Abstract
BACKGROUND:
Sunitinib and pazopanib are among the most prescribed targeted therapies for the systemic management of advanced renal cell carcinoma (RCC), but published cost comparisons between the 2 agents are few and limited by methodological and population differences. Also, sunitinib is administered on a 4-week on/2-week off cycle, and pazopanib is taken continuously. Thus, appropriate use and cost comparisons between the 2 drugs require methodological approaches to account for these differences. One way to accomplish this is to substitute expected for observed days supply. Recognizing the effects of nonrepresentative days supply values is important for assessing real-world treatment patterns and costs.
OBJECTIVES:
To (a) characterize demographic and clinical characteristics among patients with RCC newly initiating sunitinib or pazopanib, using a large administrative claims dataset; (b) characterize treatment patterns, persistence, and costs for each treatment group; and (c) assess the effect on treatment patterns and costs for sunitinib by substituting 42 days for prescriptions with 28- or 30-day supplies to account for sunitinib’s 4-week on/2-week off dosing schedule.
METHODS:
This was a retrospective cohort study using health care claims data from the Truven MarketScan Research Databases, which include enrollment information and medical and pharmacy claims. Baseline patient demographic and clinical characteristics and treatment patterns (continuation, discontinuation, switching, or interruption; days supply; and persistence) were compared. Health care costs were calculated as mean daily index medication costs and as total, medical, and medication (all-cause and RCC-related) costs over the 12 months post-index period. Inclusion criteria were continuous health plan enrollment between 6 months pre-index and 12 months post-index; no RCC medications 6 months pre-index; ≥ 2 RCC diagnoses within ±180 days of index; and age ≥ 20 years. For demographic and clinical characteristics, treatment patterns, and costs, means (± standard deviations) for continuous data and relative frequencies for categorical data were reported. Chi-square tests or Student t-tests were used to evaluate differences other than costs. A generalized linear model with gamma distribution and log link was used for evaluating costs, controlling for patient demographic and pre-index clinical characteristics, persistence days, and index medication. All statistical tests were 2-tailed with significance set at P < 0.05 for all comparisons except for interactions with significance set at P < 0.10. The effects of substituting 42 days supply for sunitinib prescription records with 28 or 30 days supply were determined.
RESULTS:
In total, 609 (15.1% of the sunitinib overall sample) sunitinib patients and 183 (8.3% of the pazopanib overall sample) pazopanib patients were included. Demographic and clinical characteristics were similar for each treatment cohort. The persistence periods and number of prescriptions filled were also similar. Without substitution, significant differences were observed between treatment groups in patterns of index medication use (overall P = 0.0409), with fewer patients taking sunitinib continuing treatment than patients taking pazopanib. However, with substitution, treatment patterns differed significantly (overall P = 0.0026), but with more sunitinib patients than pazopanib patients continuing treatment. Without substitution, unadjusted daily mean index medication costs were significantly different for sunitinib ($216) versus pazopanib ($177, P < 0.0001). Substitution of sunitinib days supply eliminated the significant differences in daily index medication costs between treatment groups. The 1-year RCC-related and all-cause medication, medical, and total unadjusted costs were not significantly different between treatment groups, and substitution had no effect on these costs. After adjustment for possible confounding factors, these cost results were similar to those found with unadjusted analyses.
CONCLUSIONS:
In this study, patients with RCC who were initiating sunitinib and pazopanib had similar demographic and clinical characteristics and drug persistence patterns. The effect of substituting days supply values was demonstrated as an approach to considering differences in dosing cycles. Substitution significantly reduced sunitinib mean daily index medication costs and eliminated or reversed the direction of significant differences in costs between drugs during the persistence period. No significant differences were observed in unadjusted or adjusted 1-year costs.
What is already known about this subject
Sunitinib and pazopanib are commonly used as first-line treatment for advanced renal cell carcinoma (RCC).
Persistence with self-administered oral oncology medications is vital to the success of treatment.
Assessment of persistence (duration of therapy) and related costs of sunitinib requires consideration of the 4-week on/2-week off dosing schedule.
What this study adds
The majority of prescription records contains days supply values that do not accurately reflect the recommended dosing schedule for sunitinib.
Substitution of days supply to account for the recommended sunitinib dosing schedule eliminated the difference between groups in the proportion of patients continuing index treatment and in the mean daily index medication cost that resulted from using observed days supply.
Total all-cause and RCC-related costs did not differ between index drug treatment groups over 1 year of follow-up.
