Abstract
BACKGROUND:
Tablet splitting is a well-established medical practice in clinical settings for multiple reasons, including cost savings and ease of swallowing. However, it does not necessarily result in weight-uniform half tablets.
OBJECTIVES:
To (a) investigate the effect of tablet characteristics on weight and content uniformity of half tablets, resulting from splitting 16 commonly used medications in the outpatient setting and (b) provide recommendations for safe tablet-splitting prescribing practices.
METHODS:
Ten random tablets from each of the selected medications were weighed and split by 5 volunteers (2 men and 3 women aged 25-44 years) using a knife. The selected medications were mirtazapine 30 mg, bromazepam 3 mg, oxcarbazepin 150 mg, sertraline 50 mg, carvedilol 25 mg, bisoprolol fumarate 10 mg, losartan 50 mg, digoxin 0.25 mg, amiodarone HCl 200 mg, metformin HCl 1,000 mg, glimepiride 4 mg, montelukast 10 mg, ibuprofen 600 mg, celecoxib 200 mg, meloxicam 15 mg, and sildenafil citrate 50 mg. The resulting half tablets were evaluated for weight and drug content uniformity in accordance with proxy United States Pharmacopeia (USP) specification (95%-105% for digoxin and 90%-110% for the other 15 drugs). Weight and drug content uniformity were assessed by comparing weight or drug content of the half tablets with one-half of the mean weight or drug content for all whole tablets in the sample. The percentages by which the weight and drug content of each whole tablet or half tablet differed from sample mean values were calculated. Other relevant physical characteristics of the 16 products were measured.
RESULTS:
A total of 52 of 320 half tablets (16.2%) and 48 of 320 half tablets (15.0%) fell outside of the proxy USP specification for weight and drug content, respectively. Bromazepam, carvedilol, bisoprolol, losartan, digoxin, and meloxicam half tablets failed the weight and content uniformity test; however, the half tablets for the rest of the medications passed the test. Mean percent weight loss after splitting was less than 1.5% for all drugs. Bromazepam, carvedilol, and digoxin showed the highest powdering loss during the tablet-splitting process.
CONCLUSIONS:
Tablet splitting could be safer and easier when drug- and patient-specific criteria have been met. Tablet size, shape, and hardness may also play a role in the decision to split a tablet or not. Tablets containing drugs with a wide therapeutic index and long half-life might be more suitable candidates for division. Dose variation exceeded a proxy USP specification for more than one-third of sampled half tablets of bromazepam, carvedilol, bisoprolol, and digoxin. Drug content variation in half tablets appeared to be attributed to weight variation due to fragment or powder loss during the splitting process.
What is already known about this subject
Tablet splitting is a well-established medical practice in clinical settings, especially within the geriatric and psychiatric communities, as a means of reducing medication dose and/or cost and providing for ease of swallowing. However, it does not necessarily result in weight-uniform half tablets.
Most studies have assessed drug content uniformity only as variation in half tablet weights. However, a few studies have explored the drug content of half tablets.
United States Pharmacopeia guidelines for the drug content of split tablets have yet to be established. To date, no available guidelines regulate the tablet-splitting practice in Egypt.
What this study adds
This research was conducted to recommend initiating a database that could be accessed electronically for safe tablet-splitting prescribing practices.
Recommendations are provided for what tablets can or cannot be divided depending on the effect of different tablet characteristics on weight and content uniformity of the selected medications.
Various cost-saving strategies have been used in order to alleviate rising prescription drug costs, including the use of generic medications, selection of more cost-effective medications, formulary restrictions, and tablet splitting.1 Tablet splitting is a well-established medical practice in clinical settings, especially within the geriatric and psychiatric communities, as a means of reducing medication dose and/or cost.2,3 Many prescription drugs are available at increased dosages for the same or similar costs as smaller dosages. Physicians frequently write prescriptions for half- and quarter-tablets in order to achieve doses less than the smallest available manufactured strength. Besides the cost-saving potential,4 tablet splitting has a number of advantages, including providing proper dosage in cases where slow dose titration and dose tapering are necessary, such as with antihyperlipidemic or antihypertensive drugs.5 Another important advantage of scored tablets for geriatric and pediatric patients is ease of swallowing.6
A score on a tablet, however, can be misleading because not all scored tablets are suitable for splitting.7 Accordingly, uneven splitting may result in the administration of an inaccurate dose, which can be of significant risk if the split medication is a narrow therapeutic index medication.3 Several studies have reported weight differences among split medications.7-13 Most of these studies have assessed drug content uniformity only as a variation in half tablet weights. However, a few studies have explored the drug content of half tablets.8 United States Pharmacopeia (USP) guidelines for the drug content of split tablets have yet to be established. These studies adapted the USP guidelines to ensure that actual drug content was equivalent to manufacturer-labeled drug content and indirectly measured half tablet drug content by measuring half tablet weight.10
Although tablet splitting may be frequent in long-term care facilities, little is known about actual patterns of tablet splitting, particularly in ambulatory settings in Egypt. In addition, tablet splitters are not commonly used and not even available in all pharmacies in Egypt. Accordingly, the objective of this study was to investigate the effect of tablet characteristics on weight and content uniformity of half tablets resulting from splitting 16 products that are commonly split and used for long-term therapy in different clinical settings in Egypt. Furthermore, this study sought to provide recommendations for safe tablet-splitting prescribing practices. Factors that affect accuracy of tablet splitting, including tablet shape, size, hardness, presence of score line, and depth of score line, were also determined.
Methods
Sixteen commonly split drugs available in the Egyptian market were studied. These products included a narrow therapeutic index medication, medications that require tapering, and medications that could be administered when needed. Medications with extended-release formulations were excluded, since altering the physical properties of these medications by splitting could negatively impact their pharmacokinetics. The products included in this study are as follows: Remeron (mirtazapine 30 millgrams (mg), Schering-Plough, Netherlands); Calmepam (bromazepam 3 mg, GlaxoSmithKline, United Kingdom); Trileptal (oxcarbazepin 150 mg, Novartis, Switzerland); Lustral (sertraline 50 mg, Pfizer, United Kingdom); Dilatrend (carvedilol 25 mg, Roche, Germany); Concor (bisoprolol fumarate 10 mg, Merck, Germany); Cozaar (losartan 50 mg, Merck Sharp & Dohme, Netherlands); Lanoxin (digoxin 0.25 mg, GlaxoSmithKline, United Kingdom); Cordarone (amiodarone HCl 200 mg, Sanofi-Synthelabo, France); Glucophage (metformin HCl 1,000 mg, Merck, Germany); Amaryl (glimepiride 4 mg, Sanofi-Aventis, Germany); Singulair (montelukast 10 mg, Merck, United States); Brufen (ibuprofen 600 mg, Abott, United States); Eurocox (celecoxib 200 mg, Amriya, Egypt); Mobic (meloxicam 15 mg, Boehringer Ingelheim, Germany); and Viagra (sildenafil citrate 50 mg, Pfizer, United States).
