Abstract
Purpose
In this study, we described a large family presenting different manifestations of cone dystrophy at different ages associated with GUCY2D gene mutation.
Method
Sixty-three individuals of a single kindred, including 23 affected with cone dystrophies, were recruited and received ocular examinations, including best corrected visual acuity, intraocular pressure, slit-lamp biomicroscopy, color fundus photograph (CFP), fundus autofluorescence, optical coherence tomography, fluorescence fundus angiography, color vision testing, full-field electroretinography, and electro-oculogram. Whole exome sequencing (WES) and Sanger sequencing were performed for underlying mutations associated with cone dystrophy.
Result
There were 23 affected family members. Clinical analysis showed that the proband and other patients had impaired visual acuity ranging from 20/800 to 20/50 with impaired color vision. Fundus photograph showed retinal pigment epithelium (RPE) granular abnormalities with depressed macular reflex in young patients and macular or retinochoriodal atrophy in older patients. OCT examination confirmed the reduced outer retinal thickness or inner retinal thickness, absence of the ellipsoid zone (EZ) and retinal atrophy to varying degrees. Electroretinography revealed a reduced cone response combined with a relatively maintained rod response. WES and Sanger sequencing revealed a heterozygous variant c.2512C>T in the GUCY2D gene of the affected family members.
Conclusions
We reported cone dystrophy in 23 affected individuals in a five-generation family and demonstrated different macular abnormalities in OCT scans and CFP at different ages. The multimodal ocular records in our study provide physicians and ophthalmologists with a better understanding of cone dystrophy associated with GUCY2D mutation.
Subject terms: Eye diseases, Eye manifestations
Introduction
Cone dystrophies (CODs) and cone-rod dystrophies (CORDs) are subgroups of inherited retinopathies that are characterized by degeneration of cones with the relative preservation of rod function [1]. The disease is characterized by an early loss of visual acuity and color discrimination associated with loss of cones from adolescence or in early adult life [2], followed by nyctalopia and progressive peripheral field loss as the rods subsequently degenerate [3]. These clinical features are typically accompanied by electroretinographic abnormalities that reveal early loss of photopic (cone) responses and later progressive loss of scotopic (rod) responses. Most CODs or CORDs are sporadic cases [4]. However, when an inheritance pattern can be reliably established, autosomal dominant inheritance is the most common, followed by autosomal recessive inheritance. Disorders of cone function can be divided into stationary and progressive patterns. Progressive cone dystrophies usually present in childhood or early adult life, with many patients developing rod photoreceptor involvement later in life [5]. Therefore, CODs have variable ophthalmoscopic appearances, the retina may appear normal or only temporal pallor of the optic disc in the early stage, and varying degrees of pigmentary disturbance or atrophy may be evident in later stages. Optical coherence tomography (OCT) and electroretinography (ERG) can detect early structural and functional abnormalities including thinning of the outer retinal layer [6], a discourteous ellipsoid zone (EZ), and severe attenuation or absence of photopic electroretinographic responses with normal scotopic electroretinographic responses [7]. The diagnosis of CODs is mainly based on clinical evaluation and ocular examination. ERG can confirm cone dysfunction much earlier than other imaging modalities; therefore, they are both an irreplaceable adjuvant diagnostic tool in the diagnosis and management of COD/CORD patients.
To date, more than 20 genes have been reported and associated with cone dystrophy in the literature [8]. GUCY2D is the major gene responsible for autosomal dominant progressive cone degeneration [9]. GUCY2D encodes the membrane-bound retinal guanylyl cyclase-1 protein (RetGC-1), which synthesizes the intracellular messenger of photoreceptor excitation cGMP and is regulated by intracellular Ca2+-sensor proteins named guanylate cyclase-activating proteins (GCAPs) [10]. This protein is expressed in both cone and rod photoreceptors but predominantly in the cone outer segments, and it has two functional domains, namely, protein kinase and guanylate cyclase [11]. Furthermore, different mutation sites in the GUCY2D gene can lead to different genetic diseases and are responsible for autosomal recessive Leber congenital amaurosis (LCA) [4], central areolar choroidal dystrophy (CACD) [12], CODs or CORDs [13].
In the present study, CODs were found in a five-generation Chinese family with 23 affected individuals, and we identified a recurrent c.2512C>T (p. Arginine 838 cysrine) mutation in GUCY2D (OMIM 600179). We presented multimodal ophthalmic findings in different stages of cone dystrophy at different ages to provide physicians and ophthalmologists with a better understanding of inherited cone dystrophies.
