Key Clinical Message
Disseminated tuberculosis (TB) resulting from lymphohematogenous dissemination of Mycobacterium tuberculosis during primary infection or reactivation of latent disease is rare among young immunocompetent patients. Central nervous system TB (CNS TB) is one of the most challenging clinical diagnoses with high fatality. Here, we describe a young immunocompetent female with no known comorbidities initially presented with military pulmonary TB and later developed CNS TB. This presentation of disseminated TB in immunocompetent patient warrant early diagnosis and treatment.
Keywords: case report, disseminated tuberculosis, miliary tuberculosis, Mycobacterium tuberculosis, tuberculous meningitis
FLAIR (Fluid Attenuated Inverse Recovery) showing features suggestive of inflammation. Post contrast MRI coronal section showing diffuse leptomeningeal enhancement in bilateral cerebral hemispheres with multiple enhancing nodular lesions in bilateral cerebral hemispheres, basal ganglia and brainstem. HRCT scan of the chest showing mild pneumomediastinum, numerous randomly distributed miliary nodules in bilateral lungs, patchy areas of consolidation, and few patches of ground glass opacities in bilateral lower lobes.
1. INTRODUCTION
Tuberculosis (TB) is one of the most common global health burdens caused by Mycobacterium tuberculosis. 1 Almost half of the TB cases remain unreported, contributing to the underdiagnosis of extrapulmonary tuberculosis. 2 The worldwide incidence of disseminated TB is also in increasing trend. Central nervous system (CNS) involvement is one of the most devastating complications of tuberculosis and is seen in 10% of all disseminated TB cases, accounting for 1% of all TB cases. 3 CNS involvement may present as meningitis, cerebral tuberculoma, tuberculoma abscess, and thoracic transverse myelopathy. 1 , 4
The predominant symptoms of disseminated TB are fever, cough, malaise, loss of appetite, weakness, and weight loss. In addition, symptoms according to system involvement are often seen, like a headache in the case of meningeal tuberculosis and abdominal pain in peritoneal or intestinal tuberculosis. 5
Although the treatment of disseminated and pulmonary TB are considered the same, CNS involvement warrants a longer duration of treatment. The four‐drug regimen of rifampicin, isoniazid, pyrazinamide, and ethambutol is administered daily for 2 months, followed by rifampicin and isoniazid for 2 months. In disseminated cases, these two drug regimens can be extended. There is no clear evidence of the effectiveness of corticosteroids in disseminated TB cases. However, steroids are often used in disseminated cases. 6 Early identification and prompt management are the cornerstones for optimal patient management in disseminated TB with CNS involvement.
We reported this case following the updated consensus‐based Surgical Case Report (SCARE) Guidelines. 7
2. CASE PRESENTATION
A 25‐year‐old female with no known comorbidities presented with complaints of fever, productive cough, generalized body weakness, vomiting, and anorexia for 5 months. However, she gave no history of evening rise in temperature, significant weight loss, blood in sputum, or contact with TB‐positive people. Physical examination, including vitals and general and systemic examination, was normal.
Chest X‐ray showed miliary nodules in bilateral lung fields, while gene Xpert sputum test revealed rifampicin‐sensitive Mycobacterium tuberculosis. Pulmonary tuberculosis was thus diagnosed on the grounds of a positive gene Xpert test and features of tuberculosis in a chest radiograph. Antitubercular therapy (ATT) with rifampicin, isoniazid, pyrazinamide, and ethambutol was started. However, as she developed increased oxygen requirements, she was admitted to the medical ICU. During ICU stay, the patient developed irrelevant talking, altered mental status, and increased shortness of breath. She also had an episode of a generalized tonic–clonic seizure. Following this, a lumbar puncture was done, which showed lymphocyte predominance, increased protein, and decreased glucose level. However, adenosine deaminase (ADA) was within normal range, and acid‐fast bacillus was not seen. Thus, TB meningitis was diagnosed on the background of pulmonary tuberculosis.
