Abstract
BACKGROUND:
The clinical trial ARISTOTLE showed that apixaban was superior to warfarin in reducing the risks of stroke and bleeding among patients with nonvalvular atrial fibrillation (NVAF). Further study of the effect of apixaban versus warfarin use on health care resource utilization (HCRU) and associated costs in the real-world setting is warranted, especially among elderly patients who are at higher risk of stroke and bleeding.
OBJECTIVE:
To compare HCRU and costs among elderly NVAF patients treated with apixaban versus warfarin in the United States.
METHODS:
Elderly patients (aged ≥ 65 years) with Medicare coverage who initiated apixaban or warfarin were identified from the Humana research database during January 1, 2013-September 30, 2015. Patients were required to have 12 months of continuous insurance coverage before drug initiation (baseline period) and an atrial fibrillation diagnosis during the baseline period or on the date of drug initiation. NVAF patients were grouped into cohorts depending on the drug initiated. Propensity score matching (PSM) was conducted to control for differences in demographics and clinical characteristics of study cohorts. Patients were followed after the index date for a variable length of follow-up. All-cause and disease-specific HCRU and costs during the follow-up were evaluated before and after PSM and reported as per patient per year.
RESULTS:
Of the overall (unmatched) population, 8,250 patients (mean age: 78.0 years) initiated apixaban and 14,051 patients (mean age: 78.2 years) initiated warfarin. Among NVAF patients who initiated apixaban versus those who initiated warfarin, mean Charlson Comorbidity Index (CCI) scores (3.0 vs. 3.4, P < 0.001); stroke risk scores, including CHADS2 (2.7 vs. 2.9, P < 0.001) and CHA2DS2-VASc (4.6 vs. 4.7, P < 0.001); and bleeding risk scores, including HAS-BLED (3.1 vs. 3.2, P < 0.001), were lower. Additionally, total annual all-cause health care costs were lower during the baseline period for patients treated with apixaban versus warfarin ($17,077 vs. $20,236, P < 0.001). After PSM, 14,214 patients were matched, with 7,107 in each cohort. Mean age, CCI score, and stroke and bleeding risks were similar between matched cohorts, as were total all-cause health care costs during the baseline period. During the follow-up among matched cohorts, apixaban versus warfarin treatment was associated with higher annual pharmacy costs ($5,159 vs. $2,867, P < 0.001) but lower annual inpatient ($8,327 vs. $14,296, P < 0.001), outpatient ($9,655 vs. $11,469, P < 0.001), and total all-cause health care costs ($23,141 vs. $28,633, P < 0.001), which were reflective of lower inpatient, outpatient, and all-cause HCRU among apixaban-treated patients. Furthermore, bleeding-related ($2,101 vs. $3,963, P < 0.001) and stroke-related ($652 vs. $1,178, P = 0.001) annual medical costs were lower for patients treated with apixaban versus warfarin.
CONCLUSIONS:
After controlling for differences in patient characteristics, in the real-world setting apixaban versus warfarin use was associated with less HCRU and lower total all-cause health care costs and costs for bleeding- and stroke-related medical services, but greater pharmacy costs, among elderly NVAF patients.
What is already known about this subject
Previous economic modeling studies, based on clinical trial event rates, predicted a cost savings to health care systems in the United States associated with apixaban versus warfarin use among non-valvular atrial fibrillation (NVAF) patients.
A study of NVAF patients hospitalized for AF showed that apixaban versus warfarin treatment was associated with a shorter hospital length of stay.
What this study adds
This study examined the effect of apixaban versus warfarin use on health care resource utilization (HCRU) and the associated costs among elderly NVAF patients in the real-world setting in the United States.
In comparison with NVAF patients who received warfarin, patients who initiated apixaban significantly differed in many patient characteristics, with age, bleeding and stroke risks, and previous HCRU and costs being lower for those who received apixaban.
After controlling for differences in patient characteristics with propensity score matching, apixaban versus warfarin use was associated with less HCRU and lower total all-cause health care costs and costs for bleeding- and stroke-related medical services, but greater pharmacy costs, among elderly NVAF patients.
Atrial fibrillation (AF) is a common cardiac rhythm disorder that is associated with up to a 5-fold increase in stroke risk.1,2 It is primarily nonvalvular, with less than 5% of AF patients having valvular heart disease.1 In 2005, there were an estimated 3 million persons with AF in the United States.3 With the growing elderly population in the United States, this number is expected to at least double by 2050.1,3 The annual direct medical cost of nonvalvular atrial fibrillation (NVAF) was estimated at $6 billion for NVAF-related costs only and $26 billion when including other concomitant cardiovascular and noncardiovascular costs in 2008 U.S. dollars.4
Vitamin K antagonists, mainly warfarin, have been used for decades to reduce stroke risk for NVAF patients.5 However, warfarin has several disadvantages, including a limited therapeutic index, potential for drug and food interactions, and bleeding risk.5,6 Furthermore, nearly half of AF patients in the United States do not receive warfarin therapy as recommended.7 Four new oral anticoagulants (NOACs) have been introduced to the U.S. market within the past several years and are alternatives to warfarin for anticoagulation therapy among NVAF patients. In the randomized clinical trial, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE), treatment with the NOAC apixaban was shown to be superior to warfarin for stroke prevention and also was associated with lower bleeding risk among NVAF patients.8 Additionally, a recent retrospective cohort analysis conducted in the United States observed that apixaban use in comparison with warfarin was associated with significantly lower stroke and major bleeding risk in the real-world setting.9
Some economic modeling studies based on clinical event rates reported in the ARISTOTLE trial predicted apixaban versus warfarin use to be associated with a cost savings to health care systems in the United States.10-13 Additionally, a study of 1,664 NVAF patients (aged ≥ 18 years) hospitalized for AF showed that apixaban versus warfarin treatment was associated with a shorter hospital length of stay.14 Further study of the effect of apixaban versus warfarin use on health care resource utilization (HCRU) and associated costs in the real-world setting is warranted, especially among elderly patients with NVAF who are at higher risk of stroke and bleeding.1,15 To address this need, we evaluated the effect of treatment with apixaban versus warfarin on HCRU and costs, specifically among elderly (aged ≥ 65 years) NVAF patients with Medicare insurance coverage, using a large retrospective database claims analysis.
