Abstract
Key Clinical Message
Fetal bradycardia and congenital complete heart block could be the presentation of neonatal lupus. A high index of suspicion of this condition helps to identify an asymptomatic mother.
Abstract
Neonatal lupus erythematosus (NLE) is a rare acquired autoimmune disease of newborns due to placental transfer of Ro/SSA or La/SSB autoantibodies. Though cardiac, cutaneous, hematological, and hepatobiliary abnormalities are detected, cardiac defects cause significant morbidity and mortality. We report a case of complete congenital heart block due to NLE.
Keywords: autoantibodies, bradycardia, heart block
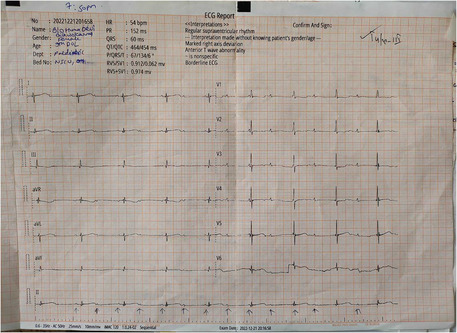
The electrocardiograph of the baby showing a heart rate of 54 beats/min and features of complete heart block.
1. INTRODUCTION
Neonatal lupus erythematosus (NLE) is a rare acquired autoimmune disease characterized by cutaneous, cardiac, and systemic abnormalities in newborn infants whose mothers bear antibodies against autoantigen type A (Ro/SSA) or B (La/SSB). 1 , 2 Passive transfer of these immunoglobulin G autoantibodies to the fetus via the placenta is the pathogenesis behind the disease; however, only 2% of babies born to mothers with Ro/SSA or La/SSB autoantibodies develop NLE with increased risk to 18%–20% in subsequent pregnancies. 3
Skin and heart are commonly affected organs in NLE. The most common skin manifestation is a non‐scarring, non‐atrophic lesion resembling subacute cutaneous lupus erythematosus (CLE), while the child may also develop other skin changes like petechiae or purpura due to thrombocytopenia, and jaundice due to liver involvement. 1 , 4 The cardiac lesions are, however, permanent and carry significant morbidity and mortality. The irreversible and complete heart block (CHB) due to NLE causes mortality in 15%–30% of cases and two‐thirds of them require permanent pacing. 5 Here, we present a rare case in Nepal of a newborn delivered by emergency LSCS (lower segment cesarean section) at 33 weeks of gestation for fetal bradycardia who later develops complete heart block, and diagnosed to have NLE.
2. CASE PRESENTATION
A female baby on her ninth day of life was referred to our center for poor feeding, respiratory distress, and persistent bradycardia since birth. She was born to a 24‐year primigravida mother at 33+6 weeks of gestation and was appropriate for gestational age (1.8 kg). The baby was delivered outside our center by emergency lower segment cesarean section (LSCS) for fetal bradycardia. The mother was on regular antenatal checkups (ANCs) with unremarkable antenatal laboratory results. She did not have a history suggesting of any rheumatological illnesses. An anomaly scan done in the second trimester revealed no gross congenital anomalies in the fetus. While she was on her regular ANC visit, the fetus was found to have bradycardia, and therefore, an emergency cesarean section was performed. The baby cried immediately after birth. Her 1 and 5‐min Apgar scores were 6/10 and 8/10 respectively, and heart rate ranged from 58 to 70 beats/min. The baby was then admitted to neonatal intensive care unit (NICU) for low birth weight and bradycardia.
The electrocardiograph (ECG) done outside our center showed a heart rate of 54 beats/min and findings suggestive of complete a heart block (Figure 1). Her echocardiography showed 5 mm ostium secundum atrial septum defect (OS‐ASD). The baby was started on dobutamine and referred to our center for further management. At the time of presentation to our center, the baby was pale but had no cyanosis, icterus, and dehydration. Her heart rate was 54 bpm (beats per minute), good volume, her temperature was 95.8°F, and she had a normal respiratory rate (36 breaths per minute) with oxygen saturation maintained at 94% in nasal prongs with a flow of 2 L/min. Extremities were warm and blood pressure was 50/30 mm of Hg. Examination findings of other systems were non‐significant.
FIGURE 1.