The patient was referred to our center 5 days after starting ATT for better management. At the time of presentation, her vitals showed blood pressure of 135/70 mmHg, pulse rate of 82 bpm, temperature of 36°C, respiratory rate of 20 breaths per minute, and oxygen saturation of 100% in 15 L per minute of oxygen. On auscultation of the chest, decreased air entry and crepitations were heard in bilateral lung fields. The patient had spontaneous eye opening, inappropriate vocalization, and localizing movement with pain (E4V3M5). Motor examination revealed the power of 4/5 in bilateral upper and lower extremities, normal tone and deep tendon reflexes in bilateral upper limbs and lower limbs, normal plantar reflex, and intact sensation. Neck rigidity was present while Brudzinki's and Kernig's signs were absent.
Lab investigations showed increased liver enzymes; AST: 78 U/L, ALT: 80 U/L, GGT: 95 U/L, increased CRP (73 mg/L), methicillin‐sensitive coagulase‐negative Staphylococcus in blood culture, Na 133 mmol/L, K‐3.6 mmol/L, Hb‐11.5 g/dL, total leukocyte count of 9489/cubic mm. Serology of HCV antibody, HBsAg, HIV 1 and 2 antibodies was negative. In the NCCT head, mild effacement of sulcal spaces in bilateral cerebral hemispheres was seen. Fluid Attenuated Inverse Recovery (FLAIR) imaging showed features suggestive of inflammation (Figure 1). MRI of coronal section of brain showed multiple enhancing nodular lesions in bilateral hemispheres (Figure 2). A high‐resolution CT (HRCT) scan of the chest showed mild pneumomediastinum, numerous randomly distributed miliary nodules in bilateral lungs, patchy areas of consolidation, and few patches of ground glass opacities in bilateral lower lobes (Figure 3). Based on findings of LP, MRI head, HRCT chest, and gene Xpert test, the final diagnosis of disseminated tuberculosis was made.
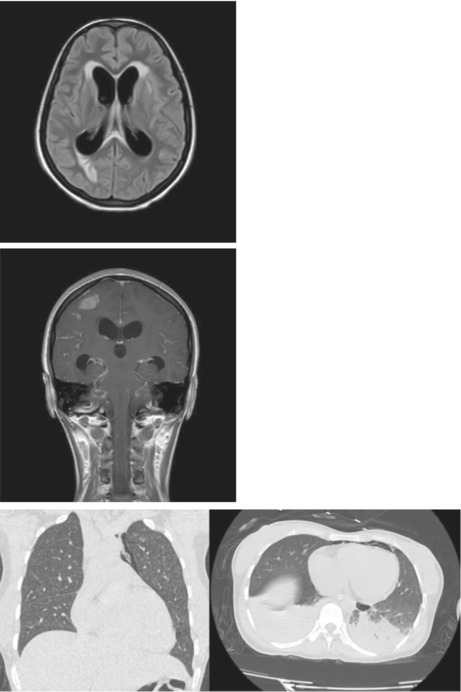
FIGURE 1.
FLAIR (Fluid Attenuated Inverse Recovery) showing features suggestive of inflammation.
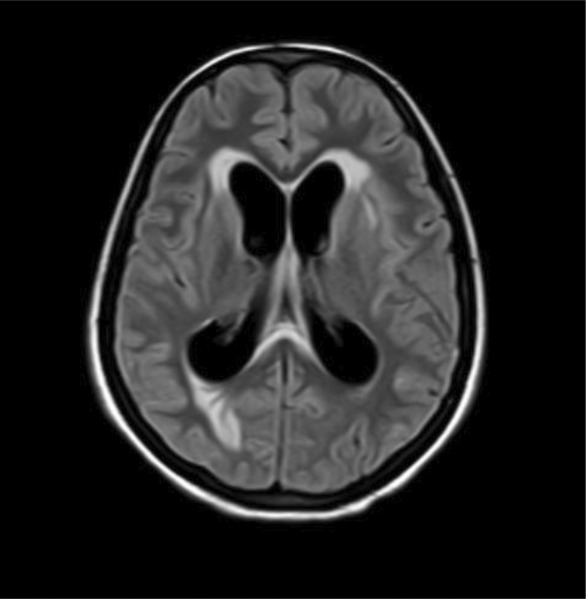
FIGURE 2.
