD patients will have relapsing disease and recovery from attacks is typically good, chronic immunotherapy is usually reserved for patients with relapsing disease or severe disease with significant residual disability after the first attack. Similar to NMOSD, MS medications have been shown to be ineffective for MOGAD [66, 99–101]. Many of the chronic therapies that have been employed in MOGAD have been extrapolated from the treatment of NMOSD, which includes rituximab, mycophenolate mofetil, and azathioprine [99–104]. Retrospective studies have suggested these are all partially beneficial in reducing relapses in MOGAD, but rituximab may be less effective in MOGAD than in NMOSD [105, 106]. Chronic prednisone has been shown to be very effective in preventing relapses in MOGAD, but its use can be limited because of the side effects of chronic steroid treatment [66]. Recent case series have suggested that targeting IL-6 with tocilizumab may be effective in patients with refractory MOGAD [107, 108]. Lastly, several retrospective studies have suggested that maintenance IVIG may be one of the most effective treatments for patients with relapsing MOGAD [73, 99, 109]. Future randomised clinical trials will be required to determine the optimal therapy for MOGAD. Upcoming randomised clinical trials for satralizumab (IL-6 inhibitor) and rozanolixizumab (neonatal Fc receptor inhibitor) are underway, which will hopefully lead to FDA approved medications for MOGAD and a better understanding of the disease.
Conclusions
The discovery of AQP4 and MOG antibodies have facilitated the recognition of NMOSD and MOGAD as separate neuroimmunological disease entities. Early diagnosis of each disorder is crucial as it impacts the course of treatment, clinical outcomes and morbidity. Because NMOSD-ON is associated with poor outcomes, early treatment with high-dose corticosteroids and plasma exchange are recommended for acute attacks. Patients with NMOSD also require long-term ISTs, such as rituximab or one of the recently FDA approved monoclonal antibody treatments. In contrast, MOGAD-ON is usually responsive to high-dose corticosteroids and has a generally favourable visual outcome. However, some cases are corticosteroid dependent or have relapsing disease and require maintenance therapy with either chronic prednisone or long-term immunotherapy, such as maintenance IVIG. The optimal long-term treatment for relapsing MOGAD will be elucidated in future randomised clinical trials.
Author contributions
JJC and NM were involved in the study conception and design, review of the literature, drafting of the manuscript, and approval of the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beck RW, Cleary PA, Anderson MM, Jr, Keltner JL, Shults WT, Kaufman DI, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326:581–8. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- 2.Beck RW. The optic neuritis treatment trial. Arch Ophthalmol. 1988;106:1051–3. doi: 10.1001/archopht.1988.01060140207023. [DOI] [PubMed] [Google Scholar]
- 3.Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65:727–32. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petzold A, Fraser CL, Abegg M, Alroughani R, Alshowaeir D, Alvarenga R, et al. Diagnosis and classification of optic neuritis. Lancet Neurol. 2022;21:1120–34. doi: 10.1016/S1474-4422(22)00200-9. [DOI] [PubMed] [Google Scholar]
- 5.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–12. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 6.Waters P, Woodhall M, O’Connor KC, Reindl M, Lang B, Sato DK, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2015;2:e89. doi: 10.1212/NXI.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JJ, Tobin WO, Majed M, Jitprapaikulsan J, Fryer JP, Leavitt JA, et al. Prevalence of myelin oligodendrocyte glycoprotein and aquaporin-4-IgG in patients in the optic neuritis treatment trial. JAMA Ophthalmol. 2018;136:419–22. doi: 10.1001/jamaophthalmol.2017.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarius S, Wildemann B. The history of neuromyelitis optica. J Neuroinflammation. 2013;10:8. doi: 10.1186/1742-2094-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinshenker BG, Wingerchuk DM. Neuromyelitis spectrum disorders. Mayo Clin Proc. 2017;92:663–79. doi: 10.1016/j.mayocp.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Frohman EM, Kerr D. Is neuromyelitis optica distinct from multiple sclerosis?: something for “lumpers” and “splitters”. Arch Neurol. 2007;64:903–5. doi: 10.1001/archneur.64.6.903. [DOI] [PubMed] [Google Scholar]
