Abstract
Background
Data regarding the safety and long-term effectiveness of percutaneous closure of paravalvular leak (PVL) after transcatheter aortic valve implantation (TAVI) are scarce.
Aims
This study aims to present a large multicentre international experience of percutaneous post-TAVI PVL closure.
Methods
All patients who underwent percutaneous post-TAVI PVL closure in 14 hospitals across Europe and North America between January 2018 and October 2022 were included.
Results
Overall, 45 patients (64% male) were enrolled. The median age was 80 years (75-84). Among them, 67% and 33% had self-expanding and balloon-expandable valve implantations, respectively. Baseline post-TAVI PVL was severe in 67% of cases and moderate in the rest. The time from index TAVI to PVL closure procedure was 16.1 (8.7-34.8) months. Most patients were in NYHA Class III and IV (73%) before the procedure, and 40% had referred hospitalisations for heart failure between TAVI and the PVL closure procedure. Successful PVL closure was achieved in 94%, reducing regurgitation to ≤mild in 91% and moderate in the rest. The Amplatzer Valvular Plug III was the most frequently used device (27 cases), followed by the Amplatzer Valvular Plug 4. The incidence of severe adverse events was 11%. None of the patients died during the index hospitalisation. During long-term follow-up (21.7±16.2 months), the all-cause mortality rate was 14%, and patients presented improvement in functional status and a significant reduction in the rate of hospitalisation for heart failure (from 40% to 6%).
Conclusions
Percutaneous PVL closure is a feasible and safe option for treating post-TAVI leaks. Successful PVL reduction to mild or less could be associated with acute and long-lasting improvements in clinical outcomes.
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as a breakthrough treatment for patients with symptomatic severe aortic stenosis (AS)1. Given its minimally invasive approach, TAVI has become the first treatment option in older patients, improving their prognosis and quality of life compared to surgical aortic valve replacement2,3. Paravalvular leakage (PVL) is among the most common complications post-TAVI4, with a prevalence that ranges from 7% to 40%5. More than mild post-TAVI PVL negatively impacts long-term outcomes, even in asymptomatic patients6. In fact, moderate to severe post-TAVI PVLs are associated with a twofold increase in overall all-cause mortality5.
Improved transcatheter valve design has resulted in a decline in PVL after TAVI5,7. Nonetheless, post-TAVI PVLs still happen, which demand potential corrective measures8,9,10. Possible causation mechanisms include valve underexpansion, valve malposition, and a high burden of annular or left ventricular outflow calcium8,9. Both balloon dilatation and valve-in-valve implantation reduce their occurrence but are associated with increased complications (need for a pacemaker and aortic annulus rupture) and procedural costs, respectively. Transcatheter post-TAVI PVL closure with vascular plugs may represent another valid option.
Data regarding the safety and long-term effectiveness of percutaneous post-TAVI PVL closure are scarce, limited to case reports, small case series, and a recently published registry10,11,12,13. This study aims to present a large, international, multicentre experience of percutaneous post-TAVI PVL closure, including feasibility, safety, and long-term outcomes.
Methods
Study population and clinical data
The PLUGinTAVI Working Group Registry retrospectively collected data from consecutive patients undergoing percutaneous PVL closure after TAVI at a total of 14 sites in Europe and North America between January 2018 and October 2022. The mean number of cases per centre per year was 200 (±50). Sites were contacted by the principal investigator (X. Freixa) and invited to participate in the registry. Patients were included in the registry irrespective of the type of TAVI, and the selection of any procedural features (device, access, imaging, implantation technique, etc.,) was at the operators’ discretion. Our cohort of patients only included patients with PVL; intravalvular leaks were excluded from the analysis. The final decision to perform PVL closure was made by the Heart Team of each centre. In general, the management of multiple PVLs corresponded to their severity. When ≥2 PVLs existed, the primary target of intervention was the largest and most significant leak, which the Heart Team considered responsible for the patient’s clinical symptoms. In case of persistent leaks after the first PVL closure, most operators adopted a tailored patient approach based on their severity and the potential impact on symptoms.
A dedicated database was used to collect baseline characteristics, periprocedural percutaneous PVL closure features, and clinical/echocardiographic in-hospital and follow-up outcomes. All patients gave written informed consent before the procedure, and the study was performed in accordance with the local ethics committee of each centre. The study conformed to the guiding principles of the Declaration of Helsinki.
