Abstract
BACKGROUND:
The median age at renal cell carcinoma (RCC) diagnosis is 64 years. However, few studies have assessed the real-world time on treatment (TOT), health resource utilization (HRU), costs, or treatment compliance associated with targeted therapy use among patients in this age group with RCC.
OBJECTIVE:
To assess the HRU, costs, and compliance during TOT among Medicare patients aged ≥ 65 years with advanced RCC (aRCC) who initiated first targeted therapy with pazopanib or sunitinib.
METHODS:
Patients with aRCC were identified in the 100% Medicare + Part D databases administered by the Centers for Medicare & Medicaid Services. Eligible patients initiated first targeted therapy with sunitinib or pazopanib (index drug) on or after their first diagnosis of secondary neoplasm between October 19, 2009, and January 1, 2014, and were aged ≥ 65 years as of 1 year before first targeted therapy initiation (index date). Included patients were stratified into pazopanib and sunitinib cohorts based on first targeted therapy and matched 1:1 on baseline characteristics using propensity scores. TOT was defined as the time from the index date to treatment discontinuation (prescription gap > 90 days) or death. Compliance was defined as the ratio of drug supply days to TOT. Monthly all-cause costs and costs associated with RCC diagnosis (medical and pharmacy in 2015 U.S. dollars) and HRU (inpatient [admissions, readmissions, and days], outpatient, and emergency room visits) were assessed in the 1-year post-index period during TOT. Matched cohorts’ TOT was compared using Kaplan-Meier analyses and univariable Cox models, and compliance, HRU, and costs were compared using Wilcoxon signed-rank tests.
RESULTS:
Of 1,711 included patients, 526 initiated pazopanib and 1,185 initiated sunitinib. Before matching, more patients in the pazopanib cohort were white, diagnosed in 2010-2014 versus 2006-2009, and had lung metastases compared with the sunitinib cohort (all P < 0.05). The pazopanib cohort also had higher mean outpatient visits and costs but lower mean total all-cause pharmacy costs, than the sunitinib cohort (all P < 0.05). After matching, the pazopanib and sunitinib cohorts had similar characteristics (mean age 75 years, 58% male, and Charlson Comorbidity Index score of 9.2 in both cohorts) and median TOT (4.8 and 4.1 months, respectively). Among the 522 matched pairs, pazopanib was associated with significantly lower total all-cause health care costs ($8,527 vs. $10,924, respectively [mean difference = $2,397]); total medical costs ($3,991 vs. $5,881, respectively, [$1,890]); and inpatient costs ($2,040 vs. $3,731, respectively, [$1,692]; all P < 0.01) compared with sunitinib. Patients receiving pazopanib had significantly fewer inpatient admissions (0.179 vs. 0.289, respectively) and days (1.063 vs. 1.904, respectively; both P < 0.01) than patients receiving sunitinib. Mean treatment compliance was lower for the pazopanib versus sunitinib cohort (0.91 vs. 0.94, respectively; P < 0.01).
CONCLUSIONS:
In this retrospective analysis of Medicare patients with aRCC from a TOT perspective, first targeted therapy with pazopanib was associated with significantly lower all-cause health care costs and HRU, but lower compliance, compared with sunitinib.
What is already known about this subject
In patients with clear-cell advanced renal-cell carcinoma (aRCC), the targeted therapies pazopanib and sunitinib have shown similar overall survival and progression-free survival, making real-world economic evaluations of these drugs useful for treatment decisions.
Pazopanib has been associated with lower health resource utilization (HRU) and monthly health care costs versus sunitinib from an intent-to-treat perspective, although a similar analysis from a time-on-treatment (TOT) perspective has not been performed.
Although more than half of patients are diagnosed with RCC after the age of 65 years, few studies have assessed real-world clinical and economic outcomes among Medicare patients with RCC.
What this study adds
This retrospective claims analysis assessed HRU and costs (all-cause and those associated with RCC diagnosis) from a TOT perspective among aRCC patients enrolled in Medicare (aged ≥ 65 years) who initiated first targeted therapy with pazopanib or sunitinib.
After propensity score matching, pazopanib was associated with significantly fewer monthly all-cause and RCC diagnosis-associated inpatient admissions, readmissions, and days, as well as fewer all-cause emergency room visits.
Pazopanib patients had significantly lower monthly all-cause (total health care, total pharmacy, medical, inpatient, emergency room, skilled nursing facility, and home health agency) and RCC diagnosis-associated (all categories but outpatient) costs compared with sunitinib patients but lower treatment compliance.
Renal cell carcinoma (RCC) is a common malignancy among adults in the United States, with over 65,000 new cases diagnosed each year.1 RCC originates in the renal cortex and represents 80%-85% of all primary renal neoplasms.2 The median age at RCC diagnosis is 64 years,3 and it is uncommon among patients aged under 40 years.4 A substantial proportion of patients are diagnosed with advanced RCC (aRCC) or progress to advanced states of the disease. Approximately 25%-33% of patients with RCC have de novo metastases, while a further 20%-40% of patients who receive treatment for localized disease eventually develop metastases.5-7 Patient outcomes are related to RCC disease severity; as severity increases, the 5-year survival rate falls from 92.5% among patients with localized disease to 11.7% among patients with distant disease.3 However, improvements in RCC treatment over the past few decades—including advances in surgery, therapies, and noninvasive diagnostic imaging—have increased the survival rate of RCC patients, despite a rise in RCC incidence.8,9
In particular, the development of targeted therapies, such as vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin (mTOR) inhibitors, has led to substantial improvements in survival among RCC patients. Compared with cytokine treatment, VEGFR-TKIs and mTOR inhibitors have doubled the mean overall survival (OS) of patients with aRCC from 10 to 20 months,10,11 and they have become the standard of care among patients who are not candidates for resection.12
Among the VEGFR-TKIs approved by the U.S. Food and Drug Administration for aRCC, pazopanib (approved in 200913) and sunitinib (approved in 200614) are 2 commonly prescribed first treatment options.15 In patients with clear-cell metastatic RCC, pazopanib has shown similar OS and progression-free survival (PFS) to sunitinib but is associated with higher quality of life (QoL) outcomes and a differentiated safety profile.15-17 Given the similar efficacy of pazopanib and sunitinib, economic evaluations of these therapies can provide important information when choosing the optimal first-line treatment for aRCC.
