Abstract
BACKGROUND:
Psoriasis is a chronic, incurable, and immune-mediated skin disorder that is characterized by erythematous scaly papules and plaques. Understanding of psoriasis at the molecular level has led to the development of biologic agents that target disease-specific inflammatory mediators in psoriatic lesions. Biologic agents have become important components of the psoriasis armamentarium, but some patients become refractory to these agents over time or fail to respond to subsequent biologics.
OBJECTIVES:
To (a) evaluate demographic and clinical characteristics of psoriasis patients who have treatment patterns suggestive of failure to a newly initiated biologic agent (treatment-regimen failures) compared with those who do not (non-treatment-regimen failures) and (b) to assess health care-related resource utilization and costs in non-treatment-regimen failures and treatment-regimen failures.
METHODS:
In this retrospective observational cohort study, patients were selected from the MarketScan claims database of commercially insured individuals and individuals with Medicare supplemental insurance. The index event was a newly initiated biologic agent for the treatment of psoriasis (etanercept, adalimumab, ustekinumab, or infliximab) between January 2010 and December 2011. The analysis included psoriasis patients aged ≥ 18 years with ≥ 1 prescription claim for a biologic and continuous enrollment 12 months pre- and post-index date. Patients with claims for a biologic in the pre-index period were excluded. Patients were divided into treatment-regimen-failure and non-treatment-regimen-failure groups based on their treatment patterns post-index date. The treatment-regimen-failure group included patients who switched to another biologic, discontinued the biologic without restarting, increased the dose of the biologic, or augmented treatment with a nontopical psoriasis medication during the post-index period. Between-group patient characteristics and medication use were compared using analysis of variance for continuous variables and chi-square tests for categorical variables without adjustment. Cost differences were compared using the propensity score-adjusted bin bootstrapping method.
RESULTS:
Overall, 2,146 patients met the enrollment criteria. The mean age was 45.1 years. Of these patients, 41.5% were considered treatment-regimen failures. Among treatment-regimen failures, 53% were females, and among non-treatment-regimen failures, 61% were male. Patients who experienced treatment-regimen failure had higher incidences of comor-bid cerebrovascular disease, hypertension, chronic pulmonary disease, depression, and anxiety in the pre-index period and were more likely to use concomitant topicals (67.0% vs. 58.4%; P < 0.001), methotrexate (20.2% vs. 7.3%; P < 0.001), and cyclosporine (3.1% vs. 1.0%; P < 0.001) in the post-index period. Mean total all-cause health care costs were higher in patients with treatment-regimen failure versus non-treatment-regimen failure during the pre-index period ($8,024 vs. $6,637; P = 0.002), but patients with non-treatment-regimen failure had higher all-cause costs ($30,759 vs. $28,012; P = 0.002) and psoriasis-related costs ($25,286 vs. $19,625; P < 0.001) during the post-index period.
CONCLUSIONS:
The results of the current study demonstrated that psoriasis patients with treatment patterns suggestive of treatment-regimen failure on an index biologic had different characteristics and incurred higher all-cause health care costs than did patients without treatment-regimen failure during the pre-index period.
This study was supported by Eli Lilly and Company. Foster, Zhu, Guo, Nikai, Malatestinic, Ojeh, and Goldblum are full-time employees and stockholders of Eli Lilly and Company. Kornberg is a full-time employee of INC Research, which was contracted by Eli Lilly to assist with medical writing. Wu has received research funding from AbbVie, Amgen, Coherus Biosciences, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Regeneron, and Sandoz; he is a consultant for AbbVie, Amgen, Celgene, Dermira, DUSA Pharmaceuticals, Eli Lilly, and Pfizer.
Study concept was developed by Foster, Ojeh, Malatestinic, and Goldblum. Zhu and Guo, along with Foster, took the lead in data collection, and data interpretation was performed by Nikai, Wu, and Foster, with assistance from the other authors. The manuscript was primarily written by Kornberg, along with Foster, with assistance from the other authors. All of the authors were involved with manuscript revision.
What is already known about this subject
Plaque psoriasis is a chronic incurable skin disorder with signs and symptoms (visible red and scaly lesions, pain, and pruritus) that can affect health-related quality of life and productivity.
Understanding of psoriasis at the molecular level has led to the development of biologic agents that target inflammatory mediators in psoriatic lesions.
Some patients can be biologic contraindicated, fail to respond to first-line biologic therapy, or become refractory to biologic agents over time.
What this study adds
A higher proportion of those identified as treatment-regimen failures were female and had a higher incidence of some comor-bidities in the pre-index period compared with non-treatment-regimen failures.
Patients who experienced treatment-regimen failure with biologic therapy had higher use of concomitant medications, including topicals, compared with those who did not experience treatment-regimen failure, suggesting that current biologic therapy may be inadequate in a significant number of patients.
Mean total health care-related costs and psoriasis-related health care costs were higher in non-treatment-regimen failures than treatment-regimen failures during the post-index period; however, non-treatment-regimen failures had lower all-cause outpatient costs in both the pre- and post-index periods compared with treatment-regimen failures.