Definitions and procedure
The patient’s functional status was evaluated before and after PVL closure and classified based on the New York Heart Association (NYHA) Functional Class. Functional Class data from successful PVL closures were obtained from medical records in routine follow-up visits, mostly at 1, 6, and 12 months after leak closure. The Society of Thoracic Surgery Predicted Risk of Mortality (STS-PROM) and the European System for Cardiac Operative Risk Evaluation (EuroSCORE II) were used to evaluate the surgical risk. A 3-class scheme (mild, moderate, severe) was used to evaluate paravalvular regurgitation before and after PVL closure following the recommendation of the American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) guidelines14. In the present study, PVL was primarily assessed by transthoracic echocardiogram (TTE)/transoesophageal echocardiogram (TOE) before the intervention. In a minority of cases, computed tomography angiography (CTA) was also used for localisation, defect sizing, and procedural planning purposes. Procedural success was defined as the successful closure of PVL with a reduction of at least one grade of paravalvular regurgitation15,16. Procedural and major adverse events (MAEs) were reported according to Valve Academic Research Consortium 3 criteria17. Serious adverse events (SAEs) were defined as intraprocedural death, cardiac tamponade, stroke, device embolisation, major vascular complications, and major bleeding events. Major bleeding events were defined as type 3 or greater on the Bleeding Academic Research Consortium (BARC) scale18. Clinical follow-up was carried out through patient visits, medical report reviews, and phone contact. Adverse events reported at follow-up included death (cardiovascular and non-cardiovascular), heart failure (HF) hospitalisation, stroke, post-TAVI endocarditis, and aortic reintervention. A reduction in hospitalisation for HF was found after comparing the number of patients with an implanted TAVI and ≥1 hospitalisation for HF before and after successful PVL closure.
Statistical analysis
Categorical variables are presented as frequencies (percentages). Continuous variables are presented as a mean±standard deviation (SD) or median (interquartile range). The Kolmogorov-Smirnov test was applied to ensure normal distribution. Follow-up was terminated at the date of the final follow-up for each patient. Analyses were performed using STATA software, version 14.0 (StataCorp).
Results
Baseline characteristics
Data on all post-TAVI transcatheter PVL closures performed in the participating centres were collected. The study included 45 patients with a median age of 80 years (75-84), of whom 64% were men. The mean STS score and EuroSCORE II were 5.8±5.3 and 6.3±5.7, respectively. PVL closure was performed at a median of 16.1 months (8.7-34.8) after the index TAVI. Most patients (91%) presented with HF symptoms, while 2% presented with isolated haemolysis, and 7% had both HF and haemolysis. Prior to the leak closure, 73% of patients were in NYHA Class III and IV. Hospitalisations for HF between the index TAVI and PVL closure occurred in 40% of patients. Single antiplatelet therapy (SAPT) (53%) was the most common antithrombotic therapy prescribed before the procedure. A self-expanding valve was used in 67% of cases, and a balloon-expandable valve was used in the remaining 33%. Replacement valve sizes ranged from 26 to 31 mm. PVL was severe in 67% of cases and moderate in the remaining cases. Baseline clinical and echocardiographic characteristics are summarised in Table 1 and Table 2. Patients who received a balloon-expandable valve had a longer time interval between TAVI and the PVL closure than those who received a self-expanding valve (p=0.02). No other significant differences were observed between the two groups. Supplementary Table 1 presents detailed information on clinical and echocardiography outcomes at maximum follow-up, based on valve type.
Table 1. Baseline demographic characteristics.
| Total (n=45) | ||
|---|---|---|
| Age, years | 80 (75-84) | |
| Male gender | 29 (64) | |
| Body mass index, kg/m2 | 26.40±4.87 | |
| Diabetes | 15 (33) | |
| Hypertension | 29 (64) | |
| Atrial fibrillation | 17 (38) | |
| Previous CAD | 22 (49) | |
| Previous ischaemic stroke | 8 (18) | |
| Previous peripheral artery disease | 9 (20) | |
| Chronic kidney disease | 18 (40) | |
| Liver disease | 0 | |
| Creatinine, mmol/L | 82.5 (67.5-97.0) | |
| Haemoglobin, mg/dL | 11.70±1.97 | |
| Platelets, 10^9/L | 193.7±60.6 | |
| Previous AT treatment | None | 0 |
| SAPT | 24 (53) | |
| DAPT | 7 (16) | |
| Warfarin | 6 (13) | |
| DOAC | 8 (18) | |
| Values are expressed as n (%), mean±SD, or median (IQR). AF: atrial fibrillation; AT: antithrombotic treatment; CAD: coronary artery disease; DAPT: dual antiplatelet therapy; DOAC: direct oral anticoagulation; IQR: interquartile range; SAPT: single antiplatelet therapy; SD: standard deviation; TAVI: transcatheter aortic valve implantation | ||
Table 2. Baseline clinical and echocardiography characteristics.