- 11.Pittock SJ. Demyelinating disease: NMO spectrum disorders: clinical or molecular classification? Nat Rev Neurol. 2016;12:129–30. doi: 10.1038/nrneurol.2016.9. [DOI] [PubMed] [Google Scholar]
- 12.Hinson SR, Lennon VA, Pittock SJ. Autoimmune AQP4 channelopathies and neuromyelitis optica spectrum disorders. Handb Clin Neurol. 2016;133:377–403. doi: 10.1016/B978-0-444-63432-0.00021-9. [DOI] [PubMed] [Google Scholar]
- 13.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandit L, D’Cunha A, Malapur PU. Incidence and prevalence of neuromyelitis optica spectrum disorders in the background of international consensus diagnostic criteria—a systematic review. Neurol India. 2022;70:1771–9. doi: 10.4103/0028-3886.359235. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan EP, Cabre P, Weinshenker BG, Sauver JS, Jacobson DJ, Majed M, et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol. 2016;79:775–83. doi: 10.1002/ana.24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SH, Mealy MA, Levy M, Schmidt F, Ruprecht K, Paul F, et al. Racial differences in neuromyelitis optica spectrum disorder. Neurology. 2018;91:e2089–99. doi: 10.1212/WNL.0000000000006574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingerchuk DM. Neuromyelitis optica: effect of gender. J Neurol Sci. 2009;286:18–23. doi: 10.1016/j.jns.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 18.Matiello M, Kim HJ, Kim W, Brum DG, Barreira AA, Kingsbury DJ, et al. Familial neuromyelitis optica. Neurology. 2010;75:310–5. doi: 10.1212/WNL.0b013e3181ea9f15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite MI, et al. Demographic and clinical features of neuromyelitis optica: a review. Mult Scler. 2015;21:845–53. doi: 10.1177/1352458515572406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CW, Lin IH, Chen TC, Jou JR, Woung LC. Clinical course and treatment response of neuromyelitis optica spectrum disease: an 8-year experience. Asia Pac J Ophthalmol. 2019;8:206–10. doi: 10.22608/APO.2018247. [DOI] [PubMed] [Google Scholar]
- 21.The clinical profile of optic neuritis. Experience of the optic neuritis treatment trial. Optic Neuritis Study Group. Arch Ophthalmol. 1991;109:1673–8. [DOI] [PubMed]
- 22.Masuda H, Mori M, Uzawa A, Muto M, Uchida T, Ohtani R, et al. Recovery from optic neuritis attack in neuromyelitis optica spectrum disorder and multiple sclerosis. J Neurol Sci. 2016;367:375–9. doi: 10.1016/j.jns.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes DB, Ramos Rde I, Falcochio C, Apostolos-Pereira S, Callegaro D, Monteiro ML. Comparison of visual acuity and automated perimetry findings in patients with neuromyelitis optica or multiple sclerosis after single or multiple attacks of optic neuritis. J Neuroophthalmol. 2012;32:102–6. doi: 10.1097/WNO.0b013e31823a9ebc. [DOI] [PubMed] [Google Scholar]
- 24.Jarius S, Frederikson J, Waters P, Paul F, Akman-Demir G, Marignier R, et al. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. J Neurol Sci. 2010;298:158–62. doi: 10.1016/j.jns.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Zhao S, Yin D, Chen X, Xu Q, Chen T, et al. Optic neuritis: a 5-year follow-up study of Chinese patients based on aquaporin-4 antibody status and ages. J Neurol. 2016;263:1382–9. doi: 10.1007/s00415-016-8155-7. [DOI] [PubMed] [Google Scholar]