Renal cell carcinoma (RCC) accounts for 2%-3% of all malignancies diagnosed in adults and approximately 85% of kidney cancers.1 Approximately 62,700 new cases and 14,240 deaths due to kidney cancer are estimated to occur in 2016.1 A substantial proportion (15%-40%) of cases are diagnosed in late stages or will develop distant metastases,1-4 and estimates of 5-year relative survival are only 8%-12% for late-stage metastatic cases.1,5 Sunitinib and pazopanib are among the most prescribed targeted treatments for RCC,6,7 and pazopanib was found to be noninferior to sunitinib in a randomized clinical trial.8 However, compliance with therapy is critical to treatment success, and safety, adverse events, quality of life, and costs are factors that may affect compliance with treatment in real-world practice. Recent studies employing health care claims data reported similar persistence and compliance patterns between these agents.9,10
In addition to adverse events,11 treatment costs are known to affect patient adherence to self-administered oncologic medication and are of increasing importance to U.S. payers and oncology professional organizations.12 In choosing between efficacious drugs, costs may influence treatment preference for payers and patients. Yet, cost comparisons between the commonly used RCC first-line drugs sunitinib and pazopanib in real-world populations are few, and methodological differences limit interpretation across studies. Hansen et al. (2015) suggested that costs were lower for pazopanib-treated patients than for patients treated with sunitinib; however, the cohorts were not compared within the context of real-world treatment, but rather in the context of patients participating in a clinical trial, which does not provide an accurate profile of costs incurred in routine clinical practice.13 Racsa et al. (2015) presented differences between patients newly treated with sunitinib and pazopanib, suggesting higher annual costs for sunitinib, yet differences did not persist with adjusted models and were only statistically significant for nonadherent patients, perhaps because of the inclusion of patients previously treated for RCC.10
A preliminary analysis of sunitinib prescription records revealed that the majority (75%) of records had 28 or 30 days supplies indicated, although 42 days is the accurate supply for 1 prescription, given the recommended dosing schedule of 4 weeks on therapy followed by 2 weeks off. Objectives of the current study were to (a) characterize demographic and clinical characteristics among treatment-naïve patients with RCC newly initiating sunitinib or pazopanib from a large administrative claims dataset; (b) characterize treatment patterns, persistence, and costs associated with each treatment group; and (c) assess the effect on treatment patterns and costs for sunitinib by substituting 42 days for prescriptions that have 28 or 30 days supplies to account for sunitinib’s dosing schedule. Also examined were differences in costs by patient age (< 65 vs. ≥ 65 years) to serve as a possible proxy for commercial versus Medicare-insured status.
Methods
Data Source
This study used data from the Truven Health MarketScan Research Database, which provides enrollment data, paid medical claims for inpatient and outpatient utilization, and pharmacy claims for over 170 million U.S. patients. Truven Health databases comprise the health care characteristics of covered populations and providers, including employer-sponsored commercial plans as well as Medicare-supplemental employer-sponsored plans (both Medicare- and employer-paid portions). This study used HIPAA-compliant, de-identified data, as specified in US 45 CFR 46 and was thus exempt from review and approval by an institutional review board.
Study Design and Sample
This was a retrospective cohort study using health care claims data to characterize demographic and clinical characteristics, treatment patterns, mean daily medication costs and 12-month follow-up costs for treatment-naïve patients with RCC who were initiating treatment with sunitinib or pazopanib. The sample included patients with at least 2 International Classifications of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes (189.0) for RCC and at least 1 prescription fill for sunitinib or pazopanib (see Appendix A for National Drug Code numbers and Healthcare Common Procedure Coding System codes, available in online article) between October 1, 2009, and September 30, 2013, with the first fill date defining the index date.
Inclusion Criteria.
Patients were required to have continuous health plan enrollment from 6 months before to 12 months after the index date; have no RCC medications for 6 months before the index date; have 2 RCC diagnosis codes at least 7 days apart within ±180 days of the index date; and be aged ≥ 20 years in the year of the index prescription fill.
Exclusion Criteria.
Patients were excluded if they had sunitinib and pazopanib prescription fills on the same index date; days supply < 28 for sunitinib or days supply < 30 for pazopanib (a priori definition); and prescription records with nonpositive days supply.
Measures and Data Captured
Patient Characteristics.
Pre-index (baseline) demographic characteristics included age, gender, geographic region of residence (by U.S. Census divisions Northeast, North Central, South, and West) and continuous health plan enrollment (days) pre-index and post-index. Pre-index (baseline) clinical characteristics included the following:
Deyo Charlson Comorbidity Index (CCI) score and number and percentage (n %) of patients with each CCI comorbidity in 6 months pre-index period (Appendix B, available in online article).14
Days from the earliest RCC diagnosis to the index date (back to January 2008).
Six-month pre-index RCC-related (records with ICD-9-CM 189.0) and all-cause inpatient admissions, number and percentage of patients with an admission and per-patient mean (± standard deviation [SD]) number of admissions.
Metastases (ICD-9-CM codes 196.xx-199.xx): Percentage of patients during 6 months pre-index and January 1, 2008-September 30, 2013.
Post-index Persistence.
The persistence period consisted of the number of days from the start of the index medication to the end date of the last prescription’s days supply or the day before the start of a non-index RCC medication prescription within the allowed gap (30 days; Figure 1). The following treatment continuation definitions were applied, and patients were categorized as having continued, switched, interrupted, or discontinued their therapy. These patterns were defined as follows:
FIGURE 1.
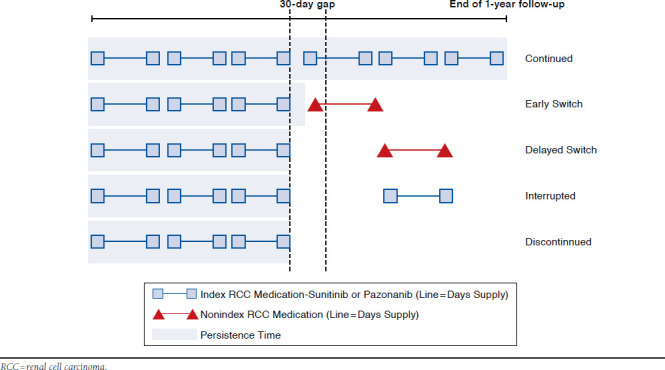
Treatment Pattern Classification
Continued: Patients did not switch or discontinue index medication (i.e., there was no > 30-day gap for index medication during 12-month follow-up).
Early Switch: Patients switched to a nonindex medication while in possession of index medication (before run-out of last prescription’s days supply). There was at least 1 > 30-day gap for index medication during follow-up and at least 1 nonindex RCC medication within the 30-day gap.
