Abstract
BACKGROUND:
Diabetes is a leading cause of morbidity, mortality, and medical resource utilization in the United States and worldwide. Treatment is aimed at keeping blood glucose levels close to normal and preventing or delaying medical complications. It has been estimated that only 50% of patients with diabetes in the United States achieve glycosylated hemoglobin A1c level < 7%. Nonadherence to antidiabetic medications has been identified as a major factor related to poor glycemic control.
OBJECTIVES:
To (a) assess adult patients with type 2 diabetes mellitus (T2DM) whose adherence status to oral antidiabetic drugs (OADs) changed from 1 year to the next and (b) identify predictors of change in adherence status.
METHODS:
This retrospective study of the Humana Medicare Advantage Database included patients with T2DM and continuous enrollment between 2010 and 2012. Proportion of days covered (PDC) by OADs was calculated for each of the 3 study years (2010, 2011, 2012). Patients were classified as adherent (PDC ≥ 80%) or nonadherent (PDC < 80%) during each year. Patient characteristics from the baseline period (2010) were used as covariates, and adherence status changes from baseline to follow-up year (2011) were used as response variables. Data from the subsequent study periods (2011 as baseline, 2012 as follow-up) were used to validate the model (final model).
RESULTS:
A total of 238,402 patients met inclusion criteria. Among them, 144,216 (60.5%) were adherent, and 94,186 (39.5%) were nonadherent during the baseline period. Change in adherence status from baseline to follow-up year was observed in 31,320 (21.7%) patients that were adherent and 39,284 (41.7%) patients that were nonadherent during the baseline year. The final model for baseline adherent patients had a receiver-operating characteristic (ROC) index of 73% and a misclassification rate of 39%. The predictors of highest importance were identified as total number of prescriptions filled with 90-day supply, diabetes-related pill burden, longest gap in OADs, total number of antidiabetic classes filled, and copay for the last OAD filled. The final model had a sensitivity value of 76.4%. The final model for baseline nonadherent patients had a ROC index of 68%, a misclassification rate of 36.4%, and sensitivity value of 52.9%. The predictors of highest importance were diabetes-related pill burden, longest gap in OADs, month-wise patient oscillation from adherent to nonadherent during baseline year, total number of prescriptions filled with a 90-day supply, and total pill burden during the baseline year.
CONCLUSIONS:
One third of the T2DM patients changed adherence status from 1 year to the next, and factors associated with adherence status changes were identified. Predictive models such as those used in this study can serve as useful and cost-effective tools for payers, helping to identify members that should be targeted for adherence enhancement programs and, ultimately, to improve patients’ long-term outcomes.
What is already known about this subject
Only 50% of patients with diabetes in the United States achieve glycemic control (hemoglobin A1c < 7%).
Poor adherence to oral antidiabetic drugs (OADs) is strongly associated with poor glycemic control as well as higher risk for micro- and macrovascular complications.
Patients with type 2 diabetes mellitus are likely to show fluctuations in level of adherence to OADs over time.
What this study adds
This retrospective claims database study showed that almost one third of patients had an adherence status change from 1 year to the next, and among them, a large proportion (21.7%) were adherent patients who became nonadherent.
For status change from adherent to nonadherent, this study found that the 5 strongest predictors during the baseline year were total number of prescriptions filled with a 90-day supply, diabetes-related pill burden, longest gap in OADs, total number of antidiabetic classes filled, and copay for the last OAD filled.
For status change from nonadherent to adherent, this study found that the 5 strongest predictors during the baseline year were diabetes-related pill burden, longest gap in OADs, month-wise patient oscillation from adherent to nonadherent, total number of prescriptions filled with a 90-day supply, and total pill burden.
Diabetes affects 29.1 million people in the United States and has been identified as a leading cause of medical resource utilization, disability, and death.1 Type 2 diabetes mellitus (T2DM) accounts for about 90%-95% of all diagnosed cases of diabetes in adults and mainly occurs in people aged over 40.2,3 T2DM is characterised by high blood glucose in the context of insulin resistance and loss of pancreatic beta cell function. The prevalence of the disease has increased in parallel with obesity over the past 20 to 30 years.
The main aims of diabetes treatment are to keep blood glucose level as close to normal as possible and prevent or delay development of medical complications. Studies have found that improved glycemic control benefits people with diabetes.4 In general, every percentage point drop (e.g., from 8% to 7%) in glycosylated hemoglobin (A1c) can reduce the risk of microvascular complications including kidney, eye, and nerve diseases by as much as 40%.2,3,5
Despite the recognized benefits of diabetes therapy, it has been estimated that only 50% of diabetes patients in the United States achieve glycemic control, defined as an A1c level of < 7%.6 Nonadherence to antidiabetic medications has been identified as 1 of the major factors related to poor glycemic control, with rates of adherence to oral antidiabetic drugs (OADs) reported as ranging from 36% to 93%.7,8 Poor adherence to OADs is strongly associated with poor glycemic control and with a higher risk for micro- and macrovascular complications,9,10 while adherence is associated with fewer emergency room and inpatient visits.7 It has been estimated, for each patient, that increases in medication adherence of 20% measured by proportion of days covered (PDC) could reduce total health care spending by as much as $1,074.11 The Health Effectiveness Data and Information Set (HEDIS) and Centers for Medicare & Medicaid Services (CMS) have recognized the importance of medication adherence and included it in health care quality measures. HEDIS recommends the use of PDC to measure adherence, and the CMS defines adherence as PDC ≥ 80%.12,13
Several studies have been conducted to identify factors related to adherence with OADs and have shown that patients with higher disease burden and total medication burden are more likely to be nonadherent.14 Additionally, African Americans, women, and patients who experienced medication switching, augmentation, or hypoglycemia appear to be less adherent to their medication regimens.10,15,16 Other factors, such as depression and amount of copayment, were also found to predict medication adherence.14,17,18
Although patients’ adherence to medication appears to be influenced by numerous patient, treatment, and environmental factors and thus is dynamic in nature and changes over time, no data are available on this phenomenon for the treatment of diabetes. More specifically, there is no information on change in adherence to OADs from 1 year to the next or on which factors may be linked to adherence change in the treatment of patients with T2DM.
To help address this information gap, this retrospective database study aimed to (a) assess the proportion of adult patients with T2DM whose adherence to OADs changed from nonadherent to adherent and vice versa and (b) identify predictors of change in adherence status from 1 year to the next.
Methods
This study was a longitudinal retrospective analysis of private health care payer data of T2DM patients enrolled in the Humana Medicare Advantage plan with prescription drug coverage. The Humana Medicare Advantage Database includes pharmacy and medical claims data, laboratory data, and consumer behavioral/socioeconomic data, along with enrollment data.
For inclusion in this study, patients were required to meet the following criteria: (a) diagnosis of T2DM (International Classification of Diseases, Ninth Revision, Clinical Modification[ICD-9-CM] codes 250.00, 250.x0, or 250.x2) during the year 2010; (b) continuous enrollment from January 1, 2010, to December 31, 2012; (c) at least aged 19 years as of January 1, 2010; and (d) 2 or more prescriptions for medications from any of the OAD classes included in the adherence measurement (i.e., biguanides, sulfonylureas, dipeptidyl peptidase-4 inhibitors [DPP-4], meglitinides, alpha-glucosidase inhibitors [AGIs], and thiazolidinediones [TZDs]) during each year of the study period.