Method
Subjects
In this study, all the subjects in the family were identified through the proband attending the Ophthalmology Department at West China Hospital. This study was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of West China Hospital. All individuals taking part in this research received written informed consent. Complete history-taking, pedigree analysis and ophthalmic examinations were performed. All the family members who participated in the study received detailed ophthalmic examinations, including best corrected visual acuity (BCVA), intraocular pressure (IOP), slit-lamp biomicroscopy, color fundus photographs (CFP), fundus autofluorescence (FAF), optical coherence tomography (OCT), fluorescence fundus angiography (FFA) and color vision testing using pseudoisochromatic charts. Retinal electrophysiological examinations included full-field electroretinography (ERG) and electro-oculogram (EOG), which were performed according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standards 27.
Whole exome sequencing (WES) and Sanger sequencing
Twelve of the 23 patients and 6 unaffected family members had undergone molecular genetic investigation. Three patients and one unaffected family member received whole exome sequencing. The genomic DNA of the 18 individuals was isolated from peripheral blood using standard protocols and the Blood Genome Column Medium Extraction Kit (Kangweishiji, China) according to the manufacturer’s instructions. The extracted DNA samples were subjected to quality control using a Qubit 2.0 fluorimeter and electrophoresis with a 0.8% agarose gel for further protocols. Protein-coding exome enrichment was performed using xGen Exome Research Panel v2.0 (IDT, Iowa, USA), which consists of 429,826 individually synthesized and quality-controlled probes, targets a 39 Mb protein-coding region (19,396 genes) of the human genome and covers 51 Mb of end-to-end tiled probe space. Bidirectional direct Sanger sequencing was performed to validate the variant identified by next-generation sequencing. Genomic DNA was amplified by PCR using T3 Super PCR Mix, KAPA2G Robust Hotstart DNA Polymerase (2500 U) and GUCY2D-specific primers (Forward: ATGGCAACCCAGGTCTTCAG, Reverse: ACGGAGGCAGCATCTGTGTA) designed with Primer 3. The PCR conditions were as follows: 94 °C for 5 min of initial denaturation, followed by 30 cycles of amplification of 30 s at 94 °C, 30 s at 60 °C, and 45 s at 72 °C. High-throughput sequencing was performed by a MGISEQ-T7 series sequencer, and no less than 99% of the target sequences were sequenced. The sequencing process was performed by Beijing Chi Gene Translational Medicine Research Center Co., Ltd, 100875, Beijing. Raw data were processed by fastq for adapter removal and low-quality read filtering. The paired-end reads were performed using Burrows-Wheeler Aligner (BWA) to the Ensemble GRCh37/hg19 reference genome. Base quality score recalibration together with SNP and short indel calling was conducted using GATK. According to the sequence depth and variant quality, SNPs and indels were screened, and high-quality and reliable variants were obtained. The online system independently developed by Chi-gene (www.chigene.org) was used to annotate database-based minor allele frequencies (MAFs) and ACMG practice guideline-based pathogenicity of every yielded gene variant, and the system also provided serial software packages for conservative analysis and protein product structure prediction. The databases for MAFs annotation included 1000 genomes, dbSNP, ESP, ExAC, and Chi-gene in-house MAFs databases. As a prioritized pathogenicity annotation to ACMG guidelines, the OMIM, HGMD and ClinVar databases were used as conferences of pathogenicity of this variant.
Result
Clinical features
There were 23 patients out of 63 members in this large family, and 12 patients were further confirmed by genetic testing. None of the patients were blind at birth, but most of them had progressively reduced visual acuity from childhood according to the family history. All affected individuals demonstrated dramatically reduced visual acuity, color vision defects and photophobia in childhood. None of the patients complained of nyctalopia. The proband (IV-7) was a 24-year-old female who complained of gradual central vison loss in the past several years and was diagnosed with amblyopia in childhood. Ocular examinations were as follows: BCVA was 20/50 in both eyes, and IOP was 14 mmHg for oculus dexter (OD) and 15 mmHg for oculus sinister (OS). Slit lamp biomicroscopy showed a transparent cornea and normal anterior segment in both eyes. Color fundus photograph demonstrated slight PRE atrophy (RPE granular abnormalities) in the macular and depressed foveal reflex for both eyes (Fig. 1A, C), and FFA showed dot hyperfluorescence corresponding to PRE atrophy in CFP both in the early and late stages (Fig. 1B, D) without any leakage or expansion. Optical coherence tomography (OCT) of the proband showed thinning of the outer retina as well as inner retinal layers and loss of the ellipsoid zone in the macular area (Fig. 2A, B). Full-field flash-ERG showed diminished photopic activity and maintained scotopic activity, consistent with cone function impairment (Fig. 3B). The proband had abnormalities in color vision testing, and the findings mentioned above supported the diagnosis of cone dystrophy. Her aunt and her mother had similar complaints. Her 42-year-old aunt (III-24) had similar fundus images as the proband (Fig. 1E–H). However, her 60-year-old mother (III-14) had patchy retinal atrophy with proliferation of RPE in both eyes (Fig. 1I–N), and the FAF for the proband’s mother showed decreased autofluorescence in the atrophy area with a ring of increased autofluorescence at the edge of atrophy (Fig. 1J, M). The FFA confirmed retinal atrophy presenting “window” defects (revealing the choroid vessel) in the retinal pigment epithelium and RPE proliferation causing local hypofluorescence (Fig. 1K, N). Four patients received EOG, and the Arden ratio was normal in both eyes.