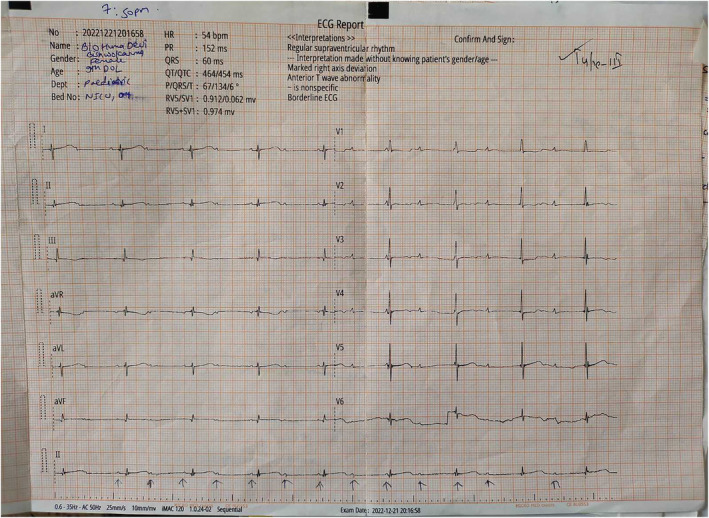
The electrocardiograph of the baby showing a heart rate of 54 beats/min and features of a complete heart block.
Initial blood investigations revealed a low platelet count of 27,000 per mm3. The rest of the investigations were within normal limits (Table 1). An ambulatory Holter recording was performed which revealed neonatal bradycardia with an average heart rates of 79 bpm during the day, 76 bpm at night, and 77 bpm overall. The maximum heart rate reached was 128 bpm and the minimum was 68 bpm.
TABLE 1.
Laboratory parameters of the baby at the time of admission.
| Parameters | Results | Normal reference used |
|---|---|---|
| Hematology | ||
|
5.7 gm% | 12.5%–15 gm% |
|
35.1% | 37.5%–45% |
|
3.51 million/cubic mm | 3.5–4.5 million/cubic mm |
|
5700 | 4000–11,000 |
|
27,000 | 150,000–400,000 |
| Renal function test | ||
|
55 μMol/L | 40–110 μMol/L |
|
3.4 mMol/L | 1.6–7.0 mMol/L |
|
140 mEq/L | 135–146 mEq/L |
|
5.2 mEq/L | 3.5–5.2 mEq/L |
| Liver function test | ||
|
6 μMol/L | 5–21 μMol/L |
|
2 μMol/L | 0–4 μMol/L |
|
15 U/L | 0–35 U/L |
|
20 U/L | 0–35 U/L |
|
52 U/L | 30–120 U/L |
|
14 U/L | 0–38 U/L |
| Others | ||
| CRP | 5.35 mg//dl | 0–6 mg/dL |
| FDP d‐dimer | 2.07 μg/mL | 0.01–0.50 μg/mL |
| Fibrinogen | 253 mg/dL | 200–400 mg/dL |
The most important cause of neonatal bradycardia is prolonged QT syndrome. However, the corrected QT interval (QTc) on ECG was 0.42 s excluding this diagnosis. The baby was closely observed for signs of hypoperfusion. Considering the possibility of pneumonia, the baby was managed with intravenous (IV) antibiotic, which was stopped after a negative blood culture report. Oral feeding was slowly started and nutritional supplementation was done including calcium gluconate. The baby was on oxygen therapy for possible respiratory distress syndrome. It was weaned off gradually and dobutamine was also tapered and then stopped, as there were no signs of hypoperfusion.
Since neonatal lupus is the most common cause of congenital complete heart block, the mother was investigated for SLE even in the absence of any symptoms, and the tests were positive (Table 2). Therefore, the mother was referred to a rheumatologist and was started on immunosuppressive therapy. At the time of discharge from our center, the baby's heart rate was between 70 and 90 bpm, and she did not have any features of heart failure. The clinical manifestation of poor perfusion were explained to the parents and were advised to attend the emergency department in such situations. The baby was planned to monitor for heart failure and growth delay during the follow‐ups.
TABLE 2.
