Abstract
BACKGROUND:
Recent clinical trials indicate that pharmacogenetic-guided treatment of major depressive disorder (MDD) results in higher treatment response rates by genetically matching patients to medications and avoiding a trial-and-error process.
OBJECTIVE:
To evaluate the cost-effectiveness of a pharmacogenetic test (IDGx) that has demonstrated effectiveness compared with standard of care (SOC) medication management among patients with varied MDD severity.
METHODS:
Data from a large prospective, randomized controlled trial of treatment-naive patients or patients with inadequately controlled MDD in general practice and psychiatric treatment settings were used to build a Markov state-transition probability model. Analyses were conducted from the societal perspective. Treatment response rates, mortality rates, direct and indirect medical costs, and utility inputs were derived from the reference study and published scientific literature. The cost of the pharmacogenetic test was $2,000. A 3% discount rate was used to discount costs and effects. Univariate one-way sensitivity analyses were performed to determine the effect of input parameters on net monetary benefit.
RESULTS:
For moderate to severe MDD, the model estimated a cumulative effect over 3 years of 2.07 quality-adjusted life-years (QALYs) for the pharmacogenetic-guided treatment group and 1.97 QALYs for the SOC group, including a lower probability of death from suicide (0.328% and 0.351%, respectively). Total costs over 3 years were $44,697 (IDGx) and $47,295 (SOC). This difference includes a savings of $2,918 in direct medical costs and $1,680 in indirect costs. Results were more pronounced when only severely depressed patients were evaluated.
CONCLUSIONS:
Pharmacogenetic testing among moderate to severe MDD patients improved QALYs and resulted in cost savings. Sensitivity analyses supported the robust nature of the current findings of the dominant IDGx test to guide treatment.
What is already known about this subject
The results of recent clinical trials suggest that pharmacogenetic-guided treatment of major depressive disorder (MDD) can improve patient outcomes compared with the standard of care, which relies on trial and error.
Prospective studies on the cost-effectiveness of pharmacogenetic-guided treatment for MDD are limited to treatment-resistant patients.
What this study adds
This study evaluates the cost-effectiveness of pharmacogenetic-guided treatment for newly diagnosed MDD or patients with inadequately controlled MDD using data from a large, randomized controlled trial.
Pharmacogenetic-guided therapeutic management of MDD appears to be a dominant treatment strategy over the standard of care, producing gains in quality-adjusted life-years and cost savings.
Major depressive disorder (MDD), one of the most prominent mental health disorders,1 can be difficult to treat, prolonging the effects of the illness and often resulting in disabling symptoms, suicide, functional impairment, lost productivity, and increased health care costs. Depressive disorders have remained a leading cause of disease burden for decades,2 with global prevalence rates estimated to be nearly 5%.3 In the United States, the 12-month prevalence of at least 1 major depressive episode is 6.7%, representing more than 16 million U.S. adults.1,4 Symptoms and functional impairment result in reduced quality of life (QOL) and lost quality-adjusted life-years (QALYs). Untreated or unsuccessful treatment of MDD can lead to attempted and completed suicide, 1 of the top 10 leading causes of death in the United States.5 In addition to diminished QOL and increased risk of suicide, there is significant economic burden associated with MDD, including increased medical costs, workplace costs, and greater expenditures for non-MDD comorbidities.6,7
Pharmacotherapy is generally recommended as a frontline treatment for MDD.8 However, predictors of treatment success have low sensitivity and specificity.9 Nearly half of patients new to antidepressants fail to remit or experience intolerable side effects, requiring an alternative medication using a trial-and-error approach.8,10 Unsuccessful pharmacological treatment increases the risk of suicide and the associated costs of MDD. In a 2012 study, Olin et al. found that the suicide rate for patients with treatment-resistant depression was 0.16% for those who received treatment as usual but 0.09% for responders.11 Additionally, the financial burden for treatment-resistant depression was found to be nearly double that of treatment-responsive depression.12
In recent years, research on individualized medicine has been able to link patient pharmacogenetic profiles to therapeutic response in terms of diagnostic criteria, as well as improved outcomes (QOL, productivity, and costs) using existing data-sets.13-16 Ongoing work continues to provide support for these findings.17,18 Pharmacogenetic-guided treatment of MDD was studied in prospective clinical trials. These tests enable prescribers to assess how a panel of medications will interact with an individual’s unique genetic variants and make recommendations for treatment based on this information. Findings suggest that pharmacogenetic-guided treatment is a promising alternative to current prescribing practices.19-22
In terms of the effect of testing on the costs of MDD care, Perlis et al. (2009) constructed a cost-effectiveness model using data from the multicenter STAR*D trial.23 Their model was conservative and concluded that pharmacogenetic-guided treatment would be cost-effective (< $50,000 per QALY) under some circumstances. In addition, a prospective study tracked pharmacy costs for patients who either received or did not receive combinatorial pharmacogenetic testing (CPGx), and found a cost savings of more than $1,000 per patient after 1 year.24 Finally, using prospective data from the 3 trials of CPGx,19-22 Hornberger et al. (2015) conducted a cost-effectiveness analysis for directing the treatment of treatment-resistant depression.25 The study concluded that CPGx produced both improved health outcomes and cost savings.
