Abstract
Approximately half of the brain’s circuits are involved in vision and control of eye movements. Therefore, visual dysfunction is a common symptom of concussion, the mildest form of traumatic brain injury (TBI). Photosensitivity, vergence dysfunction, saccadic abnormalities, and distortions in visual perception have been reported as vision-related symptoms following concussion. Impaired visual function has also been reported in populations with a lifetime history of TBI. Consequently, vision-based tools have been developed to detect and diagnose concussion in the acute setting, and characterize visual and cognitive function in those with a lifetime history of TBI. Rapid automatized naming (RAN) tasks have provided widely accessible and quantitative measures of visual-cognitive function. Laboratory-based eye tracking approaches demonstrate promise in measuring visual function and validating results from RAN tasks in patients with concussion. Optical coherence tomography (OCT) has detected neurodegeneration in patients with Alzheimer’s disease and multiple sclerosis and may provide critical insight into chronic conditions related to TBI, such as traumatic encephalopathy syndrome. Here, we review the literature and discuss the future directions of vision-based assessments of concussion and conditions related to TBI.
Subject terms: Eye manifestations, Visual system, Perception
Abstract
将近一半的大脑回路参与视觉及眼球运动的控制。因此, 视觉障碍是脑震荡的常见症状, 是最轻微的脑部创伤 (TBI) ——。光敏度下降、辐辏功能失调、扫视运动异常和视物变形已被报道为脑震荡后的视觉相关体征。在有TBI终生病史的人群中, 也有视觉功能受损的报道。因此, 用于检测和诊断急性环境中的脑震荡的视觉工具已研发出来, 用以检测有TBI终生病史的患者的视觉和认知功能。快速自动命名 (RAN) 任务提供了视觉认知功能的广泛可及的定量测量。基于实验室的眼动追踪方法在测量脑震荡患者的视觉功能和验证RAN任务的结果方面大有前景。相干光断层扫描技术 (OCT) 已检测到阿尔茨海默病和多发性硬化症患者的神经元退行性变, 并可能提供与TBI相关的慢性疾病之间的关键性证据, 如创伤性脑病综合征。本文我们对文献进行了回顾性分析, 讨论了基于视觉的脑震荡评估和TBI相关研究的未来方向。
Introduction
Concussion, the mildest form of traumatic brain injury (TBI), is a major cause of morbidity. Between 1.6 and 3.8 million sports- and activity-related concussions occur each year in the United States [1]. All age groups may be affected by concussion; however, children, adolescents, and the elderly are especially vulnerable [2]. Concussion is among the most challenging conditions for physicians to diagnose, perhaps, due to the simple definition [3]. Concussion is defined as any blow to the head or body in combination with any new neurological symptoms. This simple definition ensures that even non-clinical personnel, sports-parents and others can identify concussion, particularly when medical professionals are not readily available to make an assessment.
Concussions can cause physical, cognitive, social, emotional, and behavioral symptoms. Although most people recover from a concussion within 2–4 weeks post-injury, some will have symptoms for months or longer. Furthermore, multiple concussions may increase one’s risk of developing long-term visual, vestibular, mood and cognitive impairment. Long-term exposures to multiple head traumas have been associated with traumatic encephalopathy syndrome, the clinical condition associated with chronic traumatic encephalopathy (CTE) [4–7].
Visual dysfunction is common following concussion; approximately 70% of adolescents diagnosed with concussion will present with visual symptoms [8–10]. These symptoms include blurred vision, eye movement abnormalities, and impairment in more complex aspects of visual perception, such as scene processing [11]. Visual dysfunction has also been reported in patients who have a lifetime history of multiple head impacts. In an optical coherence tomography (OCT) study that captured visual pathway structure in former collision sport athletes, Leong and colleagues [12] determined that retinal nerve fibre layer (RNFL) thickness was a significant predictor of collision sport athlete versus non-exposed control status.
Many concussion assessment batteries rely on patients to accurately report symptoms for physicians to make a concussion diagnosis [13]. On the sports sideline, the physician’s dependence on self-reporting is especially problematic as many athletes have been shown to hide concussion symptoms to avoid removal from play [14]. The under-diagnosis of concussion may have devastating consequences given the risk of second impact syndrome, a rare but catastrophic condition that can lead to permanent disability and death [15]. The dangerous consequences of insufficient concussion detection have led to the development of novel concussion assessment tools that provide quantitative measurements. These tools were designed to limit reliance on subjective ratings and bias in concussion assessment and to expand access to concussion detection for the layperson examiner, such as a sports parent.
We have examined the association between concussion and visual dysfunction to develop vision-based diagnostic tools that are quantitative, affordable, and widely accessible. This literature review first elaborates on the myriad of visual symptoms related to concussion and then discusses how rapid automatized naming (RAN) tasks and laboratory-based eye tracking tools have demonstrated promise as concussion assessment tools. Finally, we conclude with a review of the future directions of vision-based testing in the context of concussion and the long-term sequelae of multiple TBIs.