Postcontrast MRI coronal section showing diffuse leptomeningeal enhancement in bilateral cerebral hemispheres with multiple enhancing nodular lesions in bilateral cerebral hemispheres, basal ganglia, and brainstem.
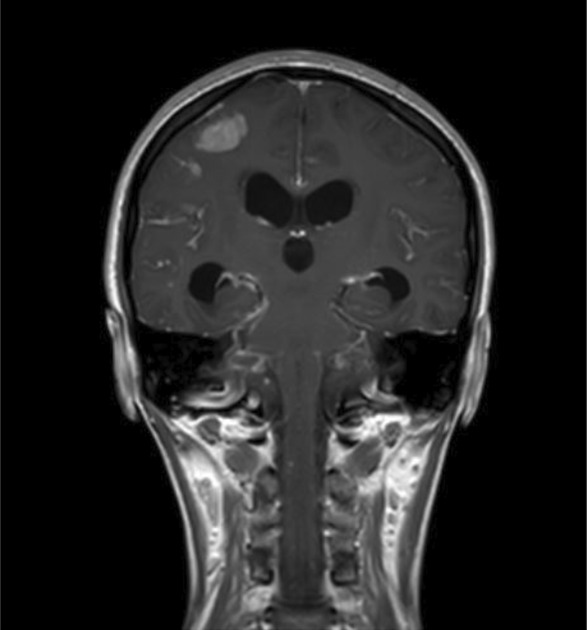
FIGURE 3.
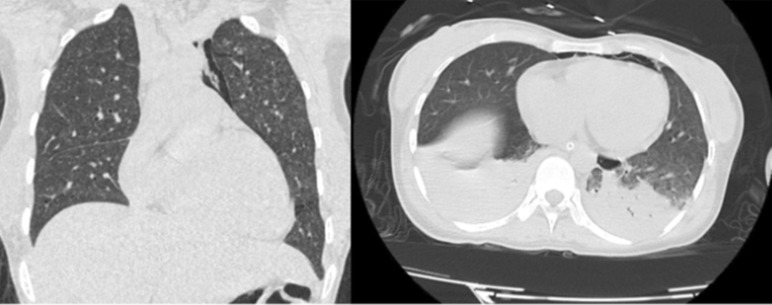
HRCT scan of the chest showing mild pneumomediastinum, numerous randomly distributed miliary nodules in bilateral lungs, patchy areas of consolidation, and few patches of ground glass opacities in bilateral lower lobes.
Along with the continuation of antitubercular therapy, the patient was started on dexamethasone 4 mg TDS for 4 weeks, which was tapered off, and stopped. During her hospital stay, she developed ATT‐induced hepatitis, for which she was kept in liver friendly regimen until recovery. She was reinitiated on ATT after improvement in liver function. The patient had developed pneumoperitoneum with pneumomediastinum, which was managed conservatively.
At the time of discharge, she could walk without support and was oriented to time, place and person. Two weeks later, she presented with fever and burning micturition, which was treated with appropriate antibiotics. Her SARS‐COV‐2 report was positive despite being previously vaccinated with two doses of Vero cell vaccine. She was admitted to the COVID‐19 isolation ward, where her stay was uneventful. On the follow‐up visit, the patient was doing fine and was taking intensive phase of ATT drugs. Her steroid dosage was gradually tapered on follow‐up visit.