Methods
Study Population
NVAF patients with Medicare insurance coverage and aged ≥ 65 years who were first prescribed apixaban or warfarin between January 1, 2013, and September 30, 2015, were identified from the Humana research database. The database comprises claims from millions of members with Medicare Advantage coverage. The database is an integrated source of managed care medical and pharmacy claims and eligibility files. The medical file contains data on diagnostic and therapeutic services rendered in both inpatient and outpatient settings, including the emergency room. The pharmacy file contains data on outpatient prescription drugs dispensed (retail and mail order) with accompanying information on the characteristics of the drug dispensed, such as quantity and days supply. For both medical and pharmacy files, the dates of service are recorded. The eligibility file also contains data on demographic characteristics and periods of insurance eligibility for each patient.
The date of the earliest apixaban or warfarin prescription to occur (index event) between January 1, 2013, and September 30, 2015, was defined as the index date. The study start date was chosen since apixaban was approved by the U.S. Food and Drug Administration in December 2012. The study end date was chosen based on the availability of the most recent data when the study was conducted.
Patients were required to have 12 months of continuous insurance coverage before drug initiation (baseline period). Patients were also required to have at least 1 inpatient or outpatient AF diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 427.31) during the baseline period or on the date of drug initiation. Patients who had medical claims indicative of diagnoses of valvular heart disease or venous thromboembolism during the baseline period were excluded, as were patients with a diagnosis or procedure code for transient AF, cardiac surgery, hyperthyroidism or thyroid toxicity, or pregnancy (Appendix A, available in online article). Additionally, patients were excluded if they had a pharmacy claim for warfarin, apixaban, dabigatran, rivaroxaban, or edoxaban during the baseline period or had claims for > 1 type of oral anticoagulant (OAC) on the index date.
Eligible patients were grouped either in the apixaban or warfarin cohort based on which drug the patients newly initiated. Patients were followed-up after the index date for a variable length of time until the earliest of the following dates: 90 days after the index OAC treatment discontinuation, which was a grace period included to ensure potential stroke and bleeding events were captured after patients discontinued OAC treatment; the date that the patient switched from the index OAC treatment to another OAC; the health plan disenrollment date; or the end of the study period (September 30, 2015).
Discontinuation was defined as no prescription refill of the index OAC within 30 days from the end date of the last filled prescription. The date of discontinuation was the end date of the last filled prescription before the treatment gap. A switch among OACs was defined as a prescription filled for nonindex OACs within ± 30 days after the discontinuation date. Figure 1 shows the process of selection of patients for study cohorts.
FIGURE 1.
Selection of Patients for Study Cohorts
Propensity Score Matching
Propensity scores were generated using a multivariate logistic regression, which controlled for patient characteristics identified from claims data: age, gender, race, U.S. geographic region, and Charlson Comorbidity Index (CCI) score—a measurement to reflect the patient’s burden of comorbidities, which are correlated with the risk of death from comorbid disease, that involves calculating a score based on the degree of mortality attributed to each comorbid condition.16 Other characteristics are CHA2DS2-VASc score—an estimate of stroke risk in patients with AF17; HAS-BLED score—an estimate of bleeding risk in patients with AF18; follow-up period duration; baseline total health care cost; baseline bleeding-related medical cost; baseline stroke-related medical cost; baseline comorbidities (thrombocytopenia, congestive heart failure, diabetes, hypertension, renal disease, myocardial infarction, dyspepsia/stomach discomfort, peripheral vascular disease, transient ischemic attack, coronary artery disease); and baseline medication use (angiotensin-converting enzyme inhibitors, amiodarone, angiotensin receptor blockers, beta blockers, H2-receptor antagonists, proton pump inhibitors, statins, and antiplatelet drugs).
As these patient characteristics were derived from claims within the database and based on diagnosis and drug codes, there is potential for miscoding. Matching was conducted 1:1 by using the nearest neighbor algorithm, which required matched patients to have propensity scores within 0.001 of each other. Additionally, matched patients were required to have HAS-BLED scores within 3 points of each other and baseline bleeding-related medical costs within $5,000 of each other in order for the matched patient cohorts to be well balanced (without statistically significant differences).
Demographics and Clinical Characteristics
Demographics, including age, gender, race, and U.S. geographic region and clinical characteristics, CCI score, CHADS2 score, CHA2DS2-VASc score, HAS-BLED score, and previous bleeding and stroke diagnoses during the 12-month baseline period, were determined for each patient in the study cohorts before and after propensity score matching (PSM).
HCRU and Cost Measurements
HCRU and costs during the baseline (including index date) and follow-up periods were evaluated for the unmatched and propensity score matched study cohorts and normalized to per patient per year (PPPY). Health care resources evaluated included number of hospitalizations, hospital length of stay, number of outpatient claims (with breakdown into office visits, ER visits, outpatient hospital claims, and other outpatient claims), and number of outpatient prescription claims for all causes. Use of bleeding- and stroke-related medical services were evaluated and identified by the corresponding ICD-9-CM codes on either inpatient or outpatient health care encounters for a primary or secondary diagnosis of the medical condition (Appendix B, available in online article). Health care costs were measured for all evaluated HCRU categories as the following: all-cause total health care costs; all-cause medical costs; all-cause inpatient costs; all-cause outpatient medical costs (with breakdown into office visit costs, ER costs, outpatient hospital costs, and other outpatient costs); all-cause prescription costs; bleeding-related medical costs; and stroke-related medical costs. All costs were inflation-adjusted to 2015 cost levels using the medical care component of the Consumer Price Index.19
Statistical Analyses
Descriptive statistics were used to evaluate differences between the study cohorts in demographics, clinical characteristics, and HCRU and costs before and after matching. T-tests and chi-square tests were used to detect statistically significant differences in continuous and categorical variables, respectively, as commonly used in similar studies evaluating such data with and without PSM.20,21 All data analyses were executed using SAS statistical software package 9.3 (SAS Institute, Cary, NC).