Delayed Switch: Patients switched to a nonindex medication after completion of the last index medication prescription’s days supply. There was at least 1 > 30-day gap for index medication and at least 1 nonindex RCC medication > 30 days after the last index prescription’s days supply before reinitiation of index medication or end of follow-up.
Interrupted: Patients’ treatments were halted but reinitiated with index medication. That is, there was reinitiation of the index medication after the last index prescription’s days supply plus at least 30 days, with no nonindex RCC medications before reinitiation.
Discontinued: Patients ceased treatment with index medication, with no switch to nonindex RCC medications from last index prescription through follow-up and no reinitiation of index medication from last index prescription’s days supply plus at least 30 days through the end of follow-up.
The RCC medications included sunitinib, pazopanib, temsirolimus, axitinib, everolimus, interferon alfa-2B recombinant, bevacizumab, sorafenib, aldesleukin, and interferon alfa-2a (Appendix A).
Costs.
Costs were categorized into the following 3 groups:
Index medication costs: Mean daily index medication cost during the persistence period was determined using persistence days as the denominator.
1-year RCC-related costs: Payer-paid costs were determined over the 12-month follow-up for RCC medical (diagnosis-related), RCC medication, and total RCC-related (medical and medication) costs. RCC-related medical costs were based on inpatient and outpatient visits with an ICD-9-CM code of 189.0. Costs related to RCC medication included administrations and prescriptions for sunitinib, pazopanib, aldesleukin, axitinib, bevacizumab, everolimus, interferon α-2a, sorafenib, interferon α-2B recombinant, and temsirolimus.
1-year all-cause costs: All-cause medical (inpatient + outpatient), all-cause medication, and all-cause total (medical and medication) costs were determined over the 12 months post-index. Any health care cost, regardless of diagnosis or type of medication, was included.
Statistical Analysis
Demographic and pre-index clinical characteristics, treatment patterns, and costs between treatment groups were compared using means [± SD] for continuous variables and relative frequencies (n, %) for categorical data.
The current starting dose schedule recommendation for sunitinib is 50 mg orally every day for 4 weeks followed by 2 weeks off therapy (i.e., a 42-day supply). The days supply value in the prescription record is integral to calculations of persistence, yet the majority of records in a preliminary analysis had 28 or 30 days supply, which does not account for the 2 weeks off therapy. Thus, we evaluated the effect of substituting 42 days for sunitinib prescription records with 28 or 30 days supply on calculations of mean days supply, treatment patterns including persistence, and costs. To serve as a possible proxy for commercial versus Medicare-insured status, differences in costs were evaluated for patients aged < 65 years versus patients aged ≥ 65 years.
Chi-square tests were used to test for differences in categorical variables, and Student t-tests were used for continuous variables other than costs. A multivariable regression model was used to examine differences in costs between sunitinib and pazopanib. Since health care costs are typically non-normally distributed, a generalized linear model with a gamma distribution and log link was used for evaluating costs (dependent variable), controlling for patient demographics and pre-index characteristics, persistence days, and index medication (as predictor variables). All statistical tests were 2-tailed with significance set at P < 0.05, except for treatment group by age interactions where significance was set at P < 0.10.
Results
Description of Study Sample
Following application of inclusion/exclusion criteria, 609 patients initiating treatment with sunitinib and 183 patients initiating treatment with pazopanib were identified as the study sample (Figure 2). Of these, 382 (65%) sunitinib patients and 115 (63%) pazopanib patients were aged < 65 years (data not shown).
FIGURE 2.
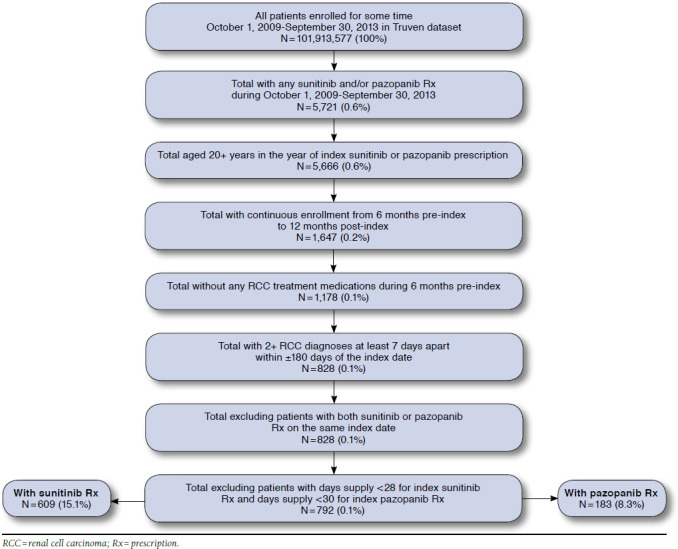
Sample Selection and Attrition Flowchart
No significant differences in mean age, gender, geographic region, pre-index inpatient admissions, or metastatic diagnosis were observed between sunitinib or pazopanib treatment groups (Table 1). Mean [SD] pre-index plan enrollment was significantly shorter for patients in the sunitinib group than for patients in the pazopanib group (857 vs. 962 days, respectively; P = 0.002). Of note, the length of time between the earliest pre-index RCC diagnosis (back to January 1, 2008) and the index date was also shorter for sunitinib (373 days) than pazopanib (491 days; P = 0.001).
TABLE 1.
