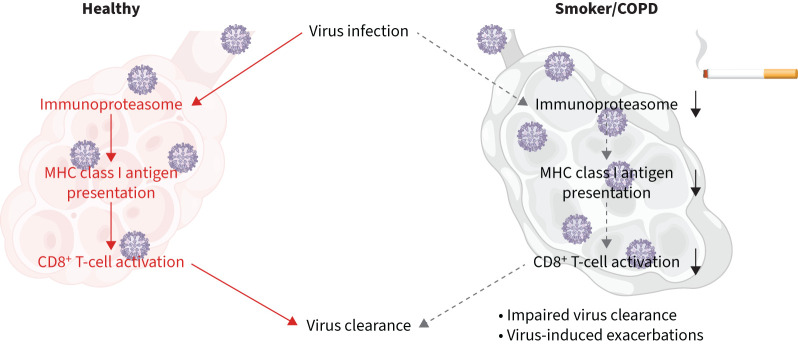
Graphical abstract
Main findings of the study. Cigarette smoke impairs virus-induced upregulation of the major histocompatibility complex (MHC) class I antigen presentation machinery resulting in reduced activation of antiviral CD8+ T-cells.
Abstract
Background
Virus infections drive COPD exacerbations and progression. Antiviral immunity centres on the activation of virus-specific CD8+ T-cells by viral epitopes presented on major histocompatibility complex (MHC) class I molecules of infected cells. These epitopes are generated by the immunoproteasome, a specialised intracellular protein degradation machine, which is induced by antiviral cytokines in infected cells.
Methods
We analysed the effects of cigarette smoke on cytokine- and virus-mediated induction of the immunoproteasome in vitro, ex vivo and in vivo using RNA and Western blot analyses. CD8+ T-cell activation was determined in co-culture assays with cigarette smoke-exposed influenza A virus (IAV)-infected cells. Mass-spectrometry-based analysis of MHC class I-bound peptides uncovered the effects of cigarette smoke on inflammatory antigen presentation in lung cells. IAV-specific CD8+ T-cell numbers were determined in patients’ peripheral blood using tetramer technology.
Results
Cigarette smoke impaired the induction of the immunoproteasome by cytokine signalling and viral infection in lung cells in vitro, ex vivo and in vivo. In addition, cigarette smoke altered the peptide repertoire of antigens presented on MHC class I molecules under inflammatory conditions. Importantly, MHC class I-mediated activation of IAV-specific CD8+ T-cells was dampened by cigarette smoke. COPD patients exhibited reduced numbers of circulating IAV-specific CD8+ T-cells compared to healthy controls and asthmatics.
Conclusion
Our data indicate that cigarette smoke interferes with MHC class I antigen generation and presentation and thereby contributes to impaired activation of CD8+ T-cells upon virus infection. This adds important mechanistic insight on how cigarette smoke mediates increased susceptibility of smokers and COPD patients to viral infections.
Extract
COPD is characterised by progressive airflow limitation and alveolar destruction, resulting in reduced lung function and severely diminished quality of life [1]. >10% of the world's population are diagnosed with COPD, with a mortality that makes COPD the third leading cause of death globally [2]. Tobacco smoke consumption is one of the main risk factors for developing COPD [1]. Exacerbations of the disease with sudden decline in lung function are related to viral infections such as rhinovirus and influenza A virus (IAV) or bacteria [3, 4] and contribute to COPD progression [5, 6].
Tweetable abstract
Cigarette smoke impairs virus-induced upregulation of the MHC class I antigen presentation machinery resulting in reduced activation of antiviral CD8+ T-cells. This may reduce viral clearance and increase susceptibility to viral exacerbations in COPD. https://bit.ly/43o0p3D
Introduction
COPD is characterised by progressive airflow limitation and alveolar destruction, resulting in reduced lung function and severely diminished quality of life [1]. >10% of the world's population are diagnosed with COPD, with a mortality that makes COPD the third leading cause of death globally [2]. Tobacco smoke consumption is one of the main risk factors for developing COPD [1]. Exacerbations of the disease with sudden decline in lung function are related to viral infections such as rhinovirus and influenza A virus (IAV) or bacteria [3, 4] and contribute to COPD progression [5, 6].
A key player in antiviral immunity is the activation of cytotoxic CD8+ T-cells which specifically kill virus-infected cells. The importance of fast and effective CD8+ T-cell activation in the control and resolution of virus infections is currently being re-discovered in coronavirus disease 2019 patients [7, 8]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) effectively impairs the ability of infected cells to produce interferons, which under physiological conditions locally restrict virus replication and instantly alarm the innate immune system [9]. Unhindered virus replication drives an overexpansion of innate immune responses that contributes to hyperinflammatory lung immunopathology while protective T-cell responses are delayed [10, 11]. Similarly, viral infections and acute exacerbations in COPD patients are characterised by impaired viral clearance, hyperinflammatory innate immune responses and ineffective T-cell immunity [3, 12–14]. In experimental mouse models, cigarette smoke exposure restricted not only antiviral innate immune responses but also impaired activation of adaptive CD8+ T-cells [15, 16], supporting the notion that smoking is a major driver of impaired antiviral immune responses in COPD [17, 18].
Activation of antiviral cytotoxic CD8+ T-cells is achieved upon recognition of virus-derived antigenic peptides complexed with major histocompatibility complex (MHC) class I molecules on the surface of virus-infected cells [19]. These viral epitopes are mainly generated upon intracellular degradation of viral proteins by the immunoproteasome [20]. The immunoproteasome is a specialised type of proteasome (the main protein-degrading machinery in the cell) that contains a set of inducible catalytic subunits, namely low molecular weight protein (LMP)2, multicatalytic endopeptidase complex subunit (MECL)1 and LMP7. These subunits are transcriptionally induced upon virus infection by type I and II interferon (IFN) signalling and contribute to the generation of antigenic MHC class I epitopes in virus-infected cells [20–22]. Experimental infection of mice with pulmonary viruses causes pronounced induction of the three proteolytic subunits of the immunoproteasome in the lung as an intrinsic part of the antiviral immune defence [21, 23, 24]. Knockout mice lacking one or several of the proteolytic subunits of the immunoproteasome are severely hampered in effectively combatting virus infections, demonstrating the essential function of the immunoproteasome in antiviral T-cell immunity [21, 25]. We have previously shown inhibition of the constitutively expressed immunoproteasome in immune cells by cigarette smoke and in COPD causing impaired MHC class I-mediated CD8+ T-cell activation in vitro and in vivo [26]. In the present study, we investigated whether cigarette smoke interferes with cytokine- and virus-mediated induction of the immunoproteasome and MHC class I antigen presentation in parenchymal cells of the lung and the consequences thereof.
Methods
For details of the materials and methods, the reader is referred to the supplementary material.
Human samples
EDTA–blood samples of human leukocyte antigen (HLA)-A2-positive stable COPD (n=11) and asthma (n=12) patients were obtained from the CPC-M bioArchive at the Comprehensive Pneumology Center (CPC; Munich, Germany). Blood samples from lung healthy donors (n=10) were allocated from the Helmholtz Center Munich. All COPD patients had received yearly IAV vaccinations. The study was approved by the local ethics committee of the Ludwig-Maximilians-University (Munich, Germany; ethic vote #382–10). EDTA–blood from HLA-A2-positive healthy cigarette smokers (ex-smokers and current smokers, n=7) and never-smokers (n=7, including one Shisha smoker) was obtained from the BioMaterialBank North and approved by the local ethics committee of the University of Lübeck (Lübeck, Germany; ethic votes 14–225 and 22–583). Written informed consent was obtained for all study participants. Details on the study participants are given in table 1 and supplementary tables S1 and S2.
TABLE 1.