- 26.Optic Neuritis Study Group. Visual function 15 years after optic neuritis: a final follow-up report from the Optic Neuritis Treatment Trial. Ophthalmology. 2008;115:1079–82.e5. doi: 10.1016/j.ophtha.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Srikajon J, Siritho S, Ngamsombat C, Prayoonwiwat N, Chirapapaisan N, Siriraj Neuroimmunology Research Group. Differences in clinical features between optic neuritis in neuromyelitis optica spectrum disorders and in multiple sclerosis. Mult Scler J Exp Transl Clin. 2018;4:2055217318791196. doi: 10.1177/2055217318791196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sotirchos ES, Filippatou A, Fitzgerald KC, Salama S, Pardo S, Wang J, et al. Aquaporin-4 IgG seropositivity is associated with worse visual outcomes after optic neuritis than MOG-IgG seropositivity and multiple sclerosis, independent of macular ganglion cell layer thinning. Mult Scler. 2020;26:1360–71. doi: 10.1177/1352458519864928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Zhou H, Wang J, Sun M, Teng D, Song H, et al. The prevalence and prognostic value of myelin oligodendrocyte glycoprotein antibody in adult optic neuritis. J Neurol Sci. 2019;396:225–31. doi: 10.1016/j.jns.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Wang Y, Xu Q, Zhang A, Zhou H, Zhao S, et al. Features of anti-aquaporin 4 antibody-seropositive Chinese patients with neuromyelitis optica spectrum optic neuritis. J Neurol. 2015;262:2293–304. doi: 10.1007/s00415-015-7844-y. [DOI] [PubMed] [Google Scholar]
- 31.Tajfirouz D, Padungkiatsagul T, Beres S, Moss HE, Pittock S, Flanagan E, et al. Optic chiasm involvement in AQP-4 antibody-positive NMO and MOG antibody-associated disorder. Mult Scler. 2022;28:149–53. doi: 10.1177/13524585211011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou H, Xu Q, Zhao S, Wang W, Wang J, Chen Z, et al. Distinct clinical characteristics of atypical optic neuritis with seronegative aquaporin-4 antibody among Chinese patients. Br J Ophthalmol. 2017;101:1720–4. doi: 10.1136/bjophthalmol-2017-310157. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima H, Hosokawa T, Sugino M, Kimura F, Sugasawa J, Hanafusa T, et al. Visual field defects of optic neuritis in neuromyelitis optica compared with multiple sclerosis. BMC Neurol. 2010;10:45. doi: 10.1186/1471-2377-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gospe SM, 3rd, Chen JJ, Bhatti MT. Neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein associated disorder-optic neuritis: a comprehensive review of diagnosis and treatment. Eye. 2021;35:753–68. doi: 10.1038/s41433-020-01334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oertel FC, Specovius S, Zimmermann HG, Chien C, Motamedi S, Bereuter C, et al. Retinal optical coherence tomography in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1068. doi: 10.1212/NXI.0000000000001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petzold A, Balcer LJ, Calabresi PA, Costello F, Frohman TC, Frohman EM, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16:797–812. doi: 10.1016/S1474-4422(17)30278-8. [DOI] [PubMed] [Google Scholar]
- 37.Filippatou AG, Mukharesh L, Saidha S, Calabresi PA, Sotirchos ES. AQP4-IgG and MOG-IgG related optic neuritis-prevalence, optical coherence tomography findings, and visual outcomes: a systematic review and meta-analysis. Front Neurol. 2020;11:540156. doi: 10.3389/fneur.2020.540156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasad S, Chen J. What you need to know about AQP4, MOG, and NMOSD. Semin Neurol. 2019;39:718–31. doi: 10.1055/s-0039-3399505. [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Paul F, Lana-Peixoto MA, Tenembaum S, Asgari N, Palace J, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84:1165–73. doi: 10.1212/WNL.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63:964–8. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- 41.Cortese R, Battaglini M, Prados F, Bianchi A, Haider L, Jacob A, et al. Clinical and MRI measures to identify non-acute MOG-antibody disease in adults. Brain. 2022;awac480. 10.1093/brain/awac480. Online ahead of print. [DOI] [PubMed]
