Abstract
Objectives
To determine 36-month efficacy and safety outcomes of the PRESERFLO Microshunt implant in the treatment of refractory uveitic glaucoma.
Methods
Consecutive patients with uncontrolled uveitic glaucoma despite maximum medical treatment received PRESERFLO Microshunt implant with mitomycin C (MMC) in a tertiary referral glaucoma practice. Efficacy and safety outcomes data were collected at month 6, 12, 24, and 36, postoperatively.
The primary outcome was surgical success, defined as intraocular pressure (IOP) between 5 and 21 mmHg or > 20% reduction from baseline, and absence of criteria for surgical failure. Secondary outcomes were IOP, visual acuity (VA), use of glaucoma medical therapy, surgical complications, rate of reoperation for glaucoma.
Results
21 eyes of 21 patients were included. The mean rate of success was 0.74 (95%CI 0.48–0.88), 0.68 (0.43–0.84), 0.47 (0.25–0.67), and 0.47 (0.25–0.67, at 6, 12, 24, and 36 months postoperatively, respectively. The mean IOP decreased by 30.7% (95% CI 13.7–47.7), 26.5% (95% CI 3.2–49.8), 33.5% (95% CI 21.8–45.3), and 30.1% (95% CI 7.2–52.9) from baseline at postoperative month 6, 12, 24, and 36, respectively (p < 0.001). The mean ± SD number of glaucoma medications decreased from 4.1 ± 0.9 to 0.9 ± 1.2 at the final follow up (p = 0.0005). No sight-threatening complications were reported by 36 months.
Conclusions
Three-year results of the PRESERFLO Microshunt implant demonstrated favourable efficacy and safety profile in the treatment of refractory uveitic glaucoma.
Subject terms: Health care, Outcomes research
Introduction
Increased intraocular pressure (IOP) leading to glaucomatous optic neuropathy is a common and potentially blinding complication of uveitis [1–3]. Chronic anterior uveitis and certain types of infectious uveitis are particularly prone to develop secondary glaucoma (UG). Both the uveitis itself and the principle treatment, corticosteroid therapy, may lead to IOP elevation. The mechanisms include acute and chronic angle closure, trabecular inflammation in addition to steroid responsiveness. The IOP tends to be higher and visual field progression faster in UG in comparison with primary open angle glaucoma (POAG). Close monitoring and aggressive IOP-lowering are essential to prevent glaucoma-related blindness, as UG can deteriorate rapidly [4, 5]. Surgery is indicated when the IOP is uncontrolled despite the maximum tolerated medical therapy, or when episodic IOP elevation leads to progression of optic neuropathy or deterioration of visual fields [3]. While angle surgery has been reported to be successful in treating younger UG patients with open angles, most patients require filtering surgery to effectively lower IOP [6–12].
Trabeculectomy in UG can be challenging. Long term and short term hypotony are more frequent due to reduced aqueous production in chronic uveitis and the younger age of these patients [13–15]. Despite a higher rate of hypotony in UG in comparison with POAG, tube shunt surgery and trabeculectomy augmented with antimetabolites remain the main surgical options for the management of UG because of their high level of IOP-lowering efficacy [16]. Subconjunctival minimally or less invasive glaucoma procedures are bleb forming filtering devices that produce IOP lowering approaching the level of traditional glaucoma filtering procedures in POAG. They are attractive options in UG as the patients often have very high IOP but not severe glaucomatous optic neuropathy and hence very low target IOP levels are not required. Both the XEN Gel Implant (Allergan, Dublin, Ireland) and PRESERFLO Microshunt (Santen Pharmaceutical Co. Ltd, Osaka, Japan; formerly known as the Innfocus Microshunt) are two commercially available subconjunctival bleb-forming devices [17]. Intraoperatively, these devices cause less tissue dissection and are quicker to implant in comparison with traditional filtering surgery. They also involve fewer post-operative visits and result in a faster visual recovery. Reasonable success has been reported with the XEN Gel implant in the surgical treatment of medically-uncontrolled UG [18], though a small risk of bleb-related complications such as ocular infection remains [19].
The purpose of the study described here is to report the safety and efficacy of the PRESERFLO in patients with refractory uveitic glaucoma 36 months after implantation.
Methods
Study design and participants
Retrospective, non-comparative, interventional case series of consecutive patients who underwent implantation of a PRESERFLO Microshunt augmented with MMC for the treatment of refractory uveitic glaucoma in a subspecialist glaucoma clinic, under the care of one surgeon (KB) from May 2016 to October 2017. All patients diagnosed with UG and uncontrolled IOP or with worsening visual field despite maximum tolerated medical treatment requiring glaucoma surgery were consecutively included in the analysis. This study was approved by the audit committee of Moorfields Eye Hospital and adhered to the tenets of the Declaration of Helsinki. All subjects provided written informed consent for the surgical procedure.
Eligible patients underwent a complete preoperative ophthalmic examination, including medical history, slit-lamp examination, gonioscopy, and IOP measurement with Goldmann applanation tonometry and could be either phakic or pseudophakic. Refractory uveitic glaucoma was defined as uncontrolled IOP or visual field progression despite maximum tolerated medical treatment in patients with past history of anterior, intermediate, and/or posterior uveitis. If both eyes of the same patient were eligible for inclusion, the first implanted eye was included.
Surgical technique
Surgery was performed under local anaesthesia. A superior conjunctival peritomy was performed and Tenon’s dissected creating a fornix-based conjunctival flap. Three MMC-soaked circular LASIK shields were placed into the sub-Tenon’s space for 3 min after which the MMC was irrigated with 20 ml of balanced salt solution. Haemostasis was achieved with gentle diathermy around the planned site of scleral entry. The sclera was marked 3 mm behind the limbus and a 1 mm-wide lamellar scleral tunnel fashioned from the 3 mm mark to the limbus. The anterior chamber (AC) was entered using a 25-gauge needle.