Results
Unmatched Study Cohorts
Table 1 presents the baseline demographics, clinical characteristics, and health care costs of study cohorts before matching. Of the overall unmatched population, 8,250 patients (mean age: 78.0 years) initiated apixaban, and 14,051 patients (mean age: 78.2 years) initiated warfarin. Before matching, among NVAF patients who initiated apixaban versus those who initiated warfarin, mean CCI score (3.0 vs. 3.4, P < 0.001); CHADS2 score (2.7 vs. 2.9, P < 0.001); CHA2DS2-VASc score (4.6 vs. 4.7, P < 0.001); and HAS-BLED score (3.1 vs. 3.2, P < 0.001) were lower. The proportions of patients with bleeding (18.9% vs. 24.0%, P < 0.001) and stroke (11.8% vs. 15.8%, P < 0.001) diagnoses during the baseline period were lower for NVAF patients treated with apixaban versus warfarin.
TABLE 1.
Baseline Demographics, Clinical Characteristics, and Health Care Costs of Study Cohorts Before and After Propensity Score Matching
| Before Matching | P Valuea | After Matching | P Valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Apixaban n = 8,250 | Warfarin n= 14,051 | Apixaban n = 7,107 | Warfarin n= 7,107 | |||||||
| Demographics | ||||||||||
| Age | ||||||||||
| Mean (SD) | 78.0 (9.0) | 78.2 (9.0) | 0.03 | 78.2 (9.1) | 78.1 (8.8) | 0.54 | ||||
| Median | 76 | 77 | 77 | 77 | ||||||
| n | % | n | % | n | % | n | % | |||
| Gender | ||||||||||
| Male | 4,249 | 51.5 | 7,757 | 55.2 | < 0.001 | 3,740 | 52.6 | 3,688 | 51.9 | 0.38 |
| U.S. geographic region | < 0.001 | 0.82 | ||||||||
| South | 5,707 | 69.2 | 7,807 | 55.6 | 4,675 | 65.8 | 4,717 | 66.4 | ||
| Midwest | 1,582 | 19.2 | 4,168 | 29.7 | 1,514 | 21.3 | 1,504 | 21.2 | ||
| West | 775 | 9.4 | 1,640 | 11.7 | 737 | 10.4 | 705 | 9.9 | ||
| Northeast | 186 | 2.3 | 436 | 3.1 | 181 | 2.6 | 181 | 2.6 | ||
| Race/ethnicity | < 0.001 | 0.85 | ||||||||
| White | 7,385 | 89.5 | 12,507 | 89.0 | 6,373 | 89.7 | 6,371 | 89.6 | ||
| Black | 481 | 5.8 | 977 | 7.0 | 440 | 6.2 | 436 | 6.1 | ||
| Other | 192 | 2.3 | 329 | 2.3 | 167 | 2.4 | 160 | 2.3 | ||
| Unknown | 192 | 2.3 | 238 | 1.7 | 127 | 1.8 | 140 | 2.0 | ||
| Clinical characteristics | ||||||||||
| Charlson Comorbidity Index score | ||||||||||
| Mean (SD) | 3.0 (2.5) | 3.4 (2.6) | < 0.001 | 3.0 (2.4) | 3.0 (2.4) | 0.97 | ||||
| Median | 3 | 3 | 3 | 3 | ||||||
| CHADS2 score | ||||||||||
| Mean (SD) | 2.7 (1.4) | 2.9 (1.4) | < 0.001 | 2.7 (1.4) | 2.7 (1.3) | 0.37 | ||||
| Median | 3 | 3 | 3 | 3 | ||||||
| CHA2DS2-Vasc score | ||||||||||
| Mean (SD) | 4.6 (1.6) | 4.7 (1.6) | < 0.001 | 4.6 (1.6) | 4.6 (1.6) | 0.66 | ||||
| Median | 4 | 5 | 4 | 4 | ||||||
| HAS-BLED score | ||||||||||
| Mean (SD) | 3.1 (1.2) | 3.2 (1.2) | < 0.001 | 3.0 (1.1) | 3.1 (1.1) | 0.22 | ||||
| Median | 3 | 3 | 3 | 3 | ||||||
| Baseline conditions | n | % | n | % | n | % | n | % | ||
| Previous bleeding | 1,561 | 18.9 | 3,375 | 24.0 | < 0.001 | 1,339 | 18.8 | 1,350 | 19.0 | 0.81 |
| Previous stroke | 970 | 11.8 | 2,216 | 15.8 | < 0.001 | 842 | 11.9 | 834 | 11.7 | 0.84 |
| Thrombocytopenia | 316 | 3.8 | 691 | 4.9 | < 0.001 | 252 | 3.6 | 274 | 3.9 | 0.33 |
| CHF | 2,580 | 31.3 | 5,045 | 35.9 | < 0.001 | 2,224 | 31.3 | 2,234 | 31.4 | 0.86 |
| Diabetes | 3,165 | 38.4 | 6,204 | 44.2 | < 0.001 | 2,775 | 39.1 | 2,717 | 38.2 | 0.32 |
| Hypertension | 7,543 | 91.4 | 12,841 | 91.4 | 0.91 | 6,469 | 91.0 | 6,513 | 91.6 | 0.19 |
| Renal disease | 2,580 | 31.3 | 5,135 | 36.6 | < 0.001 | 2,231 | 31.4 | 2,227 | 31.3 | 0.94 |