Demographic and Clinical Characteristics
| Characteristic | Sunitinib n = 609 | Pazopanib n = 183 | P Value |
|---|---|---|---|
| Age, mean [SD] | 62.61 [10.38] | 61.93 [11.27] | 0.443 |
| Age group, n (%) | |||
| 18-34 | 2 (0.33) | 2 (1.09) | 0.252 |
| 35-44 | 22 (3.61) | 6 (3.28) | |
| 45-54 | 93 (15.27) | 38 (20.77) | |
| 55-64 | 265 (43.51) | 69 (37.70) | |
| 65+ | 227 (37.27) | 68 (37.16) | |
| Gender, n (%) | |||
| Male | 443 (72.74) | 138 (75.41) | 0.474 |
| Female | 166 (27.26) | 45 (24.59) | |
| Geographic region, n (%) | |||
| Northeast | 87 (14.29) | 22 (12.02) | 0.832 |
| North Central | 162 (26.60) | 52 (28.42) | |
| South | 220 (36.12) | 68 (37.16) | |
| West | 132 (21.67) | 40 (21.86) | |
| Unknown | 8 (1.31) | 1 (0.55) | |
| Continuous enrollment days (pre-index), mean [SD] | 856.67 [399.48] | 962.21 [436.43] | 0.002a |
| CCI score, mean [SD] | 7.98 [2.90) | 8.11 [2.80] | 0.604 |
| Metastatic diagnoses during 6 months pre-index, n (%) | 495 (81.28) | 151 (82.51) | 0.706 |
| Metastatic diagnosis from January 1, 2008 through September 30, 2013 | 578 (94.91) | 179 (97.81) | 0.094 |
| Days from earliest pre-index RCC diagnosis, January 1, 2008, to index date, mean [SD] | 372.57 [397.26] | 490.75 [435.17] | < 0.001a |
| Number of all-cause inpatient admissions, during 6 months pre-index, n (%) | 322 (52.87) | 90 (49.18) | 0.381 |
| Per patient mean [SD] | 0.72 [0.82] | 0.86 [1.23] | 0.145 |
| Inpatient admissions with any RCC diagnosis, during 6 months pre-index, n (%) | 264 (43.35) | 73 (39.89) | 0.407 |
| Per patient mean [SD] | 0.55 [0.73] | 0.64 [1.04] | 0.228 |
aP < 0.05.
CCI = Charlson Comorbidity Index; RCC = renal cell carcinoma; SD = standard deviation.
Mean CCI scores were similar, as was the proportion of patients with specific CCI conditions (Appendix A), with the exception of chronic pulmonary disease, which was more prevalent in the sunitinib group (16.4%) than the pazopanib group (8.74%, P = 0.010). All characteristics were similar between patients aged < 65 years versus patients aged ≥ 65 years (data not shown).
Medication Use Patterns
Using days supply as observed in the prescription records, the persistence period was shorter for sunitinib (177 days) than pazopanib (194 days), although the difference was not statistically significant (Table 2). Significant differences in the number of index medication prescriptions (4.53 vs. 5.81, P < 0.001) and total days supply (145 vs. 183, P < 0.001) during the persistence period were observed between sunitinib and pazopanib groups, respectively. The number of unique RCC medications was not significantly different.
TABLE 2.
Medication Treatment Patterns Using Observed and Substituted Days Supplya
| Sunitinib n = 609 | Pazopanib n = 183 | P Value | |
|---|---|---|---|
| Persistence period, mean [SD] days | 176.61 [125.20] | 194.28 [129.84] | 0.097 |
| Substituted | 209.46 [126.17] | 194.28 [129.84] | 0.157 |
| Total index medication days supply during persistence period, mean [SD] | 144.78 [102.05] | 182.50 [123.82] | < 0.001b |
| Substituted | 201.59 [122.16] | 182.50 [123.82] | 0.065 |
| Number of index medication prescriptions filled during persistence period, mean [SD] | 4.53 [3.16] | 5.81 [4.06] | < 0.001b |
| Substituted | 5.14 [3.48] | 5.81 [4.06] | 0.007b |
| Number of unique RCC medications during persistence period, mean [SD] | 1.01 [0.08] | 1.02 [0.15] | 0.178 |
| Substituted | 1.02 [0.16] | 1.02 [0.15] | 0.932 |
| Pattern of index medication use, overall, n (%) | 0.041b | ||
| Substituted | 0.0023 | ||
| Continue | 137 (22.50) | 56 (30.60) | 0.025b |
| Substituted | 201 (33.0) | 56 (30.60) | 0.543 |
| Discontinue | 113 (18.56) | 21 (11.48) | 0.025b |
| Substituted | 117 (19.2) | 21 (11.48) | 0.016b |
| Switched | 186 (30.54) | 58 (31.69) | 0.767 |
| Substituted | 197 (32.3) | 58 (31.69) | 0.868 |
| Interrupted therapy | 173 (28.41) | 48 (26.23) | 0.565 |
| Substituted | 94 (15.4) | 48 (26.23) | < 0.001b |
a Substitution of 42-day supply for prescriptions with 28- or 30-day supply.
bP < 0.05.
RCC = renal cell carcinoma; SD = standard deviation.
Using the observed days supply, there were significant differences between treatment groups in continuation of the index medication (overall P = 0.041) with fewer patients in the sunitinib group continuing treatment than in the pazopanib group. These patterns were similar between patients aged < 65 years versus patients aged ≥ 65 years (data not shown).