Fig. 1. Fundus imaging and scatter diagrams for ocular characteristics of COD patients carrying the GUCY2D mutation in this family.
The fundus photograph of the proband (A, C) and her aunt (E, G) showed subtle mottling of the RPE (RPE granular abnormalities) in the macular and depressed foveal reflex for both eyes, and FFA revealed dot hyperfluorescence corresponding to PRE atrophy in CFP (B, D, F, H). CFP for her mother revealed patchy retinal atrophy with proliferation of PRE in both eyes (I, L), and the FAF showed decreased autofluorescence in the area of atrophy with increased autofluorescence at the edge of atrophy (J, M). The FFA confirmed retinal atrophy presenting “window” defects and RPE proliferation causing focal hypofluorescence (K, N). The scatter diagrams displayed the older, the worse BCVA (O), the more advanced stage in OCT (P) and CFP (Q) among those family members.
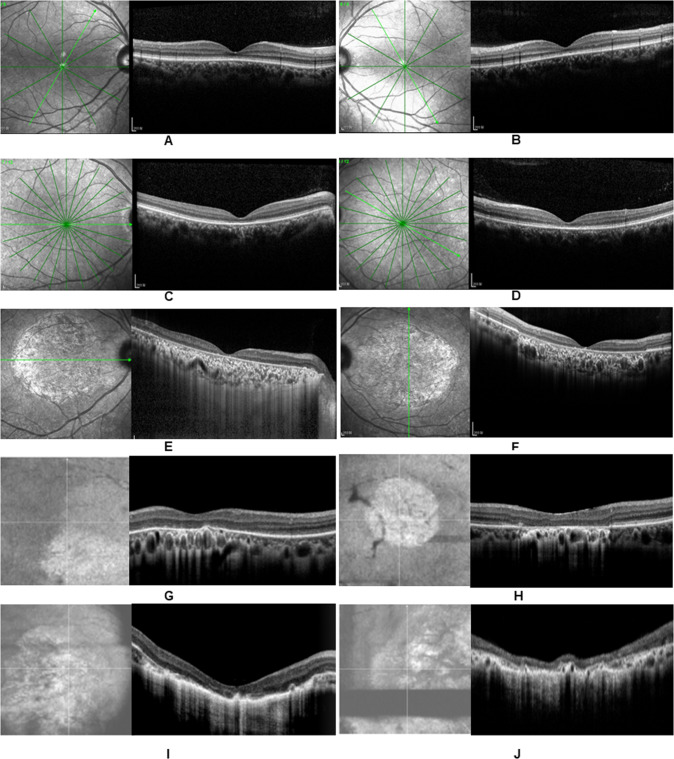
Fig. 2. OCT confirmed various degrees of retinal atrophy in the macula in these family members.
The proband had obvious thinning of the retinal layer and discontinuous ellipsoid zone (EZ) circumscribed in the macular fovea on both eyes (A, B), and the inner retinal thickness appeared normal. Her aunt (42 years old) had both decreased outer and inner retinal thickness (C, D). Her mother (60 years old) presented large-scale retinal atrophy with reduced retinal neuroepithelial thickness and a disappearance of the high reflection band for the RPE in the macula (E, F). Her other aunts (40 years old, 52 years old and 43 years old) had a larger lesion in the outer retinal layer and loss of ellipsoid zone (EZ) in the macula (G, H) as well as decreased inner retina thickness or atrophy of the RPE (I). Her 74-year-old grandfather had large-scale macular atrophy and extremely reduced retinal thickness and retinochoroid atrophy (J).