| MI | 1,362 | 16.5 | 2,597 | 18.5 | < 0.001 | 1,141 | 16.1 | 1,115 | 15.7 | 0.55 |
| Dyspepsia | 1,771 | 21.5 | 3,022 | 21.5 | 0.94 | 1,449 | 20.4 | 1,436 | 20.2 | 0.79 |
| PVD | 4,724 | 57.3 | 8,494 | 60.5 | < 0.001 | 4,076 | 57.4 | 4,010 | 56.4 | 0.26 |
| TIA | 590 | 7.2 | 1,115 | 7.9 | 0.03 | 495 | 7.0 | 504 | 7.1 | 0.77 |
| CAD | 4,075 | 49.4 | 7,167 | 51.0 | 0.02 | 3,485 | 49.0 | 3,435 | 48.3 | 0.40 |
| Baseline medications | n | % | n | % | n | % | n | % | ||
| ACE inhibitor | 3,589 | 43.5 | 6,479 | 46.1 | < 0.001 | 3,130 | 44.0 | 3,136 | 44.1 | 0.92 |
| Amiodarone | 1,115 | 13.5 | 1,638 | 11.7 | < 0.001 | 839 | 11.8 | 819 | 11.5 | 0.60 |
| ARB | 1,999 | 24.2 | 2,801 | 19.9 | < 0.001 | 1,576 | 22.2 | 1,590 | 22.4 | 0.78 |
| Beta blocker | 6,190 | 75.0 | 10,166 | 72.4 | < 0.001 | 5,235 | 73.7 | 5,276 | 74.2 | 0.43 |
| H2-receptor antagonist | 592 | 7.2 | 1,013 | 7.2 | 0.93 | 474 | 6.7 | 515 | 7.3 | 0.18 |
| PPI | 2,697 | 32.7 | 4,372 | 31.1 | 0.01 | 2,198 | 30.9 | 2,254 | 31.7 | 0.31 |
| Statin | 5,165 | 62.6 | 8,722 | 62.1 | 0.43 | 4,387 | 61.7 | 4,378 | 61.6 | 0.88 |
| Antiplatelet | 1,454 | 17.6 | 2,299 | 16.4 | 0.01 | 1,173 | 16.5 | 1,144 | 16.1 | 0.51 |
| Duration of follow-up (months) | ||||||||||
| Mean (SD) | 6.3 (5.2) | 8.3 (6.8) | < 0.001 | 6.7 (5.3) | 6.6 (5.4) | 0.67 | ||||
| Median | 5 | 6 | 5 | 5 | ||||||
| Total all-cause health care cost ($) | ||||||||||
| Mean (SD) | 17,077 (20,794) | 20,236 (27,688) | < 0.001 | 14,317 (14,314) | 13,971 (14,499) | 0.15 | ||||
| Median | 10,128 | 10,982 | 9,367 | 9,075 | ||||||
| Total all-cause medical cost ($) | ||||||||||
| Mean (SD) | 14,098 (19,277) | 17,834 (26,418) | < 0.001 | 11,816 (13,678) | 11,899 (13,885) | 0.72 | ||||
| Median | 7,436 | 8,605 | 6,936 | 6,960 | ||||||
| Bleeding-related medical cost ($) | ||||||||||
| Mean (SD) | 876 (6,073) | 1,798 (8,078) | < 0.001 | 670 (3,447) | 740 (3,695) | 0.24 | ||||
| Median | 0 | 0 | 0 | 0 | ||||||
| Stroke-related medical cost ($) | ||||||||||
| Mean (SD) | 862 (4,796) | 1,466 (6,990) | < 0.001 | 725 (3,651) | 724 (3,605) | 0.99 | ||||
| Median | 0 | 0 | 0 | 0 | ||||||
aP values were calculated for the differences in mean values.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CAD = coronary artery disease; CHF = congestive heart failure; MI = myocardial infarction; PPI = proton pump inhibitor; PVD = peripheral vascular disease; SD = standard deviation; TIA = transient ischemic attack.
Among unmatched patients during the baseline period, the total all-cause mean medical (inpatient + outpatient) and prescription costs were lower for patients treated with apixaban versus warfarin ($17,077 vs. $20,236 PPPY, P < 0.001; Table 1). In addition, mean costs for bleeding-related ($876 vs. $1,798 PPPY, P < 0.001) and stroke-related ($862 vs. $1,466 PPPY, P < 0.001) medical services were lower for patients treated with apixaban versus warfarin during the baseline period (Table 1).
During the follow-up period, the number of hospitalizations (0.8 vs. 1.2, P < 0.001); hospital length of stay (4.6 vs. 7.5 days, P < 0.001); number of outpatient claims (total: 34.4 vs. 54.6, P < 0.001); and number of outpatient prescription claims (54.8 vs. 56.8, P < 0.001) based on PPPY for all causes were lower for unmatched patients treated with apixaban versus warfarin (Table 2). Apixaban treatment was also associated with lower mean inpatient and outpatient costs based on PPPY for all causes as compared with warfarin (Table 2).
TABLE 2.