| Total (n=45) | ||
|---|---|---|
| Time from TAVI procedure, months | 16.1 (8.7-34.8) | |
| Type of TAVI | Balloon-expandable | 15 (33) |
| Self-expanding | 30 (67) | |
| Type of balloon-expandable TAVI | SAPIEN XT/3 | 13 (86) |
| Myval | 2 (14) | |
| Type of self-expanding TAVI | Evolut R/Pro | 26 (86) |
| ACURATE neo | 3 (10) | |
| Portico | 1 (4) | |
| Size of TAVI, mm | 26 (26-31) | |
| Main symptoms | Heart failure | 41 (91) |
| Haemolysis | 1 (2) | |
| Both | 3 (7) | |
| New York Heart Association (NYHA) | I | 0 |
| II | 12 (27) | |
| III | 27 (60) | |
| IV | 6 (13) | |
| HFH between TAVI and leak procedure | 18 (40) | |
| EuroSCORE II | 6.3±5.7 | |
| STS score | 5.8±5.3 | |
| Left ventricular ejection fraction | 52.0±10.7 | |
| Paravalvular aortic regurgitation | Mild | 0 |
| Moderate | 15 (33) | |
| Severe | 30 (67) | |
| Aortic gradient, mmHg | 7.0 (3.5-11.6) | |
| Mitral regurgitation | No | 9 (20) |
| Mild | 21 (46) | |
| Moderate | 12 (27) | |
| Severe | 3 (7) | |
| Tricuspid regurgitation | No | 8 (18) |
| Mild | 26 (58) | |
| Moderate | 7 (16) | |
| Severe | 4 (8) | |
| Massive | 0 | |
| Torrential | 0 | |
| Systolic pulmonary artery pressure, mmHg (n=26) | 41.5 (30.0-57.5) | |
| Values are expressed as n (%), mean±SD, or median (IQR). EuroSCORE: European System for Cardiac Operative Risk Evaluation; IQR: interquartile range; HFH: heart failure hospitalisation; SD: standard deviation; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | ||
Procedural characteristics
Successful PVL closure was achieved in most patients (94%), reducing regurgitation to ≤mild in 91% of cases, while moderate regurgitation persisted in the remaining cases. The reasons for PVL closure failure are listed in Supplementary Table 2. The majority of patients (61%) presented with a single PVL, while 35% exhibited two leaks, and only a small proportion (4%) had three. Most PVLs were found to be located at the left (49%) or non-coronary cusps (31%) and more than one leak was treated in 13% of cases.
The procedure was performed under local anaesthesia in 58% of cases and general anaesthesia in 42%, with guidance mainly by TOE (42%), followed by angiography+transthoracic echocardiography (36%) and intracardiac echocardiography (22%). A retrograde approach from the aorta was used in all patients, with femoral artery access in 78% and a radial approach in 22%. Secondary access was obtained via arterial or venous routes in 33% and 11%, respectively, and an arterio-arterial loop was utilised in 23% of cases. The use of an arteriovenous loop was infrequent (2%).
Guiding catheters were utilised in 47% of cases and diagnostic catheters in 18%, with a mother-and-child technique used in one-quarter of cases. Delivery of devices was achieved through the use of Flexor Shuttle (Cook Medical) or Destination (Terumo) guiding sheaths in 35% of cases. The most frequently used device was the Amplatzer Vascular Plug III (AVP-3; Abbott), in different sizes (27 cases), the Amplatzer Vascular Plug 4 (AVP-4; Abbott) in 15 cases and Occlutech PLD devices (Occlutech) were used in the remaining case. A summary of the procedural characteristics is presented in Table 3.
Table 3. Procedural characteristics and in-hospital outcomes.
| Total (n=45) | ||
|---|---|---|
| General anaesthesia | 19 (42) | |
| Procedural imaging | Angiography+TTE | 16 (36) |
| Standard TOE | 19 (42) | |
| Others | 10 (22) | |
| Primary access | Femoral artery | 35 (78) |
| Radial artery | 10 (22) | |
| Secondary access | No | 25 (55) |
| Arterial | 15 (33) | |
| Venous | 5 (11) | |
| Loop | No | 33 (75) |
| Arterio-arterial | 11 (25) | |
| Arteriovenous | 1 (2) | |
| Leak location | Non-coronary sinus | 14 (31) |
| Left coronary | 22 (49) | |
| Right coronary | 9 (20) | |
| Number of paravalvular leaks | 1 | 26 (61) |
| 2 | 15 (33) | |
| 3 | 2 (4) | |
| Catheter used during the procedure | Delivery (Destination, Flexor Shuttle) | 16 (35) |
| Guiding | 21 (47) | |
| Diagnostic | 8 (18) | |
| Mother-and-child technique | 12 (27) | |
| More than one leak treated | 6 (13) | |
| Closure device | ||
| Amplatzer Vascular Plug | 0 | |
| Amplatzer Vascular Plug II | 0 | |
| Amplatzer Vascular Plug III | 6/3 mm | 4 (9) |
| 10/5 mm | 13 (30) | |
| 12/5 mm | 2 (5) | |
| 14/5 mm | 8 (19) | |
| Amplatzer Vascular Plug 4 | 15 (33) | |
| Others | 1 (3) | |
| Procedural success | 42 (94) | |
| Residual shunt | No | 25 (60) |
| Intradevice | 5 (12) | |
| Peridevice | 11 (28) | |
| Residual aortic regurgitation | No | 12 (29) |
| Mild | 26 (62) | |
| Moderate | 4 (9) | |
| Severe | 0 | |
| Mean aortic gradient post-procedure, mmHg | 6.5 (4.0-10.2) | |
| Patients with procedure- or device-related SAEs ≤7 days* | 5 (11) | |
| Intraprocedural death | 0 | |
| Cardiac tamponade | 1 (3) | |
| Stroke | 1 (3) | |
| Device embolisation | 1 (3) | |
| Major vascular complications | 2 (5) | |
| Major bleeding | 0 | |
| Days until discharge after procedure | 2 (1-6) | |
| AT treatment at discharge | None | 1 (2) |
| SAPT | 14 (33) | |
| DAPT | 12 (29) | |
| Warfarin | 6 (14) | |
| DOAC | 9 (22) | |
| Values are expressed as n (%) or median (IQR). *Subjects may have had more than one type of major SAE event. AT: antithrombotic treatment; DAPT: dual antiplatelet therapy; DOAC: direct oral anticoagulation; IQR: interquartile range; SAE: serious adverse event; SAPT: single antiplatelet therapy; SD: standard deviation; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography | ||
In-hospital and long-term outcomes
The rate of SAE was 11%. Nonetheless, there were no reported deaths during the index hospitalisation. One patient experienced cardiac tamponade, which was effectively treated through percutaneous drainage. Another patient experienced device embolisation, which was also managed percutaneously. In addition, there was one case of minor stroke and two major vascular complications. Following PVL closure, the median hospital stay was 2 days1,2,3,4,5,6. In-hospital outcomes are presented in Table 3.