The Microshunt was inserted into the tunnel and AC such that its fins self-retained in the scleral tunnel to secure its position. Tenon’s capsule and conjunctiva were reapposed and secured with 10-0 nylon (Ethicon, Johnson and Johnson). In cases combined with cataract extraction, the conjunctival flap, MMC application and washout were performed first, followed by phacoemulsification and intraocular lens implant, then Microshunt implantation and conjunctiva-Tenon’s closure.
Postoperatively, all patients received topical dexamethasone 0.1%, 2 hourly tapering over 3 months to their baseline steroid regimen, and topical chloramphenicol 0.5%, 4 times daily for 1 month. Ocular hypotensive medication was discontinued after surgery in the operated eye.
Data collection
Baseline demographic and clinical information were collected for enrolled patients. Follow-up examinations were performed at 1 day, 1 week, and 1, 3, 6, 12, 24, and 36 months postoperatively. For each visit, best-corrected visual acuity, intraocular pressure, number of glaucoma medications, adverse events, and need/type of further medical or surgical treatment were collected. The re-introduction of IOP-lowering medication or the need of further surgical procedures was at the discretion of the investigator on the basis of target IOP and glaucoma severity.
Outcome measures
The primary outcome measure was the cumulative rate of surgical success at 36 months. Secondary outcome measures included IOP, visual acuity (VA), use of glaucoma medical therapy, and surgical complications.
Evaluation of the cumulative rate of surgical success was made using the criteria employed in the Primary Tube Versus Trabeculectomy (PTVT) study [20].
Surgical success was defined as IOP between 5 and 21 mmHg, or more than 20% reduction from baseline at 2 consecutive follow-up visits after 3 months, and without criteria for failure. Conversely, failure was defined as IOP more than 21 mmHg or less than 20% reduction below baseline at 2 consecutive follow-up visits after 3 months, IOP of 5 mmHg or less at 2 consecutive follow-up visits after 3 months, the need for further glaucoma surgery, or loss of light perception vision. Further glaucoma surgery was defined as intervention requiring a return to the operating theatre, including revision surgery, insertion of a further drainage implant or cyclodestructive procedures. Subconjunctival injections and needling procedures at the slit lamp were not categorized as failures. Eyes that had not failed by the above criteria and were not receiving supplemental medical therapy were considered complete successes. Patients who required supplemental medical therapy were categorized as qualified successes.
Early postoperative complications were defined as those occurring within the first month after surgery and late complications were defined as those occurring after the first postoperative month. Complications developing during the first month and persisting with longer follow-up were counted only as early postoperative complications. Cataract was considered to have progressed if there was more than 2 Snellen lines loss that was attributable to cataract at the 6-month follow-up visit or thereafter, or if cataract surgery was performed.
Statistical analysis
Statistical analysis was performed with SPSS Statistics Version 26 (IBM, Armonk, New York, USA). Outcomes were reported in accordance with the Guidelines on Design and Reporting of Glaucoma Surgical Trials published by the World Glaucoma Association.
The sample size of the study was calculated to be appropriate for a power of 75% and type I error rate (α) of 5%. Frequency histograms were used to assess the distribution of all variables. All variables are presented as mean ± standard deviation (SD) or mean and 95% confidence interval (CI). P-value < 0.05 was considered statistically significant.
The Kaplan-Meier life-table analysis was performed to calculate the overall cumulative rate of surgical success with 95% confidence interval. The Log-Rank (Mantel-Cox) and the Gehan-Breslow-Wilcoxon tests were both performed in order to evaluate whether the rate of surgical success was affected by early follow up time points.
Postoperative IOP and number of medications were assessed over time using a repeated-measures analysis of variance (ANOVA). Eyes were analysed on an intention-to-treat basis and those that required subsequent glaucoma surgery were not excluded from analysis.
Results
Baseline findings
21 eyes of 21 patients were eligible for analysis. The baseline findings are shown in Table 1. More than 70% were of non-White ethnicity, predominantly males, and the majority were phakic at the time of implantation.
Table 1.
Baseline Demographic Data.
| Variable | Value |
|---|---|
| No. of patients | 21 |
| No. of eyes | 21 |
| Age in years at the time of surgery, mean ± SD (range) | 49.3 ± 17.3 (17–84) |
| Follow-up time, mean ± SD (range) months | 30.9 ± 8.0 (17–45) |
| Ethnicity, number of patients (%) | |
| • Asian or Asian Indian | 9 (43%) |
| • Black (African or Caribbean) | 6 (29%) |
| • White British | 4 (19%) |
| • Mixed/Other | 2 (9%) |
| Gender, number of patients (%) | |
| • Male | 15 (71%) |
| • Female | 6 (29%) |
| Uveitis Diagnosis, number of patients (%) | |
| • Idiopathic | 11 (52%) |
| • Posner-Schlossman Syndrome | 2 (10%) |
| • Sarcoid/TB | 2 (10%) |
| • Other (including HSV, HLA-B27, and HLA-B51-related uveitis, Fuchs’ Heterochromic Iridocylcitis) | 6 (18%) |
| Pre-Operative Intraocular Pressure (mmHg), mean ± SD (range) | 26.0 ± 9.0 (12–45) |
| Number of Pre-Operative Glaucoma Medications, mean ± SD (range) | 4.1 ± 0.9 (2–5) |
| Lens status at the time of surgery, number of eyes (%) | |
| • Phakic | 16 (76%) |
| • Pseudophakic | 5 (24%) |
| Concentration of Intraoperative Mitomycin-C, number of eyes (%) | |
| • 0.1 mg/ml | 2 (10%) |
| • 0.2 mg/ml | 18 (86%) |
| • 0.4 mg/ml | 1 (4%) |
All patients had inactive uveitis at the time of surgery, with an inflammation-free period between 4 and 10 weeks. Some patients were on topical/systemic steroids to keep their condition inactive, as listed below: 11 out of 21 patients had no topical nor systemic steroid/immunosuppressant treatment, 6 patients were on topical prednisolone acetate 1% eye drops (average ± SD times daily 3.0 ± 1.1), 1 patient was on oral prednisolone 5 mg daily without topical treatment, 2 patients were on both systemic and topical steroid (oral prednisolone 5 mg daily + prednisolone acetate 1% eye drops 4 times daily), 1 patient was on topical prednisolone acetate 1% eye drops 3 times daily + oral mycophenolate 250 mg daily.