Unmatched Study Cohorts: HCRU and Associated Costs per Patient per Year During the Follow-up Period
| Apixaban n = 8,250 Mean (SD) Median | Warfarin n = 14,051 Mean (SD) Median | P Valuea | |
|---|---|---|---|
| All causes | |||
| Inpatient | |||
| Number of hospitalizations | 0.8 (2.3) 0 | 1.2 (2.9) 0 | < 0.001 |
| Hospital length of stay (days) | 4.6 (21.5) 0 | 7.5 (27.4) 0 | < 0.001 |
| Total inpatient cost ($) | 9,453 (38,068) 0 | 14,572 (48,344) 0 | < 0.001 |
| Outpatient | |||
| Number of outpatient claims | 34.4 (33.3) 24 | 54.6 (43.1) 43 | < 0.001 |
| Total outpatient cost ($) | 10,538 (18,968) 4,386 | 13,003 (22,083) 5,804 | < 0.001 |
| Number of office visit claims | 17.3 (15.2) 14 | 24.2 (19.7) 21 | < 0.001 |
| Office visit cost ($) | 2,292 (5,457) 1,365 | 2,390 (5,477) 1,411 | 0.20 |
| Number of emergency room claims | 1.9 (4.8) 0 | 2.4 (5.1) 0 | < 0.001 |
| Emergency room cost ($) | 912 (2,990) 0 | 1,177 (4,181) 0 | < 0.001 |
| Outpatient hospital claims | 7.3 (15.4) 3 | 14.2 (26.6) 5 | < 0.001 |
| Outpatient hospital cost ($) | 4,088 (12,705) 345 | 4,294 (12,796) 579 | 0.24 |
| Other outpatient claims | 12.8 (25.0) 4 | 22.7 (33.8) 11 | < 0.001 |
| Other outpatient cost ($) | 3,246 (10,400) 216 | 5,141 (13,993) 637 | < 0.001 |
| Prescription | |||
| Number of outpatient prescription claims | 54.8 (39.1) 48 | 56.8 (41.7) 48 | < 0.001 |
| Total outpatient prescription cost ($) | 5,619 (9,226) 4,327 | 3,198 (7,720) 1,539 | < 0.001 |
| Total medical cost ($; inpatient + outpatient) | 19,991 (47,549) 5,162 | 27,575 (59,315) 7,659 | < 0.001 |
| Total medical and prescription cost ($) | 25,611 (48,874) 10,924 | 30,772 (60,252) 10,804 | < 0.001 |
| Bleeding-related | |||
| Total medical cost ($; inpatient + outpatient) | 2,428 (20,554) 0 | 4,068 (25,632) 0 | < 0.001 |
| Stroke-related | |||
| Total medical cost ($; inpatient + outpatient) | 817 (10,038) 0 | 1,499 (14,472) 0 | < 0.001 |
aP values were calculated for the differences in mean values.
HCRU = health care resource utilization; SD = standard deviation.
Although patients treated with apixaban had higher mean prescription costs ($5,619 vs. $3,198 PPPY, P < 0.001), total mean costs (inpatient + outpatient + prescription) based on PPPY for all causes were lower for patients treated with apixaban versus warfarin ($25,611 vs. $30,772 PPPY, P < 0.001), as were inpatient costs ($9,453 vs. $14,572 PPPY, P < 0.001) and total outpatient costs ($10,538 vs. $13,003 PPPY, P < 0.001; Table 2). Also, costs for bleeding-related ($2,428 vs. $4,068 PPPY, P < 0.001) and stroke-related ($817 vs. $1,499 PPPY, P < 0.001) medical services were lower for patients treated with apixaban versus warfarin during the follow-up period (Table 2).
PSM Study Cohorts
Table 1 presents the baseline demographics, clinical characteristics, and health care costs of study cohorts after matching. By implementing PSM, 14,214 patients were matched, with 7,107 patients in each cohort. After PSM, mean ages (78.2 years vs. 78.1 years, P = 0.54); CCI scores (3.0 vs. 3.0, P = 0.97); and stroke and bleeding risks, based on CHADS2 score (2.7 vs. 2.7, P = 0.37), CHA2DS2-VASc score (4.6 vs. 4.6, P = 0.66), and HASBLED score (3.0 vs. 3.1, P = 0.22), were similar (no statistically significant differences) between matched cohorts.
The proportions of patients with bleeding (18.8% vs. 19.0%, P = 0.81) and stroke (11.9% vs. 11.7%, P = 0.84) diagnoses during the baseline period were also similar for NVAF patients treated with apixaban versus warfarin. Additionally, total all-cause medical costs ($11,816 vs. $11,899 PPPY, P = 0.72); total all-cause medical costs plus outpatient prescription costs ($14,317 vs. $13,971 PPPY, P = 0.15); bleeding-related medical services costs ($670 vs. $740 PPPY, P = 0.24); and stroke-related medical services costs ($725 vs. $724 PPPY, P = 0.99) during the baseline period were similar for matched NVAF patients treated with apixaban versus warfarin.
The mean durations of follow-up for matched study cohorts were similar (mean: 6.7 vs. 6.6 months, P = 0.67; median: 5 vs. 5 months). During the follow-up period, the number of hospitalizations (0.7 vs. 1.1, P < 0.001; difference: -0.4); hospital length of stay (4.0 vs. 7.1 days, P < 0.001; difference: -3.1 days); number of outpatient claims (32.7 vs. 51.0, P < 0.001; difference: -18.3); and number of outpatient prescription claims (53.0 vs. 54.7, P = 0.006; difference: -1.8) based on PPPY for all causes were lower for matched patients treated with apixaban versus warfarin (Table 3).
TABLE 3.