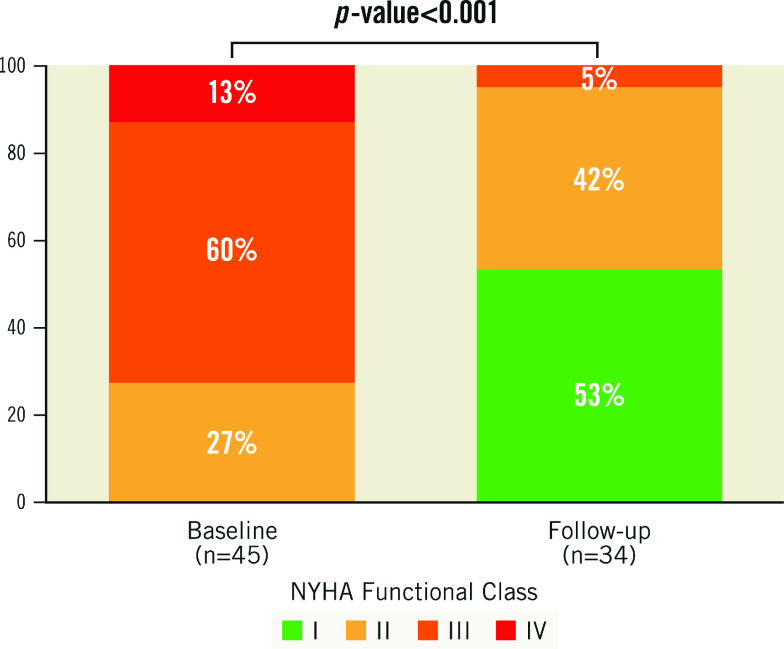
At the final follow-up (21.7±16.2 months), five patients (14%) had died. Among these deaths, two were attributed to cardiovascular causes. Table 4 presents the clinical and echocardiographic outcomes at maximum follow-up. Of the 42 patients who had successful PVL closure, long-term follow-up was available for 34 patients. During the extended follow-up, it was observed that most of the patients with previous functional impairment presented significant and sustained improvement in their functional status (NYHA I or II in 95%) following PVL closure (p<0.001) (Central illustration). The aortic regurgitation (AR) grade changed from severe (in 72%) to ≤mild (in 76%) between baseline and discharge echocardiography (p<0.001). Supplementary Figure 1 shows a maintained improvement in AR at 3-6 months and during extended follow-up. Supplementary Figure 2 illustrates that the persistent improvement in successful PVL cases, independent of the number of treated leaks, remained unchanged at 3-6 months and long-term follow-up. Figure 1 and Figure 2 illustrate the NYHA Functional Class and AR changes between baseline and maximum follow-up. Furthermore, during the follow-up period, there was a significant decrease in the rate of HF hospitalisation from 40% before PVL closure to 6% after the procedure. Only one case of infective endocarditis was noted during the follow-up period.
Table 4. Clinical and echocardiography outcomes at maximum follow-up.
| Total (n=34) | ||
|---|---|---|
| Clinical outcomes | ||
| Median final follow-up, months | 21.7±16.2 | |
| All-cause death | 5 (14) | |
| Cardiovascular death | 2 (5) | |
| New York Heart Association | I | 18 (53) |
| II | 14 (42) | |
| III | 2 (5) | |
| IV | 0 | |
| Heart failure hospitalisation | 2 (5) | |
| Stroke | 1 (3) | |
| Endocarditis | 1 (3) | |
| Reintervention | 0 | |
| Echocardiography outcomes | ||
| Residual paravalvular aortic regurgitation | No | 10 (30) |
| Mild | 15 (46) | |
| Moderate | 5 (15) | |
| Severe | 3 (9) | |
| Device embolisation | 1 (3) | |
| Device thrombosis | 0 | |
| Values are expressed as n (%) or mean±SD. SD: standard deviation | ||
Central illustration. Significant and sustained improvement in functional status following successful PVL closure.
comp: complications; hosp: hospitalision; NYHA: New York Heart Association; PVL: paravalvular leakage; SAE: serious adverse event; TAVI: transcatheter aortic valve implantation
Figure 1. Improvement in functional status during follow-up after successful percutaneous post-TAVI PVL closure.
NYHA: New York Heart Association; PVL: paravalvular leakage; TAVI: transcatheter aortic valve implantation
Figure 2. Baseline, discharge, and follow-up grades of post-TAVI PVLs in patients who underwent successful percutaneous post-TAVI PVL closure.