Several previous studies have compared the cost of treatment of aRCC with pazopanib versus sunitinib from an intent-to-treat (ITT) perspective and reported lower health care costs and superior compliance among pazopanib-treated patients. For example, a 2015 cost-effectiveness analysis compared pazopanib and sunitinib for first-line metastatic RCC treatment in the United States using data from the COMPARZ and PISCES trials,15,16 and it found that pazopanib provided more quality-adjusted life-years (QALYs) at lower cost than sunitinib.18 A subsequent letter to the editor criticized the inputs used in the model, namely those used for utilities and survival modeling.19 A 2015 analysis of health care costs using commercial claims (2008-2011) found lower costs among patients with aRCC treated with first-line pazopanib versus sunitinib,20 similar to the findings reported by an analysis of commercial and Medicare claims (2009-2012) by Racsa et al. (2015).21 Compliance with pazopanib or sunitinib as first treatments has been reported to be similar, but age is a negative predictor of compliance.22-24 However, economic outcomes and treatment compliance during time on treatment (TOT) with pazopanib versus sunitinib have not yet been assessed.
Although more than half of patients are diagnosed with RCC after age 65,3 few studies have assessed real-world clinical and economic outcomes among Medicare patients with RCC. As might be expected, overall medical costs are higher among elderly patients with RCC compared with those without RCC. A claims analysis by Hollenbeak et al. (2011), which used information from the Surveillance, Epidemiology, and End Results-Medicare database, found that the mean costs per patient per month (PPPM) were $3,673 and $793, respectively.25 However, the authors did not include or compare targeted therapies. To address this gap in the literature, this study assessed real-world health resource utilization (HRU), health care costs, and treatment compliance from a TOT perspective among aRCC patients enrolled in Medicare (aged 65 years and older) who initiated first targeted therapy with pazopanib or sunitinib.
Methods
Data Source
This study used data retrieved from the 100% Medicare database + Part D linkage (spanning January 1, 2006-December 31, 2014) provided through the Centers for Medicare & Medicaid Services. This database contains information collected by Medicare to pay for health care services provided to Medicare beneficiaries. The data included enrollment and claims history from Medicare Part A (i.e., institutional claims such as inpatient and outpatient services), Medicare Part B (i.e., noninstitutional claims such as carrier and medical equipment information), and Medicare Part D (i.e., prescription and drug data). The data were de-identified and complied with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act. This study received an exemption from institutional board review from the New England Institutional Review Board on November 6, 2015.
Sample Selection and Construction
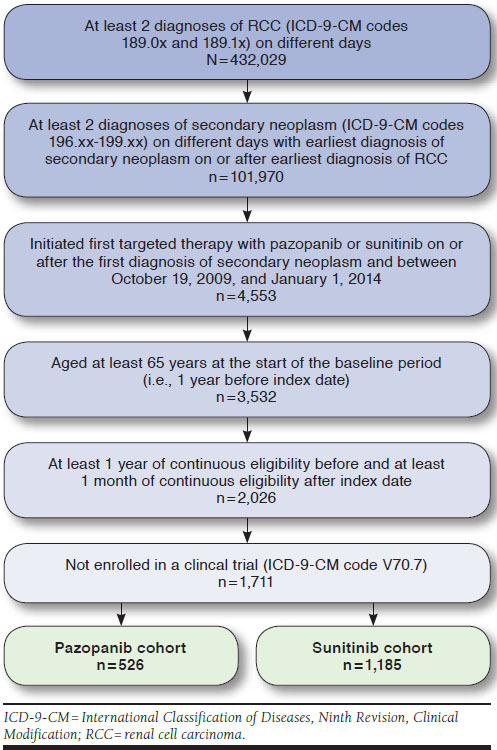
Patients eligible for inclusion in the study had at least 2 diagnoses of RCC (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 189.0x, 189.1x) on different days and at least 2 diagnoses of secondary neoplasm (ICD-9-CM codes 196.xx-199.xx) on different days, with the earliest diagnosis of secondary neoplasm on or after the earliest diagnosis of RCC. Patients were required to have initiated first targeted therapy (index drug) with pazopanib or sunitinib on or after the first diagnosis of secondary neoplasm and between October 19, 2009, the approval date of pazopanib, and January 1, 2014, to ensure at least 1 year of potential follow-up. The initiation date of first targeted therapy was defined as the index date (Appendix A, available in online article). Patients were required to be at least aged 65 years at the start of the baseline period (i.e., 1 year before the index date) and to have at least 1 year of continuous Medicare eligibility before the index date and at least 1 month of continuous eligibility after the index date. Patients enrolled in a clinical trial (ICD-9-CM code V70.7) were excluded. Included patients were stratified into 2 cohorts based on the index drug: the pazopanib cohort or the sunitinib cohort.
Baseline Characteristics
Patient demographic and clinical characteristics assessed at the index date included age, sex, race, follow-up duration from the index date, and year of RCC diagnosis. Metastatic sites (i.e., ICD-9-CM codes for lung 197.0x, lymph node 196.xx, bone 198.5x, and liver 197.7x); comorbidities (i.e., cardiovascular disease, hypertension, chronic pulmonary disease, diabetes, renal failure, and liver disease; see Appendix B in online article for diagnosis codes); and the Charlson Comorbidity Index (CCI) score were assessed during the baseline period.26
Monthly HRU and costs were also assessed during the baseline period for the purposes of propensity score matching. HRU categories included inpatient admissions, days, and readmissions (i.e., an inpatient admission within 30 days of a preceding inpatient discharge), as well as emergency room (ER) and outpatient visits. Health care costs (inflated to 2015 U.S. dollars using the medical component of the Consumer Price Index27) from the payer’s perspective were identified using the Medicare paid amount. Total all-cause medical costs, total all-cause pharmacy costs, and total all-cause health care costs (i.e., the sum of total all-cause pharmacy and total all-cause medical costs) were assessed on a PPPM basis. Total all-cause medical costs included inpatient costs, ER costs, outpatient costs, skilled nursing facility costs, home health agency costs, and other medical service costs (e.g., laboratory tests).