Concussion and visual symptoms
Concussion can result in the disruption of both afferent and efferent visual pathways [11]. Common visual signs and symptoms of concussion include blurred vision, impaired pupillary function, photosensitivity, abnormalities in the vestibular-ocular reflex, slowed or inaccurate saccades, impaired smooth pursuit, and convergence and accommodative insufficiency [16]. These symptoms may not appear immediately after injury, but may develop over hours to days or may appear when the patient is under physical or emotional stress [11].
Photophobia is considered the second most common symptom of concussion. Between 60 and 75% of soldiers who sustained concussions from blast waves suffered from photophobia [17]. Although the underlying mechanism of photophobia in concussion has yet to be elucidated, investigators have suggested that photophobia in patients with concussions may be the result of the dysfunction in the trigeminothalamic pathway [18].
The pupillary light reflex may be significantly delayed, slowed, and reduced in patients with concussions versus non-injured controls [19]. As a result, pupillary responsivity has been proposed as a marker during the acute stage of sport-related concussion [10]. In a retrospective clinical review of a mobile pupilometer app, Carrick and colleagues [20] found that patients diagnosed with concussions demonstrated abnormal constriction latencies, abnormal maximum and minimum pupillary diameters, and decreased speed of contraction and dilation after light stimulation. This effect was also modulated by gender and age.
The vestibulo-ocular reflex (VOR) is a reflex that stabilizes gaze during head movements. The VOR can be assessed with the head impulse test (HIT) where an examiner rapidly moves the patient’s head to one side while asking them to fixate on an object at distance. In an intact VOR, the patient’s gaze will remain fixed on the object of regard. However, when the VOR is compromised, patients will lose fixation as the head is rapidly rotated, requiring a catch-up saccade to re-fixate. Impaired VOR may be associated with other concussion symptoms such as movement-related dizziness, oscillopsia, blurred vision, and difficulty balancing [21].
Our eyes must converge to focus on an object at near. Convergence abnormalities have been reported in 38–49% of patients with post-concussion symptoms [22]. Additionally, 65% of concussed patients versus only 15% of controls have abnormalities in accommodative amplitude, defined as the closest point to the nasal bridge at which the eyes can focus [23]. Patients diagnosed with concussion who suffer from either convergence or accommodative insufficiency report symptoms like headache, eye pain, horizontal diplopia at near, and losing one’s place while reading [23]. These oculomotor symptoms have been attributed to the disruption of oculomotor pathways in the brainstem. A 2015 brain imaging study found that patients diagnosed with traumatic brain injuries showed a significant reduction in the activation of oculomotor nuclei in the brainstem on functional MRI. In particular, decreased activation of the supra-oculomotor area was associated with diminished convergence and divergence velocity [24].
Smooth pursuit eye movements are tracking eye movements in which the eyes remain fixated on a moving object. Smooth pursuits are governed by cerebellar and cortical input as successful tracking requires attention, anticipation, and working memory; these functions are widely known to be impaired following concussion [25]. Approximately 60% of patients with concussion have abnormalities in smooth pursuit eye movements. Injury status is correlated with decreased target prediction, increased eye position error, and variability of eye position using a circular tracking test [23, 26].
Abnormalities in saccades, eye movements that rapidly bring an object of regard onto the fovea, are also common following concussion. Approximately 30% of patients with concussion have abnormalities in saccades. These patients often generate hypometric saccades that undershoot fixation on an object of interest. Some patients may have difficulty limiting involuntary saccades or performing anti-saccade tasks [27]. In addition to the disruption of ocular motor pathways, impairment of higher executive functions such as attention and memory may also play a role in saccadic disturbances [28, 29].
Vision-based concussion testing
Vestibular/ocular-motor screening (VOMS) tool
The VOMS tool is a symptom checklist designed to assess vestibular and ocular symptoms in sports-related concussions. Subjects are asked to report symptoms of headache, nausea, fogginess, and dizziness when completing a battery of vestibular and ocular provocations. A 2014 study by Mucha and colleagues [30] found that subjects who reported symptoms while completing the VOMS battery were significantly more likely to have a concussion than those who did not. When combined with other concussion assessments in the Sport Concussion Assessment Tool 3, the VOMS battery increased the detection rate of concussion in a collegiate athlete cohort [31]. However, the VOMS’ dependence on patient symptom self-reporting and 10-minute completion time may limit its use on the athletic sideline.
Rapid automatized naming (RAN) tasks
RAN tasks have been used for over 80 years to assess cognitive, visual, and language function [32]. During RAN tasks, participants are instructed to rapidly name several stimuli, typically numbers or objects, while minimizing errors in naming. RAN tasks were originally designed to distinguish subjects with and without reading and learning disabilities but have been repurposed for the diagnosis of concussion [33–35]. RAN tasks rely on attention, language, and the precise coordination of saccadic eye movements. Therefore, successful completion of RAN tasks requires multiple neuroanatomic regions, including the frontal eye fields, dorsolateral prefrontal cortex, middle temporal area, cerebellum, brainstem, and striate cortex (Fig. 1) [16].
Fig. 1. Major cortical and subcortical pathways involved in saccadic generation.