Two eyes had Microshunt implantation combined with cataract extraction, whereas the remainder underwent standalone implantation. Only one case was treated intra-operatively with a high MMC concentration (0.4 mg/ml), while the rest had MMC ≤ 0.2 mg/ml. 9 out of 21 (42%) patients were on oral acetazolamide before surgery.
Overall cumulative rate of surgical success and possible predictive factors of surgical failure
Figure 1 shows overall cumulative rate of surgical success after Microshunt implant in patients with uveitic glaucoma. The mean cumulative probabilities of success (95% confidence interval) were 0.74 (0.48–0.88) at 6 months, 0.68 (0.43–0.84) at 1 year and 0.47 (0.25–0.67) at 2 years and also at 3 years, respectively. Out of the group defined as surgical success, all patients met the criteria for complete success apart from one patient, who was defined as qualified success after re-introduction of one topical IOP-lowering medication because of IOP above target at the month 6 post-operative visit.
Fig. 1. Kaplan-Meier survival curve for overall cumulative surgical success.
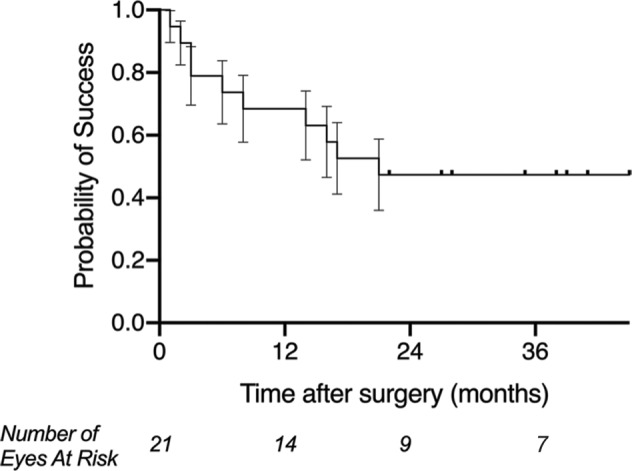
Cumulative surgical success after Preserflo Microshunt insertion in patients with uveitic glaucoma. Plotted is the Kaplan-Meier life-table analysis for each eye with the probability of success vs follow-up time (error bars represent the standard error). Mean cumulative probabilities of success (95% confidence interval) were 0.74 (0.48–0.88) at 6 months, 0.68 (0.43–0.84) at 1 year and 0.47 (0.25–0.67) at 2 years at respectively.
We observed a significant association between patients’ ethnicity and the overall cumulative rate of surgical success.
There was a statistically significant difference between White and non-White patients in the survival curves using both the Log-Rank (Mantel-Cox) and the Gehan-Breslow-Wilcoxon tests (p = 0.02), as shown in Fig. 2. The rate of surgical failure was significantly higher in patients of non-White ethnicity (Hazard Ratio 45.0 (95% CI: 1.3–18.8)).
Fig. 2. Kaplan-Meier survival curve for overall cumulative surgical success- impact of ethnicity.
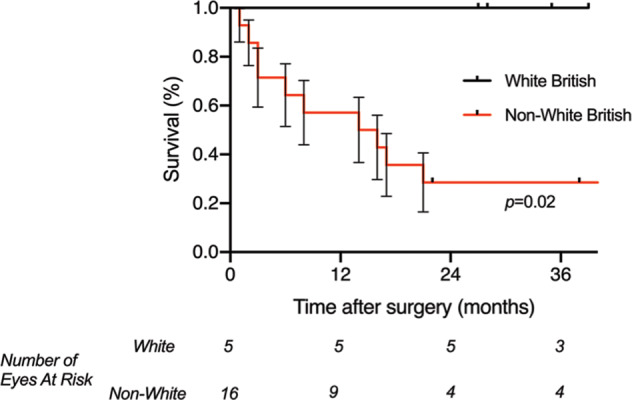
A comparison of the cumulative surgical success after Preserflo Microshunt insertion in patients with uveitic glaucoma, between patients of White British and non-White British ethnicity. Plotted is the Kaplan-Meier life-table analysis for each eye with the probability of success vs follow-up time (error bars represent the standard error). There is a statistically significant difference between the survival curves using both the Log-Rank (Mantel-Cox) and the Gehan-Breslow-Wilcoxon tests (p = 0.02). The rate of surgical failure is significantly higher in patients of non-White British ethnicity (Hazard Ratio 45.0 (95% CI: 1.3–18.8)).
No statistically significant difference was found in the overall cumulative rate of surgical success between phakic and pseudophakic eyes at baseline, either using the Log-Rank (Mantel-Cox) (p = 0.69) and the Gehan-Breslow-Wilcoxon tests (p = 0.73) (Fig. 3).