Propensity Score Matching
Patients in the pazopanib and sunitinib cohorts were matched 1:1 using propensity scores to account for observable differences at baseline between the cohorts. The matching algorithm selected the control closest to the case using the greedy method, allowing for a Euclidean distance of up to 25% of the standard deviation of the propensity score. Propensity scores were calculated using a logistic regression, and covariates included age, sex, race, year of RCC diagnosis, metastatic sites, comorbidities, CCI score, inpatient costs, outpatient costs, ER costs, and total all-cause pharmacy costs.
Outcomes
Patients from the matched first targeted therapy cohorts were followed from index date until the earliest of death, end of eligibility, or end of data (i.e., duration of follow-up). TOT was calculated as time from the index date to the earliest of treatment discontinuation (> 90-day gap in supply of the index drug from the last day of supply) or death.28,29 Patients without an event were censored at the end of continuous eligibility or end of data (December 31, 2014). The same categories of HRU and costs assessed during the baseline period were assessed during TOT. In addition, the same categories of HRU and costs during TOT were summarized among claims with an ICD-9-CM diagnosis code for RCC (i.e., HRU and costs associated with RCC diagnosis). Compliance was estimated using medication possession ratio (MPR), defined as the ratio of the days supply of the index drug to TOT. High compliance was defined as MPR ≥ 80%. Because of the recommended sunitinib dosing schedule of 4 weeks on/2 weeks off, 14 days were added to the end of sunitinib prescriptions with 28-31 days of supply in all analyses.
Statistical Analyses
Baseline characteristics in the unmatched pazopanib and sunitinib cohorts were compared using Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables. Baseline characteristics and outcomes in the matched pazopanib and sunitinib cohorts were compared using Wilcoxon signed-rank tests for continuous variables and McNemar tests for categorical variables. HRU and costs during TOT were compared between the matched pazopanib and sunitinib cohorts using Wilcoxon signed-rank tests; differences in mean costs were also computed.
TOT was compared between the matched targeted therapy cohorts using Kaplan-Meier analyses with log-rank tests and univariable Cox proportional hazards models that reported hazard ratios (HRs) with 95% confidence intervals (CIs). Compliance was compared between the matched cohorts using a Wilcoxon signed-rank test and a univariable logistic regression model to assess the odds ratio (OR) with 95% CI of achieving high compliance.
A P value of 0.05 was used to determine significant differences. All analyses were conducted using SAS Enterprise Guide, version 7.12 (SAS Institute, Cary, NC).
Results
Baseline Characteristics and Propensity Score Matching
Overall, 1,711 patients with RCC met the study criteria, with 526 patients who initiated first targeted therapy with pazopanib and 1,185 who initiated sunitinib (Figure 1). Before matching, the baseline characteristics of the pazopanib and sunitinib cohorts were generally similar. In both cohorts, the mean age was 75 years; 58% were male; and the mean CCI score was 9.2 (Table 1). The mean (standard deviation [SD]) duration of follow-up after the index date was 15.7 (± 10.7) months for the pazopanib cohort and 17.2 (± 13.7) months for the sunitinib cohort (P = 0.570). However, compared with the pazopanib cohort, smaller proportions of the sunitinib cohort were white (88.0% vs. 83.8%, respectively; P = 0.024), diagnosed with RCC during the years 2010-2014 (61.4% vs. 53.8%, respectively; P = 0.003), or had lung metastases (56.7% vs. 50.3%, respectively; P = 0.015). At baseline, patients in the pazopanib cohort had more monthly outpatient visits (0.888 vs. 0.847, respectively; P = 0.012), higher monthly outpatient costs ($404 vs. $344, respectively; P < 0.001), and lower monthly all-cause pharmacy costs ($143 vs. $161, respectively; P = 0.004) than the sunitinib cohort.
FIGURE 1.
Sample Selection
TABLE 1.
