Abstract
BACKGROUND:
Despite evidence showing the benefits of treatment intensification following an elevated hemoglobin A1c (A1c), clinical inertia, or failure to establish and/or escalate treatment to achieve treatment goals, is a concern among patients diagnosed with type 2 diabetes (T2DM). Clinical inertia may contribute to increased health care utilization and costs due to poor clinical outcomes in MCOs.
OBJECTIVES:
To (a) identify factors associated with clinical inertia in T2DM and (b) determine differences in A1c goal attainment between patients who experience clinical inertia versus treatment intensification in a commercially insured population.
METHODS:
Medical and pharmacy claims data were used to identify commercially insured patients in a regional MCO with a recorded A1c ≥ 8.0% between January 1, 2013, and December 31, 2015. In the 4 months following the first elevated A1c value (index date), patients were classified into 2 groups: treatment intensification or clinical inertia. Treatment intensification was defined as the addition of ≥ 1 new noninsulin antihyperglycemic medication, the addition of insulin, or a dose increase of any current noninsulin antihyperglycemic medication. Patients were required to have ≥ 1 follow-up A1c value 6-12 months after the index date and continuous enrollment in the health plan for 12 months before and after the index date. Patients were excluded if they had a diagnosis for gestational diabetes or type 1 diabetes or if they were on insulin in the pre-index period. The primary outcome of attaining A1c < 7.0% was compared between groups after propensity score matching (PSM). Factors associated with clinical inertia were identified using logistic regression.
RESULTS:
3,078 patients, with a mean (SD) age of 54.4 (10.6) years and a mean (SD) baseline A1c of 9.6% (1.7), were included in the study. Of these, 1,093 patients (36%) experienced clinical inertia. After PSM, 1,760 patients remained; 880 in each group. In the clinical inertia group, 23% of patients achieved an A1c < 7.0% in the post-index period, compared with 35% in the treatment intensification group (P < 0.001). A greater likelihood of experiencing clinical inertia was associated with baseline treatment with 2 (OR = 1.51, 95% CI = 1.22-2.86; P < 0.001) or ≥ 3 (OR = 1.78, 95% CI = 1.30-2.42; P < 0.001) antihyperglycemic medications (vs. none), baseline age ≥ 65 years (OR = 2.11, 95% CI = 1.63-2.74; P < 0.001), and diagnosis of coronary heart disease (OR = 1.44, 95% CI = 1.10-1.88; P = 0.007). A baseline A1c ≥ 9.0% (vs. 8.0%-8.9%) was associated with a lower likelihood of experiencing clinical inertia (OR = 0.56, 95% CI = 0.48-0.66; P < 0.001).
CONCLUSIONS:
More than a third of patients in a commercially insured population with T2DM and a baseline A1c ≥ 8% experienced clinical inertia. Clinical inertia resulted in worse A1c outcomes over the 12-month follow-up period. Results of this study suggest that treatment intensification should be monitored, with efforts made to educate health care providers on strategies aimed at improving glycemic control for high-risk patients.
What is already known about this subject
Early and intensive treatment of type 2 diabetes mellitus (T2DM) can lead to the attainment of recommended evidence-based goals of glucose control, as measured by hemoglobin A1c (A1c), which can subsequently reduce diabetes-related complications.
Clinical inertia, or failure to establish treatment goals and/or intensify treatment after a lack of A1c response or loss of A1c control, is a concern.
MCOs are faced with large T2DM populations, so clinical inertia may have a pronounced contribution to increases in health care utilization and costs due to poor clinical outcomes.
What this study adds
Among commercially insured T2DM patients with an A1c ≥ 8.0% in a regional MCO, 36% experienced clinical inertia, which led to a significantly lower proportion of patients attaining an A1c < 7.0%.
Patients using multiple antihyperglycemic medications at the time of an elevated A1c, patients aged ≥ 65 years, and patients with an A1c between 8.0% and 9.0% were more likely to experience clinical inertia.
This study adds to the growing body of evidence highlighting the need to decrease the incidence of clinical inertia in patients with T2DM and target those at risk based on patient characteristics.
In the United States, an estimated 21.9 million adults are diagnosed with type 2 diabetes mellitus (T2DM).1 The annual cost of diabetes in the United States is $327 billion, including $237 billion in direct medical costs and $90 billion in lost productivity.2 Suboptimal or poor glycemic control is manifested by elevations in hemoglobin A1c (A1c), an indicator of the average blood glucose over the previous 3 months, and increases the risk of cardiovascular disease, microvascular complications, and mortality.3,4 To reduce the risk of diabetes-related complications and poor outcomes, the American Diabetes Association (ADA) recommends an A1c treatment goal < 7.0% for most patients with T2DM.5 However, health plan quality measures may have different T2DM treatment control definitions, such as an A1c < 8.0% used by the Healthcare Effectiveness Data and Information Set (HEDIS)measures.6
Despite the clinical benefits associated with early treatment intensification, clinical inertia—defined by the Agency for Healthcare Research and Quality as failure to intensify therapy within 4 months of an uncontrolled A1c—is common.7-17 The prevalence of clinical inertia in patients with T2DM in the United States ranges from 28% to 73% and has been associated with older age and lower, yet still above-goal, baseline A1c.11-17 However, clinical inertia is multifactorial and may be driven by physician, patient, and system-related factors.10 Thus, to design strategies that can successfully target and address this problem, managed care organizations (MCOs) should understand factors associated with clinical inertia in managed care patient populations.
The primary objective of our study was to evaluate the effect of clinical inertia on the attainment of an A1c < 7.0%. To accomplish this, we identified the extent of clinical inertia in a commercially insured patient population and assessed its effect on A1c values in a 6- to 12-month follow-up period. We also aimed to identify factors associated with clinical inertia in our commercial claims data. Our main purpose was to provide MCOs with valuable information that may help identify, prevent, and address this challenge.
Methods
Study Design
We conducted a retrospective cohort study that used medical and pharmacy claims data, as well as laboratory data, between January 1, 2012, and September 30, 2016, from commercially insured SelectHealth patients (Figure 1). SelectHealth is a nonprofit regional MCO that covers approximately 850,000 lives from the Intermountain region of the United States with commercial, Medicare, and Medicaid plans available.18 The index date was defined as the date of the patient’s first A1c ≥ 8.0% between January 1, 2013, and September 30, 2015. Because patients with an uncontrolled A1c closer to 7.0% may receive nonpharmacologic recommendations (e.g., diet and exercise), we used an A1c ≥ 8.0%, which aligns with HEDIS health plan quality measures and where pharmacologic intensification is likely to be the most appropriate intervention, to identify patients at risk for experiencing clinical inertia.6,19 Patients were required to have at least 1 follow-up A1c value between 6 and 12 months after the index date. The study exposure, treatment intensification versus clinical inertia, was assessed during the 4-month post-index window (index date –7 days to +120 days).10
FIGURE 1.
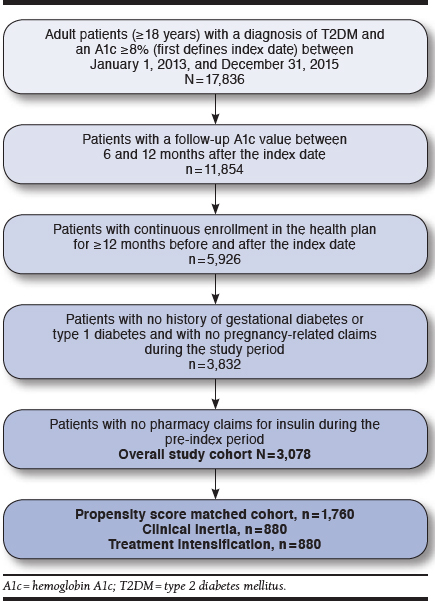
Patient Identification Flowchart
Patient Population
In addition to an A1c ≥ 8.0%, patients were required to have at least 1 medical diagnosis of T2DM (identified by International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 250.x0 and 250.x2) to be included in the study. Patients were also required to have continuous enrollment (i.e., no gaps in coverage ≥ 90 days) in their commercial health plan for at least 12 months before and after the index date.