Patients were excluded from analyses if (a) they filled any insulin product at any time during the study period; (b) they did not have all of the consumer behavioral/socioeconomic data; or (c) information on sex was unknown.
The index date for all analyses was the date of the first claim for an OAD in the measurement period for which adherence to OADs was calculated. This date was used for calculating age at baseline. The baseline and follow-up periods were each 12 months. Since the models were built in 2 steps, the baseline and follow-up periods were different for each step, as shown in Figure 1.
FIGURE 1.
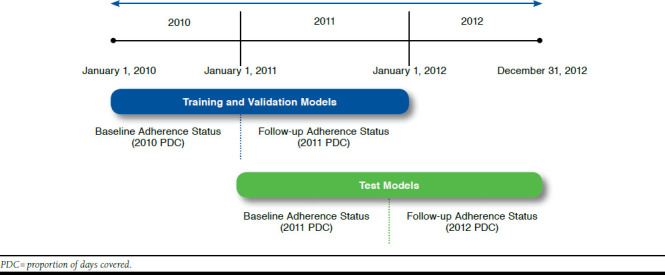
Study Period: January 1, 2010, to December 31, 2012
Measures
Baseline Measures.
For identifying the best predictors of change in adherence status from 1 year to the next, a number of demographic, behavioral, socioeconomic, clinical, and health care cost-related variables were used. The baseline variables were created separately for each baseline period except for the behavioral and socioeconomic variables, since the database containing these variables was available since July 2011, and it was assumed that the values for these variables did not change significantly from 2010 to 2011. Age at index date, sex, race/ethnicity, eligibility for low-income subsidy, dual eligibility, population density, and geographic region were determined for each subject based on the health plan enrollment data.
The clinical variables were created using medical, pharmacy, and laboratory data. Comorbidities including cardiovascular disease, nephropathy, neuropathy, retinopathy, obesity, depression and chronic kidney disease, bariatric surgery, lower extremity amputation, and documented hypoglycemic event were identified. The comorbidity scores included were from the Deyo-Charlson Comorbidity Index (DCCI), Diabetes Complications Severity Index (DCSI), Chronic Disease Score (CDS), and RxRisk-V Score.
The DCCI uses 17 categories of comorbidity to calculate a score that reflects cumulative increased likelihood of 1-year mortality.16 It predicts adverse outcomes including hospitalization and mortality based on number and severity of complications associated with diabetes and is based on a summary score derived from diagnostic and laboratory data.19 A recent implementation of the DCSI in a managed care setting demonstrated that a modified version of index-omitting laboratory data can be used to explain concurrent medical costs.19 In that study, DCSI was determined based on diagnosis codes only, and the laboratory components of the nephropathy category score were omitted.19 For our study, we created the DCSI scores based only on diagnosis codes. When controlled for health care utilization, CDS predicts subsequent mortality and hospitalization.20 CDS can serve, with certain precautions, as a readily accessible low-cost measure of health status.21 The potential range of values for CDS is 0 to 35.22 The RxRisk-V is a prescription claims-based comorbidity index originally developed as an enhancement of the RxRisk risk assessment instrument for use in the Veterans Health Administration population.23-25
Other baseline clinical variables included the use of each antidiabetic drug class, last OAD prescription filled, longest gap in OAD refill, use of mail order pharmacy, number of prescriptions with 90 days supply, total number of prescriptions filled, overall and diabetes-related pill burden, whether A1c was under control, total number of inpatient visits, total number of outpatient visits, and total number of emergency room visits.
The cost-related variables included cost for diabetic supplies (alcohol swabs, test strips, lancets, glucagon kit, insulin syringes, insulin injection pump and supplies, and syringe with needle for external insulin pump); out-of-pocket cost for diabetic supplies; copay for the last OAD filled; total medical cost (inpatient, outpatient, and emergency room visit costs); out-of-pocket medical cost; pharmacy cost; pharmacy out-of-pocket cost; and total health care cost. Total health care cost was the total of pharmacy and medical costs. Pharmacy and medical costs included member and plan-paid costs, whereas the out-of-pocket costs were the member-paid costs only. The behavioral and socioeconomic variables included estimated household income, home owner status indicator, primary language of the subjects, number of adults in the household, number of children in the household, total net worth, total number of people in the household, presence of an elderly parent in the household, education level, household with dual income, indicator for person living in a household with access to the Internet, percentage of blue-collar workers living in the area where the patient lives, and percentage of white-collar workers living in the area the patient lives. A detailed list of the socioeconomic and behavioral variables can be found in Appendix A (available in online article).
Outcome Measures.
The outcome variable was change in adherence status from baseline to the follow-up period. If a patient was adherent in the baseline year 2010 and nonadherent in the follow-up year 2011, that patient was categorized as having changed adherence status (adherent to nonadherent). Adherence to OADs was defined according to the Pharmacy Quality Alliance’s (PQA) medication adherence PDC measure as “the percent of days in the measurement period covered by prescription claims for the same medication or medications in its therapeutic category.”13 For measuring adherence, the claims for drugs from the following OAD classes were included: biguanides, sulfonylureas, TZDs, meglitinides, AGIs, and DPP-4 inhibitors. Patients were only included in the PDC calculation if the first medication fill occurred at least 91 days before the end of the measurement period. PDC was calculated for each patient for each of the baseline periods (years 2010 and 2011). Patients with a PDC ≥ 80% during a given period were assigned a status of “adherent,” and those with a PDC < 80% had a status of “nonadherent.” Patients changing from adherent in the baseline period to nonadherent in the follow-up period and vice versa were flagged as patients with “status change.”
Analyses
Separate models were generated for 2 cohorts: (1) patients adherent in the baseline period and nonadherent in the follow-up period; and (2) patients nonadherent in the baseline period and adherent in the follow-up period. The models were built stepwise in order to provide a robust analysis. First, all the baseline characteristics and adherence to OADs in 2010 were used as independent variables for identifying factors that were associated with change in adherence status from 2010 to 2011. Then the findings were tested using data from 2011 to predict adherence status change from 2011 to 2012. Figure 1 depicts the study time periods for adherence status change.
Because of the large sample size and the limitation of the software to process such large data within a reasonable time, we randomly selected 20% of the patients based on status change from the adherence to nonadherence group and 20% of the patients from the nonadherence to adherence group. Random sampling was used as a strategy for improving computational efficiency. This sample was then randomly partitioned into 2 subsets: training (50%) and validation (50%). The training dataset was used for preliminary model fitting; the validation dataset was used to assess the adequacy of the models. Models were cross-validated using the training and validation datasets. Validation misclassification rate was used for final model selection for each model generated.
The “best” model was defined by choosing an appropriate cut-point based on clinical meaningful value to balance the sensitivity and specificity. For the adherent cohort analysis, we wanted the model to enable us to correctly identify those adherent patients who became nonadherent in the follow-up year. High sensitivity and acceptable specificity were important because they helped to correctly identify a subgroup of patients as nonadherent and to correctly identify the maximum number of patients who maintained their adherence status in this subgroup. For the baseline adherent cohort model, we prioritized sensitivity over specificity and chose sensitivity around 75% as a reasonable cutpoint. For the nonadherent cohort analysis, we wanted a model with high specificity that would correctly identify patients likely to maintain nonadherence status in the follow-up year. Acceptable sensitivity guaranteed that the model had the ability to identify patients likely to be adherent in the follow-up year. Therefore, for the baseline nonadherent cohort model, we chose specificity around 70% and sensitivity around 50%.