- 42.Waters PJ, McKeon A, Leite MI, Rajasekharan S, Lennon VA, Villalobos A, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78:665–71. doi: 10.1212/WNL.0b013e318248dec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matiello M, Lennon VA, Jacob A, Pittock SJ, Lucchinetti CF, Wingerchuk DM, et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology. 2008;70:2197–200. doi: 10.1212/01.wnl.0000303817.82134.da. [DOI] [PubMed] [Google Scholar]
- 44.Majed M, Fryer JP, McKeon A, Lennon VA, Pittock SJ. Clinical utility of testing AQP4-IgG in CSF: guidance for physicians. Neurol Neuroimmunol Neuroinflamm. 2016;3:e231. doi: 10.1212/NXI.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019–32. doi: 10.1111/j.1468-1331.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 46.Takano R, Misu T, Takahashi T, Sato S, Fujihara K, Itoyama Y. Astrocytic damage is far more severe than demyelination in NMO: a clinical CSF biomarker study. Neurology. 2010;75:208–16. doi: 10.1212/WNL.0b013e3181e2414b. [DOI] [PubMed] [Google Scholar]
- 47.Aktas O, Smith MA, Rees WA, Bennett JL, She D, Katz E, et al. Serum glial fibrillary acidic protein: a neuromyelitis optica spectrum disorder biomarker. Ann Neurol. 2021;89:895–910. doi: 10.1002/ana.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinoto A, Sechi E, Flanagan EP, Ferrari S, Solla P, Mariotto S, et al. Serum and cerebrospinal fluid biomarkers in neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein associated disease. Front Neurol. 2022;13:866824. doi: 10.3389/fneur.2022.866824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura M, Nakazawa T, Doi H, Hariya T, Omodaka K, Misu T, et al. Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefes Arch Clin Exp Ophthalmol. 2010;248:1777–85. doi: 10.1007/s00417-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 50.Akaishi T, Takeshita T, Himori N, Takahashi T, Misu T, Ogawa R, et al. Rapid administration of high-dose intravenous methylprednisolone improves visual outcomes after optic neuritis in patients with AQP4-IgG-positive NMOSD. Front Neurol. 2020;11:932. doi: 10.3389/fneur.2020.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stiebel-Kalish H, Hellmann MA, Mimouni M, Paul F, Bialer O, Bach M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflamm. 2019;6:e572. doi: 10.1212/NXI.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrow SA, Fraser JA, Day C, Bowman D, Rosehart H, Kremenchutzky M, et al. Effect of treating acute optic neuritis with bioequivalent oral vs intravenous corticosteroids: a randomized clinical trial. JAMA Neurol. 2018;75:690–6. doi: 10.1001/jamaneurol.2018.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merle H, Olindo S, Jeannin S, Valentino R, Mehdaoui H, Cabot F, et al. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol. 2012;130:858–62. doi: 10.1001/archophthalmol.2012.1126. [DOI] [PubMed] [Google Scholar]
- 54.Bonnan M, Valentino R, Debeugny S, Merle H, Ferge JL, Mehdaoui H, et al. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry. 2018;89:346–51. doi: 10.1136/jnnp-2017-316286. [DOI] [PubMed] [Google Scholar]
- 55.Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, Levy M. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22:185–92. doi: 10.1177/1352458515581438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trebst C, Jarius S, Berthele A, Paul F, Schippling S, Wildemann B, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS) J Neurol. 2014;261:1–16. doi: 10.1007/s00415-013-7169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tahara M, Oeda T, Okada K, Ochi K, Maruyama H, Fukaura H, et al. Compassionate open-label use of rituximab following a randomised clinical trial against neuromyelitis optica (RIN-2 study): B cell monitoring-based administration. Mult Scler Relat Disord. 2022;60:103730. doi: 10.1016/j.msard.2022.103730. [DOI] [PubMed] [Google Scholar]
- 58.Tahara M, Oeda T, Okada K, Kiriyama T, Ochi K, Maruyama H, et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19:298–306. doi: 10.1016/S1474-4422(20)30066-1. [DOI] [PubMed] [Google Scholar]