Plaque psoriasis (psoriasis vulgaris or chronic plaque psoriasis) is a chronic, immune-mediated skin disorder characterized by erythematous scaly papules and plaques. It is a complex condition with both physical (visible red, scaly lesions; pain; pruritus) and psychosocial consequences that affect overall quality of life and productivity.1-3 Associated comorbidities, such as diabetes, cardiovascular disease, metabolic syndrome, autoimmune diseases, and depres-sion3-9 complicate the medical management of these patients. Additionally, as many as 20% to 30% of psoriasis patients have psoriatic arthritis (joint involvement),10 which further adds to the burden of these patients.
Psoriasis is incurable, so emphasis is on disease management. Treatment goals include reduction of the size, thickness, and extent of the lesions and erythema and improvement in health-related quality of life.11 Depending on disease severity, psoriasis is treated with (alone or in combination therapy) topical agents, phototherapy, oral systemic agents (e.g., metho-trexate, cyclosporine, acitretin, apremilast), and biologics (e.g., etanercept, adalimumab, infliximab, ustekinumab).11-14 Understanding of psoriasis at the molecular level has led to the development of biologic agents that inhibit T-cell function and block tumor necrosis factor (TNF)-α, interleukin (IL)-12/IL-23, and IL-17 activities.10,12,15,16
Biologic agents have become important components of the psoriasis armamentarium, but individuals can become refractory to some biologic agents over time, fail to respond to first-line treatment, or have treatment contraindications.12 In 2005, Feldman et al. reported treatment-regimen failure rates and direct medical costs associated with psoriasis therapy using claims data, but their study did not include biologic agents.17 More recent studies have evaluated the reasons for treatment changes in biologic-treated patients18 and treatment patterns/costs among biologic-treated patients19 using chart reviews and claims data, respectively, but these studies did not compare patients who responded to first biologic therapy with those who did not.
Failure to respond to therapy has both medical and economic consequences. An understanding of the characteristics of patients who fail to respond to first biologic therapy and the impact of failure to respond to first therapy on health care utilization and costs may provide important insights to clinicians and payers. We hypothesized that patients who experience treatment-regimen failure would have greater resource utilization and costs compared with non-treatment-regimen failures. Thus, the objectives of this retrospective, observational study were to (a) evaluate demographic and clinical characteristics of patients who had treatment patterns suggestive of failure to the first received biologic therapy (treatment-regimen failures) compared with those who did not (non-treatment-regimen failures) and (b) assess health care-related resource utilization and costs in treatment-regimen failures and non-treatment-regimen failures.
Methods
Data Source and Patient Selection
The study population was extracted from Truven Health Analytics’ MarketScan Commercial Claims and Encounters Database and the Medicare Supplemental and Coordination of Benefits Database. This study enrolled patients newly initiating a biologic (etanercept, adalimumab, ustekinumab, or infliximab) with ≥ 1 biologic prescription drug claim between January 1, 2010, and December 31, 2011. The biologic initiation date was the index date and patients had to have continuous enrollment (medical and pharmacy benefits) for 12 months pre-index and 12 months post-index date. Additionally, patients had to be ≥ 18 years of age on the index date and had evidence of a psoriasis diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification[ICD-9-CM] code 696.1) during the 12-month pre-index period as defined by (a) ≥ 2 outpatient visits, at least 30 days apart, with a psoriasis diagnosis and with at least 1 such diagnosis occurring in the 60 days prior to the index date or (b) ≥ 1 inpatient admission with a psoriasis diagnosis.
Because claims data were used and the authors did not have access to clinical information related to treatment responses, treatment patterns were used to define treatment failure.17 Treatment-regimen failure was defined as individuals who switched to another biologic, discontinued their biologic without restarting the original biologic, or augmented treatment with a nontopical psoriasis medication. In addition, those who had a dose increase (≥ 30%) above the recommended maintenance dose in the maintenance period were also included in the treatment-regimen-failure group. Non-treatment-regimen failure was defined as those not meeting the definition of treatment-regimen failure.
Patients with a medical claim for rheumatoid arthritis (ICD-9-CM code 714.x [rheumatoid arthritis]); Crohn’s disease (ICD-9-CM code 555.x [regional enteritis]); ulcerative colitis (ICD-9-CM code 556.x [ulcerative (chronic) enterocolitis]); osteoar-thritis (ICD-9-CM codes 715.0x [osteoarthrosis generalized], 715.1x [osteoarthrosis localized primary], 715.2x [osteoarthro-sis localized secondary], 715.8x [osteoarthrosis involving or with more than 1 site but not specified as generalized], 715.9x [osteoarthrosis unspecified whether generalized or localized], 721.0 [cervical spondylosis without myelopathy], 721.1 [cervical spondylosis with myelopathy], 721.2 [thoracic spondylosis without myelopathy], 721.3 [lumbosacral spondylosis without myelopathy], 721.41 [spondylosis with myelopathy thoracic region], 721.42 [spondylosis with myelopathy lumbar region]); reactive arthritis (ICD-9-CM code 099.3 [Reiter’s disease]); or ankylosing spondylitis (ICD-9-CM code 720.x [ankylosing spondylitis and other inflammatory spondylopathies]) during the pre-index period were excluded. Also excluded were patients with claims for a biologic (etanercept, adalimumab, ustekinumab, or infliximab) in the pre-index period. Patients with comorbid psoriatic arthritis (ICD-9-CM code 696.0 [pso-riatic arthropathy]) were included.