Patients were excluded if they met any of the following criteria: (a) female patients could not have a diagnosis of gestational diabetes or any pregnancy-related claims during the study period (Appendix A, available in online article); (b) patients could not have any insulin prescription claims in the pre-index period, as assessing insulin dose intensification is not reliable using claims data and may have resulted in misclassification of clinical inertia (Appendix B, available in online article)20; and (c) patients could not have a medical diagnosis of type 1 diabetes (Appendix A).
Study Variables
Primary Independent Variable – Clinical Inertia.
Clinical inertia was defined as no evidence of T2DM treatment intensification during the 4-month window following an A1c ≥ 8.0%.5,10 Treatment intensification was defined as the presence of at least 1 of the following ADA guideline-recommended interventions5: (a) a prescription claim for at least 1 new noninsulin antihyperglycemic medication class, (b) a prescription claim for insulin, or (c) an increase in the average daily dose of a current noninsulin antihyperglycemic medication. The average daily dose (i.e., [medication strength (e.g., milligrams) × quantity]/day supply) was calculated from the prescription claim information for each individual antihyperglycemic agent in the 4 months before and after the index date. The time to treatment intensification was quantified as the days until the first change in patients’ antihyperglycemic regimen.
A1c Outcomes.
The primary study outcome was the proportion of patients who achieved an A1c < 7.0% on the last recorded A1c between 6 and 12 months after the index date and was compared between the treatment intensification versus the clinical inertia groups. We also compared the difference in follow-up A1c and the change in A1c from baseline between the treatment intensification and clinical inertia groups. To assess for variability in the follow-up time, we described the time until the last recorded A1c in the 6- to 12-month post-index period.
Baseline Characteristics.
Patient baseline demographic and clinical characteristics were obtained using data from the 12-month pre-index period up to and including the index date. These characteristics included age, sex, race, baseline A1c at the index date, presence of key comorbidities (identified using ICD-9-CM codes, Appendix A), and receipt of diabetes counseling or education session visits (identified using Current Procedural Terminology codes, Appendix A).
Any baseline antihyperglycemic medication use, including the number and specific classes used, was assessed using pharmacy claims data from the 4-month period before the index date (Appendix B). The adapted Diabetes Complication Severity Index (aDCSI) was also calculated for each patient to assess disease severity (Appendix A).21,22 Physician practice type was identified using the physician listed on the first antihyperglycemic pharmacy claim in the post-index period. However, due to the amount of missing data resulting from this approach, we supplemented it using the physician listed on the last outpatient claim for T2DM immediately before the index date. Physician practice type was classified as primary care, specialty (i.e., cardiologists, endocrinologists, and nephrologists), other, or missing.
Other Covariates.
To examine potential difficulties in lowering A1c, we evaluated whether patients switched to or added medications with a higher glucose-lowering potential during the post-index year.23-25 Based on previous literature, dipeptidyl peptidase-4 inhibitors and alpha glucosidase inhibitors, were considered to have lower glucose-lowering potential than metformin, sulfonylureas, thiazolidinediones, glucagon-like peptide-1 receptor agonists, and meglitinides, which were considered equivalent.23-25 For example, an individual on monotherapy with a dipeptidyl peptidase-4 inhibitor at baseline who added metformin to their regimen was considered to have switched to a higher glucose-lowering potential drug. However, if an individual on metformin switched to or added a sulfonylurea, they were not considered to have switched to a higher glucose-lowering potential drug.
We also examined the potential that antihyperglycemic medication switches were due to medication-related adverse events during the pre-index period by describing the incidence of common T2DM medication side effects in the 30 days before the index date (identified using ICD-9-CM codes, Appendix A). We also captured claims for side effects in the 30 days after the index date to allow for delayed claims processing.
Data Analysis
Descriptive statistics (e.g., mean, standard deviation [SD], median, interquartile range [IQR], frequency, and percentage) were used to report all covariates, and independent Student’s t-tests, Mann-Whitney U tests, or chi-square tests were used to assess differences in covariates between the treatment intensification and the clinical inertia group in the overall cohort.
Propensity score matching was then used to balance differences in covariates between patients experiencing clinical inertia and treatment intensification. The propensity score model was created using the MatchIt package in R version 3.1 (R Foundation for Statistical Computing, Vienna, Austria) and included the covariates previously listed in the Baseline Characteristics section.26,27 Matching between the groups was carried out using a 1:1 ratio without replacement and a greedy nearest neighbor approach with calipers. A standardized mean difference (SMD) of < 0.1 was used to indicate adequate covariate balance between groups.28
The primary outcome, attainment of A1c < 7.0%, was assessed in the propensity matched cohort using descriptive statistics and compared using a chi-square test. Index and follow-up A1c, as well as the change in A1c in the 6- to 12-month post-index period, were evaluated using descriptive statistics. Independent or paired Student’s t-tests were used to assess differences between the matched groups, as appropriate.
For descriptive purposes, the proportion of patients in the unmatched overall cohort experiencing treatment intensification at any time over the post-index period was shown using a cumulative incidence curve. Finally, a multivariable logistic regression model was used in the unmatched overall cohort to assess the odds of experiencing clinical inertia versus treatment intensification. Based on previous clinical and research experience, we examined potential associations between the following baseline characteristics and clinical inertia: age, sex, race/ethnicity, baseline A1c classification (i.e., ≥ 8.0% - < 9.0%or ≥ 9.0%), previous oral antihyperglycemic medication adherence, baseline comorbidities, baseline diabetes counseling/education sessions, and physician practice type. In the final model, we only included covariates with adequate observations and excluded physician practice type due to lack of confidence in the derivation method. We assessed for multicollinearity in the model by evaluating the variance inflation factors associated with each variable.
This study was reviewed and approved by the Intermountain Healthcare Institutional Review Board. All analyses were performed using R.27
Results
Baseline Characteristics
A total of 3,078 patients were included in the overall patient cohort (Figure 1), with a mean (SD) age of 54.4 (10.6) years and a mean (SD) baseline A1c of 9.6% (1.7; Table 1). Overall, 63.9% of patients were male; 86.5% were white; 63.2% had hypertension; and 64.2% had dyslipidemia. During the 4-month post-index period, 1,093 (35.5%) patientsexperienced clinical inertia, while 1,985 (64.5%) had treatment intensification (Figure 2). However, 30% of patients in the clinical inertia group experienced treatment intensification after the initial 4-month exposure classification window (Figure 2). Among all patients who experienced treatment intensification at any time during the follow-up period, the median (IQR) time to treatment intensification was 2 (0-26) days.
TABLE 1.