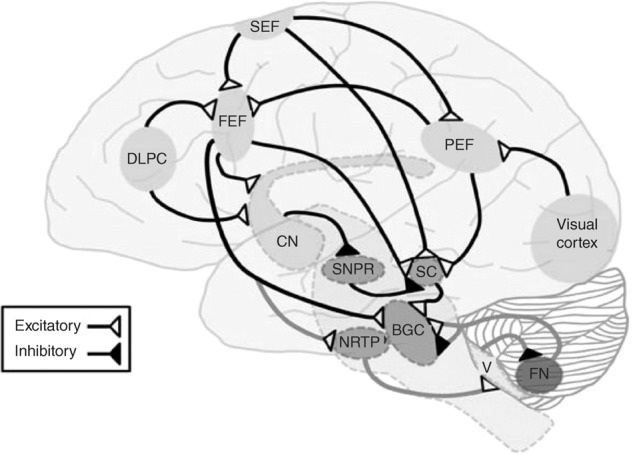
Eye fields (FEF). Striate cortex (SC). Dorsolateral prefrontal cortex (DLPC). Supplementary eye fields (SEF). Fastigial nucleus (FN). Substantia nigra pars reticulata (SNPR). Nucleus reticularis tegmenti pontis (NRTP). Brainstem Gaze Centers (BGC). Cerebellum Oculomotor Vermis (V). Reprinted with permission from: Galetta et al. [44].
Different RAN tasks also elicit activity in specific regions in functional neuroimaging studies. Pictorial tasks, which often feature images arranged on test cards, have been found to activate regions associated with object recognition and facial identification such as the bilateral fusiform gyri and area V4 [36]. Alphanumeric RAN tasks, which feature numbers and letters arranged in variable patterns on the testing cards, activate predominately left-sided brain regions. These regions include the primary left inferior temporal lobe, left motor cortex, left superior parietal gyrus, and the medial supplementary motor area [37]. Furthermore, when compared to pictorial tasks, alphanumeric RAN tasks have been shown to elicit greater activity in semantic and articulatory regions such as the precuneus, bilateral supramarginal gyrus, nucleus accumbens, and the thalamus [38].
RAN tasks have become increasingly employed as sideline concussion assessment tools. In this context, pre-season athletes complete baseline trials, which are then compared to a test taken following a head injury. Any worsening of RAN task completion time is suggestive of concussion because of anticipated learning effects. One study found that participants improved their testing times significantly across the first eight attempts of an alphanumeric RAN task, and a ceiling effect was not determined until participants completed 30 trials [39]. The continual improvement in RAN task performance may explain the results of another study where ten concussed collegiate athletes failed to show any significant changes in performance post-injury or at the time of return to play [40]. The longitudinal learning effect of RAN tasks is an obvious limitation that should be studied further. Nevertheless, several studies have demonstrated the utility of RAN tasks in the context of concussion on the athletic sideline. The Mobile Universal Lexicon Evaluation System (MULES), Staggered Uneven Number (SUN), and King-Devick (K-D) tests, discussed below, are several RAN tasks that have successfully detected concussion by measuring visual function.
The Mobile Universal Lexicon Evaluation System (MULES) test
The MULES is a pictorial RAN task that involves the rapid naming of photographs of common objects such as fruits and animals (Fig. 2). The MULES assesses object recognition, colour detection, and semantic categorization.
Fig. 2. Screenshots of the Mobile Integrated Cognitive Kit (MICK) app.
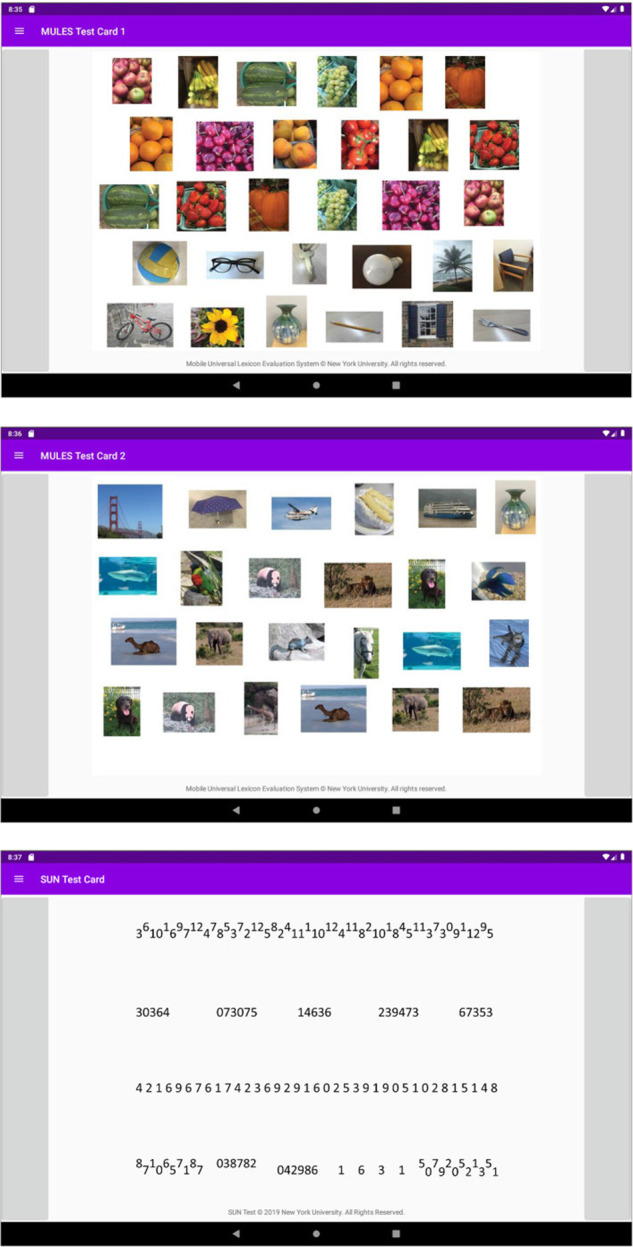
This app includes digitized versions of the MULES (upper two panels) and SUN tests (lowest panel), tailored for tablet devices to allow for greater accessibility of testing. The app is currently available for research-based testing. Trained professionals who perform testing are able to assess performance on the MULES and SUN tests in a user-friendly manner with additional features such as timers and voice recordings. Versions of the app that can be used on smartphone-based platforms are also under development. Reprinted with permission from: Park et al. [32].