- 59.Pittock SJ, Berthele A, Fujihara K, Kim HJ, Levy M, Palace J, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381:614–25. doi: 10.1056/NEJMoa1900866. [DOI] [PubMed] [Google Scholar]
- 60.Cree BAC, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394:1352–63. doi: 10.1016/S0140-6736(19)31817-3. [DOI] [PubMed] [Google Scholar]
- 61.Yamamura T, Kleiter I, Fujihara K, Palace J, Greenberg B, Zakrzewska-Pniewska B, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381:2114–24. doi: 10.1056/NEJMoa1901747. [DOI] [PubMed] [Google Scholar]
- 62.Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128–38. doi: 10.1093/brain/awx276. [DOI] [PubMed] [Google Scholar]
- 63.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15:134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–45. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 65.Lopez-Chiriboga AS, Majed M, Fryer J, Dubey D, McKeon A, Flanagan EP, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol. 2018;75:1355–63. doi: 10.1001/jamaneurol.2018.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89:127–37. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen JJ, Flanagan EP, Jitprapaikulsan J, Lopez-Chiriboga ASS, Fryer JP, Leavitt JA, et al. Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. 2018;195:8–15. doi: 10.1016/j.ajo.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Mol CL, Wong Y, van Pelt ED, Wokke B, Siepman T, Neuteboom RF, et al. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler. 2020;26:806–14. doi: 10.1177/1352458519845112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kunchok A, Chen JJ, McKeon A, Mills JR, Flanagan EP, Pittock SJ. Coexistence of myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies in adult and pediatric patients. JAMA Neurol. 2020;77:257–9. doi: 10.1001/jamaneurol.2019.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hassan MB, Stern C, Flanagan EP, Pittock SJ, Kunchok A, Foster RC, et al. Population-based incidence of optic neuritis in the era of aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies. Am J Ophthalmol. 2020;220:110–4. doi: 10.1016/j.ajo.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soelberg K, Jarius S, Skejoe H, Engberg H, Mehlsen JJ, Nilsson AC, et al. A population-based prospective study of optic neuritis. Mult Scler. 2017;23:1893–901. doi: 10.1177/1352458517734070. [DOI] [PubMed] [Google Scholar]
- 72.Chen JJ, Pineles SL, Repka MX, Pittock SJ, Henderson RJ, Liu GT, et al. MOG-IgG among participants in the pediatric optic neuritis prospective outcomes study. JAMA Ophthalmol. 2021;139:583–5. doi: 10.1001/jamaophthalmol.2021.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hacohen Y, Banwell B. Treatment approaches for MOG-Ab-associated demyelination in children. Curr Treat Options Neurol. 2019;21:2. doi: 10.1007/s11940-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song H, Zhou H, Yang M, Tan S, Wang J, Xu Q, et al. Clinical characteristics and prognosis of myelin oligodendrocyte glycoprotein antibody-seropositive paediatric optic neuritis in China. Br J Ophthalmol. 2019;103:831–6. doi: 10.1136/bjophthalmol-2018-312399. [DOI] [PubMed] [Google Scholar]
- 75.Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22:470–82. doi: 10.1177/1352458515593406. [DOI] [PubMed] [Google Scholar]
- 76.Zhao Y, Tan S, Chan TCY, Xu Q, Zhao J, Teng D, et al. Clinical features of demyelinating optic neuritis with seropositive myelin oligodendrocyte glycoprotein antibody in Chinese patients. Br J Ophthalmol. 2018;102:1372–7. doi: 10.1136/bjophthalmol-2017-311177. [DOI] [PubMed] [Google Scholar]
- 77.Akaishi T, Sato DK, Nakashima I, Takeshita T, Takahashi T, Doi H, et al. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry. 2016;87:446–8. doi: 10.1136/jnnp-2014-310206. [DOI] [PubMed] [Google Scholar]
- 78.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016;13:279. doi: 10.1186/s12974-016-0717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Asseyer S, Hamblin J, Messina S, Mariano R, Siebert N, Everett R, et al. Prodromal headache in MOG-antibody positive optic neuritis. Mult Scler Relat Disord. 2020;40:101965. doi: 10.1016/j.msard.2020.101965. [DOI] [PubMed] [Google Scholar]