Patient characteristics
| Control | COPD | Asthma | p-value# | ||||
| Sex | |||||||
| Female | 80.00 | 8/10 | 36.36 | 4/11 | 50.00 | 6/12 | |
| Male | 20.00 | 2/10 | 63.63 | 2/11 | 50.00 | 6/12 | 0.123 |
| Age, years | 55 (37–66) | 10 | 60 (54–70) | 11 | 48 (21–79) | 12 | 0.060 |
| BMI, kg·m−2 | 23.25 (19.53–35.11) | 10 | 22.77 (17.72–35.56) | 11 | 24.11 (18.17–35.06) | 12 | 0.691 |
| Comorbidities¶ | |||||||
| No | 80.00 | 8/10 | 72.72 | 8/11 | 91.67 | 11/12 | |
| Yes | 20.00 | 2/10 | 27.27 | 3/11 | 8.33 | 1/12 | 0.493 |
| Immunosuppressive medication+ | |||||||
| No | 100.00 | 10/10 | 72.72 | 8/11 | 58.33 | 7/12 | |
| Yes | 0.00 | 0/10 | 27.27 | 3/11 | 41.67 | 5/12 | 0.073 |
| Smoking status | |||||||
| Current | 10.00 | 1/10 | 0.00 | 0/11 | 8.33 | 1/12 | |
| Former | 20.00 | 2/10 | 100.00 | 11/11 | 25.00 | 3/12 | |
| Never | 70.00 | 7/10 | 0.00 | 0/11 | 66.67 | 8/12 | 0.002 |
| Pack-years | 0 (0–18) | 8/10 | 32.5 (10–50) | 10 | 0 (0–50) | 12 | <0.001 |
| GOLD stage | |||||||
| I/II/III/IV | 1/2/0/8 | ||||||
| A/B/C/D | 1/1/2/7 | ||||||
| Asthma severity | |||||||
| I/II/III/IV/V | 1/0/2/1/8 | ||||||
| FEV1/FVC, % | 80.0 (70.0–90.0) | 9 | 37.2 (20.2–69.7) | 11 | 74.6 (54.8–89.5) | 12 | <0.001 |
| FEV1/FVC % pred§ | 99.88 (87.94–110.97) | 9 | 47.77 (26.00–87.67) | 11 | 93.37 (69.59–104.25) | 12 | <0.001 |
Data are presented as %, n/N or median (range), unless otherwise stated. Bold type represents statistical significance. BMI: body mass index; GOLD: Global Initiative for Chronic Obstructive Lung Disease; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. #: differences between groups were tested using Chi-squared test for categorical variables and Kruskal–Wallis test for continuous variables; ¶: defined as hypertension, myocardial infarction or stroke; +: defined as oral immunosuppressive drugs (e.g. cortisone); §: percent predicted according to Global Lung Initiative [59].
Statistical analysis
Data are shown as described in the figure legends. Comparison between two groups was analysed as detailed in the figure legends, with statistical significance indicated as p<0.05, p<0.01, p<0.001 and p<0.0001. Statistical analysis was performed using the software GraphPad Prism (version 9.00). Statistical evaluation of the immunopeptidome analysis was performed using R studio 2021.09.1 and R version 4.1.2 (2021-11-01). Nonparametric two-way aligned rank transform ANOVA was performed using the R package ARTool (0.11.1).
Results
Cigarette smoke impairs cytokine- and virus-induced upregulation of the immunoproteasome in vitro, ex vivo and in vivo
We first validated virus-mediated induction of the immunoproteasome in lung epithelial cells using publicly available datasets of IAV-infected mice. Single-cell RNA-sequencing (seq) data analysis resolved the most prominent induction of the three immunoproteasome subunits Psmb8 (encoding LMP7), Psmb9 (encoding LMP2) and Psmb10 (encoding MECL-1) in infected alveolar epithelial cells (AECs) (supplementary figure S1a) [27]. Bulk RNA-seq data of AECs from IAV-infected mice showed highest upregulation of the immunoproteasome subunits after 3 days of IAV infection (supplementary figure S1b) [28].
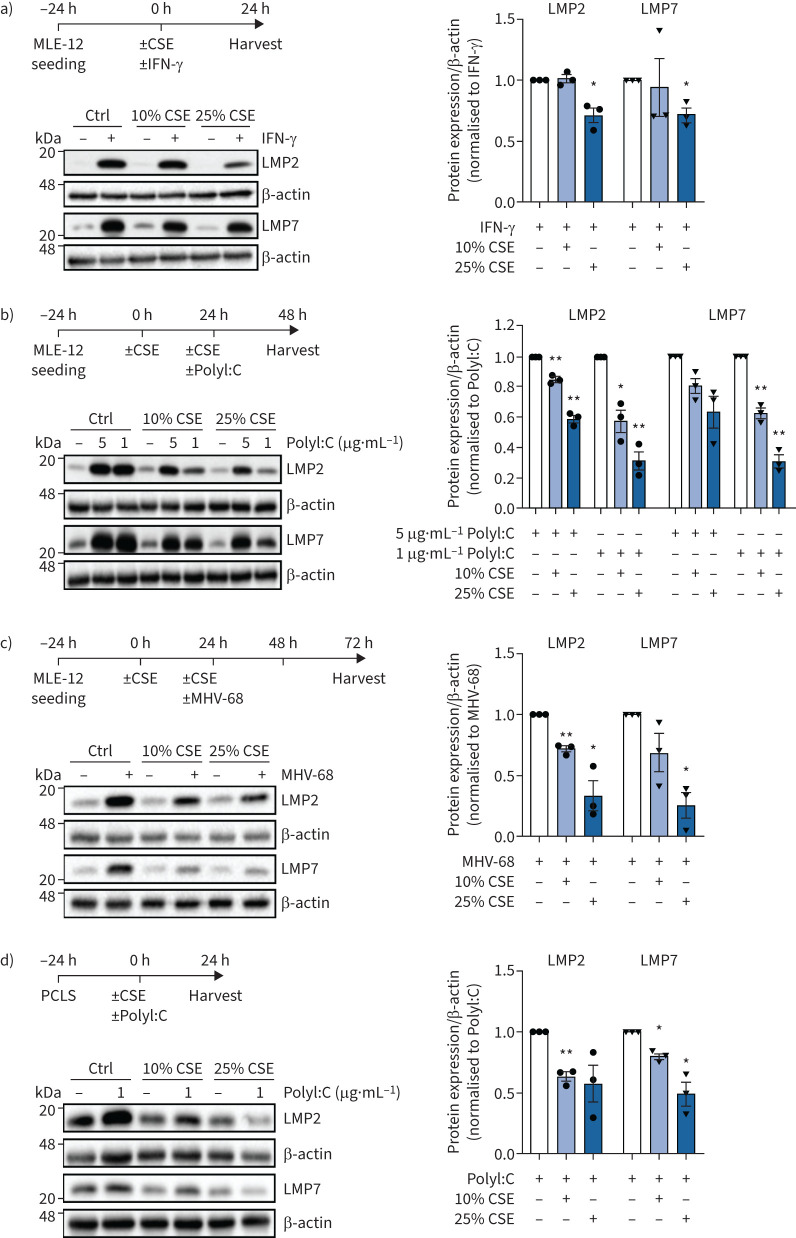
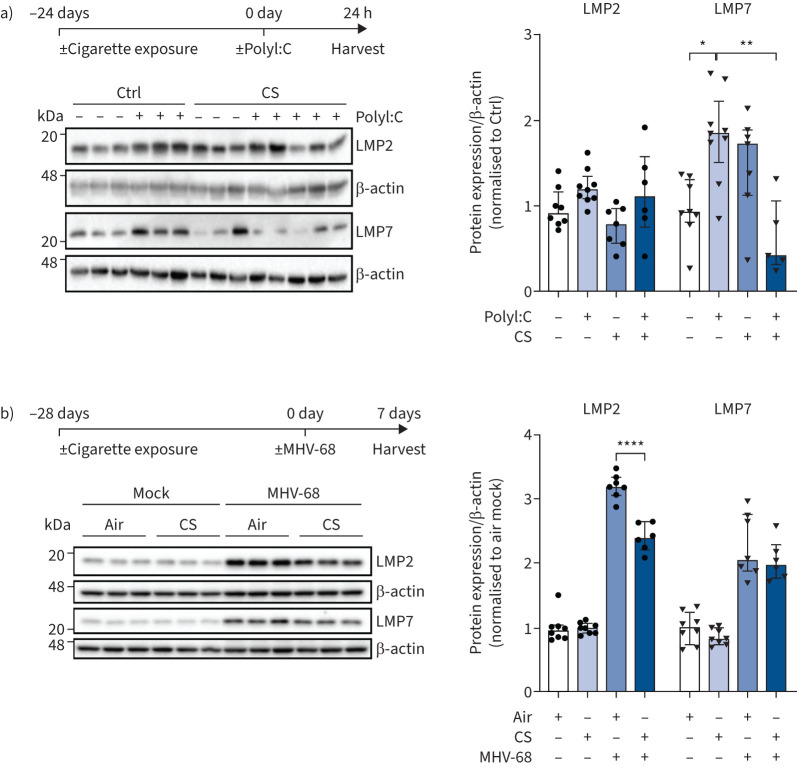
We then investigated the effects of cigarette smoke exposure on immunoproteasome induction in AECs in vitro. In a first set of experiments, mouse MLE-12 AECs were stimulated with IFN-γ and co-treated with nontoxic doses of cigarette smoke extract (CSE) (supplementary figure 1c) [29]. Of note, 25% of CSE significantly inhibited the induction of the two main catalytic subunits of the immunoproteasome LMP2 and LMP7 after 24 h (figure 1a). This inhibition was evident also at lower doses of 10% CSE for all three immunosubunits, including MECL-1, on the mRNA level (supplementary figure S1d). Next, we pre-treated MLE-12 cells with CSE and transfected the synthetic double-stranded (ds)RNA analogue PolyI:C as a surrogate for dsRNA virus infection. CSE dose-dependently inhibited immunoproteasome induction upon co-treatment with PolyI:C (1 or 5 μg·mL−1) in MLE-12 cells after 24 h as shown by Western blot analysis (figure 1b). In MLE-12 cells infected with murine γ-herpesvirus (MHV)-68, pre-treatment with 10% or 25% CSE also effectively counteracted virus-induced upregulation of the immunoproteasome after 72 h (figure 1c). A similar, albeit less pronounced, inhibitory effect of CSE was observed for PolyI:C- or IFN-γ-mediated induction of the immunoproteasome in ex vivo lung tissue after 24 h of treatment when using mouse precision-cut lung slices (figure 1d, supplementary figure S1e). Using an in vivo model of cigarette smoke exposure and PolyI:C challenge, we dissected the kinetics of transcriptional immunoproteasome activation at 2, 8 and 24 h after a single dose of 10 μg PolyI:C treatment. Cigarette smoke exposure of mice for 24 days attenuated the acute induction of Psmb8, Psmb9 and Psmb10 after 8 h of PolyI:C instillation (supplementary figure S2a). After 24 h of PolyI:C stimulation, the protein levels of the immunoproteasome subunit LMP7 were also significantly reduced in the mouse lungs (figure 2a). Staining of lung tissue sections for the immunoproteasome revealed that PolyI:C induced LMP2 mainly in endothelial capillary and bronchial epithelial cells in response to PolyI:C (supplementary figure 2b). A second mouse model confirmed these data for virus-induced activation of the immunoproteasome. In mice that had been exposed to cigarette smoke for 28 days and then infected with MHV-68 for 7 days, protein expression of LMP2 in the lung was significantly attenuated in smoke-exposed mice compared to air-treated controls (figure 2b). As body weight, virus load and number of lung-resident CD3+ and CD8+ T-cells were not significantly different in cigarette-smoke exposed mice compared to control mice (supplementary figure S2c–f), the attenuated induction of LMP2 is probably not due to an altered viral load or response to infection upon cigarette smoke exposure. Together, our data demonstrate that cigarette smoke inhibits PolyI:C- and virus-induced upregulation of the immunoproteasome in mouse lungs.