In the past year, the largest clinical trial of pharmacogenetic testing to date was conducted by Bradley et al. (2018).26 The study found improved outcomes for a generalizable sample of adults with MDD across multiple clinical settings using the IDgenetix (IDGx) pharmacogenetic test. The purpose of this study was to evaluate the cost-effectiveness of the IDGx test for a wider range of MDD severity among treatment-naive patients and patients with inadequately controlled MDD. Outcomes evaluated were total costs (direct and indirect), QALYs, and suicide rates over a 3-year time horizon.
Methods
All research methods and analyses follow the recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.27-29 Only de-identified data were used in the analyses, which followed protocol approved by an institutional review board.
Population and Perspective
This analysis targeted treatment-naive patients with MDD or patients with inadequately controlled MDD and a score of 20 or greater on the 17-item Hamilton Rating Scale for Depression (HAM-D17). Patients scoring in this range are described as having moderate or severe depression. The response rates and assumed age (48 years) were drawn from a large, multicenter clinical study conducted in a real-world setting (outpatient clinics).26 Analyses were calculated and presented from the societal perspective.
Analytic Framework
Input parameters for the analytic framework are presented in Table 1. The Markov state-transition probability analysis model was developed using TreeAge Pro software (TreeAge Pro Healthcare, Williamstown, MA). The model consisted of 3 health states for both the IDGx and standard of care (SOC) treatment groups (Figure 1). QALYs and mortality probabilities (both suicide and all other causes) for each state were entered into the model. Patients in each group could either respond to treatment or not respond to treatment, and each of those groups could then either survive or die of suicide or other-cause mortality. The probability of spontaneous transition between response and nonresponse was assumed to be equivalent in both arms and was thus left at zero. However, to simulate the typical SOC process, in which most nonresponders try different medications until finding one that is effective, a proportion of initial nonresponders transitioned to the responder state in subsequent iterations of the analysis.
TABLE 1.
Input Parameters
| Parameters | Base-Case Values | References |
|---|---|---|
| Test inputs | ||
| Response rate—SOC | 46% (SD 36%) | Bradley (2018)26 |
| Relative benefit ratio for response—IDGx | 1.39 (SD 2.02) | N/A |
| Clinical inputs | ||
| Age of patient at baseline | 48 | Bradley (2018)26 |
| Mortality rates | ||
| Suicide rate (responders) | 0.09% | Olin (2012)11 |
| Suicide rate (nonresponders) | 0.16% | Olin (2012)11 |
| Relative risk of all-cause mortality (responders) | 1.0 | Arias (2017)35 |
| Relative risk of all-cause mortality (nonresponders) | 1.0 | Arias (2017)35 |
| Cost inputs, $ | ||
| Test | 2,000 | AltheaDx |
| Direct medical costs, annual (responders) | 8,675 | Mrazek (2014)12 |
| Direct medical costs, annual (nonresponders) | 14,837 | Mrazek (2014)12 |
| Indirect medical costs, annual (responders) | 3,234 | Mrazek (2014)12 |
| Indirect medical costs, annual (nonresponders) | 7,785 | Mrazek (2014)12 |
| Utility inputs | ||
| Responders | 0.81 | Sobocki (2006)53 |
| Nonresponders | 0.57 | Sobocki (2006)53 |
| Policy inputs | ||
| Time horizon (years) | 3 | N/A |
| Discount rate | 3% | Neumann (2016)27 |
| Catch-up year | 3 | Geddes (2003)30 |
IDGx = IDgenetix; N/A = not applicable; SD = standard deviation; SOC = standard of care.
FIGURE 1.