Gradient boosting trees were used to generate models to identify the factors predictive of adherence status change. This method is widely used, and it is considered one of the most robust methods to identify predictors.26 From 91 factors, we identified the top 5 predictors ranked by their ability to predict adherence status change. Univariate logistic regression models for each of these top 5 variables were applied to each cohort to generate parameter estimates (odds ratios) for each of the variables to provide insight into the directionality and magnitude of association the respective variable had on adherence status change. Receiver operating characteristic (ROC) curves from the training and validation models were plotted. Area under the curve (AUC) indices, which are based on ROC curves and measure the predictive accuracy of the model, were computed. The AUC index assesses overall model performance for a range of cutoff values. The ROC curve was also examined to determine several potential cutpoint values. Each cutoff represents a trade-off between misclassification rate, sensitivity, and specificity. The operating characteristics at cutoff points based on ROC were reported for each model.
Various sensitivity analyses were conducted for testing the robustness of the methods. These analyses included running models after (a) dropping the variables for consumer/behavioral characteristics; (b) excluding patients taking noninsulin injectable antidiabetic medications (GLP-1) at any time during the baseline year or the follow-up year; and (c) including patients with claims for insulin.
All data analyses for this study were conducted using SAS 9.2/SAS EG (Enterprise Guide) and SAS EM (Enterprise Miner) 12.1 software (SAS Institute, Cary, NC). The a priori alpha level for all inferential analyses was set at 0.05, and all statistical tests were 2-tailed, unless otherwise specified.
Results
Sample Derivation
At the beginning of this study, over 1.4 million T2DM patients were identified. However, sample size dropped significantly after applying the inclusion and exclusion criteria.
Figure 2 presents the sample selection process. The arm “Total Patients” indicates the total number of patients (with and without insulin during the study period) that were eligible for enrollment in the study. There were 305,043 patients enrolled in this study. Among them, 238,402 patients did not have any insulin dispensed during the study period.
FIGURE 2.
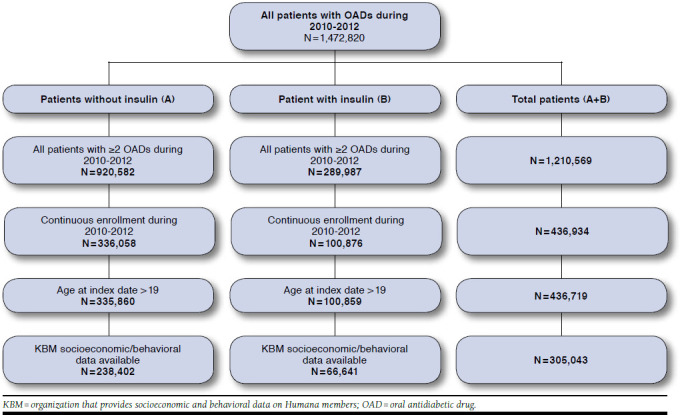
Study Sample Derivation
Patient Characteristics
Demographic Characteristics.
For the main analysis, the patients who had pharmacy claims for insulin were excluded. From the remaining sample, patients were divided into 2 groups based on adherence or nonadherence to OADs during the baseline period.
Baseline adherent group. As shown in Table 1, there were 144,216 patients adherent during the period from January 2010 to December 2010. These adherent patients were further divided into patients who changed adherence status (21.7%) in the follow-up period (January 2011-December 2011) and those whose adherence status did not change (78.3%). The mean age of the 2 groups was 72.0 and 72.3 years. Females (54.8% and 54.0%) and white race (81.4% and 81.7%) were in the majority in each group. A slightly higher proportion of patients with no adherence change in the follow-up period resided in the southern region of the United States (55.0% vs. 57.0%), while the proportion of patients living in urban areas (58.3% vs. 57.0%) was a little lower compared with patients without change in adherence status.
TABLE 1.
Baseline Demographics (January 2010-December 2010)
| Characteristic | Baseline Adherent | Baseline Nonadherent | ||||
|---|---|---|---|---|---|---|
| Status Change n = 31,320 | No Status Change n = 112,896 | P Value | Status Change n = 39,284 | No Status Change n = 54,902 | P Value | |
| Age, years, mean (SD) | 72.0 (8.6) | 72.3 (8.6) | < 0.001a | 71.4 (9.1) | 70.8 (9.7) | < 0.001a |
| Age category, n (%) | ||||||
| 19-29 | < 10 (0.0) | 20 (0.0) | < 0.001b | 15 (0.0) | 48 (0.1) | < 0.001b |
| 30-39 | 53 (0.2) | 211 (0.2) | 107 (0.3) | 302 (0.6) | ||
| 40-49 | 364 (1.2) | 1,269 (1.1) | 680 (1.7) | 1,206 (2.2) | ||
| 50-59 | 1,522 (4.9) | 5,138 (4.6) | 2,370 (6.0) | 3,839 (7.0) | ||
| 60-69 | 6,459 (20.6) | 22,194 (19.7) | 9,043 (23.0) | 11,846 (21.6) | ||
| 70-79 | 15,534 (49.6) | 56,664 (50.2) | 18,802 (47.9) | 26,098 (47.5) | ||
| 80-89 | 6,604 (21.1) | 24,434 (21.6) | 7,400 (18.8) | 10,363 (18.9) | ||
| ≥ 90 | 777 (2.5) | 2,966 (2.6) | 867 (2.2) | 1,200 (2.2) | ||
| Sex, n (%) | ||||||
| Male | 14,168 (45.2) | 51,976 (46.0) | < 0.012b | 18,261 (46.5) | 24,153 (44.0) | < 0.001b |
| Female | 17,152 (54.8) | 60,920 (54.0) | 21,023 (53.5) | 30,749 (56.0) | ||
| Race, n (%) | ||||||
| White | 25,497 (81.4) | 92,284 (81.7) | < 0.001b | 31,586 (80.4) | 40,840 (74.4) | < 0.001b |
| Black | 3,264 (10.4) | 11,719 (10.4) | 4,012 (10.2) | 7,751 (14.1) | ||
| Hispanic | 551 (1.8) | 2,225 (2.0) | 748 (1.9) | 1,204 (2.2) | ||
| Other | 944 (3.0) | 3,231 (2.9) | 1,222 (3.1) | 1,718 (3.1) | ||
| Unknown | 1,064 (3.4) | 3,437 (3.0) | 1,716 (4.4) | 3,389 (6.2) | ||
| Geographic region, n (%) | ||||||
| Northeast | 1,894 (6.1) | 6,598 (5.8) | < 0.001b | 2,089 (5.3) | 2,854 (5.2) | < 0.001b |
| Midwest | 8,509 (27.2) | 30,160 (26.7) | 10,380 (26.4) | 13,403 (24.4) | ||
| South | 17,218 (55.0) | 64,287 (57.0) | 22,302 (56.8) | 32,306 (58.8) | ||
| West | 3,698 (11.8) | 11,842 (10.5) | 4,511 (11.5) | 6,336 (11.5) | ||
| Unknown | < 10 (0.0) | < 10 (0.0) | < 10 (0.0) | < 10 (0.0) | ||
| Population density, n (%) | ||||||
| Urban | 18,247 (58.3) | 64,389 (57.0) | < 0.001b | 22,975 (58.5) | 33,023 (60.2) | < 0.001b |
| Suburban | 8,191 (26.2) | 29,393 (26.0) | 10,062 (25.6) | 13,838 (25.2) | ||
| Rural | 4,682 (15.0) | 18,521 (16.4) | 6,009 (15.3) | 7,692 (14.0) | ||
| Unknown | 200 (0.6) | 593 (0.5) | 238 (0.6) | 349 (0.6) | ||
| Plan characteristics, n (%) | ||||||
| LIS status only | 2,238 (7.2) | 10,011 (8.9) | < 0.001b | 2,893 (7.4) | 4,128 (7.5) | 0.373b |
| Dual eligibility only | 86 (0.3) | 348 (0.3) | 0.336b | 116 (0.3) | 154 (0.3) | 0.676b |
| LIS status and dual eligibility | 2,666 (8.5) | 12,579 (11.1) | < 0.001b | 3,529 (9.0) | 5,027 (9.7) | 0.362b |
a Wilcoxon rank sum test was used for the continuous variables for all the tables.
b Chi-square test was used for the categorical variables for all the tables.