Twenty whole tablets were randomly selected from each medication lot for each of the 16 products. All of them were weighed individually using a sensitive balance (Sartorius, Goettingen, Germany), and the average weight per tablet was calculated. Tablet characteristics including diameter, thickness, and score depth were measured using a micrometer. Tablet hardness was measured using a hardness tester (Erweka, Heusenstamm, Germany).
Ten of the 20 randomly selected tablets were split using a knife with a sharp stainless steel blade that was commonly available in pharmacies and houses. The dimensions of the blade were measured at the midpoint using a micrometer; the length of the blade was 10.3 centimeters (cm), and the width of the blade at the nonsharpened end was 0.13 cm. The length of the edge of the sharpened end was 0.1 cm. Tablets were split on a glassine weighing paper placed on a flat surface.
Five volunteers (2 men and 3 women aged 25-44 years) were recruited to perform the splitting. Volunteer details are shown in Table 1. All of the volunteers were right handed with no physical disability affecting the ability to split tablets. Each volunteer split 4 randomly selected tablets of each medication. They were instructed to hold the knife in their right hands, place the sharp end along the middle of the tablet, and apply incremental force on the nonsharpened end of the knife using the left hand until the tablet split.13 The weights of the half tablets were then measured.
TABLE 1.
Characteristics of Study Volunteers
| Volunteer | Gender | Age (years) | Training Level | Splitting Experience |
|---|---|---|---|---|
| 1 | F | 32 | Physician | No |
| 2 | F | 42 | Nurse | Yes |
| 3 | F | 44 | Laboratory technician | No |
| 4 | M | 19 | Pharmacy student | No |
| 5 | M | 25 | Community pharmacist | Yes |
The 10 whole tablets and 20 half tablets for each of the 16 products were then dissolved separately in an appropriate diluent adapted from respective USP official monographs. All tablets were assayed for content uniformity in accordance with USP methodology14 via an ultraviolet spectrophotometer (JASCO V-530 UV/VIS spectrophotometer, Tokyo, Japan).
The criteria for assessing weight and content uniformity were adapted from Hill et al. (2009).8 Hill et al. adapted their methodology from USP Chapter 905 (2005) and hypothesized that the drug content and weight of half tablets would deviate from USP specifications for drug content and weight of whole tablets (proxy USP specification).15 In the present study, the target drug content and weight of a half tablet was defined as equal to half of the mean drug content and weight, respectively, for all whole tablets in a sample of 16 commonly split medications. Furthermore, the acceptability of variation in the half tablets was assessed as the percentage by which each individual whole tablet and half tablet differed from the sample mean values.8
Measured Weight
The weight of each whole tablet (n = 10) was compared with the target weight for whole tablets, defined as the mean measured weight for all whole tablets in the sample. Target weight for individual tablets (measured mean weight per tablet) was found using the following equation:
The target weight of each half tablet (n = 20) was compared with one-half of the target weight for whole tablets, defined as one-half of the mean measured weight for all whole tablets in the sample.
The measured weight expressed as a percentage of the target weight was calculated for each tablet or half tablet using the following equation:
The proxy USP specification for weight is the measured weight of whole or half tablets within 95%-105% of the target weight for half tablets for digoxin and within 90%-110% of target weight for half tablets for the other medications.
The percentage of weight loss due to fragmenting and/or powdering during the splitting process was calculated for each tablet using the following equation:
Measured Drug Content
The drug content for each whole tablet (n = 10) was compared with the target drug content for whole tablets, defined as the mean measured drug content for all whole tablets in the sample.
The target drug content for each half tablet (n = 20) was compared with the target drug content for whole tablets, defined as one-half of the mean measured drug content for all whole tablets in the sample.
To account for tablet powdering or fragmenting and the inability to split tablets into perfectly equal halves, the target drug content for each half tablet (n = 20) was adjusted for the weight of the fragment. The adjustment formula assumed that within a single half tablet of known weight, the half tablet’s proportion of the whole tablet drug content should equal the half tablet’s proportion of the whole tablet weight.
Nonscored drug tablets (n = 60; montelukast, ibuprofen, and sildenafil citrate) were compared with the 13 other scored drug tablets (n =260) on 2 outcome measures: half tablet drug content and half tablet weight. The measured drug content expressed as a percentage of target drug content was calculated for each tablet or half tablet using the following equation:
The percentage by which weight-adjusted drug content differed from target drug content was calculated using the following equation:
Because no USP criteria for drug content uniformity of half tablets have yet been established, this study applied the proxy USP specification for whole tablets to half tablets. Proxy USP specifications were chosen for weight and content uniformity: 95%-105% of target weight and content for half tablets for digoxin and within 90%-110% of target weight and content for half tablets for the other medications, rather than 85%-115% used in other studies.11,13
Relative standard deviation expressed as a percentage (%RSD), which is a ratio of the standard deviation (SD) to the mean of the variable being analyzed, was calculated for whole tablets (drug content and weight) and for half tablets (drug content, weight-adjusted drug content, and weight). The %RSD is widely used to assess the repeatability and precision of the assays used to analyze drug content. Individual medication lots for whole tablets are targeted to have a %RSD less than 6% (proxy USP specification for %RSD).
Results
This study identified 16 commonly split medications in outpatient settings. Of these medications, many are used for treatment of psychiatric disorders, hypertension, cardiovascular diseases, diabetes, asthma, and pain. In addition, sildenafil, a drug for erectile dysfunction, was included.
Basic Characteristics of Products
The basic characteristics of the 16 products studied are listed in Table 2. Of the 16 medications, 8 tablets were oblong; 1 tablet was oval; 3 tablets were round; 1 tablet was rounded and diamond shaped; 1 tablet was heart shaped, 1 tablet was rectangular; and 1 tablet was pentagonal. The 16 medications comprised scored (81.25%) and unscored (18.75%) tablets. The unscored medications were montelukast, ibuprofen, and sildenafil. Among the scored tablets, 5 had a score line along 2 faces of the tablet (oxcarbazepin, carvedilol, bisoprolol, metformin, and glimepiride).
TABLE 2.