Baseline Characteristics and Select Post-Index Treatment Characteristics
| Unmatched Cohort | Matched Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 3,078) | Clinical Inertia (n = 1,093) | Treatment Intensification(n = 1,985) | P Valuea | SMD | Overall (N = 1,760) | Clinical Inertia (n = 880) | Treatment Intensification(n = 880) | P Valuea | SMD | |
| Baseline characteristics | ||||||||||
| Age, years (mean, SD) | 54.4 (10.6) | 56.7 (10.9) | 53.2 (10.2) | <0.001 | 0.33 | 55.8 (10.1) | 55.7 (10.2) | 55.4 (9.9) | 0.568 | 0.03 |
| Age categories (%, n) | <0.001 | 0.31 | 0.940 | 0.04 | ||||||
| <50 years | 30.3 (932) | 24.7 (270) | 33.4 (662) | 25.3 (446) | 25.5 (224) | 25.2 (222) | ||||
| 50-54 years | 17.5 (539) | 16.8 (184) | 17.9 (355) | 17.9 (315) | 18.6 (164) | 17.2 (151) | ||||
| 55-59 years | 20.6 (633) | 20.6 (225) | 20.6 (408) | 21.9 (385) | 21.6 (190) | 22.2 (195) | ||||
| 60-64 years | 18.6 (573) | 18.5 (202) | 18.7 (371) | 19.2 (338) | 19.0 (167) | 19.4 (171) | ||||
| ≥ 65 years | 13.0 (401) | 19.4 (212) | 9.5 (189) | 15.7 (276) | 15.3 (135) | 16.0 (141) | ||||
| Male (%, n) | 63.9 (1,966) | 63.5 (694) | 64.1 (1,272) | 0.776 | 0.01 | 62.5 (1,100) | 62.8 (553) | 62.2 (547) | 0.806 | 0.01 |
| Race/ethnicity (%, n) | 0.428 | 0.05 | 0.496 | 0.06 | ||||||
| White | 86.5 (2,663) | 85.9 (939) | 86.9 (1,724) | 87.0 (1,531) | 86.2 (759) | 87.7 (772) | ||||
| Nonwhite | 5.3 (164) | 6.0 (66) | 4.9 (98) | 5.6 (98) | 5.6 (49) | 5.6 (49) | ||||
| Missing | 8.2 (251) | 8.1 (88) | 8.2 (163) | 7.4 (131) | 8.2 (72) | 6.7 (59) | ||||
| Baseline A1c | ||||||||||
| Overall (mean, SD) | 9.6 (1.7) | 9.3 (1.5) | 9.8 (1.8) | <0.001 | 0.33 | 9.4 (1.6) | 9.3 (1.5) | 9.4 (1.7) | 0.089 | 0.08 |
| A1c ≥ 9.0 (%, n) | 50.0 (1,538) | 39.5 (432) | 55.7 (1,106) | <0.001 | 0.33 | 40.6 (715) | 41.1 (362) | 40.1 (353) | 0.698 | 0.02 |
| Physician practice type (%, n) | <0.001 | 0.54 | 0.850 | 0.04 | ||||||
| Primary care | 86.4 (2,658) | 75.3 (823) | 92.4 (1,835) | 87.2 (1,535) | 87.2 (767) | 87.3 (768) | ||||
| Specialty | 3.7 (113) | 9.3 (102) | 0.6 (11) | 2.8 (49) | 2.6 (23) | 3.0 (26) | ||||
| Other | 7.5 (230) | 13.0 (142) | 4.4 (88) | 8.5 (150) | 8.5 (75) | 8.5 (75) | ||||
| Missing | 2.5 (77) | 2.4 (26) | 2.6 (51) | 1.5 (26) | 1.7 (15) | 1.2 (11) | ||||
| Adapted DCSI (mean, SD) | 0.4 (0.8) | 0.5 (0.9) | 0.3 (0.8) | <0.001 | 0.16 | 0.42 (0.88) | 0.41 (0.85) | 0.44 (0.90) | 0.463 | 0.04 |
| Score, range 0-13 (%, n) | <0.001 | 0.19 | 0.884 | 0.06 | ||||||
| 0 | 78.2 (2,406) | 73.7 (806) | 80.6 (1,600) | 74.8 (1,316) | 75.3 (663) | 74.2 (653) | ||||
| 1 | 12.6 (388) | 14.3 (156) | 11.7 (232) | 14.3 (252) | 14.2 (125) | 14.4 (127) | ||||
| 2 | 5.8 (178) | 7.5 (82) | 4.8 (96) | 6.9 (122) | 6.8 (60) | 7.0 (62) | ||||
| 3+ | 3.4 (106) | 4.4 (49) | 2.9 (57) | 3.0 (70) | 3.7 (32) | 4.3 (38) | ||||
| Comorbidities (%, n) | ||||||||||
| Anxiety | 9.7 (299) | 9.4 (103) | 9.9 (196) | 0.734 | 0.02 | 9.8 (172) | 9.9 (87) | 9.7 (85) | 0.936 | 0.01 |
| Depression | 13.6 (419) | 14.0 (153) | 13.4 (266) | 0.683 | 0.02 | 14.7 (258) | 14.7 (129) | 14.7 (129) | 1.000 | <0.001 |
| Severe mental illnessb | 7.0 (216) | 7.7 (84) | 6.6 (132) | 0.316 | 0.04 | 7.7 (136) | 7.6 (67) | 7.8 (69) | 0.929 | 0.01 |
| Hypertension | 63.2 (1,944) | 67.2 (734) | 61.0 (1,210) | 0.001 | 0.13 | 68.3 (1,202) | 67.3 (592) | 69.3 (610) | 0.384 | 0.04 |
| Dyslipidemia | 64.2 (1,977) | 67.7 (740) | 62.3 (1,237) | 0.003 | 0.11 | 69.6 (1,225) | 69.0 (607) | 70.2 (618) | 0.604 | 0.03 |
| Overweight | 1.1 (34) | 1.1 (12) | 1.1 (22) | 1.000 | 0.00 | 1.2 (21) | 1.1 (10) | 1.2 (11) | 1.000 | 0.01 |
| Obesity | 16.8 (518) | 15.6 (171) | 17.5 (347) | 0.210 | 0.05 | 17.5 (308) | 16.5 (145) | 18.5 (163) | 0.286 | 0.05 |
| Metabolic syndrome | 1.5 (45) | 1.7 (19) | 1.3 (26) | 0.429 | 0.04 | 1.9 (34) | 2.0 (18) | 1.8 (16) | 0.863 | 0.02 |
| Coronary heart disease | 9.4 (290) | 12.4 (135) | 7.8 (155) | <0.001 | 0.15 | 11.4 (200) | 11.5 (101) | 11.2 (99) | 0.940 | 0.01 |
| Heart failure | 2.7 (82) | 3.6 (39) | 2.2 (43) | 0.028 | 0.08 | 3.2 (57) | 3.4 (30) | 3.1 (27) | 0.788 | 0.02 |
| Peripheral artery disease | 0.9 (28) | 1.5 (16) | 0.6 (12) | 0.027 | 0.09 | 1.0 (17) | 0.9 (8) | 1.0 (9) | 1.000 | 0.01 |
| Cerebrovascular disease | 1.5 (47) | 1.6 (17) | 1.5 (30) | 1.000 | 0.00 | 1.5 (26) | 1.5 (13) | 1.5 (13) | 1.000 | <0.001 |
| CKD | 4.0 (122) | 5.1 (56) | 3.3 (66) | 0.019 | 0.09 | 4.3 (76) | 4.3 (38) | 4.3 (38) | 1.000 | <0.001 |
| ESRD | 0.4 (12) | 0.6 (7) | 0.3 (5) | 0.176 | 0.06 | 0.6 (10) | 0.6 (5) | 0.6 (5) | 1.000 | <0.001 |
| Nephropathy | 2.5 (78) | 3.8 (41) | 1.9 (37) | 0.002 | 0.11 | 3.0 (52) | 2.8 (25) | 3.1 (27) | 0.888 | 0.01 |
| Neuropathy | 3.7 (113) | 4.3 (47) | 3.3 (66) | 0.202 | 0.05 | 4.5 (80) | 4.4 (39) | 4.7 (41) | 0.909 | 0.01 |
| Retinopathy | 2.4 (73) | 3.1 (34) | 2.0 (39) | 0.061 | 0.07 | 2.7 (47) | 2.4 (21) | 3.0 (26) | 0.554 | 0.04 |
| Treatment characteristics | ||||||||||
