Abstract
BACKGROUND:
Chronic pain is a significant health problem that affects an estimated 100 million American adults (aged ≥ 18 years). Chronic pain affects more individuals than heart disease, stroke, diabetes, and cancer combined. Chronic pain sufferers cost up to $635 billion annually in medical treatment and lost productivity. Opioids are commonly used to treat chronic pain, but their metabolic interactions with concurrently prescribed medications for concomitant disease burdens can affect potency and efficacy of pain therapy. Additionally, misuse of short-acting opioids (SAOs) for chronic pain versus breakthrough pain can create gaps in pain relief. These potentially suboptimal prescribing practices may contribute to the high economic impact associated with chronic pain.
OBJECTIVE:
To examine the prevalence of suboptimal opioid therapy and the associated health care costs resulting from these prescribing practices in real-world patients presenting for all-causes to the emergency department (ED).
METHODS:
This retrospective observational database cohort analysis used the linked Premier-Optum database and included patients with ED visits from 2006 to 2010 having ≥60 days supply of opioids in the 75 days prior to the visit. Suboptimal prescribing practices were identified as patients with (a) drug-drug exposures (DDEs), defined as cytochrome P-450 (CYP-450)-metabolized opioids prescribed concurrently with CYP-450 inhibitors or inducers and/or (b) monotherapy with SAOs. Comorbid conditions and principal diagnoses were documented. Readmission rates to the ED and hospital within 72 hours as well as ≤ 30, ≤ 45, ≤60, and ≤ 90 days were computed. Total costs for health care were calculated, and reimbursement rates were normalized using 2011 Medicare severity diagnosis-related group (MS-DRG) and CPT-4 information. Nonparametric bootstrapping to adjust for patient comorbidities was applied to cost data.
RESULTS:
Of the 9,214 patients identified with chronic pain, potentially suboptimal medication practices prior to the index ED visit were found for 8,539 (92.6%) patients. These appeared to be corrected in 345 (4.0%) patients before leaving the ED. Of 675 (7.3%) patients without prior DDE or exclusive use of SAOs, 345 (51.1%) patients were discharged with one of these. Of the 8,352 patients who left the ED with DDE or exclusive use of SAOs, 1,525 (18.3%) left with a DDE without exclusive SAO use; 4,812 (57.6%) left with both DDE and exclusive SAO use; and 2,015 (24.1%) left with only exclusive SAO use. Only 862 (9.3%) patients from the entire cohort left the ED without DDE or exclusive SAO use. Patients identified with suboptimal opioid use were aged 50 ± 13.5 years and were predominantly female (64.0%). Hypertension (44.0%), fluid and electrolyte disorders (32.7%), chronic pulmonary disease (22.8%), depression (19.6%), diabetes without chronic complications (16.2%), and drug abuse (15.6%) were the most prevalent comorbid conditions identified. The most prevalent principal diagnoses involved symptoms and signs of ill-defined conditions (36.5%), injury and poisoning (18.2%), and diseases of the musculoskeletal system (13.2%). The majority of revisits to the ED and hospital admissions occurred within 72 hours (73.6%) of the index visit and within 30 days (70%), respectively. When adjusted total costs were compared for all patients whose opioid use included DDE versus those without, a significantly greater cost (P < 0.05) was observed at every time period except ≤ 72 hours. Respective mean increases in costs were $581, $689, $773, and $1,275 at 30, 45, 60, and 90 days. Exclusive SAO use with or without DDE resulted in a significant increase (P < 0.05) in mean costs at all times: $214 at 72 hours; $836 at 30 days; $1,023 at 45 days; $1,022 at 60 days; and $1,536 at 90 days.
CONCLUSIONS:
This study identified potentially suboptimal opioid prescribing practices in a real-world population presenting for all-causes to the ED. The observed rate of ED revisits and inpatient admissions in these patients was associated with increased health care costs. These findings suggest that the ED has the future potential to serve as an ideal setting to identify and correct such practices, thereby improving patient care and reducing resource use and beneficiary costs.
What is already known about this subject
Chronic pain is a significant health problem that affects more than 100 million American adults, and chronic pain sufferers are considered to be heavy users of health care services.
Chronic opioid use includes patients being medically managed for chronic conditions such as end-stage chronic obstructive pulmonary disease, osteoarthritis, rheumatoid arthritis, heart failure, and stroke.
Exclusive use of short-acting opioids (SAOs) may predispose the patient to gaps in analgesia and adversely affect the patient experience compared with the appropriate use of controlled-release or long-acting opioids.
What this study adds
Approximately one third of patients having potentially suboptimal medication practices (medications known to interfere with opioid metabolism—drug-drug exposure [DDE]—and exclusive use of SAOs) were more likely to be seen in the ED a second time and in the inpatient setting if a DDE was involved.
Average adjusted total costs for patients with DDE were higher than for patients without DDE. Total costs for patients with exclusive SAOs and DDE were consistently higher.
Patients with exclusive Class-II SAOs, regardless of present DDE, had significantly more ED visits within 72 hours and through time intervals up to 90 days than patients with Class-III SAOs, although odds were higher when accompanied by DDE.
Chronic pain is a significant health problem that affects more than 100 million American adults.1,2 More patients suffer from chronic pain than those with heart disease, stroke, diabetes, and cancer combined.1-3 Chronic pain sufferers are heavy users of health care services, costing the nation up to $635 billion annually in medical treatment and lost productivity.1-4 The true economic impact of chronic pain management is likely underestimated due to the use, misuse, and abuse of opioid analgesics.4 State prescription monitoring programs, in addition to federal programs and policies, have been developed to attempt to address this problem (e.g., Prescription Drug Abuse Prevention Plan [National Survey on Drug Use and Health] and U.S. Food and Drug Administration [FDA] Guidances on Abuse-Deterrent Opioids). Chronic noncancer pain has been defined as pain lasting longer than 3 months or beyond the expected period of healing of tissue pathology,4 although variation in practical application of the definition may exist to varying degrees. Based on clinician input in the design of this study, use of opioids for the majority of a 75-day period through the date of the index emergency department (ED) visit presupposes that persistent chronic pain was present.
Despite well-known serious adverse events of acute respiratory depression, nocturnal hypoventilation, hypogonadism, physical dependence, and abuse, prescriptions for opioids in the treatment of chronic pain syndromes continue to rise disproportionately to the increase in the size of the U.S. population.5 Chronic opioids are commonly used for as-needed pain relief in medically managed chronic conditions such as end-stage chronic obstructive pulmonary disease, osteoarthritis, rheumatoid arthritis, heart failure, and stroke.6 Recognition of inappropriate use of opioids and the consequences of addiction and overdosing is reflected in the recent FDA labeling changes for controlled-release and long-acting (CR/LA) opioids.7,8
Patients taking opioids and medications for concomitant medical conditions (e.g., depression and anxiety) are at increased risk for simultaneous drug-drug exposures (DDEs) and for interactions that can adversely alter opioid metabolism or amplify the adverse effect profile of these analgesics.3,9 Exclusive use of short-acting opioids (SAOs) may predispose the patient to gaps in analgesia, poor sleep hygiene, and adverse effects (namely, nausea and somnolence) compared with the use of CR/LA opioids. In addition to having a higher risk of abuse, Schedule II opioids have practical disadvantages compared with Schedule III opioids. Short-acting formulations of Schedule II and Schedule III opioids may result in different outcomes associated with their perceived analgesic “strength” and the likelihood that chronic or long-lasting pain may not be appropriately controlled due to their short-acting nature.
The magnitude of the problem associated with chronic pain and its treatment is evident in EDs nationwide.10 Often patients without continuity or plans of care rely on the ED for maintenance of their pain management and treatment in times of “flare.” This pattern of ED use, as well as the possibility of hospital admission/readmission, is associated with a staggering price tag. Capitated reimbursements are problematic for health care systems managing these high-cost patients, and the burden of illness is especially high to accountable care organizations (ACOs) and their hospitals. These high costs serve as incentives among third-party and Centers for Medicare & Medicaid Services (CMS) payers to reduce the cost of caring for patients with chronic diseases.