This study was exempt from informed consent requirements, and institutional/ethical review board approval was not required because the patient records were deidentified.
The study period was January 1, 2009, to December 31, 2012. The index period was January 1, 2010, to December 31, 2011. The index date was defined as the first claim for a biologic with no claims for biologics in the pre-index period.
Outcomes Measures
The recommended maintenance doses were defined by the respective package inserts.20-23 A dose increase was defined as having a mean observed average daily dose ≥ 30% larger than the recommended U.S. Food and Drug Administration-approved maintenance dose (during the maintenance period).24,25
Switching was defined as a claim for a different biologic at any time during the post-index period after the index medication was discontinued. Discontinuation was defined as a gap of at least 120 days between prescriptions with no restarts to the end of the post-index period. The 120-day gap was used to account for the recommended dosing regimen for ustekinumab. Augmentation was defined as the proportion of patients starting a new nontopical psoriasis-related medication in the post-index period that was not used in the pre-index period. Concomitant medications were defined as any psoriasis-related medications (including topicals) that were used in the postindex period regardless of whether they were initiated in the pre-index period or newly initiated in the post-index period.
Cost was defined as the total payment to the provider. Resource utilization and costs (outpatient, inpatient, and pharmacy prescriptions) were measured in non-treatment-regimen failures and treatment-regimen failures during the pre- and post-index periods. Total health care costs were the sum of inpatient, outpatient, and pharmacy costs.
Statistical Analysis
Patient characteristics and medication use between the treatment-regimen-failure and non-treatment-regimen-failure cohorts were compared using analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables without adjustment. Cost differences between the 2 cohorts during the post-index period were compared using the propensity score-adjusted bin bootstrapping (PSBB) method. PSBB was used to minimize the potential bias between the cohorts and address the skewed distribution problems of cost data. The PSBB involves the following steps: (1) the propensity score for each patient was computed using logistic regression with the comparison group and other adjustment as the dependent variable; (2) patients were grouped into 5 strata of equal size based on the propensity score quantiles; (3) within each comparison group, 5,000 bootstrapped resamples of fixed sizes were drawn within each stratum, with the total number of samples equaling the total number of patients; and (4) the difference in mean costs between groups was computed for each of the 5,000 replications. Means, medians, standard deviations, and P values were computed from the 5,000 bootstrapped resa-mples. The variables used for the PSBB adjustment included age, gender, insurance type, Charlson Comorbidity Index, total net cost in pre-index period, comorbidities, medications, and provider type.
Results
Patient Characteristics
The final population consisted of 2,146 patients (Figure 1). Patients in this study were a mean age of 45.1 years, with 93.8% having commercial insurance, 6.2% having Medicare supplemental insurance, and 71.1% being treated by a dermatologist (Table 1). A higher proportion of non-treatment-regimen failures than treatment-regimen failures were male (61.0% vs. 47.0%; P < 0.001), whereas a higher proportion of treatment-regimen failures than non-treatment-regimen failures were female (53% vs. 39%; P < 0.001). Patients who experienced treatment-regimen failure had higher incidences of comorbid cerebrovascular disease (2.5% vs. 1.2%), myocardial infarction (1.1% vs. 0.4%), hypertension (26.9% vs. 23.2%), chronic pulmonary disease (8.4% vs. 5.4%), depression (5.6% vs. 3.0%), and anxiety (7.0% vs. 4.9%) than did non-treatment-regimen failures in the pre-index period.
FIGURE 1.
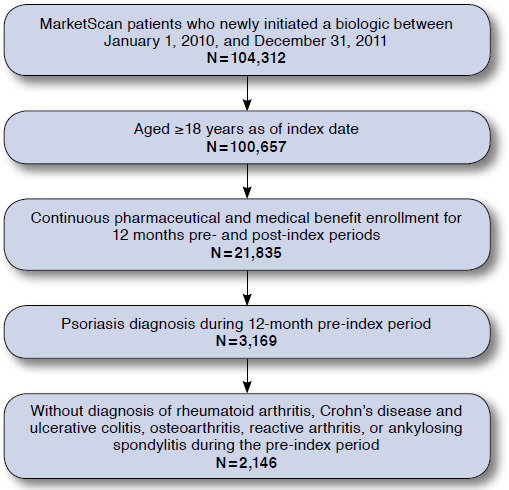
Patient Selection and Flow
TABLE 1.