| Index date antihyperglycemic regimen (%, n) | ||||||||||
| Noninsulin antihyperglycemics (mean, SD) | 0.8 (1.0) | 0.9 (1.1) | 0.7 (0.9) | <0.001 | 0.23 | 0.93 (0.98) | 0.92 (0.99) | 0.95 (0.98) | 0.561 | 0.03 |
| Noninsulin antihyperglycemic count (%, n) | <0.001 | 0.29 | 0.080 | 0.425 | ||||||
| 0 | 52.8 (1,625) | 49.1 (537) | 54.8 (1,088) | 44.0 (774) | 45.2 (398) | 42.7 (376) | ||||
| 1 | 24.3 (748) | 20.3 (222) | 26.5 (526) | 26.1 (460) | 24.7 (217) | 27.6 (243) | ||||
| 2 | 16.5 (508) | 21.2 (232) | 13.9 (276) | 22.8 (401) | 23.4 (206) | 22.2 (195) | ||||
| ≥ 3 | 6.4 (197) | 9.3 (102) | 4.8 (95) | 7.1 (125) | 6.7 (59) | 7.5 (66) | ||||
| Noninsulin antihyperglycemic classes (%, n) | ||||||||||
| Metformin | 40.1 (1,235) | 45.9 (502) | 36.9 (733) | <0.001 | 0.18 | 49.9 (878) | 48.9 (430) | 50.9 (448) | 0.418 | 0.04 |
| Sulfonylurea | 19.5 (599) | 24.7 (270) | 16.6 (329) | <0.001 | 0.20 | 23.4 (412) | 23.8 (209) | 23.1 (203) | 0.778 | 0.02 |
| GLP-1 receptor agonists | 4.1 (126) | 3.1 (34) | 4.6 (92) | 0.052 | 0.08 | 3.8 (66) | 3.6 (32) | 3.9 (34) | 0.900 | 0.01 |
| TZD | 3.0 (93) | 4.0 (44) | 2.5 (49) | 0.021 | 0.09 | 3.8 (66) | 3.9 (34) | 3.6 (32) | 0.900 | 0.01 |
| DPP-4 inhibitor | 9.7 (298) | 13.0 (142) | 7.9 (156) | <0.001 | 0.17 | 11.9 (209) | 11.4 (100) | 12.4 (109) | 0.556 | 0.03 |
| SGLT2 inhibitors | 0.5 (15) | 0.5 (5) | 0.5 (10) | 1.000 | 0.01 | 0.7 (13) | 0.6 (5) | 0.9 (8) | 0.578 | 0.04 |
| Alpha-glucosidase inhibitors | 0.1 (3) | 0.1 (1) | 0.1 (2) | 1.000 | 0.00 | 0.0 (0) | 0.0 (0) | 0.0 (0) | - | - |
| Meglitinide | 0.1 (4) | 0.0 (0) | 0.2 (4) | 0.336 | 0.06 | 0.0 (0) | 0.0 (0) | 0.0 (0) | - | - |
| Patients on ≥ 1 fixed-dose combination antihyperglycemic medication (%, n) | 6.3 (193) | 9.6 (105) | 4.4 (88) | <0.001 | 0.20 | 8.1 (143) | 8.1 (71) | 8.2 (72) | 1.000 | 0.00 |
| Patients receiving diabetic counseling (%, n)c | 6.5 (201) | 6.5 (71) | 6.5 (130) | 1.000 | 0.00 | 6.8 (119) | 6.5 (57) | 7.0 (62) | 0.704 | 0.02 |
| Antihyperglycemic-related adverse events within ±30 days of index date (%, n) | ||||||||||
| Hypoglycemia | 0.1 (4) | 0.1 (1) | 0.2 (3) | 1.000 | - | 0.2 (3) | 0.1 (1) | 0.2 (2) | 1.000 | - |
| Nausea/vomiting | 1.8 (56) | 1.6 (17) | 2.0 (39) | 0.501 | - | 1.4 (25) | 1.7 (15) | 1.1 (10) | 0.420 | - |
| Edema | 0.2 (7) | 0.5 (5) | 0.1 (2) | 0.111 | - | 0.3 (6) | 0.6 (5) | 0.1 (1) | 0.220 | - |
| Urinary tract infections | 3.2 (98) | 3.3 (36) | 3.1 (62) | 0.881 | - | 3.5 (62) | 3.6 (32) | 3.4 (30) | 0.897 | - |
aP values for statistical significance were obtained using independent Student’s t-tests for continuous variables or Mann-Whitney U or chi-square tests for categorical variables, as appropriate; bolded P values indicate statistical significance.
bIncludes schizophrenia, bipolar disorder, and substance abuse.
cDiabetic counseling identified by CPT code from medical claims.
A1c = hemoglobin A1c; aDCSI = adapted Disease Complication Severity Index; CKD = chronic kidney disease; CPT-4 = Common Procedural Terminology, 4th Edition, DPP-4 = dipeptidyl peptidase-4; ESRD = end-stage renal disease; GLP-1 = glucagon-like peptide-1; SD = standard deviation; SGLT2 = sodium-glucose cotransporter-2; SMD = standardized mean difference; TZD = thiazolidinedione.
FIGURE 2.
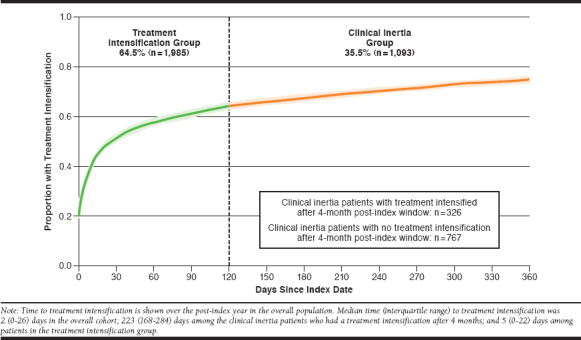
Time to Treatment Intensification in Days from Elevated A1c (≥ 8%; N = 3,078)
Patients with clinical inertia appeared to be later in the diabetes disease process than patients in the treatment intensification group. Compared with those with treatment intensification, patients with clinical inertia were older (56.7 [SD 10.9] years vs. 53.2 [SD 10.2] years; P < 0.001), had a higher aDCSI (0.5 [SD 0.9] vs. 0.3 [SD 0.8]; P < 0.001), and a greater proportion of them received ≥ 2 noninsulin antihyperglycemic medications (30.5% vs. 18.7%; P < 0.001) at baseline (Table 1). Similarly, more patients in the clinical inertia group had a diagnosis of hypertension (67.2% vs. 61.0%; P = 0.001) and dyslipidemia (67.7% vs. 62.3%; P = 0.003) than patients in the treatment intensification group. However, compared with the treatment intensification group, clinical inertia patients had a lower baseline A1c (9.3% [SD 1.5] vs. 9.8% [SD 1.8]; P < 0.001).
After propensity score matching, 1,760 patients remained, with 880 patients each in the clinical inertia and treatment intensification groups (Table 1). Similar to the overall cohort, patients in the propensity score matched cohort had a mean (SD) age of 55.8 (10.1) years and a mean (SD) baseline A1c of 9.4% (1.6). Furthermore, 62.5% of patients were male; 87.0% were white; 68.3% had hypertension; and 69.6% had dyslipidemia. Covariate balance was achieved, as evidenced by an SMD < 0.1 for each covariate (Table 1), indicating no statistically significant differences in baseline characteristics between groups after propensity score matching.