- 80.Zhao G, Chen Q, Huang Y, Li Z, Sun X, Lu P, et al. Clinical characteristics of myelin oligodendrocyte glycoprotein seropositive optic neuritis: a cohort study in Shanghai, China. J Neurol. 2018;265:33–40. doi: 10.1007/s00415-017-8651-4. [DOI] [PubMed] [Google Scholar]
- 81.Cobo-Calvo A, Ruiz A, Rollot F, Arrambide G, Deschamps R, Maillart E, et al. Clinical features and risk of relapse in children and adults with myelin oligodendrocyte glycoprotein antibody-associated disease. Ann Neurol. 2021;89:30–41. doi: 10.1002/ana.25909. [DOI] [PubMed] [Google Scholar]
- 82.Liu H, Zhou H, Wang J, Xu Q, Wei S. Antibodies to myelin oligodendrocyte glycoprotein in chronic relapsing inflammatory optic neuropathy. Br J Ophthalmol. 2019;103:1423–8. doi: 10.1136/bjophthalmol-2018-313142. [DOI] [PubMed] [Google Scholar]
- 83.Lee HJ, Kim B, Waters P, Woodhall M, Irani S, Ahn S, et al. Chronic relapsing inflammatory optic neuropathy (CRION): a manifestation of myelin oligodendrocyte glycoprotein antibodies. J Neuroinflammation. 2018;15:302. doi: 10.1186/s12974-018-1335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petzold A, Woodhall M, Khaleeli Z, Tobin WO, Pittock SJ, Weinshenker BG, et al. Aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies in immune-mediated optic neuritis at long-term follow-up. J Neurol Neurosurg Psychiatry. 2019;90:1021–6. doi: 10.1136/jnnp-2019-320493. [DOI] [PubMed] [Google Scholar]
- 85.Vosoughi AR, Ling J, Tam KT, Blackwood J, Micieli JA. Ophthalmic manifestations of myelin oligodendrocyte glycoprotein-IgG-associated disorder other than optic neuritis: a systematic review. Br J Ophthalmol. 2021;105:1591–8. doi: 10.1136/bjophthalmol-2020-317267. [DOI] [PubMed] [Google Scholar]
- 86.Havla J, Kumpfel T, Schinner R, Spadaro M, Schuh E, Meinl E, et al. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol. 2017;264:139–51.. doi: 10.1007/s00415-016-8333-7. [DOI] [PubMed] [Google Scholar]
- 87.Pache F, Zimmermann H, Mikolajczak J, Schumacher S, Lacheta A, Oertel FC, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation. 2016;13:282. doi: 10.1186/s12974-016-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen JJ, Sotirchos ES, Henderson AD, Vasileiou ES, Flanagan EP, Bhatti MT, et al. OCT retinal nerve fiber layer thickness differentiates acute optic neuritis from MOG antibody-associated disease and Multiple Sclerosis: RNFL thickening in acute optic neuritis from MOGAD vs MS. Mult Scler Relat Disord. 2022;58:103525. doi: 10.1016/j.msard.2022.103525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian G, Li Z, Zhao G, Feng C, Li M, Huang Y, et al. Evaluation of retinal nerve fiber layer and ganglion cell complex in patients with optic neuritis or neuromyelitis optica spectrum disorders using optical coherence tomography in a Chinese cohort. J Ophthalmol. 2015;2015:832784. doi: 10.1155/2015/832784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bartels F, Lu A, Oertel FC, Finke C, Paul F, Chien C. Clinical and neuroimaging findings in MOGAD-MRI and OCT. Clin Exp Immunol. 2021;206:266–81. doi: 10.1111/cei.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waters PJ, Komorowski L, Woodhall M, Lederer S, Majed M, Fryer J, et al. A multicenter comparison of MOG-IgG cell-based assays. Neurology. 2019;92:e1250–e5. doi: 10.1212/WNL.0000000000007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reindl M, Schanda K, Woodhall M, Tea F, Ramanathan S, Sagen J, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. 2020;7:e674. doi: 10.1212/NXI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sechi E, Buciuc M, Pittock SJ, Chen JJ, Fryer JP, Jenkins SM, et al. Positive predictive value of myelin oligodendrocyte glycoprotein autoantibody testing. JAMA Neurol. 2021;78:741–6. doi: 10.1001/jamaneurol.2021.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carta S, Cobo Calvo A, Armangue T, Saiz A, Lechner C, Rostasy K, et al. Significance of myelin oligodendrocyte glycoprotein antibodies in CSF: a retrospective multicenter study. Neurology. 2023;100:e1095–e1108. [DOI] [PMC free article] [PubMed]