Characteristics of Wet OAB Versus Non-OAB Cohorts
| Patient Characteristic | Total N = 2,146 | Non-Treatment-Regimen Failures n = 1,255 | Treatment-Regimen Failures n = 891 | Difference P Valuea |
|---|---|---|---|---|
| Demographics (at index) | ||||
| Age at index | 0.350 | |||
| Mean (SD) | 45.1 (13.21) | 44.9 (12.93) | 45.5 (13.60) | - |
| Gender, n (%) | < 0.001 | |||
| Male | 1,185 (55.2) | 766 (61.0) | 419 (47.0) | - |
| Female | 961 (44.8) | 489 (39.0) | 472 (53.0) | - |
| Primary payer, n (%) | 0.061 | |||
| Commercial | 2,012 (93.8) | 1,187 (94.6) | 825 (92.6) | - |
| Medicare | 134 (6.2) | 68 (5.4) | 66 (7.4) | - |
| Clinical characteristics (pre-index) | ||||
| Comorbidities, n (%)b | ||||
| Anxiety | 123 (5.7) | 61 (4.9) | 62 (7.0) | 0.039 |
| Cerebrovascular disease | 37 (1.7) | 15 (1.2) | 22 (2.5) | 0.026 |
| Chronic pulmonary disease | 143 (6.7) | 68 (5.4) | 75 (8.4) | 0.006 |
| Congestive heart failure | 19 (0.9) | 13 (1.0) | 6 (0.7) | 0.377 |
| Coronary atherosclerosis | 86 (4.0) | 47 (3.7) | 39 (4.4) | 0.462 |
| Depression | 88 (4.1) | 38 (3.0) | 50 (5.6) | 0.003 |
| Diabetes | 266 (12.4) | 156 (12.4) | 110 (12.3) | 0.953 |
| Dyslipidemia | 571 (26.6) | 322 (25.7) | 249 (27.9) | 0.237 |
| Hypertension | 531 (24.7) | 291 (23.2) | 240 (26.9) | 0.047 |
| Obesity | 88 (4.1) | 51 (4.1) | 37 (4.2) | 0.919 |
| Mild liver disease | 69 (3.2) | 45 (3.6) | 24 (2.7) | 0.248 |
| Moderate or severe liver disease | 3 (0.1) | 2 (0.2) | 1 (0.1) | 0.773 |
| Myocardial infarction | 15 (0.7) | 5 (0.4) | 10 (1.1) | 0.047 |
| Psoriatic arthritis | 277 (12.9) | 156 (12.4) | 121 (13.6) | 0.434 |
| Renal disease | 28 (1.3) | 17 (1.4) | 11 (1.2) | 0.809 |
| TI A/stroke | 32 (1.5) | 14 (1.1) | 18 (2.0) | 0.088 |
| Unstable angina | 8 (0.4) | 3 (0.2) | 5 (0.6) | 0.228 |
| Charlson Comorbidity Index | 0.180 | |||
| Mean (SD) | 0.4 (0.86) | 0.3 (0.83) | 0.4 (0.89) | - |
| Medications, n (%) | ||||
| Topicals | 1,758 (81.9) | 1,024 (81.6) | 734 (82.4) | 0.641 |
| Methotrexate | 518 (24.1) | 295 (23.5) | 223 (25.0) | 0.417 |
| Cyclosporine | 65 (3.0) | 39 (3.1) | 26 (2.9) | 0.801 |
| Acitretin | 198 (9.2) | 108 (8.6) | 90 (10.1) | 0.238 |
| Methoxsalen | 10 (0.5) | 5 (0.4) | 5 (0.6) | 0.585 |
| Phototherapy | 269 (12.5) | 157 (12.5) | 112 (12.6) | 0.967 |
| Provider specialty, n (%) | 0.354 | |||
| Primary care, internal medicine, general practice | 252 (11.7) | 144 (11.5) | 108 (12.1) | - |
| Dermatologist | 1,525 (71.1) | 906 (72.2) | 619 (69.5) | - |
| Rheum atolo gist | 81 (3.8) | 39 (3.1) | 42 (4.7) | - |
| Other | 221 (10.3) | 128 (10.2) | 93 (10.4) | - |
| Missing | 67 (3.1) | 38 (3.0) | 29 (3.3) | - |
| Concomitant medication use (post-index) | ||||
| Medication use, n (%) | ||||
| Topic a l s | 1,330 (62.0) | 733 (58.4) | 597 (67.0) | < 0.001 |
| Methotrexate | 272 (12.7) | 92 (7.3) | 180 (20.2) | < 0.001 |
| Cyclosporine | 40 (1.9) | 12 (1.0) | 28 (3.1) | < 0.001 |
| Acitretin | 68 (3.2) | 12 (1.0) | 56 (6.3) | < 0.001 |
| Concomitant phototherapy | 127 (5.9) | 67 (5.3) | 60 (6.7) | 0.177 |
aP value is from analysis of variance for continuous variables and chi-square test for categorical variables.
bSee Appendix for ICD-9-CM codes (available in online article).
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; SD = standard deviation; TIA = transient ischemic attack.
Patterns of Biologic Therapies
Overall, 41.5% of patients were defined as treatment-regimen failures. Among treatment-regimen failures, 22.4% switched biologic agents (Figure 2). The mean time to switch was 173.0 days (data not shown). The mean length of therapy was 185.2 days (data not shown) among treatment-regimen failures, with 49.9% of treatment-regimen failures discontinuing therapy. Of 891 treatment-regimen-failure patients, 228 (25.6%) augmented their therapy with a new nontopical therapy and 39 (4.4%) had a dose increase.
FIGURE 2.