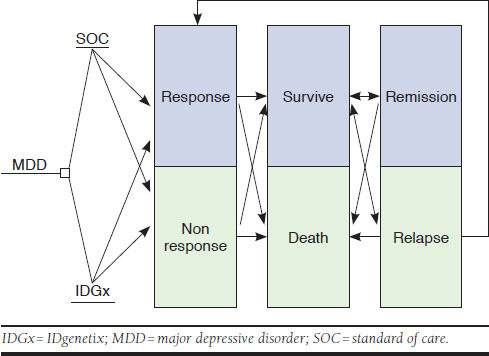
Markov Model
The primary advantage of pharmacogenetic testing to direct depression pharmacotherapy is the ability to find an effective treatment faster, thus avoiding side effects and potential suicides, increasing QOL, and reducing medical costs. Therefore, it is important that the analysis models the future course of responders and nonresponders, and for the latter group, their trajectory toward eventual response regardless of their group assignment (i.e., IDGx or SOC). In order to accomplish this, the model assumes that the SOC group would catch up to the response rates of genetic testing after 3 years based on data from STAR*D trials. This projection follows the same method used by Hornberger et al. and is based on a systematic review by Geddes et al. (2003), who reported that continued antidepressant therapy consistently reduced the risk of relapse, and that after 36 months treatment, effects were found to be persistent.25,30 Once the SOC group caught up, there were minimal differences in future years consisting mainly of small changes in accrual of costs and QALYs from the slightly higher suicide rates in the first 3 years. Following this assumption, and in accordance with Good Research Practices recommendations,31 a 3-year time horizon was chosen because minimal differences exist between the groups after 3 years, and shorter time horizons (2-3 years) are commonly used in cost-effectiveness analyses for depression medications.32,33 The shorter time frame may provide a more accurate and tangible expression of the relative costs and benefits provided. Finally, a standard discount rate of 3% per year was used to discount costs and effects. This rate is the most commonly used discount rate in medical cost-effectiveness analysis.27 Following recommendations, a half-cycle correction was applied in the model.31
Response Rates
Response rates were taken from Bradley et al.26 The prospective, randomized, double-blind study included 20 independent clinical sites in psychiatry, internal medicine, obstetrics and gynecology, and family medicine. Patients in the depression arm with moderate to severe depression (n = 261)—diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) or other SOC procedures—were randomly assigned to the experimental group or a control group. Patients in the study were either new to treatment or had inadequately controlled MDD. The severity of MDD was graded using the HAM-D17 interview.
All patients provided buccal cell swabs for the IDGx pharmacogenetic test to detect and interpret the effect of an individual patient’s genetic variants associated with pharmacokinetic and pharmacodynamic response, as well as metabolic interactions related to concurrent medications, over-the-counter drugs, herbal supplements, and diet. According to Bradley et al., the IDGx test “uses a genetic variant panel of 10 genes, along with concomitant medications, to make medication management recommendations based on gene-drug and drug-drug interactions for over 40 medications used in the treatment of depression and anxiety.” Turnaround time for test results was 48 to 72 hours after receipt of the sample by AltheaDx. Patients in the experimental group had their results released to participating clinics to help guide medication selection. For the control group, results of the IDGx test were not made available, and clinicians were asked to manage the case using the existing standard of care.
Patients were monitored and assessed for depression symptoms using HAM-D17 interviews by trained, blinded raters at study initiation (baseline) and at the 4-, 8-, and 12-week follow-up visits. The efficacy analysis was conducted for patients with a disease severity of moderate or severe. The endpoints included HAM-D17 changes from baseline at the 4-, 8-, and 12-week follow-up visits measurement points, with response to treatment defined as ≥50% reduction in HAM-D17 scores. Patients with HAM-D17 scores of 20 or greater at baseline were included in the efficacy analysis (moderate to severe depression). For patients with HAM-D17 scores of 20 or greater, response rates after 12 weeks were 64% for the experimental group and 46% for the control group. For patients with severe depression, defined as HAM-D17 scores greater than 24, response rates after 12 weeks were 73% for the experimental group and 36% for the control group. No differences in adverse events were observed between groups.
Mortality Rates
Mortality rates for individuals with MDD were divided into rates for those who experienced death from suicide and for all other-cause mortality. Estimates of suicide rates for individuals with MDD were drawn from a study by Olin et al. with treatment-resistant MDD patients.11 The experimental group received vagus nerve stimulation while a control group received usual care over a 5-year period. As these were treatment-resistant patients, the suicide rate over 5 years of 0.16% was used as the rate for nonresponders. As vagus nerve stimulation in addition to usual care has been shown to be an effective treatment for MDD patients,34 the experimental group’s suicide rate of 0.09% was used for responders. These rates were used previously in a similar published analysis.25 Age-adjusted nonsuicide mortality rates were derived from the National Center for Health Statistics database.35 With a lack of generalizable data on differences in nonsuicide mortality in people with MDD, we assumed an equal nonsuicide mortality rate in both treatment groups.
Utility of Major Depressive Disorder
Utility scores for patients with MDD were taken from a previous study in which patients at 56 primary care centers were tracked over 6 months while being treated for depression.32 Patients completed the EuroQol 5D (EQ-5D) instrument during general practitioner outpatient visits. Health-state utilities for treatment responders and nonresponders were 0.81 and 0.57, respectively. These rates were used in a similar previously published analysis examining the cost-effectiveness of new treatments for depression.