LIS = low-income subsidy; SD = standard deviation.
Baseline nonadherent group. As Table 1 shows, there were 94,186 nonadherent patients identified during the period from January 2010 to December 2010, among whom adherence status changed for 41.7% of patients in the follow-up period (January 2011-December 2011), and adherence status did not change for 58.3% of patients (Table 1). The mean age of the 2 groups was 71.4 vs. 70.8 years. Females (53.5% vs. 56.0%) and white race (80.4% vs. 74.4%) were in the majority in the 2 groups. More than half of the population in each group lived in the southern region (56.8% vs. 58.8%) and urban areas (58.5% vs. 60.2%).
Baseline Clinical Characteristics.
The clinical characteristics were measured at the baseline for adherent and nonadherent cohorts. These were included in the models as covariates.
Baseline adherent group. All of the descriptions (numbers) in Table 2 are the baseline clinical characteristics for the 2 groups—status change and no status change, respectively. The mean PDC of the 2 groups was 0.90 vs. 0.92. Only 18.6% vs. 17.3% patients had most recent baseline A1c below 7%. More patients in the status-change group had cardiovascular disease compared with patients in the no-status-change group (20.1% vs. 18.7%, P < 0.001). The absolute DCCI (1.08 vs. 1.06), DCSI (0.94 vs. 0.93), and CDS (8.31 vs. 8.34) scores were similar in the 2 groups. The most frequently used antidiabetic classes were biguanides (78.1% vs. 79.2%), sulfonylureas (52.4%, 63.1%), and TZDs (16.2% vs. 22.3%). A high percentage of the population in the status-change group used mail order pharmacy services (74.7% vs. 50.8%, P < 0.001). The average daily diabetes-related pill burden (1.19 vs. 1.35, P < 0.001) and total pill burden (5.52 vs. 5.78, P < 0.001) in the status-change group were a little smaller than the no-status-change group.
TABLE 2.
Baseline Clinical Characteristics (January 2010-December 2010)
| Characteristic | Baseline Adherent | Baseline Nonadherent | ||||
|---|---|---|---|---|---|---|
| Status Change n = 31,320 | No Status Change n = 112,896 | P Value | Status Change n = 39,284 | No Status Change n = 54,902 | P Value | |
| Adherence to OAD medications | ||||||
| PDC, mean (SD) | 0.90 (0.1) | 0.92 (0.1) | < 0.001a | 0.59 (0.2) | 0.54 (0.2) | < 0.001b |
| Adherent (PDC ≥ 80%), n (%) | 31,320 (21.7) | 112,896 (78.3) | < 0.001b | 39,284 (41.7) | 54,902 (58.3) | < 0.001b |
| Copay of last OAD during baseline period, $ | ||||||
| Mean (SD) | 15 (54) | 18 (60) | < 0.001a | 13 (43) | 14 (42) | < 0.001a |
| Median (IQR) | 3 (10) | 4 (8) | – | 4 (8) | 4 (10) | – |
| Final A1c laboratory value, n (%) | ||||||
| Under control | 5,814 (18.6) | 19,542 (17.3) | < 0.001b | 6,914 (17.6) | 9,874 (18.0) | < 0.001b |
| Uncontrolled | 2,167 (6.9) | 8,675 (7.7) | 3,163 (8.1) | 4,391 (8.0) | < 0.001b | |
| Unknown | 23,339 (74.5) | 84,679 (75.0) | 29,207 (74.4) | 40,637 (74.0) | < 0.001b | |
| Comorbid conditions, n (%) | ||||||
| Cardiovascular disease | 6,295 (20.1) | 21,066 (18.7) | < 0.001b | 7,722 (19.7) | 10,171 (18.5) | < 0.001b |
| Nephropathy | 3,625 (11.6) | 13,878 (12.3) | < 0.001b | 4,422 (11.3) | 6,007 (10.9) | < 0.001b |
| Neuropathy | 4,024 (12.9) | 14,512 (12.9) | < 0.001b | 4,613 (11.7) | 6,540 (11.9) | < 0.001b |
| Retinopathy | 2,071 (6.6) | 7,831 (6.9) | < 0.001b | 2,366 (6.0) | 3,121 (5.7) | < 0.001b |
| Obesity | 1,934 (6.2) | 6,449 (5.7) | < 0.001b | 2,650 (6.8) | 3,563 (6.5) | < 0.001b |
| Depression | 1,649 (5.3) | 5,271 (4.7) | < 0.001b | 2,327 (5.9) | 3,218 (5.9) | < 0.001b |
| Chronic kidney disease | 2,794 (8.9) | 10,779 (9.6) | < 0.001b | 3,328 (8.5) | 4,550 (8.3) | < 0.001b |
| Diabetes-related complications, n (%) | ||||||
| Bariatric surgery | 0 | 0 | – | 0 | 0 | – |
| Lower extremity amputation | 0 | 0 | – | 0 | 0 | – |
| Emergent hypoglycemic event | 606 (1.9) | 1,846 (1.6) | < 0.001b | 669 (1.7) | 972 (1.8) | < 0.001b |
| Comorbidity scores, mean (SD) | ||||||
| DCCI | 1.08 (1.7) | 1.06 (1.7) | < 0.001a | 1.05 (1.6) | 1.03 (1.6) | 0.351a |
| DCSI | 0.94 (1.6) | 0.93 (1.7) | < 0.001a | 0.91 (1.6) | 0.87 (1.6) | 0.003a |
| CDS | 8.31 (2.9) | 8.34 (2.9) | 0.094a | 8.22 (3.0) | 7.99 (3.0) | < 0.001a |
| RxRisk-V | 2.16 (2.2) | 2.35 (2.3) | < 0.001a | 2.04 (2.2) | 1.95 (2.1) | < 0.001a |
| Oscillation status, n (%) | 13,347 (42.9) | 30,506 (27.0) | < 0.001b | 42,250 (77.0) | 24,866 (63.3) | < 0.001b |
| Medication use | ||||||
| Number of antidiabetic classes used, mean (SD) | 1.45 (0.6) | 1.63 (0.7) | < 0.001a | 1.37 (0.6) | 1.29 (0.5) | < 0.001a |
| Use of each antidiabetic class, n (%) | ||||||
| Biguanides | 24,456 (78.1) | 89,409 (79.2) | < 0.001b | 30,612 (77.9) | 42,227 (76.9) | < 0.001b |
| Sulfonylureas | 16,417 (52.4) | 71,265 (63.1) | < 0.001b | 18,419 (46.9) | 22,235 (40.5) | < 0.001b |
| Thiazolidinediones | 5,062 (16.2) | 25,191 (22.3) | < 0.001b | 5,380 (13.7) | 7,975 (14.5) | < 0.001b |
| Amylin agonists | 0 | 0 | – | 0 | < 10 (0.0) | < 0.001b |
| Meglitinides | 314 (1.0) | 472 (0.4) | < 0.001b | 527 (1.3) | 1,454 (2.7) | < 0.001b |
| Alpha-glucosidase inhibitors | 179 (0.6) | 313 (0.3) | < 0.001b | 172 (0.4) | 436 (0.8) | < 0.001b |