Baseline Characteristics Among the First Targeted Therapy Cohorts Before and After Propensity Score Matching
| Before Matchinga | After Matchingb | |||||
|---|---|---|---|---|---|---|
| Pazopanib n = 526 | Sunitinib n = 1,185 | P Value | Pazopanib n = 522 | Sunitinib n = 522 | P Value | |
| Demographics at index date | ||||||
| Age, years, mean ± SD | 74.8 ± 6.0 | 74.7 ± 5.9 | 0.733 | 74.8 ± 6.0 | 75.2 ± 6.3 | 0.354 |
| Male, n (%) | 307 (58.4) | 682 (57.6) | 0.754 | 305 (58.4) | 303 (58.0) | 0.900 |
| Race, n (%) | ||||||
| White | 463 (88.0) | 993 (83.8) | 0.024c | 459 (87.9) | 460 (88.1) | 0.917 |
| Black | 29 (5.5) | 85 (7.2) | 0.204 | 29 (5.6) | 25 (4.8) | 0.572 |
| Other or unknown | 34 (6.5) | 107 (9.0) | 0.075 | 34 (6.5) | 37 (7.1) | 0.705 |
| Follow-up duration after index date, months, mean ± SD | 15.7 ± 10.7 | 17.2 ± 13.7 | 0.570 | 15.7 ± 10.7 | 15.7 ± 12.5 | 0.919 |
| Year of RCC diagnosis, n (%) | ||||||
| 2006-2009 | 203 (38.6) | 548 (46.2) | 0.003c | 202 (38.7) | 200 (38.3) | 0.888 |
| 2010-2014 | 323 (61.4) | 637 (53.8) | 0.003c | 320 (61.3) | 322 (61.7) | 0.888 |
| Metastatic sites, n (%) | ||||||
| Lung | 298 (56.7) | 596 (50.3) | 0.015c | 294 (56.3) | 280 (53.6) | 0.358 |
| Lymph node | 102 (19.4) | 187 (15.8) | 0.066 | 100 (19.2) | 102 (19.5) | 0.876 |
| Bone | 168 (31.9) | 405 (34.2) | 0.365 | 167 (32.0) | 171 (32.8) | 0.792 |
| Liver | 75 (14.3) | 175 (14.8) | 0.783 | 75 (14.4) | 76 (14.6) | 0.931 |
| CCI, mean ± SD | 9.2 ± 2.4 | 9.2 ± 2.4 | 0.811 | 9.2 ± 2.4 | 9.2 ± 2.4 | 0.956 |
| Comorbidities, n (%) | ||||||
| Cardiovascular disease | 343 (65.2) | 737 (62.2) | 0.233 | 341 (65.3) | 338 (64.8) | 0.842 |
| Hypertension | 474 (90.1) | 1,072 (90.5) | 0.821 | 470 (90.0) | 471 (90.2) | 0.916 |
| Chronic pulmonary disease | 181 (34.4) | 438 (37.0) | 0.311 | 181 (34.7) | 177 (33.9) | 0.791 |
| Diabetes | 227 (43.2) | 569 (48.0) | 0.063 | 227 (43.5) | 223 (42.7) | 0.797 |
| Renal failure | 248 (47.1) | 547 (46.2) | 0.705 | 246 (47.1) | 264 (50.6) | 0.279 |
| Liver disease | 27 (5.1) | 78 (6.6) | 0.249 | 27 (5.2) | 19 (3.6) | 0.228 |
| Monthly per patient health care resource use, mean ± SD | ||||||
| Inpatient admissions | 0.086 ± 0.105 | 0.088 ± 0.097 | 0.244 | 0.086 ± 0.105 | 0.094 ± 0.095 | 0.120 |
| Inpatient days | 0.591 ± 0.898 | 0.630 ± 0.925 | 0.163 | 0.593 ± 0.901 | 0.653 ± 0.843 | 0.214 |
| Inpatient readmissions | 0.014 ± 0.051 | 0.016 ± 0.047 | 0.141 | 0.014 ± 0.051 | 0.016 ± 0.046 | 0.321 |
| Emergency room visits | 0.048 ± 0.079 | 0.053 ± 0.110 | 0.609 | 0.049 ± 0.079 | 0.049 ± 0.085 | 0.892 |
| Outpatient visits | 0.888 ± 0.681 | 0.847 ± 0.874 | 0.012c | 0.879 ± 0.672 | 0.912 ± 1.045 | 0.420 |
| Monthly per patient health care costs to the payer (2015 USD), mean ± SD | ||||||
| Total all-cause health care costs | 3,005 ± 3,133 | 2,952 ± 3,074 | 0.797 | 2,998 ± 3,142 | 3,114 ± 2,792 | 0.244 |
| Total all-cause medical costs | 2,862 ± 3,081 | 2,791 ± 3,035 | 0.968 | 2,855 ± 3,090 | 2,960 ± 2,747 | 0.243 |
| Inpatient costs | 1,629 ± 2,498 | 1,601 ± 2,355 | 0.394 | 1,633 ± 2,506 | 1,700 ± 2,194 | 0.290 |
| Emergency room costs | 37 ± 76 | 43 ± 103 | 0.631 | 37 ± 76 | 39 ± 80 | 0.927 |
| Outpatient costs | 404 ± 414 | 344 ± 395 | < 0.001c | 393 ± 369 | 388 ± 448 | 0.146 |
| Skilled nursing facility costs | 117 ± 497 | 146 ± 604 | 0.258 | 118 ± 499 | 138 ± 557 | 0.577 |
| Home health agency costs | 106 ± 264 | 100 ± 265 | 0.581 | 106 ± 265 | 103 ± 256 | 0.884 |
| Other medical service costs | 569 ± 723 | 557 ± 784 | 0.459 | 568 ± 723 | 593 ± 798 | 0.700 |
| Total all-cause pharmacy costs | 143 ± 261 | 161 ± 324 | 0.004c | 143 ± 262 | 154 ± 311 | 0.108 |
aBefore matching, Wilcoxon rank-sum tests were used for continuous variables, and chi-square tests were used for categorical variables.
bAfter matching, Wilcoxon signed-rank tests were used for continuous variables, and McNemar tests were used for categorical variables.
cP < 0.05 for pairwise comparison of the pazopanib cohort with the sunitinib cohort.
CCI = Charlson Comorbidity Index; RCC = renal cell carcinoma; SD = standard deviation; USD = U.S. dollars.
Propensity score matching of the pazopanib and sunitinib patients yielded 522 matched pairs with balanced baseline characteristics and similar median follow-up durations (15.7 months for both cohorts; Table 1).
TOT and Treatment Compliance
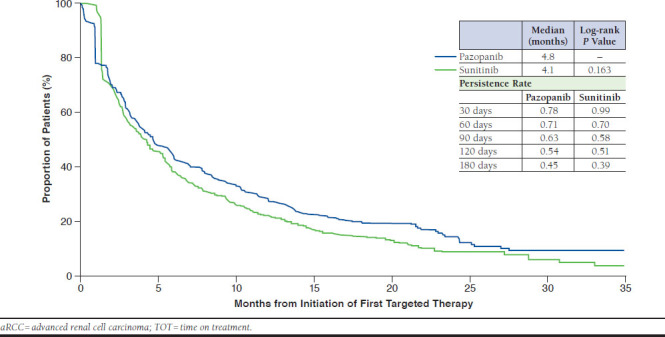
After matching, pazopanib was associated with similar TOT compared with sunitinib (HR = 0.90, 95% CI = 0.78-1.04; P = 0.169), with a median TOT of 4.8 and 4.1 months, respectively (log-rank P = 0.163; Figure 2).29 At 30 days on treatment, the persistence rates for pazopanib and sunitinib were 0.78 and 0.99, respectively, and at 180 days, the rates were 0.45 and 0.39, respectively. Mean (SD) MPR was lower among the pazopanib cohort (0.91 [± 0.13]) compared with the sunitinib cohort (0.94 [± 0.10]; mean difference = -0.03; P < 0.001), and pazopanib was associated with a lower likelihood of high compliance (OR = 0.54, 95% CI = 0.37-0.77; P < 0.001).