Tablet Characteristics of Study Medications (Mean ± SD)
| Drug | Weight (gm) n = 20 | Dimensions (mm), n = 20 | Score (mm), n = 20 | Score Depth/Total Thickness × 100 (%) | Flat-Faced Tablet | Hardness (kg/inch 2 ) n = 5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diameter | Thickness | Width | Length | Score Depth | Score Mark | |||||
| Mirtazapine | 0.32 ± 0.012 | — | 3.4 ± 0.004 | 6.2 ± 0.001 | 12.1 ± 0.01 | 0.12 ± 0.03 | 1-sided | 3.52 | No | 8.7 ± 0.06 |
| Bromazepam | 0.236 ± 0.004 | — | 3.8 ± 0.01 | 9.0 ± 0.01 | — | 0.31 ± 0.07 | 1-sided | 8.15 | No | 4.7 ± 0.02 |
| Oxcarbazepin | 0.209 ± 0.004 | — | 3.2 ± 0.0 | 5.8 ± 0.001 | 11.8 ± 0.005 | 0.82 ± 0.06 | 2-sided | 51.25 | No | 7.8 ± 0.01 |
| Sertraline | 0.151 ± 0.001 | — | 2.9 ± 0.001 | 4.9 ± 0.0 | 11.0 ± 0.0 | 0.11 ± 0.02 | 1-sided | 3.79 | No | 6.8 ± 0.03 |
| Carvedilol | 0.093 ± 0.001 | 7.5 ± 0.002 | 2.1 ± 0.0 | — | — | 0.18 ± 0.03 | 2-sided | 8.57 | Yes | 4.5 ± 0.1 |
| Bisoprolol | 0.173 ± 0.002 | 7.5 ± 0.001 | 2.2 ± 0.001 | — | — | 0.10 ± 0.03 | 2-sided | 9.09 | No | 4.0 ± 0.02 |
| Losartan | 0.17 ± 0.02 | — | 3.3 ± 0.0 | 5.7 ± 0.00 | 10.5 ± 0.02 | 0.16 ± 0.04 | 1-sided | 4.84 | No | 8.21 ± 0.2 |
| Digoxin | 0.112 ± 0.002 | 7.0 ± 0.01 | 2.7 ± 0.02 | — | — | 0.17 ± 0.05 | 1-sided | 6.29 | No | 6.0 ± 0.12 |
| Amiodarone | 0.346 ± 0.004 | 10.5 ± 0.02 | 2.8 ± 0.001 | — | — | 0.48 ± 0.1 | 1-sided | 17.14 | No | 9.15 ± 0.48 |
| Metformin | 1.071 ± 0.015 | — | 5.1 ± 0.01 | 11.1 ± 0.03 | 18.9 ± 0.1 | 0.92 ± 0.1 | 2-sided | 36.07 | No | 10.1 ± 0.48 |
| Glimepiride | 0.170 ± 0.004 | — | 3.2 ± 0.0 | 5.6 ± 0.0 | 11.0 ± 0.02 | 0.85 ± 0.09 | 2-sided | 53.12 | Yes | 7.5 ± 0.3 |
| Montelukast | 0.21 ± 0.0 | — | 3.2 ± 0.001 | 7.9 ± 0.002 | 7.9 ± 0.004 | — | — | — | No | 12.0 ± 0.5 |
| Ibuprofen | 0.981 ± 0.005 | — | 3.9 ± 0.01 | 9.9 ± 0.005 | 21.1 ± 0.02 | — | — | — | No | 12.2 ± 0.48 |
| Celecoxib | 0.614 ± 0.006 | — | 5.2 ± 0.0 | 8.5 ± 0.1 | 17.0 ± 0.0 | 0.19 ± 0.03 | 1-sided | 3.65 | No | 12.1 ± 0.88 |
| Meloxicam | 0.191 ± 0.01 | 10.4 ± 0.0 | 4.8 ± 0.0 | — | — | 0.21 ± 0.02 | 1-sided | 4.37 | Yes | 6.0 ± 0.35 |
| Sildenafil | 0.305 ± 0.004 | — | 3.5 ± 0.001 | 8.3 ± 0.002 | 11.0 ± 0.003 | — | — | — | No | 10.5 ± 0.1 |
gm = gram; kg = kilogram; mm = millimeter; SD = standard deviation.
Bromazepam, carvedilol, and bisoprolol tablets had the lowest hardness values (approximately 4 kilogram [kg]/inch2). Metformin, montelukast, ibuprofen, celecoxib, and sildenafil tablets had the highest hardness values (approximately 10-12 kg/inch2). Metformin, ibuprofen, and celecoxib had the highest weight (1.071, 0.981, and 0.614 grams [gm], respectively) and the highest hardness (>10 kg/inch2). Oxcarbazepin, metformin, and glimepiride had the highest score depth, which represented 51.25%, 36.07%, and 53.12% of the total thickness, respectively.
Weight Uniformity
The results of the weight uniformity test performed on whole and half tablets of the 16 products are shown in Table 3. For all whole tablets studied, measured weight expressed as a percentage of target weight fell within the proxy USP specification for weight and met the proxy USP specification for %RSD (Table 3). All half tablets passed the weight uniformity test except bromazepam, carvedilol, bisoprolol, losartan, digoxin, and meloxicam. At least 5 half tablets for each of these medications fell outside the proxy USP specification. A total of 52 of 320 half tablets (16.2%) fell outside of the proxy USP specification for weight; these included bromazepam (45%), carvedilol (60%), bisoprolol (40%), losartan (30%), digoxin (60%), and meloxicam (25%). Mean percent weight loss, after splitting, was less than 1.5% for all drugs (Table 3). Bromazepam, carvedilol, and digoxin showed the highest powdering loss during the tablet-splitting process. Amiodarone, montelukast, and celecoxib were split with the lowest powdering loss.
TABLE 3.