Fig. 3. Kaplan-Meier survival curve for overall cumulative surgical success- impact of prior cataract surgery.
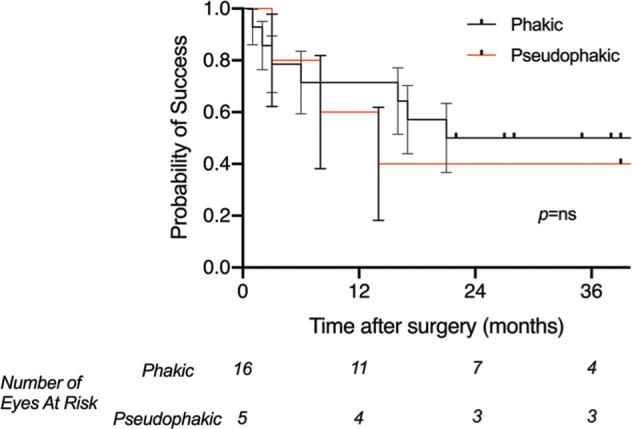
A comparison of the cumulative surgical success after Preserflo Microshunt insertion in patients with uveitic glaucoma, between phakic patients and those who have undergone prior cataract surgery. Plotted is the Kaplan-Meier life-table analysis for each eye with the probability of success vs follow-up time (error bars represent the standard error). No significant difference between the survival curves for each group was found using the Log-Rank (Mantel-Cox) (p = 0.69) and the Gehan-Breslow-Wilcoxon tests (p = 0.73).
Intraocular pressure, IOP-lowering medications, topical and systemic steroids and/or immunomodulators
Mean (±SD) IOP (mmHg) decreased significantly from 26.0 ± 9.0 at baseline to 15.2 ± 5.4 at the final follow-up visit (p = 0.0005). Similarly, the number of IOP-lowering medications decreased significantly from 4.1 ± 0.9 at baseline to 0.9 ± 1.2 at 3 years (p = 0.0005). Conversely, the visual acuity (logMAR) did not change significantly over time (0.18 ± 0.3 preoperatively, 0.16 ± 0.34 at 3 years, p = 0.73).
Figure 4 shows scatterplots relative to the relationship between preoperative and postoperative IOP values at month 6, year 1, 2, and 3, respectively.
Fig. 4. Intraocular pressure over follow-up.
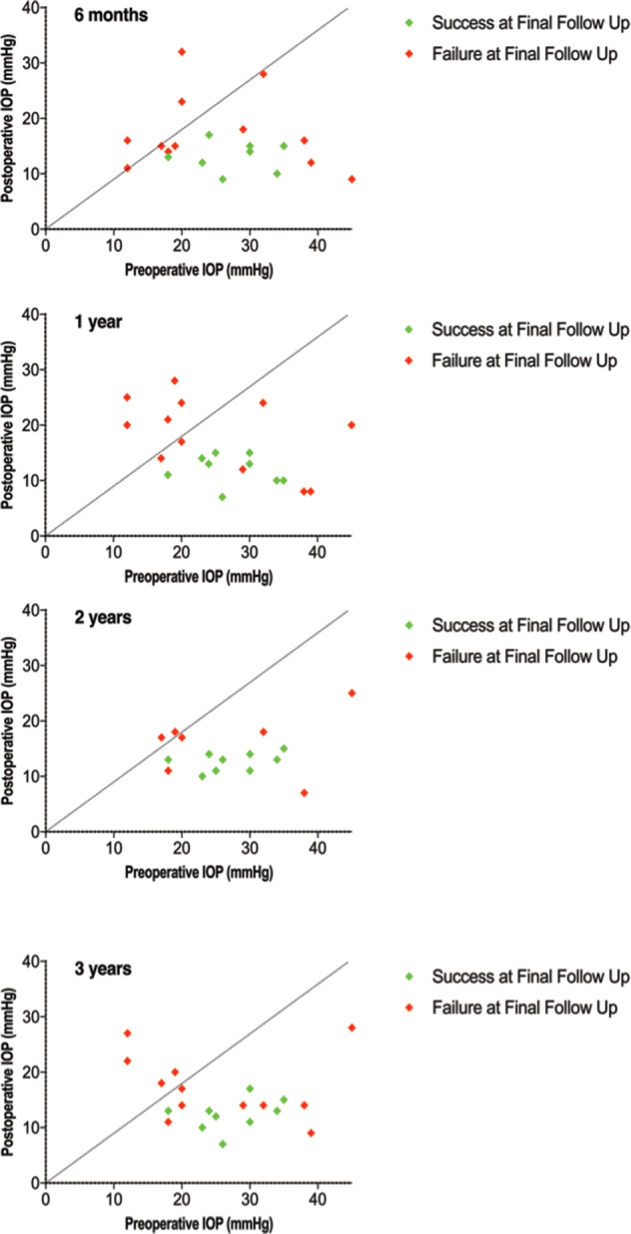
Scattergram of preoperative vs postoperative intraocular pressure at 6 months, 1 year, 2 year and 3 years after surgery. Each point represents one individual study eye. Eyes classified as success at final follow up are indicated by a green point with eyes classified as failure at final follow up are indicated by a red point. The dotted line represents equivalence; that is, points below the line show a reduction in IOP and points above show an increase.