Costs
Costs included in the analyses consisted of the cost of the IDGx testing, direct medical costs, and indirect costs related to MDD, such as lost productivity.36 The $2,000 cost of the test was provided by the test developer and owner, AltheaDx. The direct medical costs and indirect costs of having MDD were derived from Mrazek et al. (2014)12 and adjusted to 2016 U.S. dollars, based on the Bureau of Labor Statistics medical care component of the Consumer Price Index (CPI),12,37 and following methods used by Hornberger et al.25 The total costs of MDD over the 3-year time window were $22,622 for nonresponders and $11,909 for responders. Direct medical costs were composed of medications for depression and all other conditions, hospitalizations, physician visits, and psychotherapy.12
Sensitivity Analyses
Univariate, one-way sensitivity analyses were performed using TreeAge software. When available, parameter ranges were based on 95% confidence intervals (CI) from the scientific literature; 95% CIs were available for response rates, suicide rates, and utility values for health states. In the absence of published 95% CIs, ranges were based on values equal to ± 25% of the estimated value, as used in similar high-quality cost-effectiveness analyses.25,38 The discount rate varied between 2.5% and 3.5% based on commonly used values in scientific literature and recommendations.27 Sensitivity analyses expressed the model’s output in terms of net monetary benefit (NMB), which integrates QALYs, and through the willingness-to-pay function, which is expressed in terms of cost per QALY.27,39 A willingness-to-pay threshold of $50,000 was used, as it is the most commonly used threshold in cost-effectiveness analysis.39,40 This threshold is widely cited and is conservative because it has not been adjusted for inflation since being proposed more than 30 years ago.40,41
Results
Results of the Markov modeling over the 3-year time horizon for patients with moderate to severe MDD are shown in Table 2. After 3 years, the Markov model estimated a cumulative effect of 2.07 QALYs for the IDGx group compared with 1.97 QALYs for SOC, a difference of 0.10 QALYs. Part of this difference in QALYs includes a lower probability of death from suicide for the IDGx group compared with MDD patients receiving SOC (0.328% vs. 0.351%). For a suicide death to be prevented, approximately 4,300 patients would need to be tested. With 6.7% of the U.S. population experiencing MDD in a given year, full implementation of the test could potentially prevent 5,000 deaths (per year for U.S. population), a 12% reduction based on 42,826 deaths by suicide per year.42
TABLE 2.
Moderate to Severe MDD Compared with Severe-Only MDD over a 3-Year Time Horizon
| Moderately to Severely Depressed | Severely Depressed | |||||
|---|---|---|---|---|---|---|
| SOC | IDGx | Difference | SOC | IDGx | Difference | |
| Outcome | ||||||
| Probability of death from suicide, % | 0.351 | 0.328 | –0.023 | 0.356 | 0.311 | –0.045 |
| QALYs | 1.97 | 2.07 | 0.10 | 1.98 | 2.15 | 0.17 |
| Costs, $ | ||||||
| Test | 0 | 2,000 | 2,000 | 0 | 2,000 | 2,000 |
| Direct medical costs | 32,908 | 29,990 | –2,918 | 33,345 | 27,258 | –6,087 |
| Indirect medical costs | 14,387 | 12,707 | –1,680 | 13,680 | 11,957 | –1,723 |
| Total costs (including test) | 47,295 | 44,697 | –2,598 | 47,025 | 41,215 | –5,810 |
IDGx = IDgenetix; MDD = major depressive disorder; QALYs = quality-adjusted life-years; SOC = standard of care.
The model found that the total cost over 3 years was less for moderate to severe MDD patients whose clinicians had been guided by the IDGx test compared with patients in the SOC group ($44,697 vs. $47,295 respectively) despite the $2,000 initial cost of testing. As shown in Table 2, after 3 years, the IDGx group projected $2,918 less in direct medical costs, $1,680 less in indirect medical costs, and $2,598 less in total costs. Thus, with the IDGx-guided treatment producing both QALYs gained and cost savings, the treatment “dominates” the SOC treatment.
When modeling response rates for those with severe depression only (HAM-D17 > 24), the results were even more pronounced. Total costs at 3 years were $41,215 for the IDGx group compared with $47,025 for those receiving SOC. This represents a total difference of $5,810 including the cost of the test ($2,000), easily offsetting this cost after 3 years. QALYs for the IDGx group were 2.15 compared with 1.98 for SOC, resulting in a 3-year difference of 0.17 QALYs.
With dominant treatments, the calculation of an incremental cost-effectiveness ratio is not meaningful. Thus, a series of one-way sensitivity analyses were conducted to determine the effect of the input parameters on the NMB of IDGx-guided treatment for moderate to severe MDD patients. As shown in Figure 2, the response rate with IDGx had the highest effect on NMB, followed by the cost associated with treatment response and cost of nonresponse. However, none of the parameter values tested produced an NMB less than $50,000, reflecting the robust nature of the current findings of the dominant IDGx test.