FIGURE 1.
Cigarette smoke impairs interferon (IFN)-γ and virus-induced upregulation of the immunoproteasome in vitro and ex vivo. a) Immunoproteasome subunits low molecular weight protein (LMP)2 and LMP7 expression in MLE-12 treated with 10% or 25% cigarette smoke extract (CSE) and 75 IU·mL−1 IFN-γ for 24 h. Densitometric analysis of LMP2 and LMP7 expression normalised to β-actin with control set to 1 in IFN-γ-treated cells. b) LMP2 and LMP7 expression in MLE-12 treated with 10% or 25% CSE for 24 h and then electroporated with 1 or 5 μg·mL−1 PolyI:C for 24 h. Densitometric analysis of LMP2 and LMP7 expression normalised to β-actin with PolyI:C only group set to 1. c) LMP2 and LMP7 expression in MLE-12 treated with 10% or 25% CSE for 24 h and then infected with murine γ-herpesvirus (MHV)-68 (multiplicity of infection (MOI) 1) for 48 h. Densitometric analysis of LMP2 and LMP7 expression normalised to β-actin with MHV-68 infection group set to 1. d) LMP2 and LMP7 expression in mouse precision-cut lung slices (PCLS) treated with 10% or 25% CSE and co-cultured with 1 μg·mL−1 PolyI:C for 24 h. Densitometric analysis of LMP2 and LMP7 expression normalised to β-actin with PolyI:C only group set to 1. Data are presented as mean±sem, one-sample t-test; *: p<0.05, **: p<0.01.
FIGURE 2.
Cigarette smoke impairs PolyI:C and virus-induced upregulation of the immunoproteasome in vivo. a) Low molecular-weight protein (LMP)2 and LMP7 expression in mouse lungs 24 h after treatment with 10 μg PolyI:C following 24 days of cigarette smoke (CS) exposure. Densitometric analysis of LMP2 and LMP7 expression normalised to β-actin loading with mean of controls (Ctrl) without PolyI:C treatment set to 1 (median, interquartile range). b) LMP2 and LMP7 expression in mouse lungs after 28 days of cigarette exposure and subsequent infection with murine γ-herpesvirus (MHV)-68 for 7 days. Densitometric analysis of LMP2 and LMP7 expression normalised to β-actin with air mock control set to 1 (median, interquartile range). Kruskal–Wallis test with Dunn's post-test; *: p<0.05, **: p<0.01, ****: p<0.0001.
Cigarette smoke impairs virus-mediated induction of the immunoproteasome and the class I MHC in human epithelial cells
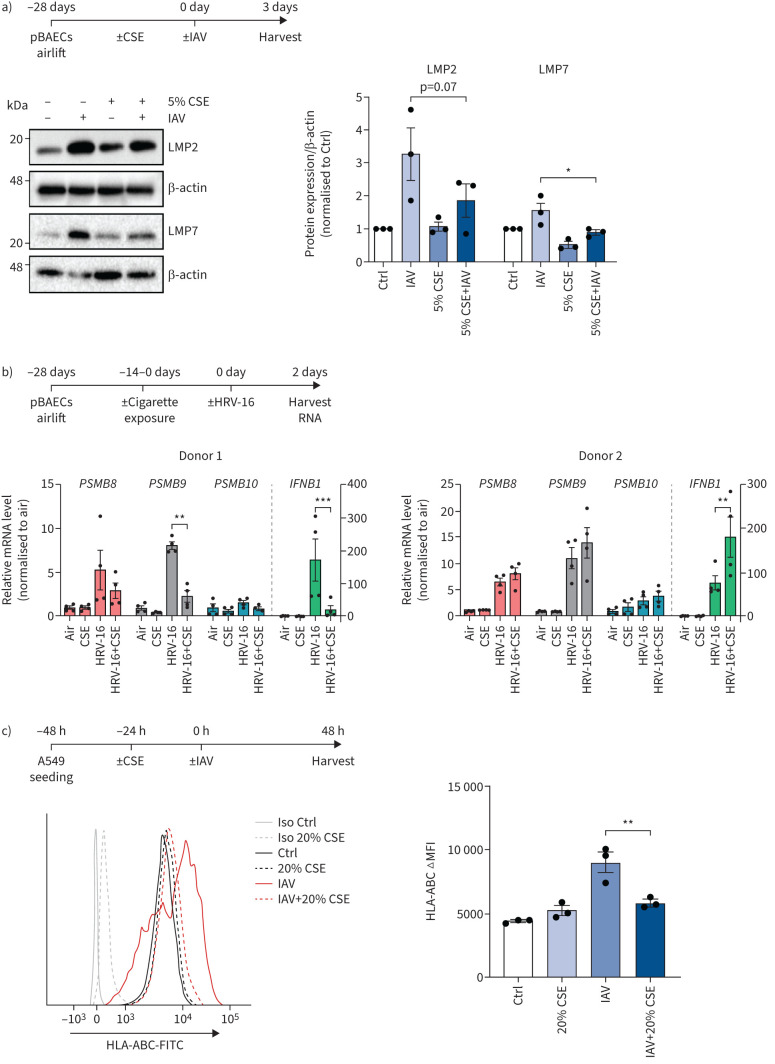
We further analysed the effect of cigarette smoke on virus-mediated induction of the immunoproteasome in human cells. For that, we cultured primary human bronchial epithelial cells (pBAECs) at air–liquid interface and infected them with clinically relevant virus strains, i.e. IAV or human rhinovirus (HRV)-16. In a first set of experiments, pBAECs from three different donors were chronically exposed to low doses of cigarette smoke extract (CSE; 5%) in the basolateral medium during the whole course of differentiation for 28 days [30]. Upon infection with IAV, we observed highest induction of the immunoproteasome after 3 days (supplementary figure S3a). Of note, CSE significantly impaired the IAV-mediated induction of the immunoproteasome subunits LMP2 and LMP7 by IAV (figure 3a). In a second set of experiments, we exposed airlifted pBAECs from two different donors (supplementary table S2) to two cigarettes a day between days 14 and 28 of differentiation [31]. Cells were subsequently infected with HRV-16 and immunoproteasomal gene expression was analysed 48 h after virus infection using reverse transcriptase quantitative PCR. Cigarette smoke attenuated HRV-16-mediated induction of LMP2, but not LMP7 and MECL-1 in donor 1 but not in donor 2 (figure 3b). The donor-specific response coincided with differential regulation of IFN-β: donor 1 responded to cigarette smoke with reduced IFN-β induction, while donor 2 was unaffected by cigarette smoke exposure (figure 3b). Our data thus demonstrate that cigarette smoke can impair induction of the immunoproteasome in pBAECs in response to virus infection which can impair virus-specific MHC class I antigen presentation and CD8+ T-cell activation. We further analysed the human alveolar-derived carcinoma cell line A549 and tested whether cigarette smoke impairs virus-driven induction of MHC class I molecules. IAV-infection of A549 cells not only strongly induced the immunoproteasome, but also upregulated surface expression of MHC class I by almost two-fold as determined by flow cytometry (figure 3c and supplementary figure S3b). Nontoxic doses of CSE counteracted virus-induced upregulation of the MHC class I and the immunoproteasome (figure 3c and supplementary figure S3b and c).