FIGURE 2.
Kaplan-Meier Curve of TOT by First Targeted Therapy Among Propensity Score-Matched Elderly Patients with aRCC
All-Cause and RCC Diagnosis-Associated HRU During TOT
Among the matched cohorts, first targeted therapy with pazopanib was associated with significantly fewer all-cause monthly inpatient admissions (0.179 vs. 0.289, respectively [mean difference = 0.110]; P < 0.001) and days (1.063 vs. 1.904, respectively [0.840]; P < 0.001) compared with first targeted therapy with sunitinib (Table 2). In addition, pazopanib patients had fewer all-cause inpatient readmissions (0.031 vs. 0.061, respectively [0.03]; P = 0.009) and ER visits (0.096 vs. 0.122, respectively [0.026]; P =0.004) than sunitinib patients.
TABLE 2.
After Propensity Score Matching: Comparison of HRU by First Targeted Therapy Among Cohorts During TOT
| Monthly per Patient Resource Use | Pazopanib, n = 522 | Sunitinib, n = 522 | Mean Difference [A] - [B] | P Value | ||
|---|---|---|---|---|---|---|
| Mean [A] ± SD | Median [A] | Mean [B] ± SD | Median [B] | |||
| All-cause | ||||||
| Inpatient admissions | 0.179 ± 0.426 | 0.000 | 0.289 ± 0.449 | 0.000 | -0.110 | < 0.001a |
| Inpatient days | 1.063 ± 2.614 | 0.000 | 1.904 ± 3.675 | 0.000 | -0.840 | < 0.001a |
| Inpatient readmissions | 0.031 ± 0.145 | 0.000 | 0.061 ± 0.226 | 0.000 | -0.030 | 0.009a |
| Emergency room visits | 0.096 ± 0.303 | 0.000 | 0.122 ± 0.248 | 0.000 | -0.026 | 0.004a |
| Outpatient visits | 1.396 ± 1.499 | 1.052 | 1.415 ± 1.447 | 0.978 | -0.019 | 0.432 |
| RCC diagnosis-associated | ||||||
| Inpatient admissions | 0.167 ± 0.416 | 0.000 | 0.270 ± 0.445 | 0.000 | -0.103 | < 0.001a |
| Inpatient days | 0.827 ± 2.111 | 0.000 | 1.687 ± 3.539 | 0.000 | -0.860 | < 0.001a |
| Inpatient readmissions | 0.032 ± 0.143 | 0.000 | 0.059 ± 0.224 | 0.000 | -0.028 | 0.028a |
| Emergency room visits | 0.048 ± 0.217 | 0.000 | 0.056 ± 0.167 | 0.000 | -0.008 | 0.097 |
| Outpatient visits | 0.987 ± 1.206 | 0.719 | 0.903 ± 1.055 | 0.686 | 0.083 | 0.455 |
Note: HRU was measured from treatment initiation to the earliest of treatment discontinuation or death (TOT perspective).
aP < 0.05 for pairwise comparison of pazopanib cohort with sunitinib cohort using Wilcoxon signed-rank tests.
HRU = health resource use; RCC = renal cell carcinoma; SD = standard deviation; TOT = time on treatment.
In the analysis of HRU associated with an RCC diagnosis, the trend was similar to the all-cause HRU results. The pazopanib cohort had significantly fewer mean monthly RCC-diagnosis associated inpatient admissions (0.167 vs. 0.270, respectively [mean difference = 0.103]; P < 0.001) and days (0.827 vs. 1.687, respectively [0.860]; P < 0.001) compared with the sunitinib cohort (Table 2). In addition, the pazopanib cohort experienced fewer monthly RCC diagnosis-associated inpatient readmissions compared with the sunitinib cohort (0.032 vs. 0.059, respectively [0.028]; P = 0.028).
All-Cause and RCC Diagnosis-Associated Monthly Health Care Costs During TOT
The pazopanib cohort incurred significantly lower monthly total all-cause health care costs ($8,527 vs. $10,924, respectively [mean difference = $2,397]), total all-cause medical costs ($3,991 vs. $5,881, respectively [$1,890]), and all-cause inpatient costs ($2,040 vs. $3,731, respectively [$1,692]; all P < 0.001) compared with the sunitinib cohort (Table 3). In addition, pazopanib patients had lower all-cause ER ($73 vs. $115, respectively [$42]; P = 0.005), skilled nursing facility ($156 vs. $369, respectively [$213]; P = 0.002), home health agency ($230 vs. $303, respectively [$74]; P = 0.028), and total all-cause pharmacy ($4,536 vs. $5,043, respectively [$506]; P < 0.001) costs compared with the sunitinib cohort.
TABLE 3.