FIGURE 2.
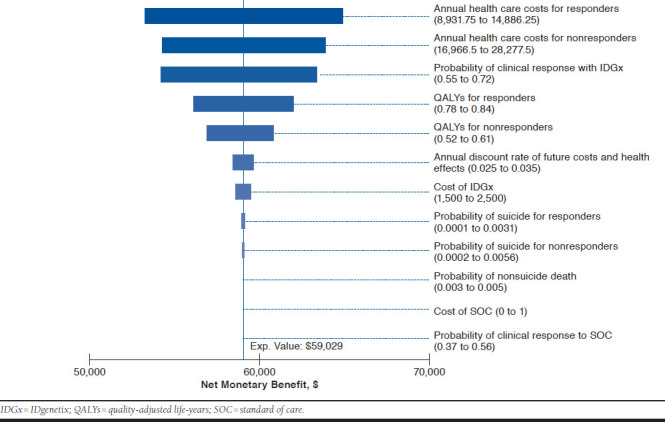
One-Way Sensitivity Analysis of Model Inputs
Discussion
MDD is a condition associated with substantial economic burden as well as increased risk of suicide.5-7 Many patients with MDD do not respond initially to standard treatments, leading to a trial-and-error process where multiple medications must be tried sequentially, causing significant delays in response for many people with MDD. These delays result in suffering, higher direct and indirect medical costs, and a greater risk of suicide. Growing evidence supports the use of pharmacogenetic-guided treatment to shorten the length of time required to identify an effective treatment regimen.20,22,26 In this study, a cost-effectiveness analysis using recent data from a large prospective trial of pharmacogenetic-guided MDD treatment was conducted. The analysis found that pharmacogenetic-guided treatment both reduced health care costs and was more effective after 3 years compared with the current standard of care.
Our results are consistent with those of other recently published analyses on pharmacogenetic-guided treatment. In a systematic review of economic evaluations of pharmacogenetic and pharmacogenomic screening tests across all medical conditions, Berm et al. (2016) reported that the majority of the 80 studies reviewed reported favorable economic outcomes.43 However, the general nature of this review does not facilitate depression-specific comparisons with our results. Also, the costs for many of the screening tests included in the Berm et al. analysis were considerably lower ($72 to $575) than the IDGx test that is $2,000, making it more likely that the tests would be found to be cost-effective.
In a more relevant study examining the effect of pharmacogenomic testing on costs, Winner et al. (2013) retrospectively compared claims data for MDD patients whose treatment included a medication identified as potentially problematic by pharmacogenomic testing (i.e., use with increased caution and frequent monitoring) versus medication suitable to use as directed versus medication suitable to use with caution.21 They found that the mean health care utilization costs associated with those patients using the nonrecommended medication were significantly higher over 1 year compared with either of the comparison groups. Although the 1-year time window of the study makes direct comparisons with the current study difficult, the largest cost differences are attributable to the first year of treatment. Therefore, the health care utilization cost difference of more than $5,000 per year may be seen as similar to the 3-year direct medical cost difference of $6,087 for the severe depression group in the current study. The Winner et al. study was limited by retrospective data.
A recent study of prospective trial data similarly modeled the cost-effectiveness of a pharmacogenetic test to guide treatment management for treatment-resistant depression. The study conducted by Hornberger et al. found that improved response rates as a result of pharmacogenetic-guided treatment resulted in reduced costs and increased QALYs over the average patient lifetime.25 However, that study and the current study differ in some ways. The current study uses results from a single large, randomized controlled trial, while Hornberger et al. used response rates from a meta-analysis they conducted of 3 smaller studies (sample sizes of 49, 44, and 165). In addition, we analyzed data from a broader range of depression, including treatment-naive patients, while the prior study focused only on depression that was treatment resistant. Although the exact effect of studying treatment-naive as opposed to treatment-resistant patients on the cost-effectiveness results is unclear, there is solid evidence that treatment-resistant patients become less likely to achieve remission with every failure.12 This suggests that the cost savings and health benefits may be a little higher in the treatment-resistant group. However, depending on the definition, treatment-resistant patients comprise a much smaller subset of all people with depression.12,44,45 Thus, although the per-person benefit of pharmacogenetic-guided treatment may be smaller, this approach to treatment can benefit virtually all people with moderate or severe depression, and likely have a greater overall effect on the health of our society and cost of care. Thus, using pharmacogenetic treatment with treatment-naive patients reduces the number that could become treatment resistant by matching them with an appropriate medication from the beginning.