Propensity Score Matched Study Cohorts: HCRU and Associated Costs per Patient per Year During the Follow-up Period
| Apixaban n = 7,107 Mean (SD) Median | Warfarin n = 7,107 Mean (SD) Median | P Valuea | |
|---|---|---|---|
| All causes | |||
| Inpatient | |||
| Number of hospitalizations | 0.7 (2.1) 0 | 1.1 (2.9) 0 | < 0.001 |
| Hospital length of stay (days) | 4.0 (19.8) 0 | 7.1 (25.6) 0 | < 0.001 |
| Total inpatient cost ($) | 8,327 (34,325) 0 | 14,296 (49,994) 0 | < 0.001 |
| Outpatient | |||
| Number of outpatient claims | 32.7 (31.0) 24 | 51.0 (37.7) 42 | < 0.001 |
| Total outpatient cost ($) | 9,655 (16,922) 4,158 | 11,469 (19,582) 5,295 | < 0.001 |
| Number of office visit claims | 16.9 (14.4) 14 | 24.9 (20.4) 21 | < 0.001 |
| Office visit cost ($) | 2,150 (4,583) 1,333 | 2,358 (5,168) 1,433 | 0.01 |
| Number of emergency room claims | 1.8 (4.3) 0 | 2.4 (5.3) 0 | < 0.001 |
| Emergency room cost ($) | 864 (2,715) 0 | 1,155 (3,817) 0 | < 0.001 |
| Outpatient hospital claims | 6.8 (14.0) 3 | 11.4 (19.3) 4 | < 0.001 |
| Outpatient hospital cost ($) | 3,782 (11,751) 354 | 3,515 (11,264) 384 | 0.17 |
| Other outpatient claims | 11.7 (22.8) 4 | 20.4 (30.8) 9 | < 0.001 |
| Other outpatient cost ($) | 2,858 (9,159) 204 | 4,441 (12,723) 458 | < 0.001 |
| Prescription | |||
| Number of outpatient prescription claims | 53.0 (36.4) 46 | 54.7 (39.2) 48 | 0.006 |
| Total outpatient prescription cost ($) | 5,159 (5,909) 4,296 | 2,867 (4,857) 1,545 | < 0.001 |
| Total medical cost ($; inpatient + outpatient) | 17,981 (43,039) 4,878 | 25,766 (59,220) 6,793 | < 0.001 |
| Total medical and prescription cost | 23,141 (43,595) 10,452 | 28,633 (59,650) 9,967 | < 0.001 |
| Bleeding-related | |||
| Total medical cost ($; inpatient + outpatient) | 2,101 (17,867) 0 | 3,963 (27,424) 0 | < 0.001 |
| Stroke-related | |||
| Total medical cost ($; inpatient + outpatient) | 652 (7,192) 0 | 1,178 (11,695) 0 | 0.001 |
aP values were calculated for the differences in mean values.
HCRU = health care resource utilization; SD = standard deviation.
Apixaban treatment was also associated with lower mean inpatient and outpatient costs versus warfarin (Table 3 and Figure 2). While patients treated with apixaban had higher mean prescription costs ($5,159 vs. $2,867 PPPY, P < 0.001), total mean costs (inpatient + outpatient + prescription) based on PPPY for all causes were $5,493 lower for patients treated with apixaban versus warfarin ($23,141 vs. $28,633 PPPY, P < 0.001; Table 3 and Figure 2). Inpatient costs ($8,327 vs. $14,296 PPPY, P < 0.001) and total outpatient costs ($9,655 vs. $11,469 PPPY, P < 0.001) were also lower (Table 3). Additionally, costs for bleeding-related ($2,101 vs. $3,963 PPPY, P < 0.001; difference: -$1,862) and stroke-related ($652 vs. $1,178 PPPY, P = 0.001; difference: -$525) medical services were lower for patients treated with apixaban versus warfarin during the follow-up period (Table 3).
FIGURE 2.
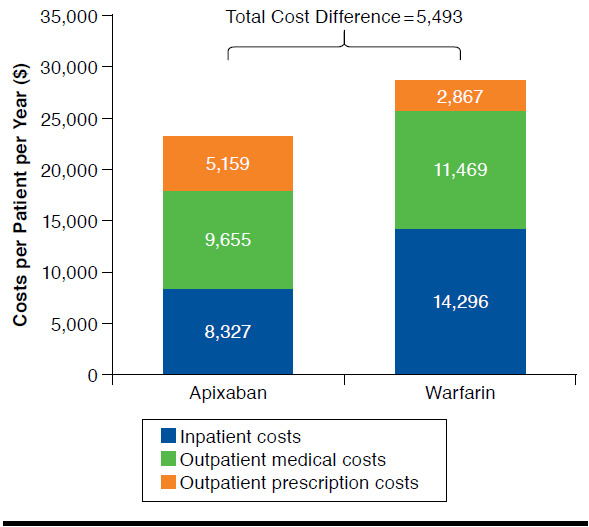
Propensity Score Matched Study Cohorts: Health Care Costs per Patient per Year During the Follow-up Period Among Nonvalvular Atrial Fibrillation Patients Treated with Apixaban Versus Warfarin
Discussion
The current study analyzed claims data from the Humana research database to evaluate the effect of treatment with apixaban versus warfarin on HCRU and costs among elderly NVAF patients. The results of the study show that elderly NVAF patients who initiated apixaban significantly differed in many patient characteristics compared with those who received warfarin, with age, bleeding and stroke risks, prevalence of comorbidities, and previous HCRU and costs being lower for those who received apixaban. These differences may in part be attributed to the fact that apixaban is a new drug and clinicians might be more inclined to “play safe” and prescribe the new drug to healthier patients first until they gain more experience with it. After controlling for key significant differences in patient characteristics with PSM, including age, bleeding and stroke risks, and comorbidities, our study demonstrated that apixaban treatment was associated with greater pharmacy costs than treatment with warfarin but less HCRU, as well as lower total all-cause health care costs and bleeding- and stroke-related medical service costs, in comparison with warfarin treatment. The difference in mean total health care costs based on PPPY for all causes between apixaban and warfarin treatment reached $5,493 (Figure 2).
For the statistical analysis after the post hoc PSM, as in this current analysis, most of the analyses in the published literature used unpaired statistical methods.22 While some researchers advocate that paired statistical methods can be used in the PSM cohort comparison to use the higher statistical sensitivity of paired method, simulation analysis has shown that the paired methods in comparison with nonpaired methods tend to have higher type I (false positive) error.23 Additionally, other researchers recommend that the paired methods should not be used in the cohorts generated by post hoc matching methods such as PSM, as there is little theoretical foundation to support that the PSM cohorts are truly paired cohorts and that “matching” erroneously suggests that the resulting data should be analyzed as if they were matched pairs.24 Since there are many confounding factors in the data analysis of retrospective real-world data analyses already, we chose to use the more conservative methods of unpaired statistical analyses to avoid the higher false positive rate associated with the paired methods.