Other Covariates
As expected, more patients in the treatment intensification group switched to or added a medication with a higher glucose-lowering potential in the post-index period than those in the clinical inertia group (9.0% vs. 3.1%; P = 0.001; data not shown). The incidence of potential medication-related adverse events common in antihyper-glycemic medications was low overall (ranged from 0.1% to 3.2%), and no differences were identified between groups (Table 1).
A1c Outcomes
After propensity score matching, fewer matched patients in the clinical inertia group attained A1c < 7.0% in the post-index period than in the treatment intensification group (22.6% vs. 34.4%; P < 0.001; Table 2). Similarly, despite no statistically significant differences in mean (SD) baseline A1c (clinical inertia 9.3% [1.5] vs. treatment intensification 9.4% [1.7]; P = 0.089), matched patients with clinical inertia had a higher mean (SD) follow-up A1c compared with patients with treatment intensification (8.3% [1.8] vs. 7.8% [1.8]; P < 0.001; Table 2). Although both groups had significant reductions in A1c 6-12 months after the index date (paired Student’s t-test P < 0.001 for each group), patients in the treatment intensification group experienced a greater A1c lowering in the 6- to 12-month follow-up period than patients in the clinical inertia group (–1.6% [SD 2.2] vs. –1.0% [SD 2.2]; P < 0.001; Table 2). Finally, there was no difference in the time to the last A1c value between the groups (Table 2).
TABLE 2.
Unadjusted and Adjusted A1c Outcomes 6-12 Months After Index Date
| Overall Cohort | Matched Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall (N = 3,078) | Clinical Inertia (n = 1,093) | Treatment Intensification (n = 1,985) | P Valuea | Overall (N = 1,760) | Clinical Inertia (n = 880) | Treatment Intensification (n = 880) | P Valuea | |
| Primary outcome | ||||||||
| Attainment of A1c goal (%, n) | ||||||||
| A1c < 7.0% | 31.9 (981) | 22.8 (249) | 36.9 (732) | < 0.001 | 28.5 (502) | 22.6 (199) | 34.4 (303) | < 0.001 |
| Other A1c outcomes | ||||||||
| Other follow-up A1c categories (%, n) | ||||||||
| A1c 7.0%-7.9% | 26.6 (818) | 28.6 (313) | 25.4 (505) | 0.060 | 27.2 (478) | 26.7 (235) | 27.6 (243) | 0.708 |
| A1c 8.0%-8.9% | 18.5 (570) | 21.8 (238) | 16.7 (332) | 0.001 | 19.9 (350) | 21.6 (190) | 18.2 (160) | 0.083 |
| A1c ≥ 9.0% | 23.0 (709) | 26.8 (293) | 21.0 (416) | < 0.001 | 24.4 (430) | 29.1 (256) | 19.8 (174) | < 0.001 |
| Overall A1c measurements (mean, SD) | ||||||||
| Baseline A1c | 9.6 (1.7) | 9.3 (1.5) | 9.8 (1.8) | < 0.001 | 9.4 (1.6) | 9.3 (1.5) | 9.4 (1.7) | 0.089 |
| Follow-up A1c | 8.0 (1.8) | 8.2 (1.8) | 7.8 (1.8) | < 0.001 | 8.1 (1.8) | 8.3 (1.8) | 7.8 (1.7) | < 0.001 |
| Change in A1c | -1.6 (2.4) | -1.1 (2.2) | -2.0 (2.4) | < 0.001 | -1.3 (2.2) | -1.0 (2.2) | -1.6 (2.2) | < 0.001 |
| Time of last follow-up A1c, months (mean [SD]) | 8.4 (1.7) | 8.4 (1.8) | 8.4 (1.7) | 0.972 | 8.4 (1.7) | 8.4 (1.8) | 8.3 (1.7) | 0.427 |
| Last follow-up A1c ≥ 9 months after index date (%, n) | 35.5 (1,093) | 35.1 (384) | 35.7 (709) | 0.775 | 35.3 (621) | 35.6 (313) | 35.0 (308) | 0.842 |
aP values for statistical significance were obtained using independent Student’s t-tests for continuous variables or Mann-Whitney U or chi-squared tests for categorical variables, as appropriate; bolded P values indicate statistical significance.
A1c = hemoglobin A1c; SD = standard deviation.
Factors Associated with Clinical Inertia
In the overall cohort, several factors were associated with a greater likelihood of experiencing clinical inertia (Table 3). Compared with having no claims for antihyperglycemic medications at baseline, having 2 (odds ratio [OR] = 1.51, 95% CI [confidence interval] = 1.22-1.86; P < 0.001) or ≥ 3 anti-hyperglycemic medications (OR = 1.78, 95% CI = 1.30-2.42; P < 0.001) was associated with a higher odds of clinical inertia. Conversely, having a claim for 1 antihyperglycemic medication (vs. none) at baseline was associated with a lower odds of experiencing clinical inertia (OR = 0.75, 95% CI = 0.61-0.91; P = 0.004). The presence of a coronary heart disease (OR = 1.44, 95% CI = 1.10-1.88; P = 0.007) was associated with a greater odds of experiencing clinical inertia, as was a baseline age ≥ 65 years versus age < 50 years (OR = 2.11, 95% CI = 1.63-2.74; P < 0.001). Conversely, having a baseline A1c ≥ 9.0% was associated with a lower odds of experiencing clinical inertia than having a baseline A1c between 8.0% and < 9.0% (OR = 0.56, 95% CI = 0.48-0.66; P < 0.001). In sensitivity analyses, the results of the regression were minimally affected by inclusion of variables with a small number of observations and physician practice type (data not shown).
TABLE 3.
Logistic Regression Predicting Odds of Experiencing Clinical Inertia in the Overall Unmatched Cohort (N = 3,078)
| Variables | Odds Ratio | 95% CI | P Valuea |
|---|---|---|---|
| Clinical inertia(ref: treatment intensification) | - | - | - |
| Baseline age category, years (ref: < 50 years) | - | - | - |
| 50-54 years | 1.16 | (0.92-1.47) | 0.211 |
| 55-59 years | 1.20 | (0.96-1.50) | 0.113 |
| 60-64 years | 1.08 | (0.86-1.37) | 0.511 |
| ≥ 65 years | 2.11 | (1.63-2.74) | < 0.001 |
| Male (ref: female) | 1.01 | (0.86-1.19) | 0.902 |
| Race (ref: white) | - | - | - |
| Nonwhite | 1.32 | (0.94-1.84) | 0.105 |
| Missing | 0.99 | (0.75-1.31) | 0.953 |
| Baseline A1c ≥ 9.0%(Ref: A1c ≥ 8.0% to < 9.0%) | 0.56 | (0.48-0.66) | < 0.001 |
| Baseline number of antihyperglycemic medications (ref: 0) | - | - | - |
| 1 | 0.75 | (0.61-0.91) | 0.004 |
| 2 | 1.51 | (1.22-1.86) | <0.001 |
| ≥ 3 | 1.78 | (1.30-2.42) | <0.001 |
| Receiving diabetic counseling at baseline | 0.91 | (0.66-1.24) | 0.547 |
| Baseline comorbidities | - | - | - |
| Anxiety | 0.95 | (0.72-1.26) | 0.733 |
| Depression | 1.10 | (0.86-1.39) | 0.451 |
| Severe mental illness | 1.11 | (0.81-1.50) | 0.516 |
| Hypertension | 1.09 | (0.92-1.30) | 0.326 |
| Dyslipidemia | 1.03 | (0.86-1.22) | 0.766 |
| Obesity | 0.81 | (0.65-1.00) | 0.054 |
| Coronary heart disease | 1.44 | (1.10-1.88) | 0.007 |
| Chronic kidney disease | 0.99 | (0.64-1.51) | 0.953 |
| Nephropathy | 1.47 | (0.87-2.48) | 0.150 |
| Neuropathy | 1.00 | (0.66-1.50) | 0.993 |
| Retinopathy | 1.20 | (0.73-1.96) | 0.464 |
aP values for statistical significance were obtained from the logistic regression model; bolded P values indicate statistical significance.