3. DISCUSSION
Disseminated tuberculosis (TB) resulting from lymphohematogenous dissemination of Mycobacterium tuberculosis during primary infection or reactivation of latent disease is rare among young immunocompetent patients. 8 , 9 Central nervous system TB (CNS TB) is one of the most challenging clinical diagnoses, with a prevalence of 4.55% of all cases of TB. The spectrum of CNS TB includes TB meningitis, tuberculoma, and spinal arachnoiditis, where TB meningitis is the most predominant form. 10 Small tuberculous lesions known as Rich's foci, formed in the cerebral cortex, cerebral parenchyma, or spinal cord, may remain dormant for several years. Rupture of these foci leads to the spread of the bacilli into the subarachnoid space, resulting in tuberculous meningitis. 11 Risk factors of miliary TB and extrapulmonary TB include HIV, diabetes mellitus, smoking, alcohol abuse, malnutrition, and overcrowding, which were absent in our patient. 8 , 12 Recent research has suggested that this disease form may be predisposed by a genetic abnormality in the interleukin‐12 (IL‐12) and interferon‐gamma pathways. The pathophysiology and genetic components driving immunocompetent hosts' susceptibility to Mycobacterium TB infection are yet unclear. 13
Due to the variable and nonspecific presentation of the disease, CNS TB is difficult and challenging to diagnose. The clinical course is typically subacute or chronic. 14 About one‐third of patients on presentation have underlying miliary TB. Careful fundoscopy may reveal choroidal tubercles, offering a valuable clue to the etiology. 15
A definitive diagnosis of tuberculous meningitis may be established with detection of AFB either by positive smear, culture, or with a positive nucleic acid amplification test (NAAT). 16 The poor sensitivity and specificity of diagnostic tests, however, can make it difficult to make a definitive diagnosis. Typically, the CSF in tuberculous meningitis shows an elevated protein level, predominant lymphocytosis, and a decreased glucose concentration.
Adenosine deaminase (ADA) testing has not been helpful in diagnosing TB meningitis, as an ADA cut‐off value that optimizes both sensitivity and specificity has been difficult to establish. In 2013, WHO recommended Xpert MTB/Rif assay, a commercial NAAT, preferentially over conventional microscopy and culture for initial TB meningitis testing in resource‐limited settings, which is highly specific for TB meningitis but is variable in specificity. Brain imaging in TB meningitis commonly shows basilar meningeal enhancement, hydrocephalus, basal ganglion enhancement, cerebral parenchymal tuberculomas, and infarcts. MRI is superior to CT for detecting TB meningitis. 17 Pulmonary tuberculosis (PTB) gives clues to suspect bacillary etiology of CNS infection. Therefore, this patient's CNS diagnosis was made based on specific CSF findings, CT/MRI findings, and simultaneous pulmonary TB diagnosis.
The current WHO recommendations for treating TB meningitis are based on those made to treat PTB and recommend that all patients receive therapy with 2 months of rifampicin (RMP), isoniazid (INH), pyrazinamide (PZE), and ethambutol (ETB), followed by up to 10 months of RMP and INH. This regimen ignores the many anti‐tuberculosis medications' varying capacities for brain penetration. Dexamethasone lowers mortality in HIV‐negative TB meningitis; however, there is not enough data to support its usage in HIV co‐infection. 18 This patient was started on 2 months of HRZE, 2 months of dexamethasone, and continued with HR. Timely diagnosis and treatment are essential since prognosis largely depends on neurological status at presentation, time‐to‐treatment initiation, HIV co‐infection, and drug resistance. Therefore, empiric treatment should be initiated as soon as the diagnosis is suspected. This patient, with no immunocompromised conditions and drug resistance, received timely treatment for TB meningitis and, thus, showed total recovery.
Disseminated TB involving CNS in immunocompetent adults is challenging to diagnose and often delayed or missed. As there is little evidence on the pathophysiology of disseminated TB in the immunocompetent host, further research is essential.
AUTHOR CONTRIBUTIONS
Ram Chandra Subedi: Conceptualization; data curation; resources; supervision; writing – original draft; writing – review and editing. Subi Acharya: Conceptualization; data curation; resources; supervision; writing – original draft. Ayush Adhikari: Writing – original draft; writing – review and editing. Sabin Banmala: Conceptualization; supervision; writing – original draft; writing – review and editing. Tibbin Kumar Shiwakoti: Conceptualization; methodology; writing – original draft; writing – review and editing. Pearlbiga Karki: Conceptualization; methodology; writing – original draft; writing – review and editing. Shekhar Gurung: Validation; writing – original draft; writing – review and editing. Bhuwan Bhatta: Validation; writing – original draft; writing – review and editing. Naresh Kharbuja: Validation; writing – original draft; writing – review and editing. Raju Paudel: Validation; writing – original draft; writing – review and editing.