The need to more safely and effectively treat pain is reflected in the 2010 Patient Protection and Affordable Care Act that required the Department of Health and Human Services to enlist the Institute of Medicine to examine pain as a public health problem.11 Additionally, the American Pain Society and the American Academy of Pain Medicine jointly published clinical guidelines to assist in the safe use of chronic opioid therapy in persistent noncancer pain in 2009.9 Because of the paucity of data concerning quality and cost within integrated care/accountable care health system models for chronic pain treatment, we sought to gain insights into opioid use in real-world patients presenting for all-causes to the ED. Specifically, our objectives were to (a) describe and quantify opioid drugs and concomitant medications known to interfere with opioid metabolism, which indicate potentially suboptimal medication practice; (b) describe and quantify comorbid conditions in this patient population; (c) determine the rate of admission for any cause to either the ED or the hospital within 72 hours and up to 90 days following an index ED visit; and (d) determine costs associated with these ED and hospital admissions. We hypothesized that among patients receiving opioids on a chronic basis who present for all-causes to the ED, there is an opportunity to identify and correct potentially suboptimal medication practices via medication reconciliation, which may result in improved patient care and safety, cost, and resource use.
Methods
Study Design and Data Sources
This retrospective observational database cohort analysis used the Premier research database,12 a large U.S. hospital-based, all-payer, comparative database containing information on approximately 6 million annual hospital discharges (approximately one fourth of all hospitalizations in the United States) from primarily nonprofit, nongovernmental community and teaching hospitals, and health systems. Premier data include hospitalizations from more than 600 hospitals for the period 2000 to 2014 from all regions of the United States. In addition to the service-level data recorded in most standard hospital discharge files, the Premier database provides a daily log of all billed items, including procedures, medications, and laboratory tests, as well as diagnostic and therapeutic services at the individual patient level. The Premier data also provide information on demographic characteristics, discharge diagnoses, and discharge status (including death, but not its cause). The data are not identified and comply with the Health Insurance Portability and Accountability Act of 1996.
The Premier database was linked to the Optum Clinformatics database13 to form a single combined dataset for this study. The Optum database contains administrative claims data for an employed, commercially insured population and their dependents enrolled in select health insurance plans affiliated with United Health Group. It is representative of the nonelderly, commercially insured, managed care population of the United States, although it also contains information on several hundred thousand managed Medicaid and Medicare Advantage members. The database consists of aggregated enrollment data and inpatient, outpatient, pharmacy, and laboratory test claims, which when matched to the Premier hospital database permits continuum of care analyses.
Records from the Premier and Optum databases relating to a common hospital discharge were linked by matching inpatient hospitals to health plan hospitals and episodes of care in the two settings, based on hospital identification, patient admission/discharge dates, gender, date of birth, and diagnosis-related group (DRG) or Medicare severity diagnosis-related group (MS-DRG) assignment. The Premier-Optum linked dataset comprises aggregated (longitudinal) enrollment data and inpatient, outpatient, pharmacy, and laboratory test claims from approximately 100,000 patients annually, and it covers a geographically diverse membership. Patients in the linked dataset predominantly present with the same demographic profile as the Optum dataset on its own.
Study Population
Patients aged ≥ 18 years with hospital ED visits between January 2006 and September 2010 were included; the first ED visit for an individual meeting all selection criteria was considered the index visit; and each individual was followed for 90 days thereafter. Patients also had to have filled prescriptions for a minimum 60-day supply of at least one opioid medication within 75 days immediately prior to the index ED visit to be considered a chronic user of opioids as defined by both study design and definition of chronic pain. Comorbid conditions were obtained from current and prior visits via International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and MS-DRG coding.14 ED and inpatient admissions following the index ED visit were identified from claims so as to not be limited to the Premier set of hospitals. Patients with missing demographics, comorbidities, and principal diagnoses were excluded.
Potentially suboptimal opioid medication practices were defined as patients either (a) having used at least one cytochrome P-450 (CYP-450)-metabolized opioid with at least one CYP-450 nonopioid medication matched for opioid substrate, which indicated CYP-450 DDE; or (b) having only SAO agents for chronic pain. The DDE group only was divided into those with long-acting opioids (LAOs) and those with exclusive use of SAO. Thus, four major groups were assessed: (a) no DDE or exclusive use of SAO, (b) DDE only with or without LAO use; (c) DDE with exclusive SAO use; (d) exclusive SAO use and comparison by class as Schedule II and III.
The precedent for identifying DDEs comes from published sources.15 CYP-450 inhibitors and inducers identified for this study were drawn from lists available publicly,16 excluding non-medication dietary items. SAOs included certain formulations of codeine, hydrocodone, hydromorphone, and opioids that are not specifically formulated to be long-acting. LAOs included oxycodone formulated as Oxycontin, morphine as MS-Contin or Kadian, methadone as Dolophine, fentanyl as Duragesic, and oxymorphone as Opana ER. Exclusively used Schedule II and III SAOs were separately compared in acknowledgment of different risk of abuse because of practical considerations in prescribing that may affect costs and outcomes, and because chronic or long-lasting pain that is not appropriately controlled due to the drugs’ short-acting nature could be associated with outcomes differently depending on the Schedule.
Statistical Analysis and Data Assessment
Descriptive analyses were conducted, including baseline demographics and clinical characteristics of the total population and opioid groups. For continuous variables mean, standard deviation, median values, and minimum and maximum values were examined. Analysis of variance was used to determine differences observed between groups. For categorical variables, frequency percentages were examined and chi-square tests used to determine differences between groups.
Rates for all-cause readmission to the ED and hospital admission occurring within 72 hours, 30 days, and up to 90 days were computed as well as total costs. Multivariable hierarchical logistic regression models were developed to account for varying hospital costs while adjusting for 29 comorbid conditions (Table 1) and principal ICD-9-CM codes of the initial ED visit. Models were developed for each of the following time periods: ≤ 72 hours, ≤ 30 days, ≤ 45 days, ≤ 60 days, and ≤ 90 days. These analyses were replicated for comparing the exclusive SAO Class II (C-II) and Class III (C-III) subgroups. These adjusted analyses computed odds ratios (ORs) and 95% confidence intervals (CIs) for the likelihood of returning to the ED or being admitted to the hospital following the index ED visit.
TABLE 1.