| Glucagone-like peptide-1 receptor agonists | 388 (1.2) | 610 (0.5) | < 0.001b | 356 (0.9) | 619 (1.1) | < 0.001b |
| DPP-4 inhibitors | 754 (2.4) | 4,793 (4.3) | < 0.001b | 1,140 (2.9) | 994 (1.8) | < 0.001b |
| Insulin (subgroup analysis only) | 0 | 0 | – | 0 | 0 | – |
| Last OAD class used during the baseline period, n (%) | ||||||
| Biguanides | 15,978 (51.0) | 52,283 (46.3) | < 0.001b | 21,612 (55.0) | 30,643 (55.8) | < 0.001b |
| Sulfonylureas | 10,705 (34.2) | 40,896 (36.2) | < 0.001b | 12,235 (31.1) | 14,305 (26.1) | < 0.001b |
| Thiazolidinediones | 2,099 (6.7) | 10,629 (9.4) | < 0.001b | 2,437 (6.2) | 3,732 (6.8) | < 0.001b |
| Meglitinides | 90 (0.3) | 132 (0.1) | < 0.001b | 120 (0.3) | 925 (1.7) | < 0.001b |
| Alpha-glucosidase inhibitors | 43 (0.1) | 113 (0.1) | < 0.001b | 57 (0.2) | 232 (0.4) | < 0.001b |
| DPP-4 inhibitors | 70 (0.2) | 355 (0.3) | < 0.001b | 127 (0.3) | 203 (0.4) | < 0.001b |
| Biguanide + DPP-4 inhibitor | 0 | 0 | – | 1 (0.0) | 1 (0.0) | < 0.001b |
| Biguanide+ meglitinide | 1,590 (5.1) | 5,297 (4.7) | < 0.001b | 1,569 (4.0) | 2,923 (5.3) | < 0.001b |
| Biguanide + sulfonylurea | 570 (1.8) | 2,020 (1.8) | < 0.001b | 708 (1.8) | 1,609 (2.9) | < 0.001b |
| Biguanide + thiazolidinediones | 175 (0.6) | 1,171 (1.0) | < 0.001b | 418 (1.1) | 329 (0.6) | < 0.001b |
| Use of mail order pharmacy for fills of antidiabetic medications, n (%) | 23,409 (74.7) | 57,345 (50.8) | < 0.001b | 22,282 (56.7) | 30,471 (55.5) | < 0.001b |
| Number of claims for 90-day supply of antidiabetic medications, mean (SD) | 3.41 (2.9) | 2.35 (3.0) | < 0.001a | 1.76 (2.1) | 1.64 (2.0) | < 0.001a |
| Pill burden, mean (SD) | ||||||
| Diabetes-related pill burden | 1.19 (0.4) | 1.35 (0.5) | < 0.001a | 0.75 (0.4) | 0.67 (0.3) | < 0.001a |
| Total pill burden | 5.52 (2.5) | 5.78 (2.5) | < 0.001a | 4.60 (2.3) | 4.07 (2.2) | < 0.001a |
| Longest medication gap, mean (SD) | 16.53 (15.9) | 11.42 (12.5) | < 0.001a | 34.52 (39.7) | 45.78 (43.1) | < 0.001a |
| Number of pharmacies used, mean (SD) | 28.02 (16.7) | 32.91 (18.6) | < 0.001a | 27.03 (16.7) | 24.61 (15.6) | < 0.001a |
| Enrollment in Humana program that may impact adherence, n (%) | 3,230 (10.3) | 1,1350 (10.1) | < 0.001b | 3,800 (9.7) | 5,579 (10.2) | < 0.001b |
a Wilcoxon rank sum test was used for the continuous variables for all the tables.
b Chi-square test was used for the categorical variables for all the tables.
A1c = glycated hemoglobin; CDS = Chronic Disease Score; DCCI = Deyo-Charlson Comorbidity Index; DCSI = Diabetes Complications Severity Index; DPP-4 = dipeptidyl peptidase-4; IQR = interquartile range; OAD = oral antidiabetic drug; PDC = proportion of days covered; SD = standard deviation.
Baseline nonadherent group. As shown in Table 2, the mean PDC of the 2 groups, status change and no status change, was 0.59 vs. 0.54, respectively. Only 17.6% vs. 18.0% of patients had A1c below 7%. More patients in the status-change group had cardiovascular disease than patients in the no-status-change group (19.7% vs. 18.5%, P < 0.05). The absolute DCCI (1.05 vs. 1.03), DCSI (0.91 vs. 0.87), and CDS (8.22 vs. 7.99) scores were similar in the 2 groups. The antidiabetic classes most frequently used were biguanides (77.9% vs. 76.9%), sulfonylureas (46.9% vs. 40.5%), and TZDs (13.7% vs. 14.5%). Similar proportions of patients in the 2 groups (56.7% vs. 55.5%) used mail order pharmacy services. The diabetes-related pill burden (0.75 vs. 0.67) and the total pill burden (4.60 vs. 4.07) in the status-change group were a little higher than the no-status-change group. As expected, the antidiabetic drug use in the nonadherent patients was lower at baseline than for adherent patients.
Predictors of Adherence Status Change
Figure 3A represents the results for a subset of members who were adherent in the 2010 baseline period (Model 1A), while Figure 3B represents the results for a subset of members who were nonadherent in the 2010 baseline period (Model 1B).
FIGURE 3.
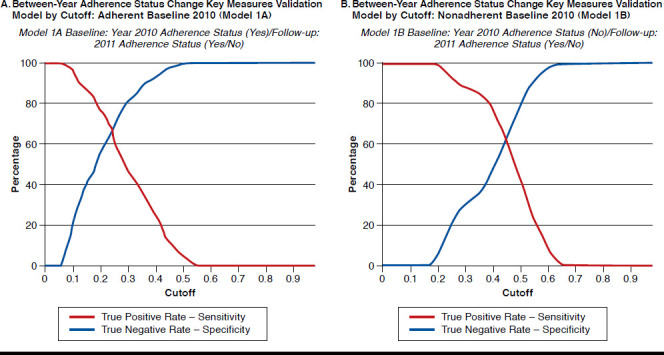
Validation Model Summary
Figure 3A provides the key measures by cutoff and was used to select the target cutoff. The misclassification rate for the validation model was 39% with a sensitivity and specificity of 76% and 57%, respectively, using a cutoff of 0.20. The c-statistic for the validation model was 0.73. The top 5 predictors identified by the gradient boosted classification tree were subsequently used in a univariate logistic regression model to calculate odds ratios (ORs), which could be used to quantify the direction and magnitude of the association between the predictor and becoming nonadherent in the 2011 follow-up period (see Appendix B, available in online article). The predictors were the number of 90-day prescriptions filled in the baseline period (OR = 1.107, 95% confidence interval [CI] = 1.093-1.120); diabetes-related pill burden (OR = 0.450, 95% CI = 0.410-0.494); longest gap in OAD therapy (7-day increments, OR = 1.183, 95% CI = 1.161-1.205); total number of antidiabetic classes filled (OR = 0.625, 95% CI = 0.586-0.666); and copay for the last OAD ($4 increments, OR = 0.712, 95% CI = 0.657-0.771; Appendix B).