After Propensity Score Matching: Comparison of Health Care Costs by First Targeted Therapy Among Cohorts During TOT
| Monthly per Patient Costs to Payer (2015 USD) | Pazopanib, n = 522 | Sunitinib, n = 522 | Mean Difference [A] - [B] | P Value | ||
|---|---|---|---|---|---|---|
| Mean [A] ± SD | Median [A] | Mean [B] ± SD | Median [B] | |||
| All-cause | ||||||
| Total all-cause health care costs | 8,527 ± 6,264 | 7,013 | 10,924 ± 9,365 | 8,357 | -2,397 | < 0.001a |
| Total all-cause medical costs | 3,991 ± 5,971 | 1,562 | 5,881 ± 9,057 | 2,499 | -1,890 | < 0.001a |
| Inpatient costs | 2,040 ± 5,084 | 0 | 3,731 ± 8,316 | 0 | -1,692 | < 0.001a |
| Emergency room costs | 73 ± 239 | 0 | 115 ± 315 | 0 | -42 | 0.005a |
| Outpatient costs | 477 ± 722 | 272 | 385 ± 509 | 219 | 92 | 0.116 |
| Skilled nursing facility costs | 156 ± 832 | 0 | 369 ± 1,500 | 0 | -213 | 0.002a |
| Home health agency costs | 230 ± 590 | 0 | 303 ± 662 | 0 | -74 | 0.028a |
| Other medical service costs | 1,016 ± 2,160 | 382 | 977 ± 1,370 | 425 | 39 | 0.226 |
| Total all-cause pharmacy costs | 4,536 ± 2,171 | 4,510 | 5,043 ± 2,413 | 4,838 | -506 | < 0.001a |
| RCC diagnosis-associated | ||||||
| Inpatient costs | 1,166 ± 3,509 | 0 | 2,495 ± 7,116 | 0 | -1,329 | < 0.001a |
| Emergency room costs | 27 ± 137 | 0 | 46 ± 197 | 0 | -19 | 0.047a |
| Outpatient costs | 309 ± 506 | 153 | 232 ± 415 | 108 | 77 | 0.002a |
Note: Costs were measured from treatment initiation to the earliest of treatment discontinuation or death (TOT perspective).
aP < 0.05 for pairwise comparison of the pazopanib cohort with the sunitinib cohort using Wilcoxon signed-rank tests.
RCC= renal cell carcinoma; SD = standard deviation; TOT = time on treatment; USD = U.S. dollars.
In the analysis of health care costs associated with an RCC diagnosis, the pazopanib cohort had lower monthly inpatient costs ($1,166 vs. $2,495, respectively [mean difference = $1,329]; P < 0.001) and ER costs ($27 vs. $46, respectively [$19]; P = 0.047), but slightly higher outpatient costs ($309 vs. $232, respectively [$77]; P = 0.002) than the sunitinib cohort (Table 3).
Discussion
Research incorporating real-world evidence on HRU and costs among patients initiating pazopanib versus sunitinib for aRCC can provide insight into the comparative economic benefits of these therapies, given their association with similar patient outcomes. While pazopanib has been previously associated with lower costs and HRU from an ITT perspective, similar analyses have not been conducted from a TOT perspective. Thus, this study assessed HRU, costs, and treatment compliance from a TOT perspective among a large cohort of Medicare-enrolled aRCC patients who initiated first targeted therapy with pazopanib or sunitinib. After propensity score matching, TOT was similar, and pazopanib was associated with significantly fewer monthly all-cause and RCC diagnosis-associated inpatient admissions, readmissions, and days, as well as fewer all-cause ER visits during TOT.29 In addition, pazopanib patients had lower monthly all-cause (health care, medical, inpatient, ER, skilled nursing facility, home health agency, and total pharmacy costs) and RCC diagnosis-associated costs (in all categories but outpatient) compared with sunitinib patients. Treatment compliance was observed to be lower among patients initiating pazopanib compared with sunitinib.
The number of inpatient admissions was lower for patients treated with pazopanib versus sunitinib, which is an important clinical benefit for patients with aRCC, along with other previously reported benefits. While pazopanib and sunitinib have demonstrated comparable OS and PFS in clinical trials, pazopanib has been associated with higher QoL outcomes and a differentiated safety profile compared with sunitinib.15,17 For example, in PISCES, a randomized crossover trial comparing the effects of toxicity and tolerability of pazopanib and sunitinib on preferences of patients with aRCC, pazopanib was preferred by 70% of patients and 61% of clinicians.16 Less fatigue and better overall QoL were the primary reasons cited by patients preferring pazopanib, and these factors could have potentially contributed to the lower rates of HRU and according health care costs among the pazopanib cohort in the current study.
There is limited research on TOT and compliance with pazopanib and sunitinib among patients with aRCC in the real world. A 2014 study reported a TOT of 11.6 months for pazopanib and 6.2 months for sunitinib among a small cohort of patients with RCC and end-stage renal disease (N = 17).30 A 2015 study reported a mean time to treatment discontinuation as 133.4 days and 139.9 days among matched pazopanib and sunitinib patients with RCC, respectively.22 Previous analyses have found that TOT was similar for elderly patients with aRCC initiating either first-line pazopanib or sunitinib, which is consistent with the current findings.31 As with 2 previous real-world studies that reported similar rates of high compliance for these therapies as first treatments, older age has been noted to be a strong predictor of poor pazopanib compliance.22-24 The current study found high compliance with pazopanib and sunitinib, although compliance was somewhat higher for sunitinib (MPR difference of 0.03). In this study, due to sunitinib’s recommended dosing schedule of 4 weeks on/2 weeks off, the 14-day off period was added to sunitinib prescriptions with 28-31 days of supply, and patients were considered treatment-compliant during the off days. The assumption that patients on sunitinib were completely adherent during the 2 weeks off may have elevated the calculation of compliance on sunitinib.
Although the median age of RCC diagnosis is 64 years,3 few previous studies comparing pazopanib and sunitinib have focused on this age group, and none have compared real-world economic outcomes during TOT among Medicare beneficiaries initiating targeted therapies as first-line treatment for aRCC.