FIGURE 3.
Cigarette smoke impairs virus-mediated induction of the immunoproteasome and major histocompatibility complex (MHC) class I in human epithelial cells. a) Low molecular weight protein (LMP)2 and LMP7 expression in primary human bronchial epithelial cells (pBAECs) from three different donors that had been cultured at air–liquid interface conditions with or without cigarette smoke extract (CSE) in their medium [30] and infected with influenza A virus (IAV) for 3 days [60]. Densitometric analysis of LMP2 and LMP7 expression normalised to β-actin with untreated group set to 1. b) pBAECs from two male healthy donors were infected with human rhinovirus (HRV)-16 for 2 days after exposure to two cigarettes per day for 14 days during the air–liquid interface differentiation phase. mRNA levels for PSMB8 (LMP7), PSMB9 (LMP2) and PSMB10 (multicatalytic endopeptidase complex subunit (MECL)-1) and IFNB1 (interferon-β) were determined using reverse transcriptase quantitative PCR and related to housekeeping gene expression (HPRT and RPL32). Data are shown as four replicates per donor (mix of two technical and two independent cultures) with mean±sem. c) Human leukocyte antigen (HLA)-ABC surface expression of A549 cells treated with 20% CSE for 24 h and infected with IAV (multiplicity of infection 1) for 24 h. Fluorescence intensity is shown as mean±sem of three independent experiments. Ctrl: control; Iso: isotype; FITC: fluorescein isothiocyanate; ΔMFI: change in mean fluorescence intensity. One-way ANOVA with Bonferroni post-test. *: p<0.05, **: p<0.01, ***: p<0.001.
Cigarette smoke impairs activation of IAV-specific CD8+ T-cells
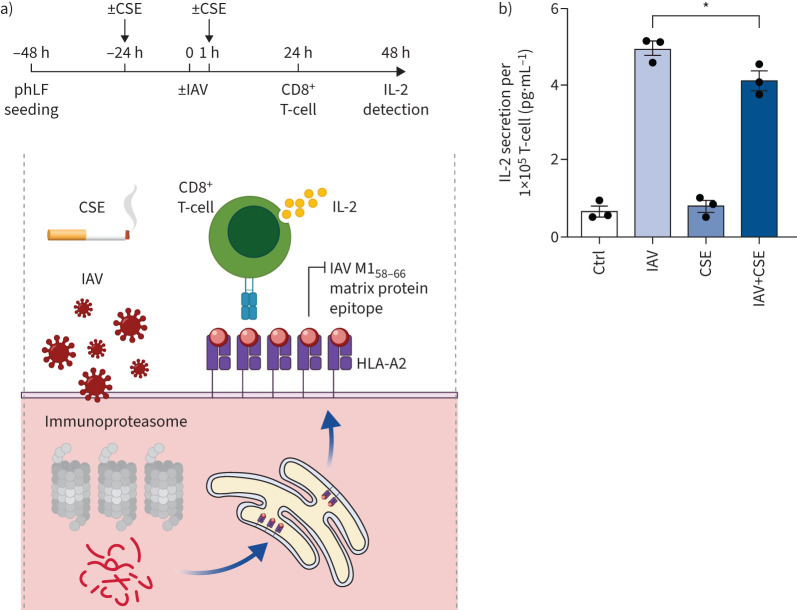
Next, we analysed whether cigarette smoke also dampened virus-mediated activation of CD8+ T-cells. For that we employed an IAV-specific CD8+ T-cell activation assay which involved a CD8+ T-cell clone that recognises the IAV M158–66 matrix protein epitope presented by HLA-A2 (figure 4a) [32]. The cognate IAV epitope is generated by the immunoproteasome. Therefore, activation of the T-cell clone depends on the efficient activity of the immunoproteasome upon IAV infection [33]. Primary human lung fibroblasts (phLF) isolated from HLA-A2 positive donors were exposed to CSE or air, then infected with IAV and co-cultured with the IAV-specific T-cell clone. The secretion of interleukin (IL)-2 was quantified to determine antigen-specific T-cell activation (figure 4a) [32]. We confirmed that the applied dose of CSE was nontoxic to the phLFs using MTT assays (supplementary figure S4a). IAV infection strongly induced the expression of the immunoproteasome and activated the CD8+ T-cells as indicated by a five-fold increase in IL-2 secretion (figure 4b and supplementary figure S4b). Specificity of the T-cell clone towards M1 peptide recognition was confirmed by external loading of phLFs with the M158–66 peptide (GILGFVFTL) (supplementary figure S4c). CSE exposure significantly attenuated the capacity of IAV-infected phLFs to activate the IAV-specific T-cell clone, but did not alter the T-cell response to non-IAV-infected cells (figure 4b). These proof-of-concept data thus indicate that cigarette smoke mediated inhibition of the immunoproteasome and MHC class I antigen presentation can result in impaired activation of virus-specific CD8+ T-cells upon virus infection of lung cells.
FIGURE 4.
Cigarette smoke impairs activation of influenza A virus (IAV)-specific CD8+ T-cells. a) Schematic depiction of the IAV antigen presentation assay: immunoproteasome-dependent processing of the IAV M1 matrix protein generates the M158–66 peptide, which is loaded onto human leukocyte antigen (HLA)-A2 major histocompatibility complex (MHC) I molecules in the endoplasmic reticulum. HLA-A2/M1-peptide complexes are transported to the cell surface where they activate the specific T-cell hybridoma cell line 4VA1 to secrete interleukin (IL)-2, which can be detected by ELISA. b) IAV-specific CD8+ T-cell activation upon co-culture with IAV-infected HLA-A2+ primary human lung fibroblasts. Primary human lung fibroblasts (phLFs) were treated with 10% cigarette smoke extract (CSE) in 1% fetal bovine serum for 24 h, infected with IAV (multiplicity of infection 1) for 1 h and further cultured for 24 h with or without CSE to be then co-cultured with the CD8+ ifluenza-M1 protein specific T-cells at the ratio of 1:2 for 24 h. IL-2 secreted by the mouse-derived T-cell clone was quantified from the supernatant (mean±sem). Ctrl: control. Significance was analysed with one-way ANOVA with Bonferroni post-test. *: p<0.05.
Cigarette smoke profoundly alters the inflammatory MHC class I presented antigenic repertoire
These results of impaired MHC class I restricted T-cell activation prompted us to investigate the effect of cigarette smoke on the pan-MHC class I antigenic repertoire using mass-spectrometry based immunopeptidomics [34]. This peptidomics approach involves the pulldown of MHC class I molecules, subsequent elution of MHC I-bound peptides and mass spectrometry based peptide identification. To meet the requirements of large cell numbers, we employed the A549 human lung epithelial cell line and investigated the effect of CSE on IFN-γ-induced MHC class I restricted antigen presentation. A549 cells were pre-treated with 20% CSE for 48 h and then co-treated for another 24 h with medium containing CSE with/without IFN-γ. The experiment was performed in three independent replicates with technical duplications, i.e. mass spectrometry runs. While the chosen dose of CSE did not consistently inhibit IFN-γ-mediated induction of immunoproteasome expression after 24 h (supplementary figure S5a), we noted attenuated induction of MHC class I molecules (HLA-ABC) in flow cytometry analysis (supplementary figure S5b).