Patient Characteristics
| Total (N = 9,214) | No DDE or Exclusive SAO Use (n = 862) | Exclusive SAO Use Only (n = 2,015) | DDE Only (n = 1,525) | DDE + Exclusive SAO Use (n = 862) | P Valuea | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years, mean (SD) | 50.0 (13.5) | 49.9 (14.7) | 49.1 (13.6) | 49.3 (12.5) | 50.6 (13.5) | < 0.001 |
| Gender, n (%) | < 0.001 | |||||
| Female | 5,893 (64.0) | 465 (53.9) | 1,196 (59.4) | 922 (60.5) | 3,310 (68.8) | |
| Male | 3,321 (36.0) | 397 (46.1) | 819 (40.6) | 603 (39.5) | 1,502 (31.2) | |
| Payer, n (%) | < 0.001 | |||||
| Medicaid | 633 (6.9) | 32 (3.7) | 145 (7.2) | 74 (4.9) | 382 (7.9) | |
| Commercial | 8,581 (93.1) | 830 (96.3) | 1,870 (92.8) | 1,451 (95.1) | 4,430 (92.1) | |
| Census region, n (%) | < 0.001 | |||||
| East North Central | 1,416 (15.4) | 141 (16.4) | 328 (16.3) | 223 (14.6) | 724 (15.0) | |
| East South Central | 363 (3.9) | 25 (2.9) | 90 (4.5) | 41 (2.7) | 207 (4.3) | |
| Middle Atlantic | 385 (4.2) | 56 (6.5) | 82 (4.1) | 63 (4.1) | 184 (3.8) | |
| Mountain | 588 (6.4) | 63 (7.3) | 129 (6.4) | 93 (6.1) | 303 (6.3) | |
| New England | 76 (0.8) | 11 (1.3) | 15 (0.7) | 11 (0.7) | 39 (0.8) | |
| Pacific | 495 (5.4) | 51 (5.9) | 97 (4.8) | 92 (6.0) | 255 (5.3) | |
| South Atlantic | 3,078 (33.4) | 294 (34.1) | 710 (35.2) | 537 (35.2) | 1,537 (31.9) | |
| West North Central | 1,601 (17.4) | 128 (14.8) | 305 (15.1) | 286 (18.8) | 882 (18.3) | |
| West South Central | 1,211 (13.1) | 93 (10.8) | 259 (12.9) | 179 (11.7) | 680 (14.1) | |
| Comorbidities, n (%) | ||||||
| AIDS | 35 (0.4) | 1 (0.1) | 4 (0.2) | 6 (0.4) | 24 (0.5) | 0.162 |
| Alcohol abuse | 638 (6.9) | 72 (8.4) | 123 (6.1) | 84 (5.5) | 359 (7.5) | 0.008 |
| Coagulopathy | 524 (5.7) | 59 (6.8) | 93 (4.6) | 100 (6.6) | 272 (5.7) | 0.035 |
| Congestive heart failure | 822 (8.9) | 81 (9.4) | 162 (8.0) | 123 (8.1) | 456 (9.5) | 0.147 |
| Chronic blood loss anemia | 270 (2.9) | 26 (3.0) | 56 (2.8) | 40 (2.6) | 148 (3.1) | 0.790 |
| Chronic pulmonary disease | 2,097 (22.8) | 193 (22.4) | 379 (18.8) | 365 (23.9) | 1,160 (24.1) | < 0.001 |
| Depression | 1,804 (19.6) | 159 (18.4) | 273 (13.5) | 372 (24.4) | 1,000 (20.8) | < 0.001 |
| Diabetes with chronic complications | 468 (5.1) | 44 (5.1) | 54 (2.7) | 91 (6.0) | 279 (5.8) | < 0.001 |
| Diabetes w/o chronic complications | 1,493 (16.2) | 108 (12.5) | 270 (13.4) | 253 (16.6) | 862 (17.9) | < 0.001 |
| Deficiency anemia | 1,159 (12.6) | 101 (11.7) | 198 (9.8) | 226 (14.8) | 634 (13.2) | < 0.001 |
| Drug abuse | 1,436 (15.6) | 167 (19.4) | 249 (12.4) | 316 (20.7) | 704 (14.6) | < 0.001 |
| Fluid and electrolyte disorders | 3,015 (32.7) | 256 (29.7) | 567 (28.1) | 547 (35.9) | 1,645 (34.2) | < 0.001 |
| Hypertension | 4,050 (44.0) | 336 (39.0) | 812 (40.3) | 640 (42.0) | 2,262 (47.0) | < 0.001 |
| Hypothyroidism | 818 (8.9) | 71 (8.2) | 138 (6.8) | 150 (9.8) | 459 (9.5) | 0.002 |
| Liver disease | 434 (4.7) | 41 (4.8) | 84 (4.2) | 76 (5.0) | 233 (4.8) | 0.624 |
| Lymphoma | 100 (1.1) | 7 (0.8) | 17 (0.8) | 24 (1.6) | 52 (1.1) | 0.166 |
| Metastatic cancer | 432 (4.7) | 51 (5.9) | 74 (3.7) | 95 (6.2) | 212 (4.4) | < 0.001 |
| Obesity | 1,157 (12.6) | 113 (13.1) | 233 (11.6) | 181 (11.9) | 630 (13.1) | 0.265 |
| Other neurologic disorders | 906 (9.8) | 96 (11.1) | 148 (7.3) | 169 (11.1) | 493 (10.2) | < 0.001 |
| Paralysis | 248 (2.7) | 35 (4.1) | 41 (2.0) | 58 (3.8) | 114 (2.4) | < 0.001 |
| Peptic ulcer disease | 18 (0.2) | 0 (0.0) | 2 (0.1) | 4 (0.3) | 12 (0.2) | 0.294 |
| Peripheral vascular disease | 308 (3.3) | 32 (3.7) | 55 (2.7) | 56 (3.7) | 165 (3.4) | 0.343 |
| Psychoses | 1,028 (11.2) | 93 (10.8) | 153 (7.6) | 205 (13.4) | 577 (12.0) | < 0.001 |
| Pulmonary circulation disease | 282 (3.1) | 39 (4.5) | 46 (2.3) | 46 (3.0) | 151 (3.1) | 0.015 |
| Renal failure | 460 (5.0) | 36 (4.2) | 68 (3.4) | 75 (4.9) | 281 (5.8) | < 0.001 |
| Rheumatoid arthritis/collagen vas | 559 (6.1) | 37 (4.3) | 67 (3.3) | 102 (6.7) | 353 (7.3) | < 0.001 |
| Solid tumor without metastasis | 350 (3.8) | 34 (3.9) | 61 (3.0) | 74 (4.9) | 181 (3.8) | 0.046 |
| Valvular heart disease | 472 (5.1) | 48 (5.6) | 89 (4.4) | 74 (4.9) | 261 (5.4) | 0.315 |
| Weight loss | 593 (6.4) | 53 (6.1) | 97 (4.8) | 114 (7.5) | 329 (6.8) | 0.005 |
| Primary diagnosis category, n (%) | ||||||
| Complications of pregnancy | 31 (0.3) | 6 (0.7) | 15 (0.7) | 2 (0.1) | 8 (0.2) | < 0.001 |
| Congenital anomalies | 2 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.0) | 0.742 |
| Diseases of the blood and organs | 56 (0.6) | 7 (0.8) | 17 (0.8) | 7 (0.5) | 25 (0.5) | 0.304 |
| Diseases of the circulatory system | 301 (3.3) | 31 (3.6) | 70 (3.5) | 43 (2.8) | 157 (3.3) | 0.674 |
| Diseases of the digestive system | 442 (4.8) | 37 (4.3) | 94 (4.7) | 68 (4.5) | 243 (5.0) | 0.656 |
| Diseases of the genitourinary system | 341 (3.7) | 22 (2.6) | 76 (3.8) | 65 (4.3) | 178 (3.7) | 0.206 |
| Diseases of the musculoskeletal system | 1,213 (13.6) | 132 (15.3) | 315 (15.6) | 200 (13.1) | 566 (11.8) | < 0.001 |
| Diseases of the nervous system | 453 (4.9) | 34 (3.9) | 92 (4.6) | 90 (5.9) | 237 (4.9) | 0.142 |
| Diseases of the respiratory system | 455 (4.9) | 49 (5.7) | 78 (3.9) | 83 (5.4) | 245 (5.1) | 0.073 |
| Diseases of the skin | 244 (2.6) | 26 (3.0) | 41 (2.0) | 40 (2.6) | 137 (2.8) | 0.247 |
| Endocrine metabolic/immune disorders | 201 (2.2) | 21 (2.4) | 30 (1.5) | 34 (2.2) | 116 (2.4) | 0.112 |
| Infectious and parasitic diseases | 114 (1.2) | 12 (1.4) | 28 (1.4) | 19 (1.2) | 55 (1.1) | 0.825 |
| Injury and poisoning | 1,681 (18.2) | 151 (17.5) | 397 (19.7) | 267 (17.5) | 866 (18.0) | 0.269 |
| Mental illness | 252 (2.7) | 38 (4.4) | 39 (1.9) | 55 (3.6) | 120 (2.5) | < 0.001 |
| Neoplasms | 19 (0.2) | 0 (0.0) | 3 (0.1) | 2 (0.1) | 14 (0.3) | 0.241 |
| Symptoms/signs ill-defined conditions | 3,359 (36.5) | 287 (33.3) | 709 (35.2) | 538 (35.3) | 1,825 (37.9) | 0.015 |
| Other | 50 (0.5) | 9 (1.0) | 10 (0.5) | 12 (0.8) | 19 (0.4) | 0.052 |
aContinuous variables compared using one-way analysis of variance; categorical variables compared using chi-square test. Values in bold signify statistical significance with P < 0.05.