Weight Variation Analysis for Study Medication Whole and Half Tablets
| Drug | Whole or Half Tablets | Target Weight (gm) | Measured Weight Mean (gm) | %RSD | Mean Percent Weight Loss (SD) | Percentage of Target Weight Range | Outside of Proxy USP Specification a | Result |
|---|---|---|---|---|---|---|---|---|
| Mirtazapine | Whole (n = 10) | — | 0.320 | 3.75 | — | 98.8-101.1 | 0 | Accept |
| Bromazepam | Whole (n = 10) | — | 0.236 | 1.694 | — | 97.4-102.1 | 0 | Accept |
| Oxcarbazepin | Whole (n = 10) | — | 0.209 | 1.913 | — | 99.4-103.3 | 0 | Accept |
| Sertraline | Whole (n = 10) | — | 0.151 | 0.662 | — | 99.8-102.0 | 0 | Accept |
| Carvedilol | Whole (n = 10) | — | 0.093 | 1.075 | — | 97.8-102.1 | 0 | Accept |
| Bisoprolol | Whole (n = 10) | — | 0.173 | 1.156 | — | 98.8-102.3 | 0 | Accept |
| Losartan | Whole (n = 10) | — | 0.170 | 1.176 | — | 99.1-102.1 | 0 | Accept |
| Digoxin | Whole (n = 10) | — | 0.112 | 1.785 | — | 98.2-103.5 | 0 | Accept |
| Amiodarone | Whole (n = 10) | — | 0.346 | 1.156 | — | 98.5-102.0 | 0 | Accept |
| Metformin | Whole (n = 10) | — | 1.071 | 1.437 | — | 99.1-101.2 | 0 | Accept |
| Glimepiride | Whole (n = 10) | — | 0.170 | 2.352 | — | 99.4-103.0 | 0 | Accept |
| Montelukast | Whole (n = 10) | — | 0.210 | 0.0 | — | 99.5-100.9 | 0 | Accept |
| Ibuprofen | Whole (n = 10) | — | 0.981 | 0.509 | — | 99.1-103.1 | 0 | Accept |
| Celecoxib | Whole (n = 10) | — | 0.614 | 0.977 | — | 98.1-102.3 | 0 | Accept |
| Meloxicam | Whole (n = 10) | — | 0.191 | 5.235 | — | 98.2-101.1 | 0 | Accept |
| Sildenafil | Whole (n = 10) | — | 0.305 | 1.311 | — | 99.0-101.9 | 0 | Accept |
| Mirtazapine | Half (n = 20) | 0.160 | 0.151 | 6.0 | 0.17 (0.42) | 97.3-107.5 | 0 | Accept |
| Bromazepam | Half (n = 20) | 0.118 | 0.108 | 12.0 | 1.40 (1.2) | 90.9-118.1 | 9 (45%) | Reject |
| Oxcarbazepin | Half (n = 20) | 0.104 | 0.101 | 5.7 | 0.25 (0.2) | 91.1-108.9 | 0 | Accept |
| Sertraline | Half (n = 20) | 0.075 | 0.074 | 3.9 | 0.20 (0.1) | 101.4-109.7 | 0 | Accept |
| Carvedilol | Half (n = 20) | 0.046 | 0.040 | 17.6 | 1.50 (1.0) | 80.0-112.5 | 12 (60%) | Reject |
| Bisoprolol | Half (n = 20) | 0.086 | 0.082 | 12.9 | 0.58 (0.33) | 86.4-112.0 | 8 (40%) | Reject |
| Losartan | Half (n = 20) | 0.085 | 0.081 | 11.0 | 0.47 (0.21) | 87.5-114.7 | 6 (30%) | Reject |
| Digoxin | Half (n = 20) | 0.056 | 0.051 | 12.3 | 1.30 (0.05) | 89.2-117.8 | 12 (60%) | Reject |
| Amiodarone | Half (n = 20) | 0.173 | 0.173 | 2.1 | 0.03 (0.02) | 98.2-104.0 | 0 | Accept |
| Metformin | Half (n = 20) | 0.535 | 0.531 | 2.3 | 0.21 (0.01) | 99.7-103.1 | 0 | Accept |
| Glimepiride | Half (n = 20) | 0.085 | 0.083 | 4.2 | 0.10 (0.03) | 96.3-108.4 | 0 | Accept |
| Montelukast | Half (n = 20) | 0.105 | 0.101 | 5.9 | 0.02 (0.01) | 92.3-105.7 | 0 | Accept |
| Ibuprofen | Half (n = 20) | 0.490 | 0.489 | 4.8 | 0.31 (0.02) | 97.1-106.1 | 0 | Accept |
| Celecoxib | Half (n = 20) | 0.307 | 0.307 | 5.8 | 0.04 (0.02) | 97.2-103.0 | 0 | Accept |
| Meloxicam | Half (n = 20) | 0.095 | 0.091 | 12.1 | 0.24 (0.12) | 88.2-116.2 | 5 (25%) | Reject |
| Sildenafil | Half (n = 20) | 0.152 | 0.153 | 5.2 | 0.30 (0.06) | 96.0-110.0 | 0 | Accept |
a Number of whole or half tablets with measured weight NOT within 95%-105% of target weight for digoxin or 90%-110% of target weight for the other medications and NOT within %RSD < 6.
gm = gram; SD = standard deviation; USP = United States Pharmacopeia; %RSD = percentage of relative standard deviation.
Content Uniformity
For all whole tablets studied, measured drug content expressed as a percentage of target drug content fell within the proxy USP specifications (Table 4). The measured drug content expressed as a percentage of target drug content for half tablets fell outside of the proxy USP specification for at least 5 half tablets of bromazepam, carvedilol, bisoprolol, losartan, digoxin, and meloxicam. A total of 48 of 320 half tablets (15.0%) fell outside of the proxy USP specifications for drug content: bromazepam (40%), carvedilol (60%), bisoprolol (40%), losartan (25%), digoxin (50%), and meloxicam (25%). These results coincided with the weight uniformity results.
TABLE 4.
Drug Content for Study Medication Whole and Half Tablets
| Drug | Whole or Half Tablets | Target Drug Content (mg) | Measured Drug Content Mean (mg) | %RSD | Percentage of Target Drug Content-Range | Outside of Proxy USP Specification a | Results |
|---|---|---|---|---|---|---|---|
| Mirtazapine | Whole (n = 10) | — | 29.83 | 2.32 | 95.0-102.5 | 0 | Accept |
| Bromazepam | Whole (n = 10) | — | 3.014 | 2.54 | 96.3-104.0 | 0 | Accept |
| Oxcarbazepin | Whole (n = 10) | — | 151.8 | 1.218 | 100.0-103.3 | 0 | Accept |
| Sertraline | Whole (n = 10) | — | 50.4 | 2.1 | 98.4-104.8 | 0 | Accept |
| Carvedilol | Whole (n = 10) | — | 25.02 | 3.64 | 96.1-108.2 | 0 | Accept |
| Bisoprolol | Whole (n = 10) | — | 10.23 | 3.7 | 99.0-109.0 | 0 | Accept |
| Losartan | Whole (n = 10) | — | 51.3 | 3.64 | 97.6-16.2 | 0 | Accept |
| Digoxin | Whole (n = 10) | — | 0.253 | 3.12 | 98.0-108.1 | 0 | Accept |
| Amiodarone | Whole (n = 10) | — | 200.6 | 1.11 | 99.2-102.1 | 0 | Accept |
| Metformin | Whole (n = 10) | — | 1000.1 | 3.1 | 99.9-104.2 | 0 | Accept |
| Glimepiride | Whole (n = 10) | — | 4.05 | 1.97 | 99.7-105.0 | 0 | Accept |
| Montelukast | Whole (n = 10) | — | 10.1 | 1.71 | 99.8-104.0 | 0 | Accept |
| Ibuprofen | Whole (n = 10) | — | 599.9 | 2.4 | 99.8-103.3 | 0 | Accept |