The MULES test has previously demonstrated utility in detecting neurologic deficits in patients with concussion as well as neurodegenerative diseases. The presence of a concussion impairs performance on the MULES test [41, 42]. The MULES test also shows decrement in performance in individuals with Parkinson’s disease, multiple sclerosis, and disorders along the spectrum of Alzheimer’s disease [32, 43].
The staggered uneven naming (SUN) test
The SUN test is an alphanumeric RAN task that consists of four rows of single and two-digit numbers arranged in various patterns: numbers are diagonally placed and variably crowded to the degree that represents reading (Fig. 2). Participants are asked to read the numbers verbally from left to right, starting with the first row, as quickly as possible without making errors.
The Mobile Integrated Cognitive Kit (MICK) app
The MICK app presents the MULES and SUN in a tablet-based format (Fig. 2). The MICK app records testing times and creates an audio recording of the subject as they complete the RAN tasks. Initial studies have demonstrated excellent agreement of time scores between the MICK app and paper versions of the SUN and MULES [32]. The combination of the alphanumeric SUN test and pictorial MULES on the MICK app enables an assessment of a wide variety of neural networks.
Consistent with previous studies on paper/pencil versions of the MULES and SUN, MICK app users have demonstrated learning effects [32]. Such learning effects were greater for the MULES compared to the SUN (Fig. 3). One explanation is that numeric naming requires less extensive recruitment of neural networks, enabling participants to score closer to their peak optimal performance on their first trial. Meanwhile, pictorial tasks require greater cognitive effort as participants must identify an object in a colour photo within context. During this task, participants may be distinguishing the pictured objects from related ones stored in visual memory. The learning effects demonstrated on both alphanumeric and non-alphanumeric RAN tasks highlight the need for pre-season testing in athletic cohorts to establish the best pre-injury baseline score.
Fig. 3. Box plot showing MULES and SUN time scores with learning effects (n = 59).
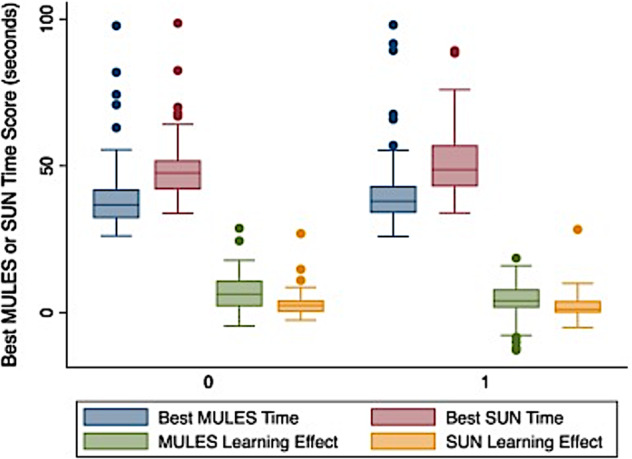
Lines in the boxes represent medians; boxes delineate the interquartile range (25th to 75th percentiles). Whiskers represent ranges of observations; outliers are represented by dots. No significant differences were noted between MICK app-based MULES and SUN vs. the previously developed paper/ pencil versions. These analyses accounted for whether MICK app versions or paper/ pencil versions were presented first (P = 0.45 for MULES, P = 0.50 for SUN, linear regression models). Percentage learning effects (difference between two trials divided by best time for each test) were significantly greater for the paper/pencil version compared with the MICK app only for the MULES (17.8% vs. 11.5%, P = 0.03, linear regression accounting simultaneously for which platform was presented first). MULES learning effects were significantly greater than those for SUN, as demonstrated in previous studies [32, 36]. Reprinted with permission from: Park et al. [32].