AIDS = acquired immune deficiency syndrome; DDE = drug-drug exposure; SAO = short-acting opioid; SD = standard deviation.
Total costs for health care over a defined period following the index ED visit that were associated with return to the ED or in-hospital readmissions were calculated as the sum of costs to the payer and out of the pocket of the patient. Commercially insured patients’ reimbursement rates were normalized to the Medicare fee schedule after applying the national daily average for MS-DRG and American Medical Association Common Procedural Terminology (CPT) reimbursement rates for 2011. Estimated inpatient hospital costs were calculated by multiplying the length of stay by the average Medicare daily reimbursement rate (average reimbursement rate ÷ average length of stay = average daily reimbursement). All-cause physician office visits, ED visits, and other facility charges were assigned costs by using national Medicare CPT reimbursement rates. Inpatient medication costs from the Premier hospital database and medication copay amounts from the Optum data were used for prescription costs. Patients with total costs above the 99th percentile were trimmed to eliminate outliers.17
To account for variation in costs across hospitals, multivariable mixed regression models were developed with a hierarchical modeling structure using hospital site as a random effect. Additional covariates included the 29 comorbid conditions and the primary ICD-9-CM diagnosis code from the index ED visit. Nonparametric bootstrapping was applied using 1,000 replicates with replacement to compare arithmetic means for costs between groups while accounting for the skewness in the cost data.17,18 Median, 2.5%, and 97.5% estimates were used to determine the average cost and 95% CIs, respectively. Where the 95% CI of the difference between comparison group costs did not include zero (no difference), the difference was determined to be statistically significant.
All data aggregation and statistical analysis were performed using WinSQL (Synametrics Technologies, Inc., Plainview, NJ) and SAS version 9.3 (SAS Institute, Cary, NC). Statistical significance was defined as P ≤ 0.05.
Results
Overall Study Population
Of 379,614 patients with available data, 9,214 patients fit all inclusion criteria (Figure 1). Potentially suboptimal medication practices prior to the index ED visit were found for 8,539 (92.6%) patients. These treatments appeared to be corrected in 345 (4.0%) patients before they left the ED. Of 675 (7.3%) patients without prior DDE or exclusive use of SAOs, 345 (51.1%) patients were discharged with a DDE or with SAOs but no LAOs. A total of 8,352 (90.6%) left the ED with a DDE or exclusive SAOs, and 862 (9.4%) left without either. Of these 8,352 patients, 1,525 (18.3%) left with a DDE without exclusive SAO use; 4,812 (57.6%) left with both DDE and exclusive SAO use (3,683, C-II opioids; 1,129, C-III opioids); and 2,015 (24.1%) left with only exclusive SAO use (1,557, C-II opioids; 458, C-III opioids).
FIGURE 1.
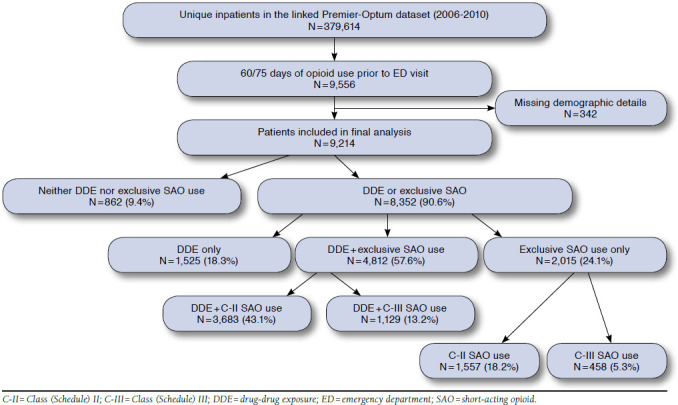
Schematic of the Study Population
Patient Population Characteristics
Patients in the overall study population were aged 50.0 ± 13.5 (mean ± SD) years, and were predominantly female (64.0%), commercially insured (93.1%), and mostly treated in hospitals in the South and North Central United States (Table 1). Hypertension (44.0%), fluid and electrolyte disorders (32.7%), chronic pulmonary disease (22.8%), depression (19.6%), diabetes without chronic complications (16.2%), and drug abuse (15.6%) were the most prevalent of the 29 comorbid conditions identified (Table 1). The most prevalent primary diagnoses (Table 1) involved symptoms and signs of ill-defined conditions (36.5%), injury and poisoning (18.2%), and diseases of the musculoskeletal system (13.6%). Demographics, payer status, hospital census regions, comorbidities, and primary diagnoses for all opioid use groups were similar to that observed for the overall study population (Table 1).
Emergency Department Revisits and Inpatient Admissions
Overall Study Population.
Of 9,214 patients, 14% (1,293) returned within 72 hours to the ED, 16.6% (1,531) within 30 days, 17.3% (1,597) within 45 days, 18.0% (1,661) within 60 days, and 19.0% (1,754) within 90 days. Inpatient admissions for the same respective time periods were 12.4% (1,146), 23.5% (2,167), 27.1% (2,497), 30.4% (2.802), and 33.6% (3,096). For the total population, the majority of revisits to the ED and the majority of hospitalizations occurred within 72 hours (73.6%) of the index visit and within 30 days (70%), respectively. A similar pattern for ED revisits at 72 hours and inpatient admissions at 30 days was observed for all opioid medication groups.
Without DDE.
As shown in Table 2 and Appendix A (available in online article), 10.3% of patients whose opioid use did not include DDE or exclusive SAO use returned to the ED within 72 hours. A small increase in returns from 13.2% to 15.4% was observed between 30 and 90 days. With exclusive SAO use, 13.7% of patients returned to the ED within 72 hours, increasing steadily throughout 90 days to 18.0%. When these two opioid use groups were compared, more ED revisits were observed with exclusive SAO use at 72 hours (P = 0.013) and at 60 days (P = 0.043). Although significance was not reached at the other time points, a trend toward more ED revisits among exclusive SAO patients than among other patients was observed (P = 0.090-0.099). The adjusted odds of a return visit within 45 days and within 90 days were about 1.4 times greater for exclusive SAO patients (Figure 2). Similar ORs were observed for other intervals but only approached statistical significance (P > 0.05).
TABLE 2.