PVL: paravalvular leakage; TAVI: transcatheter aortic valve implantation
Discussion
The key findings of the present study, representing one of the largest multicentre international experiences of percutaneous post-TAVI PVL closure, were the following: 1) percutaneous PVL closure after self-expanding or balloon-expandable TAVI was a technically feasible procedure with a high success rate; 2) the rate of significant adverse periprocedural events in the post-TAVI PVL closure population was low; 3) a maintained reduction in AR post-TAVI PVL closure was observed in the present cohort, resulting in a sustained improvement in NYHA Functional Class; 4) a high survival rate was observed during long-term follow-up post-TAVI PVL closure.
Percutaneous post-TAVI PVL closure has already been explored, but the evidence is scarce10,11,12,13,19. Percutaneous post-TAVI PVL closure is more demanding than the treatment of leaks in postsurgical conventional valves8. Native cusps are not resected during TAVI, and a high calcium volume may persist in the TAVI device landing zone, limiting accessibility and navigation of equipment between the prosthesis frame and native tissue9,19. Additionally, catheter progression through the valve struts may be required, and identifying the correct path to cross the defect can be extremely challenging. Furthermore, percutaneous post-TAVI PVL closure in a self-expanding valve can be more demanding than in a balloon-expandable valve, as strut avoidance is more complicated8,9.
Preprocedural planning is an essential aspect of percutaneous post-TAVI PVL closure. In our series, most cases were assessed by TTE/TOE. Only in a minority of cases was CTA used for procedural purposes. In any case, CTA might represent a valid tool to locate, size, and define the optimal working projection for PVL closure.
Despite all the technical difficulties mentioned, we observed a high success rate (94%) in our cohort, higher than reported in previously published studies10,13. There are some plausible explanations for such a high procedural success rate. First, this is a more contemporary series, demonstrating the increasing experience of operators with complex structural procedures. Second, the use of mother-and-child techniques in one-quarter of patients and arterio-arterial loops in 25% of cases20 were paramount in this setting as delivery catheters are generally difficult to advance. Third, in the presence of small leaks, lower profile AVP-III and AVP-4 devices were delivered via 5 Fr, 6 Fr, or even 4 Fr diagnostic catheters, reducing the need for larger catheters and sheaths and subsequently avoiding excessive manipulation8. Fourth, in this systematic review of 14 studies, the definition of successful PVL closure following TAVI varied widely, ranging from mild or trivial AR to a significant reduction of AR post-procedure10. However, the largest series included in this systematic review, conducted by Saia et al12, utilised a definition of procedural success with a final grade AR ≤2, which corresponds to mild-to-moderate PVL severity and is consistent with the definition used in our study.
The use of devices designed for other settings such as ventricular septal defects, atrial septal defects, or patent ductus arteriosus closure devices (i.e., Amplatzer Septal Occluder device, Amplatzer Duct Occluder, Amplatzer Vascular Plug II or the Amplatzer Muscular VSD Occluder; all Abbott) has been extrapolated to the percutaneous PVL closure scenario. These devices present several potential limitations in this context, mainly derived from the shape and the need for large delivery catheters, resulting in a lower success rate8. The Occlutech Paravalvular Leak Device (PLD) and the AVP-III obtained the CE mark (European conformity) in 2014 and 2020, respectively, for percutaneous PVL closure. However, neither have yet obtained U.S. Food and Drug Administration approval8,21.
Saia et al12 reported a high rate of periprocedural complications (29.7%) in 27 patients as compared to the 12.5% observed in our cohort. Again, the increasing experience of operators, the use of vascular closure devices, and/or the minimalistic approach using radial access resulted in a lower rate of general anaesthesia use and major bleedings compared to this previous series12 (48% vs 40% and 3.7% vs 0%, respectively). The advantages of radial over femoral access have been shown in other clinical scenarios, such as PCI or conventional post-surgical PVL closure22,23.
The potential clinical benefit from aortic PVL closure corresponds to the amount of PVL reduction achieved8,15,19,21. A percutaneous leak reduction to ≤mild has been associated with a significant mortality reduction during long-term follow-up in patients with surgically implanted prostheses15,19. However, in previous studies focusing on the percutaneous post-TAVI PVL closure population, the prognosis remained relatively poor despite a high success rate10,12. In the ReDo-TAVI registry13, the largest series that describes the efficacy of different transcatheter treatments for PVL after TAVI, patients with residual PVL ≥moderate, despite reintervention, had higher mortality at 1 year compared with patients in whom AR was reduced to ≤mild (21% vs 8%; respectively). A sustained reduction in AR post-TAVI PVL closure, resulting in a sustained improvement in NYHA Functional Class and a high survival rate (85%) during long-term follow-up, was observed in our study. The positive impact in survival benefits related to percutaneous post-TAVI PVL closure reported in our cohort could be related to the actual trend in the TAVI population: a stable proportion of elderly patients at intermediate surgical risk24, contrary to previous studies, which included inoperable patients with a high burden of comorbidities10.