Currently free to download and with a user interface designed to be universally accessible, the MICK app takes advantage of the growing prevalence of digital technology to increase access to a reliable and low-cost neurologic assessment. Increased accessibility of a digital concussion assessment tool will hopefully improve screening for concussions on the athletic sideline, thereby preventing long-term complications of undiagnosed traumatic brain injuries. The MICK app may also enable a more comprehensive screening via outpatient tele-neurology that can be compared across clinical visits in the setting of post-concussion recovery. Future smartphone-accessible versions of the MICK may further broaden accessibility. However, it is currently unknown how the change in screen size will impact performance on the MULES and whether scores will be comparable across devices. Future studies will need to evaluate the inter-rater reliability of the MICK app on different delivery platforms.
King–Devick (K–D) test
The K–D test is an alphanumeric RAN task with three cards with numbers arranged in rows with variable horizontal and vertical spacing. Participants are instructed to read cards as quickly as possible and scores are primarily determined based on time to completion, where longer times are considered worse.
The K–D test has been studied extensively in the context of sports concussion injuries. A meta-analysis conducted by Galetta and colleagues found that the K–D test was highly sensitive (86%) and specific (90%) in distinguishing concussed athletes from controls [44]. Prior research showed the utility of the K–D test in multiple sports and athletic teams, including mixed martial arts, boxing, hockey, rugby, and collegiate sports [44–47]. These studies have consistently revealed that concussed athletes score an average of 5–7 seconds worse than their respective baseline scores immediately after injury [44–47]. Performance on the K–D test has also been shown to predict the number of subspecialty referrals and the total number of clinical visits with moderate to high reliability [48].
Laboratory-based eye tracking
Developments in eye movement tracking tools have demonstrated promise in capturing specific vision-related symptoms of concussion. A 2015 study by Cifu and colleagues [49] reported that an eye-tracking device captured larger saccadic position errors, smaller saccadic amplitudes, decreased peak velocities and accelerations, and longer saccadic durations in a cohort of military subjects with post-concussive syndrome. New developments in eye-tracking technology may also improve the diagnostic accuracy for concussions. The computer-based eye-tracking software RightEye offered a sensitivity of 0.77 and specificity of 0.78 when diagnosing TBI using a horizontal saccade test and a sensitivity of 0.64 and a specificity of 0.65 when diagnosing TBI based on a vertical saccade test [50].
Eye tracking devices have also captured eye movement abnormalities and provided crucial quantitative data regarding error types in patients performing RAN tasks. Video oculography studies in patients with concussion demonstrated prolonged inter-saccadic intervals and increased numbers of saccades while completing the K-D test and MULES test respectively [51, 52]. Additionally, video-oculography may validate scores on RAN tasks. Rizzo and colleagues found that analysis of eye movement metrics from infrared video-oculography could readily identify deliberate underperforming efforts on RAN tasks. To subvert concussion detection, athletes may intentionally perform poorly on baseline RAN tests in a pre-season setting. However, saccadic patterns in so-called ‘sandbagging’ are easily differentiated from poor physiologic performance [53].
Further research is necessary to determine how acutely eye movement changes occur. Approximately 5–10% of healthy controls can have abnormal pursuits and saccades, 20% can have symptoms of convergence insufficiency, 5–10% can have abnormal convergence on examination, and 10–15% can have abnormal accommodation [54, 55]. Furthermore, eye tracking technology with the resolution necessary to reliably detect saccadic and pursuit changes is not widely accessible beyond rare laboratory-based techniques at specialized centres. This limits its use on the sideline and may be cost-prohibitive to most athletic programs, particularly those for youth. Therefore, current iterations of eye-tracking technology may not be ideal as performance-based sideline tools.
Afferent vision-based evaluation for tbi related diseases
OCT has demonstrated promise in detecting neurodegeneration via visualization of neuronal tissue in vivo in the eye. OCT uses light to image the cornea, lens, anterior chamber, retinal tissue, and retinal vasculature. The two most commonly studied OCT measures are the parapapillary retinal nerve fibre layer (RNFL) and the macular ganglion cell/inner plexiform layer (GCIPL). OCT measures have effectively demonstrated abnormalities in non-TBI neurologic conditions such as multiple sclerosis, mild cognitive impairment due to Alzheimer’s disease, and Friedreich ataxia [56–58]. Since the cerebral cortex and retina are both of neuroepithelial origin, retinal thinning is hypothesized to be a result of retrograde atrophy and/or parallel neurodegenerative disease processes. For example, inner retinal thinning measured by OCT has been correlated with cognitive performance, hippocampal atrophy, and disease status in patients with Alzheimer’s disease and related disorders [59]. OCT can also identify multiple sclerosis early in the course of the disease and before other detection methods, including fundoscopy [60].
In terms of TBI, a study by Leong and colleagues used OCT in contact sport athletes; former boxers showed RNFL thinning while football players did not [12]. OCT analysis also showed abnormalities in the GCIPL in veterans with a history of TBI [61]. Future studies should involve a comprehensive assessment of visual function in a larger cohort of individuals with known history of TBI.