Accounting for sunitinib dosing schedule through substitution of days supply had an effect on medication use patterns. Although the mean persistence period did not differ significantly between groups with substitution, the mean days supply reversed direction from being significantly lower with sunitinib (144.8 days vs. 182.5 days for pazopanib, P < 0.001) to trending higher (201.6 days with sunitinib vs. 182.5 days for pazopanib, P = 0.065) with substitution. Treatment patterns also changed with substitution for the numbers of patients who continued with therapy and those whose therapy was interrupted. Without substitution, a significant difference was observed in patients who continued sunitinib versus pazopanib (22.5% vs. 30.6%, respectively, P = 0.025); with substitution the difference became nonsignificant (33.0% vs. 30.6%, respectively, P = 0.543). However, substitution had a larger effect with regard to interrupted therapy, with significantly fewer sunitinib patients having interrupted therapy (28.4% of sunitinib vs. 26.2% of pazopanib without substitution, P = 0.565; 15.4% vs. 26.2%, respectively, P = 0.001, with substitution).
RCC-Related and All-Cause Costs
As shown in Table 3, unadjusted 1-year total RCC-related and all-cause total costs were not significantly different between treatment groups. Because these costs are not specific to the persistence period, substitution had no effect. All cost components were similar by age group, with the exception of all-cause medical cost, which was significantly higher in the sunitinib group in patients aged ≥ 65 years ($46,859.42 and $32,321.80, respectively, P = 0.010).
TABLE 3.
Unadjusted Health Care Costsa
| Sunitinib Mean [SD] | Pazopanib Mean [SD] | P Value | |
|---|---|---|---|
| Average daily cost for index medication during persistence period | |||
| Aged < 65 years | 182.59 [52.33] | 172.78 [52.53] | 0.079 |
| Aged ≥ 65 years | 171.58 [50.50] | 183.17 [41.70] | 0.086 |
| RCC diagnosis cost in 12 months post-index | |||
| Aged < 65 years | 37,465.39 [115,596.71] | 31,530.71 [39,019.37] | 0.189 |
| Aged ≥ 65 years | 17,126.76 [24,026.93] | 13,292.10 [16,697.30] | 0.126 |
| RCC medicationb cost in 12 months post-index | |||
| Aged < 65 years | 59,884.14 [27,250.96] | 54,790.31 [29,787.99] | 0.388 |
| Aged ≥ 65 years | 46,333.61 [27,635.49] | 49,215.15 [26,962.09] | 0.586 |
| RCC-related total cost (RCC diagnosis cost + RCC medication cost) in 12 months post-index | |||
| Aged < 65 years | 97,349.53 [116,782.36] | 86,321.03 [48,606.30] | 0.072 |
| Aged ≥ 65 years | 63,460.37 [35,258.34] | 62,507.25 [32,143.48] | 0.862 |
| All-cause medical cost (IP + OP) in 12 months post-index | |||
| Aged < 65 years | 69,987.68 [170,408.04] | 67,499.81 [69,852.38] | 0.754 |
| Aged ≥ 65 years | 46,859.42 [62,186.99] | 32,321.80 [35,269.38] | 0.010 |
| All-cause medication cost (Rx) in 12 months post-index | |||
| Aged < 65 years | 61,134.88 [28,641.89] | 55,561.92 [31,591.63] | 0.357 |
| Aged ≥ 65 years | 46,812.58 [28,369.01] | 52,398.83 [29,156.07] | 0.293 |
| All-cause total cost (IP + OP + Rx) in 12 months post-index | |||
| Aged < 65 years | 131,122.56 [168,767.89] | 123,061.73 [73,631.26] | 0.344 |
| Aged ≥ 65 years | 93,672.00 [64,833.49] | 84,720.63 [44,705.62] | 0.238 |
aAll amounts are in U.S. dollars.
bRCC-related medication costs include index and nonindex medications and included administration costs for RCC medications.
IP = inpatient; OP = outpatient; RCC = renal cell carcinoma; Rx = prescription; SD = standard deviation.
The unadjusted mean daily index medication costs using observed days supply was significantly different in the < 65 age group ($219.22 sunitinib and $172.88 pazopanib, P < 0.001) and nonsignificant in the ≥ 65 age group ($209.24 sunitinib and $183.17 pazopanib, P = 0.055). Substitution of days supply for sunitinib resulted in lower mean daily medication cost for this agent, eliminating the difference compared with pazopanib in patients aged < 65 years ($182.59 sunitinib and $172.88 pazopanib, P = 0.400) or aged ≥ 65 years ($171.58 sunitinib and $183.17 pazopanib, P = 0.320; Table 3).
After adjusting for possible confounding factors (Figure 3), we found similar results as with unadjusted costs; however, treatment group comparisons within age groups were not different (aged < 65 years P = 0.340, aged ≥ 65 years P = 0.110). Similar findings with substitution were observed (data not shown). Similar to unadjusted cost results, substituting days supply affected the mean daily adjusted index medication costs by eliminating the significant differences between treatment groups. After substitution, the mean [SD] daily costs were similar between comparators: $178.49 [51.89] for sunitinib versus 176.65 [48.93] for pazopanib (P = 0.669). The adjusted 1-year RCC-related and all-cause costs were not significantly different between index medication treatment groups, and substitution had no effect on these costs.
FIGURE 3.
