ABSTRACT.
Cedecea lapagei is a gram-negative, non-encapsulated, facultative anaerobic bacterium that has been reported in only a few cases with varying clinical presentations, drug susceptibility, and treatment since its first isolation in 1981. This study aimed to describe a case report of C. lapagei in Peru and systematically review the documented case reports of individuals infected with C. lapagei. A 59-year-old man who had become bedridden with Parkinson’s disease and had epilepsy presented with a 1-week history of fever and sore throat and was admitted. Physical examination revealed an obtundation state and abolished vesicular murmur in the right hemithorax. During hospitalization, the patient was diagnosed with various infections, including tuberculosis, for which he received broad-spectrum antibiotics. In the absence of clinical improvement, a urine culture was performed showing C. lapagei (detected by BD Phoenix M50 system, Vernon Hills, IL). The patient received amoxicillin/clavulanate and was discharged. Case reports of C. lapagei were also searched in five databases on January 28, 2023. Twenty cases of C. lapagei were reported worldwide between 2006 and 2022, 16 of which involved adults. Fever was the most common manifestation (75%), and pneumonia was the primary form of presentation (45%). Moreover, 90% of the patients had at least one comorbidity, and 15% died. Also, most of the isolates were sensitive to ciprofloxacin (81%), meropenem (62%), and amikacin (60%). Overall, C. lapagei should be suspected in compromised hosts, particularly those with pneumonia. Although the bacterium can affect various organs and the antibiotic susceptibility pattern is variable, quinolones, tetracyclines, and carbapenems seem to be the first therapeutic option.
INTRODUCTION
Cedecea lapagei is a gram-negative, non-encapsulated, facultative anaerobic, lactose-negative, catalase-positive, and non–spore-forming bacterium that belongs to the Enterobacteriaceae family. It was first isolated in 1981.1 The Cedecea genus consists of five species: Cedecea davisae, Cedecea neteri, Cedecea lapagei, and two other unnamed species.1,2 The members of the Cedecea genus are usually motile and grow well on nonselective laboratory media, producing convex colonies at 37.1°C. Cedecea lapagei can be distinguished from other Cedecea strains by its growing ability in media lacking thiamine.3 Although it was discovered more than 40 years ago, it was designated a human pathogen in 2006,4 and few cases have been reported worldwide.
The distribution of organ involvement is highly variable. According to reports, the infections produced include bacterial peritonitis, pneumonia, bacteremia, and wound infection2,4–6 and may even lead to septic shock.7,8 However, C. lapagei does not exhibit a clear tropism and affects different age groups. This bacterium belongs to the gastrointestinal and urinary microbiota and grows in acidic and alkaline conditions (pH 4–10).3 Its transmission mechanism is unknown, even though it could be related to the environment, hospital contacts, host immunity status, use of antimicrobials, or other factors that have not yet been studied.2
The treatment of infections caused by Cedecea strains represents both a challenge and a threat to health systems, especially in low- and middle-income countries owing to their drug resistance patterns. In Peru, where the health system is simultaneously dealing with multiple epidemics (arbovirosis and tuberculosis, among others) and has high antibiotic resistance rates,9 the emergence of new bacterial genera with a potentially high resistance profile is important. Hence, this study aimed to systematically review the documented case reports of individuals with C. lapagei. Secondarily, we describe the first case report of C. lapagei in Peru in a patient co-infected with pulmonary tuberculosis.
CASE REPORT
A 59-year-old man, a resident of Lima (the capital of Peru) who had a history of epilepsy and was bedridden secondary to Parkinson’s disease treated with valproate and methyldopa, was admitted to the emergency department. The patient presented with a 1-week history of fever and sore throat, according to a relative.
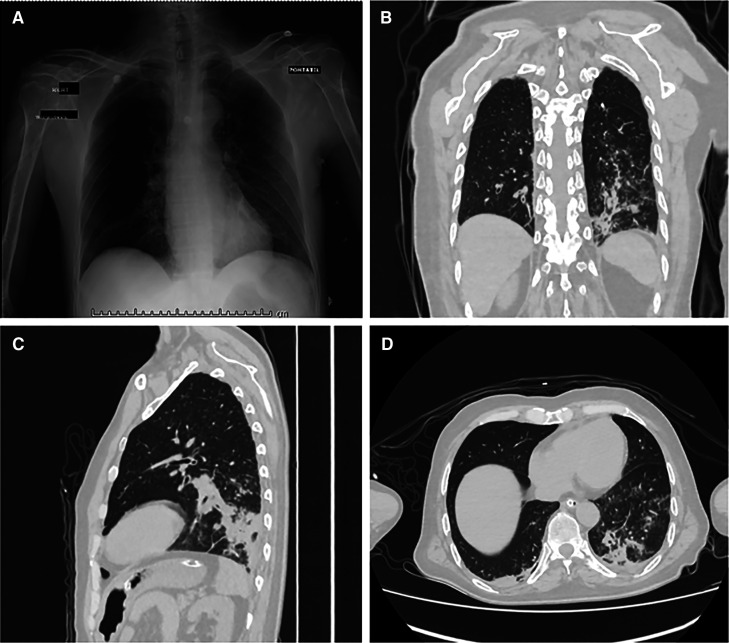
On admission, his vital signs showed a temperature of 36°C, heart rate of 72 beats/minute, respiratory rate of 24/minute, blood pressure of 82/46 mm Hg, and oxygen saturation of 92%. Physical examination revealed a sacral pressure ulcer grade IV, abolished vesicular murmur in the lower third and mid-third of the right hemithorax, rales in the pulmonary apex and left hemithorax, and diffuse rhonchi. The patient was obtunded, and his Glasgow coma scale score was 11/15. Laboratory results showed mild anemia (hemoglobin 11.4 g/dL), leukocytes 8.11 × 103/m, platelets 179 × 103/m, and hyponatremia (sodium 122 mmol/L). An arterial blood gas test revealed metabolic alkalosis with respiratory alkalosis. In addition, the urinalysis showed hematuria and leukocyturia. The blood culture was negative, and the urine culture presented the growth of Proteus mirabilis wild-type. The sputum smear was positive for acid-fast bacilli. A chest x-ray showed reinforcement of the pulmonary interstitium, a micronodular pattern with right predominance, elevation of both hemidiaphragms with right predominance, and the usual placement of a central venous catheter (Figure 1A). Computed tomography of the lung in a tree-in-bud pattern involving the left lower lobe, in addition to bibasilar atelectasis and mediastinal adenopathy (Figures 1B–D).