Table 3 provides the results when the model was applied to a separate dataset where the member was adherent during 2011 (baseline), and 2012 was the follow-up period. Using a cutoff of 0.20, the test model had a misclassification rate of 46% and sensitivity and specificity of 80% and 44%, respectively.
TABLE 3.
Between-Year Adherence Status Change Test Model Summary: Primary Analyses
| Predictive Model Name | Adherent Baseline 2011 (Model 1A) | Nonadherent Baseline 2011 (Model 1B) |
|---|---|---|
| Cutoff | 0.20 | 0.47 |
| Sensitivity | 79.7% | 43.8% |
| Specificity | 44.5% | 76.8% |
| Positive Predictive Value | 34.7% | 51.8% |
| Negative Predictive Value | 85.5% | 70.6% |
| Misclassification Rate | 46.0% | 35.1% |
Figure 3B provides the key measures by cutoff and was used to select the target cutoff. The misclassification rate for the validation model was 36% with a sensitivity and specificity of 53% and 71%, respectively, using a cutoff of 0.47. The c-statistic for the validation model was 0.68. The top 5 predictors that increase the chances of being adherent in the 2011 follow-up period and their ORs from univariate logistic regression model were diabetes-related pill burden (OR = 2.122, 95% CI = 1.883-2.391); longest gap in OAD therapy (7-day increments, OR = 0.958, 95% CI = 0.951-0.965); month-wise oscillation between adherence statuses (OR = 0.531, 95% CI = 0.4850.581); the number of 90-day prescriptions filled in the baseline period (OR = 1.030, 95% CI = 1.010-1.051); and total pill burden (OR = 1.103, 95% CI = 1.082-1.123; Appendix B).
Table 3 provides the results when the model was applied to a separate dataset where the member was nonadherent during 2011 (baseline), and 2012 was the follow-up period. Using a cutoff of 0.47, the test model had a misclassification rate of 35.1% and sensitivity and specificity of 43.8% and 76.8%, respectively.
Figure 4A-B represents the ORs and their 95% CIs for baseline adherent and nonadherent cohorts, respectively. Details are shown in Appendix B.
FIGURE 4.
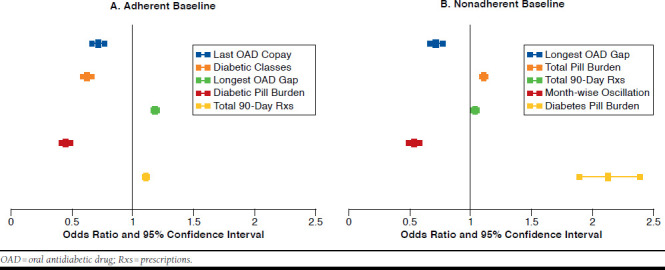
Predictors for Adherence Status Change in the Follow-up Period
Sensitivity Analyses Findings
Sensitivity analysis was conducted by adding patients who had filled at least 1 claim for insulin during the study period. All these patients were further divided into separate cohorts of patients who were adherent at baseline and those who were nonadherent. Using the same approach as in the main analyses, we developed predictive models for these cohorts to identify those patients likely to have a change in adherence status during the follow-up year. The predictors obtained were similar to the main analyses discussed above. For the cohort adherent at baseline, the top 5 predictors identified by the gradient boosted classification tree were use of mail order pharmacy in the baseline period, 90-day prescriptions filled, longest gap in OAD therapy (7-day increments), diabetes-related pill burden, and use of the sulfonylurea drug class.
For the nonadherent cohort, the top 5 predictors identified by the gradient boosted classification tree at baseline were diabetes-related pill burden in the baseline period, longest gap in OAD therapy (7-day increments), month-wise oscillation between adherence statuses, 90-day prescriptions filled, and use of the sulfonylurea drug class.
Discussion
It is worth noting that the 5 most significant predictors for adherence status change among the adherence and nonadherence baseline groups were treatment-related variables; none were patient related or disease related. Almost one-third of the OAD users had an adherence status change from 1 year to the next, with almost 22% of patients adherent in the baseline year shifting to a nonadherent status in the follow-up year. These findings represent an opportunity for health plans to impact the medication utilization behavior of members who are pre-scribed medications for chronic conditions.
Current findings demonstrated that predictive models can provide a valuable method of identifying those patients likely to have an adherence status change from a large pool of health plan members using OADs for the treatment of diabetes. Subsequent sensitivity analysis, which added patients who were treated with insulin, also confirmed the results. The models demonstrated a fair amount of consistency in the predictors identified as being important for identifying changes in adherence status. In particular, for patients changing status from adherent to nonadherent, those with higher diabetes-related pill burden were less likely to become nonadherent (OR = 0.45, P < 0.001) and were consistently and positively associated with becoming adherent (OR = 2.122, P < 0.001) among the nonadherence baseline group. These results indicate that patients with higher pill burden are likely to be more adherent.
The predictive models used in this study (see Table 3) were set up to identify a change in status. Most likely, the members with higher pill burden and higher copays experienced treatment intensification by using more therapeutic agents and possibly newer/branded therapies in the baseline period. Patients were engaged in their treatment because they knew it was important to control their diabetes, and that engagement lasted into the follow-up period.
This study used the PQA method for calculating adherence, which aggregates the therapeutic categories into a single measure of adherence (multiple therapeutic categories could translate into higher pill burden). Also, adherence was measured only for OADs. This study required patients to have ≥ 2 prescriptions filled in each year of the study period (baseline and follow-up). Since patients already have a higher pill burden with their OADs, and 78.3% of patients did not change from adherent status to nonadherent status in the follow-up period, it is likely that patients did not become nonadherent in the follow-up period. Either the patients were motivated to be adherent to their OADs or if there was any intervention by the insurance company, then the results may have been from the the follow-up period.
Furthermore, the models developed as a part of this study are meant to be used in the context of business activities. They are not set up to make inferences about the relationship between factors such as pill burden and copayment. The reason for using the univariate regression models was to get an idea of the direction of the association. Consequently, the results of the regression analyses may not be generalizable.
During the follow-up period, an increase in the longest gap in OADs predicted nonadherence (OR = 1.183, P < 0.001) among the adherence baseline group and was negatively associated with adherence (OR = 0.958, P < 0.001) among the nonadherence baseline group. The results for the longest gap in OADs playing different roles in the 2 groups may be because patient populations were different for these 2 groups.
Results of the this study complement findings of other publications. For example, a number of studies found higher copayments to be associated with lower medication (including OADs) adherence,14,27,28 but none evaluated the association of higher copayments with change in adherence status. Our study showed that a higher copay for the last OAD filled was negatively associated with becoming nonadherent (OR = 0.712, P < 0.001). The effect of higher copays on adherence status change was consistent with that of higher diabetes-related pill burden and total number of antidiabetic classes filled.