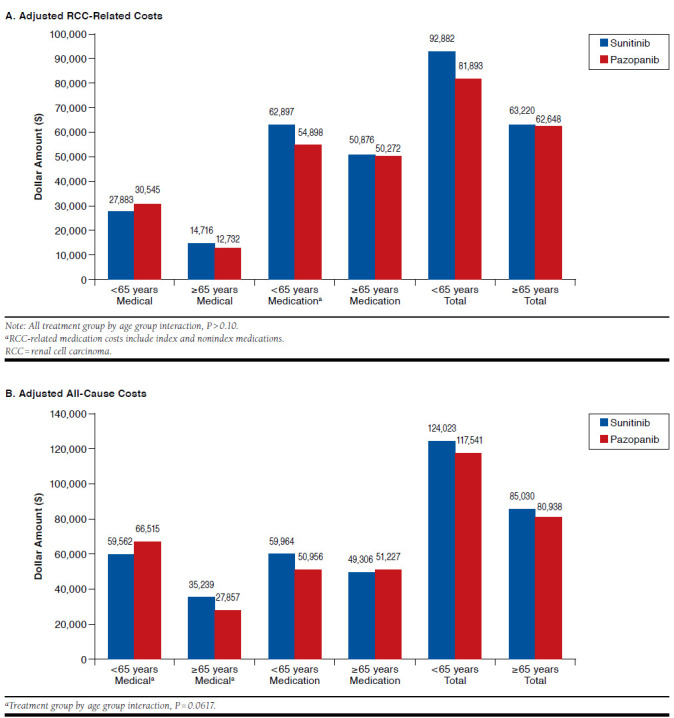
Adjusted RCC-Related and All-Cause Costs
Discussion
Accurate understanding of treatment persistence and costs in a real-world setting is an important contributor to decision making regarding the first-line treatment of RCC. Existing literature describing cost differences between sunitinib and pazopanib include a study applying costs to a clinical trial population and a real-world study of pazopanib and sunitinib in first or later lines of therapy for RCC.10,13 Using health care claims, we compared patient characteristics and medication use patterns between treatment-naïve RCC patients initiating sunitinib and pazopanib as first-line therapy. This study contributes unique health care cost information and demonstrates the effect of using substitution of days supply to address the difference in dosing schedules of these 2 drugs. There are few studies comparing first-line RCC targeted treatment groups, but we highlight key similarities and differences with this study.
Demographic and Pre-index Clinical Characteristics
Mean age, the predominance of males, and geographic distribution of patients were similar between treatment groups and comparable to national statistics and previous studies of various targeted RCC treatments using health care claims.7,9,13,15 Similar to our results, the results of the only other real-world study comparing sunitinib and pazopanib treatment patterns using CCI scores found a similar extent of comorbidity between treatment groups.9 In the current study, a significantly longer time from RCC diagnosis to index date and longer pre-index enrollment period for pazopanib patients than sunitinib patients was observed, although reasons for these differences are not clear. It is possible that the baseline health of patients or physician experience influenced the choice of index treatment. Because pazopanib was approved for advanced RCC late in 2009, physicians may have been more inclined to prescribe sunitinib as first-line treatment in the early years of the study period. This might also explain the larger proportion of sunitinib patients in the study overall.
Treatment Patterns
Increasing use of oral oncology agents provides convenience advantages over clinic-delivered treatments, but it has been reported that persistence to self-administered drugs may be problematic.15,16 A few retrospective studies have assessed persistence to tyrosine kinase inhibitors in RCC, but comparisons are hampered by dissimilar measures and methodology. Important factors that must be considered when comparing treatment patterns and costs over time are similarities or differences in dosing schedules. The problem of drug exposure misclassification with inaccurate pharmacist-recorded days supply has been described, and correction of days supply by substitution has been used in disease states where noncontinuous daily drug dosing occurs.18,19 Packaging of sunitinib as 28 count and U.S. health plan dispensing requirements likely contribute to pharmacist entry of 28 count or 30 count in the days supply field of the prescription record.
For the current study, the need to accommodate for non-continuous daily dosing through correction of days supply by substitution was informed through preliminary analysis. This analysis revealed that most sunitinib prescriptions had days supply values of 28 or 30, both less than the dosing cycle of 42 days, but prescription date intervals were consistent with a dosing cycle of 42 days. The effect of substitution was evident through changes in persistence results and consequent conclusions regarding differences between treatment groups that involve this measure. In their studies comparing treatment patterns between sunitinib and pazopanib, DaCosta Byfield et al. (2015) adjusted for sunitinib’s dosing schedule using different methods, and Racsa et al. used substitution methods similar to the current study.9,10 Despite different methods and patient selection criteria, both studies yielded similar conclusions as the current study, that is, no significant differences in persistence between treatment groups. Taken together, all studies further emphasize that consideration of dosing schedule is needed when comparing sunitinib to other first-line RCC treatments.
Health Care Costs
The final objective of this study was to compare mean daily index medication costs and all-cause and RCC-related health care costs during the 12-month follow-up period. It was hypothesized that substitution would affect cost calculations because days supply affects calculations of the persistence period and, as a result, costs calculated over the persistence period. Without substitution, sunitinib had a significantly higher average daily medication cost, but substitution eliminated the difference. No significant differences were observed between treatment groups stratified by the age 65 threshold in adjusted daily costs, RCC-related costs, or all-cause 12-month costs. Before adjustment for demographic and clinical characteristics, all-cause medical costs were significantly higher in the sunitinib group in patients aged ≥ 65 years but were not different after adjustment.