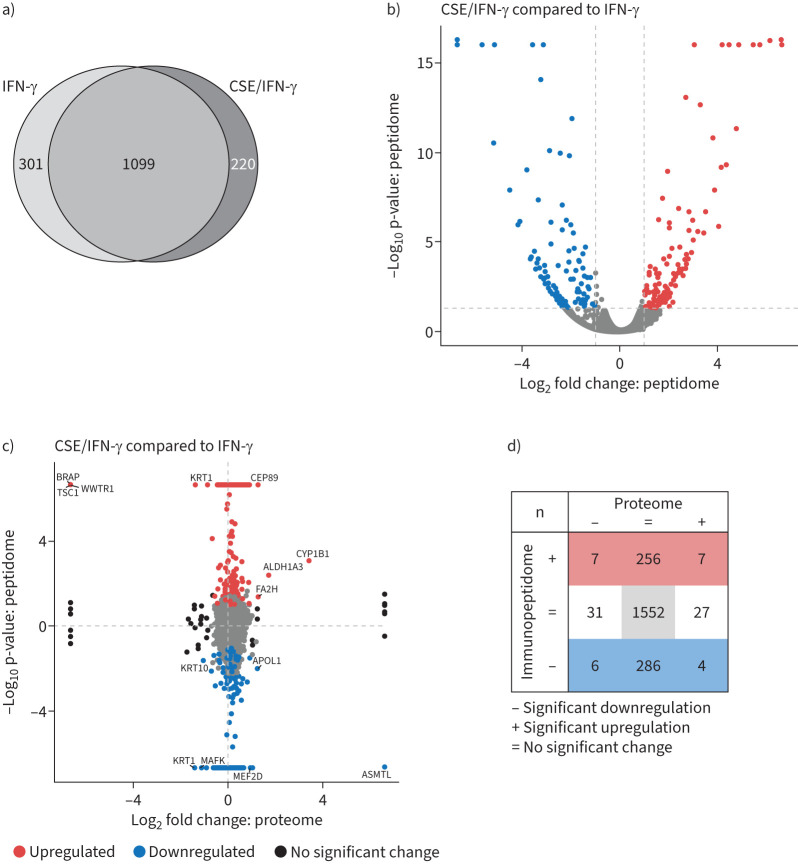
In our immunopeptidome analysis, we first determined the number of peptides that fulfil the requirements of HLA-binding. With the outlined stringent selection criteria (see supplementary methods for details), we identified between 400 and 1500 HLA-bound peptides per treatment (supplementary figure S6a). The number of identified antigenic peptides was higher in IFN-γ-treated cells, which is in line with previous observations [35]. CSE-treatment of the cells did not grossly change the number of HLA-peptides at baseline nor upon IFN-γ-treatment (supplementary figure S6a). The distribution of peptides derived from the different HLA class I alleles was affected by IFN-γ, as shown before [36], but not by CSE treatment (supplementary figure S6b). As IFN-γ treatment induced the most pronounced changes in the overall antigenic repertoire, we focused our analysis on the effect of CSE treatment on the IFN-γ-induced immunopeptidome. We detected 220 new MHC class I antigenic peptides after CSE/IFN-γ-treatment compared to IFN-γ as visualised by Venn (figure 5a) and volcano plots (figure 5b). The new antigenic peptides were derived from multiple proteins and related to diverse ontology terms with various cellular localisation and molecular functions without enrichment for specific pathways (data not shown). Moreover, 301 peptides reproducibly identified in IFN-γ-treated cells were less prevalent after CSE/IFN-γ-treatment (figure 5a and b). These were also derived from proteins of diverse ontology, functions and pathways.
FIGURE 5.
Effect of cigarette smoke extract (CSE) on the inflammatory major histocompatibility complex (MHC) class I immunopeptidome. a) Venn diagram of overlapping and unique MHC class I peptides identified in the immunopeptidome of A549 cells (binding rank ≤2%, confidence score ≥4.2, n=3). Cells had either been stimulated with 75 U·mL−1 interferon (IFN)-γ for 24 h or pre-treated with 20% CSE for 48 h and then co-stimulated with 75 U·mL−1 IFN-γ for the past 24 h. b) Volcano plot of the identified MHC class I peptides from a). Significantly upregulated peptides from CSE and IFN-γ-treated cells compared to IFN-γ treatment alone are depicted in red, significantly downregulated peptides are depicted in blue. A dashed horizontal line indicates the significance threshold of 0.05. The two dashed vertical lines indicate the log2 abundance ratio thresholds of −1 and 1. c) Abundance comparison of peptides identified in the immunopeptidome and proteins detected in the proteome from CSE and IFN-γ-treated cells compared to IFN-γ treatment alone. Significantly upregulated MHC class I peptides are depicted in red, while significantly downregulated MHC class I peptides are blue. Proteins significantly regulated in the proteome without significant changes in the peptidome are depicted in black. Peptides significantly regulated in the proteome and the immunopeptidome are labelled with their gene symbol. d) Enumeration of significantly regulated peptides from c).
Next, we investigated how differences in cellular protein abundance translated into altered MHC class I antigen presentation upon combined CSE and IFN-γ treatment. Therefore, we performed additional shotgun proteomic analysis for each treatment conditions and then plotted the log2-fold change of MHC class I antigenic peptides on the y-axis to the log2-fold change of protein abundance on the x-axis for each treatment pair (figure 5c and supplementary figure S6c). CSE/IFN-γ-treatment upregulated seven antigenic peptides due to elevated protein levels (upper right quartile, figure 5c and d). Seven antigenic peptides were presented from proteins that were downregulated compared to IFN-γ-treated controls (upper left quartile, figure 5c and d). Most upregulated antigenic peptides were derived from proteins that were not altered in expression by CSE/IFN-γ compared to only IFN-γ treatment (figure 5c and d). These changes in abundance ratios of immunopeptidome and proteome were less pronounced when we compared CSE-treated cells to untreated cells indicating that the initial proteome remodelling is driven predominantly by IFN-γ and not CSE treatment (supplementary figure S6c). We conclude that CSE shapes the immunopeptidome of A549 cells under inflammatory stimulation with IFN-γ, but has only a minor impact on the steady-state proteome. This remodelling of the inflammatory antigenic peptide repertoire by cigarette smoke has the potential to affect CD8+ T-cell activation and adaptive immune responses to virus infections.
COPD patients have reduced numbers of circulating IAV-specific CD8+ T-cells
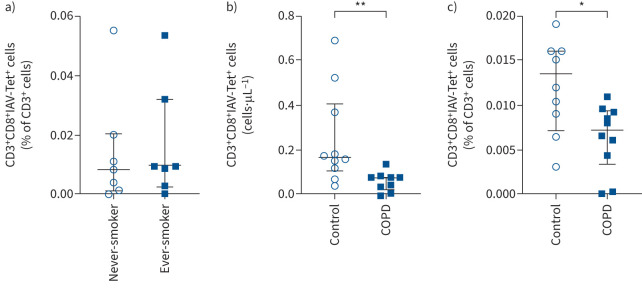
Considering the results of reduced virus-specific CD8+ T-cell activation by cigarette smoke, we reasoned that extended cigarette smoke exposure in smokers and COPD patients contributes to impaired activation of antiviral adaptive immune responses. This might be detectable as reduced numbers of virus-specific CD8+ T-cells in the peripheral blood. We tested this concept by analysing the number of IAV-specific CD8+ T-cells in isolated peripheral blood mononuclear cells (PBMCs), comparing never-smokers to ever-smokers (study cohort 1) (supplementary table S1 for study cohort) and comparing severe COPD patients to lung healthy and asthma disease controls (study cohort 2). All COPD patients were former smokers with duration ranging from 10 to 50 pack-years (table 1). To determine the number of IAV-specific T-cells, we used the tetramer technology and focused on the immunodominant HLA-A2-restricted IAV M158–66 epitope described earlier [37] restricted to HLA-A2. HLA-A2 tetramers loaded with the M158–66 bind specifically to IAV-specific CD8+ T-lymphocytes, which can then be quantified by flow cytometry (supplementary figure S7 for gating strategy and controls). As this IAV epitope is HLA-A2 restricted, our study participants were first selected for HLA-A2 expression (table 1 and supplementary table S1). In our first study cohort, IAV-specific CD8+ T-cells (as percentage of total CD3+ cells) were not significantly different between never-smokers (n=7, including one Shisha smoker) and ever-smokers (n=7) (figure 6a). There was no correlation between the percentage of CD3+CD8+IAV-tetramer+ T-cells and pack-years (data not shown). However, in the COPD patient cohort, the absolute number of CD3+CD8+IAV-tetramer+ T-cells was lower in severe COPD patients compared to lung-healthy controls and compared to asthmatics (figure 6b, supplementary figure S8a). Similarly, the frequency of IAV-specific CD8+ T-cells was significantly lower in COPD patients when related to the total number of CD3+ cells present in PBMC samples of these patients (figure 6c, supplementary figure S8b). These data support the concept that COPD patients have an impaired antiviral CD8+ T-cell response [17, 18]. However, the small number of study participants and their heterogeneous smoking habits (supplementary table S1) limit the conclusion and warrant further analysis.
FIGURE 6.
Determination of influenza A virus (IAV)-specific CD8+ T-cells in blood of lung-healthy controls and COPD patients. a) Percentage of CD3+CD8+IAV-tetramer(Tet)+ T-cells within the fraction of all CD3+ cells in peripheral blood mononuclear cells (PBMCs) of lung-healthy controls who are never-smokers (n=7, including one Shisha smoker) compared with ex-smokers or current smokers (n=7). b) Absolute numbers of CD3+CD8+IAV-tetramer+ T-cells (cells·μL−1) in isolated PBMCs of COPD patients (n=9) and age-matched lung-healthy controls (n=10). Clinical characteristics can be found in table 1. c) Percentage of CD3+CD8+IAV-tetramer+ T-cells within the fraction of all CD3+ cells in PBMCs of lung healthy controls (n=9) and COPD patients (n=10). Data are presented as median (interquartile range); significance was tested using Mann–Whitney U-test. *: p<0.05, **: p<0.01.