Figure 1.
Chest x-ray and computed tomography (CT). (A) Chest x-ray in antero-posterior view. (B) Chest CT in coronal view. (C) Chest CT in sagittal view. (D) Chest CT in axial view.
Broad-spectrum antibiotic therapy was initiated with ceftriaxone and clindamycin, which was administered for 12 days. Likewise, the patient received the basic tuberculosis treatment regimen (isoniazid, rifampicin, ethambutol, and pyrazinamide) from the Peruvian Ministry of Health throughout his hospital stay. The patient also received valproate for seizure prevention, and a nasogastric tube and a Foley catheter were inserted. After the initial antibiotic therapy was suspended for 13 days owing to clinical improvement, the patient presented with a fever and decreased consciousness; therefore, a new urine culture was performed and empirical treatment with ceftriaxone was reinitiated. The urine culture showed the growth of extended-spectrum beta-lactamase–producing Escherichia coli (detected by the BD Phoenix M50 system), and the treatment was changed to meropenem.
Nevertheless, in the absence of clinical improvement and persistent fever, another urine culture was performed, showing the growth of C. lapagei. The strain was resistant to cefazolin, cefuroxime, ceftriaxone, cefepime, cefoxitin, imipenem, meropenem, ertapenem, gentamicin, amikacin, trimethoprim/sulfamethoxazole, ciprofloxacin, and levofloxacin. The identification was performed in CLED agar using the Phoenix M50 system (Table 1). According to the susceptibility pattern, the patient received amoxicillin/clavulanate. After 14 days of treatment, the antibiotic was changed to piperacillin/tazobactam plus clindamycin for 5 days to widen antibiotic coverage. The patient was finally discharged and continued outpatient treatment of tuberculosis.
Table 1.
Antimicrobial susceptibility of Cedecea lapagei
| Antibiotic | MIC (μg/mL) | Interpretation |
|---|---|---|
| Amoxicillin/clavulanate | ≤ 8/4 | Sensitive |
| Piperacillin/tazobactam | 32/4–64/4 | Intermediate |
| Cefazolin | ≥ 8 | Resistant |
| Cefuroxime | ≥ 32 | Resistant |
| Ceftriaxone | ≥ 4 | Resistant |
| Cefepime | ≥ 16 | Resistant |
| Cefoxitin | ≥ 32 | Resistant |
| Imipenem | ≥ 4 | Resistant |
| Meropenem | ≥ 4 | Resistant |
| Ertapenem | ≥ 4 | Resistant |
| Gentamicin | ≥ 16 | Resistant |
| Amikacin | ≥ 64 | Resistant |
| Trimethoprim/sulfamethoxazole | ≥ 4/76 | Resistant |
| Nitrofurantoin | 64 | Intermediate |
| Ciprofloxacin | ≥ 2 | Resistant |
| Levofloxacin | ≥ 4 | Resistant |
MIC = minimum inhibitory concentration.
Mechanism of resistance: serine-based carbapenemases; phenotype MLSb.
MATERIALS AND METHODS
Registration and reporting.
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines, and the Cochrane Handbook for Systematic Reviews was used to draft this manuscript.10 A short version of the protocol was submitted to the International Prospective Register of Systematic Reviews database (ID: CRD42023404648).
Search strategy and databases.
We followed the guidelines of the Peer Review of Electronic Search Strategies for building the search strategy.11 The search formula was based on MeSH, Emtree, and free terms. The search term was “Cedecea lapagei.” The search strategy was modified as needed for all databases.
The systematic search was simultaneously run in all databases from inception to January 28, 2023. We did not apply language restrictions for this systematic review. We searched through the following databases: PubMed, Web of Science, Scielo, Embase, and Scopus, and a manual search was also performed. We have attached the complete search strategy as supplementary material (Supplemental Table 1).
Eligibility criteria.
The case reports of C. lapagei infection in patients of any age were included. Studies that were published as abstracts or letters were also included, provided the available information made data collection possible.
Study selection process and data extraction.
After records were retrieved from all the databases, duplicates were removed using Rayyan QCRI (Rayyan Systems Inc., Cambridge, MA).12 This software was used for screening by titles and abstracts. Three researchers independently performed this process (P. V.-P., A. A.-C., and J. R. U.-B.). The same researchers independently reviewed the full-text articles from the remaining records. Afterward, two authors (A. A.-C. and J. R. U.-B.) independently extracted the data from each of the included studies in pre-set Google Sheets. We resolved any conflict or discrepancy concerning the inclusion of articles at any stage of the selection process by consensus. The following information was extracted: author; publication date; country; patient’s age, sex, medical diagnosis, clinical presentation, and comorbidities; diagnostic method; antimicrobial susceptibility; antibiotic treatment; and outcome.
Quality assessment.