Fig. 3. Electroretinography, Sanger sequencing and pedigree in this family.
Dramatic decrease in amplitudes of both a- and b-waves on photopic electroretinogram and reduced 30 Hz photopic responses and normal scotopic responses for the proband (B) and her mother (C) compared with representative normal traces (A). A heterozygous variant c.2512C>T was identified in exon 13 of GUCY2D in the affected members. The chromatogram of the DNA sequence from the affected subjects showed both C and T at the indicated position (red arrows in E–G), and unaffected individual (IV-6) showed only C in this alle(D). Figure H shows the pedigree of this large family. Roman numerals indicate generations. Squares and circles represent males and females, respectively. Solid symbols indicate affected members, and empty symbols indicate unaffected individuals. The diagonal line indicates a deceased family member, and the arrow indicates the proband.
The other 20 affected individuals had impaired color vision and reduced BCVA ranging from 20/800 to 20/63 without nystagmus. OCT examination revealed various degrees of macular or retinochoriodal atrophy. Those patients with different ages presented different alterations on OCT, as displayed in Fig. 2. The youngest patient was 13 years old, and his fundus photograph appeared normal, while the OCT scan showed a slightly reduced outer retinal thickness with an impaired BCVA of 20/100. The oldest patient was 74 years old, and his fundus imaging demonstrated large-scale macular atrophy. An OCT scan showed extremely reduced retinal thickness and retinochoriodal atrophy (Fig. 2F). Alterations on OCT and color fundus images were more obvious in the older generations. We classified the progression of cone dystrophy into four stages on OCT and two types on CFP (Table 1). The main clinical and electrophysiological features of these patients are summarized in Table 2. We drew scatter diagrams to display the relationship between age and the severity of the disease. We can also roughly conclude from the graphs that the older patients had worse BCVA (Fig. 1O) and a more advanced stage on OCT (Fig. 1P) and CFP (Fig. 1Q) in this family. 80.8% (9 of 11 patients) had suffered low vision (BCVA between 0.05 and 0.3). All the patients over the age of 40 suffered greater vision loss and more severe structural damage on OCT.
Table 1.
Proposed stages and classifications for OCT and CFP.
| OCT staging | Abnormality of different stages in OCT |
| Stage 1 | Mild to moderate thinning of outer retinal thickness with or without discontinuity of ellipsoid band |
| Stage 2 | Moderate-severe thinning of outer retina with continuous ellipsoid band loss, but no RPE involvement |
| Stage 3 | the whole retinal thickness was severely decreased, accompanied by continuous loss of ellipsoid band, continuous damage of RPE |
| Stage 4 | Extensive atrophy of full-layer retina, RPE and choroid. |
| Classification in CFP | Abnormality in CFP |
| I | no obvious fundus changes or a few small yellow-white spots or granular pigment abnormalities in the macula |
| II | a well-defined degeneration or atrophy area, presenting as a “bull’s eye” or “fried cake” changes. |
Table 2.
Clinical characteristics and electrophysiological results in this family.
| NO. | Gender | Age (year) | VA for OD | VA for OS | GUCY2D Mutation | color vision | OCT stages | CFP subtypes | AMP for ERG | |
|---|---|---|---|---|---|---|---|---|---|---|
| Photopic response | Scotopic response | |||||||||
| II-2 | Male | 74 | 20/250 | 20/500 | + | AB | 4 | II | NP | NP |
| II-3 | Male | NA | NA | NA | + | NA | NP | NP | NP | NP |
| III-3 | Female | 55 | 20/500 | 20/500 | + | AB | 4 | II | NP | NP |
| III-6 | Male | 60 | 20/20 | 20/20 | − | N | N | N | N | N |
| III-12 | Female | 52 | 20/200 | 20/200 | + | AB | 3 | II | Decreased | N |
| III-14 | Female | 60 | 20/500 | 20/500 | + | AB | 3 | II | Decreased | N |
| III-16 | Female | 43 | 20/667 | 20/800 | + | AB | 4 | II | Decreased | N |
| III-18 | Female | 40 | 20/100 | 20/100 | + | AB | 4 | II | Decreased | N |
| III-24 | Female | 42 | 20/160 | 20/160 | + | AB | 2 | I | Decreased | N |
| III-29 | Female | 48 | 20/20 | 20/20 | − | N | N | N | N | N |