Revisits to Emergency Department and Inpatient Hospital Admissions by Time Period in Patients Without DDE and with DDE
| Total Population N (%) | Neither DDE nor Exclusive SAO n (%) | Exclusive SAO n (%) | P Value | Exclusive C-II SAO, n (%) | Exclusive C-III SAO, n (%) | P Valuea | |
|---|---|---|---|---|---|---|---|
| Without DDE | |||||||
| Emergency department visits | |||||||
| ≤ 72 hours | 365 (12.7) | 89 (10.3) | 276 (13.7) | 0.013 | 228 (14.6) | 48 (10.5) | 0.023 |
| ≤ 30 days | 430 (14.9) | 114 (13.2) | 316 (15.7) | 0.090 | 261 (16.8) | 55 (12.0) | 0.014 |
| ≤ 45 days | 450 (15.6) | 120 (13.9) | 330 (16.4) | 0.097 | 274 (17.6) | 56 (12.2) | 0.006 |
| ≤ 60 days | 472 (16.4) | 123 (14.3) | 349 (17.3) | 0.043 | 288 (18.5) | 61 (13.3) | 0.010 |
| ≤ 90 days | 495 (17.2) | 133 (15.4) | 362 (18.0) | 0.099 | 301 (19.3) | 61 (13.3) | 0.003 |
| Inpatient admissions | |||||||
| ≤ 72 hours | 321 (11.2) | 129 (15.0) | 192 (9.5) | < 0.001 | 150 (9.6) | 42 (9.2) | 0.766 |
| ≤ 30 days | 578 (20.1) | 223 (25.9) | 355 (17.6) | < 0.001 | 281 (18.0) | 74 (16.2) | 0.351 |
| ≤ 45 days | 668 (23.2) | 246 (28.5) | 422 (20.9) | < 0.001 | 335 (21.5) | 87 (19.0) | 0.244 |
| ≤ 60 days | 736 (25.6) | 263 (30.5) | 473 (23.5) | < 0.001 | 375 (24.1) | 98 (21.4) | 0.233 |
| ≤ 90 days | 823 (28.6) | 292 (33.9) | 531 (26.4) | < 0.001 | 432 (27.7) | 99 (21.6) | 0.009 |
| With DDE | |||||||
| Emergency department visits | |||||||
| ≤ 72 hours | 928 (14.6) | 214 (14.0) | 714 (14.8) | 0.438 | 596 (16.2) | 118 (10.5) | < 0.001 |
| ≤ 30 days | 1,101 (17.4) | 262 (17.2) | 839 (17.4) | 0.819 | 692 (18.8) | 147 (13.0) | < 0.001 |
| ≤ 45 days | 1,147 (18.1) | 275 (18.0) | 872 (18.1) | 0.938 | 715 (19.4) | 157 (13.9) | < 0.001 |
| ≤ 60 days | 1,189 (18.8) | 286 (18.8) | 903 (18.8) | 0.992 | 740 (20.1) | 163 (14.4) | < 0.001 |
| ≤ 90 days | 1,259 (19.9) | 305 (20.0) | 954 (19.8) | 0.882 | 781 (21.2) | 173 (15.3) | < 0.001 |
| Inpatient admissions | |||||||
| ≤ 72 hours | 825 (13.0) | 189 (12.4) | 636 (13.2) | 0.405 | 475 (12.9) | 161 (14.3) | 0.237 |
| ≤ 30 days | 1,589 (25.1) | 379 (24.9) | 1,210 (25.1) | 0.818 | 949 (25.8) | 261 (23.1) | 0.073 |
| ≤ 45 days | 1,829 (28.9) | 446 (29.2) | 1,383 (28.7) | 0.704 | 1,096 (29.8) | 287 (25.4) | 0.005 |
| ≤ 60 days | 2,066 (32.6) | 520 (34.1) | 1,546 (32.1) | 0.153 | 1,234 (33.5) | 312 (27.6) | < 0.001 |
| ≤ 90 days | 2,273 (35.9) | 565 (37.0) | 1,708 (35.5) | 0.270 | 1,369 (37.2) | 339 (30.0) | < 0.001 |
aValues in bold signify statistical significance with P < 0.05.
C-II = Class (Schedule) II; C-III-Class (Schedule) III; DDE = drug-drug exposure; SAO = short-acting opioid.
FIGURE 2.
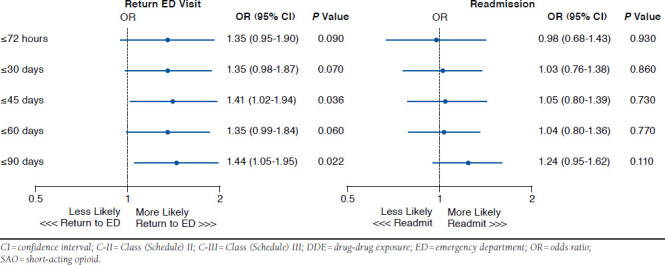
Exclusive C-II SAO Versus Exclusive C-III SAO Without DDE
As shown in Table 2 and Appendix B (available in online article), at ≤72 hours, hospital admissions for patients with no DDE or exclusive use of SAO were 15%, reaching 25.9% by 30 days and 28.5% by 45 days. Between 60 days (30.5%) and 90 days (33.9%), the rates of admission were twice that observed by 72 hours. With exclusive SAO use, inpatient admissions at ≤ 72 hours were 9.5%. The percent of hospitalizations nearly doubled and tripled through 30 days (17.6%) and 90 days (26.4%), respectively. Compared with no DDE or exclusive SAO use, the rate of inpatient admissions for exclusive SAOs was significantly lower (P ≤0.001) at every time period assessed. The adjusted odds of a readmission were not significantly different for exclusive C-II SAO patients without DDEs, regardless of the time interval (Figure 2).
Exclusive C-II SAO use in the absence of DDE had significantly higher rates for return ED visits than exclusive C-III SAO without DDE within every time period (P < 0.05; Table 2). Patients from these groups were not more likely to be hospitalized between 72 hours and 60 days. By 90 days, more admissions were noted with exclusive C-II SAO use than with exclusive C-III SAO use (27.7% vs. 21.6%; P = 0.009; Table 2).
With DDE.
As depicted in Table 2 and Appendix C (available in online article), at ≤72 hours, 14.0% of patients with only DDE returned to the ED. Within 30, 45, 60, and 90 days, the rate of ED visits was 17.2%, 18.0%, 18.8%, and 20%, respectively. When compared with this group, rates of return to the ED were nearly identical for DDE patients with exclusive SAOs at every time point (P > 0.05).
As depicted in Table 2 and Appendix D (available in online article), inpatient admissions for DDE were 12.4% within 72 hours. This increased to 24.9% at 30 days and to 29.2% at 45 days. Within 60 and 90 days, 34.1% and 37.0% respective hospitalizations were observed. DDE with exclusive SAO use had similar rates of admissions to the DDE-only group (P > 0.05).
In the presence of a DDE, 16.2% of patients with exclusive C-II SAO use returned to the ED by 72 hours (Table 2 and Appendix C). Within 90 days, this rate increased to 21.2%. For those with DDE and exclusive C-III SAO use, return visits to the ED ranged between 10.5% and 15.3% within 72 hours and 90 days. At every time point assessed, the rate of revisits was significantly greater with DDE and exclusive C-II SAO use (P < 0.001). The adjusted odds of returning to the ED among these patients was 1.37-1.50 times greater than for exclusive C-II SAO patients (all P ≤ 0.002; Figure 3).
FIGURE 3.
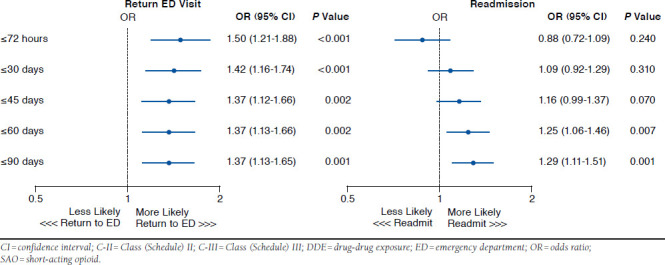
Exclusive C-II SAO Versus Exclusive C-III SAO with DDE
The rate of inpatient readmissions was similar between these DDE and exclusive SAO groups at 72 hours and 30 days (Table 2 and Appendix D). Hospitalizations with DDE and exclusive C-II SAO use versus DDE and exclusive C-III SAO use were significantly greater at 45 days (29.8% vs. 25.4%, P = 0.005), 60 days (33.5% vs. 27.6%, P < 0.001), and 90 days (37.2% vs. 30.0%, P < 0.001). The adjusted odds of readmission varied between 0.88 and 1.29 times greater for exclusive C-II SAO patients, but were only significantly different at 60 and 90 days (OR = 1.25, P = 0.007; and OR = 1.29, P = 0.001, respectively; Figure 3).
Total Risk-Adjusted Costs
When total adjusted costs were compared for all patients whose opioid use included DDE versus no DDE (Appendix E, available in online article), a significantly greater average cost (P < 0.05) was observed only after 72 hours. The mean increases in costs (95% CI) were $581 (95% CI = $193-$972), $689 (95% CI = $173-$1,164), $773 (95% CI = $189-$1,328), and $1,275 (95% CI = $559-$1,994) at 30 days, 45 days, 60 days, and 90 days. Exclusive SAO use in the absence and presence of DDE (Appendix F, available in online article) resulted in a significant increase (P < 0.05) in mean costs (95% CI) at all times: $214 (95% CI = $22-$395) at 72 hours; $836 (95% CI = $403-$1,257) at 30 days; $1,023 (95% CI = $486-$1,554) at 45 days; $1,022 (95% CI = $313-$1,665) at 60 days; and $1,536 (95% CI = $658-$2,311) at 90 days. Comparisons between exclusive C-II versus C-III SAO use in the overall patient population, as well as in the absence and presence of a DDE, revealed a significant increase (P < 0.05) in costs between 30 and 90 days but not at 72 hours (Appendix G, available in online article), which was consistent across all group comparisons.