The quality assessment of case reports was performed with the Joanna Briggs Institute’s critical appraisal tool for case reports.13 A score of seven or more stars was deemed to have a low risk of bias, whereas a score of six or fewer stars was deemed to have a high risk of bias.
RESULTS
Search results.
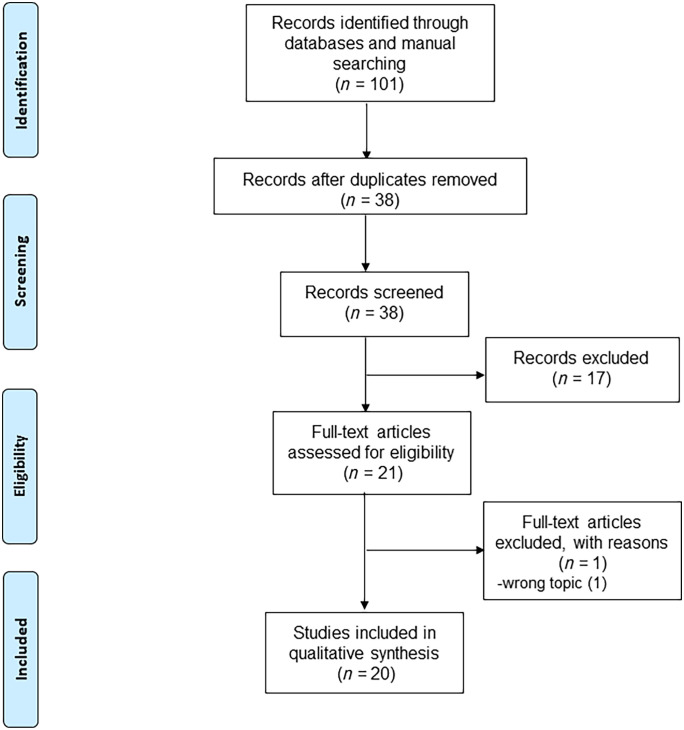
The systematic search retrieved a total of 101 records, of which 63 duplicates were removed. According to the inclusion criteria, after articles were excluded by title/abstracts and full-text articles were assessed, 20 articles were identified as eligible for this systematic review.4–8,14–28 The detailed flow chart of the literature selection is shown in Figure 2.
Figure 2.
Flowchart of included studies.
Quality assessment.
In the quality assessment of the case reports, all studies were found to have a low risk of bias (Supplemental Table 2).
Study characteristics.
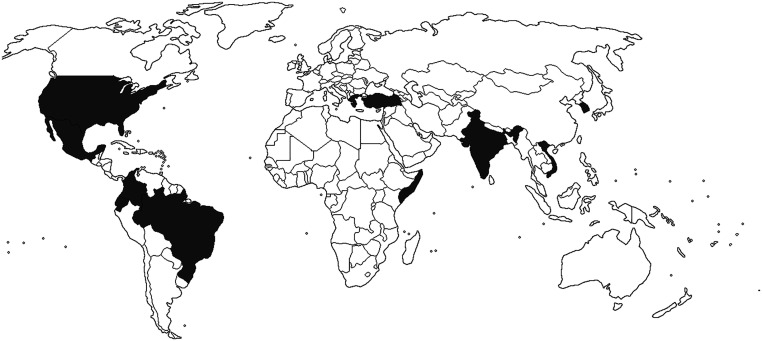
The characteristics of the case reports are summarized in Table 2. Of the 20 case reports included (17 males and 3 females), 4 cases were reported in children under 1 year and the rest in adults (> 18 years). The reports were published between 2006 and 2022. Most of the cases were reported in countries from Asian and American continents (Figure 3).
Table 2.
Summary of clinical cases of Cedecea lapagei
| Author | Year | Country | Age | Sex | Medical diagnosis | Clinical presentation | Comorbidities | Diagnostic method | Antimicrobial susceptibility testing (antibiogram) | Antibiotic treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duperret et al.14 | 2020 | United States | 45 | Male | Sinusitis | Foul rhinorrhea, sinus headache, and fever | Asthma | Culture from purulent nasal secretions | Resistant to: amoxicillin/clavulanate, ampicillin, and cefazolin Sensitive to: ciprofloxacin, gentamicin, meropenem, tetracycline, and tobramycin Intermediate sensitivity to: cefuroxime | Oral ciprofloxacin + nasal gentamicin/dexamethasone | Patient recovered (discharged) |
| Hai et al.7 | 2020 | Vietnam | 38 | Male | Pneumonia and septic shock | Fatigue, cough, sputum, myalgia, fever, and shortness of breath | Diabetes mellitus type 2 | Sputum culture | Resistant to: trimethoprim/ sulfamethoxazole Sensitive to: piperacillin/ tazobactam, ceftazidime, ceftriaxone, cefazolin, cefoxitin, aztreonam, imipenem, meropenem, amikacin, gentamicin, tobramycin, levofloxacin, and ciprofloxacin Intermediate sensitivity to: piperacillin | IV meropenem + IV ciprofloxacin | Patient recovered (discharged) |
| Ramaswamy et al.15 | 2019 | India | 35 days | Male | Nosocomial pneumonia and sepsis | Poor peripheral pulses, lethargy, fever, and severe respiratory distress | Late preterm | Blood culture | Resistant to: meropenem, colistin, amikacin, gentamycin, and ceftazidime Sensitive to: piperacillin, piperacillin/ tazobactam, trimethoprim/ sulfamethoxazole, ciprofloxacin, and levofloxacin | IV piperacillin/ tazobactam | Patient recovered (discharged) |
| Chavez Herrera et al.16 | 2018 | Mexico | 52 | Male | Infection of hemorrhagic bullae causing necrotizing fasciitis and septic shock | Fever, pain, edema in his right lower limb, and multiple organ failure | Liver cirrhosis and treated hypertension | Bullae fluid culture | Resistant to: ampicillin, cefazolin, and imipenem Sensitive to: amikacin, aztreonam, ceftriaxone, ceftazidime, cefotaxime, ciprofloxacin, cefepime, cefuroxime, cefotetan, gentamicin, levofloxacin, meropenem, moxifloxacin, and piperacillin/ tazobactam Intermediate sensitivity to: ampicillin/sulbactam | IV clindamycin + IV ceftriaxone | Death |
| Kury et al.17 | 2017 | Brazil | 110 days | Male | Ventilator-associated pneumonia and sepsis | Fever and yellow sputum | Ischemic encephalopathy sequelae and chronic lung disease | Cultures from blood and tracheal aspirates | Resistant to: NR Sensitive to: piperacillin, imipenem, amikacin, gentamycin, ciprofloxacin, levofloxacin, meropenem, and trimethoprim/ sulfamethoxazole | IV meropenem | Patient recovered (discharged) |