Limitations
The present report has several limitations that should be acknowledged. First, the main limitation of this study is its observational design, which implies an inherent bias for several of the analysed variables. No comparison between the successful PVL closure and the failed group was possible as the subgroup sample sizes were too small. Therefore, our results should be considered hypothesis-generating. Second, the clinical and imaging results were self reported, and there was no independent adjudication. Third, there is no standard definition of procedure success for PVL closure. However, the definition used in the present study is derived from prior large retrospective observational registry studies15,16. Fourth, the times between index TAVI and PVL intervention and maximum follow-up after PVL closure were unequal.
Conclusions
Percutaneous PVL closure is a feasible and safe option for treating post-TAVI leaks. Successful PVL reduction to mild or less than mild could be associated with acute and long-lasting improvements in clinical outcomes. Adequate patient selection, knowledge of equipment, techniques, and comprehensive cardiac imaging intraprocedural guidance during the procedure are imperative for achieving good results and limiting periprocedural complications.
Impact on daily practice
Despite current TAVI developments, relevant post-TAVI PVLs may still occur, potentially requiring corrective measures. Percutaneous post-TAVI PVL closure with vascular plugs might represent a valid therapeutic option. Data regarding the safety and long-term effectiveness of percutaneous post-TAVI PVL closure are scarce. Percutaneous post-TAVI PVL closure is a feasible and safe option for treating post-TAVI leaks. A high successful procedural rate was observed, which resulted in acute and long-lasting improvements in clinical outcomes.
Supplementary data
Clinical and echocardiography outcomes at maximum follow-up according to valve type.
Reasons for paravalvular leak closure failure.
Consistent improvement of AR in cases where isolated PVLs were successfully treated.
Comparison of successful PVL closure grade at 3-6 months and during long-term follow-up in cases where follow-up was available.
Acknowledgments
Acknowledgements
We are grateful to Mathieu Albertini for his support in this study.
Conflict of interest statement
I.Cruz-González is aproctor for Abbott. The other authors have no conflicts of interest to declare relevant to the content of this manuscript.
Abbreviations
- AS
aortic stenosis
- HF
heart failure
- PVL
paravalvular leak
- TAVI
transcatheter aortic valve implantation
Contributor Information
Eduardo Flores-Umanzor, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network (UHN), Toronto, ON, Canada.
Jason Nogic, Department of Cardiology, Royal Papworth Hospital, Cambridge, UK.
Pedro Cepas-Guillén, Hospital Clinic de Barcelona, Institut Clinic Cardiovascular, IDIBAPS, Barcelona, Spain.
Sebastian Hascoet, Department of Congenital Heart Diseases, Marie Lannelongue Hospital, M3C Network, INSERM UMR-S 999, Paris-Saclay University, Plessis-Robinson, Paris, France.
Piotr Pysz, Medical University of Silesia, Katowice, Poland.
Jose Antonio Baz, Cardiovascular Research Group, Department of Cardiology, University Hospital Alvaro Cunqueiro, Galicia Sur Health Research Institute (IIS Galicia Sur), Servizo Galego de Saude, University of Vigo, Vigo, Spain.
Ignacio Cruz-González, Hospital Universitario de Salamanca, IBSAL, CIBER-CV, Salamanca, Spain.
Ignacio J. Amat-Santos, Hospital Universitario de Valladolid, Valladolid, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain.
Pablo Antúnez-Muiños, Hospital Universitario de Salamanca, IBSAL, CIBER-CV, Salamanca, Spain.
Jose Carlos González, Hospital Universitario de Valladolid, Valladolid, Spain.
Valeriano Ruíz-Quevedo, Hospital Universitario de Navarra, Navarra, Spain.
Rodrigo Estevez-Loureiro, Cardiovascular Research Group, Department of Cardiology, University Hospital Alvaro Cunqueiro, Galicia Sur Health Research Institute (IIS Galicia Sur), Servizo Galego de Saude, University of Vigo, Vigo, Spain.
Benoit Gerardin, Department of Congenital Heart Diseases, Marie Lannelongue Hospital, M3C Network, INSERM UMR-S 999, Paris-Saclay University, Plessis-Robinson, Paris, France.
Xavier Millan, Hospital Santa Creu i Sant Pau, Barcelona, Spain.
Marcel Santaló-Corcoy, Montreal Heart Institute, Montreal, QC, Canada.
Ander Regueiro, Hospital Clinic de Barcelona, Institut Clinic Cardiovascular, IDIBAPS, Barcelona, Spain.
Réda Ibrahim, Montreal Heart Institute, Montreal, QC, Canada.
Dabit Arzamendi, Hospital Universitario de Valladolid, Valladolid, Spain.
Eustaquio Maria Onorato, Cardiologia Universitaria, Ospedale Galeazzi - Sant’Ambrogio, IRCCS, Milan, Italy.
Josep Rodés-Cabau, Quebec Heart and Lung Institute, Laval University, Quebec City, QC, Canada.
Eric Horlick, Peter Munk Cardiac Centre, Toronto General Hospital, University Health Network (UHN), Toronto, ON, Canada.
Patrick A. Calvert, Department of Cardiology, Royal Papworth Hospital, Cambridge, UK.
Xavier Freixa, Hospital Clinic de Barcelona, Institut Clinic Cardiovascular, IDIBAPS, Barcelona, Spain.