Conclusion
Photosensitivity, vergence dysfunction, saccadic abnormalities, and distortions in visual perception have been reported as vision-related symptoms following concussion, making visual assessments critically important following mild traumatic brain injury. RAN tasks offer quantitative, quick, and low-cost methods for the evaluation of visual function in concussion. Eye-tracking technology has demonstrated promise as a complement to RAN tasks by objectively validating diagnostic accuracy of clinically-recognized eye movement abnormalities that follow concussion. However, cost, availability, and interpretation remain a barrier to wide spread use of eye trackers on the sidelines. The long-term impact of TBI on visual function is not well understood. Prior studies have demonstrated an association between TBI history and retinal thinning on OCT. Future longitudinal studies will be necessary to illuminate the long-term impact of concussion on visual function.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: SLG; literature review: CAB; draft manuscript preparation: CAB; LJB, SLG, SNG, MB. All authors reviewed and approved the final version or the manuscript.
Funding
This study was supported in part by the NYU Grossman School of Medicine.
Data availability
There are no primary data for this manuscript.
Competing interests
LJB is editor-in-chief of the Journal of Neuro-Ophthalmology. The remaining authors have no disclosures.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Thurman DJ. The epidemiology of traumatic brain injury in children and youths: a review of research since 1990. J Child Neurol. 2016;31:20–7. doi: 10.1177/0883073814544363. [DOI] [PubMed] [Google Scholar]
- 3.McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838–47. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 4.McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain J Neurol. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAllister T, McCrea M. Long-term cognitive and neuropsychiatric consequences of repetitive concussion and head-impact exposure. J Athl Train. 2017;52:309–17. doi: 10.4085/1062-6050-52.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzanero S, Elkington LJ, Praet SF, Lovell G, Waddington G, Hughes DC. Post-concussion recovery in children and adolescents: a narrative review. J Concussion. 2017;1:2059700217726874. doi: 10.1177/2059700217726874. [DOI] [Google Scholar]
- 7.Doroszkiewicz C, Gold D, Green R, Tartaglia MC, Ma J, Tator CH. Anxiety, depression, and quality of life: a long-term follow-up study of patients with persisting concussion symptoms. J Neurotrauma. 2021;38:493–505. doi: 10.1089/neu.2020.7313. [DOI] [PubMed] [Google Scholar]
- 8.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Marinides Z, Galetta KM, Andrews CN, Wilson JA, Herman DC, Robinson CD, et al. Vision testing is additive to the sideline assessment of sports-related concussion. Neurol Clin Pract. 2015;5:25–34. doi: 10.1212/CPJ.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Master CL, Podolak OE, Ciuffreda KJ, Metzger KB, Joshi NR, McDonald CC, et al. Utility of pupillary light reflex metrics as a physiologic biomarker for adolescent sport-related concussion. JAMA Ophthalmol. 2020;138:1135–41. doi: 10.1001/jamaophthalmol.2020.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong RA. Visual problems associated with traumatic brain injury. Clin Exp Optom. 2018;101:716–26. doi: 10.1111/cxo.12670. [DOI] [PubMed] [Google Scholar]
- 12.Leong D, Morettin C, Messner LV, Steinmetz RJ, Pang Y, Galetta SL, et al. Visual structure and function in collision sport athletes. J Neuro-Ophthalmol. 2018;38:285–91. doi: 10.1097/WNO.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 13.Lempke LB, Schmidt JD, Lynall RC. Athletic trainers’ concussion-assessment and concussion-management practices: an update. J Athl Train. 2020;55:17–26. doi: 10.4085/1062-6050-322-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres DM, Galetta KM, Phillips HW, Dziemianowicz EMS, Wilson JA, Dorman ES, et al. Sports-related concussion. Neurol Clin Pract. 2013;3:279–87. doi: 10.1212/CPJ.0b013e3182a1ba22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May T, Foris LA, Donnally III CJ. Second impact syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK448119/. [PubMed]
- 16.Ventura RE, Jancuska JM, Balcer LJ, Galetta SL. Diagnostic tests for concussion: is vision part of the puzzle? J Neuroophthalmol. 2015;35:73–81. doi: 10.1097/WNO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 17.Abusamak M, Alrawashdeh HM. Post-concussion syndrome light sensitivity: a case report and review of the literature. Neuro-Ophthalmol Aeolus Press. 2022;46:85–90. doi: 10.1080/01658107.2021.1983612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diel RJ, Mehra D, Kardon R, Buse DC, Moulton E, Galor A. Photophobia: shared pathophysiology underlying dry eye disease, migraine and traumatic brain injury leading to central neuroplasticity of the trigeminothalamic pathway. Br J Ophthalmol. 2021;105:751–60. doi: 10.1136/bjophthalmol-2020-316417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciuffreda KJ, Joshi NR, Truong JQ. Understanding the effects of mild traumatic brain injury on the pupillary light reflex. Concussion. 2017;2:CNC36. doi: 10.2217/cnc-2016-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrick FR, Azzolino SF, Hunfalvay M, Pagnacco G, Oggero E, D’Arcy RCN, et al. The pupillary light reflex as a biomarker of concussion. Life. 2021;11:1104. doi: 10.3390/life11101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkathiry AA, Kontos AP, Furman JM, Whitney SL, Anson ER, Sparto PJ. Vestibulo-ocular reflex function in adolescents with sport-related concussion: preliminary results. Sports Health. 2019;11:479–85. doi: 10.1177/1941738119865262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez DTL. Functional mechanism of neural control in persistent post-concussion symptoms convergence insufficiency. clinicaltrials.gov; 2022. Report No.: NCT05262361. https://clinicaltrials.gov/ct2/show/NCT05262361.