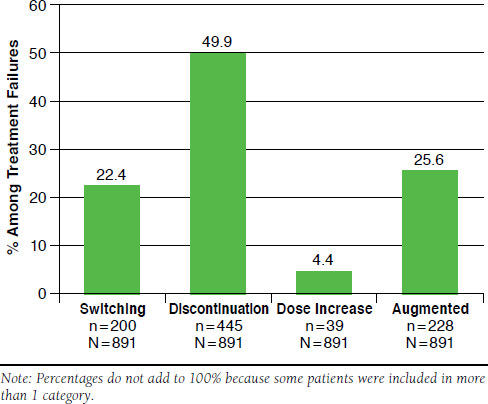
Patterns Defining Treatment-Regimen Failures
During the post-index period, fewer non-treatment-regimen failures than treatment-regimen failures used concomitant topicals (58.4% vs. 67.0%; P < 0.001), methotrexate (7.3% vs. 20.2%; P < 0.001), and cyclosporine (1.0% vs. 3.1%; P < 0.001). There was no difference in concomitant phototherapy usage (5.3% vs. 6.7%; P = 0.177; Table 1).
All-Cause Health Care Resource Utilization and Costs
Non-treatment-regimen failures had, on average, fewer outpatient office visits during both the pre-index period (16.2 vs. 1 7 . 9 ; P = 0.013) and post-index period (13.4 vs. 17.2; P < 0.001) than treatment-regimen failures (Table 2). Mean outpatient costs were lower among non-treatment-regimen failures than treatment-regimen failures during both the pre- and post-index periods. Likewise, non-treatment-regimen failures had fewer mean inpatient admissions than treatment-regimen failures during the pre- (0.2 vs. 0.3; P = 0.050) and post-index periods (0.3 vs. 0.6; P = 0.012). For pharmacy costs, no significant differences were observed during the pre-index period between non-treatment-regimen failures and treatment-regimen failures ($2,580 vs. $2,508; P = 0.651). However, non-treatment-regimen failures had higher costs during the post-index period ($25,641 vs. $19,398; P < 0.001). Mean total health care costs were lower in non-treatment-regimen failures than in treatment-regimen failures during the pre-index period ($6,637 vs. $8,024; P = 0.002) but were higher in non-treatment-regimen failures during the post-index period ($30,759 vs. $28,012; P = 0.0 02).
TABLE 2.
All-Cause Utilization and Costs
| Pre-index Period | Post-index Period | |||||
| Non-Treatment-Regimen Failures n = 1,255 | Treatment-Regimen Failures n = 891 | Difference P Valuea | Non-Treatment-Regimen Failures n = 1,255 | Treatment-Regimen Failures n = 891 | Difference P Valueb | |
|---|---|---|---|---|---|---|
| Outpatient | ||||||
| Patients with outpatient physician visit, n (%) | 1,254 (99.9) | 891 (100.0) | 0.399 | 1,250 (99.6) | 879 (98.7) | 0.015 |
| Outpatient visits (all), mean (SD) | 16.2 (16.1) | 17.9 (16.2) | 0.013 | 13.4 (12.3) | 17.2 (15.0) | < 0.001 |
| Outpatient costs, US $ | - | - | 0.002 | - | - | < 0.001 |
| Mean (SD) | 3,549 (6,298) | 4,470 (7,387) | - | 4,026 (8,126) | 6,926 (14,535) | - |
| Median | 1,682 | 2,121 | - | 1,473 | 2,435 | - |
| Inpatient | ||||||
| Patients with inpatient admission, n (%) | 44 (3.5) | 46 (5.2) | 0.059 | 62 (4.9) | 66 (7.4) | 0.017 |
| Inpatient admissions, mean (SD) | 0.2 (1.1) | 0.3 (1.5) | 0.050 | 0.3 (2.1) | 0.6 (2.7) | 0.012 |
| Inpatient costs, US $ | - | - | 0.024 | - | - | 0.301 |
| Mean (SD) | 507 (3,694) | 1,046 (7,198) | - | 1,092 (13,856) | 1,688 (8,588) | - |
| Median | 0 | 0 | - | 0 | 0 | - |
| Pharmacy, U.S. $ | ||||||
| Pharmacy costs | - | - | 0.651 | - | - | < 0.001 |
| Mean (SD) | 2,580 (3,757) | 2,508 (3,550) | - | 25,641 (11,712) | 19,398 (13,291) | - |
| Median | 1,389 | 1,388 | - | 25,070 | 17,405 | - |
| Total health care costs, U.S. $ | - | - | 0.002 | - | - | 0.002 |
| Mean (SD) | 6,637 (8,908) | 8,024 (11,576) | - | 30,759 (19,866) | 28,012 (21,583) | - |
| Median | 3,938 | 4,583 | - | 27,943 | 24,494 | - |
aCosts during pre-index period were compared using analysis of variance.
bCosts during post-index period were analyzed using propensity score bin bootstrapping adjusted for age, gender, insurance type, Charlson Comorbidity Index, total net cost in prior period, comorbidities, medications, and provider types.
SD = standard deviation; U.S. = United States.