Also, the current study uses a 3-year time horizon in order to focus on differences between groups in the catch-up period, during which the standard of care might have comparable response rates to care guided by pharmacogenetics testing. The Hornberger et al. study addressed the cumulative cost-effectiveness over the lifetime of a patient (38 years).
When considering these differences, and the much shorter time horizon, the results of the current study provide additional evidence that pharmacogenetic-guided treatment both improves the health and QOL of MDD patients and reduces health care expenditures, because of the lower medical and indirect costs associated with greater response. In the model, we found it took only 2 years for the $2,000 cost of the test to be offset by reduced direct medical costs of –$2,189.
Limitations
This study has some limitations to consider. One limitation is that the effects of medical treatments for depression are estimated in QALYs. Although most effectiveness measures have their own inherent limitations, it is important to note that QALYs are measured in different ways across studies, and thus are not always comparable.46 In addition, utility scores may be based on data from samples that are different from the populations that they are applied to. The Pettitt et al. (2016) literature review provides additional detail on limitations of the QALY methodology, including ethical concerns about how QALYs are applied in decision making.46
Like many clinical trials of medication for MDD,19,20,47,48 the trial on which the current analysis is based is limited to 12-week follow-up data. This results in uncertainty regarding the true trajectory of patient response over the first year and beyond. However, the 12-week follow-up period provided additional benefit beyond the initial 8-week follow-up, indicating an increasing effect over 12 weeks. At some point, this effect may decline slightly because of relapse to depression in responders, but relapse could be expected to occur at the same rate in both groups. To minimize the effect of changes after 12 weeks through the first year, we applied a half-cycle correction to avoid overestimating the treatment effect. Between 12 and 36 months, we have applied a catch-up calculation to account for an expected reduction in the response difference as the trial-and-error process progresses in the SOC group. In a systematic review of 31 randomized trials, Geddes et al. found similar relapse rates during the first 12 months of treatment compared with 12-36 months, providing evidence that most of the effectiveness is accounted for within at least the first year of treatment.30
A possible minor limitation was the choice of a 3-year time window. This makes comparisons to a recent similar study more challenging, but there are advantages to shorter time horizons, which are frequently used in economic evaluations of depression medication studies.33,49-51
Additionally, our model assumes a 3-year catch-up in which the SOC group response becomes equal to the IDGx group. Thus, once response rates match, outcomes for both groups from that point forward are quite similar, although they would be added onto any differences realized in the first 3 years. Finally, the shorter window provides results that are less affected by discount rates and may be easier to understand from a health policy or payer perspective.
As is often the case with decision modeling and cost-effectiveness analyses, data that are specific to the exact disease population being studied may be insufficient, requiring the use of the best available estimates. For example, suicide rates for both responders and nonresponders were derived from a study focusing on treatment-resistant MDD.11 This may lead to a slight overestimation of mortality for a broader and less severe range of depression in the current study. However, because rates for both responders and nonresponders come from the same study, any overestimation occurring in both groups is unlikely to have a significant effect on our results. This conclusion is supported by the robustness of results when these rates were varied in sensitivity analyses. When estimating costs, we considered using estimates of incremental costs of broader MDD provided in Greenberg et al. (2015).6 However, in that publication, the estimates of direct medical costs of people with MDD were $10,000 (2010 U.S. dollars) more than people without MDD. Thus, we used a more conservative estimate, in which costs were $6,162 more in the nonresponse group despite being converted to 2016 U.S. dollars. The Greenberg et al. data also had less detail on indirect costs. In summary, we sought to identify and use the most accurate estimates for model inputs when available while also keeping estimates conservative to avoid overestimation.
Conclusions
A much-cited barrier for the implementation of personalized medicine is the lack of cost-effectiveness studies to assess the economic benefit of pharmacogenetic-guided medication management. This study combines response rates from the largest randomized clinical trial of pharmacogenetic-guided medication management for depression to date and previously published data to model the cost-effectiveness of implementing pharmacogenetics as a standard tool to improve response rates in depression. The Markov model found that, at 3 years, IDGx pharmacogenetic-guided treatment was both cost saving and more effective compared with the current SOC. The cost of the IDGx test was more than offset by the savings in direct medical costs after 3 years. This result coincides well with the results from a prior study, and is particularly impressive because the test has the potential to reduce health care costs while still providing a clinically important improvement in QALYs over time.52
Furthermore, the clinical outcomes data from Bradley et al. and the cost-effectiveness model presented here indicate that wider use of pharmacogenetic testing with a broader range of depression severity is warranted. Given the increased need for a variety of health care providers to prescribe and manage antidepressants, pharmacogenetic tests are a valuable tool that demonstrate improved patient outcomes in real-world settings and are strongly positioned to help reduce the economic burden of depression.
REFERENCES
- 1.National Institute of Mental Health. Major depression among adults. 2017. Available at: https://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml. Accessed June 5, 2018.