Similar to this analysis, pazopanib has previously been reported to be more cost-effective than sunitinib (although from an ITT perspective) among clinical- and real-population patients with aRCC. A 2015 cost-effectiveness analysis compared pazopanib and sunitinib for first-line metastatic RCC treatment in the United States using data from pivotal trials,15,16 and it found that pazopanib was associated with more QALYs at lower cost compared to sunitinib.18 However, that model used clinical data for utility and survival inputs, and because of the design of the PISCES trial and potentially noncomparable patient populations, these inputs may be unreliable.19 A 2015 study using real-world data from the Truven MarketScan database came to similar conclusions as those in the current analysis regarding lower HRU and costs among patients with aRCC initiating first-line pazopanib versus sunitinib.20 However, that study was constrained by the use of older data (2008-2011) and a smaller sample size than the current study. In addition, Racsa et al. found higher medication and health care costs among sunitinib- versus pazopanib-treated aRCC patients with commercial insurance or Medicare.21
Contrary to the findings of the previously mentioned studies, a 2016 study using claims of commercially insured patients in the Truven MarketScan database reported that after substituting a 42-day supply of sunitinib (vs. 28 or 32 days) there were no significant differences in costs compared with pazopanib.31 However, before substitution, pazopanib was associated with significantly lower medication costs in that study. The study design assessed patients over a shorter baseline period (6 vs. 12 months in the present study), used an ITT perspective, did not adjust for differences in patient baseline characteristics, and assessed outcomes over a fixed 12-month follow-up period, which excluded patients who died within 1 year of treatment initiation.
Limitations
Although claims data comprise a large and valid data sample from a real-world setting, this study is subject to the limitations inherent to retrospective observational studies using claims data. First, administrative claims data only contain diagnostic and procedure codes recorded for reimbursement purposes. Second, retrospective databases may be subject to coding errors or data omissions, and patients may not have used the recorded medication as prescribed after filling a prescription. For this reason, treatment initiation dates are approximate. Third, this study only included adult patients covered by Medicare. Thus, the results may not generalize to other populations, such as Medicaid enrollees, uninsured or commercially insured patients, or patients aged younger than 65 years. Fourth, patients may not have used the recorded medication as prescribed, so treatment initiation dates (and thus TOT dates) are approximate. Finally, included patients were not required to have continuous Medicare enrollment before and after the first RCC diagnosis; thus, the date of RCC diagnosis is approximate.
Conclusions
In this retrospective analysis of Medicare patients with aRCC using a TOT perspective, first targeted therapy with pazopanib was associated with significantly lower all-cause and RCC diagnosis-associated health care costs and resource use compared with first targeted therapy with sunitinib. TOT was similar among the cohorts, and treatment compliance was lower for the pazopanib versus sunitinib cohort. Given the comparable patient outcomes of first-line pazopanib and sunitinib in the treatment of aRCC, this economic comparison may provide useful evidence from a new perspective to inform decisions regarding the choice of first-line therapy.
ACKNOWLEDGMENTS
Medical writing assistance was provided by Shelley Batts, PhD, an employee of Analysis Group.
APPENDIX A. Drug Codes
| Targeted Therapy | NDC | GPI | CPT/HCPCS |
|---|---|---|---|
| Axitinib | 00069014501, 00069015111 |
21534008000320, 21534008000340 |
|
| Bevacizumab | 50242006001, 50242006002, 50242006101 |
21335020002020, 21335020002025, 21335020002030 |
J9035 |
| Everolimus | 00078056651, 00078056661, 00078056751, 00078056761, 00078059451, 00078059461, 00078062051, 00078062061 |
21532530000320, 21532530000330, 21532530000310, 21532530000325 |
|
| Pazopanib | 00173080409 | 21534070100320 | |
| Sorafenib | 00026848858, 50419048858 |
21533060400320 | |
| Sunitinib | 00069055030, 00069055038, 00069077030, 00069077038, 00069083038, 00069098030, 00069098038, 54569598200, 54569598300 |
21533070300120, 21533070300130, 21533070300135, 21533070300140 |
|
| Temsirolimus | 00008117901 | 21532570002020 | C9239, J9330 |
CPT/HCPCS = Common Procedural Terminology/Healthcare Common Procedure Coding System; GPI = General Product Identifier; NDC = National Drug Code.
APPENDIX B. Diagnosis Codes
| Condition | ICD-9-CM Diagnosis Codes |
|---|---|
| Lung | 197.0x |
| Lymph node | 196.xx |
| Bone | 198.5x |
| Liver | 197.7x |
| Cardiovascular disease | 398.91, 402.11, 402.91, 404.11, 404.13, 404.91, 404.93, 428.xx, 426.10, 426.11, 426.13, 426.2x, 426.3x, 426.4x, 426.50, 426.51, 426.52, 426.53, 426.6x, 426.7x, 426.8x, 427.0x, 427.2x, 427.31, 427.60, 427.9x, 785.0x, V45.0x, V53.3x, 093.2x, 394.xx, 395.xx, 396.xx, 397.0x, 397.1x, 424.0x, 424.1x, 424.2x, 424.3x, 424.90, 424.91, 746.3x, 746.4x, 746.5x, 746.6x, V42.2x, V43.3x, 416.xx, 417.9x, 440.xx, 441.2x, 441.4x, 441.7x, 441.9x, 443.1x, 443.2x, 443.8x, 443.9x, 447.1x, 557.1x, 557.9x, V43.4x |
| Hypertension | 401.1x, 401.9x, 402.10, 402.90, 404.10, 404.90, 405.1x, 405.9x |
| Chronic pulmonary disease | 490.xx, 491.xx, 492.xx, 493.0x, 493.1x, 493.2x, 493.8x, 493.90, 493.91, 494.xx, 495.xx, 496.xx, 500.xx, 501.xx, 502.xx, 503.xx, 504.xx, 505.xx, 506.4x |
| Diabetes | 250.0x, 250.1x, 250.2x, 250.3x, 250.4x, 250.5x, 250.6x, 250.7x, 250.9x |
| Renal failure | 403.11, 403.91, 404.12, 404.92, 585.xx, 586.xx, V42.0x, V45.1x, V56.0x, V56.8x |
| Liver disease | 070.32, 070.33, 070.54, 456.0x, 456.1x, 456.2x, 571.0x, 571.2x, 571.3x, 571.4x, 571.5x, 571.6x, 571.8x, 571.9x, 572.3x, 572.8x, V42.7x |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A.. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30. [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK. Prognostic factors in patients with renal cell carcinoma. In: Richie JP, Atkins MB, Ross ME, eds. UpToDate. Retrieved June 9, 2016. Updated January 1, 2018. Available at: https://www.uptodate.com/contents/prognostic-factors-in-patients-with-renal-cell-carcinoma. Accessed April 19, 2018.