Compared to baseline values, the overall mean IOP decreased by 30.7% (95%CI 13.7–47.7), 26.5% (95%CI 3.2–49.8), 33.5% (95%CI 21.8–45.3), and 30.1% (95%CI 7.2–52.9) at postoperative month 6, 12, 24, and 36, respectively (p < 0.001). Of eyes classified as successful at final follow-up, the mean IOP reduction from baseline was 53.4% (95%CI 43.3–63.6), three-quarters (9/12 eyes) were medication free and considered complete successes. Eyes in this group used a mean of −3.8 fewer medications at final follow-up compared to before surgery (95% CI −4.5 to −3.0).
Patients’ ethnicity seemed to play a role in the reduction of IOP and number of IOP-lowering medications from baseline. White and non-White patients had 60.4% (95%CI 50.3–70.5) and 20.6% (95% CI −8.4 to 49.5) final IOP reduction from baseline, respectively (p < 0.001). The final number of IOP-lowering medications was 0.0 ± 0.0 in the White British group, and 1.13 ± 1.26 in the non-White British group (p = 0.003).
The overall average ± SD number of steroid eye drops did not change significantly from baseline to the final follow-up appointment (1.32 ± 1.75 and 1.13 ± 1.70, respectively; p = 0.21).
On average, more patients in the failure group than in the success group required either topical and/or systemic anti-inflammatory treatment both at baseline and at the final follow up visit (7 vs. 3 patients, respectively, numbers unchanged for both groups from baseline to 36 months). The baseline average ± SD number of steroid drops was 1.11 ± 1.69 and 1.58 ± 1.83 for success and failure groups, respectively (p = 0.55). The final average ± SD number of steroid drops was 0.78 ± 1.39 and 1.18 ± 1.53 for success and failure groups, respectively (p = 0.54).
Complications
Overall, there were no cases of significant hyphaema (>10% of the anterior chamber). One patient only had less than 10% of the anterior chamber hyphaema on day 1, which resolved completely within one week after prescribing topical cycloplegic treatment (atropine 1% eye drops once nightly for 1 week). Recurrent anterior uveitis occurred in 4 out of 21 eyes (19.0%), and in 2 of these cases it was associated with cystoid macular oedema (CMO) requiring a Dexamethasone 700 micrograms intravitreal implant (Ozurdex). These 2 patients already had a pre-operative history of CMO due to their baseline condition. One eye showed early (postoperative week 1) exposure of the Microshunt through a conjunctival buttonhole.
No bleb-related infection was recorded during the 36-month follow up.
Glaucoma re-operation and cataract extraction
Of the eyes classified as failures, all were based on the requirement for further glaucoma surgery. Over the 3-year follow-up period, 12 out of 21 eyes (57.1%) required re-intervention for glaucoma. Eight eyes showed significant subconjunctival fibrosis, 1 required early revision for exposure through a conjunctival buttonhole and 3 Microshunts did not demonstrate any aqueous outflow at revision surgery and were replaced.
In 8 out of 12 eyes, the revision surgery consisted of re-opening of the conjunctiva and Tenon’s capsule, application of MMC, removal of sub-Tenon’s fibrotic tissues, re-establishment of aqueous outflow through the Microshunt (as mentioned above, that was not possible in 3 eyes, in which cases the Microshunts were replaced), and closure of the Tenon’s capsule and conjunctiva with 10-0 nylon sutures. Four out of 12 eyes required a Baerveldt Glaucoma Implant. Three out of 21 eyes (14.3%) needed cataract extraction during the 36-month follow-up.
Discussion
In this exploratory interventional case series, we report the 3-year efficacy and safety of the PRESERFLO Microshunt implant in the treatment of uveitic glaucoma recalcitrant to maximum tolerated medical treatment. An overall cumulative rate of surgical success of 47.0% was reported after 36-months. The overall mean IOP decreased by about 30% from baseline at this time-point, whereas the final number of IOP-lowering medications dropped by an average of 3.8 medications from baseline. Furthermore, the safety profile was favourable, with no cases of sight-threatening complications, such as loss of vision, hypotony with sequelae, and bleb-related ocular infections.
After 36-months, 47% eyes were classified as surgical successes according to the Tube Versus Trabeculectomy (TVT) and Primary TVT (PTVT) studies criteria [20, 21]. 42.9% eyes were free of IOP-lowering medications at the end of follow up, and therefore considered complete successes.
The success rate reported here is lower than that of two case series of PRESERFLO Microshunt implantation in POAG patients. Batlle et al. reported a complete success rate (defined as IOP ≤ 14 mmHg and IOP reduction ≥ 20%) of 64% and a qualified success rate of 95% at three years in 23 eyes of POAG patients [22]. Schlenker et al. published a larger series of 132 POAG patients who underwent Microshunts implantation. They reported a 76.9% complete success and 92.5% qualified success at one year [23].
Slightly lower success rates were recently published by Bhayani et al. [24]. In this study, the authors reported 74% and 58% qualified and complete success rates (respectively) 12 months after Preserflo implant in patients with mixed forms of glaucoma (70% POAG), according with the Criterion-A for definition of surgical success by the World Glaucoma Association consensus guidelines (IOP ≤ 21 and reduction ≥20% from baseline).
The criteria for defining surgical success (either complete or qualified) employed by Battle et al. and Schlenker et al. are different, using 14 mmHg and 17 mmHg as upper limit for success, respectively. Failure was not specifically defined in either series. In this regard, we thought it was reasonable to use the same criteria for surgical success as in previous randomized controlled clinical trials (RCT), such as the TVT study and the PTVT study [20, 21]. Although we used a higher IOP of 21 mmHg as upper limit of success, patients in our series who qualified as surgical success had a final mean ± SD IOP less than 17 mmHg (12.3 ± 2.9, analysis not shown).