- 2.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari A, Somerville A, Baxter A, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43(3):471-81. [DOI] [PubMed] [Google Scholar]
- 4.Pratt LA, Brody DJ.. Depression in the U.S. household population, 2009-2012. NCHS Data Brief. 2014;(172):1-8. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 10 leading causes of death by age group, United States—2014. 2016. Available at: https://www.cdc.gov/injury/images/lc-charts/leading_causes_of_death_age_group_2014_1050w760h.gif. Accessed June 5, 2018.
- 6.Greenberg PE, Fournier A-A, Sisitsky T, Pike CT, Kessler RC.. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155-62. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. Available at: https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed June 12, 2018.
- 9.Kemp AH, Gordon E, Rush AJ, Williams LM.. Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, and genetic measures. CNS Spectr. 2008;13(12):1066-86; quiz 1087-88. [DOI] [PubMed] [Google Scholar]
- 10.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-17. [DOI] [PubMed] [Google Scholar]
- 11.Olin B, Jayewardene AK, Bunker M, Moreno F.. Mortality and suicide risk in treatment-resistant depression: an observational study of the long-term impact of intervention. PLoS One. 2012;7(10):e48002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrazek DA, Hornberger JC, Altar CA, Degtiar I.. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65(8):977-87. [DOI] [PubMed] [Google Scholar]
- 13.Altar CA, Hornberger J, Shewade A, Cruz V, Garrison J, Mrazek D.. Clinical validity of cytochrome P450 metabolism and serotonin gene variants in psychiatric pharmacotherapy. Int Rev Psychiatry. 2013;25(5):509-33. [DOI] [PubMed] [Google Scholar]
- 14.Laje G, Perlis RH, Rush AJ, McMahon FJ.. Pharmacogenetics studies in STAR*D: strengths, limitations, and results. Psychiatr Serv. 2009;60(11):1446-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon FJ, Buervenich S, Charney D, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78(5):804-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niitsu T, Fabbri C, Bentini F, Serretti A.. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:183-94. [DOI] [PubMed] [Google Scholar]
- 17.Altar CA, Carhart J, Allen JD, Hall-Flavin D, Winner J, Dechairo B.. Clinical utility of combinatorial pharmacogenomics-guided antidepressant therapy: evidence from three clinical studies. Mol Neuropsychiatry. 2015;1(3):145-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altar CA, Carhart JM, Allen JD, Hall-Flavin DK, Dechairo BM, Winner JG.. Clinical validity: combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. Pharmacogenomics J. 2015;15(5):443-51. [DOI] [PubMed] [Google Scholar]
- 19.Hall-Flavin DK, Winner JG, Allen JD, et al. Utility of integrated pharmacogenomic testing to support the treatment of major depressive disorder in a psychiatric outpatient setting. Pharmacogenet Genomics. 2013;23(10):535-48. [DOI] [PubMed] [Google Scholar]
- 20.Hall-Flavin DK, Winner JG, Allen JD, et al. Using a pharmacogenomic algorithm to guide the treatment of depression. Transl Psychiatry. 2012;2:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winner J, Allen JD, Altar CA, Spahic-Mihajlovic A.. Psychiatric pharmacogenomics predicts health resource utilization of outpatients with anxiety and depression. Transl Psychiatry. 2013;3(3):e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winner JG, Carhart JM, Altar CA, Allen JD, Dechairo BM.. A prospective, randomized, double-blind study assessing the clinical impact of integrated pharmacogenomic testing for major depressive disorder. Discov Med. 2013;16(89):219-27. [PubMed] [Google Scholar]
- 23.Perlis RH, Patrick A, Smoller JW, Wang PS.. When is pharmacogenetic testing for antidepressant response ready for the clinic? A cost-effectiveness analysis based on data from the STAR*D study. Neuropsychopharmacology. 2009;34(10):2227-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winner JG, Carhart JM, Altar CA, et al. Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr Med Res Opin. 2015;31(9):1633-43. [DOI] [PubMed] [Google Scholar]
- 25.Hornberger J, Li Q, Quinn B.. Cost-effectiveness of combinatorial pharmacogenomic testing for treatment-resistant major depressive disorder patients. Am J Manag Care. 2015;21(6):e357-65. [PubMed] [Google Scholar]
- 26.Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psychiatr Res. 2018;96:100-07. September 23, 2017 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG.. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 2016. [Google Scholar]
- 28.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-103. [DOI] [PubMed] [Google Scholar]
- 29.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29(2):117-22. [DOI] [PubMed] [Google Scholar]
- 30.Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361(9358):653-61. [DOI] [PubMed] [Google Scholar]
- 31.Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Value Health. 2012;15(6):812-20. [DOI] [PubMed] [Google Scholar]
- 32.Maniadakis N, Kourlaba G, Mougiakos T, Chatzimanolis I, Jonsson L.. Economic evaluation of agomelatine relative to other antidepressants for treatment of major depressive disorders in Greece. BMC Health Serv Res. 2013;13:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodward TC, Tafesse E, Quon P, Lazarus A.. Cost effectiveness of adjunctive quetiapine fumarate extended-release tablets with mood stabilizers in the maintenance treatment of bipolar I disorder. Pharmacoeconomics. 2010;28(9):751-64. [DOI] [PubMed] [Google Scholar]
- 34.Feldman RL, Dunner DL, Muller JS, Stone DA.. Medicare patient experience with vagus nerve stimulation for treatment-resistant depression. J Med Econ. 2013;16(1):62-74. [DOI] [PubMed] [Google Scholar]
- 35.Arias E, Heron M, Xu J.. United States life tables, 2013. Natl Vital Stat Rep. 2017;66(3)1-64. [PubMed] [Google Scholar]
- 36.Krol M, Brouwer W, Rutten F.. Productivity costs in economic evaluations: past, present, future. Pharmacoeconomics. 2013;31(7):537-49. [DOI] [PubMed] [Google Scholar]
- 37.Bureau of Labor Statistics. Medical care in U.S. city average, all urban consumers, not seasonally adjusted. 2017. Available at: https://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths. Accessed June 11, 2018.