| Biswal et al.18 | 2015 | India | 50 | Male | Ulcer and pus formation at the cancer site | Fever and pain at the local site of ulcer, radiating to ear and forehead | Squamous cell carcinoma of the right buccal mucosa undergoing chemotherapy | Culture of pus samples from the ulcer site | Resistant to: ampicillin/sulbactam, tetracycline, and tigecycline Sensitive to: amikacin, gentamicin, ceftazidime, ceftriaxone, cefepime, ciprofloxacin, meropenem, and trimethoprim/ sulfamethoxazole | IV ciprofloxacin | Patient recovered (discharged) |
| Salazar et al.6 | 2013 | Ecuador | 24 | Male | Traumatic wound infection | Fever, poor condition of healing tissue, increased discharge, and bad odor | No comorbidities | Culture of secretion obtained from granulation tissue | Resistant to: ampicillin and ampicillin/ sulbactam Sensitive to: cefuroxime, amikacin, trimethoprim/ sulfamethoxazole, ciprofloxacin, cefotaxime, carbapenems | IV ampicillin/sulbactam | Patient recovered (discharged) |
| Hong et al.19 | 2015 | South Korea | 76 | Male | Pneumonia | Fever and dyspnea | Chronic obstructive pulmonary disease | Sputum culture | Resistant to: amoxicillin/clavulanate and cefoxitin Sensitive to: piperacillin, cefotaxime, ceftazidime, cefepime, imipenem, amikacin, ciprofloxacin, tetracycline, and trimethoprim/ sulfamethoxazole | IV cefpodoxime | Patient recovered (discharged) |
| Sanchez-Lopez et al.5 | 2013 | Mexico | 34 | Male | Pneumonia | Fever, dyspnea, bilateral alveolar infiltrates, copious yellow sputum, and disseminated intravascular coagulation | Acute promyelocytic leukemia | Sputum culture | Resistant to: amikacin, ampicillin, aztreonam, cefazolin, cefepime, ceftriaxone, ciprofloxacin, ertapenem, imipenem, meropenem, moxifloxacin, nitrofurantoin, piperacillin/ tazobactam, and trimethoprim/ sulfamethoxazole Sensitive to: tigecycline Intermediate sensitivity to: ampicillin/sulbactam, gentamicin, and tobramycin | IV cefepime + IV vancomycin | Patient recovered (discharged) |
| Yetkin et al.20 | 2008 | Turkey | 38 | Male | Pneumonia from the aspiration of upper airway secretion | Fever, dyspnea | Subarachnoid hemorrhage and chronic obstructive pulmonary disease | Culture from bronchoalveolar lavage fluid | Resistant to: amoxicillin, ampicillin, amoxicillin/clavulonate, and cephalothin Sensitive to: amikacin, ciprofloxacin, trimethoprim/ sulfamethoxazole, imipenem, aztreonam, cefepime, ceftriaxone, ceftazidime, cefotaxime, cefuroxime, piperacillin / tazobactam, gentamicin, piperacillin, and tetracycline | IV amikacin + IV vancomycin + IV meropenem | Death |
| Dalamaga et al.21 | 2008 | Greece | 47 | Male | Wound infection | Fever, full-thickness burns in the right knee, and erythema, vesicles, and ulcerations in the left knee | Diabetes mellitus type 2 | Blood and wound cultures | Resistant to: gentamicin, tobramycin, cephalothin, cefuroxime, cefoxitin, ceftazidime, tetracycline, and ampicillin Sensitive to: amikacin, meropenem, cefotaxime, cefepime, aztreonam, amoxicillin/clavulanate, piperacillin/tazobactam, trimethoprim/ sulfamethoxazole, ciprofloxacin, and levofloxacin | IV cefotaxime + IV amikacin | Patient recovered (discharged) |
| Davis and Wall4 | 2006 | United States | 55 | Male | Peritonitis | Fever, abdominal pain, and cloudy dialysis effluent | Hypertension, orthotopic liver transplantation secondary to cirrhosis related to hepatitis C, continuous peritoneal dialysis, and end-stage renal disease secondary to glomerular disease | Culture from peritoneal fluid | NR | IV vancomycin + IV gentamicin | Patient recovered (discharged) |
| Mohamud et al.22 | 2022 | Somalia | 55 | Male | Urinary tract infection | Acute exacerbations of renal failure and irritative voiding symptoms | Chronic renal failure, uncontrolled diabetes mellitus type 2, and hypertension | Culture from urine | Resistant to: ceftriaxone, cefazolin, ceftazidime, cefixime, ampicillin, and amoxicillin/clavulanate Sensitive to: amikacin, gentamicin, trimethoprim/ sulfamethoxazole, ciprofloxacin, levofloxacin, imipenem, and ertapenem | Oral levofloxacin | Patient recovered (discharged) |
| Cárdenas et al.23 | 2015 | Colombia | 47 | Male | Acute osteomyelitis | Fever, edema, and erythema in the surgical area | No comorbidities | Culture from bone biopsy | Sensitive to: ertapenem | IV oxacillin + rifampicin empirically, switched to ertapenem after culture | Patient recovered (discharged) |