If more similar criteria for surgical success compared to ours were used, Bhayani et al. found lower success rates than Battle et al. and Schlenker et al., although still higher than ours. However, it is important to notice that the abovementioned studies were carried out totally (Battle et al. and Schlenker et al.) or predominantly (Bhayani et al.) on POAG patients.
In addition to the baseline diagnosis of uveitic glaucoma that may increase the risk of surgical failure [13, 15], in our series, ethnicity appeared to play an important part on the rate of surgical failure with 100% success in whites and 25% success in non-white ethnicity at 3 years. We previously reported a similar series of 24 XEN Gel Implants in uveitic glaucoma, observing a success rate of 79.2% with 62.5% medication free at 1 year which is also higher than our success rate of 68% at one year [18]. However, in that study the patients were predominantly white (Sng CC, personal correspondence), so a direct comparison with regards to ethnicity between the study reported here and that by Sng et al. may be inaccurate.
On the other hand, and similarly to our study, Bhayani et al. in their recent report found that, even in an heterogenous cohort of patients (70% POAG, 13% pseudoesfoliative glaucoma, 17% pigmentary, uveitic, normal tension, angle-closure glaucoma), non-Caucasian ethnicity was the only variable significantly associated with increased risk of failure at 1 year after Preserflo Microshunt implant [24].
Second, aqueous humour and subconjunctival tissues in UG eyes differ from POAG eyes which could explain higher rate of filtering surgery failure [25, 26]. This explains the popular preference for tube shunt surgery rather than trabeculectomy in UG eyes [27, 28].
However, in the present case series the uveitis status did not seem to affect the final surgical outcome, given that all patients had their surgery in a period of uveitis inactivity, their uveitis status did not change significantly during follow up between success and failure groups, and no significant differences were found between success and failure groups in the number of topical/systemic steroid/immunosuppressant medications both at baseline and at the end of follow up. The only difference that we found is that, on average, more patients in the failure group compared to the success group required steroid/immunosuppressant treatment to keep their condition inactive both at baseline and at 36 months (7 vs. 3, respectively). While such small numbers do not allow to perform an appropriate statistical analysis, it may be possible to speculate whether patients ending up failing had more aggressive baseline condition requiring stronger anti-inflammatory treatment.
Third, both Batlle and Schlenker’s series used a higher dose of MMC than our series: 0.4 mg/ml versus 0.2 mg/ml. 31.1% eyes in the study by Schlenker et al. were treated with intraoperative MMC concentration of 0.2 mg/ml, while the vast majority received a higher dose (0.4 or 0.5 mg/ml). Conversely, virtually all of our patients had intraoperative MMC exposure of 0.2 mg/ml irrespective of ethnicity which was our standard practice at the time of the enrolled procedures. This concentration was consistent with that used in the randomized controlled trial on the efficacy of MicroShunt versus trabeculectomy. (clinicaltrials.gov, trial No. INN-005 (NCT01881425)) Interestingly, in the study by Schlenker, an MMC concentration of 0.2 mg/ml represented a significant risk factor for failure on multivariable analysis [23].
The advent of less invasive glaucoma surgery has increased interest in these procedures for treating several types of uncontrolled glaucoma, other than POAG. The Microshunt implant augmented with MMC, although similar to the trabeculectomy in terms of the ab externo approach, can be considered a less-invasive procedure, considering its less tissue invasive nature and faster visual recovery [29]. Before the PRESERFLO implant was introduced into the market, Xen-45 collagen implant was the only commercially available bleb-forming MIGS. We have previously reported 12-month outcomes of Xen-45 implant in the treatment of UG [18]. Although the follow-up was short and sample size small, our results were surprisingly encouraging for a MIGS procedure considering that filtering surgery in UG tends to be less effective. The Xen gel implant was designed as an ab interno procedure, avoiding opening of the conjunctiva-Tenon’s capsule. Consequently, the sub-Tenon’s capsule and sub-conjunctival fibrosis occurred quite frequently. We reported a 41.7% rate of bleb needling and 20.8% of bleb revision at 12 months, i.e., a 60% rate of bleb manipulation in order to achieve functioning blebs.
In contrast, while a significant number of patients required MicroShunt revision in our series (8/21), none underwent bleb needlings. The MicroShunt is implanted via an ab externo technique and hence positioned under Tenon’s in every case, in contrast to the XEN which must be positioned superficially in order to avoid obstruction with Tenon’s as the ab interno implantation technique does not permit precise manipulation of the XEN into the sub-tenon’s space free of Tenon’s obstruction. The deeper positioning of the MicroShunt relative to the XEN renders effective needling less likely and for this reason, no bleb needlings were performed in this cohort of patients. In the study by Schlenker, a relatively low proportion of POAG eyes (8.5%) implanted with PRESERFLO underwent needling within 1 year [23].
In the present study, a 57% overall rate of reoperation was reported. The majority of reoperations were Microshunt revisions. 8 out of 21 eyes (38.1%) were revised revealing sub-conjunctival fibrosis occluding the Microshunt. In 1 out of 21 eyes (4.8%), the Microshunt was revised early (1 week) due to extrusion through a conjunctival buttonhole. In this case, the probable cause of erosion was a partial tenonectomy that was performed during the original surgery.
3 out of 21 eyes in the present study required early revision because of lack of IOP lowering in the early postoperative period. In all 3 cases, no aqueous flow was visualized through the Microshunt after removing fibrous tissue. In all 3 cases, flow was re-established by flushing the Microshunt with balanced salt solution via a thin walled 23 gauge (G) cannula. All 3 eyes were controlled and medication free at final follow-up. Overall, 4 of the 8 revised were controlled without IOP-lowering medication or further surgery at 3 years, whereas 4 were controlled after further Baerveldt Glaucoma Implant insertion (2 after 6 months, 1 at one year and 1 after 2 years).