| Celecoxib | Whole (n = 10) | — | 201.2 | 1.2 | 99.2-102.5 | 0 | Accept |
| Meloxicam | Whole (n = 10) | — | 15.1 | 1.25 | 99.3-103.3 | 0 | Accept |
| Sildenafil | Whole (n = 10) | — | 50.6 | 2.43 | 97.6-106.0 | 0 | Accept |
| Mirtazapine | Half (n = 20) | 14.91 | 15.4 | 5.76 | 90.6-110.0 | 0 | Accept |
| Bromazepam | Half (n = 20) | 1.507 | 1.315 | 11.41 | 86.6-113.3 | 8 (40%) | Reject |
| Oxcarbazepin | Half (n = 20) | 75.9 | 76.4 | 4.23 | 92.0-109.3 | 0 | Accept |
| Sertraline | Half (n = 20) | 25.2 | 24.85 | 4.1 | 94.6-104.0 | 0 | Accept |
| Carvedilol | Half (n = 20) | 12.51 | 11.84 | 12.4 | 80.8-116.3 | 12 (60%) | Reject |
| Bisoprolol | Half (n = 20) | 5.11 | 4.72 | 9.64 | 82.5-106.9 | 8 (40%) | Reject |
| Losartan | Half (n = 20) | 25.65 | 24.02 | 10.5 | 84.2-112.0 | 5 (25%) | Reject |
| Digoxin | Half (n = 20) | 0.126 | 0.131 | 12.3 | 80.0-132.0 | 10 (50%) | Reject |
| Amiodarone | Half (n = 20) | 100.3 | 100.2 | 2.97 | 95.0-104.1 | 0 | Accept |
| Metformin | Half (n = 20) | 500.05 | 499.0 | 3.1 | 98.9-107.5 | 0 | Accept |
| Glimepiride | Half (n = 20) | 2.02 | 2.03 | 4.7 | 94.5-109.0 | 0 | Accept |
| Montelukast | Half (n = 20) | 5.06 | 5.01 | 4.61 | 94.0-107.1 | 0 | Accept |
| Ibuprofen | Half (n = 20) | 299.95 | 298.7 | 5.1 | 92.1-108.3 | 0 | Accept |
| Celecoxib | Half (n = 20) | 100.6 | 100.2 | 4.9 | 95.2-104.0 | 0 | Accept |
| Meloxicam | Half (n = 20) | 7.55 | 73.8 | 10.6 | 80.1-120.0 | 5 (25%) | Reject |
| Sildenafil | Half (n = 20) | 25.3 | 25.2 | 5.2 | 95.4-108.1 | 0 | Accept |
| Mirtazapine | Half wt adj (n = 20) | — | 14.09 | 5.14 | 92.1-108.7 | 0 | Accept |
| Bromazepam | Half wt adj (n = 20) | — | 1.37 | 9.13 | 89.0.1-99.1 | 1 (5%) | Reject |
| Oxcarbazepin | Half wt adj (n = 20) | — | 73.35 | 3.1 | 96.6-102.8 | 0 | Accept |
| Sertraline | Half wt adj (n = 20) | — | 24.69 | 3.9 | 96.4-102.7 | 0 | Accept |
| Carvedilol | Half wt adj (n = 20) | — | 10.76 | 10.5 | 86.5-110.9 | 2 (10%) | Reject |
| Bisoprolol | Half wt adj (n = 20) | — | 4.84 | 8.8 | 88.8-104.2 | 2 (10%) | Reject |
| Losartan | Half wt adj (n = 20) | — | 24.44 | 7.1 | 93.0-104.8 | 0 | Reject |
| Digoxin | Half wt adj (n = 20) | — | 0.126 | 10.1 | 88.0-108.0 | 2 (10%) | Reject |
| Amiodarone | Half wt adj (n = 20) | — | 100.3 | 2.0 | 99.2-102.1 | 0 | Accept |
| Metformin | Half wt adj (n = 20) | — | 500.0 | 2.7 | 99.2-104.1 | 0 | Accept |
| Glimepiride | Half wt adj (n = 20) | — | 1.97 | 4.1 | 97.4-102.5 | 0 | Accept |
| Montelukast | Half wt adj (n = 20) | — | 4.88 | 3.91 | 95.9-103.0 | 0 | Accept |
| Ibuprofen | Half wt adj (n = 20) | — | 299.1 | 4.5 | 95.3-104.1 | 0 | Accept |
| Celecoxib | Half wt adj (n = 20) | — | 100.6 | 3.11 | 99.2-102.5 | 0 | Accept |
| Meloxicam | Half wt adj (n = 20) | — | 7.19 | 9.8 | 89.5-113.4 | 1 (10%) | Reject |
| Sildenafil | Half wt adj (n = 20) | — | 25.38 | 4.1 | 97.9-104.3 | 0 | Accept |
a Number of whole or half tablets with measured drug content NOT within 95%-105% of target drug content for digoxin or 90%-110% of target drug content for the other medications and NOT within %RSD < 6.
mg = milligram; USP = United States Pharmacopeia; wt adj = weight adjusted; %RSD = percentage of relative standard deviation.
Weight-adjusted drug content, expressed as a percentage of target drug content for half tablets, fell outside of the proxy USP specification for at most 2 half tablets of bromazepam, carvedilol, bisoprolol, losartan, digoxin, and meloxicam (Table 4). After weight adjustment, a total of 8 of 320 half tablets (2.5%) fell outside of the proxy USP specification for drug content; these included bromazepam (5%), carvedilol (10%), bisoprolol (10%), losartan (0%), digoxin (10%), and meloxicam (5%).
Scored Versus Nonscored Tablets
For the selected nonscored medications, all half tablets passed the proxy USP specifications for weight and drug content (Table 5). However, 52 of 260 (20.0%) half tablets and 48 of 260 (18.4%) half tablets of scored medications fell outside of the proxy USP specifications for weight and drug content, respectively (Table 5). The number of half tablets for scored (nonscored) drugs falling outside of the range for weight were 71 (15) for 95%-105%, 47 (0) for 90%-110%, 35 (0) for 85%-115%, and 12 (0) for 75%-125%. The numbers of half tablets for scored (nonscored) drugs falling outside of range for drug content were 68 (13) for 95%-105%, 44 (0) for 90%-110%, 34 (0) for 85%-115%, and 10 (0) for 75%-125%.
TABLE 5.
Comparison of Scored and Nonscored Half tablets: Weight and Drug Content
| Tablet Type | Percentage of Mean-Range | Outside of Proxy USP Specification a | Number (%) of Half tablets with Measured Weight/Drug Content | |||
|---|---|---|---|---|---|---|
| Out of Range (95%-105%) | Out of Range (90%-110%) | Out of Range (85%-115%) | Out of Range (75%-125%) | |||
| Weight | ||||||
| Scored (n = 260) | 80.0-118.1 | 52 (20.0%) | 71 (29.5%) | 47 (19.5%) | 35 (14.5%) | 12 (5.0%) |
| Nonscoredb (n = 60) | 92.3-110.0 | 0 | 15 (25.0%) | 0 | 0 | 0 |
| Drug Content | ||||||
| Scored (n = 260) | 80.0-132.0 | 48 (18.4%) | 68 (28.3%) | 44 (18.3%) | 34 (14.1%) | 10 (16.6%) |
| Nonscored (n = 60) | 92.1-108.3 | 0 | 13 (21.6%) | 0 | 0 | 0 |
a Number of half tablets with measured weight or drug content NOT within 95%-105% of target weight or drug content for digoxin or 90%-110% of target weight or drug content for the other medications and NOT within %RSD < 6.
b The unscored medications were montelukast, ibuprofen, and sildenafil.