Psoriasis-Related Health Care Resource Utilization and Costs
On average, non-treatment-regimen failures and treatment-regimen failures had the same number of psoriasis-related outpatient office visits (7.7 vs. 7.9; P = 0.783) during the pre-index period, but non-treatment-regimen failures had fewer psoriasis-related outpatient office visits (5.0 vs. 5.7; P = 0.036) during the post-index period (Table 3). Non-treatment-regimen failures had lower mean outpatient costs than treatment-regimen failures during the post-index period. A numerically lower proportion of non-treatment-regimen failures than treatment-regimen failures had psoriasis-related inpatient admissions during the pre- (0.2% vs. 1.2%; P = 0.002) and post-index periods (0.9% vs. 1.6%; P = 0.139), but this difference was only significant during the pre-index period. Mean psoriasis-related pharmacy costs were the same in non-treatment-regimen failures and in treatment-regimen failures during the pre-index period ($7 vs. $4; P < 0.627) but were higher in non-treatment-regimen failures during the post-index period ($23,801 vs. $17,000; P < 0.001). Likewise, psoriasis-related total health care costs were higher in non-reatment-regimen failures than in treatment-regimen failures ($25,286 vs. $19,625; P < 0.001) during the post-index period.
TABLE 3.
Psoriasis-Specific Utilization and Costs
| Pre-index Period | Post-index Period | |||||
| Non-Treatment-Regimen Failures n = 1,255 | Treatment-Regimen Failures n = 891 | Difference P Valuea | Non-Treatment-Regimen Failures n = 1,255 | Treatment-Regimen Failures n = 891 | Difference P Valueb | |
|---|---|---|---|---|---|---|
| Outpatient | ||||||
| Patients with outpatient physician visit, n (%) | 1,253 (99.8) | 889 (99.8) | 0.730 | 1,198 (95.5) | 801 (89.9) | < 0.001 |
| Outpatient visits, mean (SD) | 7.7 (12.6) | 7.9 (12.4) | 0.783 | 5.0 (7.0) | 5.7 (7.5) | 0.036 |
| Outpatient costs, US $ | - | - | 0.313 | - | - | 0.026 |
| Mean (SD) | 1,126 (4,286) | 1,315 (4,237) | - | 1,456 (5,444) | 2,524 (10,822) | - |
| Median | 392 | 402 | - | 294 | 317 | - |
| Inpatient | ||||||
| Patients with inpatient admission, n (%) | 2 (0.2) | 11 (1.2) | 0.002 | 11 (0.9) | 14 (1.6) | 0.139 |
| Inpatient admissions, mean (SD) | 0.0 (0.1) | 0.0 (0.4) | 0.0 13 | 0.0 (0.2) | 0.0 (0.4) | 0.062 |
| Inpatient costs, US $ | - | - | 0.041 | - | - | 0.107 |
| Mean (SD) | 11 (2 75) | 83 (1,210) | - | 29 (437) | 101 (1,330) | - |
| Median | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| Pharmacy | ||||||
| Pharmacy costs, U.S. $ | - | - | 0.627 | - | - | < 0.001 |
| Mean (SD) | 7 (140) | 4 (115) | - | 23,801 (10,801) | 17,000 (12,539) | - |
| Median | 0 | 0 | - | 23,679 | 14,656 | - |
| Total health care costs, U.S. $ | - | - | 0.174 | - | - | < 0.001 |
| Mean (SD) | 1,144 (4,294) | 1,402 (4,388) | - | 25,286 (10,294) | 19,625 (15,394) | - |
| Median | 394 | 404 | - | 24,372 | 17,521 | - |
aCosts during pre-index period were compared using analysis of variance.
bCosts during post-index period were analyzed using propensity score bin bootstrapping adjusted for age, gender, insurance type, Charlson Comorbidity Index, total net cost in prior period, comorbidities, medications, and provider types.
SD = standard deviation; U.S. = United States.
Discussion
The purpose of this retrospective observational cohort study was to describe characteristics and health care utilization and costs in psoriasis patients who had treatment patterns suggestive of treatment-regimen failure with their first biologic compared with non-treatment-regimen-failure patients. In our study, 41.5% of patients were considered treatment-regimen failures with an average length of therapy of 185.2 days. Of note, a higher proportion of females than males were treatment-regimen failures, and there were higher incidences of some comorbidities among treatment-regimen failures in the pre-index period.
The proportion of treatment-regimen failures in our cohort is consistent with a retrospective cross-sectional study reported by Levin et al. (2014) that analyzed the clinical practice of 2 dermatologists in the U.S. between January 2008 and January 2012.26 This study reported that 48% of patients failed biologic therapy (as defined by the frequency of discontinuation) in an average of 242 days. Unfortunately, the reasons for failure/discontinuation of biologic treatment in our study are not known. Levin et al. reported that 42% of patients discontinued biologic therapy due to lack/loss of efficacy, 7.5% discontinued due to adverse events, and 44% continued treatment after 4 years.26 Other studies reported loss of efficacy, drug no longer needed, and adverse events to be the predominant reasons for biologic discontinuation.27,28 An inability to afford the copayment or coinsurance is another potential reason for discontinuation.
Treatment-regimen failures used more concomitant medications than non-treatment-regimen failures during the postindex period. Other than the nontopical concomitant medication used to define treatment failure (methotrexate, cyclospo-rine, etc.), it is notable that concomitant topical use was also higher in the treatment-regimen-failure group in the post-index period. The use of these medications appeared to decline in both groups from the pre- to post-index periods, which suggests that biologic treatment may result in a reduction in the use of nonbiologic psoriasis medications in at least some patients; however, in other patients, the use of biologics only may be inadequate.