Discussion
In this study, we demonstrate that cigarette smoke impairs cytokine- and virus-induced upregulation of the immunoproteasome/MHC class I antigen presentation machinery which associates with reduced activation of antiviral CD8+ T-cells. We used different model systems ranging from in vitro mouse cell lines, ex vivo lung cultures, in vivo mouse experiments to the use of primary human cells and the testing of clinically relevant viruses, i.e. IAV and HRV-16. Moreover, mass-spectrometry based immunopeptidome analysis unravelled a major remodelling of the inflammatory MHC class I antigenic repertoire by cigarette smoke. The concept that cigarette smoke interferes with effective antiviral CD8+ T-cell immunity was further corroborated by the finding that severe COPD patients with a smoking history had reduced numbers of influenza-specific peripheral CD8+ T-cells compared to lung healthy controls and asthma patients.
Cigarette smoke interferes with IFN-mediated antiviral immune responses
While our previous studies analysed the effects of cigarette smoke on immunoproteasome function in lung immune cells, lung tissue [26, 38] and peripheral blood cells [39], here we focused on the effect of cigarette smoke on cytokine- and virus-mediated induction of the immunoproteasome in nonimmune cells. Our data are in line with and extend previous studies reporting reduced responsiveness of epithelial cells to IFNs or viruses when exposed to cigarette smoke extract [40–42]. Furthermore, our results are supported by recent studies on cigarette smoke exposure of SARS-CoV-2 infected airway epithelial cells [43] and ex vivo human lung cultures [31]. While the focus of these studies was on the suppression of antiviral innate immunity by CSE and how CSE interfered with IFN signalling [44, 45], our data reveal an association between CSE-induced impairment of IFN responses in infected cells and adaptive CD8+ T-cell activation via the immunoproteasome/MHC class I antigen presentation machinery. We thereby provide a mechanistic link to the previously reported impairment of CD8+ T-cell activation in cigarette smoke-exposed mice upon viral infection [14, 46]. We observed varied effects of cigarette smoke on the expression of individual immunoproteasomal catalytic subunits. The underlying mechanism requires further investigation. However, it is appreciated that the catalytic subunits of the immunoproteasome are differentially regulated under inflammatory and stress conditions [21, 47]. To this end, we recently demonstrated in a large population-based cohort that the activity of the catalytic subunits of the proteasome in peripheral blood cells are differentially regulated depending on sex and age and chronic inflammatory diseases [48]. Overall, the immunoproteasome function is not only regulated by the expression of the catalytic subunits, but also by their proteolytic activity, which is impaired by cigarette smoke [26, 29, 38]. Of note, cigarette smoke not only suppressed cytokine- and virus-mediated induction of the immunoproteasome in multiple models including different viruses, but also counteracted virus-induced upregulation of MHC class I molecules, which accords with previous reports [26, 49]. Our immunopeptidomic analysis revealed an additional layer of regulation by cigarette smoke, inducing qualitative changes of the inflammatory MHC class I immunopeptidome. We observed >200 novel antigenic peptides that were presented in CSE/IFN-γ co-treated compared to IFN-γ stimulated cells while several antigens were lost upon CSE treatment. These antigens were derived from proteins across multiple cellular pathways and not enriched for specific molecular functions. Most of these peptides were not regulated on the protein level. Taken together, we here demonstrate that cigarette smoke interferes with MHC class I mediated antigen presentation on multiple levels ranging from reduced immunoproteasome induction, altered antigenic epitope generation and diminished MHC class I upregulation. All these mechanisms add to reduced CD8+ T-cell activation as observed in this study. Our data thus provide important mechanistic insight on how cigarette smoke impairs CD8+ T-cell immune responses which hinder viral clearance.
Defective antiviral immunity in COPD patients
Our in vitro data showing defective MHC class I antigen presentation and antiviral CD8+ T-cell activation are supported by our observation of reduced frequencies of influenza-specific peripheral CD8+ T-cells in COPD patients compared to healthy controls and asthmatics. Our data accord with a study from 2005 reporting lower levels of RSV-specific CD8+ T-cells in the blood of COPD patients [50]. The functionality of IAV-specific and total CD8+ T-cells in COPD patients as assessed by surface expression of the degranulation marker CD107a and intracellular IFN-γ staining was not altered (data not shown). This finding is consistent with published results reporting similar cytokine production of healthy and COPD-derived CD8+ T-cells after in vitro vaccine stimulation [51], but contrasts with observed elevated inflammatory capacity of CD8+ T-cells from COPD patients when activated in vitro[52]. Conflicting data are further reported regarding the numbers, phenotypes and functionality of circulating and lung-resident CD8+ T-cells in stable COPD patients [53, 54]. However, a recent meta-analysis on this topic seemingly supports the notion of elevated levels of peripheral CD8+ T-cells in COPD that lack full functionality [53]. For lung-resident CD8+ T-cells, upregulation of programmed cell death protein (PD)-1 and reduced functionality was reported [55]: the Wilkinson lab demonstrated that ex vivo infection of COPD lung tissue with IAV upregulated PD-1 on CD8+ T-cells derived from COPD tissue more strongly compared to control tissue which was associated with reduced degranulation capacity of these cells. Biton et al. [56] reported exhaustion of lung tumour resident CD8+ T-cells to be more prevalent in patients with accompanying COPD Global Initiative for Chronic Obstructive Lung Disease stages II+III. An elegant mouse experiment showed that cigarette smoke-induced emphysema functionally impaired early cytotoxic CD8+ T-cell responses against lung implanted cancer cells [15]. These observations raise the intriguing question whether ineffective clearing of virus infections by defective CD8+ T-cell immunity might not only contribute to the increased susceptibility of COPD patients to viral exacerbations, but also drive disease development when latent viral infections and cigarette smoke synergistically add to chronic inflammation and tissue damage in COPD, as suggested previously [3].
Strength and limitations of this study
In this study, we analysed the effect of cigarette smoke on several components of the complex process of MHC class I antigen presentation, i.e. altered virus-mediated immunoproteasome induction, reduced MHC class I surface expression and generation of novel antigenic epitopes. For that we used in vitro, ex vivo and in vivo models that allowed us to obtain proof-of-concept evidence for the effect of cigarette smoke on the single components. It is most likely that the diminished activation of CD8+ T-cells by cigarette smoke involves the combined impairment of the entire immunoproteasome/MHC class I antigen presentation machinery. Our study is limited to the use of a single HLA-A2-restricted IAV epitope, namely the M158–66 antigen. We have focused on this epitope as it is well known that the generation of this antigen depends on the catalytic activity of the immunoproteasome [33, 57]. We can thereby relate CD8+ T-cell activation to the activity of the immunoproteasome. While there are multiple other well-known IAV antigens and corresponding CD8+ T-cell clones available [32], it is unknown whether the generation of these viral antigens depends on the immunoproteasome. Another reason to focus our tetramer analysis on an HLA-A2-restricted IAV epitope is that HLA-A2 the most prevalent allele family in all ethnic populations [58]. Despite these limitations, our data provide important mechanistic insight into how cigarette smoke might hamper antiviral CD8+ T-cell responses. Still, the number of study participants in our two study arms is rather small. However, incorporation of a lung healthy control and an asthma disease control group strengthen our conclusions. Importantly, our study provides conceptual evidence for altered MHC class I antigen presentation in COPD disease exacerbation, a topic that has been under-studied so far.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods and tables ERJ-01374-2022.Supplement (286.8KB, pdf)
Supplementary figures ERJ-01374-2022.Figures (4.8MB, pdf)
Shareable PDF
Acknowledgements
We kindly acknowledge the generous gift of the IAV strain PR8 from Susanne Herold (Justus Liebig University, Excellence Cluster Cardio-Pulmonary Institute (CPI), Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Gießen, Germany) and the provision of the IAV-specific T-cell hybridoma clone 4VA1 by David Canaday (Case Western Reserve University, Cleveland, OH, USA). Moreover, we are grateful for the expert support by Beatrix Steer (Research Center Borstel, Leibniz Lung Center, Airway Research Center North (ARCN), Member of the German Center for Lung Research (DZL), Borstel, Germany). We thank the NIH Tetramer Core Facility (contract number 75N93020D00005) for providing the IAV tetramers. The graphical abstract was generated with BioRender. We gratefully acknowledge the provision of human biomaterial and clinical data from the CPC-M bioArchive and its partners at the Asklepios Biobank Gauting, the LMU Hospital and the Ludwig-Maximilians-Universität München. We are grateful to the excellent technical assistance of Frauke Koops, Gesine Rode and Christian Rosero (Research Center Borstel). We also like to thank Margrit Kernbach and Stefanie Fox from the BioMaterialBank North (Borstel, Germany), for their excellent technical assistance, and the study centre of the Research Center Borstel for their help. We thank the patients and their families as well as the healthy blood donors for their support.