| IV-3 | Male | 20 | 20/63 | 20/200 | + | AB | 1 | I | Decreased | N |
| IV-5 | Female | NA | NA | NA | NA | NA | NP | NP | NP | NP |
| IV-6 | Male | 25 | 20/20 | 20/20 | − | N | N | N | N | N |
| IV-7 | Female | 24 | 20/50 | 20/50 | + | AB | 1 | I | Decreased | N |
| IV-9 | Male | 23 | 20/333 | 20/333 | + | AB | 2 | I | Decreased | N |
| IV-11 | Male | 13 | 20/100 | 20/100 | + | AB | 1 | I | Decreased | N |
| IV-12 | Female | 18 | NA | NA | NA | N | N | N | N | N |
| IV-13 | Female | NA | NA | NA | NA | AB | NP | NP | NP | NP |
| IV-14 | Female | NA | NA | NA | NA | AB | NP | NP | NP | NP |
| IV-16 | Male | NA | NA | NA | − | AB | NP | NP | NP | NP |
| IV-17 | Female | 7 | 20/25 | 20/25 | − | N | N | NP | NP | NP |
| V-1 | Female | 8 | 20/20 | 20/25 | − | N | N | NP | NP | NP |
VA visual acuity, OD oculus dexter, OS oculus sinister, OCT optical coherence tomography, CFP color fundus photographs, ERG electroretinogram, AMP amplitude, N normal, AB abnormal, NP not performed, NA not available, + is for positive GUCY2D mutation, − is for negative GUCY2D mutation.
Genetic result
Three of the affected patients (III-22, IV-3, IV-9) and one unaffected family member (IV-6) received whole exome sequencing. We screened the possible mutations that were related to the manifestations of retinal dystrophy and shared by all three patients. Then, we performed Sanger sequencing to identify the causative gene. We finally found the GUCY2D gene. Sanger sequencing analysis of GUCY2D detected a heterozygous mutation, c.2512C>T (p.R838C). and this mutation appeared in all 12 patients (Supplementary file 1) with cone dystrophies but did not appear in six unaffected family members (Fig. 3D–G). This mutation is a recurrent mutation in GUCY2D [14] for cone dystrophies (http://www.hgmd.org/), and it is co-segregated with the disease in all members in this five-generation family (Fig. 3H).
Discussion
In this study, we reported autosomal dominant cone dystrophy caused by a recurrent mutation in the GUCY2D gene in 23 affected individuals in a five-generation family. The GUCY2D gene, comprising 18 coding exons, encodes a protein containing 1103 amino acids that belongs to one of the isoforms of guanylate cyclase (retinal guanylyl cyclase-1 protein, RetGC-1). The guanylate cyclase protein, mostly located in the marginal region of the cone’s outer segments, plays an important role in restoring photoreceptor sensitivity due to its involvement in the synthesis of cyclic guanosine. Over 240 disease-causing mutations have been described so far in GUCY2D. Eighty-eight percent of these mutations cause autosomal recessive Leber congenital amaurosis (LCA, MIM 204000) [15], while heterozygous missense mutations cause autosomal dominant cone dystrophies or cone-rod dystrophies (CORD6, MIM 601777) [13]. In addition, the GUCY2D gene mutation is responsible for central areolar choroidal dystrophy (CACD, MIM 215500) [12] and congenital stationary night blindness [16] (CSNB, MIM618555). Usher syndrome [17] Senior-Loken, retinitis pigmentosa [18] and familial exudative vitreoretinopathy (FEVR) were also suspected to be associated with GUCY2D mutation and need to be further explored. Indeed, variants in GUCY2D are associated with a large disease spectrum. Sometimes, it is difficult to make the correct diagnosis, especially in the late stage of the disease. The fundus appearances in this family for older patients may also be considered central areolar choroidal dystrophy (CACD). However, photophobia and temporal pallor of the optic disc are usually absent in CACD. Although LCA mutations have been identified in several locations throughout the GUCY2D gene, no case has been reported localized to exon 13, and it seems likely that the mutations associated with LCA have a more severe effect on protein function than the mutations resulting in COD. On the other hand, some animal models have been established for exploring the underlying mechanisms for CODs [10, 11, 19], and the results suggested that alterations in the activity and affinity of the variants lead to reduced sensitivity of the protein to Ca2+ inhibition, which might be potential explanations for the different severities of different variants at codon 838 [20].