- 95.Jarius S, Lechner C, Wendel EM, Baumann M, Breu M, Schimmel M, et al. Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 2: results from 108 lumbar punctures in 80 pediatric patients. J Neuroinflammation. 2020;17:262. doi: 10.1186/s12974-020-01825-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sechi E, Buciuc M, Flanagan EP, Pittock SJ, Banks SA, Lopez-Chiriboga AS, et al. Variability of cerebrospinal fluid findings by attack phenotype in myelin oligodendrocyte glycoprotein-IgG-associated disorder. Mult Scler Relat Disord. 2021;47:102638. doi: 10.1016/j.msard.2020.102638. [DOI] [PubMed] [Google Scholar]
- 97.Chen JJ, Flanagan EP, Bhatti MT, Tisavipat N, Jamali S, Kunchok A, et al. Details and outcomes of a large cohort of MOG-IgG associated optic neuritis. Mult Scler Relat Disord. 2022;68:104237. doi: 10.1016/j.msard.2022.104237. [DOI] [PubMed] [Google Scholar]
- 98.Vosoughi AR, Muccilli A, Schneider R, Rotstein D, Micieli JA. Recovery of vision in myelin oligodendrocyte glycoprotein-IgG optic neuritis without treatment: a case series. J Neuroophthalmol. 10.1097/WNO.0000000000001583. Online ahead of print. [DOI] [PubMed]
- 99.Chen JJ, Flanagan EP, Bhatti MT, Jitprapaikulsan J, Dubey D, Lopez Chiriboga ASS, et al. Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology. 2020;95:e111–20. doi: 10.1212/WNL.0000000000009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cobo-Calvo A, Sepulveda M, Rollot F, Armangue T, Ruiz A, Maillart E, et al. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J Neuroinflammation. 2019;16:134. doi: 10.1186/s12974-019-1525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie L, Zhou H, Song H, Sun M, Yang M, Lai YM, et al. Comparative analysis of immunosuppressive therapies for myelin oligodendrocyte glycoprotein antibody-associated optic neuritis: a cohort study. Br J Ophthalmol. 2022;106:1587–95. doi: 10.1136/bjophthalmol-2020-318769. [DOI] [PubMed] [Google Scholar]
- 103.Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018;90:e1858–69. doi: 10.1212/WNL.0000000000005560. [DOI] [PubMed] [Google Scholar]
- 104.Hacohen Y, Wong YY, Lechner C, Jurynczyk M, Wright S, Konuskan B, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2018;75:478–87. doi: 10.1001/jamaneurol.2017.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Whittam DH, Cobo-Calvo A, Lopez-Chiriboga AS, Pardo S, Gornall M, Cicconi S, et al. Treatment of MOG-IgG-associated disorder with rituximab: an international study of 121 patients. Mult Scler Relat Disord. 2020;44:102251. doi: 10.1016/j.msard.2020.102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Durozard P, Rico A, Boutiere C, Maarouf A, Lacroix R, Cointe S, et al. Comparison of the response to rituximab between myelin oligodendrocyte glycoprotein and aquaporin-4 antibody diseases. Ann Neurol. 2020;87:256–66. doi: 10.1002/ana.25648. [DOI] [PubMed] [Google Scholar]
- 107.Elsbernd PM, Hoffman WR, Carter JL, Wingerchuk DM. Interleukin-6 inhibition with tocilizumab for relapsing MOG-IgG associated disorder (MOGAD): a case-series and review. Mult Scler Relat Disord. 2021;48:102696. doi: 10.1016/j.msard.2020.102696. [DOI] [PubMed] [Google Scholar]
- 108.Ringelstein M, Ayzenberg I, Lindenblatt G, Fischer K, Gahlen A, Novi G, et al. Interleukin-6 receptor blockade in treatment-refractory MOG-IgG-associated disease and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1100. doi: 10.1212/NXI.0000000000001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen JJ, Huda S, Hacohen Y, Levy M, Lotan I, Wilf-Yarkoni A, et al. Association of maintenance intravenous immunoglobulin with prevention of relapse in adult myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2022;79:518–25. doi: 10.1001/jamaneurol.2022.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]