To our knowledge, the current study is one of the first comprehensive analyses to compare all types (inpatient, outpatient, and prescriptions) of HCRU and associated costs between apixaban and warfarin treatment among elderly NVAF patients. Recently, Xie et al. (2016) evaluated hospitalized NVAF patients treated with apixaban or warfarin using a large U.S. claims database and observed that apixaban treatment was associated with shorter length of stay and lower hospital costs versus warfarin.25 Similarly, an earlier study by Farr et al. (2015) found that patients hospitalized for NVAF and treated with apixaban had a shorter length of stay than patients treated with warfarin.14 Our findings are generally consistent with these other published studies. Additionally, our study provides data on other types of HCRU, such as outpatient and prescriptions, and evaluated the costs of NVAF patients treated with apixaban and warfarin for a longer period after initiating treatment than the earlier studies did.
Despite having higher prescription costs, apixaban treatment was associated with lower total health care costs versus warfarin. Most of the cost savings associated with apixaban versus warfarin treatment was attributed to less hospital resource use (inpatient cost difference = $5,970). The less resource use for hospital as well as outpatient services among patients treated with apixaban may be due to fewer bleeding and stroke events associated with apixaban treatment versus warfarin, as both bleeding-related and stroke-related medical costs were lower for patients treated with apixaban versus warfarin. Since bleeding and stroke-related medical costs only captured inpatient and outpatient services that could be identified by relevant bleeding and stroke diagnosis codes, some of the economic benefits associated with apixaban versus warfarin treatment, such as the lack of need for warfarin monitoring, were not included in these costs.
The health care and economic burdens of AF-related stroke, especially among the elderly, are projected to increase.1-4 Furthermore, the risk for bleeding also increases with age.15,26 Hylek et al. (2007) additionally reported that among AF patients aged ≥ 80 years, 26% stopped taking warfarin within the first year.26 Concerns related to safety were responsible for most of the discontinuations.26 New pharmacotherapies provide alternative options for anticoagulation therapy and stroke prevention for NVAF patients. Apixaban demonstrated superior efficacy to warfarin and a significant reduction in risk for major bleeding in both a clinical trial and the real-world setting.8,9 Our study additionally shows that the superior efficacy and safety of apixaban versus warfarin use among elderly NVAF patients translate to a reduced health care and economic burden. Future additional studies with larger sample sizes and longer follow-up may be needed to further confirm the results of this early evaluation of the effect of apixaban versus warfarin on HCRU and costs. Also, although we comprehensively evaluated all HCRU and associated costs, including inpatient, outpatient, and pharmacy use of elderly patients treated with apixaban versus warfarin, we believe that further assessment of the costs related to caregiver burden among elderly NVAF patients is warranted.
Limitations
Retrospective, observational analyses using claims databases have certain inherent limitations, as the claims are collected for the purpose of payment and not research. First, presence of a claim for a filled prescription does not indicate that the medication was consumed or that it was taken as prescribed. Second, medications filled over the counter or provided as samples by the physician are not captured in the claims data. Third, presence of a diagnosis code on a medical claim is not positive presence of disease, as the diagnosis code may be incorrectly coded or included as a rule-out criterion rather than indicate actual disease. Fourth, although we used PSM to control for multiple confounders, there is potential for residual bias and no causal relationship can be inferred from this study.
Fifth, since apixaban was a newly approved drug and warfarin has been used for many years, NVAF patients treated with apixaban had shorter follow-up durations than did warfarin-treated patients. While we aimed to adjust for the follow-up duration by calculating PPPY data, this may not fully adjust for the effect of the follow-up durations. For instance, in the occurence of a bleeding event, HCRU and cost data may not be linearly (uniformally) distributed over time, as it is possible that more events and costs may occur during the early months of the follow-up periods. In our study, the follow-up durations were similar between the patient cohorts after the PSM to minimize such potential effects.
Finally, the Humana research database comprises claims of persons primarily residing in the southern and midwestern regions of the United States, and therefore the results of this study may not be representative of the entire U.S. population.
Conclusions
In comparison with NVAF patients who received warfarin, patients who initiated apixaban significantly differed in many patient characteristics. After controlling for these differences, we found that although treatment with apixaban was associated with higher pharmacy costs than treatment with warfarin, NVAF patients treated with apixaban had less HCRU, as well as lower total all-cause health care costs and bleeding- and stroke-related medical service costs, in comparison with patients treated with warfarin.
APPENDIX A. Claim Codes for Excluded Conditions
| Condition | ICD-9-CM Code/Procedure Code/HCPCS Code |
|---|---|
| Valvular heart disease | 394.0, 394.1, 394.2, 394.9, 396.0, 396.1, 396.8, 396.9, 424.0, 745.xx, V42.2, V43.3 |
| Venous thromboembolism | DVT: 451-453, 671.3, 671.4, 671.9 PE: 415.1, 673.2, 673.8 |
| Transient atrial fibrillation or cardiac surgery | V422, V433, 35.05-35.09, 35.20-35.28 and 35.97; Pericarditis: 006.8, 017.9, 036.41, 074.21, 093.81, 098.83, 115.93, 390, 391, 392.0, 393, 411.0, 420.90, 420.91, 420.99, 423.0, 423.1, 423.2, 423.8, 423.9 |
| Hyperthyroidism or thyroid toxicity | 242.0, 242.1, 242.2, 242.3, 242.4, 242.8, 242.9 |
| Pregnancy | 630-679, V22, V23, V24, V27, V28, V61.6, V61.7, 792.3, 796.5, ICD-9-CM procedure code 72-75.99, or HCPCS codes 59000-59350, 76801-76828, 83661-83664 |
DVT = deep vein thrombosis; HCPCS = Healthcare Common Procedure Coding System; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; PE = pulmonary embolism.