During the pre-index period, mean total all-cause health care costs were lower in non-treatment-regimen failures than in treatment-regimen failures, whereas there was no difference in total psoriasis-related health care costs. Mean outpatient costs were lower in non-treatment-regimen failures than in treatment-regimen failures, which is consistent with there being a lower incidence of some comorbidities among non-treatment-regimen failures.
During the post-index period, treatment-regimen failures had lower all-cause health care costs and psoriasis-related health care costs, most of which were pharmacy-related. Because 49.9% of treatment-regimen failures discontinued biologic therapy during the post-index period, it is not unexpected that pharmacy-related, and by extension, total health care costs would be reduced. However, this seems to be partially offset by higher outpatient costs among treatment-regimen failures during the post-index period. It should be noted that treatment-regimen failures had higher all-cause outpatient costs during the pre-index period as well, perhaps because they had more comorbidities.
Failing initial treatment with biologics may reduce patient satisfaction and result in the unwillingness to try alternative biologics. This could result in greater resource utilization and costs for the payer. Understanding the characteristics of patients who may be more likely to fail treatment and the potential consequences of that failure would be important information for clinicians when treating their patients and for payers as they make decisions about potential cost-containment efforts (e.g., step edits).
Limitations
Because only patients with employee-based health insurance or Medicare supplemental insurance were included, patients in this cohort may not have been representative of all psoriasis patients. Data were limited to those that are commonly reported in administrative health care claims, so the possibility of miscoding, errors, and omissions exists, and details regarding the patients’ treatment and clinical picture were limited. Another limitation is that it is possible that the 12-month follow-up period was too short to completely capture treatment patterns. Also, the lack of patient randomization may have led to between-group systematic and outcome differences because of unavoidable imbalances between groups.
In addition, a limitation lies in the definition of “non-treatment-regimen failure” and “treatment-regimen failure.” Given that this was a retrospective administrative claims analysis, clinical measures were not available to determine whether or not patients did in fact fail treatment. There was no information on the reasons for changes in treatment. It is possible that patients may have switched or discontinued their biologic for reasons unrelated to clinical efficacy, such as formulary changes or an inability to pay for medications. Dose increases or augmentation may not be a result of treatment-regimen failure but rather just part of usual physician prescribing practice. Treatment-regimen failure in this study was defined using treatment patterns similar to that used by Feldman et al.17 Although both studies define treatment-related failures by treatment patterns, there were some differences. Feldman et al. used the terminology “treatment failure,” which was defined as switching, augmentation with a nontopical agent, discontinuation after uptitration, or discontinuation after hos-pitalization.17 In this study, “treatment-regimen failure” was defined as switching, augmentation with a nontopical medication, discontinuation, or an increase in dose. The differences in definitions are due to the drugs used in the respective studies. The Feldman study evaluated conventional systematic agents,17 whereas this study evaluated biologics. It would be uncommon for patients to be hospitalized for psoriasis-related symptoms, and hospitalization related to adverse events associated with biologic use is rare; thus, our definition did not include discontinuation after hospitalization. Similarly, given the costs of biologics, the decision to uptitrate in and of itself is most likely suggestive of a lack of response or loss of response with biologics, so that was used as part of our definition, whereas uptitration with systemic therapies may be more common, and, thus, discontinuation after uptitration may have been more appropriate for the analysis done by Feldman.
Conclusions
In this study, there were significant differences found in demographic and clinical characteristics and health care-related resource utilization and costs among non-treatment-regimen failures and treatment-regimen failures. Understanding these differences may provide important insights to clinicians and payers in the treatment of psoriasis.
APPENDIX. ICD-9-CM Codes Used for Comorbidities
| Comorbidity | ICD-9-CM Code |
|---|---|
| Anxiety | 300.x |
| Cerebrovascular disease | 362.34, 430.x-438.x |
| Congestive heart failure | 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4-425.9, 428.x |
| Coronary atherosclerosis | 414.x |
| Chronic pulmonary disease | 416.8, 416.9, 490.X-505.X, 506.4, 508.1, 508.8 |
| Depression | 311.x, 296.2x, 296.3x, 300.4x |
| Diabetes | 250.0-250.3, 250.8, 250.9, 250.4-250.7 |
| Dyslipidemia | 272.0-272.4, 272.9 |
| Hypertension | 401.x, 405.x, 642.lx |
| Myocardial infarction | 410.x, 412.x |