The strength of the current study is the large number of patient-, treatment-, and disease-related variables that were evaluated. Among patient-related variables were patient demographic, behavioral, psychosocial, and socioeconomic characteristics. Treatment-related variables included treatment complexity, costs, pill burden, and month-wise oscillation from adherent to nonadherent and vice versa. Among disease-related variables were a number of proxies for illness severity measured via comorbidity indices.
The modest predictive accuracy of the models was similar to a study performed by Steiner et al. (2009) that evaluated sociodemographic and clinical predictors of patients with chronic disease (hypertension) and showed that socioeconomic variables were not strong predictors of adherence.29 This modest performance could have been due to several underlying causes that were not captured in the data. Medication adherence can be impacted by several key factors not available in an administrative claims database (e.g., patient attitudes toward the disease and its treatment). Furthermore, although we used the same medication adherence measurement methodology used by the PQA, that methodology can be biased when attempting to calculate adherence for a condition similar to diabetes when multiple therapies may be utilized for glucose control. Since PQA methodology only includes OADs in the calculation of PDC, if patients are taking injectable OADs, those will not be included, which leads to bias in identifying patients as adherent or nonadherent. Therapies may frequently be adjusted or temporarily discontinued so members may appear to not be refilling their medications in a consistent manner. Group-based trajectory models may provide a better measure of adherence status given the dynamic nature of adherence over time.30
The predictors that were deemed to be important factors for classifying adherence status between the time periods are readily available in most health plans’ data platforms and can be measured with relative ease. Furthermore, the predictors that consistently demonstrated importance in the primary and sensitivity analysis models could serve as areas of focus for health plans. For example, the number of prescriptions filled with a 90-day supply and gap in OAD therapy are predictors that could be influenced by health plans through benefit designs and provider messaging. Predictors such as diabetes-related pill burden could be impacted by a manufacturer by developing formulations that require once-daily or even less-frequent dosing.
Limitations
The predictive models were built using the retrospective Humana Medicare Advantage Database and have certain limitations. All patients included in this study were required to be continuously enrolled in Humana health plans for at least 3 years and have at least 2 prescriptions in each year of the study period. Because of this requirement, patients who died or unenrolled from Humana Medicare Advantage plans during the study period may have gotten excluded. Patients excluded for not having continuous enrollment in Humana Medicare Advantage plans could be different from those with continuous enrollment, particularly in socioeconomic and behavioral characteristics. Also, excluded patients could have had different levels of access to medications and hence adherence. Similarly, patients who died could have had severe conditions that needed focus on other therapies, which would have affected adherence to OADs. Since we do not know the situations of the excluded patients, we feel that the results should be interpreted cautiously.
Retrospective claim study design has several limitations in general (e.g., missing data, coding error, and selection bias) and restrain the ability to assess the causal relationship between the predictors and outcomes.31 More research needs to be done to understand the causal relationships between medication adherence and the identified factors and to clarify which specific interventions may help maintain adherence among initially adherent patients and improve medication adherence among patients who are initially nonadherent.
Conclusions
Patient adherence to OADs appears to dynamically change over time, with about one-third of T2DM patients changing adherence status from adherent to nonadherent and vice versa from 1 year to the next. Our methods identified a set of distinct factors associated with such changes in adherence status, which could help identify patients who should be targeted for adherence enhancement programs to help improve long-term treatment outcomes. The identified factors could also be used by health plans to improve allocation of resources used for addressing medication adherence measures.
ACKNOWLEDGMENTS
The authors would like to thank Radhika Nair, PhD, Research Lead, Comprehensive Health Insights, for her assistance with writing this manuscript.
Appendix A. List of Socioeconomic and Behavioral Characteristics
| Religion |
| Protestant |
| Catholic |
| Jewish |
| Unknown |
| Other |
| Ethnic indicator |
| English (British) |
| Hispanic (Spanish) |
| African American |
| Unknown |
| All others |
| Language preference |
| English |
| Hispanic |
| Other |
| Estimated household income |
| < $15,000-$19,999 |
| $20,000-$49,999 |
| $50,000-$99,999 |
| ≥ $10,0000 |
| Dual Income Index |
| A statistical model predicting a household's likelihood to have more than 1 income. The value of 0 indicates a household to most likely have dual incomes |
| and 9 being the most likely to have a single income |
| Census 2010 socioeconomic score |
| A socioeconomic score indicating whether a particular geographic unit (i.e., block group) is higher than or equal to the U.S. norm, which is 100, as |
| reported by the Census Bureau. The scores range from 60-170 and are created by assigning weights to the household income, educational attainment, |
| occupation and home value |
| 0- ≤ 69 |
| 70- ≤ 79 |
| 80- ≤ 89 |
| 90- ≤ 99 |
| ≥ 100 |
| Net worth indicator |
| Estimate of a household's total financial assets minus liabilities |
| $0- ≤ $69,000 |
| $70,000- ≤ $79,000 |
| $80,000- ≤ $89,000 |
| $90,000- ≤ $99,000 |
| ≥ $100,000 |
| Census 2010 percentage of mobile homes |
| Percentage of persons living in a mobile home reported by the Census Bureau |
| 0-9 |
| 10-19 |
| 20-29 |
| 30-39 |
| ≥ 40 |
| Home Value at ZIP 4 level |
| An average value of the properties in the area with an assessment of area-level comparisons coded at a ZIP+4 level |
| $0 |
| $1-$99 |
| $100-$199 |
| $200-$299 |
| ≥ $300 |
| Homeowner status |
| Compilation of known and modeled fields indicating the property ownership status of the household |
| Probable homeowner |
| Renter |
| Probable renter |
| Unknown |
| Homeowner |
| Number of children in household |
| Number of adults in household |
| Number of persons in household |
| Presence of elderly parent |
| Presence of college graduate |
| Census 2010 education level |
| An indicator derived from the highest level of education attained by individuals 25 years or older as reported by the Census Bureau |
| Uncoded |
| Less than 9th grade |
| Less than 12th grade |
| High school diploma |
| Some college |
| Associate degree |
| Bachelor degree |
| Graduate degree |
| Professional school degree |
| Doctoral degree |
| Family position |
| Indicator of an individual's position in the household based on age and gender |
| Spouse, husband or wife |
| Head of household, male/female |
| Grandmother |
| Grandfather |
| Child |
| Unknown |
| Length of residence |
| Compilation of fields indicating the length of time the household has been reported at the address |
| 0-9 |
| 10-19 |
| 20-29 |
| 30-39 |
| ≥ 40 |
| Online access |
| Field indicating the household has online access |
| Buyer and interest categories |
| Health & fitness |
| An individual within the household purchased health products from online or offline catalogs |
| Travel |
| An individual within the household purchased travel from online or offline catalogs or a retail store and has interest in international as well as domestic |
| travel |
| Gambling |
| Indicates an individual within the household has an interest in casino gambling |
| Pets |
| An individual within the household purchased pets or pet products from online or offline catalogs or a retail store or owns a cat or a dog |
| Leisure activity |
| Indicates an individual within the household has an interest in boating/sailing/fishing/gardening/golf/gourmet/foods/hunting/motorcycling/photography/wines, recreational vehicles. |
| Living well |
| A demographic-based analytical model which predicts an individual's attitude or perception about their health |
| Leading the way |
| In it for fun |
| Value independence |
| I need a plan |
| Not right now |
| Get through the day |
| Mindbase 2.0 Group |
| Variable that categorizes a person's buying habits |
| Expressive |
| Driven |
| At capacity |
| Rock steady |
| Down to earth |
| Sophisticated |
| Measure twice |
| Devoted |
| Census 2010 percentage of blue collar employed |
| Percentage of the population which is employed in a blue-collar industry as reported by the Census Bureau |
| 0-19 |
| 20-39 |
| ≥ 40 |
| Census 2010 percentage of white collar employed |
| Percentage of the population which is employed in a white-collar industry as reported by the Census Bureau |
| 0-39 |
| 40-59 |
| 60-79 |
| ≥ 80 |
| Diet model rank |
| Over-indulgent (alcohol) model rank |
| Health model rank |
| No exercise model rank |
Appendix B. Predictors for Adherence Status Change in the Follow-up Period
| Odds Ratio | 95% Lower CI | 95% Upper CI | P Value | |
|---|---|---|---|---|
| Adherent Baseline 2010 (Model 1A) | ||||
| Total number of Rxs filled with 90-day supply | 1.107 | 1.093 | 1.120 | < 0.001 |
| Diabetes-related pill burden | 0.450 | 0.410 | 0.494 | < 0.001 |
| Longest gap in OADs (7-day increments) | 1.183 | 1.161 | 1.205 | < 0.001 |
| Total number of antidiabetic classes filled | 0.625 | 0.586 | 0.666 | < 0.001 |
| Copay for the last OAD filled ($4 increments) | 0.712 | 0.657 | 0.771 | < 0.001 |
| Nonadherent Baseline 2010 (Model 1B) | ||||
| Diabetes-related pill burden | 2.122 | 1.883 | 2.391 | < 0.001 |
| Longest gap in OADs (7-day increments) | 0.958 | 0.951 | 0.965 | < 0.001 |
| Month-wise patient oscillation from adherent to nonadherent and vice versa | 0.531 | 0.485 | 0.581 | < 0.001 |
| Total number of Rxs filled with 90-day supply | 1.030 | 1.010 | 1.051 | 0.003 |
| Total pill burden | 1.103 | 1.082 | 1.123 | < 0.001 |
CI = confidence interval; OAD = oral antidiabetic drug; Rxs = prescriptions.