References
- Pilgrim T, Windecker S. Expansion of transcatheter aortic valve implantation: new indications and socio-economic considerations. Eur Heart J. 2018;39:2643–5. doi: 10.1093/eurheartj/ehy228. [DOI] [PubMed] [Google Scholar]
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis, De Paulis, Delgado V, Freemantle N, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022;17:e1126–96. doi: 10.4244/EIJ-E-21-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM, 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- Pibarot P, Hahn RT, Weissman NJ, Arsenault M, Beaudoin J, Bernier M, Dahou A, Khalique OK, Asch FM, Toubal O, Leipsic J, Blanke P, Zhang F, Parvataneni R, Alu M, Herrmann H, Makkar R, Mack M, Smalling R, Leon M, Thourani VH, Kodali S. Association of Paravalvular Regurgitation With 1-Year Outcomes After Transcatheter Aortic Valve Replacement With the SAPIEN 3 Valve. JAMA Cardiol. 2017;2:1208–16. doi: 10.1001/jamacardio.2017.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan S, Huang X, Li Y, He S, Mao L, Hong W, Xiao Z. Paravalvular Leak After Transcatheter Aortic Valve Implantation Its Incidence, Diagnosis, Clinical Implications, Prevention, Management, and Future Perspectives: A Review Article. Curr Probl Cardiol. 2022;47:100957. doi: 10.1016/j.cpcardiol.2021.100957. [DOI] [PubMed] [Google Scholar]
- Laakso T, Laine M, Moriyama N, Dahlbacka S, Airaksinen J, Virtanen M, Husso A, Tauriainen T, Niemelä M, Mäkikallio T, Valtola A, Eskola M, Juvonen T, Biancari F, Raivio P. Impact of paravalvular regurgitation on the mid-term outcome after transcatheter and surgical aortic valve replacement. Eur J Cardiothoracic Surg. 2020;58:1145–52. doi: 10.1093/ejcts/ezaa254. [DOI] [PubMed] [Google Scholar]
- Welle GA, El-Sabawi B, Thaden JJ, Greason KL, Klarich KW, Nkomo VT, Alkhouli MA, Guerrero ME, Crestanello JA, Holmes DR, Rihal CS, Eleid MF. Effect of a fourth-generation transcatheter valve enhanced skirt on paravalvular leak. Catheter Cardiovasc Interv. 2021;97:895–902. doi: 10.1002/ccd.29317. [DOI] [PubMed] [Google Scholar]
- Freixa X, Gabani R, Cepas-Guillén P, Flores-Umanzor E, Estévez-Loureiro R, Onorato EM. Paravalvular Leakages after Surgical Aortic-Valve Replacement and after Transcatheter Aortic-Valve Implantation: Strategies to Increase the Success Rate of Percutaneous Closure. J Clin Med. 2022;11:2989. doi: 10.3390/jcm11112989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker RS, Cleveland JC, Jr, Messenger JC. Mechanical Complications of Transcatheter Aortic Valve Replacement. Interv Cardiol Clin. 2021;10:465–80. doi: 10.1016/j.iccl.2021.06.007. [DOI] [PubMed] [Google Scholar]
- Ando T, Takagi H. Percutaneous Closure of Paravalvular Regurgitation After Transcatheter Aortic Valve Implantation: A Systematic Review. Clin Cardiol. 2016;39:608–14. doi: 10.1002/clc.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérardin B, Champagnac D, Smolka G, Bouvaist H, Jakamy R, Ghostine S, Naël J, Garcia C, Kloeckner M, Potier A, Isorni MA, Brenot P, Hascoet S. [Para valvular leak closure in TAVI]. Ann Cardiol Angeiol (Paris) 2019;68:453–61. doi: 10.1016/j.ancard.2019.09.027. [DOI] [PubMed] [Google Scholar]
- Saia F, Martinez C, Gafoor S, Singh V, Ciuca C, Hofmann I, Marrozzini C, Tan J, Webb J, Sievert H, Marzocchi A, O’Neill WW. Long-term outcomes of percutaneous paravalvular regurgitation closure after transcatheter aortic valve replacement: a multi experience. JACC Cardiovasc Interv. 2015;8:681–8. doi: 10.1016/j.jcin.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Landes U, Hochstadt A, Manevich L, Webb JG, Sathananthan J, Sievert H, Piayda K, Leon MB, Nazif TM, Blusztein D, Hildick-Smith D, Pavitt C, Thiele H, Abdel-Wahab M, Van Mieghem NM, Adrichem R, Sondergaard L, De Backer O, Makkar RR, Koren O, Pilgrim T, Okuno T, Kornowski R, Codner P, Finkelstein A, Loewenstein I, Barbash I, Sharon A, De Marco F, Montorfano M, Buzzatti N, Latib A, Scotti A, Kim WK, Hamm C, Nombela Franco L, Mangieri A, Schoels WH, Barbanti M, Bunc M, Akodad M, Rubinshtein R, Danenberg H. Treatment of late paravalvular regurgitation after transcatheter aortic valve implantation: prognostic implications. Eur Heart J. 2023;44:1331–9. doi: 10.1093/eurheartj/ehad146. [DOI] [PubMed] [Google Scholar]