| Mild liver disease | 070.22, 070.23, 070.32, 070.33, 070.44, 070.54, 070.6, 070.9, 570.x, 571.x, 573.3, 573.4, 573.8, 573.9, V42.7 |
| Moderate or severe liver disease | 456.0-456.2, 572.2-572.8 |
| Obesity | 278.00, 278.01, 278.02, 278.03 |
| Psoriatic arthritis | 696.0 |
| Renal disease | 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 582.x, 583.0-583.7, 585.x, 586.x, 588.0, V42.0, V45.1, V56.x |
| Transient ischemic attack/stroke | 433.xx, 434.xx, 436, 435.9 |
| Unstable angina | 411.1 |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
References
- 1. Griffiths CE, Barker JN.. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583);263-71. [DOI] [PubMed] [Google Scholar]
- 2. Nash AS, McAteer H, Schofield J, Penzer R, Gilbert AK. Psoriasis today: experiences of healthcare and impact on quality of life in a major UK cohort. Prim Health Care Res Dev. 2015;16(4):415-23. [DOI] [PubMed] [Google Scholar]
- 3. Fried RG, Friedman S, Paradis C, et al. Trivial or terrible? The psychosocial impact of psoriasis. Int J Dermatol. 1995;34(2):101-05. [DOI] [PubMed] [Google Scholar]
- 4. Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298(7):321-28. [DOI] [PubMed] [Google Scholar]
- 5. Edson-Heredia E, Zhu B, Lefevre C, et al.. Prevalence and incidence rates of cardiovascular, autoimmune, and other diseases in patients with psoriatic or psoriatic arthritis: a retrospective study using Clinical Practice Research Datalink. J Eur Acad Dermatol Venereol. 2015;29(5):955-63. [DOI] [PubMed] [Google Scholar]
- 6. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15(1):45-50. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18(1):49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26(3):230-34. [DOI] [PubMed] [Google Scholar]
- 9. Wu JJ, Nguyen TU, Poon KY, Herrinton LJ. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67(5):924-30. [DOI] [PubMed] [Google Scholar]
- 10. Belge K, Brück J, Ghoreschi K.. Advances in treating psoriasis. F1000Prime Rep. 2014;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826-50. [DOI] [PubMed] [Google Scholar]
- 12. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137-74. [DOI] [PubMed] [Google Scholar]
- 13. Hsu S, Papp KA, Lebwohl MG, et al.; National Psoriasis Foundation Medical Board. Consensus guidelines for the management of plaque psoriasis. Arch Dermatol. 2012;148(1):95-102. [DOI] [PubMed] [Google Scholar]
- 14. Nast A, Jacobs A, Rosumeck S, et al. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: a systemic review and meta-analysis. J Invest Dermatol. 2015;135(11):2641-48. [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Wang YM, Ahn HY. Biological products for the treatment of psoriasis: therapeutic targets, pharmacodynamics and disease-drug-drug interaction implications. AAPS J. 2014;16(5):938-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mease PJ.. Inhibition of interleukin-17, interleukin-23 and the TH17 cell pathway in the treatment of psoriatic arthritis and psoriasis. Curr Opin Rheumatol. 2015;27(2):127-33. [DOI] [PubMed] [Google Scholar]
- 17. Feldman SR, Evans C, Russell MW. Systemic treatment for moderate to severe psoriasis: estimates of failure rates and direct medical costs in a north-eastern US managed care plan. J Dermatolog Treat. 2005;16(1):37-42. [DOI] [PubMed] [Google Scholar]
- 18. Anderson KL, Feldman SR. Reasons for treatment changes in patients with moderate to severe psoriasis. J Cutan Med Surg. 2015;19(4):361-66. [DOI] [PubMed] [Google Scholar]
- 19. Feldman SR, Zhao Y, Navaratnam P, Friedman HS, Lu J, Tran MH. Patterns of medication utilization and costs associated with the use of etan-ercept, adalimumab, and ustekinumab in the management of moderate-to-severe psoriasis. J Manag Care Spec Pharm. 2015;21(3):201-09. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2015.21.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Humira [package insert]. AbbVie Inc. January 2013. Available at: http://www.rxabbvie.com/pdf/humira.pdf. Accessed December 26, 2015.
- 21. Enbrel [package insert]. Amgen, September 2013. Available at: pi.amgen.com/united_states/enbrel/derm/enbrel_pi.pdf. Accessed December 26, 2015.
- 22. Remicade [package insert]. Janssen Biotech Inc. November 2013. Available at: http://www.remicade.com/shared/product/remicade/prescrib-ing-information.pdf. Accessed December 26, 2015.
- 23. Stelara [package insert]. Janssen Biotech Inc. May 2013. Available at: http://www.stelarainfo.com/pdf/PrescribingInformation.pdf. Accessed December 26, 2015.
- 24. Chastek B, Watson C, Fox K, Gandra S.. Dose escalation of TNF-blocker therapy among patients with psoriasis in a large managed care plan. J Am Acad Dermatol. 2012;66(4): AB190. [Google Scholar]
- 25. Bonafede M, Watson C, Fox K, Gandra S. Dose escalation of TNF-blockers therapy among US managed care patients with psoriasis. J Am Acad Dermatol. 2012;66(4): AB190. [Google Scholar]
- 26. Levin AA, Gottlieb AB, Au SC. A comparison of psoriasis drug failure rates and reasons for discontinuation in biologics vs. conventional systemic therapies. J Drugs Dermatol. 2014;13(7):848-53. [PubMed] [Google Scholar]
- 27. Gniadecki R, Kragballe K, Dam TN, Skov L. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol. 2011;164(5):1091-96. [DOI] [PubMed] [Google Scholar]
- 28. Khalid JM, Fox KM, Globe G, Maguire A, Chau D. Treatment patterns and therapy effectiveness in psoriasis patients initiating biologic therapy in England. J Dermatolog Treat. 2014;25(1):67-72. [DOI] [PubMed] [Google Scholar]