We observed a good safety profile in this study. No patient suffered vision loss, hypotony requiring intervention (such as anterior chamber (AC) reformation or surgical revision), or bleb-related infection. The Microshunt appears to be a safe surgical option for patients with uncontrolled uveitic glaucoma. Although we previously reported good efficacy with the Xen Gel Implant in uveitic glaucoma, approximately 20% of eyes requiring AC reformation with viscoelastic for transient hypotony and AC shallowing [18]. Although PRESERFLO microshunts have a larger lumen size as compared to the XEN gel implants (PRESERFLO: 75 um; XEN: 45 um), they are made of different material (SIBS versus collagen) and of different length (PRESERFLO: 8.5 mm; XEN: 6 mm). While luminal diameter changes have a greater effect on flow than length, the Preserflo is also hydrophobic and the Xen hydrophilic. As mentioned above, the ab externo technique of PRESERFLO implantation permits more precise positioning under Tenon’s than the ab interno implantation of XEN. In addition, the XEN needle track (25 G) is larger than the XEN Gel Implant itself (27 G) resulting in early peritubular flow and more transient hypotony than with the Microshunt. This effect also varies according to tunnel length which is more variable with ab interno implantation. The level of hypotony that we have observed after PRESERFLO implantation in UG seems consistent with that reported in POAG, which is an important finding, given that uveitic patients have a higher tendency to hypotony after trabeculectomy than do POAG patients in general [22, 23]. The very low rate of sight-threatening complications following microshunt implants confirms its safety relative to other bleb forming procedures [28].
Our study is limited by the small sample size, the lack of comparative arms, and the high attrition rate. Further comparative studies with larger sample size, including larger number of individuals of different ethnicities, are ideal to confirm our results. Although we presented 36-month outcomes following the Microshunt implant, longer follow up would allow to report later complications such cataract formation or glaucoma progression, which was not an outcome measure in the present study.
In conclusion, the present study provides novel data on the clinical efficacy and safety profile of the Preserflo Microshunt implant in the treatment of uveitic glaucoma. Our findings suggest that PMS is an effective and highly safe option for the treatment of medically uncontrolled uveitic glaucoma.
Summary
What was known before
Short-/Mid-term safety and efficacy outcomes of Preserflo Microshunt in primary open angle glaucoma.
What this study adds
Mid-term safety and efficacy outcomes of Preserflo Microshunt for the treatment of refractory uveitic glaucoma.
Author contributions
GT: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – Original draft, Writing – Review & Editing; JW: Conceptualization, Investigation; Soledad SA-M: Methodology, Investigation, Data curation; HJ: Formal analysis, Writing – Review & Editing; KB: Conceptualization, Writing – Review & Editing; Supervision.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
The data that support the findings of this study are available from the corresponding author, KB, upon reasonable request.
Competing interests
Keith Barton lectured and consulted for Santen, receiving compensation. The rest of co-authors declare no competing financial interests related to the topic of this paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al-Ani HH, Sims JL, Tomkins-Netzer O, Lightman S, Niederer RL. Vision loss in anterior uveitis. Br J Ophthalmol. 2020;104:1652–7. doi: 10.1136/bjophthalmol-2019-315551. [DOI] [PubMed] [Google Scholar]
- 2.Aman R, Engelhard SB, Bajwa A, Patrie J, Reddy AK. Ocular hypertension and hypotony as determinates of outcomes in uveitis. Clin Ophthalmol. 2015;9:2291–8. doi: 10.2147/OPTH.S90636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sng CCA, Ang M, Barton K. Uveitis and glaucoma: new insights in the pathogenesis and treatment. Prog Brain Res. 2015;221:243–69. doi: 10.1016/bs.pbr.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Kelly SR, Montesano G, Bryan SR, Barry RJ, Keane PA, et al. Evaluating the Impact of Uveitis on Visual Field Progression Using Large-Scale Real-World Data. Am J Ophthalmol. 2019;207:144–50. doi: 10.1016/j.ajo.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Din NM, Talat L, Isa H, Tomkins-Netzer O, Barton K, Lightman S. Difference in glaucoma progression between the first and second eye after consecutive bilateral glaucoma surgery in patients with bilateral uveitic glaucoma. Graefe’s Arch Clin Exp Ophthalmol. 2016;254:2439–48. doi: 10.1007/s00417-016-3460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho CL, Wong EYM, Walton DS. Goniosurgery for glaucoma complicating chronic childhood uveitis. Arch Ophthalmol (Chic, Ill 1960) 2004;122:838–44. doi: 10.1001/archopht.122.6.838. [DOI] [PubMed] [Google Scholar]