- 23.Capó-Aponte JE, Urosevich TG, Temme LA, Tarbett AK, Sanghera NK. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil Med. 2012;177:804–13. doi: 10.7205/MILMED-D-12-00061. [DOI] [PubMed] [Google Scholar]
- 24.Tyler CW, Likova LT, Mineff KN, Nicholas SC. Deficits in the activation of human oculomotor nuclei in chronic traumatic brain injury. Front Neurol. 2015;6:173. doi: 10.3389/fneur.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes GR. Cognitive processes involved in smooth pursuit eye movements. Brain Cogn. 2008;68:309–26. doi: 10.1016/j.bandc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Suh M, Kolster R, Sarkar R, McCandliss B, Ghajar J. Deficits in predictive smooth pursuit after mild traumatic brain injury. Neurosci Lett. 2006;401:108–13. doi: 10.1016/j.neulet.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 27.Maruta J, Suh M, Niogi SN, Mukherjee P, Ghajar J. Visual tracking synchronization as a metric for concussion screening. J Head Trauma Rehabil. 2010;25:293–305. doi: 10.1097/HTR.0b013e3181e67936. [DOI] [PubMed] [Google Scholar]
- 28.Drew AS, Langan J, Halterman C, Osternig LR, Chou LS, van Donkelaar P. Attentional disengagement dysfunction following mTBI assessed with the gap saccade task. Neurosci Lett. 2007;417:61–5. doi: 10.1016/j.neulet.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Heitger MH, Jones RD, Macleod AD, Snell DL, Frampton CM, Anderson TJ. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain. 2009;132:2850–70. doi: 10.1093/brain/awp181. [DOI] [PubMed] [Google Scholar]
- 30.Mucha A, Collins MW, Elbin RJ, Furman JM, Troutman-Enseki C, DeWolf RM, et al. A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42:2479–86. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferris LM, Kontos AP, Eagle SR, Elbin RJ, Collins MW, Mucha A, et al. Predictive accuracy of the sport concussion assessment tool 3 and vestibular/ocular-motor screening, individually and in combination: A National Collegiate Athletic Association-Department of Defense Concussion Assessment, Research and Education Consortium Analysis. Am J Sports Med. 2021;49:1040–8. doi: 10.1177/0363546520988098. [DOI] [PubMed] [Google Scholar]
- 32.Park G, Balcer MJ, Colcombe JR, Hasanaj L, Joseph B, Kenney R, et al. The MICK (Mobile integrated cognitive kit) app: Digital rapid automatized naming for visual assessment across the spectrum of neurological disorders. J Neurol Sci. 2022;434:120150. doi: 10.1016/j.jns.2022.120150. [DOI] [PubMed] [Google Scholar]
- 33.Denckla MB, Rudel RG. Rapid ‘automatized’ naming (R.A.N.): dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976;14:471–9. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- 34.Geschwind N, Fusillo M. Color-naming defects in association with alexia. Arch Neurol. 1966;15:137–46. doi: 10.1001/archneur.1966.00470140027004. [DOI] [PubMed] [Google Scholar]
- 35.Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annu Rev Psychol. 2012;63:427–52. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- 36.Cobbs L, Hasanaj L, Amorapanth P, Rizzo JR, Nolan R, Serrano L, et al. Mobile Universal Lexicon Evaluation System (MULES) test: a new measure of rapid picture naming for concussion. J Neurol Sci. 2017;372:393–8. doi: 10.1016/j.jns.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahan N, Moehringer N, Hasanaj L, Serrano L, Joseph B, Wu S, et al. The SUN test of vision: Investigation in healthy volunteers and comparison to the mobile universal lexicon evaluation system (MULES) J Neurol Sci. 2020;415:116953. doi: 10.1016/j.jns.2020.116953. [DOI] [PubMed] [Google Scholar]
- 38.Cummine J, Szepesvari E, Chouinard B, Hanif W, Georgiou GK. A functional investigation of RAN letters, digits, and objects: How similar are they? Behav Brain Res. 2014;275:157–65. doi: 10.1016/j.bbr.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 39.Gunasekaran P, Fraser CL, Hodge C. The learning effect of the King-Devick test in semi-professional rugby union athletes. J Neurol Sci. 2020;419:117168. doi: 10.1016/j.jns.2020.117168. [DOI] [PubMed] [Google Scholar]
- 40.DiFabio M, Oldham J, DeWolf R, Kaminski T, Buckley T. Evaluating concussion recovery through oculomotor testing (I13.004). Neurology. 2016;86. https://n.neurology.org/content/86/16_Supplement/I13.004.