APPENDIX B. Stroke and Bleeding Claim Codes
| Type of Stroke | ICD-9-CM Code |
|---|---|
| Hemorrhagic stroke | 430.xx-432.xx Cases excluded if traumatic brain injury (ICD-9: 800-804, 850-854) was present during hospitalization |
| Ischemic stroke | 433.x1, 434.x1, 436.xx |
| Systemic embolism | 444.x, 445.x |
| Type of Bleeding | ICD-9-CM Code |
| Gastrointestinal bleeding | 456.0x, 456.20, 530.82, 531.0x, 531.2x, 531.4x, 531.6x, 532.0x, 532.2x, 532.4x, 532.6x, 533.0x, 533.2x, 533.4x, 533.6x, 534.0x, 534.2x, 534.4x, 534.6x, 535.01, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 537.83, 562.02, 562.03, 562.12, 562.13, 568.81, 569.3, 569.85, 578.x |
| Intracranial bleeding | 430.xx, 431.xx, 432.0x, 432.1x, 432.9x, 852.0x, 852.2x, 852.4x, 853.0x |
| Other bleeding | 285.1, 360.43, 362.43, 362.81, 363.61, 363.62, 363.72, 364.41, 372.72, 374.81, 376.32, 377.42, 379.23, 423.0x, 596.7x, 599.7x, 602.1x, 620.1, 621.4, 626.2, 626.5, 626.7, 626.8, 626.9, 719.1x, 782.7, 784.7, 784.8, 786.3x, 958.2, 997.02, 998.11 Procedure codes: 99.04, 44.43 |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-75. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119-25. [DOI] [PubMed] [Google Scholar]
- 3.Naccarelli GV, Varker H, Lin J, Schulman KL.. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104(11):1534-39. [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL.. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313-20. [DOI] [PubMed] [Google Scholar]
- 5.American College of Cardiology Foundation, American Heart Association, European Society of Cardiology, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2013;127(18):1916-26. [DOI] [PubMed] [Google Scholar]
- 6.Mercaldi CJ, Ciarametaro M, Hahn B, et al. Cost efficiency of anticoagulation with warfarin to prevent stroke in Medicare beneficiaries with nonvalvular atrial fibrillation. Stroke. 2011;42(1):112-18. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE.. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131(12):927-34. [DOI] [PubMed] [Google Scholar]
- 8.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. New Engl J Med. 2011;365(11):981-92. [DOI] [PubMed] [Google Scholar]
- 9.Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5(6):e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deitelzweig S, Amin A, Jing Y, et al. Medical cost reductions associated with the usage of novel oral anticoagulants vs warfarin among atrial fibrillation patients, based on the RE-LY, ROCKET-AF, and ARISTOTLE trials. J Med Econ. 2012;15(4):776-85. [DOI] [PubMed] [Google Scholar]
- 11.Deitelzweig S, Amin A, Jing Y, et al. Medical costs in the U.S. of clinical events associated with oral anticoagulant (OAC) use compared to warfarin among non-valvular atrial fibrillation patients ≥ 75 and < 75 years of age, based on the ARISTOTLE, RE-LY, and ROCKET-AF trials. J Med Econ. 2013;16(9):1163-68. [DOI] [PubMed] [Google Scholar]
- 12.Deitelzweig S, Amin A, Jing Y, et al. Medical costs of oral anticoagulants vs warfarin for atrial fibrillation patients with different stroke risks. Cardiol Ther. 2013;2(2):165-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin A, Bruno A, Trocio J, Lin J, Lingohr-Smith M.. Comparison of differences in medical costs when new oral anticoagulants are used for the treatment of patients with non-valvular atrial fibrillation and venous thromboembolism vs warfarin or placebo in the U.S. J Med Econ. 2015;18(6):399-409. [DOI] [PubMed] [Google Scholar]
- 14.Farr AM, Jing Y, Johnston S, et al. Comparison of hospital length of stay between hospitalized non-valvular atrial fibrillation patients treated with either apixaban or warfarin. Hosp Pract (1995). 2015;43(3):172-79. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L.. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke. 2015;46(1):143-50. [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-39. [DOI] [PubMed] [Google Scholar]
- 17.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ.. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137(2):263-72. [DOI] [PubMed] [Google Scholar]
- 18.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY.. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-100. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Labor. Bureau of Labor Statistics. Consumer Price Index–December 2015. News Release. USDL-16-0109. Available at: https://www.bls.gov/news.release/archives/cpi_01202016.pdf. Accessed August 3, 2017.
- 20.Lin J, Chow W, Kim MS, Rupnow MF.. Real-world treatment pattern and outcomes among patients who took tapentadol IR or oxycodone IR. J Med Econ. 2013;16(5):685-90. [DOI] [PubMed] [Google Scholar]
- 21.Obeid T, Alshaikh H, Nejim B, Arhuidese I, Locham S, Malas M.. Fixed and variable cost of carotid endarterectomy and stenting in the United States: a comparative study. J Vasc Surg. 2017;65(5):1398-1406.e1. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg. 2007;134(5):1128-35. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med. 2011;30(11):1292-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer JL, Kang J.. Average causal effects from nonrandomized studies: a practical guide and simulated example. Psychol Methods. 2008;13(4):279-313. [DOI] [PubMed] [Google Scholar]
- 25.Xie L, Vo L, Keshishian A, et al. Comparison of hospital length of stay and hospitalization costs among patients with non-valvular atrial fibrillation treated with apixaban or warfarin: an early view. J Med Econ. 2016;19(8):769-76. [DOI] [PubMed] [Google Scholar]
- 26.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S.. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115(21):2689-96. [DOI] [PubMed] [Google Scholar]