- 38.Forde GK, Hornberger J, Michalopoulos S, Bristow RE.. Cost-effectiveness analysis of a multivariate index assay compared to modified American College of Obstetricians and Gynecologists criteria and CA-125 in the triage of women with adnexal masses. Curr Med Res Opin. 2016;32(2):321-29. [DOI] [PubMed] [Google Scholar]
- 39.King JT Jr, Tsevat J, Lave JR, Roberts MS.. Willingness to pay for a quality-adjusted life year: implications for societal health care resource allocation. Med Decis Making. 2005;25(6):667-77. [DOI] [PubMed] [Google Scholar]
- 40.Neumann PJ, Cohen JT, Weinstein MC.. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-97. [DOI] [PubMed] [Google Scholar]
- 41.Laupacis A, Feeny D, Detsky AS, Tugwell PX.. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146(4):473-81. [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention Center, National Center for Health Statistics. Suicide and self-inflicted injury. 2017. Available at: https://www.cdc.gov/nchs/fastats/suicide.htm. Accessed June 5, 2018.
- 43.Berm EJ, Looff M, Wilffert B, et al. Economic evaluations of pharmacogenetic and pharmacogenomic screening tests: a systematic review. Second update of the literature. PLoS One. 2016;11(1):e0146262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGrath PJ, Stewart JW, Quitkin FM, et al. Predictors of relapse in a prospective study of fluoxetine treatment of major depression. Am J Psychiatry. 2006;163(9):1542-48. [DOI] [PubMed] [Google Scholar]
- 45.Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68 Suppl 8:17-25. [PubMed] [Google Scholar]
- 46.Pettitt DA, Raza S, Naughton B, et al. The limitations of QALY: a literature review. J Stem Cell Res Ther. 2016;6(4):1-7. [Google Scholar]
- 47.Hale A, Corral RM, Mencacci C, Ruiz JS, Severo CA, Gentil V.. Superior antidepressant efficacy results of agomelatine versus fluoxetine in severe MDD patients: a randomized, double-blind study. Int Clin Psychopharmacol. 2010;25(6):305-14. [DOI] [PubMed] [Google Scholar]
- 48.Kasper S, Hajak G, Wulff K, et al. Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J Clin Psychiatry. 2010;71(2):109-20. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong EP, Malone DC, Erder MH.. A Markov cost-utility analysis of escitalopram and duloxetine for the treatment of major depressive disorder. Curr Med Res Opin. 2008;24(4):1115-21. [DOI] [PubMed] [Google Scholar]
- 50.Beil H, Beeber LS, Schwartz TA, Lewis G.. Cost-effectiveness of alternative treatments for depression in low-income women. J Ment Health Policy Econ. 2013;16(2):55-65. [PubMed] [Google Scholar]
- 51.Choi SE, Brignone M, Cho SJ, et al. Cost-effectiveness of vortioxetine versus venlafaxine (extended release) in the treatment of major depressive disorder in South Korea. Expert Rev Pharmacoecon Outcomes Res. 2016;16(5):629-38. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan RM. The minimally clinically important difference in generic utility-based measures. COPD. 2005;2(1):91-97. [DOI] [PubMed] [Google Scholar]
- 53.Sobocki P, Ekman M, Ågren, H, et al. Model to assess the cost-effectiveness of new treatments for depression. Int J Technol Assess Health Care. 2006;22(4):469-77. [DOI] [PubMed] [Google Scholar]