Footnotes
Author contributions: I.E. Kammerl, J. Chen, X. Wang and S. Meiners are responsible for conception and design of research; M. Frankenberger, K.I. Gaede, K. Milger, J.S. Lee, J. Brands and J. Behr provided (clinical) samples and reagents; J. Chen, X. Wang, S. Haines, A. Schmalen, M.G. Stoleriu, M. Nakayama, M. Bueno, J. Brands, M. Wolff, H. Ma, A. Dmitrieva, J. Nowak and I.E. Kammerl performed experiments; J. Chen, X. Wang, S. Haines, A. Schmalen, H. Ma, J. Behrends, T. Goldmann, C.A. Staab-Weijnitz, A.L. Mora, M. Bueno, J.S. Lee, M. Wolff, H. Zhang, S. Krauss-Etschmann, H. Adler, S.M. Hauck, T.P. Hofer, E. Noessner, C.A. Deeg, A. Moosmann, I.E. Kammerl and S. Meiners analysed data; I.E. Kammerl, A.L. Mora, E. Noessner, A. Moosmann, H. Adler, T. Goldmann, J. Behrends and S. Meiners interpreted results; I.E. Kammerl, X. Wang, S. Meiners and J. Chen prepared tables and figures; I.E. Kammerl, X. Wang, S. Meiners, A. Schmalen and J. Chen drafted the manuscript; I.E. Kammerl, X. Wang and S. Meiners edited and revised the manuscript; all authors approved the final version.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01030-2023
Conflict of Interest: H. Ma reports support for the present manuscript from the German Center for Lung Research (DZL). M. Nakayama reports overseas grant from Uehara Memorial Foundation (Japan) and overseas grant from Shiga university of Medical Science, outside the submitted work. A.L. Mora reports support for the present manuscript from NIH (NIH U01 HL1455550-01 and NIH NHLBI R01 HL149825). J.S. Lee reports participation on clinical adjudication committee with Janssen R&D, outside the submitted work. S. Krauss-Etschmann reports support for the present manuscript from the German Center for Lung Research. K. Milger reports consulting fees and lecture honoraria from AstraZeneca, GSK, Janssen, Novartis and Sanofi, outside the submitted work. C.A. Staab-Weijnitz reports support for the present manuscript from Helmholtz Association, German Center for Lung Research (DZL) and Deutsche Forschungsgemeinschaft (DFG) within the Research Training Group GRK2338. K.I. Gaede reports support for the present manuscript from Research Center Borstel – Leibniz Lung Center – BioMaterialBank North, Airway Research Center North, German Center for Lung Research (DZL), PopGen 2.0 Network (P2N). K.I. Gaede also holds a leadership role as member of the Board of Directors of the TMF (www.tmf-ev.de), outside the submitted work. I.E. Kammerl reports support for the present manuscript from ERS (Short Term Fellowship). All other authors have no potential conflicts of interest to declare.
Support statement: The study was supported by funding from the Faculty of Medicine at LMU (FöFoLe program for S. Haines), the European Respiratory Society (ERS) (short-term fellowship for I.E. Kammerl), intramural funding by the Helmholtz Center Munich, and the German Federal Ministry of Education and Research, Germany (BMBF) grant EXASENS (13N13856) (funding M. Wolff) to S. Krauss-Etschmann. J. Chen was supported by the National Natural Science Foundation of China (81600063). H. Zhang is supported by China Postdoctoral Science Foundation (2022M720916). M. Nakayama was supported by the Uehara Memorial Foundation and Shiga University of Medical Science. S. Meiners is funded through a personal grant by the Leibniz foundation. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012; 379: 1341–1351. doi: 10.1016/S0140-6736(11)60968-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soriano JB, Kendrick PJ, Paulson KR, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020; 8: 585–596. doi: 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo-Parke H, Linden D, Weldon S, et al. Mechanisms of virus-induced airway immunity dysfunction in the pathogenesis of COPD disease, progression, and exacerbation. Front Immunol 2020; 11: 1205. doi: 10.3389/fimmu.2020.01205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 1618–1623. doi: 10.1164/ajrccm.164.9.2105011 [DOI] [PubMed] [Google Scholar]
- 5.Ritchie AI, Wedzicha JA. Definition, causes, pathogenesis, and consequences of chronic obstructive pulmonary disease exacerbations. Clin Chest Med 2020; 41: 421–438. doi: 10.1016/j.ccm.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agustí A, Celli BR, Criner GJ, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 report: GOLD executive summary. Eur Respir J 2023; 61: 2300239. doi: 10.1183/13993003.00239-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song J-W, Zhang C, Fan X, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun 2020; 11: 3410. doi: 10.1038/s41467-020-17240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kared H, Redd AD, Bloch EM, et al. SARS-CoV-2-specific CD8+ T cell responses in convalescent COVID-19 individuals. J Clin Invest 2021; 131: e145476. doi: 10.1172/JCI145476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon DE, Hiatt J, Bouhaddou M, et al. Comparative host–coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science 2020; 370: eabe9403. doi: 10.1126/science.abe9403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultze JL, Aschenbrenner AC. COVID-19 and the human innate immune system. Cell 2021; 184: 1671–1692. doi: 10.1016/j.cell.2021.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco-Melo D, Nilsson-Payant BE, Liu W-CC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181: 1036–1045. doi: 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geerdink JX, Simons SO, Pike R, et al. Differences in systemic adaptive immunity contribute to the ‘frequent exacerbator’ COPD phenotype. Respir Res 2016; 17: 140. doi: 10.1186/s12931-016-0456-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider D, Ganesan S, Comstock AT, et al. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 182: 332–340. doi: 10.1164/rccm.200911-1673OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer CMT, Morissette MC, Stämpfli MR. The influence of cigarette smoking on viral infections: translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinically. Chest 2013; 143: 196–206. doi: 10.1378/chest.12-0930 [DOI] [PubMed] [Google Scholar]
- 15.Kerdidani D, Magkouta S, Chouvardas P, et al. Cigarette smoke-induced emphysema exhausts early cytotoxic CD8+ T cell responses against nascent lung cancer cells. J Immunol 2018; 201: 1558–1569. doi: 10.4049/jimmunol.1700700 [DOI] [PubMed] [Google Scholar]
- 16.Robbins CS, Franco F, Mouded M, et al. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J Immunol 2008; 180: 6623–6628. doi: 10.4049/jimmunol.180.10.6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011; 378: 1015–1026. doi: 10.1016/S0140-6736(11)60988-4 [DOI] [PubMed] [Google Scholar]
- 18.Curtis JL, Freeman CM, Hogg JC. The immunopathogenesis of chronic obstructive pulmonary disease: insights from recent research. Proc Am Thorac Soc 2007; 4: 512–521. doi: 10.1513/pats.200701-002FM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy MK, Weinberg JB. The immunoproteasome and viral infection: a complex regulator of inflammation. Front Microbiol 2015; 6: 21. doi: 10.3389/fmicb.2015.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sijts EJAM, Kloetzel PM. The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell Mol Life Sci 2011; 68: 1491–1502. doi: 10.1007/s00018-011-0657-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kammerl IE, Meiners S. Proteasome function shapes innate and adaptive immune responses. Am J Physiol Lung Cell Mol Physiol 2016; 311: L328–L336. doi: 10.1152/ajplung.00156.2016 [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Zhang H, Wang Y, et al. DNA sensing via the cGAS/STING pathway activates the immunoproteasome and adaptive T-cell immunity. EMBO J 2023; 42: e110597. doi: 10.15252/embj.2022110597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller IE, Vosyka O, Takenaka S, et al. Regulation of immunoproteasome function in the lung. Sci Rep 2015; 5: 10230. doi: 10.1038/srep10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimasuay KG, Schaunaman N, Berg B, et al. Airway epithelial immunoproteasome subunit LMP7 protects against rhinovirus infection. Sci Rep 2022; 12: 14507. doi: 10.1038/s41598-022-18807-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kincaid EZ, Che JW, York I, et al. Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat Immunol 2012; 13: 129–135. doi: 10.1038/ni.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kammerl IE, Dann A, Mossina A, et al. Impairment of immunoproteasome function by cigarette smoke and in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2016; 193: 1230–1241. doi: 10.1164/rccm.201506-1122OC [DOI] [PubMed] [Google Scholar]