Laboratory parameters of the mother.
| Parameters | Results | Normal reference used |
|---|---|---|
| ANA HEp‐2 | Positive | − |
| CRP | 2.9 mg/L | <1 mg/mL |
| Anti‐Ds antibody | 175.93 IU/mL | >100 IU/mL: positive |
| Complement C3 | 78.89 | 90‐180 mg/dL |
| Compliment C4 | 0.13 | 0.1–0.4 g/L |
| RNP/Sm | +++ | − |
| Sm | +++ | − |
| RNP | +++ | − |
| SSA | +++ | − |
| Ro‐52 | +++ | − |
3. DISCUSSION
NLE is a rare autoimmune disease with unknown actual incidence worldwide as there are numerous undiagnosed occurrences. 4 However, its incidence in the USA is one in every 20,000 live births. 1 It was first reported in 1954 in a baby of an ANA‐positive mother. 6 The passive transfer of the maternal autoantibodies directed to antigen targets within the ribonucleoprotein complex (60 kDa Ro, 52 kDa Ro, 48 kDa La) via the placenta is the mechanism behind NLE. 7
Almost half of the mothers, including the one being presented here, with autoantibodies SSA/Ro–SSB/La are asymptomatic and are not diagnosed with SLE until their children are born with features of NLE, for example, CHB. 8 This suggests that the causes of incidental fetal bradycardia, whenever detected in the ANC (antenatal care) clinic, should be sought to anticipate and prevent the complications of NLE.
The baby may develop one or more of a constellation of manifestations involving cardiovascular, cutaneous, hepatobiliary, and hematological systems. 7 , 9 The common cardiovascular presentation is bradycardia (heart rate less than 100 per minute), rarely associated with signs of congestive heart failure (diaphoresis, pallor, peripheral edema, prominent jugular veins, and crackles on auscultation of the lungs). The other cardiac manifestations include QT‐interval prolongation, cardiomyopathy, congestive heart failure, myocarditis, and structural defects like ventricular septal defects (VSD), atrial septal defects (ASD), patent foramen ovale (PFO), patent ductus arteriosus (PDA), and pulmonary stenosis. 3 Antenatal diagnosis of cardiac abnormalities in structure, rhythm, and function is done using fetal echocardiography after 18 weeks of gestation. 3 The case presented here started developing conduction abnormality in‐utero, hence was delivered by emergency LSCS for fetal bradycardia. The anti‐Ro52/SSA autoantibodies in the fetus trigger an inflammatory response in the sinoatrial (SA) node, atrioventricular (AV) node, and bundle of His. This inhibits impulse transmission and ultimately leads to fibrosis and scarring of these conducting channels, thus causing congenital heart block (CHB), and other conduction abnormalities of NLE. 10 Since there were no known risk factors, and an antenatal anomaly scan done in the second trimester detected no gross fetal anomalies, fetal echocardiography was not done in our case.
The hematologic manifestations of NLE include thrombocytopenia, neutropenia, and anemia. The mechanism of pancytopenia is unclear and thought to be an outcome of an autoimmune reaction. 7 The baby had thrombocytopenia (platelet count 27,000 per mm3), but other hematological parameters were within normal range. As the meantime of appearance of cutaneous manifestations of NLE is around 6 weeks, the nine‐day‐old baby in our case did not have any dermatologic symptoms. It has been proven that exposure to ultraviolet (UV) rays triggers skin manifestations via immune complex deposition and tissue injury. Thus, the mother was counseled regarding the UV protection of her baby. 7
For non‐cardiac manifestations, which are usually self‐limiting, only symptomatic treatment modalities are practiced. The thrombocytopenia gradually improved over 1 week time and she did not have any bleeding manifestation. Therefore, she did not require any treatment for it. As CHB is a major factor causing high morbidity and mortality among newborns, priority should be laid on its early treatment. Some studies suggest the use of antenatal betamethasone/dexamethasone to prevent CHB; however, the actual benefit has not been confirmed yet. 3 , 11 After birth, around two‐thirds of the babies with congenital complete heart block (CCHB) require a pacemaker, and they might have a good prognosis if the ventricular function is preserved. 12 , 13 Decreased ventricular rate to less than 55 bpm, increased atrial rate to more than 140 bpm, wide QRS complexes, congenital heart disease, heart failure, and prolonged QT interval are the indications of implantations for permanent pacemaker (PPM) in infants. 14 The baby did not have any indication of pacemaker implantation during neonatal life, so she was discharged with plan for pacemaker implantation if any of these features appears during follow‐up visits. A high index of suspicion is required to detect congenital NLE, a rare entity among newborn babies of asymptomatic mothers to prevent complications like CCHB and neonatal mortality.