| Arellano Aguilar et al.28 | 2016 | Mexico | 67 | Female | Bacteremia and burns on 20% of body surface | Burns in the granulation stage with moderate edema | Diabetes mellitus type 1, hypertension, and dyslipidemia | Culture from bronchial aspirate and blood culture | Resistant to: ampicillin, amikacin, and piperacillin/ tazobactam Sensitive to: meropenem and imipenem | IV piperacillin/ tazobactam + caspofungin initially, switched to linezolid on day 20 + imipenem/cilastatin on day 24 | Patient recovered (discharged) |
| Çekin et al.24 | 2014 | Turkey | 40 | Male | Urinary tract infection | Fever | Quadriplegia and use of urinary catheter | Culture from urine | Resistant to: ampicillin, gentamicin, tobramycin, cefazolin, cephalothin, amikacin, ampicillin/sulbactam, ertapenem, imipenem, meropenem, piperacillin, piperacillin/ tazobactam, cefepime, cefoxitin, cefotaxime, cefuroxime, and aztreonam Sensitive to: ciprofloxacin, levofloxacin, and trimethoprim/ sulfamethoxazole | IV ciprofloxacin | Patient recovered (discharged) |
| Islam et al.25 | 2016 | India | First hours of life | Female | Sepsis with pneumonia | Respiratory distress immediately after birth | Meconium-stained amniotic fluid and low birth weight | Blood culture | Resistant to: amoxicillin/clavulonate, ceftazidime, ceftriaxone, trimethoprim/sulfamethoxazole, gentamycin, cefuroxime, and piperacillin/tazobactam Sensitive to: ciprofloxacin and imipenem | IV amikacin + IV ciprofloxacin | Patient recovered (discharged) |
| Ahmad et al.8 | 2017 | India | 26 days | Female | Late-onset sepsis | NR | Preterm, apnea | Blood culture | Resistant to: imipenem, meropenem, aztreonam, ceftazidime, cefotaxime, cefoxitin, cefepime, and cefoperazone/sulbactam | IV cefotaxime + IV amikacin | Patient recovered (discharged) |
| Deveci and Arpag26 | 2021 | Turkey | 76 | Male | Pneumonia | Fever, shortness of breath, cough, sputum, wheezing, and impaired consciousness | COVID-19 2 weeks ago | Sputum culture | Resistant to: amoxicillin/clavulanate, piperacillin/ tazobactam, cefuroxime, cefuroxime acetil, ceftriaxone, ceftazidime, cefepime, amikacin, gentamicin, ciprofloxacin, tigecycline, and colistin Intermediate sensitivity to: meropenem and trimethoprim/ sulfamethoxazole | IV meropenem + IV moxifloxacin | Patient recovered (discharged) |
| González Rivera et al.27 | 2022 | Mexico | 57 | Male | Pneumonia and septic shock | Fever, dyspnea, decreased amplection, use of accessory musculature with oxygen through nasal prongs at 5 L per minute, increased vocal vibrations on palpation, and bilateral infrascapular crepitant rales | HIV treatment discontinuation for the last 3 years | Culture from bronchial aspirate | Resistant to: ampicillin, gentamicin, tobramycin, cefazolin, cephalothin, amikacin, ampicillin/sulbactam, ertapenem, imipenem, meropenem, piperacillin, cefepime, cefotaxime, cefuroxime, aztreonam, ciprofloxacin, levofloxacin, and trimethoprim/ sulfamethoxazole | NR | Death |
IV = intravenous; NR = not reported. Age is shown as years unless otherwise noted.
Figure 3.
Geographical distribution of Cedecea lapagei studies. Countries where C. lapagei was isolated are shaded in dark grey. (Source: Blank map political world territories.png [June 10, 2017]. Wikimedia Commons, the free media repository. Available at: https://commons.wikimedia.org/wiki/File:Blank_map_political_world_territories.png. Accessed March 8, 2023.)
Fever was the most common manifestation (75%). There were many forms of presentation, the most common being pneumonia (45%), followed by sepsis (20%) and septic shock (15%), whereas skin involvement was present in four cases and one case reported osteomyelitis. Cedecea lapagei was most frequently isolated from blood culture (30%), followed by sputum (20%) and bronchoalveolar lavage (20%). Although comorbidities were variable, 90% of cases had at least one comorbidity. All reports of children under 6 months of age were associated with septic shock or sepsis. Death was reported in 15% of cases (Table 2).