Few studies have examined health care costs associated with RCC treatment. Geynisman et al. (2015) reported similar first-year, first-line oral drug costs for patients aged < 65 years.7The authors reported an average across all drugs based on data from 2004-2010, but the results are comparable to the 1-year post-index RCC medication costs for sunitinib and pazopanib observed in the current study. Racsa et al. reported higher total health care costs than in the current study (with no significant differences between treatment groups) and also found that adjusted pharmacy costs for sunitinib and pazopanib patients were not significantly different, including index and non-index RCC medications.10 However, the magnitude of cost results are not comparable to the current study because of the much shorter treatment duration in the Racsa et al. study and because that study’s sample had more Medicare patients, whereas the current study had more commercial patients.10 Our examination by age group was intended to serve as a possible proxy for commercial versus Medicare insured status. Gross covered payments for both treatment groups were higher in patients aged < 65 years compared with those patients aged ≥ 65 years, but no significant differences were observed between treatment groups for either age group.
Any economic comparison must have accurate cost data as its foundation, and so far, real-world studies using actual costs are lacking. To date, only 2 studies have directly compared health care costs between patients receiving sunitinib and pazopanib.10,13 Hansen et al. used a clinical trial population and applied wholesale acquisition drug costs to treatment and assigned price weights from insurance data to select adverse events rather than study patients from routine clinical practice and the actual costs associated with their care.13 The authors’ conclusion that overall costs were greater for patients treated with sunitinib was based solely on differences observed in drug costs, and it is important to note that the reference dates for drug costs used were from different time points: preceding a price increase for pazopanib and following a price increase for sunitinib. Racsa et al. observed no difference in overall annual costs between the 2 treatment groups but did observe significantly higher annual costs in nonadherent sunitinib patients compared with nonadherent pazopanib patients.10 This difference may be explained by unreported factors and/or patient selection, since patients previously treated for RCC were included, and there was a significantly higher rate of previous treatment in the pazopanib group.10 Comparisons of cost in the published literature are few, and understanding the methodologies employed, results observed, and conclusions made is critical. Accurate data are vital in cost studies, as well as patient, physician, and payer considerations regarding treatment.
Limitations
Possible limitations to this study should be considered in the interpretation of the findings, in part because health care claims data were not created specifically for research purposes. Claims may contain errors in coding or may be incomplete. The presence of prescription fill claims cannot be considered to represent proper self-administration or adherence or include any drugs obtained outside of the pharmacies filing the claims, such as physician samples, or patients’ participation in a clinical trial. Nevertheless, there is no reason to believe these limitations would be present to differing degrees in patients taking sunitinib versus pazopanib. This study also did not assess survival or adverse events, which might influence persistence results. Also, this study’s results are not necessarily generalizable to patients who are not enrolled in similar health plans as those in this study, since the characteristics and gross covered payments may differ among different insured samples.
Conclusions
This analysis of persistence and actual costs represents the experience of real-world clinical practice of first-line treatment of RCC with sunitinib and pazopanib. Accounting for sunitinib’s unique dosing cycle for persistence and cost calculations is critical when comparing first-line RCC targeted treatments. The substitution of sunitinib days supply corrected for the values not reflecting actual patient experience. Calculations affected by this measure include daily index medication costs. There were no significant differences in persistence and similar RCC-related and all-cause costs over the 12-month period following the initiation of sunitinib or pazopanib as first-line therapy for RCC. The results of this study support selection of initial systemic treatment for RCC based on patients’ individual clinical factors and physician experience in using these agents.
Acknowledgments
Medical writing support was provided by Caroline Jennermann, an employee of Optum, with funding by Pfizer.
APPENDIX A. NDC Numbers and HCPS/CPT Codes for RCC Medications
| NDC Numbers | Brand Name | Generic Name | HCPS/CPT Codes |
|---|---|---|---|
| 00008-1179-01, 00008-1179-05 | Torisel | Temsirolimus | J9330, C9239 |
| 00069-0145-01, 00069-0151-11 | Inlyta | Axitinib | N/A |
| 00069-0550-38, 00069-0770-38, 00069-0980-38, 69055030, 69055038, 69077030, 69077038, 69098030, 69098038, 54569598200, 54569598300, 54868557300 | Sutent | Sunitinib malate | N/A |
| 00078-0566-51, 00078-0566-61, 00078-0567-51, 00078-0567-61, 00078-0594-51, 00078-0594-61, 00078-0620-51, 00078-0620-61 | Afinitor | Everolimus | J7527, J8561 |
| 00085-0571-02, 00085-1133-01 | Intron A | Interferon alfa-2B, recombinant | J9214 CPT 100282 |
| 00173-0804-09 | Votrient | Pazopanib HCL | N/A |
| 50242-0061-01 | Avastin | Bevacizumab | C9214, C9257, J9035, Q2024, S0116 |
| 50419-0488-58 | Nexavar | Sorafenib tosylate | N/A |
| 65483-0116-07 | Proleukin | Aldesleukin | J9015 |
| 4201109, 4201607, 4201209, 4200909, 4201507, 4201509, 4201609, 4198701, 4201709, 4201707, 4690033, 4201009, 4200709, 4199309, 4201509, 4200909, 4201109, 4199309, 4201609, 4201507, 4201009, 4200709, 4201209, 4201707, 4201607, 4198701, 4690033, 4201709 | Roferon-A | Interferon Alfa-2a | J9213 |
CPT = Current Procedural Terminology; HCPCS = Healthcare Common Procedure Coding System; N/A = not available; NDC = National Drug Code.