REFERENCES
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31(3):596-615. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed January 26, 2016. [Google Scholar]
- 3.Centers for Disease Control and Prevention. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Atlanta, GA: U.S. Department of Health and Human Services, 2014. Available at: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 26, 2016. [Google Scholar]
- 4.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-53. [PubMed] [Google Scholar]
- 5.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract. 2011;65(3):314-22. [DOI] [PubMed] [Google Scholar]
- 7.Hepke KL, Martus MT, Share DA. Costs and utilization associated with pharmaceutical adherence in a diabetic population. Am J Manag Care. 2004;10(2 Pt 2):144-51. [PubMed] [Google Scholar]
- 8.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-24. [DOI] [PubMed] [Google Scholar]
- 9.Feldman BS, Cohen-Stavi CJ, Leibowitz M, et al. Defining the role of medication adherence in poor glycemic control among a general adult population with diabetes. PLoS One. 2014;9(9):e108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trinacty CM, Adams AS, Soumerai SB, et al. Racial differences in long-term adherence to oral antidiabetic drug therapy: a longitudinal cohort study. BMC Health Serv Res. 2009;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521-30. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Quality Assurance (NCQA). HEDIS & Quality Measurement. HEDIS 2014, Volume 2. Summary table of measures, product lines and changes. Available at: http://www.ncqa.org/Portals/0/HEDISQM/HEDIS2014/List_of_HEDIS_2014_Measures.pdf. Accessed February 6, 2016.
- 13.Centers for Medicare & Medicaid Services (CMS). Quality rating system measure technical specifications. September 2014. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Specifications.pdf. Accessed February 6, 2016.
- 14.Maciejewski ML, Bryson CL, Perkins M, et al. Increasing copayments and adherence to diabetes, hypertension, and hyperlipidemic medications. Am J Manag Care. 2010;16(1):e20-34. [PubMed] [Google Scholar]
- 15.Polonsky WH, Anderson BJ, Lohrer PA, Aponte JE, Jacobson AM, Cole CF. Insulin omission in women with IDDM. Diabetes Care. 1994;17(10):1178-85. [DOI] [PubMed] [Google Scholar]
- 16.Quilliam BJ, Ozbay AB, Sill BE, Kogut SJ. The association between adherence to oral anti-diabetic drugs and hypoglycaemia in persons with Type 2 diabetes. Diabet Med. 2013;30(11):1305-13. [DOI] [PubMed] [Google Scholar]
- 17.Kalsekar ID, Madhavan SS, Amonkar MM, Makela EH, Scott VG, Douglas SM. Depression in patients with type 2 diabetes: impact on adherence to oral hypoglycemic agents. Ann Pharmacother. 2006;40(4):605-11. [DOI] [PubMed] [Google Scholar]
- 18.Barron J, Wahl P, Fisher M, Plauschinat C. Effect of prescription copayments on adherence and treatment failure with oral antidiabetic medications. PT. 2008;33(9):532-53. [PMC free article] [PubMed] [Google Scholar]
- 19.Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012;18(11):721-26. [PubMed] [Google Scholar]
- 20.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45(2):197-203. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RE, Hornbrook MC, Nichols GA. Replicating the chronic disease score (CDS) from automated pharmacy data. J Clin Epidemiol. 1994;47(10):1191-99. [DOI] [PubMed] [Google Scholar]
- 22.McGregor JC, Kim PW, Perencevich EN, et al. Utility of the Chronic Disease Score and Charlson Comorbidity Index as comorbidity measures for use in epidemiologic studies of antibiotic-resistant organisms. Am J Epidemiol. 2005;161(5):483-93. [DOI] [PubMed] [Google Scholar]
- 23.Sloan KL, Sales AE, Liu CF, et al. Construction and characteristics of the RxRisk-V: a VA-adapted pharmacy-based case-mix instrument. Med Care. 2003;41(6):761-74. [DOI] [PubMed] [Google Scholar]
- 24.Sales AE, Liu CF, Sloan KL, et al. Predicting costs of care using a pharmacy-based measure risk adjustment in a veteran population. Med Care. 2003;41(6):753-60. [DOI] [PubMed] [Google Scholar]
- 25.Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O’Keeffe Rosetti MC. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care. 2003;41(1):84-99. [DOI] [PubMed] [Google Scholar]
- 26.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. New York: Springer; 2009. [Google Scholar]
- 27.Sinnott SJ, Buckley C, O’Riordan D, Bradley C, Whelton H. The effect of copayments for prescriptions on adherence to prescription medicines in publicly insured populations; a systematic review and meta-analysis. PLoS One. 2013;8(5):e64914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombi AM, Yu-Isenberg K, Priest J. The effects of health plan copayments on adherence to oral diabetes medication and health resource utilization. J Occup Environ Med. 2008;50(5):535-41. [DOI] [PubMed] [Google Scholar]
- 29.Steiner JF, Ho PM, Beaty BL, et al. Socio-demographic and clinical characteristics are not clinically useful predictors of refill adherence in patients with hypertension. Circ Cardiovasc Qual Outcomes. 2009;2(5):451-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franklin JM, Shrank WH, Pakes J, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789-96. [DOI] [PubMed] [Google Scholar]
- 31.Peterson AM, Nau P, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3-12. [DOI] [PubMed] [Google Scholar]