- 7.Heinz C, Koch JM, Zurek-Imhoff B, Heiligenhaus A. Prevalence of uveitic secondary glaucoma and success of nonsurgical treatment inadults and children in a tertiary referral center. Ocul Immunol Inflamm. 2009;17:243–8. doi: 10.1080/09273940902913035. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Wang J, Fortin E, Hamel P. Trabeculotomy in the Treatment of Pediatric Uveitic Glaucoma. J Glaucoma. 2016;25:744–9. doi: 10.1097/IJG.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 9.Ceballos EM, Beck AD, Lynn MJ. Trabeculectomy with antiproliferative agents in uveitic glaucoma. J Glaucoma. 2002;11:189–96. doi: 10.1097/00061198-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kaburaki T, Koshino T, Kawashima H, Numaga J, Tomidokoro A, Shirato S, et al. Initial trabeculectomy with mitomycin C in eyes with uveitic glaucoma with inactive uveitis. Eye. 2009;23:1509–17. doi: 10.1038/eye.2009.117. [DOI] [PubMed] [Google Scholar]
- 11.Papadaki TG, Zacharopoulos IP, Pasquale LR, Christen WB, Netland PA, Foster CS. Long-term results of Ahmed glaucoma valve implantation for uveitic glaucoma. Am J Ophthalmol. 2007;144:62–69. doi: 10.1016/j.ajo.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Ceballos EM, Parrish RK, Schiffman JC. Outcome of Baerveldt glaucoma drainage implants for the treatment of uveitic glaucoma. Ophthalmology. 2002;109:2256–60. doi: 10.1016/S0161-6420(02)01294-0. [DOI] [PubMed] [Google Scholar]
- 13.Borisuth NS, Phillips B, Krupin T. The risk profile of glaucoma filtration surgery. Curr Opin Ophthalmol. 1999;10:112–6. doi: 10.1097/00055735-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Krishna R, Godfrey DG, Budenz DL, Escalona-Camaano E, Gedde SJ, Greenfield DS, et al. Intermediate-term outcomes of 350-mm(2) Baerveldt glaucoma implants. Ophthalmology. 2001;108:621–6. doi: 10.1016/S0161-6420(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 15.Landers J, Martin K, Sarkies N, Bourne R, Watson P. A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology. 2012;119:694–702. doi: 10.1016/j.ophtha.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Chaku M, Bajwa A, Lee JK, Netland PA. Treatment of Uveitis and Outcomes of Glaucoma Drainage Implant Surgery: A Meta-Analysis. Ocul Immunol Inflamm. 2020;28:833–8. doi: 10.1080/09273948.2019.1629602. [DOI] [PubMed] [Google Scholar]
- 17.Kerr NM, Wang J, Barton K. Minimally invasive glaucoma surgery as primary stand-alone surgery for glaucoma. Clin Exp Ophthalmol. 2017;45:393–400. doi: 10.1111/ceo.12888. [DOI] [PubMed] [Google Scholar]
- 18.Sng CC, Wang J, Hau S, Htoon HM, Barton K. XEN-45 collagen implant for the treatment of uveitic glaucoma. Clin Exp Ophthalmol. 2018;46:339–45. doi: 10.1111/ceo.13087. [DOI] [PubMed] [Google Scholar]
- 19.Kerr NM, Wang J, Sandhu A, Harasymowycz PJ, Barton K. Ab Interno Gel Implant-associated Bleb-related Infection. Am J Ophthalmol. 2018;189:96–101. doi: 10.1016/j.ajo.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Gedde SJ, Chen PP, Heuer DK, Singh K, Wright MM, Feuer WJ, et al. The primary tube versus trabeculectomy study: Methodology of a multicenter randomized clinical trial comparing tube shunt surgery and trabeculectomy with Mitomycin C. Ophthalmology. 2018;125:774–81. doi: 10.1016/j.ophtha.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gedde SJ, Schiffman JC, Feuer WJ, Parrish RK, 2nd, Heuer DK, Brandt JD. The tube versus trabeculectomy study: design and baseline characteristics of study patients. Am J Ophthalmol. 2005;140:275–87. doi: 10.1016/j.ajo.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Batlle JF, Fantes F, Riss I, Pinchuk L, Alburquerque R, Kato YP, et al. Three-year follow-up of a novel aqueous humor microshunt. J Glaucoma. 2016;25:e58–65. doi: 10.1097/IJG.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 23.Schlenker MB, Durr GM, Michaelov E, Ahmed IIK. Intermediate outcomes of a novel standalone Ab Externo SIBS microshunt with Mitomycin C. Am J Ophthalmol. 2020;215:141–53. doi: 10.1016/j.ajo.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Bhayani R, Martinez de la Casa JM, Figus M, Klabe K, Rabiolo A, Mercieca K, et al. Short-term safety and efficacy of Preserflo™ Microshunt in glaucoma patients: A multicentre retrospective cohort study. Eye (Lond) 2022;12:1–6. doi: 10.1038/s41433-022-01995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broadway DC, Bates AK, Lightman SL, Grierson I, Hitchings RA. The importance of cellular changes in the conjunctiva of patients with uveitic glaucoma undergoing trabeculectomy. Eye (Lond) 1993;7:495–501. doi: 10.1038/eye.1993.108. [DOI] [PubMed] [Google Scholar]
- 26.Ni M, Chan CC, Nussenblatt RB, Li SZ, Mao W. Iris inflammatory cells, fibronectin, fibrinogen, and immunoglobulin in various ocular diseases. Arch Ophthalmol (Chic, Ill 1960) 1988;106:392–5. doi: 10.1001/archopht.1988.01060130418033. [DOI] [PubMed] [Google Scholar]
- 27.Chow A, Burkemper B, Varma R, Rodger DC, Rao N, Richter GM. Comparison of surgical outcomes of trabeculectomy, Ahmed shunt, and Baerveldt shunt in uveitic glaucoma. J Ophthalmic Inflamm Infect. 2018;8:9. doi: 10.1186/s12348-018-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinod K, Gedde SJ, Feuer WJ, Panarelli JF, Chang TC, Chen PP, et al. Practice preferences for glaucoma surgery: A survey of the American glaucoma society. J Glaucoma. 2017;26:687–93. doi: 10.1097/IJG.0000000000000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Do AT, Parikh H, Panarelli JF. Subconjunctival microinvasive glaucoma surgeries: An update on the Xen gel stent and the PreserFlo MicroShunt. Curr Opin Ophthalmol. 2020;31:132–8. doi: 10.1097/ICU.0000000000000643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, KB, upon reasonable request.