- 3.Surveillance Epidemiology and End Results Program . SEER stat fact sheets: kidney and renal pelvis cancer. October 31, 2016. Available at: http://seer.cancer.gov/statfacts/html/kidrp.html. Accessed April 19, 2018.
- 4.Thompson RH, Ordonez MA, Iasonos A, et al. Renal cell carcinoma in young and old patients—is there a difference? J Urol. 2008;180(4):1262-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam JS, Leppert JT, Belldegrun AS, Figlin RA.. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23(3):202-12. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Bander NH, Nanus DM.. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865-75. [DOI] [PubMed] [Google Scholar]
- 7.Janzen NK, Kim HL, Figlin RA, Belldegrun AS.. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30(4):843-52. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society . Cancer facts & figures 2016. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2016/cancer-facts-and-fig-ures-2016.pdf. Accessed May 3, 2018.
- 9.Atkins MB, Choueiri TK.. Epidemiology, pathology, and pathogenesis of renal cell carcinoma. In: Richie JP, Ross ME, eds. UpToDate. Retrieved June 9, 2016. Updated March 27, 2018. Available at: https://www.uptodate.com/contents/epidemiology-pathology-and-pathogenesis-of-renal-cell-carcinoma. Accessed April 19, 2018.
- 10.Pal SK, Nelson RA, Vogelzang N.. Disease-specific survival in de novo metastatic renal cell carcinoma in the cytokine and targeted therapy era. PloS One. 2013;8(5):e63341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network . Kidney cancer. 2014. Retrieved October 31, 2016. Available at: https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf. Accessed April 19, 2018.
- 12.Randall JM, Millard F, Kurzrock R.. Molecular aberrations, targeted therapy, and renal cell carcinoma: current state-of-the-art. Cancer Metastasis Rev. 2014;33(4):1109-24. [DOI] [PubMed] [Google Scholar]
- 13.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061-68. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115-24. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722-31. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32(14):1412-18. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK.. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib [letter]. N Engl J Med. 2014;370(18):1769-70. [DOI] [PubMed] [Google Scholar]
- 18.Delea TE, Amdahl J, Diaz J, Nakhaipour HR, Hackshaw MD.. Cost-effectiveness of pazopanib versus sunitinib for renal cancer in the United States. J Manag Care Spec Pharm. 2015;21(1):46-54, 54a-b. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2015.21.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedict A, Ramaswamy K, Sandin R.. Cost-effectiveness of pazopanib versus sunitinib for renal cancer in the United States [letter]. J Manag Care Spec Pharm. 2015;21(9):834-40. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2015.21.9.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen RN, Hackshaw MD, Nagar SP, et al. Health care costs among renal cancer patients using pazopanib and sunitinib. J Manag Care Spec Pharm. 2015;21(1):37-44, 44a-d. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2015.21.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racsa PN, Whisman TR, Worley K.. Comparing two tyrosine kinase inhibitors for treatment of advanced renal cell carcinoma in Medicare and commercially insured patients. Curr Med Res Opin. 2015;31(10):1933-40. [DOI] [PubMed] [Google Scholar]
- 22.Byfield SA, McPheeters JT, Burton TM, Nagar SP, Hackshaw MD.. Persistence and compliance among U.S. patients receiving pazopanib or sunitinib as first-line therapy for advanced renal cell carcinoma: a retrospective claims analysis. J Manag Care Spec Pharm. 2015;21(6):515-22. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2015.21.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller LA, Stemkowski S, Saverno K, et al. Patterns of care in patients with metastatic renal cell carcinoma among a U.S. payer population with commercial or Medicare Advantage membership. J Manag Care Spec Pharm. 2016;22(3):219-26. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2016.22.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackshaw MD, Nagar SP, Parks DC, Miller LA.. Persistence and compliance with pazopanib in patients with advanced renal cell carcinoma within a U.S. administrative claims database. J Manag Care Spec Pharm. 2014;20(6):603-10. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2014.20.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollenbeak CS, Nikkel LE, Schaefer EW, Alemao E, Ghahramani N, Raman JD.. Determinants of Medicare all-cause costs among elderly patients with renal cell carcinoma. J Manag Care Spec Pharm. 2011;17(8):610-20. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2011.17.8.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-39. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Bureau of Labor Statistics. Consumer Price Index. Medical care component . 2015. Retrieved October 31, 2016. Available at: https://www.bls.gov/cpi/factsheets/medical-care.htm. Accessed May 3, 2018.
- 28.Vinogradova Y, Coupland C, Brindle P, Hippisley-Cox J.. Discontinuation and restarting in patients on statin treatment: prospective open cohort study using a primary care database. BMJ. 2016;353:i3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogelzang NJ, Pal SK, Ghate SR, et al. Clinical and economic outcomes in elderly advanced renal cell carcinoma patients starting pazopanib or sunitinib treatment: a retrospective medicare claims analysis. Adv Ther. 2017;34(11):2452-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shetty AV, Matrana MR, Atkinson BJ, Flaherty AL, Jonasch E, Tannir NM.. Outcomes of patients with metastatic renal cell carcinoma and end-stage renal disease receiving dialysis and targeted therapies: a single institution experience. Clin Genitourin Cancer. 2014;12(5):348-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLean E, Mardekian J, Cisar LA, Hoang CJ, Harnett J.. Real-world treatment patterns and costs for patients with renal cell carcinoma initiating treatment with sunitinib and pazopanib. J Manag Care Spec Pharm. 2016;22(8):979-90. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2016.22.8.979. [DOI] [PMC free article] [PubMed] [Google Scholar]