A1c = hemoglobin A1c; CI = confidence interval; ref = reference.
Discussion
In this claims-based analysis, 36% of commercially insured patients did not have their treatment intensified within 4 months of an A1c ≥ 8.0%. Of these patients with clinical inertia, only 30% of patients experienced treatment intensification between 4 and 12 months after the index date (i.e., after the window used to define treatment intensification). Compared with the 64% of patients with treatment intensification, patients with clinical inertia had a higher mean follow-up A1c, and a lower proportion attained an A1c < 7.0% in the 6-12 months following the elevated A1c. Baseline characteristics associated with an increased likelihood of experiencing clinical inertia included the use of ≥ 2 antihyperglycemic medications at baseline, a baseline age ≥ 65 years, and a diagnosis of coronary heart disease. Conversely, characteristics associated with a lower likelihood of experiencing clinical inertia included a baseline A1c ≥ 9.0% and the use of 1 antihyperglycemic medication at baseline.
Several previous retrospective studies evaluating clinical inertia in the United States have been published.13-17 Of these, only the study by Pantalone et al. (2016) had a similar A1c threshold as our study when defining clinical inertia (A1c > 8.0%).16 In that study, 28% of patients experienced clinical inertia at 6 months.16 Other studies reported 50%-73% of patients experiencing clinical inertia within either a 6- or 12-month post-index period.13-15 However, these studies used different criteria to define patients at risk for clinical inertia (e.g., A1c ≥ 7.0%) and also required patients to be on metformin at baseline.13-15 Conversely, our study included all patients with an A1c ≥ 8.0% regardless of their previous oral antihyperglycemic treatment regimen. Patients in our study may have been more likely to experience treatment intensification due to the higher baseline A1c required to determine if an individual was at risk for clinical inertia. We used this threshold to reduce the risk of misclassifying behaviors not identifiable in claims databases (e.g., diet and exercise) as clinical inertia, since they are more likely to occur when A1c is closer to 7.0%.19
Despite differences in inclusion criteria, our study and the other U.S. studies found clinical inertia to be associated with older age and a higher number of baseline antihyperglycemic medications.13-15 With increasing age and medication use, patients and providers may have concerns about an increased risk of medication-related adverse events, polypharmacy, and burden of disease management. In patients with complications and shorter life expectancy, such as older adult patients, treatment guidelines, therefore, recommend less aggressive A1ctargets (e.g., < 8.0% vs. < 7.0%).5 However, our study used the higher A1c threshold to identify patients at risk for clinical inertia and results indicate that provider and MCO efforts to address clinical inertia in patients ≥ 65 years and those on ≥ 2 antihyper-glycemic medications would be beneficial and could potentially improve patient outcomes and health plan quality performance measures. Thus, this study contributes to a growing body of evidence that supports targeting patients who are at risk of diabetes-related clinical inertia based on patient characteristics. However, some patient characteristics, such as coronary heart disease and hypertension, have had mixed results in published literature and warrant further examination in future research.
Overall, our results show that the majority of patients with T2DM and an elevated A1c experienced treatment intensification within 4 months. However, many patients are not receiving timely treatment intensification and may be at higher risk for long-term complications.4 At the population level, this failure to intensify therapy may also translate to MCOs’ lower performance on diabetes-related quality measures (e.g., HEDIS scores).6 Because clinical inertia is multifactorial, it is important for health care decision makers to understand the physician, patient, and system factors that may contribute to clinical inertia within their systems and develop interventions to overcome them. As many MCOs have existing T2DM care management teams and processes, patients with characteristics associated with clinical inertia may be targeted and prioritized within these systems. Regardless of the method used, to optimize the management of patients with T2DM, it is important for MCOs and health care decision makers to use information such as that provided in this study to identify patients at risk for clinical inertia and intervene as appropriate.
Limitations
Our study has several limitations that should be taken into consideration. We excluded patients with insulin use in the pre-index period because insulin dose is not included in claims data and estimations of dose based on quantity dispensed are unreliable (i.e., issues with vial expiration dates, variable or sliding scale insulin dosing, and improper syringe use).20 We intentionally did this to avoid potential misclassification bias in patients with insulin claims. However, this may have limited the number and type of patients considered in the analysis. In addition, this study included a substantial number of patients without prescription claims for antihyperglycemic therapy in the previous year. Thus, our cohort likely included newly diagnosed patients. This approach increased the generalizability of our findings but may have also led to a higher estimate of intensification rates versus other studies, as barriers to initiating first-line therapy were likely lower than adding other agents or insulin.29
As with all claims-based analyses, the recommendation of and adherence to nonpharmacologic interventions was not captured in our study. After receiving an A1c lab result ≥ 8.0%, management strategies other than antihyperglycemic medication intensification, such as counseling on lifestyle changes and adherence, may be used by clinicians. Similarly, health plan formulary, a factor that may affect the incidence of clinical inertia, cannot be captured using claims data. These items may represent a source of unmeasured confounding. Additionally, many antihyperglycemic medications are available at a reduced price on pharmacy generic drug lists, which may prompt patients to pay for their medications using cash, which would not have been captured in any claims dataset.
Furthermore, pharmacy claims data provide information on medications that are prescribed by physicians and filled by a pharmacy. Prescriptions that patients failed to fill were not included, thus, these data represented treatment intensification associated with patient follow-through with the physician prescribing recommendations. Similarly, the presence of prescription claims does not guarantee that patients actually took their medication, which may have potentially misclassified some nonadherent patients as having experienced treatment intensification. Although we examined some variables that could identify potential physician motivations surrounding changes in therapy (e.g., common side effects), future analyses should consider the added use of electronic medical record data, which contain prescription orders, laboratory results, and clinical notes, to further account for potential confounders.
In addition, this study was designed to measure the effect on surrogate measures over a 12-month time horizon. Future studies should consider the effect of clinical inertia on long-term health outcomes. Finally, while we used A1c < 7.0% as the outcome threshold, that level may not be appropriate for all patients with T2DM, especially those with a history of severe hypoglycemia and limited life expectancy.4 However, this goal is considered adequate for many patients with T2DM and represents a clinically significant improvement in glycemic control.
Conclusions
Clinical inertia occurred in over a third of patients with uncontrolled T2DM and was most likely to occur in patients aged ≥ 65 years, were prescribed ≥ 2 antihyperglycemic medications, had an A1c 8.0%-9.0%, and had coronary heart disease. As expected, patients with clinical inertia had worse follow-up A1c outcomes over the study period, but when treatment was intensified, follow-up A1c was lower than baseline. These data provide compelling evidence for MCOs to promote treatment intensification and target patients at high risk of experiencing clinical inertia. These results may help MCOs create interventions that may help better manage T2DM and reduce health care utilization.