- 27.Steuerman Y, Cohen M, Peshes-Yaloz N, et al. Dissection of influenza infection in vivo by single-cell RNA sequencing. Cell Syst 2018; 6: 679–691. doi: 10.1016/j.cels.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stegemann-Koniszewski S, Jeron A, Gereke M, et al. Alveolar type II epithelial cells contribute to the anti-influenza A virus response in the lung by integrating pathogen- and microenvironment-derived signals. mBio 2016; 7: e00276-16. doi: 10.1128/mBio.00276-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Rijt SH, Keller IE, John G, et al. Acute cigarette smoke exposure impairs proteasome function in the lung. Am J Physiol Lung Cell Mol Physiol 2012; 303: L814–L823. doi: 10.1152/ajplung.00128.2012 [DOI] [PubMed] [Google Scholar]
- 30.Mastalerz M, Dick E, Chakraborty A, et al. Validation of in vitro models for smoke exposure of primary human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2022; 322: L129–L148. doi: 10.1152/ajplung.00091.2021 [DOI] [PubMed] [Google Scholar]
- 31.Danov O, Wolff M, Bartel S, et al. Cigarette smoke affects dendritic cell populations, epithelial barrier function, and the immune response to viral infection with H1N1. Front Med 2020; 7: 571003. doi: 10.3389/fmed.2020.571003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canaday DH, Gehring A, Leonard EG, et al. T-cell hybridomas from HLA-transgenic mice as tools for analysis of human antigen processing. J Immunol Methods 2003; 281: 129–142. doi: 10.1016/j.jim.2003.07.004 [DOI] [PubMed] [Google Scholar]
- 33.Basler M, Lauer C, Moebius J, et al. Why the structure but not the activity of the immunoproteasome subunit low molecular mass polypeptide 2 rescues antigen presentation. J Immunol 2012; 189: 1868–1877. doi: 10.4049/jimmunol.1103592 [DOI] [PubMed] [Google Scholar]
- 34.Purcell AW, Ramarathinam SH, Ternette N. Mass spectrometry-based identification of MHC-bound peptides for immunopeptidomics. Nat Protoc 2019; 14: 1687–1707. doi: 10.1038/s41596-019-0133-y [DOI] [PubMed] [Google Scholar]
- 35.Komov L, Kadosh DM, Barnea E, et al. Cell surface MHC class I expression is limited by the availability of peptide-receptive “empty” molecules rather than by the supply of peptide ligands. Proteomics 2018; 18: 1700248. doi: 10.1002/pmic.201700248 [DOI] [PubMed] [Google Scholar]
- 36.Javitt A, Barnea E, Kramer MP, et al. Pro-inflammatory cytokines alter the immunopeptidome landscape by modulation of HLA-B expression. Front Immunol 2019; 10: 141. doi: 10.3389/fimmu.2019.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C, Zanker D, Valkenburg S, et al. Systematic identification of immunodominant CD8+ T-cell responses to influenza A virus in HLA-A2 individuals. Proc Natl Acad Sci USA 2011; 108: 9178–9183. doi: 10.1073/pnas.1105624108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kammerl IE, Caniard A, Merl-Pham J, et al. Dissecting the molecular effects of cigarette smoke on proteasome function. J Proteomics 2019; 193: 1–9. doi: 10.1016/j.jprot.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 39.Kammerl IE, Hardy S, Flexeder C, et al. Activation of immune cell proteasomes in peripheral blood of smokers and COPD patients: implications for therapy. Eur Respir J 2022; 59: 2101798. doi: 10.1183/13993003.01798-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer CMT, Dewitte-Orr SJ, Hornby KR, et al. Cigarette smoke suppresses type I interferon-mediated antiviral immunity in lung fibroblast and epithelial cells. J Interferon Cytokine Res 2008; 28: 167–179. doi: 10.1089/jir.2007.0054 [DOI] [PubMed] [Google Scholar]
- 41.Modestou MA, Manzel LJ, El-Mahdy S, et al. Inhibition of IFN-γ-dependent antiviral airway epithelial defense by cigarette smoke. Respir Res 2010; 11: 64. doi: 10.1186/1465-9921-11-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proud D, Hudy MH, Wiehler S, et al. Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PLoS One 2012; 7: e40762. doi: 10.1371/journal.pone.0040762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purkayastha A, Sen C, Garcia G, et al. Direct exposure to SARS-CoV-2 and cigarette smoke increases infection severity and alters the stem cell-derived airway repair response. Cell Stem Cell 2020; 27: 869–875. doi: 10.1016/j.stem.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.HuangFu W-C, Liu J, Harty RN, et al. Cigarette smoking products suppress anti-viral effects of type I interferon via phosphorylation-dependent downregulation of its receptor. FEBS Lett 2008; 582: 3206–3210. doi: 10.1016/j.febslet.2008.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin H, Huang G, Gao F, et al. Diminished stimulator of interferon genes production with cigarette smoke-exposure contributes to weakened anti-adenovirus vectors response and destruction of lung in chronic obstructive pulmonary disease model. Exp Cell Res 2019; 384: 111545. doi: 10.1016/j.yexcr.2019.111545 [DOI] [PubMed] [Google Scholar]
- 46.Feng Y, Kong Y, Barnes PF, et al. Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect Immun 2011; 79: 229–237. doi: 10.1128/IAI.00709-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bramasole L, Meiners S. Profiling proteasome activities in peripheral blood – a novel biomarker approach. J Cell Immunol 2022; 4: 171–179. doi: 10.33696/immunology.4.147 [DOI] [Google Scholar]
- 48.Kammerl IE, Flexeder C, Karrasch S, et al. Blood immunoproteasome activity is regulated by sex, age and in chronic inflammatory diseases: a first population-based study. Cells 2021; 10: 3336. doi: 10.3390/cells10123336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fine CI, Han CD, Sun X, et al. Tobacco reduces membrane HLA class I that is restored by transfection with transporter associated with antigen processing 1 cDNA. J Immunol 2002; 169: 6012–6019. doi: 10.4049/jimmunol.169.10.6012 [DOI] [PubMed] [Google Scholar]
- 50.de Bree GJ, Heidema J, van Leeuwen EMM, et al. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J Infect Dis 2005; 191: 1710–1718. doi: 10.1086/429695 [DOI] [PubMed] [Google Scholar]
- 51.Parpaleix A, Boyer L, Wiedemann A, et al. Impaired humoral and cellular immune responses to influenza vaccination in chronic obstructive pulmonary disease patients. J Allergy Clin Immunol 2017; 140: 1754–1757. doi: 10.1016/j.jaci.2017.07.038 [DOI] [PubMed] [Google Scholar]
- 52.Freeman CM, Han MK, Martinez FJ, et al. Cytotoxic potential of lung CD8+ T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J Immunol 2010; 184: 6504–6513. doi: 10.4049/jimmunol.1000006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams M, Todd I, Fairclough LC. The role of CD8+ T lymphocytes in chronic obstructive pulmonary disease: a systematic review. Inflamm Res 2021; 70: 11–18. doi: 10.1007/s00011-020-01408-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 2009; 360: 2445–2454. doi: 10.1056/NEJMra0804752 [DOI] [PubMed] [Google Scholar]
- 55.McKendry RT, Spalluto CM, Burke H, et al. Dysregulation of antiviral function of CD8+ T cells in the chronic obstructive pulmonary disease lung. Role of the PD-1–PD-L1 axis. Am J Respir Crit Care Med 2016; 193: 642–651. doi: 10.1164/rccm.201504-0782OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biton J, Ouakrim H, Dechartres A, et al. Impaired tumor-infiltrating T cells in patients with chronic obstructive pulmonary disease impact lung cancer response to PD-1 blockade. Am J Respir Crit Care Med 2018; 198: 928–940. doi: 10.1164/rccm.201706-1110OC [DOI] [PubMed] [Google Scholar]
- 57.Gileadi U, Moins-Teisserenc HT, Correa I, et al. Generation of an immunodominant CTL epitope is affected by proteasome subunit composition and stability of the antigenic protein. J Immunol 1999; 163: 6045–6052. [PubMed] [Google Scholar]
- 58.Song S, Han M, Zhang H, et al. Full screening and accurate subtyping of HLA-A*02 alleles through group-specific amplification and mono-allelic sequencing. Cell Mol Immunol 2013; 10: 490–496. doi: 10.1038/cmi.2013.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the Global Lung Function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakayama M, Marchi H, Dmitrieva AM, et al. Quantitative proteomics of differentiated primary bronchial epithelial cells from chronic obstructive pulmonary disease and control identifies potential novel host factors post-influenza A virus infection. Front Microbiol 2023; 13: 957830. doi: 10.3389/fmicb.2022.957830 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods and tables ERJ-01374-2022.Supplement (286.8KB, pdf)
Supplementary figures ERJ-01374-2022.Figures (4.8MB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01374-2022.Shareable (1.4MB, pdf)