FUNDING INFORMATION
The study did not receive any grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest.
ETHICS STATEMENT
This is a case report; therefore, it did not require ethical approval from ethics committee.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGEMENTS
The authors thank Grande International Hospital for providing the MRI images.
Subedi RC, Acharya S, Adhikari A, et al. Disseminated tuberculosis in an immunocompetent woman from the Himalayan region of Nepal: A case report. Clin Case Rep. 2023;11:e7754. doi: 10.1002/ccr3.7754
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5(7):415‐430. [DOI] [PubMed] [Google Scholar]
- 2. Gaifer Z. Epidemiology of extrapulmonary and disseminated tuberculosis in a tertiary care center in Oman. Int J Mycobacteriology. 2017;6(2):162‐166. [DOI] [PubMed] [Google Scholar]
- 3. Cherian A, Thomas SV. Central nervous system tuberculosis. Afr Health Sci. 2011;11(1):116‐127. [PMC free article] [PubMed] [Google Scholar]
- 4. Garg RK, Sharma R, Kar AM, et al. Neurological complications of miliary tuberculosis. Clin Neurol Neurosurg. 2010;112(3):188‐192. [DOI] [PubMed] [Google Scholar]
- 5. Prout S, Benatar SR. Disseminated tuberculosis. A study of 62 cases. S Afr Med J. 1980;58(21):835‐842. [PubMed] [Google Scholar]
- 6. Khan FY. Review of literature on disseminated tuberculosis with emphasis on the focused diagnostic workup. J Family Community Med. 2019;26(2):83‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int J Surg. 2020;84:226‐230. [DOI] [PubMed] [Google Scholar]
- 8. Rosenthal AH, Rothfield LD, Chamorro DL. Disseminated tuberculosis in a healthy adolescent female. Cureus. 2019;11:e4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hilal T, Hurley P, McCormick M. Disseminated tuberculosis with tuberculous meningitis in an immunocompetent host. Oxford Med Case Reports. 2014;2014:125‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navarro‐Flores A, Fernandez‐Chinguel JE, Pacheco‐Barrios N, Soriano‐Moreno DR, Pacheco‐Barrios K. Global morbidity and mortality of central nervous system tuberculosis: a systematic review and meta‐analysis. J Neurol. 2022;269(7):3482‐3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dian S, Ganiem AR, van Laarhoven A. Central nervous system tuberculosis. Curr Opin Neurol. 2021;34(3):396‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vasconcelos G, Santos L, Couto C, Cruz M, Castro A. Miliary brain tuberculomas and meningitis: tuberculosis beyond the lungs. Eur J Case Reports Intern Med. 2020;7(12):001931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donovan ML, Schultz TE, Duke TJ, Blumenthal A. Type I interferons in the pathogenesis of tuberculosis: molecular drivers and immunological consequences. Front Immunol. 2017;8:1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kent SJ, Crowe SM, Yung A, Lucas CR, Mijch AM. Tuberculous meningitis: a 30‐year review. Clin Infect Dis. 1993;17(6):987‐994. [DOI] [PubMed] [Google Scholar]
- 15. Dunphy L, Shetty P, Randhawa R, Rani KA, Duodu Y. Tuberculous meningitis in an immunocompetent male complicated by hydrocephalus. BMJ Case Rep. 2016;2016:bcr2015213916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803‐812. [DOI] [PubMed] [Google Scholar]
- 17. Bourgi K, Fiske C, Sterling TR. Tuberculosis meningitis. Curr Infect Dis Rep. 2017;19(11):39. [DOI] [PubMed] [Google Scholar]
- 18. Davis A, Meintjes G, Wilkinson RJ. Treatment of tuberculous meningitis and its complications in adults. Curr Treat Options Neurol. 2018;20(3):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