GUCY2D is a major gene responsible for autosomal dominant cone degeneration [9]. There are 34 variants in the GUCY2D gene causing COD or CORD (http://www.hgmd.cf.ac.uk/ac/), and almost all GUCY2D gene mutations identified so far in patients with CODs are located at codon 838 or the two adjacent codons 837 and 839. Moreover, the majority of the mutations in exon 13 are clustered at codon 838, in which five distinct variants have been identified [14] (c.2512C>T, p. R838C, c.2512C>A, p. R838S, c.2513G>A, p. R838H, c.2512C>G, p. R838G, c.2513G>C, p. R838P [21]). R838C was the most common mutation site, with a proportion of 58%, followed by R838H, with a proportion of 29%. It is important to identify the phenotype-genotype correlations in cone-rod dystrophies. Increased understanding of the varying phenotypes associated with different genetic mutations allows appropriate counseling of patients. Although the common feature of this disease is a mutation at codon 838, the families with additional mutations in the adjacent codons do seem to exhibit a severe phenotype with earlier onset and more pronounced rod involvement. These additional changes may have a cumulative effect on protein function [22, 23]. Other research suggested that the manifestation of R838C in patients is relatively mild, especially in young patients, and that the R838H variant can cause severe retinal features [13].
Our description of the manifestations of a large oriental family of Chinese origin with COD is in accordance with the typical manifestation of c.2512C>T (p. R838C) mutation in the GUCY2D gene [24]. In our five generations, the youngest was 13 years old, and the CFP was almost normal, but she had color vision abnormalities. The second youngest was 20 years old. Her retinal morphology on OCT showed slightly reduced outer retinal thickness, and the proband was 24 years old. She had more severe outer retinal thickness loss than her 20-year-old sister. The mother of the proband was 60 years old, and her OCT scan showed large-scale atrophy of the entire retinal thickness corresponding to macular patchy atrophy in the CFP. Her 74-year-old grandfather had extensive chorioretinal atrophy on OCT. All the affected participants had an almost normal rod response and decreased cone response in ERG, and rod dysfunction was not a predominant feature in this family, which was consistent with other reports [25]. Interestingly, we found her two aunts in their forties with different severities of COD in the OCT scan, which are shown in Fig. 2C, I. It has been reported that visual symptoms usually commence in childhood, and by the age of 40 years, most of them will have poor visual acuity. This is in accordance with previous reports in the manifestation of a large Caucasian family of British origin with CODs [24].
Fundus autofluorescence (FAF) imaging is a noninvasive modality that allows physicians to evaluate the healthy status of photoreceptor cells and the retinal pigment epithelium. An increased FAF signal is thought to reflect the abnormal accumulation of fluorophores, whereas a decreased FAF signal seems to result from atrophy of the RPE. The FAF for her 60-year-old mother had an annular increased FAF around the margin of the lesion on both eyes. In some patients, this hyperfluorescent ring around a dark macula indicated the cellular compensatory function of the RPE and expansion of macular atrophy. Fundus images are noninvasive and useful tools to monitor the progression of the disease. Two stages of cone dystrophy were recommended in CFP, and the lesions progressed from the early stage (faint granular abnormality) to the late or advanced stage (obvious macular atrophy). Optical coherence tomography (OCT), a quick and reproducible imaging technique to visualize cross-sectional images of the retina, has become one of the most valuable and powerful tools employed in the assessment of macular diseases. With the innovation of OCT, technological revolutions have promoted a better understanding of the nature of disease, and the outer five layers of the retina have been thoroughly studied. An American author reported detailed changes of four high reflectance bands in OCT for macular dystrophy patients [26] instead of the cone dystrophy associated with GUCY2D gene. And in our case, we classified the progression on OCT into four stages successively (shown in Table 1) in the five-generation members. We collected cross section ocular records and displayed varying manifestations in OCT. The older, the more severe alterations on OCT. As we only observed the different patients in different ages, it would be better if we could track this family for ophthalmic examinations every five years. In that way, we can clearly show the progression of the disease in each patients and provide ophthalmologists with detailed medical records.
In summary, we described diverse manifestations for cone dystrophy individuals at different ages with multimodal fundus examinations in a five-generation family. The ocular records of this family demonstrated different macular atrophy from fine pigment change in the early stages to advanced macular atrophy later in CFP and attenuation of the outer or inner retinal thickness and retinal atrophy in OCT. We believe this report provides physicians and ophthalmologists with a better understanding of cone dystrophy in GUCY2D mutation. In addition, increased understanding of the varying phenotypes associated with different genetic mutations allows appropriate counseling of patients.