- Zoghbi WA, Asch FM, Bruce C, Gillam LD, Grayburn PA, Hahn RT, Inglessis I, Islam AM, Lerakis S, Little SH, Siegel RJ, Skubas N, Slesnick TC, Stewart WJ, Thavendiranathan P, Weissman NJ, Yasukochi S, Zimmerman KG. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2019;32:431–75. doi: 10.1016/j.echo.2019.01.003. [DOI] [PubMed] [Google Scholar]
- García E, Arzamendi D, Jimenez-Quevedo P, Sarnago F, Martí G, Sanchez-Recalde A, Lasa-Larraya G, Sancho M, Iñiguez A, Goicolea J, Garcia-San Roman, Alonso-Briales JH, Molina E, Calabuig J, Freixa X, Berenguer A, Valdes-Chavarri M, Vazquez N, Diaz JF, Cruz-Gonzalez I. Outcomes and predictors of success and complications for paravalvular leak closure: an analysis of the SpanisH real-wOrld paravalvular LEaks closure (HOLE) registry. EuroIntervention. 2017;12:1962–8. doi: 10.4244/EIJ-D-16-00581. [DOI] [PubMed] [Google Scholar]
- Onorato EM, Alamanni F, Muratori M, Smolka G, Wojakowski W, Pysz P, Zorinas A, Zakarkaite D, Eltchaninoff H, Litzer PY, Godart F, Calvert P, Christou C, Mussayev A, Missiroli B, Buzaev I, Curello S, Tesorio T, Bartorelli AL. Safety, Efficacy and Long-Term Outcomes of Patients Treated with the Occlutech Paravalvular Leak Device for Significant Paravalvular Regurgitation. J Clin Med. 2022;11:1978. doi: 10.3390/jcm11071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARC-3 WRITING COMMITTEE: Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Van Mieghem NM, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021;77:2717–46. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- Calvert PA, Northridge DB, Malik IS, Shapiro L, Ludman P, Qureshi SA, Mullen M, Henderson R, Turner M, Been M, Walsh KP, Casserly I, Morrison L, Walker NL, Thomson J, Spence MS, Mahadevan VS, Hoye A, MacCarthy PA, Daniels MJ, Clift P, Davies WR, Adamson PD, Morgan G, Aggarwal SK, Ismail Y, Ormerod JO, Khan HR, Chandran SS, de Giovanni, Rana BS, Ormerod O, Hildick-Smith D. Percutaneous Device Closure of Paravalvular Leak: Combined Experience From the United Kingdom and Ireland. Circulation. 2016;134:934–44. doi: 10.1161/CIRCULATIONAHA.116.022684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez-Loureiro R, Benito-González T, Gualis J, Pérez de, Cuellas C, Fernandez-Vazquez F. Percutaneous paravalvular leak closure after CoreValve transcatheter aortic valve implantation using an arterio-arterial loop. J Thorac Dis. 2017;9:E103–8. doi: 10.21037/jtd.2017.02.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascoët S, Smolka G, Blanchard D, Kloëckner M, Brochet E, Bouisset F, Leurent G, Thambo JB, Combes N, Dumonteil N, Bauer F, Nejjari M, Pillière R, Dauphin C, Bonnet G, Ciobotaru V, Kételers R, Gallet R, Hammoudi N, Mangin L, Bouvaist H, Spaulding C, Aminian A, Kilic T, Popovic B, Armero S, Champagnac D, Gérardin B. Predictors of Clinical Success After Transcatheter Paravalvular Leak Closure: An International Prospective Multicenter Registry. Circ Cardiovasc Interv. 2022;15:e012193. doi: 10.1161/CIRCINTERVENTIONS.122.012193. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Andò G, Repetto A, Limbruno U, Cortese B, Sganzerla P, Lupi A, Galli M, Colangelo S, Ierna S, Ausiello A, Presbitero P, Sardella G, Varbella F, Esposito G, Santarelli A, Tresoldi S, Nazzaro M, Zingarelli A, de Cesare, Rigattieri S, Tosi P, Palmieri C, Brugaletta S, Rao SV, Heg D, Rothenbühler M, Vranckx P, Jüni P MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–76. doi: 10.1016/S0140-6736(15)60292-6. [DOI] [PubMed] [Google Scholar]
- Ortega-Paz L, Regueiro A, Perdomo JM, Sanchis L, Sabaté M, Freixa X. Minimally Invasive Transradial Percutaneous Closure of an Aortic Paravalvular Leak After Transcatheter Aortic Valve Replacement. Can J Cardiol. 2019;35:941.e1–2. doi: 10.1016/j.cjca.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Mauri V, Abdel-Wahab M, Bleiziffer S, Veulemans V, Sedaghat A, Adam M, Nickenig G, Kelm M, Thiele H, Baldus S, Rudolph TK. Temporal trends of TAVI treatment characteristics in high volume centers in Germany 2013-2020. Clin Res Cardiol. 2022;111:881–8. doi: 10.1007/s00392-021-01963-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical and echocardiography outcomes at maximum follow-up according to valve type.
Reasons for paravalvular leak closure failure.
Consistent improvement of AR in cases where isolated PVLs were successfully treated.
Comparison of successful PVL closure grade at 3-6 months and during long-term follow-up in cases where follow-up was available.