4. CONCLUSION
NLE is a rare neonatal disease caused due to passive transfer of maternal autoantibodies that commonly manifests as bradycardia and heart block. Though other systemic manifestations are self‐limiting, cardiac abnormalities carry high morbidity and mortality. A high index of suspicion is necessary to identify this disease among babies of asymptomatic mothers. Therefore, early identification and treatment of NLE in symptomatic babies and SLE among asymptomatic mothers help to prevent hazardous outcomes in both mothers and babies.
AUTHOR CONTRIBUTIONS
Siddinath Gyawali: Conceptualization; investigation; methodology; resources; supervision; validation; visualization; writing – original draft; writing – review and editing. Suraj Prasad Gupta Rauniyar: Conceptualization; investigation; writing – original draft; writing – review and editing. Bindu Gyawali: Conceptualization; investigation; writing – original draft; writing – review and editing. Tapasya Bhusal: Conceptualization; investigation; writing – original draft; writing – review and editing. Srijana Basnet: Conceptualization; formal analysis; investigation; resources; visualization; writing – original draft; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest regarding the publication of this paper.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent will be made available for review to the editor‐in‐chief of this journal if asked.
Gyawali S, Rauniyar SPG, Gyawali B, Bhusal T, Basnet S. Neonatal lupus erythematosus manifested as a complete heart block: A case report. Clin Case Rep. 2023;11:e7758. doi: 10.1002/ccr3.7758
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia S, Campos‐de‐Carvalho AC. Neonatal lupus syndrome: the heart as a target of the immune system. An Acad Bras Cienc. 2000;72(1):83‐89. [DOI] [PubMed] [Google Scholar]
- 3. Diaz‐Frias J, Badri T. Neonatal Lupus Erythematosus. StatPearls. Treasure Island; 2022. [PubMed] [Google Scholar]
- 4. Wei S, Yuan TM, Chen LH, Yu HM. Neonatal lupus erythematosus: three case reports and review of the Chinese literature. Clin Pediatr (Phila). 2010;49(7):627‐634. [DOI] [PubMed] [Google Scholar]
- 5. Buyon JP, Clancy RM. Neonatal lupus syndromes. Curr Opin Rheumatol. 2003;15(5):535‐541. [DOI] [PubMed] [Google Scholar]
- 6. Mc CC, Schoch EP Jr. Possible discoid lupus erythematosus in newborn infant; report of a case with subsequent development of acute systemic lupus erythematosus in mother. AMA Arch Derm Syphilol. 1954;70(6):782‐785. [DOI] [PubMed] [Google Scholar]
- 7. Buyon JP, Saxena A, Izmirly PM, Cuneo B, Wainwright B. Neonatal lupus: clinical spectrum, biomarkers, pathogenesis, and approach to treatment. Systemic Lupus Erythematosus: Elsevier p. 507–19 2021.
- 8. Li X, Huang X, Lu H. Two case reports of neonatal autoantibody‐associated congenital heart block. Medicine (Baltimore). 2018;97(45):e13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li YQ, Wang Q, Luo Y, Zhao Y. Neonatal lupus erythematosus: a review of 123 cases in China. Int J Rheum Dis. 2015;18(7):761‐767. [DOI] [PubMed] [Google Scholar]
- 10. Eftekhari P, Salle L, Lezoualc'h F, et al. Anti‐SSA/Ro52 autoantibodies blocking the cardiac 5‐HT4 serotoninergic receptor could explain neonatal lupus congenital heart block. Eur J Immunol. 2000;30(10):2782‐2790. [DOI] [PubMed] [Google Scholar]
- 11. Lateef A, Petri M. Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol. 2013;27(3):435‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pruetz JD, Miller JC, Loeb GE, Silka MJ, Bar‐Cohen Y, Chmait RH. Prenatal diagnosis and management of congenital complete heart block. Birth Defects Res. 2019;111(8):380‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eliasson H, Sonesson SE, Sharland G, et al. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation. 2011;124(18):1919‐1926. [DOI] [PubMed] [Google Scholar]
- 14. Tanriverdi S, Ulger Z, Siyah Bilgin B, et al. Treatment of congenital complete atrioventricular heart block with permanent Epicardial pacemaker in neonatal lupus syndrome. Iran Red Crescent Med J. 2015;17(9):e16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