Summary
What was known before
Cone dystrophies (CODs) and cone-rod dystrophies (CORDs) are subgroups of inherited retinal degenerations and rare diseases. GUCY2D is the major gene responsible for autosomal dominant progressive cone degeneration.
What this study adds
We described diverse clinical manifestations for cone dystrophy individuals of different ages with multimodal ophthalmic records in a large five-generation family. These ocular examinations of this family demonstrated different abnormalities from slight pigment change in the early stages to advanced macular atrophy later in CFP and decreased retinal thickness and atrophy of retina in OCT.
Supplementary information
Acknowledgements
We wish to thank this family for participating in our study.
Author contributions
YG, and MZ designed the study, directed the project, and interpreted the data. KL, LX, HL, ZZ, XW, RR, YT and YG performed the experiments. NY provided guidance for this project. XR wrote the paper, and YL, XF, NY, and MZ contributed to editing. All authors contributed to the paper and approved the submitted version.
Funding
This work was financially supported by The Project of National Key Research and Development (No. 2018YFC1106103) to MZ and Post-Doctor Research Project, West China Hospital, Sichuan University (No.2021HXBH030), Natural Science Foundation of Sichuan Province (No. 2022NSFSC1285) to XR.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yunxia Gao, Xiang Ren.
Contributor Information
Naihong Yan, Email: yannaihong@126.com.
Ming Zhang, Email: zhangmingscu0905@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-02355-1.
References
- 1.Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prokofyeva E, Troeger E, Bernd A, Zrenner E. Visual acuity changes in cone and cone-rod dystrophies. Ophthalmic Physiol Opt. 2012;32:53–59. doi: 10.1111/j.1475-1313.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger-Scemama E, Mohand-Said S, El Shamieh S, Demontant V, Condroyer C, Antonio A, et al., Phenotype analysis of retinal dystrophies in light of the underlying genetic defects: application to cone and cone-rod dystrophies. Int J Mol Sci. 2019;20:4854. [DOI] [PMC free article] [PubMed]
- 4.Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis. Surv Ophthalmol. 2006;51:232–58. doi: 10.1016/j.survophthal.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Gill JS, Georgiou M, Kalitzeos A, Moore AT, Michaelides M. Progressive cone and cone-rod dystrophies: clinical features, molecular genetics and prospects for therapy. Br J Ophthalmol. 2019;103:711–20. [DOI] [PMC free article] [PubMed]
- 6.Toto L, Battaglia Parodi M, D’Aloisio R, Mercuri S, Senatore A, Di Antonio L, et al. Cone dystrophies: an optical coherence tomography angiography study. J Clin Med. 2020;9:1500. [DOI] [PMC free article] [PubMed]
- 7.Oishi M, Oishi A, Gotoh N, Ogino K, Higasa K, Iida K, et al. Next-generation sequencing-based comprehensive molecular analysis of 43 Japanese patients with cone and cone-rod dystrophies. Mol Vis. 2016;22:150–60. [PMC free article] [PubMed] [Google Scholar]
- 8.Watson CM, El-Asrag M, Parry DA, Morgan JE, Logan CV, Carr IM, et al. Mutation screening of retinal dystrophy patients by targeted capture from tagged pooled DNAs and next generation sequencing. PloS one. 2014;9:e104281. doi: 10.1371/journal.pone.0104281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitiratschky VB, Wilke R, Renner AB, Kellner U, Vadala M, Birch DG, et al. Mutation analysis identifies GUCY2D as the major gene responsible for autosomal dominant progressive cone degeneration. Invest Ophthalmol Vis Sci. 2008;49:5015–23. doi: 10.1167/iovs.08-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dizhoor AM, Olshevskaya EV, Peshenko IV. The R838S mutation in retinal guanylyl cyclase 1 (RetGC1) alters calcium sensitivity of cGMP synthesis in the retina and causes blindness in transgenic mice. J Biol Chem. 2016;291:24504–16. doi: 10.1074/jbc.M116.755553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato S, Peshenko IV, Olshevskaya EV, Kefalov VJ, Dizhoor AM, Cone-Rod GUCY2D. Dystrophy-6 is a “phototransduction disease” triggered by abnormal calcium feedback on retinal membrane guanylyl cyclase 1. J Neurosci. 2018;38:2990–3000. doi: 10.1523/JNEUROSCI.2985-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes AE, Meng W, Lotery AJ, Bradley DT. A novel GUCY2D mutation, V933A, causes central areolar choroidal dystrophy. Invest Ophthalmol Vis Sci. 2012;53:4748–53. doi: 10.1167/iovs.12-10061. [DOI] [PubMed] [Google Scholar]