Discussion
The analysis presented reports extremely high rates (93%) of potentially suboptimal opioid prescribing practices in patients presenting to the ED. Both DDE and exclusive use of SAOs were identified in half (52%) of all patients. Almost 16% of the population had only DDEs, and 22% had only SAOs. Of patients treated exclusively with SAOs, three times as many Schedule II SAOs were prescribed as Class III SAOs. No beneficial changes to these reported potentially suboptimal prescribing practices were affected by hospital contact. Furthermore, these theorized suboptimal practices were associated with ED readmissions or inpatient admissions, and increased costs and poorer performance on resource use were observed.
Risk of drug interactions with an opioid is largely determined by the metabolizing enzyme system. Opioid metabolism primarily occurs in the liver through Phase 1 oxidation reactions involving the CYP-450 enzyme system and through Phase 2 glucuronidation by uridine diphosphate glucuronosyltransferases.19,20 The FDA now requires information regarding a drug’s CYP-450 metabolism and its potential for inhibition or induction to be on the drug label.20 The degree to which a patient responds to a drug is determined by genetic variability of drug metabolism20,21 as well as demographic and medical factors.18 These interactions may result in either reduced efficacy or increased toxicity of the target analgesic in question.
Use of SAOs to treat chronic pain either alone or in combination with CYP-450 metabolized opioids contributed significantly to the identified suboptimal prescribing practices observed in this study. SAOs had the greatest association with return visits to the ED and hospital admissions. When further assessed by class, patients treated with Schedule II opioids incurred higher costs and had significantly more visits to the ED across all time periods, possibly due to their seeking drugs. In the presence of DDE, more revisits to the ED and hospital occurred at all time points except 72 hours. While clear guidelines on optimal prescribing are lacking, CR/LA opioids are frequently used in patients requiring longer treatment durations. Anecdotally, SAOs result in larger risk for analgesic gaps and a higher risk for aberrant drug-taking behaviors. While SAOs are commonly prescribed, these agents are frequently considered appropriate for acute, breakthrough, or chronic intermittent pain.3,22 However, many clinicians use them around the clock to relieve chronic persistent pain due to ease in prescribing, perceived lower cost, or lack of comfort with managing CR/LA opioids.3 Many are available in combination with acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID), which limits the maximum daily dose due to the risk of hepatic, renal, or gastrointestinal toxicity.3 Nearly all of the SAOs used by this study population were CYP-450 substrates. The vast majority was fixed-dose combinations of SAOs with acetaminophen.
Although medication reconciliation was not part of the major study objectives, 4% of patients did receive new prescriptions for opioid medication at the time of ED discharge. Lack of corrective practices underscores the need to optimize opioid dosing to eliminate the risk that is associated with inappropriate use of opioid medications. Multidisciplinary approaches have been suggested and include both patients and health care practitioners.24,25 A recent survey of clinicians revealed differences among disciplines and specialties on impediments and concerns of prescribing, perceived effectiveness, Schedule II versus Schedule III selection, professional education, and understanding of tamper-resistant opioid formulations.26
It is well documented that patients requiring chronic opioid analgesia with CYP-450 DDEs/interactions impact adversely on health care costs and resource use. During a six-month observational period, patients at risk for developing a pharmacokinetic drug-drug interaction had higher medical and pharmacy costs than their matched controls without DDE.27 In this study, results did not examine the type of CYP-450 enzyme or whether the interaction involved exposure to substrates, inhibitors, or inducers. The increased costs resulted from a higher number of office and outpatient visits as well as ED visits and inpatient hospitalizations, the subject of this report. In a study of a managed care database that assessed health care use and costs of patients on chronic opioid therapy, a one-year follow-up revealed that chronic opioid users had more ambulatory, emergency, and hospital visits and higher annual costs than their nonuser matched controls.28 Total health care costs more than quadrupled, with respective medical and pharmacy costs noted to be about 5 and 3.5 times greater. Moreover, patients exhibiting nonadherence with chronic opioids identified by urine drug monitoring had more hospital days and were 14% more expensive compared with adherent patients. Unlike our study, this analysis did not specifically relate health care burden to suboptimal opioid prescribing practices.
Readmission rates were assessed at ≤72 hours and from 30 to 90 days. Readmissions prior to 72 hours may not bring additional reimbursement to the health care provider, so this is important from the hospital finance perspective. Medicare has previously proposed calculating the costs by combining a patient’s hospital expenses with fees incurred up to 90 days after discharge, and previous studies have also used 90 days as a cut-off point.29 This study considered costs at several intervals up to 90 days to ensure that variation in patients’ access to primary care physicians and clinics, to local and mail-order prescription refills, and to other unexpected resource use would be captured.
ACOs provide an opportunity to promote responsible pain management and reduce costs in chronic pain sufferers. The Joint Commission recently released several Sentinel Event Alerts specifically noting the importance of both medication reconciliation and the safe use of opioids in hospitals. While medication reconciliation is defined by the CMS as “the process of identifying the most accurate list of all medications that the patient is taking, including name, dosage, frequency, and route, by comparing the medical record to an external list of medications obtained from a patient, hospital or other provider,”30 interpretation of this data and identification of shortfalls of optimal therapy may be beyond traditional medication reconciliation.31,32 This role would be ideally suited for clinically trained pharmacists within either the ACO environment or via third-party payer drug utilization review.32 These pharmacists often serve on pain management teams, are familiar with computerized surveillance systems, and are experienced in the development of disease management optimization programs.33 The financial barriers associated with dedicating a clinical pharmacy specialist to providing medication therapy management recommendations real-time during the medication reconciliation process may be insurmountable, but they are potentially overcome by the cost data presented here.34
This study represents a real-world population providing data from all geographical areas of the United States with significant representation from South and North Central regions. Four groups of opioid medication prescribing patterns were identified and highlight ongoing multiple suboptimal prescribing practices by providers. These statistical analyses include generalized linear models for cost analysis, which help account for differences in patient and hospital characteristics between comparison groups, and the use of bootstrap analysis for comparing arithmetic mean costs.
Limitations
Use of the Optum database represents only commercially insured patients and their dependents aged 18-64 years. Medicare Advantage patient data were not available for this study. In addition, the lack of uninsured or state Medicaid-covered patients likely skews the presented data more favorably than is actually realized in the real-world health system setting. Given the nature of medical claims data, it is unclear whether observed changes to potentially suboptimal opioid practices were physician directed or the result of patient nonadherence. Potential for miscoded or missing data and the inability to control for uncaptured elements such as outpatient over-the-counter analgesics (aspirin, acetaminophen, NSAIDs) may influence outcomes. Associations with DDEs and/or exclusive SAOs are evaluated at the population level rather than on an individual patient level, so extrapolation to any individual patient prospectively might be problematic.
Costs were estimated using average 2011 MS-DRG payment amounts, so because amounts in prior years might have been less, the use of the 2011 amounts potentially produces values higher than were necessarily true. Readmissions from all-causes are not necessarily due to opioid medication, but this is indicative of the population-level view taken by the analysis. Reliance on pharmacy claims data assumes that patients are adherent with their medications for the full course of their prescriptions, although their actual duration of treatment and levels of day-to-day adherence are unknown. This limitation is offset by evidence that outpatient pharmaceutical claims data provide a more precise measure of patient medication use than self-reported medication use. Finally, this study presents statistical associations, not “cause and effect.”
Since the design and completion of this study, the DEA has rescheduled hydrocodone combination medications from C-III to C-II. Although dosage of hydrocodone in these combination products is lower, its mechanism of action is identical to oxycodone and morphine.35 Its reclassification highlights the problem of drug abuse and dependence and the need for more restrictive prescribing practices. While our study includes hydrocodone combination medications as Schedule III, the health care outcomes and associated costs of C-II SAOs to treat chronic pain are significant and remain relevant and pertinent to opioid use practices.