- 41.Akhand O, Galetta MS, Cobbs L, Hasanaj L, Webb N, Drattell J, et al. The new Mobile Universal Lexicon Evaluation System (MULES): A test of rapid picture naming for concussion sized for the sidelines. J Neurol Sci. 2018;387:199–204. doi: 10.1016/j.jns.2018.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockbridge MD, Doran A, King K, Newman RS. The effects of concussion on rapid picture naming in children. Brain Inj. 2018;32:506–14. doi: 10.1080/02699052.2018.1429660. [DOI] [PubMed] [Google Scholar]
- 43.Wu SZ, Nolan-Kenney R, Moehringer NJ, Hasanaj LF, Joseph BM, Clayton AM, et al. Exploration of rapid automatized naming and standard visual tests in prodromal Alzheimer disease detection. J Neuroophthalmol. 2022;42:79–87. doi: 10.1097/WNO.0000000000001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galetta KM, Liu M, Leong DF, Ventura RE, Galetta SL, Balcer LJ. The King-Devick test of rapid number naming for concussion detection: meta-analysis and systematic review of the literature. Concussion. 2015;1:CNC8. doi: 10.2217/cnc.15.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galetta KM, Brandes LE, Maki K, Dziemianowicz MS, Laudano E, Allen M, et al. The King–Devick test and sports-related concussion: Study of a rapid visual screening tool in a collegiate cohort. J Neurol Sci. 2011;309:34–9. doi: 10.1016/j.jns.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 46.Galetta KM, Barrett J, Allen M, Madda F, Delicata D, Tennant AT, et al. The King-Devick test as a determinant of head trauma and concussion in boxers and MMA fighters. Neurology. 2011;76:1456–62. doi: 10.1212/WNL.0b013e31821184c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King D, Clark T, Gissane C. Use of a rapid visual screening tool for the assessment of concussion in amateur rugby league: A pilot study. J Neurol Sci. 2012;320:16–21. doi: 10.1016/j.jns.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 48.Kyle Harrold G, Hasanaj L, Moehringer N, Zhang I, Nolan R, Serrano L, et al. Rapid sideline performance meets outpatient clinic: Results from a multidisciplinary concussion center registry. J Neurol Sci. 2017;379:312–7. doi: 10.1016/j.jns.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 49.Cifu DX, Wares JR, Hoke KW, Wetzel PA, Gitchel G, Carne W. Differential eye movements in mild traumatic brain injury versus normal controls. J Head Trauma Rehabil. 2015;30:21–8. doi: 10.1097/HTR.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 50.Hunfalvay M, Roberts CM, Murray N, Tyagi A, Kelly H, Bolte T. Horizontal and vertical self-paced saccades as a diagnostic marker of traumatic brain injury. Concussion. 2019;4:CNC60. doi: 10.2217/cnc-2019-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizzo JR, Hudson TE, Dai W, Birkemeier J, Pasculli RM, Selesnick I, et al. Rapid number naming in chronic concussion: eye movements in the King–Devick test. Ann Clin Transl Neurol. 2016;3:801–11. doi: 10.1002/acn3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudson TE, Conway J, Rizzo JR, Martone J, Chou LT, Balcer LJ, et al. Rapid automatized picture naming in an outpatient concussion center: quantitative eye movements during the Mobile Universal Lexicon Evaluation System (MULES) Test. Clin. Transl Neurosci. 2022;6:18. [Google Scholar]
- 53.Rizzo JR, Hudson TE, Martone J, Dai W, Ihionu O, Chaudhry Y, et al. How sandbag-able are concussion sideline assessments? A close look at eye movements to uncover strategies. Brain Inj. 2021;35:426–35. doi: 10.1080/02699052.2021.1878554. [DOI] [PubMed] [Google Scholar]
- 54.Moss HE, McCluskey L, Elman L, Hoskins K, Talman L, Grossman M, et al. Cross-sectional evaluation of clinical neuro-ophthalmic abnormalities in an amyotrophic lateral sclerosis population. J Neurol Sci. 2012;314:97–101. doi: 10.1016/j.jns.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheiman M, Mitchell GL, Cotter S, Cooper J, Kulp M, Rouse M, et al. A randomized clinical trial of treatments for convergence insufficiency in children. Arch Ophthalmol. 2005;123:14–24. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 56.Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 57.Wu SZ, Masurkar AV, Balcer LJ. Afferent and efferent visual markers of Alzheimer’s disease: a review and update in early stage disease. Front Aging Neurosci. 2020;12:572337. doi: 10.3389/fnagi.2020.572337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seyer LA, Galetta K, Wilson J, Sakai R, Perlman S, Mathews K, et al. Analysis of the visual system in Friedreich ataxia. J Neurol. 2013;260:2362–9. doi: 10.1007/s00415-013-6978-z. [DOI] [PubMed] [Google Scholar]
- 59.Galvin JE, Kleiman MJ, Walker M. Using optical coherence tomography to screen for cognitive impairment and dementia. J Alzheimers Dis. 2021;84:723–36. doi: 10.3233/JAD-210328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9:921–32. doi: 10.1016/S1474-4422(10)70168-X. [DOI] [PubMed] [Google Scholar]
- 61.Gilmore CS, Lim KO, Garvin MK, Wang JK, Ledolter J, Fenske AL, et al. Association of optical coherence tomography with longitudinal neurodegeneration in veterans with chronic mild traumatic brain injury. JAMA Netw Open. 2020;3:e2030824. doi: 10.1001/jamanetworkopen.2020.30824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no primary data for this manuscript.