- 13.Sun Z, Wu S, Zhu T, Li H, Wei X, Du H, et al. Variants at codon 838 in the GUCY2D gene result in different phenotypes of cone rod dystrophy. Ophthalmic Genet. 2020;41:548–55. doi: 10.1080/13816810.2020.1807026. [DOI] [PubMed] [Google Scholar]
- 14.Sharon D, Wimberg H, Kinarty Y, Koch KW. Genotype-functional-phenotype correlations in photoreceptor guanylate cyclase (GC-E) encoded by GUCY2D. Prog retinal eye Res. 2018;63:69–91. doi: 10.1016/j.preteyeres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Hosono K, Harada Y, Kurata K, Hikoya A, Sato M, Minoshima S, et al. Novel GUCY2D gene mutations in japanese male twins with leber congenital amaurosis. J Ophthalmol. 2015;2015:693468. doi: 10.1155/2015/693468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stunkel ML, Brodie SE, Cideciyan AV, Pfeifer WL, Kennedy EL, Stone EM, et al. Expanded retinal disease spectrum associated with autosomal recessive mutations in GUCY2D. Am J Ophthalmol. 2018;190:58–68. doi: 10.1016/j.ajo.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Smaoui N, Ayyagari R, Stiles D, Benhamed S, MacDonald IM, et al. High-throughput retina-array for screening 93 genes involved in inherited retinal dystrophy. Invest Ophthalmol Vis Sci. 2011;52:9053–60. doi: 10.1167/iovs.11-7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Tao T, Zhao L, Li G, Yang L. Molecular diagnosis based on comprehensive genetic testing in 800 Chinese families with non-syndromic inherited retinal dystrophies. Clin Exp Ophthalmol. 2021;49(1):46–59. doi: 10.1111/ceo.13875. [DOI] [PubMed] [Google Scholar]
- 19.Collery RF, Cederlund ML, Kennedy BN. Transgenic zebrafish expressing mutant human RETGC-1 exhibit aberrant cone and rod morphology. Exp eye Res. 2013;108:120–8. doi: 10.1016/j.exer.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Wilkie SE, Newbold RJ, Deery E, Walker CE, Stinton I, Ramamurthy V, et al. Functional characterization of missense mutations at codon 838 in retinal guanylate cyclase correlates with disease severity in patients with autosomal dominant cone-rod dystrophy. Hum Mol Genet. 2000;9:3065–73. doi: 10.1093/hmg/9.20.3065. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Hoyos M, Auz-Alexandre CL, Almoguera B, Cantalapiedra D, Riveiro-Alvarez R, Lopez-Martinez MA, et al. Mutation analysis at codon 838 of the Guanylate Cyclase 2D gene in Spanish families with autosomal dominant cone, cone-rod, and macular dystrophies. Mol Vis. 2011;17:1103–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Perrault I, Rozet JM, Gerber S, Kelsell RE, Souied E, Cabot A, et al. A retGC-1 mutation in autosomal dominant cone-rod dystrophy. Am J Hum Genet. 1998;63:651–4. doi: 10.1086/301985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne AM, Morris AG, Downes SM, Johnson S, Bird AC, Moore AT, et al. Clustering and frequency of mutations in the retinal guanylate cyclase (GUCY2D) gene in patients with dominant cone-rod dystrophies. J Med Genet. 2001;38:611–4. doi: 10.1136/jmg.38.9.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith M, Whittock N, Searle A, Croft M, Brewer C, Cole M. Phenotype of autosomal dominant cone-rod dystrophy due to the R838C mutation of the GUCY2D gene encoding retinal guanylate cyclase-1. Eye. 2007;21:1220–5. doi: 10.1038/sj.eye.6702612. [DOI] [PubMed] [Google Scholar]
- 25.Van Ghelue M, Eriksen HL, Ponjavic V, Fagerheim T, Andreasson S, Forsman-Semb K, et al. Autosomal dominant cone-rod dystrophy due to a missense mutation (R838C) in the guanylate cyclase 2D gene (GUCY2D) with preserved rod function in one branch of the family. Ophthalmic Genet. 2000;21:197–209. doi: 10.1076/1381-6810(200012)2141-HFT197. [DOI] [PubMed] [Google Scholar]
- 26.Lima LH, Sallum JM, Spaide RF. Outer retina analysis by optical coherence tomography in cone-rod dystrophy patients. Retina. 2013;33:1877–80. doi: 10.1097/IAE.0b013e31829234e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.