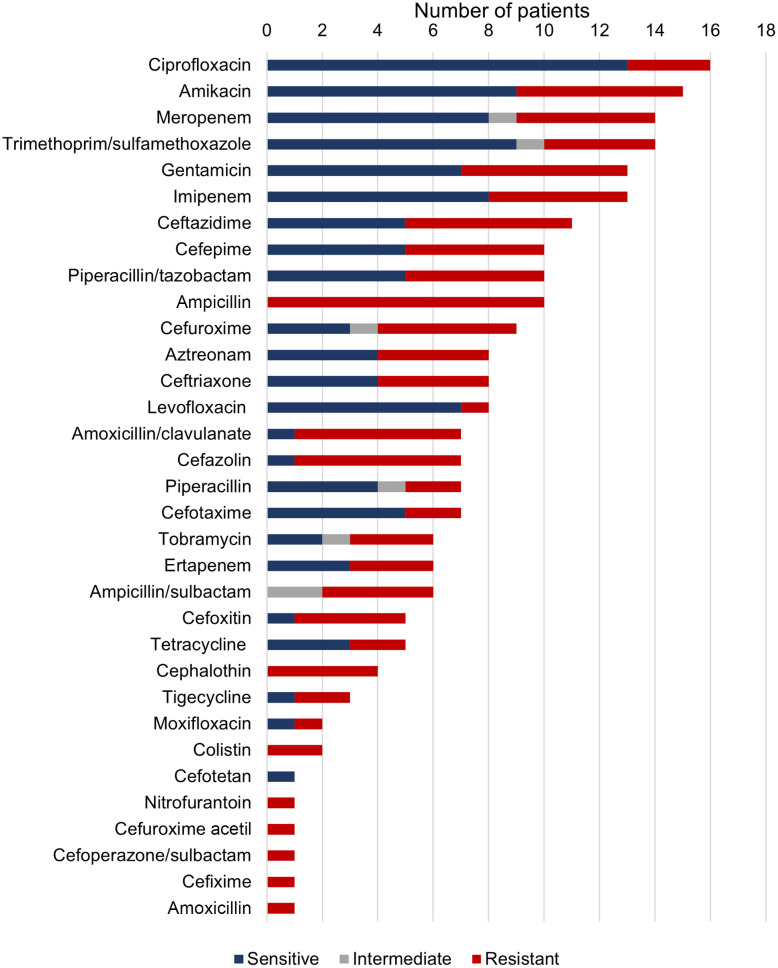
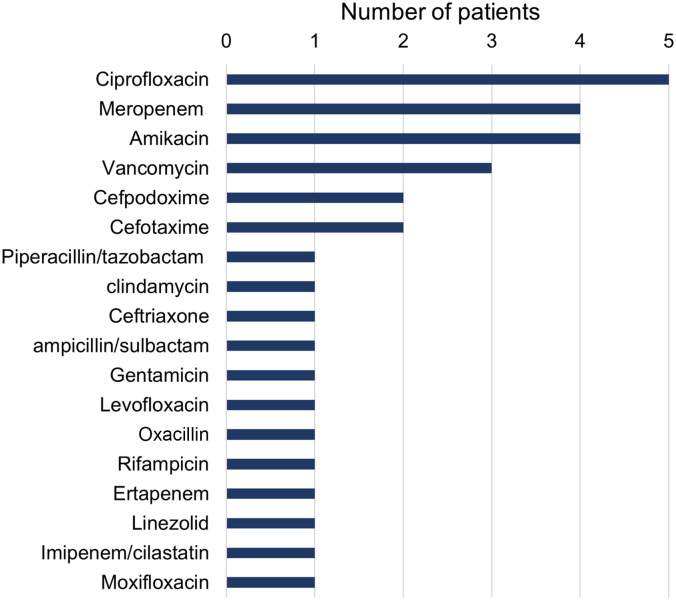
Antibiotic susceptibility was tested in 95% of cases. Most isolates were tested for susceptibility to ciprofloxacin: of 16 isolates, 3 showed resistance (18.8%). Most of the isolates were sensitive to levofloxacin (87.5%), ciprofloxacin (81.3%), cefotaxime (71.4%), trimethoprim/sulfamethoxazole (69.2%), meropenem (61.5%), imipenem (61.5%), amikacin (60%), and gentamicin (53.8%). On the other hand, all isolates were resistant to ampicillin (N = 10), cephalothin (N = 4), colistin (N = 2), nitrofurantoin (N = 1), cefuroxime acetil (N = 1), cefoperazone/sulbactam (N = 1), cefixime (N = 1), and amoxicillin (N = 1) (Figure 4). Regarding antibiotic treatment, most patients received ciprofloxacin (25%), followed by meropenem (20%) and amikacin (20%) (Figure 5).
Figure 4.
Susceptibility of clinical isolates of Cedecea lapagei to tested antibiotics.
Figure 5.
Antibiotics used for treatment of Cedecea lapagei infections.
DISCUSSION
This is the first case report of C. lapagei in Peru, involving a patient who was coinfected with tuberculosis and had epilepsy and a bedridden state secondary to Parkinson’s disease. The bacterium strain was extensively drug resistant, and the phenotype was MLSb. In addition, this is the first systematic review of cases of C. lapagei. A total of 20 cases were reported worldwide between 2006 and 2022, mainly in the Asian and American continents. Most patients had comorbidities, and 15% died.
Our case report revealed an extensive antibiotic resistance pattern, which is in line with the high rates of antimicrobial drug resistance reported in Peru.9 The patient had a lengthy hospital stay and was exposed to several broad-spectrum antibiotics before the isolation of C. lapagei. Both of these factors have been associated with an increased risk of antibiotic resistance, mainly the use of beta-lactams such as ceftriaxone or carbapenems, which our patient received.29,30 The most concerning aspect of the clinical case was the presence of pulmonary tuberculosis, which is an endemic public health concern in Peru.31 This raises concern because the emergence of new bacterium outbreaks in low- and middle-income countries represents a latent threat owing to the vulnerability of their health care systems.
Most patients in our review had comorbidities that pose a significant risk factor for opportunistic infections. This is a common characteristic of the Cedecea species.32 Although it is too early to determine with certainty which comorbidities are associated with infection by this bacterium, it is clear that it affects mainly immunocompromised individuals, and further studies are needed to clarify this topic. Likewise, different species of Cedecea have been isolated from invasive medical devices such as central venous catheters and urinary catheters.33–35 This suggests the possible role of biofilm in its pathogenicity; thus, the isolation of C. lapagei from an invasive device should not be underestimated, mainly in immunocompromised hosts or those with multiple comorbidities, such as our patient who had a urinary catheter in a nosocomial environment.