USP = United States Pharmacopeia.
Discussion
Tablet splitting is a widespread, international practice in all sectors of health care.14,16 The practice of tablet splitting is considered compounding of a medication that is not commercially available in the desired dosage by a pharmacist.17,18 Although cost savings might be accomplished, the tablet-splitting technique used could result in unpredictable effects on the stability of the drug, loss of drug due to powdering, uneven doses, lack of physical strength, and dexterity.19 Different splitting techniques can be used to cut a tablet into 2 halves, such as hand, splitting device, scissors, razor blades, or kitchen knife. Less weight loss can be achieved by using a splitting device compared with the other methods.20 With greater precision and accuracy, tablet-splitting devices generally provide more consistency in half tablet doses. However, tablet splitters are not commonly used and are not even available in all pharmacies in Egypt. Splitting by hand or with sharp instruments, such as knives and razor blades, are also commonly used techniques in the outpatient setting. Although splitting tablets by hand produce cleaner splits with less tablet crumbling, tablets split by hand show less uniformity than tablets split using knives and razor blades.9,20 Accordingly, splitting with a knife was used in this study.
To date, no available guidelines regulate tablet splitting in Egypt. Accordingly, this research was conducted to recommend initiating a database that could be accessed electronically and that would specify what tablets could or could not be divided, depending on the presence of score lines, depth of the score lines, tablet hardness, and other relevant characteristics. Many medications available in Egypt are imported from the United States and Europe. Therefore, tablet-splitting information could be quoted and applied to such products. In addition, the U.S. Food and Drug Administration issued a draft guidance for industry regarding tablet scoring that should be applied by Egyptian drug manufacturers.21
The USP has not created a method for assessing half tablet drug content uniformity; thus, previous studies assessing half tablet drug content uniformity used adapted USP methods for assessing weight variability as a means of estimating drug content uniformity.8,11,13 The selected medications in this study are commonly split during the dosage titration or tapering process either for unavailability or for cost-saving reasons. They shared relatively wide therapeutic windows except digoxin, long half-lives, and potential for cost savings.
Physical properties of medications such as scoring, shape, and size can affect the ease and accuracy of splitting.22 Metformin, glimepiride, and oxcarbazepin tablets were ideal for accurate and uniform splitting. This might be due to large tablet thickness (3.2-5.1 millimeters [mm]), high crushing strength (7.5-10.1 kg/inch2), and the deepest score line on the 2 tablet surfaces with a flat face, in case of glimepiride (Table 2). Also, the amiodarone tablet showed an excellent splitting uniformity among the studied round tablets. This could be due to the large diameter (10.5 mm), large size (0.346 gm), suitable crushing strength (9.15 kg/inch2), and the obvious score line (Table 2). The large size of the celecoxib tablet (0.614 gm) and the oblong shape of the mirtazapine and sertraline tablets might explain their good splitting behavior.
Conversely, carvedilol, bisoprolol, and digoxin tablets showed the lowest splitting uniformity and accuracy. They easily crumbled upon splitting. Digoxin tablets had the smallest diameter (7.04 mm), low weight (0.112 gm), and a biconvex face, along with the score line only on 1 face (Table 2). In addition to the low crushing strength (6 kg/inch2), these characteristics seem to provide digoxin tablets with poor splitting accuracy and uniformity. The carvedilol tablet was unsuitable for splitting because of small weight (0.093 gm) and diameter (7.5 mm) and low crushing strength (4.5 kg/inch2; Table 2). These characteristics could lead to tablet fracture at the score with moderate powdering upon splitting, despite the presence of a flat surface and double score lines on the 2 faces of the tablet. The irregular shape, small diameter, and the low crushing strength (4-4.7 kg/inch2) of the bromazepam and bisoprolol tablets might also contribute to the poor splitting accuracy and uniformity of those tablets. The meloxicam tablet was expected to split accurately because of the large tablet diameter (10.4 mm); however, the opposite happened, which could be due to its circular shape and a relatively low crushing strength (6 kg/inch2; Table 2). Also, the small size (0.17 gm) and length (10.5 mm) of the losartan tablet might cause the poor splitting behavior it exhibited (Table 2).
The relationship between tablet characteristics and splitting behavior has been previously studied.8,11,13 The effect of resistance to crushing on predicting the ease of subdivision of scored tablets has also been reported.13 The results from these previous studies suggest that crushing strength is the most important contributor to good splitting behavior, followed by diameter, score mark (1- or 2-sided), and shape (flat or biconvex).13 These findings coincided with the finding that tablets with high crushing strength values (approximately 10-12 kg/inch2), such as metformin, montelukast, ibuprofen, celecoxib, and sildenafil tablets, showed a much better splitting uniformity than tablets with low crushing strength values (approximately 4 kg/inch2), such as bromazepam, carvedilol, and bisoprolol tablets. Accordingly, a large crushing strength was expected to improve the accuracy and uniformity of tablet splitting.23 Conversely, other researchers found opposite results regarding the effect of crushing strength on tablet-splitting behavior.11 Thus, achieving a high degree of splitting accuracy and uniformity was not a result of a single characteristic but, rather, depended upon a number of tablet characteristics. Such results can be of clinical significance in cases of narrow therapeutic index medications such as digoxin, where small dose changes might result in sub- or supratherapeutic doses.24
Six of the 16 (37.5%) tested split medications (bromazepam, carvedilol, bisoprolol, losartan, digoxin, and meloxicam) fell outside of the proxy USP specification for weight and content (Tables 3 and 4). There was a wide variation of weight among these 6 medications (%RSD > 6%), despite the presence of score lines that could improve the accuracy of splitting.7 Carvedilol had the greatest degree of drug content variability (%RSD = 2.4%), which could be attributed to the greatest amount of weight loss from splitting (1.5%).
Dose variation exceeded a proxy USP specification for more than one-third of the sampled half tablets of bromazepam, carvedilol, bisoprolol, and digoxin. This variation might have been affected by the inability of the tablet-splitting device to accurately split medications into 2 equal halves. Additionally, a greater percentage of drug content variation could be attributed to tablet formulation, especially content, shape, and coating.
Variation in half tablet drug content was greatest with bromazepam, digoxin, and carvedilol, which had tablet halves ranging from 80%-132% of the target drug content for half tablets. Thus, when tablet splitting was performed for these 3 products, patients might have received daily doses that varied by as much as 50%. This finding was likely a result of weight loss due to tablet powdering and inaccuracy of tablet splitting devices and persons operating the devices. This argument is supported by the weight-adjusted data (Tables 3 and 4).