Conclusions
This study represents a retrospective observational analysis of the occurrence and prevalence of potentially suboptimal opioid medication prescribing and the cost implications associated with these practices. The in-depth opioid drug review demonstrates the magnitude of the problem and the need for targeted medication reconciliation and education. EDs provide an ideal setting to uncover these practices and initiate action that could include medication reconciliation and therapy recommendations by clinical pharmacy specialists. This resultant practice should reduce medication errors and admissions, improve patient outcomes, and be cost-effective for ACOs and other health care payers.
Acknowledgments
The authors would like to thank Ann Hartry for her contributions to the initial design of portions for this study; Teresa Davis and Julie Gayle for their contributions to the creation of datasets used in the analysis; and Carol Cohen for review and editorial assistance.
APPENDIX A. Revisits to Emergency Department by Time Period in Patients Without DDE
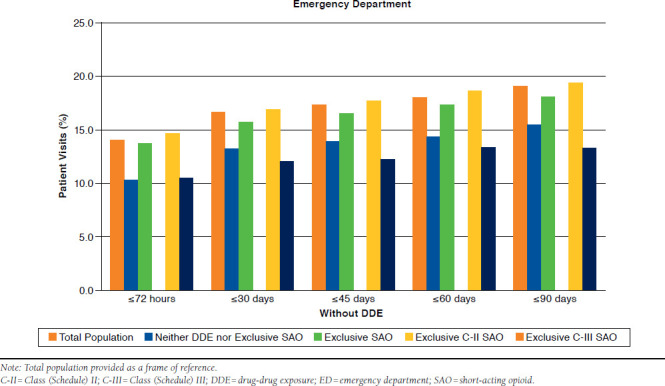
APPENDIX B. Inpatient Hospitalizations by Time Period in Patients Without DDE
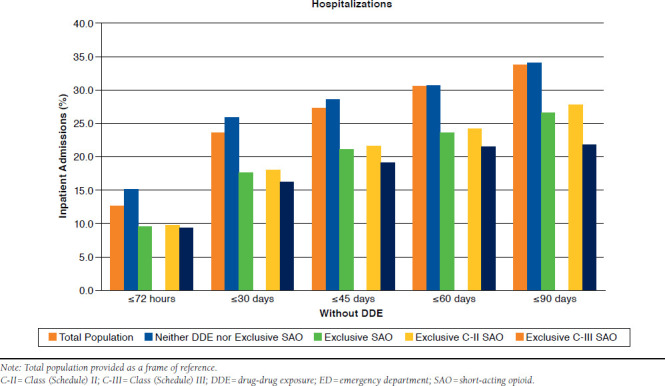
APPENDIX C. Revisits to Emergency Department by Time Period in Patients with DDE
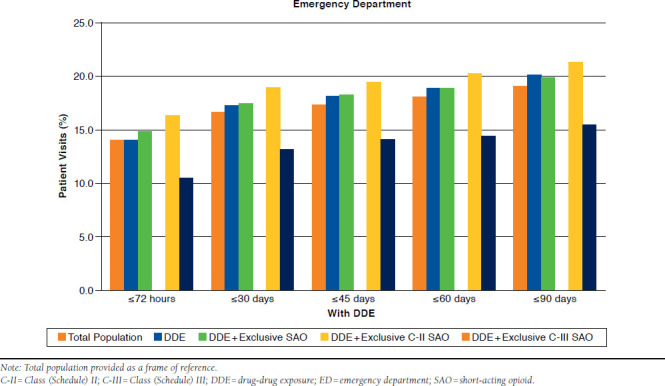
APPENDIX D. Inpatient Hospitalizations by Time Period in Patients with DDE
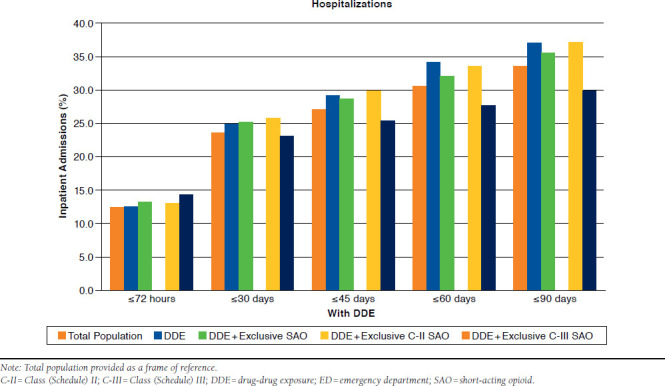
APPENDIX E. Total Adjusted Costs: No DDE Versus DDE
| Time | No DDE n = 2,877 Mean (SD) $ | DDE n = 6,337 Mean (SD) $ | Differencea Mean (95% CI) $ |
| ≤ 72 hours | 2,345 (4,475) | 2,520 (4,129) | 91 (-106-266) |
| ≤ 30 days | 4,946 (8,477) | 5,925 (9,378) | 581 (193-972)b |
| ≤ 45 days | 6,208 (11,060) | 7,452 (11,439) | 689 (173-1,164)b |
| ≤ 60 days | 7,380 (13,141) | 8,912 (13,226) | 773 (189-1,328)b |
| ≤ 90 days | 9,439 (16,218) | 11,818 (16,839) | 1,275 (559-1,994)b |
aBootstrapped analyses.
bP < 0.05.
CI = confidence interval; DDE = drug-drug exposure; SD = standard deviation.
APPENDIX F. Total Adjusted Costs Among Exclusive SAO Patients: No DDE Versus DDE
| Time | Exclusive SAO Without DDE n = 2,015 Mean (SD) $ | Exclusive SAO with DDE n = 4,812 Mean (SD) $ | Differencea Mean (95% CI) $ |
|---|---|---|---|
| ≤ 72 hours | 2,172 (3,309) | 2,492 (4,119) | 214 (22-395)b |
| ≤ 30 days | 4,493 (7,600) | 5,823 (9,458) | 836 (403-1,257)b |
| ≤ 45 days | 5,637 (9,744) | 7,342 (11,698) | 1,023 (468-1,554)b |
| ≤ 60 days | 6,785 (12,184) | 8,710 (13,428) | 1,022 (313-1,665)b |
| ≤ 90 days | 8,723 (14,811) | 11,565 (17,350) | 1,536 (658-2,311)b |
aBootstrapped analyses.
bP < 0.05.
CI = confidence interval; DDE = drug-drug exposure; SAO = short-acting opioid; SD = standard deviation.
APPENDIX G. Total Adjusted Costs Among Exclusive SAO Patients Comparing C-II and C-III Use: No DDE Versus DDE
| Time | All | No DDE | DDE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exclusive C-II SAO n = 5,240 Mean (SD) $ | Exclusive C-III SAO n = 1,587 Mean (SD) $ | Differencea Mean (95% CI) $ | Exclusive C-II SAO n = 1,557 Mean (SD) $ | Exclusive C-III SAO n = 458 Mean (SD) $ | Differencea Mean (95% CI) $ | Exclusive C-II SAO n = 3,683 Mean (SD) $ | Exclusive C-III SAO n = 1,129 Mean (SD) $ | Differencea Mean (95% CI) $ | |
| ≤ 72 hours | 2,411 (4,023) | 2,354 (3,461) | 48 (-143-252) | 2,189 (3,324) | 2,117 (3,257) | 0 (-347-334) | 2,505 (4,281) | 2,451 (3,538) | 84 (-172-340) |
| ≤ 30 days | 5,694 (9,210) | 4,561 (8,069) | 731 (314-1,158)b | 4,737 (8,114) | 3,662 (5,429) | 653 (55-1,315)b | 6,098 (9,608) | 4,926 (8,896) | 824 (211-1,383)b |
| ≤ 45 days | 7,244 (11,580) | 5,500 (9,640) | 1,152 (610-1,685)b | 5,985 (10,404) | 4,453 (6,927) | 1,026 (265-1,860)b | 7,777 (12,004) | 5,924 (10,517) | 1,296 (573-1,988)b |
| ≤ 60 days | 8,678 (13,601) | 6,369 (11,120) | 1,586 (939-2,192)b | 7,279 (13,122) | 5,106 (8,016) | 1,522 (548-2,436)b | 9,270 (13,758) | 6,882 (12,121) | 1,708 (860-2,496)b |
| ≤ 90 days | 11,571 (17,400) | 7,938 (13,736) | 2,457 (1,698-3,217)b | 9,478 (16,010) | 6,157 (9,245) | 2,230 (1,135-3,341)b | 12,456 (17,883) | 8,661 (15,127) | 2,762 (1,727-3,730)b |
aBootstrapped analyses.
bP < 0.05.