The antibiotic susceptibility pattern was variable among the isolates of C. lapagei. Although some antibiotic classes such as quinolones, tetracyclines, and carbapenems were the most sensitive, most isolates were resistant to penicillin and cephalosporins. This pattern is in line with the results of another review of Cedecea species.32 Consequently, most patients in our review received quinolones, tetracyclines, and carbapenems. Interestingly, the patient in our case report was resistant to all these groups except for amoxicillin/clavulanate. This highlights the importance of antibiotic susceptibility testing in directing therapy because of this bacterium’s variability.
There is little information on the mechanisms of resistance to antibiotics in Cedecea. Beta-lactamases have been reported as the main resistance mechanism in C. lapagei. Some of the genes detected were blaNDM-1, blaCTX-M, blaSHV, and blaTEM. A new ampC beta-lactamase–named CDA-1 combined with porin deficiency (with electrophoretic properties of E. coli ompC and ompF porins) has also been reported in C. davisae, which produced a carbapenem-resistant phenotype.36
Likewise, sequencing of the complete genome of C. neteri35 has shown the presence of multiple open reading frames with sequence similarity to ampC-type beta-lactamases (probably inducible) and four metallo-beta-lactamase–related proteins. Hence, the presence of some of these chromosomal enzymes in C. lapagei is not ruled out. It has also been observed that isolates of Cedecea possess natural resistance to colistin (Polymyxin E), probably associated with modifications of lipopolysaccharides by cationic substitution in their genome containing mgrB, phoP, and phoQ. However, no plasmid DNA has been found, which decreases the possibility of MCR-1 (mobilized colistin resistance) derived from the plasmid.37
Our study’s limitations should be taken into account when interpreting the results. The main limitation of our case report is the inability to identify the bacterium by 16S ribosomal RNA gene sequencing and resistance genes, as this molecular technique is not available in our hospital. Nevertheless, a recent study reported that the BD Phoenix M50 system performs reliably for antibiotic susceptibility testing in clinical microbiology laboratories.38 Regarding the systematic review, the case reports were not standardized; thus, some variables were not reported. In addition, because the reports were not representative of the population, it was not possible to gather data or report rates, ratios, incidence, or prevalence. Thus, our findings do not imply a cause-effect relationship and cannot be generalized.
CONCLUSION
Cedecea lapagei should be suspected in compromised hosts, particularly in those with pneumonia. Nevertheless, further reports on this bacterium are needed as it can affect several organs and its antibiotic susceptibility pattern is variable. Thus, individualized therapeutic management is required for each case. Given the emergence of this bacterium in new latitudes, microbiology laboratories need to be properly equipped to isolate it. Quinolones, tetracyclines, and carbapenems seem to be the first therapeutic option, and our findings suggest that mortality due to this bacterium is low.
Supplemental Material
ACKNOWLEDGMENT
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1. Grimont PAD, Grimont F, Farmer JJ, Asbury MA, 1981. Cedecea davisae gen. nov., sp. nov. and Cedecea lapagei sp. nov., new Enterobacteriaceae from clinical specimens. Int J Syst Bacteriol 31: 317–326. [Google Scholar]
- 2. Akinosoglou K, Perperis A, Siagris D, Goutou P, Spiliopoulou I, Gogos CA, Marangos M, 2012. Bacteraemia due to Cedecea davisae in a patient with sigmoid colon cancer: a case report and brief review of the literature. Diagn Microbiol Infect Dis 74: 303–306. [DOI] [PubMed] [Google Scholar]
- 3. Cooney S, O’Brien S, Iversen C, Fanning S, 2014. Bacteria: other pathogenic Enterobacteriaceae – Enterobacter and other genera. Motarjemi Y, ed. Encyclopedia of Food Safety. San Diego, CA: Academic Press, 433–441. [Google Scholar]
- 4. Davis O, Wall BM, 2006. “Broom straw peritonitis” secondary to Cedecea lapagei in a liver transplant recipient. Perit Dial Int 26: 512–513. [PubMed] [Google Scholar]
- 5. Sanchez-Lopez LA, Ibarra BS, de la Garza JAC, Rada F de JMP, Nuñez AIS, López MGR, 2013. First reported case of pneumonia caused by Cedecea lapagei in America. Braz J Infect Dis 17: 626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salazar G, Almeida A, Gómez M, 2013. Cedecea lapagei traumatic wound infection: case report and literature review [in Spanish]. Rev Chilena Infectol 30: 86–89. [DOI] [PubMed] [Google Scholar]
- 7. Hai PD, Dung NM, Tot NH, Chinh NX, Thuyet BT, Hoa LTV, Son PN, Thanh LV, 2020. First report of pneumonia and septic shock caused by Cedecea lapagei in Vietnam. New Microbes New Infect 36: 100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmad N, Ali SM, Khan AU, 2017. First reported New Delhi metallo-β-lactamase-1-producing Cedecea lapagei . Int J Antimicrob Agents 49: 118–119. [DOI] [PubMed] [Google Scholar]
- 9. García C. et al. , 2012. Antimicrobial drug resistance in Peru. Emerg Infect Dis 18: 520–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D, 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C, 2016. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 75: 40–46. [DOI] [PubMed] [Google Scholar]