APPENDIX B. Charlson Comorbidity Index Conditions Identified
| Comorbid Condition, n (%) | Sunitinib n = 609 | Pazopanib n = 183 | P Value |
|---|---|---|---|
| Any comorbid condition | 607 (99.7) | 182 (99.5) | 0.674 |
| Myocardial infarction | 21 (3.4) | 9 (4.9) | 0.361 |
| Congestive heart failure | 23 (3.8) | 13 (7.1) | 0.058 |
| Peripheral vascular disease | 21 (3.4) | 8 (4.4) | 0.560 |
| Cerebrovascular disease | 47 (7.7) | 12 (6.6) | 0.600 |
| Dementia | 3 (0.5) | 0 (0.0) | 0.342 |
| Chronic pulmonary disease | 100 (16.4) | 16 (8.7) | 0.010 |
| Rheumatologic disease | 7 (1.1) | 2 (1.1) | 0.950 |
| Peptic ulcer disease | 8 (1.3) | 2 (1.1) | 0.815 |
| Mild liver disease | 4 (0.7) | 2 (1.1) | 0.551 |
| Diabetes | 162 (26.6) | 40 (21.9) | 0.197 |
| Diabetes with chronic complications | 25 (4.1) | 11 (6.0) | 0.278 |
| Hemiplegia or paraplegia | 3 (0.5) | 2 (1.1) | 0.369 |
| Renal disease | 112 (18.4) | 42 (23.0) | 0.172 |
| Malignancy | 599 (98.4) | 182 (99.5) | 0.267 |
| Moderate or severe liver disease | 4 (0.7) | 0 (0.0) | 0.272 |
| Metastatic solid tumor | 495 (81.3) | 151 (82.5) | 0.706 |
| AIDS | 1 (0.2) | 0 (0.0) | 0.583 |
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29. Available at: http://onlinelibrary.wiley.com/doi/10.3322/caac.21254/pdf. Accessed June 10, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Kirchner H, Strumberg D, Bahl A, Overcamp F.. Patient-based strategy systemic treatment of metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2010;10(4):585-96. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Kidney cancer (adult)-renal cell carcinoma. Revised February 10, 2016. Available at: http://www.cancer.org/cancer/kidneycancer/detailedguide/kidney-cancer-adult-key-statistics. Accessed June 10, 2016.
- 4.Bhat S. Role of surgery in advanced/metastatic renal cell carcinoma. Indian J Urol. 2010;26(2):167-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute, Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: kidney and renal pelvis cancer. Available at: http://seer.cancer.gov/statfacts/html/kidrp.html. Accessed June 10, 2016.
- 6.Sun M, Larcher A, Karakiewicz PI.. Optimal first-line and second-line treatments for metastatic renal cell carcinoma: current evidence. Int J Nephrol Renovascul Dis. 2014;7:401-07. Available at: https://www.dovepress.com/optimal-first-line-and-second-line-treatments-for-metastatic-renal-cel-peer-reviewed-article-IJNRD. Accessed June 10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geynisman DM, Hu JC, Liu L, Tina Shih YC.. Treatment patterns and costs for metastatic renal cell carcinoma patients with private insurance in the United States. Clin Genitourin Cancer. 2015;13(2):e93-100. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722-31. Available at: http://www.nejm.org/doi/full/10.1056/NEJMoa1303989. Accessed June 10, 2016. [DOI] [PubMed] [Google Scholar]
- 9.DaCosta Byfield SA, McPheeters JT, Burton TM, Nagar SP, Hackshaw MD.. Persistence and compliance among U.S. patients receiving pazopanib orsunitinib as first-line therapy for advanced renal cell carcinoma: a retrospective claims analysis. J Manag Care Spec Pharm. 2015;21(6):515-22. Availableat: http://www.jmcp.org/doi/abs/10.18553/jmcp.2015.21.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racsa PN, Whisman TR, Worley K.. Comparing two tyrosine kinase inhibitors for treatment of advanced renal cell carcinoma in Medicare and commercially insured patients. Curr Med Res Opin. 2015;31(10):1933-40. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell CC, Parikh OA.. Factors involved in treatment preference in patients with renal cancer: pazopanib versus sunitinib. Patient Pref Adhere. 2014;8:503-11. Available at https://www.dovepress.com/factors-involved-in-treatment-preference-in-patients-with-renal-cancer-peer-reviewed-article-PPA. Accessed June 10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaisaeng N, Harpe SE, Carroll NV.. Out-of-pocket costs and oral cancer medication discontinuation in the elderly. J Manag Care Spec Pharm. 2014;20(7):669-75. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2014.20.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen RN, Hackshaw MD, Nagar SP, et al. Health care costs among renal cell cancer patients using pazopanib and sunitinib. J Manag Care Spec Pharm. 2015;21(1):37-44. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2015.21.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-19. [DOI] [PubMed] [Google Scholar]
- 15.Hackshaw MD, Nagar SP, Parks DC, Miller LA.. Persistence and compliance with pazopanib in patients with advanced renal cell carcinoma within a U.S. administrative claims database. J Manag Care Spec Pharm. 2014;20(6):603-10. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2014.20.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foulon V, Schoffski P, Wolter P.. Patient adherence to oral anticancer drugs: an emerging issue in modern oncology. Acta Clin Belg. 2011;66(2):85-96. [DOI] [PubMed] [Google Scholar]
- 17.Ruddy K, Mayer E, Partridge A.. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clinic. 2009;59(1):56-66. Available at: http://onlinelibrary.wiley.com/doi/10.3322/caac.20004/abstract;jsessionid=602DA0ED1F1F4237E65C122754E0E83F.f01t01. Accessed June 10, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Schneeweis S, Avorn J.. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323-37. [DOI] [PubMed] [Google Scholar]
- 19.Burden AM, Huang A, Tadrous M, Cadarette SM.. Variation in the days supply field for osteoporosis medications in Ontario. Arch Osteoporosis. 2013;8:128. [DOI] [PubMed] [Google Scholar]