When half tablet drug content was adjusted for weight, a large reduction in drug content variation was found. Thus, half tablet weight appeared to be directly correlated with drug content. When compared with the target drug content of a perfectly split tablet half, 48 of 320 half tablets (15.0%)—but only 8 of 320 weight-adjusted half tablets (2.5%)—fell outside of proxy USP specifications for drug content.
It was also observed that the %RSD for weight-adjusted drug content for all medications was reduced in comparison with nonweight-adjusted drug content. Carvedilol, bisoprolol, and digoxin accounted for the majority of weight-adjusted half tablets falling outside of proxy USP specifications for drug content (2 of the 20 half tablets). This finding could be explained by the nonuniform dispersion of drug content within a single whole tablet. Thus, drug content variation in half tablets appeared to be attributable primarily to weight variation occurring when tablets fragmented during the splitting process. As such, equal daily doses could be determined by the ability of patients to split tablets perfectly in half.
Conversely, in the selected medications, the data suggested greater variability in half tablet drug content and weight for scored medications than for nonscored medications. More scored half tablets were found to have drug content and weight out of the ranges of 85%-115% and 75%-125%. Although montelukast, ibuprofen, and sildenafil tablets are unscored, they exhibited good splitting with minimal powder loss and less tablet crumbling upon fracture into 2 equal halves. The mean percent weight loss values were 0.02%, 0.31%, and 0.3% for montelukast, ibuprofen, and sildenafil tablets, respectively. These findings suggest that when a tablet-splitting knife is used, dose administration might be more accurate and consistent, depending on not only the score line but also other characteristics, such as hardness, size, thickness, and shape of the tablets. The selected nonscored tablets were ideal for accurate and uniform splitting. The crushing strength and tablet thickness might explain their good splitting behavior. The studied nonscored tablets had the highest crushing strength (approximately 10-12 kg/inch2) compared with other scored tablets, such as carvedilol, bisoprolol, bromazepam, and digoxin tablets (< 6 kg/inch2). The relatively large thickness (3.2-3.9 mm) and the high tablet weight, especially with ibuprofen—which had the highest weight (0.981 gm) among all the tested medications—seemed to provide the nonscored tablets with good splitting accuracy and uniformity. However, a larger sample of scored and nonscored tablets is needed to determine if there is a significant difference between scored and nonscored tablets.
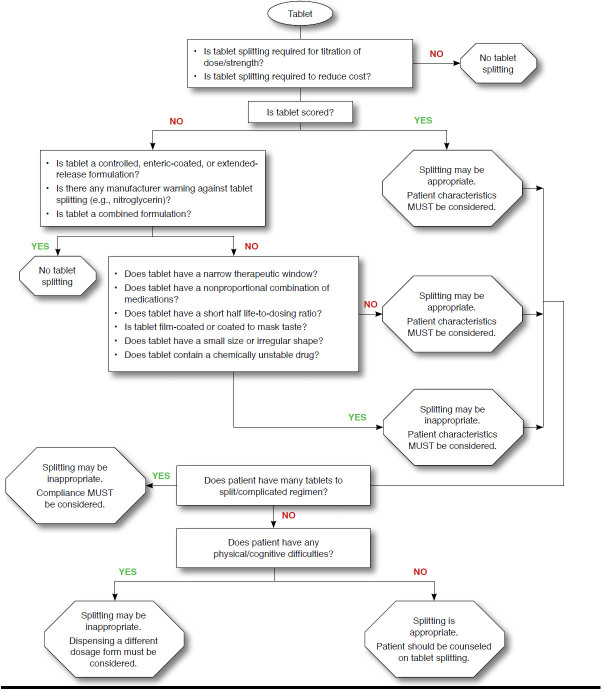
Tablet splitting is safe when drug- and patient-specific criteria have been met.1 Although cost savings might be achieved, fears of inaccurate dosing, noncompliance, poor cognitive function or memory, and physical inability to effectively split tablets might discourage physicians and patients from adopting this practice.8,25 A tool for evaluation of the appropriateness of tablet splitting, taking product and patient characteristics into account, is presented in Figure 1.
FIGURE 1.
Is Tablet Splitting Appropriate? Tablet and Patient Characteristics
Not all of the tablets used in this study were suitable for splitting. Medications should not be split if there is potential for adverse pharmacologic outcomes. Splitting of enteric-coated, sustained, and controlled-release formulations could increase the risk of side effects and compromise effectiveness.26 The pharmacokinetics seem to dictate if splitting will have a clinical impact on long-term patient outcomes. Medications with short half-lives should not be split if inaccurate splitting could result in fluctuations in plasma concentrations. Once-daily sertraline, with a half-life of 25 to 26 hours, is an example of a medication with a substantial pharmacokinetic buffer against inaccurate tablet splitting.27 Mirtazapine, bromazepam, sertraline, and montelukast are agents with long durations of action, in which minor dose variation should have no significant impact on steady state plasma concentrations. The splitting of montelukast was appropriate as long as the split tablet was used within a week of splitting. Lastly, antihypertensive drugs are administered over an extended period of time. Thus, daily fluctuations in dose would not be expected to affect blood pressure measurements and side effects and long-term clinical end points. In contrast, caution should be used when splitting narrow therapeutic index medications such as digoxin because of the potential for significant adverse events with minimal change or fluctuations in daily dose.
Tablet splitting is an accepted practice in managed care pharmacy for suitable drugs if performed by patients without physical disabilities under a pharmacist’s guidance.28 A patient’s state of health might affect the ability to properly split tablets.29 In particular, certain patients might have increased difficulty splitting tablets, such as the elderly and patients with arthritis, movement disorders, poor eyesight, or poor cognitive function.17,30 These patients should be instructed by pharmacists in how to accurately split tablets manually or how to use a tablet-splitting device.
Concerns also have been expressed regarding patient adherence. There is a fear that patients may not be willing to take the time to split a tablet before taking it. However, 1 study reported that splitting tablets had no effect on adherence.4 It was further suggested that tablet splitting might increase adherence by reducing the cost barrier faced by some patients.4
Limitations
The only tablet-splitting technique used in this study was a knife. However, splitting by hand or with sharp instruments such as splitting devices or razor blades are commonly used techniques in the outpatient setting and may lead to greater variability than that observed in this study. This research did not permit clinical conclusions, since no clinical end points were assessed.