- 12. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A, 2016. Rayyan – a web and mobile app for systematic reviews. Syst Rev 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moola S, Aromataris E, Munn Z. JBI Manual for Evidence Synthesis. North Adelaide, Australia: JBI. Available at: https://synthesismanual.jbi.global. Accessed June 05, 2023. [Google Scholar]
- 14. Duperret ME, 2020. Sinusitis caused by a rare organism, Cedecea lapagei . BMJ Case Rep 13: e.235331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramaswamy VV, Gummadapu S, Suryanarayana N, 2019. Nosocomial pneumonia and sepsis caused by a rare organism Cedecea lapagei in an infant and a review of literature. BMJ Case Rep 12: e229854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chavez Herrera VR, Rosas De Silva MF, Orendain Alcaraz H, Ceja Espiritu G, Carrazco Peña K, Melnikov V, 2018. Death related to Cedecea lapagei in a soft tissue bullae infection: a case report. J Med Case Reports 12: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kury CMH, Yabrudi AA, de Souza TB, de Souza EC, E Silva Costa LT, Soares CB, Calixto GA, Gramático MR, 2017. First reported case of ventilator-associated pneumonia and sepsis caused by Cedecea lapagei in a Brazilian neonatal intensive care unit. J Pediatric Infect Dis Soc 6: 209–210. [DOI] [PubMed] [Google Scholar]
- 18. Biswal I, Hussain NA, Grover RK, 2015. Cedecea lapagei in a patient with malignancy: report of a rare case. J Cancer Res Ther 11: 646. [DOI] [PubMed] [Google Scholar]
- 19. Hong SK, Lee JS, Kim EC, 2015. First Korean case of Cedecea lapagei pneumonia in a patient with chronic obstructive pulmonary disease. Ann Lab Med 35: 266–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yetkin G, Ay S, Kayabaş U, Gedik E, Güçlüer N, Calişkan A, 2008. A pneumonia case caused by Cedecea lapagei [Article in Turkish]. Mikrobiyol Bul 42: 681–684. [PubMed] [Google Scholar]
- 21. Dalamaga M, Karmaniolas K, Arsenis G, Pantelaki M, Daskalopoulou K, Papadavid E, Migdalis I, 2008. Cedecea lapagei bacteremia following cement-related chemical burn injury. Burns 34: 1205–1207. [DOI] [PubMed] [Google Scholar]
- 22. Mohamud HA, Mohamud RYH, 2022. Cedecea lapagei an extremely rare uropathogen: a case report. J Pharm Res Int 34: 1–5. [Google Scholar]
- 23. Cárdenas JL, 2015. Acute osteomyelitis by Cedecea lapagei . Acta Med Colomb 40: 246–248. [Google Scholar]
- 24. Çekin Y, Kızılateş F, Süleyman D, Öztoprak N, Hilmi Çekin A, 2014. The first urinary tract infection caused by Cedecea lapagei: a case report and review of the literature. Gaziantep Med J 20: 193–195. [Google Scholar]
- 25. Islam AKS, Bora R, Ahmed R, Borah AK, Ramasamy S, 2016. A case of neonatal sepsis with pneumonia due to Cedecea lapagei . IOSR-JDMS 15: 84–85. [Google Scholar]
- 26. Deveci O, Arpag H, 2021. Post-Covid-19 “Cedecea lapagei” pneumonia. Biomed Res Clin Prac 6, doi: 10.15761/BRCP.1000226. [DOI] [Google Scholar]
- 27. González Rivera G, Nava UR, Cano Díalmayer AL, 2022. Reporte de caso en México de neumonía por Cedecea lapagei multidrogorresistente. Respirar 14: 228–233. [Google Scholar]
- 28. Arellano Aguilar G, Basurto AL, Luna AG, Esquivel VH, Castilla Barajas JA, 2016. Bacteriemia por Cedecea lapagei . Acta Med Grupo Ángeles 14: 176–178. [Google Scholar]
- 29. Falagas ME, Kopterides P, 2006. Risk factors for the isolation of multi-drug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: a systematic review of the literature. J Hosp Infect 64: 7–15. [DOI] [PubMed] [Google Scholar]
- 30. Raman G, Avendano EE, Chan J, Merchant S, Puzniak L, 2018. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alarcón V, Alarcón E, Figueroa C, Mendoza-Ticona A, 2017. Tuberculosis in Peru: epidemiological situation, progress and challenges for its control [Article in Spanish]. Rev Peru Med Exp Salud Publica 34: 299–310. [DOI] [PubMed] [Google Scholar]
- 32. Thompson DK, Sharkady SM, 2020. Expanding spectrum of opportunistic Cedecea infections: current clinical status and multidrug resistance. Int J Infect Dis 100: 461–469. [DOI] [PubMed] [Google Scholar]
- 33. Peretz A, Simsolo C, Farber E, Roth A, Brodsky D, Nakhoul F, 2013. A rare bacteremia caused by Cedecea davisae in patient with chronic renal disease. Am J Case Rep 24: 216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perkins SR, Beckett TA, Bump CM, 1986. Cedecea davisae bacteremia. J Clin Microbiol 24: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ginn PS, Tart SB, Sharkady SM, Thompson DK, 2018. Urinary catheter colonization by multidrug-resistant Cedecea neteri in patient with benign prostatic hyperplasia. Case Rep Infect Dis 2018: 7529526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ammenouche N, Dupont H, Mammeri H, 2014. Characterization of a novel AmpC β-lactamase produced by a carbapenem-resistant Cedecea davisae clinical isolate. Antimicrob Agents Chemother 58: 6942–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poirel L, Jayol A, Nordmanna P, 2017. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev 30: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong JS, Kim D, Kang DY, Park BY, Yang S, Yoon EJ, Lee H, Jeong SH, 2019. Evaluation of the BD Phoenix M50 Automated Microbiology System for antimicrobial susceptibility testing with clinical isolates in Korea. Microb Drug Resist 25: 1142–1148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.