C-II = Class (Schedule) II; C-III = Class (Schedule) III; CI = confidence interval; DDE = drug-drug exposure; SAO = short-acting opioid; SD = standard deviation.
References
- 1. Gaskin DJ, Richard P.. The economic costs of pain in the United States. J Pain. 2012; 13(8): 715-24. [DOI] [PubMed] [Google Scholar]
- 2. Institute of Medicine. . Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. Available at: books.nap.edu/openbook.php?record_id=13172. Accessed September 1, 2015. [PubMed] [Google Scholar]
- 3. Rauck RL. What is the case for prescribing long-acting opioids over short-acting opioids for patients with chronic pain? A critical review. Pain Pract. 2009; 9(6): 468-79. [DOI] [PubMed] [Google Scholar]
- 4. Turk DC, Wilson HD, Cahana A.. Treatment of chronic non-cancer pain. Lancet. 2011; 377(9784): 2226-35. [DOI] [PubMed] [Google Scholar]
- 5. Manchikanti L, Fellows B, Ailinani H, Pampati V.. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010; 13(5): 401-35. [PubMed] [Google Scholar]
- 6. U.S. Food and Drug Administration. . Goal of labeling changes: better prescribing, safer use of opioids. FDA Consumer Health Information. September 2013. Available at: http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM367795.pdf. Accessed September 1, 2015. [Google Scholar]
- 7. Schedules of controlled substances: rescheduling of hydrocodone combination products from Schedule III to Schedule II. . A rule by the Drug Enforcement Administration on 08/22/2014. 21 CFR 1308 (2014). Available at: https://federalregister.gov/a/2014-19922. Accessed September 1, 2015. [PubMed]
- 8. U.S. Food and Drug Administration. . New safety measures announced for extended-release and long-acting opioids. Available at: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm363722.htm. Accessed September 1, 2015.
- 9. Chou R, Fanciullo GJ, Fine PG, et al. . Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009; 10(2): 113-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Motov SM, Khan A.. Problems and barriers of pain management in the emergency department: are we ever going to get better? J Pain Res. 2008; 2: 5-11. [PMC free article] [PubMed] [Google Scholar]
- 11. Institute of Medicine. . Committee on Advancing Pain Research, Care, and Education. Preface. In: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011: R9-R12. Available at: books.nap.edu/openbook.php?record_id=13172. Accessed September 1, 2015. [PubMed] [Google Scholar]
- 12. Premier Research Services. . Charlotte, NC: Premier, Inc. Available at: https://www.premierinc.com/transforming-healthcare/healthcare-performance-improvement/premier-research-services/. Accessed September 1, 2015. [Google Scholar]
- 13. Clinformatics Data Mart. . Eden Prairie, MN: Optum Insights. Available at: https://www.optum.com/content/dam/optum/resources/productSheets/Clinformatics_for_Data_Mart.pdf. Accessed September 1, 2015. [Google Scholar]
- 14. Elixhauser A, Steiner C, Harris DR, Coffey RM.. Comorbidity measures for use with administrative data. Med Care. 1998; 36(1): 8-27. [DOI] [PubMed] [Google Scholar]
- 15. Pergolizzi JV, Labhsetwar SA, Puenpatom RA, Joo S, Ben-Joseph RH, Summers KH.. Prevalence of exposure to potential CYP450 pharmacokinetic drug-drug interactions among patients with chronic low back pain taking opioids. Pain Pract. 2011; 11(3): 230-39. [DOI] [PubMed] [Google Scholar]
- 16. Flockhart DA. Drug interactions: P450 drug interaction table. Indiana University Department of Medicine. 2007. Available at: http://medicine.iupui.edu/clinpharm/ddis/clinical-table/. Accessed September 1, 2015. [Google Scholar]
- 17. Mahoney EM. Cost-effectiveness analysis alongside clinical trial: statistical and methodological issues. In: Weintraub WS, ed. . Contemporary Cardiology: Cardiovascular Health Economics. Totowa, NJ: Humana Press; 2003:123-56. [Google Scholar]
- 18. Efron B. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 19. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009; 84(7): 613-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holmquist GL. Opioid metabolism and effects of cytochrome P450. Pain Med. 2009; 10: S20-29. [Google Scholar]
- 21. Lynch T, Price A.. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007; 76(3): 391-96. [PubMed] [Google Scholar]
- 22. Argoff CE, Silvershein DI.. A comparison of long- and short-acting opioids for the treatment of chronic noncancer pain: tailoring therapy to meet patient needs. Mayo Clin Proc. 2009;84(7);602-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Von Korff M, Merrill JO, Rutter CM, Sullivan M, Campbell CI, Weisner C.. Time-scheduled vs. pain-contingent opioid dosing in chronic opioid therapy. Pain. 2011; 152(6): 1256-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saad M, Harisingani R, Katinas L.. Impact of geriatric consultation on the number of medications in hospitalized older patients. Consult Pharm. 2012; 27(1): 42-48. [DOI] [PubMed] [Google Scholar]
- 25. Academy of Managed Care Pharmacy. . Pharmacists as vital members of accountable care organizations: illustrating the important role that pharmacists play on health care teams. 2011. Available at: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=9728. Accessed September 1, 2015.
- 26. Wilson HD, Dansie ET, Kim MS, Moscovitz BL, Turk DC.. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013; 14(6): 1613-27. [DOI] [PubMed] [Google Scholar]
- 27. Leider HL, Dhaliwal J, Davis EJ, Kulakodlu M, Buikema AR.. Healthcare costs and nonadherence among chronic opioid users. Am J Managed Care. 2011; 17(1): 32-40. [PubMed] [Google Scholar]
- 28. Summers KH, Puenpatom RA, Rajan N, Ben-Joseph R, Ohsfeldt R.. Economic impact of potential drug-drug interactions in opioid analgesics. J Med Econ. 2011; 14(4): 390-96. [DOI] [PubMed] [Google Scholar]
- 29. Encinosa WE, Hellinger FJ.. The impact of medical errors on ninety-day costs and outcomes: an examination of surgical patients. Health Serv Res. 2008; 43(6): 2067-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Department of Health and Human Services, Centers for Medicare & Medicaid Services. . Federal Register. 75(144):44362, July 28, 2010. Available at: http://www.gpo.gov/fdsys/pkg/FR-2010-07-28/pdf/2010-17207.pdf. Accessed September 1, 2015. [Google Scholar]
- 31. Fanciullo GJ, Washington T.. Best practices to reduce the risk of drug-drug interactions: opportunities for managed care. Am J Manag Care. 2011; 17(Suppl 11): S299-304. [PubMed] [Google Scholar]
- 32. Kent AJ, Harrington L, Skinner J.. Medication reconciliation by a pharmacist in the emergency department: a pilot project. Can J Hosp Pharm. 2009; 62(3): 238-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chaffee BW, Zimmerman CR.. Developing and implementing clinical decision support for use in a computerized prescriber-order-entry system. Am J Health Syst Pharm. 2010; 67(5): 391-400. [DOI] [PubMed] [Google Scholar]
- 34. Pedersen CA, Schneider PJ, Scheckelhoffet DJ.. ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education—2009. Am J Health Syst Pharm. 2010; 67(7): 542-58. [DOI] [PubMed] [Google Scholar]
- 35. Drug Enforcement Administration. . Rescheduling of hydrocodone combination products from Schedule III to Schedule II. 79 FR 49661. August 22, 2014. Available at: http://www.gpo.gov/fdsys/pkg/FR-2014-08-22/pdf/FR-2014-08-22.pdf. Accessed September 1, 2015.