Conclusions
Tablet splitting can be a cost-saving practice when implemented judiciously using drug- and patient-specific criteria aimed at clinical safety. A patient’s state of health might affect the ability to properly split tablets. A special precaution should be written on a medication’s package indicating if dividing tablets is considered appropriate. In addition, pharmacists should instruct patients in how to accurately split tablets manually or how to use a tablet-splitting device. The criteria used to evaluate weight and drug content uniformity were derived from the criteria set for whole tablets and were applied for half tablets. Not all tablets were suitable for splitting. Medication characteristics suitable for tablet splitting include long half-life; scored; flat, oblong, or oval; large size; and broad therapeutic window. Medication characteristics unsuitable for tablet splitting include enteric-coated or extended-release formulations, frequent dosing changes, small size, easily crumbles or breaks, bitter taste, and narrow therapeutic window. More studies should be performed that assess the clinical impact of half tablet regimens for the selected 16 medications.
REFERENCES
- 1.Stafford RS, Radley DC. The potential of pill splitting to achieve cost savings. Am J Manag Care. 2002;8(8):706-12. [PubMed] [Google Scholar]
- 2.Quinzler R, Gasse C, Schneider A, Kaufmann-Kolle P, Szecsenyi J, Haefeli WE. The frequency of inappropriate tablet splitting in primary care. Eur J Clin Pharmacol. 2006;62(12):1065-73. [DOI] [PubMed] [Google Scholar]
- 3.Rodenhuis N, De Smet PA, Barends DM. The rational of scored tablets as dosage form. Eur J Pharm Sci. 2004;21(2-3):305-08. [DOI] [PubMed] [Google Scholar]
- 4.Fawell NG, Cookson TL, Scranton SS. Relationship between tablet splitting and compliance, drug acquisition cost, and patient acceptance. Am J Health Syst Pharm. 1999;56(24):2542-45. [DOI] [PubMed] [Google Scholar]
- 5.Gee M, Hasson NK, Hahn T, Ryono R. Effects of a tablet-splitting program in patients taking HMG-CoA reductase inhibitors: analysis of clinical effects, patient satisfaction, compliance, and cost avoidance. J Manag Care Pharm. 2002;8(6):453-58. Available at: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=6686. [DOI] [PubMed] [Google Scholar]
- 6.Carr-Lopez SM, Mallett MS, Morse T. The tablet splitter: barrier to compliance or cost-saving instrument? Am J Health Syst Pharm. 1995;52(23):2707-08. [DOI] [PubMed] [Google Scholar]
- 7.Arnet I, Hersberger KE. Misleading score-lines on tablets: facilitated intake or fractional dosing? Swiss Med Wkly. 2010;140(7-8):105-10. [DOI] [PubMed] [Google Scholar]
- 8.Hill SW, Varker AS, Karlage K, Myrdal PB. Analysis of drug content and weight uniformity for half tablets of 6 commonly split medications. J Manag Care Pharm. 2009;15(3):253-61. Available at: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng J, Song CK, Williams RL, Polli JE. Lack of medication dose uniformity in commonly split tablets. J Am Pharm Assoc (Wash). 2002;42(2):195-99. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg JM, Nathan JP, Plakogiannis F. Weight variability of pharmacist-dispensed split tablets. J Am Pharm Assoc (Wash). 2002;42(2):200-05. [DOI] [PubMed] [Google Scholar]
- 11.Polli JE, Kim S, Martin BR. Weight uniformity of split tablets required by a Veterans Affairs policy. J Manag Care Pharm. 2003;9(5):401-07. Available at: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook TJ, Edwards S, Gyemah C, Shah M, Shah I, Fox T. Variability in tablet fragment weights when splitting unscored cyclobenzaprine 10 mg tablets. J Am Pharm Assoc (2003). 2004;44(5):583-86. [DOI] [PubMed] [Google Scholar]
- 13.Tahaineh LM, Gharaibeh SF. Tablet splitting and weight uniformity of half tablets of 4 medications in pharmacy practice. J Pharm Pract. 2012;25(4):471-76. [DOI] [PubMed] [Google Scholar]
- 14.Grissinger M. Tablet splitting—only if you “half” to. PT. 2010;35(2):69-70. [Google Scholar]
- 15.United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary, USP28-NF23. Chapter 905: Uniformity of dosage units. Rockville, MD: United States Pharmacopeial Convention, Inc.; 2005. [Google Scholar]
- 16.Crawford DJ. Is tablet splitting safe? Nursing Made Incredibly Easy. 2011;9(5):18-20. [Google Scholar]
- 17.Noviasky J, Lo V, Luft DD. Clinical inquiries. Which medications can be split without compromising efficacy and safety? J Fam Pract. 2006;55(8):707-08. [PubMed] [Google Scholar]
- 18.van Santen E, Barends DM, Frijlink HW. Breaking of scored tablets: a review. Eur J Pharm Biopharm. 2002;53(2):139-45. [DOI] [PubMed] [Google Scholar]
- 19.Bachynsky J, Wiens C, Melnychuk K. The practice of splitting tablets: cost and therapeutic aspects. Pharmacoeconomics. 2002;20(5):339-46. [DOI] [PubMed] [Google Scholar]
- 20.Verrue C, Mehuys E, Boussery K, Remon JP, Petrovic M. Tablet-splitting: a common yet not so innocent practice. J Adv Nurs. 2011;67(1):26-32. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. Guidance for industry. Tablet scoring: nomenclature, labeling, and data for evaluation. March 2013. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM269921.pdf. Accessed November 8, 2014.
- 22.Gupta P, Gupta K. Broken tablets: does the sum of the parts equal the whole? Am J Hosp Pharm. 1988;45(7):1498. [PubMed] [Google Scholar]
- 23.van der Steen KC, Frijlink HW, Schipper CM, Barends DM. Prediction of the ease of subdivision of scored tablets from their physical parameters. AAPS PharmSciTech. 2010;11(1):126-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vivo RP, Krim SR, Perez J, Inklab M, Tenner T Jr, Hodgson J. Digoxin: current use and approach to toxicity. Am J Med Sci. 2008;336(5):423-28. [DOI] [PubMed] [Google Scholar]
- 25.Cohen CI, Cohen SI. Potential savings from splitting newer antidepressant medications. CNS Drugs. 2002;16(5):353-58. [DOI] [PubMed] [Google Scholar]
- 26.Cornish P. “Avoid the crush”: hazards of medications administration in patients with dysphagia or a feeding tube. CMAJ. 2005;172(7):871-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axelson DA, Perel JM, Birmaher B, et al. Sertraline pharmacokinetics and dynamics in adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41(9):1037-44. [DOI] [PubMed] [Google Scholar]
- 28.Navarro RP. Tablet splitting: much ado about nothing? J Manag Care Pharm. 2009;15(3):272-74. Available at: http://www.amcp.org/data/jmcp/272-274.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDevitt JT, Gurst AH, Chen Y. Accuracy of tablet splitting. Pharmacotherapy. 1998;18(1):193-97. [PubMed] [Google Scholar]
- 30.Miller DP, Furberg CD, Small RH, et al. Controlling prescription drug expenditures: a report of success. Am J Manag Care. 2007;13(8):473-80. [PubMed] [Google Scholar]