APPENDIX A. List of ICD-9/10-CM and CPT Codes
| ICD-9-CM Codesa | Equivalent ICD-10-CM Codesa | |
|---|---|---|
| For exclusion from study | ||
| Gestational diabetes | 648.8x | O99.81x |
| Pregnancy-related claims | V22.x-V24.x, V27.x, 630.xx-679.xx | Z33.x, Z34.xx, Z37.xx, Z39.x, O09.xxx, O60. xxxx-O77.x, O80, O82, O85-O92.xxx |
| Type 1 diabetes | 250.x1, 250.x3 | E10.xxxx |
| Comorbidities | ||
| Type 2 diabetes | 250.x0, 250.x2 | E11.xxxx |
| Anxiety | 300.0x | F41.x |
| Depression | 296.2x, 296.3x, 311 | F32.xx, F33.xx |
| Severe mental illness | ||
| Bipolar disorder | 296.0x, 296.4x - 296.7x, 296.80, 296.89 | F30.1, F31.xx |
| Psychotic disorders (organic) | 292.xx-294.xx | F19.xx, F05, F04 |
| Psychotic disorders (nonorganic, nonaffective) | 295.xx, 297.xx, 298.xx | F20.xx, F22, F23, F28, F29, F33.3, F44.89 |
| Hypertension | 401.xx-405.xx | I10-I.15x |
| Hyperlipidemia | 272.0 - 272.4, 272.8, 272.9 | E78.xx |
| Metabolic syndrome | 277.7 | E88.81 |
| Coronary heart disease | 410.xx-411.xx, 412, 413.x-417.x, 427.xx, 429.2, 429.9 | I20.xx-I25.xxx, I50.xx |
| Heart failure | 428.xx | I50.xx |
| Diabetic retinopathy | 362.0x | E11.31x-E11.35xx |
| Diabetic neuropathy | 357.2 | E11.4x |
| Cerebrovascular disease | 433.x1, 434.x1, 435.9, 436, 437.1, 437.9 | |
| Peripheral artery disease | 440.2x, 440.3x, 440.4, 440.8, 440.9 | I70.2xx-I70.9x |
| Diabetic nephropathy | 250.40, 250.42 | E11.2xx |
| Chronic kidney disease | 403.xx, 404.xx, 585.1-585.5, 585.9 | |
| Chronic kidney disease | 585.x, 403.xx, 404.xx | N18.x, I12.x, I13.xx |
| End-stage kidney disease | 585.6 | N18.6 |
| Hypoglycemia | 251.0-251.2 | E11.64x |
| Overweight | 278.02 | E66.3 |
| Obesity | 278.00, 278.01 | E66.0x-E66.2, E66.8-E66.9 |
| Diabetic counseling | ||
| Lifestyle and medication counseling | V65.3, V58.67, V53.91, Z79.84 | Z71.3, Z79.4 |
| CPT codes | G0108, G0109, 98960-98962, 97802-97804, 99078, S9140, S9141, S9145, S9455, S9460, S9465, S9470 | |
| For aDCSI calculation | ||
| Retinopathy | 250.5x, 361.xx, 362.01, 362.1, 362.83, 362.53, 362.81, 362.82, 362.02, 369.xx, 379.23 | E08.3x, E09.3x, E11.3x, E13.3x, H35.0x, H35.35x, H35.6x, H35.8x, H35.9, H33.x, H54.x, H43.1x |
| Nephropathy | 250.4, 580, 581, 581.81, 582, 583, 585, 586, 593.9 | E08.21, E08.22, E08.29, E09.21, E09.22, E09.29, E11.21, E11.22, E11.29, E13.21, E13.22, E13.29, N00.x, N03.x-N05.x, N18.1, N18.2-N18.6, N18.9, N19 |
| Neuropathy | 356.9, 250.6, 358.1, 951.0, 951.1, 951.3, 357.2 | E08.4x, E09.4x, E11.4x, E13.4x, G56.x, G57.x, G60.9, G73.3, G90.09, G90.1, G90.8, G90.9, G99.0, H49.x, I95.1, K31.84, K59.1, N31.9, M14.6x, S04.x |
| Cerebrovascular | 431, 433, 434-436 | G45.x, I61.x, I63.x, I65.x, I66.x, I67.81 |
| Cardiovascular | 410-414, 427.1, 427.3-427.5, 428, 429.2, 440.xx, 441 | I20.x-I25.x, I46.x-I50.x, I70.x, I71.x |
| Peripheral vascular disease | 040.0, 250.7, 442.3, 443.81, 443.9, 444.22, 785.4, 707.1, 892.1 | E08.51, E08.59, E08.621, E09.51, E09.59, E09.621, E11.51, E11.59, E11.621, E13.51, E13.59, E13.621, I72.4, I70.21, I73.89, I73.9, I74.3, S91.3x, A48.0, L97.x |
| Metabolic | 250.1-250.3 | E08.00, E08.10, E08.649, E09.00, E09.10, E09.649, E11.00, E11.10, E11.649, E13.00, E13.10, E13.649, E08.01, E08.11, E08.641, E09.01, E09.11, E09.641, E11.01, E11.11, E11.641, E13.01, E13.11, E13.641 |
| To assess for possible medication-related adverse events | ||
| Hypoglycemia | 251.0, 251.2 | E11.64 |
| Nausea | 787.0x | R11.xx |
| Edema | 782 | R60.x |
| Urinary tract infection | 599.0 | N39.0 |
aUnless otherwise specified.
aDCSI = adapted Diabetes Complications Severity Index; CPT-4 = Current Procedural Terminology, 4th Edition; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM = International Classification of Diseases, Tenth Revision, Clinical Modification
APPENDIX B. List of Generic Product Identifiers
| Generic Drug Name | Brand Drug Name | Generic Product Identifier |
|---|---|---|
| Biguanide | 2725xxxx | |
| Metformin | Fortamet, Glucophage, Glumetza, Riomet | 27250050 |
| Biguanide/nutritional supplement combinations | Appformin Pak, Appformin-D Pak | 279990xx |
| Sulfonylureas (SU) | 2720xxxx | |
| Glipizide | Glucotrol, Glucotrol XL, Glipizide XL | 27200030 |
| Glyburide | Diabeta, Glynase | 27200040 |
| Glimipiride | Amaryl | 27200027 |
| Tolazamide | Tolinase | 27200050 |
| Tolbutamide | 27200060 | |
| Sulfonylurea/metformin combinations | 279970xx | |
| Glipizide/metformin | Metaglip | 2799700235 |
| Glyburide/metformin | Glucovance | 2799700240 |
| Thiazolidinediones (TZDs) | 276070xx | |
| Pioglitazone | Actos | 27607050 |
| Rosiglitazone | Avandia | 27607060 |
| TZD/metformin combinations | 279980xx | |
| Pioglitazone/metformin | Actoplus Met, Actoplus Met XR | 279980024 |
| Rosiglitazone/metformin | Avandamet | 279980026 |
| TZD/SU combination | 279978xx | |
| Pioglitazone/glimepiride | Duetact | 27997802 |
| Glucagon-like peptide-1 receptor agonist | 2717xxxx | |
| Albiglutide | Tanzeum | 27170010 |
| Dulaglutide | Trulicity | 27170015 |
| Exenatide | Bydureon, Byetta | 27170020 |
| Liraglutide | Victoza | 27170050 |
| Sodium-glucose co-transporter-2 inhibitors (SGLT2) | 2770xxxx | |
| Canagliflozin | Invokana | 27700020 |
| Dapagliflozin | Farxiga | 27700040 |
| Empagliflozin | Jardiance | 27700050 |
| SGLT2/metformin combinations | 279960xx | |
| Canagliflozin/metformin | Invokamet | 279960022 |
| Dapagliflozin/metformin | Xigduo XR | 279960023 |
| Empagliflozin/metformin | Synjardy | 279960024 |
| Dipeptidyl peptidase-IV inhibitors (DPP-IV) | 2755xxxx | |
| Alogliptin | Nesina | 27550010 |
| Linagliptin | Tradjenta | 27550050 |
| Saxagliptin | Onglyza | 27550065 |
| Sitagliptin | Januvia | 27550070 |
| DPP-IV/metformin combinations | 279925xx | |
| Alogliptin/metformin | Kazano | 27992502021 |
| Sitagliptin/metformin | Janumet | 27992502024 |
| Linagliptin/metformin | Jentadueto | 27992502026 |
| Saxagliptin/metformin | Kombiglyze XR | 27992502027 |
| DPP-IV/HMG CoA reductase combination | 279930xx | |
| Sitagliptin/simvastatin | Juvisync | 279930xx |
| DPP-IV/TZD combination | 279940xx | |
| Alogliptin/pioglitazone | Oseni | 27994002 |
| DPP-IV/SGLT2 combination | 279965xx | |
| Empagliflozin/linagliptin | Glyxambi | 27996505 |
| Alpha-glucosidase inhibitors | 2750xxxx | |
| Miglitol | Glyset | 27500050 |
| Acarbose | Precose | 27500010 |
| Meglitinides | 2728xxxx | |
| Nateglinide | Starlix | 27280040 |
| Repaglinide | Prandin | 27280060 |
| Meglitinide/metformin combination | 279950xx | |
| Repaglinide/metformin | Prandimet | 279950xx |
| Amylin mimetics | 2715xxxx | |
| Pramlintide | Symlin | 2715xxxx |
| Insulins | 271040xx | |
| Insulin lispro | Humalog | 27104005 |
| Insulin aspart | Novolog | 27104002 |
| Insulin glulisine | Apidra | 27104004 |
| Insulin aspart protamine/aspart | Novolog Mix 70/30 | 27104070 |
| Insulin lispro protamine/lispro | Humalog Mix 50/50, Humalog Mix 75/25 | 27104080 |
| Insulin NPH | Humulin N, Novolin N, Novolin ReliOn N | 27104020 |
| Insulin regular | Humulin R U-500, Humulin R, Novolin R, Relion R, Afrezza | 27104010 |
| Insulin NPH/regular | Humulin 70/30, Novolin 70/30 | 27104090 |
| Insulin degludec | Tresiba | 27104007 |
| Insulin glargine | Lantus, Toujeo | 27104003 |
| Insulin detemir | Levemir | 27104006 |
NPH = neutral protamine Hagedorn; XL = extended release; XR = extended release.
REFERENCES
- 1.American Diabetes Association. Statistics about diabetes. 2017. Available at: http://www.diabetes.org/diabetes-basics/statistics/. Accessed December 22, 2018.
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413-20. [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-65. [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes 2018. Diabetes Care. 2018;41(Suppl 1):S1-S155. [DOI] [PubMed] [Google Scholar]
- 6.National Committee for Quality Assurance. HEDIS measures. 2018. Available at: http://www.ncqa.org/hedis-quality-measurement/hedis-measures. Accessed December 22, 2018.
- 7.Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-72. [DOI] [PubMed] [Google Scholar]
- 8.van Dieren S, Kengne AP, Chalmers J, et al. Intensification of medication and glycaemic control among patients with type 2 diabetes – the ADVANCE trial. Diabetes Obes Metab. 2014;16(5):426-32. [DOI] [PubMed] [Google Scholar]
- 9.Romanelli RJ, Chung S, Pu J, Nimbal V, Zhao B, Palaniappan L. Comparative effectiveness of early versus delayed metformin in the treatment of type 2 diabetes. Diabetes Res Clin Pract. 2015;108(1):170-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor PJ, Sperl-Hillen JM, Johnson PE, Rush WA, Blitz, G. Clinical inertia and outpatient medical errors. In: Henriksen K, ed. Advances in patient safety: from research to implementation. Vol. 2: Concepts and methodology. February 2005. Agency for Healthcare Research and Quality. Rockville, MD. Available at: https://www.ncbi.nlm.nih.gov/books/NBK20513/pdf/Bookshelf_NBK20513.pdf. Accessed December 22, 2018.
- 11.Rajpathak SN, Rajgopalan S, Engel SS. Impact of time to treatment intensification on glycemic goal attainment among patients with type 2 diabetes failing metformin monotherapy. J Diabetes Complications. 2014;28(6):831-35. [DOI] [PubMed] [Google Scholar]
- 12.Davis J, Chavez B, Juarez DT. Adjustments to diabetes medications in response to increases in hemoglobin a1c: An epidemiologic study. Ann Pharmacother. 2014;48(1):41-47. [DOI] [PubMed] [Google Scholar]
- 13.Fu AZ, Qiu Y, Davies MJ, Radican L, Engel SS. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab. 2011;13(8):765-69. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Zhou S, Wei W, Pan C, Lingohr-Smith M, Levin P. Does clinical inertia vary by personalized A1c goal? A study of predictors and prevalence of clinical inertia in a U.S. managed-care setting. Endocr Pract. 2016;22(2):151-61. [DOI] [PubMed] [Google Scholar]
- 15.Yu S, Schwab P, Bian B, Radican L, Tunceli K. Use of add-on treatment to metformin monotherapy for patients with type 2 diabetes and suboptimal glycemic control: a U.S. database study. J Manag Care Spec Pharm. 2016; 22(3): 272-80. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2016.22.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantalone KM, Wells BJ, Chagin KM, et al. Intensification of diabetes therapy and time until A1C goal attainment among patients with newly diagnosed type 2 diabetes who fail metformin monotherapy within a large integrated health system. Diabetes Care. 2016;39(9):1527-34. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Rajagopalan S, Marrett E, Davies MJ, Radican L, Engel SS. Time to treatment initiation with oral antihyperglycaemic therapy in U.S. patients with newly diagnosed type 2 diabetes. Diabetes Obes Metab. 2012;14(2):149-54. [DOI] [PubMed] [Google Scholar]
- 18.SelectHealth. About SelectHealth. 2018. Available at: http://selecthealth.org/about/Pages/home.aspx. Accessed December 22, 2018.
- 19.Marrett E, Zhang Q, Kanitscheider C, Davies MJ, Radican L, Feinglos MN. Physician reasons for nonpharmacologic treatment of hyperglycemia in older patients newly diagnosed with type 2 diabetes mellitus. Diabetes Ther. 2012;3(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stolpe S, Kroes MA, Webb N, Wisniewski T. A. systematic review of insulin adherence measures in patients with diabetes. J Manag Care Spec Pharm. 2016; 22(11): 1224-46. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2016.22.11.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted diabetes complications severity index in claims data. Am J Manag Care. 2012;18(11):721-26. [PubMed] [Google Scholar]
- 22.Hazel-Fernandez L, Li Y, Nero D, et al. Relationship of diabetes complications severity to healthcare utilization and costs among Medicare Advantage beneficiaries. Am J Manag Care. 2015;21(1):e62-70. [PubMed] [Google Scholar]
- 23.Agency for Healthcare Research and Quality. Diabetes medications for adults with type 2 diabetes: an update. Comparative Effectiveness Review 173. AHRQ Publication No. 16-EHC013-EF. April 2016. Agency for Healthcare Research and Quality. Rockville, MD. Available at: https://www.ncbi.nlm.nih.gov/books/NBK362863/. Accessed December 22, 2018. [Google Scholar]
- 24.American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment. Standards of medical care in diabetes. Diabetes Care. 2018;41(Suppl 1):S75-S85. [DOI] [PubMed] [Google Scholar]
- 25.Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA. 2016;316(3):313-24. [DOI] [PubMed] [Google Scholar]
- 26.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric pausal pnference. J Stat Software. 2011;42(8):1-28. [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. 2018. Available at: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing. Accessed January 2, 2019.
- 28.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15(3):234-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3-12. [DOI] [PubMed] [Google Scholar]


